Abstract
Finch trichomonosis, caused by the protozoal parasite Trichomonas gallinae, was first recognized as an emerging infectious disease of British passerines in 2005. The first year of seasonal epidemic mortality occurred in 2006 with significant declines of greenfinch Carduelis chloris and chaffinch Fringilla coelebs populations. Here, we demonstrate that large-scale mortality, principally of greenfinch, continued in subsequent years, 2007–2009, with a shifting geographical distribution across the British Isles over time. Consequent to the emergence of finch trichomonosis, the breeding greenfinch population in Great Britain has declined from ca 4.3 million to ca 2.8 million birds and the maximum mean number of greenfinches (a proxy for flock size) visiting gardens has declined by 50 per cent. The annual rate of decline of the breeding greenfinch population within England has exceeded 7 per cent since the initial epidemic. Although initially chaffinch populations were regionally diminished by the disease, this has not continued. Retrospective analyses of disease surveillance data showed a rapid, widespread emergence of finch trichomonosis across Great Britain in 2005 and we hypothesize that the disease emerged by T. gallinae jumping from columbiforms to passeriforms. Further investigation is required to determine the continuing impact of finch trichomonosis and to develop our understanding of how protozoal diseases jump host species.
Keywords: Trichomonas gallinae, trichomonosis, greenfinch, Carduelis chloris, emerging infectious disease, population decline
1. Introduction
Emerging infectious diseases (EIDs) are increasingly recognized as threats to public health, livestock production and wildlife conservation [1,2]. Such disease emergence usually occurs via the introduction of a novel pathogen into a naive population, resulting in increased morbidity and mortality [1,3]. In wild birds, novel bacterial and viral pathogens have been shown to cause large-scale disease outbreaks [4–6], but few studies have evaluated the longer term effects of disease emergence, such as the extent of population declines [5] and recovery [7], or the effects on species ecology and behaviour [8,9].
Historically, trichomonosis, caused by infection with the protozoal parasite Trichomonas gallinae [10], is a well-known disease of pigeons and doves, and of raptors that predate infected columbiform prey. Trichomonas gallinae infects the upper alimentary tract and causes the lesions of necrotic pharyngitis or ingluvitis that can interfere with the bird's ability to swallow [10]. The parasite is transmitted either directly between birds, when conspecifics feed one another during courtship or when feeding young, or indirectly, through shared food and water sources [10]. The parasite is labile and does not remain viable for long in the environment external to the host [10].
Trichomonosis of British passerines was first recognized in 2005 [11], when sick and dead greenfinches and chaffinches were found with the disease. This EID was subsequently termed ‘finch trichomonosis’ [12]. A single, clonal strain of T. gallinae was responsible for the epidemic passerine mortality that subsequently occurred [13]. The first occurrence of epidemic mortality due to finch trichomonosis occurred in the late summer of 2006, leading to a significant decline of around 35 per cent of the breeding greenfinch Carduelis chloris population and a 20 per cent reduction of the breeding chaffinch Fringilla coelebs population in the region of England with the greatest disease incidence [12]. Both greenfinch and chaffinch occur widely throughout Britain and commonly visit garden feeders, especially during the autumn and winter months [14]; it was estimated that approximately 500 000 greenfinches alone died from this EID in the first year of the epidemic [12].
Epidemic mortality continued in 2007, affecting a similar range of species with the same pronounced seasonal peak (August/September) in mortality as in the previous year [15]. Both garden bird mortality and greenfinch ring recovery data confirmed expansion of the geographical distribution of the finch epidemic from the central and western counties of England and Wales in 2006 to include the eastern counties of England in 2007 [15]. Seasonal (late summer) epidemic mortality due to finch trichomonosis has affected the British wild passerine population each year since its emergence. Although there are many well-known wildlife EIDs, there are few examples where the spread and impact of a disease on free-ranging populations at regional and national scales have been documented from the time of emergence. Here, we interrogate available data in an attempt to determine when and where finch trichomonosis might have initially emerged and we present evidence of substantial continued finch population declines beyond the original outbreak in 2006 as a consequence of the persistence and spread of finch trichomonosis within the British Isles from 2007 to 2009.
2. Methods
(a). Surveillance for finch trichomonosis
Reports of garden bird morbidity and mortality across Great Britain were collected since 2005 using two independent, complementary national surveillance schemes that comprised the Garden Bird Health Initiative (GBHI), as described by Robinson et al. [12]. Briefly, opportunistic reports were received from the public via the Royal Society for the Protection of Birds (RSPB) Wildlife Enquiries Unit, or directly from each of four participating veterinary diagnostic laboratories. Systematic surveillance was achieved through a network of ca 750 participants in the British Trust for Ornithology's (BTO) Garden BirdWatch (GBW) scheme [14]. Each participant reported the numbers and species of dead birds observed in their garden on a weekly basis over the period 2006–2008; in addition, the numbers and species of sick birds seen in each participant's garden were reported in both 2007 and 2008. Importantly, if no sick or dead birds were found, these negative reports were also submitted through this systematic weekly recording.
Following Robinson et al. [12], a finch trichomonosis incident was defined as occurring within the period 1 April to 30 September and if: (i) two or more dead finches (greenfinch or chaffinch) were found at the same site; (ii) one or more sick finch(es) (greenfinch or chaffinch) with typical signs of disease (lethargy, malaise, difficulty swallowing, wet feathers around the beak) were observed; or (iii) if trichomonosis was confirmed on post-mortem examination (PME) in a greenfinch or chaffinch. The period from 1 April to 30 September was selected to minimize the likelihood of confounding trichomonosis data with mortality due to salmonellosis, outbreaks of which occur almost exclusively during the winter months and which, like finch trichomonosis, result in non-specific signs of malaise in finches [12,16]. Finch trichomonosis incidents, however, do occur to some degree throughout the calendar year; application of these criteria, therefore, underestimates the frequency of this disease. This definition was also used to gauge the scale of the epidemic each year from the percentage of opportunistic garden bird mortality reports that was due to finch trichomonosis.
Bird carcasses were submitted for PME from a subset of mortality reports from each surveillance scheme. PMEs and follow-up diagnostic tests were performed at one of a network of collaborating veterinary laboratories following a standardized protocol, as described by Robinson et al. [12]. Briefly, the case definition of finch trichomonosis involved passerines with characteristic necrotic ingluvitis lesions in combination with either (i) the detection of motile trichomonads by direct light microscopic examination of lesions or following culture or (ii) positive amplification, using nested PCR, of part of the T. gallinae single subunit rRNA gene. Suspected, but not confirmed, finch trichomonosis cases were defined as passerines with necrotic ingluvitis lesions that were not positive for Trichomonas sp. using light microscopy or culture—the labile nature of the parasite makes detection difficult following the death of the host [17]—for which nested PCR results were not available, and which were negative on culture for Salmonella sp. (salmonellosis can cause similar necrotic ingluvitis lesions in birds, and the strain typically encountered, S. Typhimurium, is environmentally resistant and comparatively simple to culture [16]).
The percentage of passerine trichomonosis cases that were diagnosed in each of greenfinch, chaffinch and dunnock Prunella modularis was determined. Greenfinch and chaffinch were the species most commonly affected by the initial epidemic in 2006 [12], whereas the dunnock, a bird of similar size, occurrence and habitat use, was relatively unaffected and was used by Robinson et al. [12] to show that the 2006 finch declines were unlikely to be due to other factors, such as extreme weather. To evaluate whether the finch trichomonosis incident criteria, based on non-specific signs of malaise in finches, used by Robinson et al. [12] remained appropriate for the current study, all greenfinch and chaffinch PMEs where infectious disease was diagnosed as the cause of death were reviewed and the percentage due to trichomonosis was determined. In addition, the percentage of passerines diagnosed at PME with trichomonosis that died during the period from 1 July to 30 September was determined for each calendar year, 2006–2009, to assess whether the late summer seasonality of the epidemic observed in 2006 continued.
To give a measure of the annual relative frequency of finch trichomonosis, we expressed the total number of trichomonosis incidents reported opportunistically in each country per year (from 1 April to 30 September) per thousand households according to the 2001 UK National Census [18]. We used opportunistically collected reports to determine the relative frequency, as this surveillance method provided a much larger dataset with greater spatial coverage than the systematic surveillance participant network. The opportunistic reports were validated by spatial and temporal comparison with the percentage of systematic surveillance scheme participants, who reported cases of finch trichomonosis according to our finch trichomonosis incident rule. The systematic scheme included only Great Britain (England, Scotland and Wales). For Northern Ireland and the Republic of Ireland, only a limited number of opportunistic reports of garden bird mortality were available and a much lower intensity of disease surveillance occurred in this region.
To investigate the initial emergence of finch trichomonosis, we interrogated available reports of GBHI passerine morbidity and mortality from 2005 that were consistent with the disease. Also, we reviewed ad hoc reports collected by the RSPB's Wildlife Enquiries Unit since 2001 and ad hoc wild passerine PME results performed at the Institute of Zoology (IoZ) since 1990, the Scottish Agricultural College (SAC) since 1995 and the Wildlife Veterinary Investigation Centre (WVIC) since 2001 to determine whether earlier finch trichomonosis incidents might have occurred.
(b). Impact of finch trichomonosis on wild bird populations
Changes in the relative abundances of selected species of passerines were examined using three independent datasets. The first was compiled from all (ca 10 000) GBW participants, who annually recorded the number and species of wild birds visiting their gardens on a weekly basis throughout the calendar year [14]. Using these data, we examined the reporting rates for greenfinch, chaffinch and dunnock to determine trends in the proportion of gardens visited by each species across Great Britain during the period 1995–2009.
Data from the BTO Garden Bird Feeding Survey (GBFS) were used to provide a measure of flock size within individual gardens. The GBFS participants (ca 275) have monitored birds visiting gardens where supplementary food is provided since the scheme began in 1970 [19]. Participants recorded the maximum number of each species, including greenfinch, chaffinch and dunnock, observed on a weekly basis for the six-month period October–March. As this dataset covers a longer time period (1970–2009) than the GBW, we used the GBFS data as a surrogate for flock size within garden habitats to compare trends for each of our study species against a longer term context.
To assess change in the wider breeding population of our study species, we used data from the BTO/RSPB/Joint Nature Conservation Committee (JNCC) Breeding Bird Survey (BBS), which has monitored the numbers of birds present throughout the United Kingdom during the breeding season across a sample of ca 3000 randomly selected 1 × 1 km squares since 1994 [20]. Similar data were obtained from the Republic of Ireland through the Countryside Bird Survey (CBS) [21], which commenced in 1998 and comprises ca 300 1 × 1 km squares per annum. The BBS and CBS use an identical line-transect-based methodology involving observers counting birds within 100 m of two parallel transects through each square on two visits during the breeding season. Because of the random nature of the survey design, transects are undertaken in all habitats rather than being restricted to gardens, as with the GBW scheme. An index of relative abundance based on a generalized linear Poisson model with categorical site and year fixed-effects is produced annually [22]; we identified significant changes as presented in the annual reports of the schemes. We obtained BBS/CBS indices for each of the three constituent countries of Great Britain and for the island of Ireland (by combining indices for the Republic of Ireland and Northern Ireland weighted by geographical area) to measure the relative annual change in breeding populations of greenfinch, chaffinch and dunnock, 2006–2010.
Since the finch trichomonosis epidemic in 2006 led to declines in regional greenfinch and chaffinch populations, we retrospectively examined GBW and BBS/CBS data for declines in either, or both, of these species in the previous year. We then compared any data on finch declines with our retrospective reports of morbidity/mortality consistent with finch trichomonosis in order to look for evidence of spatial or temporal associations.
3. Results
(a). Surveillance for finch trichomonosis
Trichomonosis was most frequently diagnosed in greenfinches (mean 65%, range 58–73% of passerine carcass submissions) on PME, and chaffinches (mean 23%, range 18–30%), in each of the years 2006–2009 (table 1). By contrast, the disease was seen very infrequently in dunnocks (table 1). Trichomonosis was the most frequently diagnosed infectious disease in finches at PME, accounting for a mean of 89% (range 80–100%) of finch cases each year. The seasonal peak of mortality due to finch trichomonosis described by Robinson et al. [12] in 2006, when 55 per cent of the finches diagnosed as having trichomonosis on PME died during the period from 1 July to 30 September, was also seen in subsequent years (mean 53%, range 49–56%), and when the opportunistic (mean 55%, range 48–60%) and systematic (mean 48%, range 42–56%) PME submissions were considered independently.
Table 1.
Number (and %) of passerine trichomonosis cases diagnosed in each study species at post-mortem examination for calendar years 2006–2009.
| species |
total number of passerine trichomonosis cases | |||
|---|---|---|---|---|
| year | greenfinch | chaffinch | dunnock | |
| 2006 | 90 (58.1%) | 47 (30.3%) | 1 (0.6%) | 155 |
| 2007 | 107 (72.8%) | 27 (18.4%) | 2 (1.4%) | 147 |
| 2008 | 45 (67.2%) | 13 (19.4%) | 0 (0%) | 67 |
| 2009 | 33 (62.3%) | 12 (22.6%) | 0 (0%) | 53 |
Of the reports of wild bird mortality (of all species) received by the opportunistic surveillance scheme, and according to our definition of a finch trichomonosis incident, finch trichomonosis accounted for a large proportion (mean 62%, range 56–66%) in each calendar year. Finch trichomonosis incidents involved morbidity/mortality of greenfinches alone in 65–66% of incidents, greenfinches and chaffinches in 27–30% of incidents and chaffinches alone in 5–7% of incidents across the 4-year period 2006–2009. There was no significant difference in this pattern across the years 2006–2009 (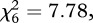 p = 0.25). For the finch trichomonosis incidents, concurrent dunnock morbidity or mortality was reported in less than 1 per cent of incidents (37 of 6024).
p = 0.25). For the finch trichomonosis incidents, concurrent dunnock morbidity or mortality was reported in less than 1 per cent of incidents (37 of 6024).
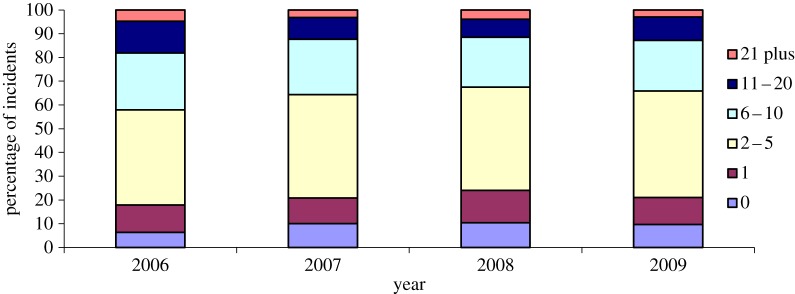
Large-scale mortality was reported from a considerable percentage of finch trichomonosis incidents; six or more dead finches were found at 32–42% of sites across the 4-year period 2006–2009 (figure 1). There were fewer reports with only sick, but no dead, greenfinches or chaffinches (6% versus 10%) and more reports with 11 or more dead greenfinches or chaffinches (18% versus 12%) in 2006 compared with subsequent years (2007–2009, 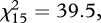 p < 0.001), but there was no significant variation in the number of dead birds reported per incident from 2007 to 2009 (
p < 0.001), but there was no significant variation in the number of dead birds reported per incident from 2007 to 2009 (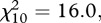 p = 0.10).
p = 0.10).
Figure 1.
The number of dead greenfinches and/or chaffinches reported per finch trichomonosis incident.
In the period from 2001 to 2004, the RSPB logged 710 ad hoc reports of garden bird mortality and morbidity where the history indicated that infectious disease might be involved (RSPB, unpublished data). Of these, 49 met our finch trichomonosis incident criteria (mean 12, range 4–20 per annum); 24 incidents had onset during the period from 1 April to 31 May and 25 from 1 June to 30 September, across years. For each year, these incidents were reported from a wide region from multiple counties with no evidence of spatial or temporal clustering. Greenfinches and chaffinches were reported with non-specific signs of malaise (i.e. lethargy and fluffed-up plumage), but there were no signs more indicative of trichomonosis described in any of these reports. Concurrent columbiform mortality was reported from one of these incidents in May 2004, with a single collared dove (Streptopelia decaocto) being seen sick at a site where seven dead greenfinches were also found.
Seven hundred and nine wild passerines (including 277 Fringillidae) were examined post-mortem at the IoZ between 1 January 1990 and 31 March 2005. Five incidents were identified where upper alimentary tract lesions were noted in greenfinches or chaffinches and from which Salmonella sp. was not isolated on microbiological examination and no other cause was determined (table 2). No examinations for T. gallinae were performed, so it is possible that these were unconfirmed finch trichomonosis cases. No concurrent morbidity or mortality of columbiform species was reported at any of these five sites. An additional incident was identified from February 1993, in which three greenfinches had been found dead at a site in Oxfordshire and where multiple collared doves had been observed with signs of difficulty in swallowing and neck swelling (signs consistent with trichomonosis). Necrotic ingluvitis, but no other significant gross lesions, was observed in all three greenfinches examined. Microbiological examination of oesophageal lesions failed to detect Salmonella sp., but flagellated protozoa morphologically characteristic of trichomonads were observed on light microscopic examination of the lesions in one of the birds. Trichomonosis was considered to be the cause of death of these finches.
Table 2.
Historical passerine mortality incidents for which the post-mortem examination results fitted our definition of ‘finch trichomonosis’ or of ‘suspected finch trichomonosis’ (see text).
| date | location | species affected, no. found dead (no. seen sick) | number examined post-mortem | summary of findings (no. birds in which these were detected) |
|---|---|---|---|---|
| February 1993 | Oxfordshire | greenfinch, multiple (0) | 3 | crop lesions (3) |
| collared dove, 0 (multiple) | flagellated protozoa observed on wet preparation (1) | |||
| December 1995 | Suffolk | greenfinch, 4 (multiple) | 1 | diffuse crop wall thickening, predator injuries |
| January 1996 | Suffolk | greenfinch, 2 (unknown) | 2 | diffuse crop wall thickening (2) |
| June 1996 | Lincolnshire | greenfinch, 20 (unknown) chaffinch, 1 (unknown) | 3 | pale cream-coloured coalescing foci widespread in crop mucosa(1) |
| crop wall thickening (2) | ||||
| May 2000 | Gloucestershire | greenfinch, 1 (unknown) | 1 | multiple firm caseous lesions (1–3 mm diameter) in crop and pharyngeal mucosa |
| September 2002 | Wiltshire | greenfinch, 1 (unknown)chaffinch, 1 (unknown) | 2 | crop wall thickened with longitudinal dull yellow-coloured lesions (1) |
| large firm caseous white/cream-coloured lesion obstructing crop (1) |
Of 110 wild passerines (including 57 Fringillidae) examined post-mortem at the WVIC between November 2001 and 31 March 2005, one incident met our finch trichomonosis criteria. In October 2004, an immature greenfinch from Cornwall was examined with necrotic ingluvitis lesions that were negative for Salmonella sp. on culture; no samples were retained for further diagnostics. The householder reported greenfinches dying in the garden over the two previous months. Of 723 wild passerines (including 374 Fringillidae) examined post-mortem at the SAC between 1 January 1995 and 31 March 2005, no cases of finch trichomonosis were either suspected or confirmed.
In 2005, reports of garden bird (predominantly greenfinch) mortality incidents consistent with finch trichomonosis first occurred in April. Within that same month, such reports were obtained from a wide geographical region, including Scotland, Wales and the English counties of Essex, West Sussex and Shropshire (figure 2). Additional reports consistent with finch trichomonosis continued to be obtained in small numbers across Great Britain throughout the rest of 2005.
Figure 2.
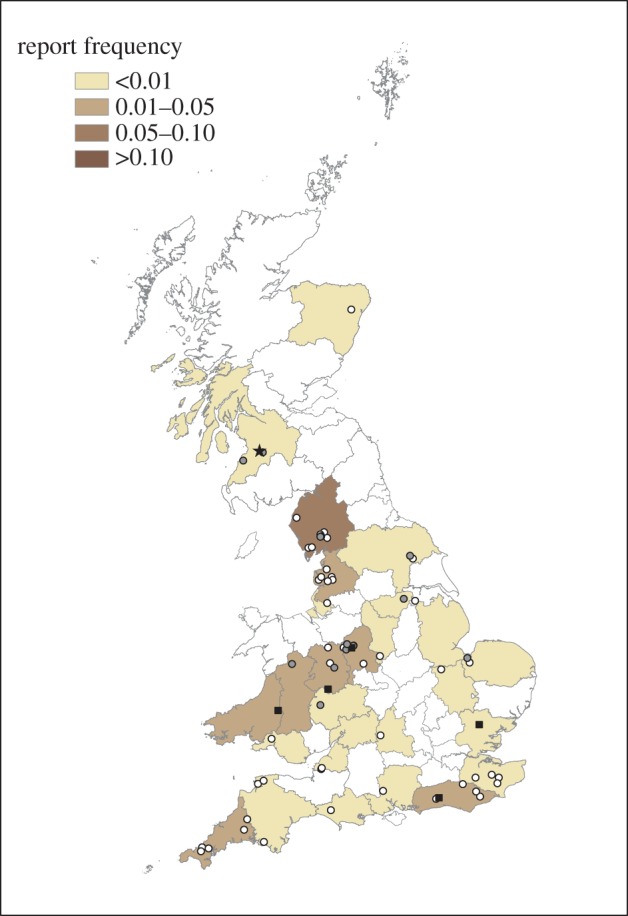
Distribution of finch trichomonosis incidents in Great Britain, 2005, obtained from opportunistic surveillance reports. The shading by county indicates relative frequency of finch trichomonosis (incidents per thousand households) during the period from 1 April to 30 September. The index case is shown with a black star. Incidents with onset in April and May (black squares), June and July (grey circles), August and September (white circles) are shown.
The index case of finch trichomonosis occurred in a chaffinch in Ayrshire, Scotland, April 2005 [12]. Finch trichomonosis was diagnosed on a PME of finches from a further five sites in three counties in southern Scotland and from across much of England (17 counties, 45 sites) in 2005: the earliest cases were from Hampshire in June 2005, Cumbria and Shropshire in July 2005 and West Sussex in August 2005. Finch trichomonosis was first confirmed in Wales in a greenfinch in Glamorgan, October 2005, and was diagnosed in finches submitted from a further two Welsh counties (two sites) in 2005. The disease was not diagnosed on PME in Northern Ireland until 2008. The authors are not aware of any PME surveillance of passerines in the Republic of Ireland.
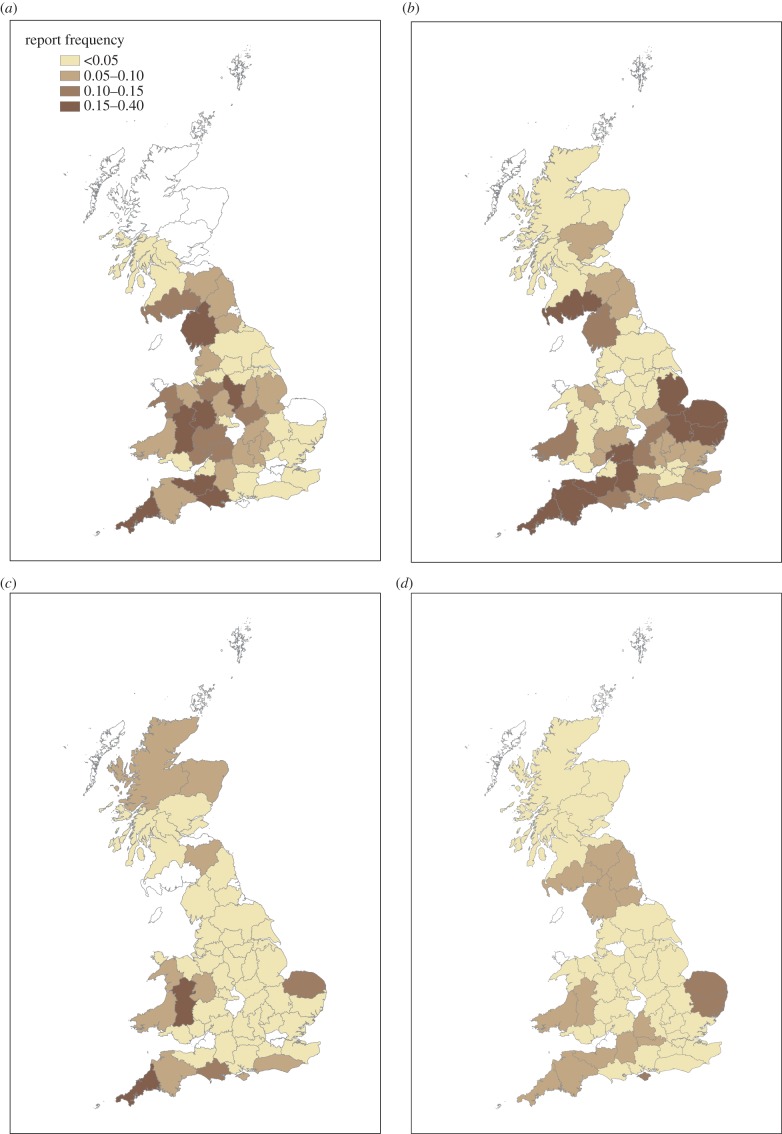
In subsequent years (2006–2009), when epidemic mortality occurred, the percentage of opportunistically obtained reports that met our finch trichomonosis incident criteria remained relatively stable in England (mean 62%, range 57–67%) and in Wales (mean 66%, range 63–70%), but increased in Scotland from 28% of reports in 2006 to 52% in 2007, 66% in 2008 and to 72% in 2009. There was variation in the distribution of the epidemic across Great Britain over this period. For the opportunistic reports, a large number of trichomonosis incidents were reported in England (mean 808, range 450–1335) in each of these years, although the distribution of finch trichomonosis reports within this country changed over time (figure 3). Most reports were from the central and western counties of England in 2006, but there was a pronounced expansion towards eastern England in 2007. In subsequent years, reports tended to be either from western or eastern England, with a lower apparent incidence in the central region. In Wales, the peak number of reports due to finch trichomonosis was recorded in 2006. In Scotland, the frequency of reports increased in 2007 with further northward spread in 2008 (figure 3). Throughout this period (2006–2009), small numbers of opportunistic reports were obtained from Northern Ireland and the Republic of Ireland: two in 2006, 11 in 2007, 29 in 2008 and 10 in 2009. Additionally, ad hoc reports of finch morbidity or mortality consistent with our finch trichomonosis incident criteria were received from across the Republic of Ireland by BirdWatch Ireland; these reports were first received in autumn 2007, continued throughout 2008 and were notably reduced in frequency in 2009.
Figure 3.
Distribution of finch trichomonosis incidents in Great Britain, 2006–2009, obtained on opportunistic surveillance reports. The shading by county indicates relative frequency of finch trichomonosis (incidents per thousand households) during the period from 1 April to 30 September for each year, (a) 2006, (b) 2007, (c) 2008 and (d) 2009.
Disease incidences from the systematic surveillance reports broadly support the results obtained from the opportunistic data, with a stable percentage of monitored sites reporting finch trichomonosis in England in 2006–2008, and an increasing percentage of monitored sites reporting finch trichomonosis in Scotland over the same time period (table 3). In Wales, however, there was an increase in the percentage of monitored sites reporting finch trichomonosis in 2008 compared with previous years (table 3).
Table 3.
Percentage of systematically monitored sites that reported finch trichomonosis incidents by country, 2006–2008.
| country |
||||
|---|---|---|---|---|
| year | dataset for incident definition | England | Wales | Scotland |
| 2006a | dead only | 5.8% (32/550) | 9.0% (6/67) | 1.8% (1/57) |
| 2007a | dead only | 6.7% (34/508) | 9.1% (6/66) | 12.3% (7/57) |
| sick or dead | 15.2% (77/508) | 12.1% (8/66) | 24.6% (14/57) | |
| 2008b | dead only | 5.6% (18/324) | 15.6% (5/32) | 32.6% (15/46) |
| sick or dead | 13.9% (45/324) | 28.1% (9/32) | 41.3% (19/46) | |
aSurveillance dataset available for quarters 2 and 3 (April–September inclusive).
bSurveillance dataset available for quarter 3 only (July–September inclusive).
(b). Impact of finch trichomonosis on wild bird populations
The GBW dataset shows continued year-on-year declines in the use of gardens by greenfinches across the period 2006–2009 (figure 4a). By contrast, for chaffinch (the second most commonly affected species), the reporting rates for 2006–2009 remained within the values recorded throughout the previous decade, with no evidence of a sustained decline (figure 4b). Similarly, there was no evidence for a decline in the reporting rate of the dunnock, our control species, during this 4-year period (figure 4c).
Figure 4.
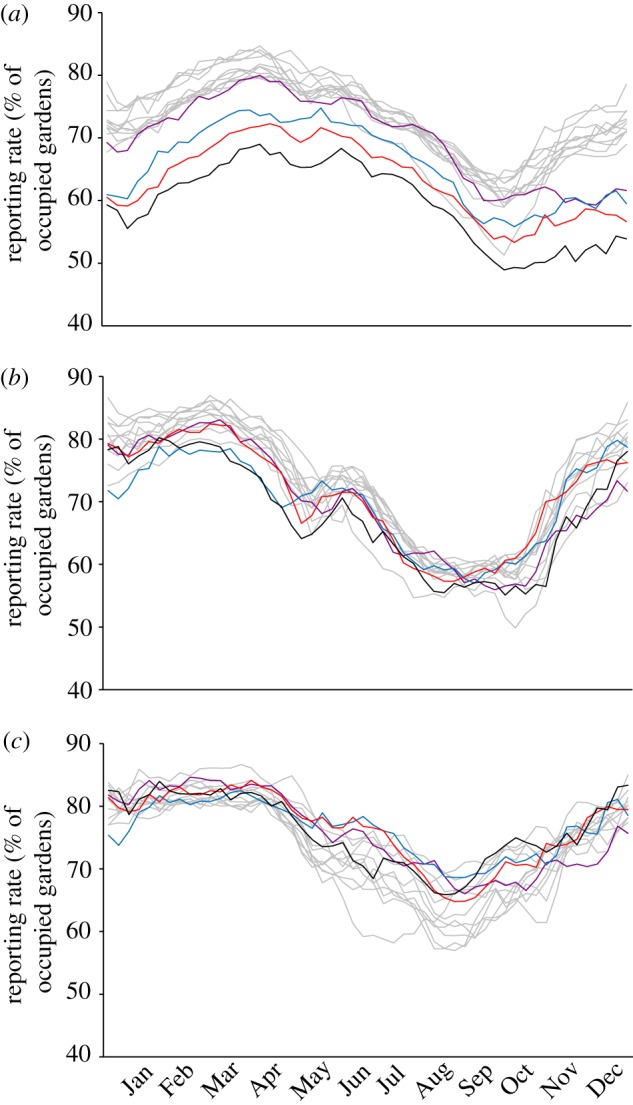
Reporting rate for (a) greenfinch, (b) chaffinch and (c) dunnock in all GBW gardens for the years 1996–2005 (grey lines), 2006 (purple), 2007 (blue), 2008 (red) and 2009 (black).
The GBFS data showed a marked year-on-year decline in the mean maximum count of greenfinches in gardens since 2005 (figure 5a), whereas the mean maximum number of chaffinches (ca 6) and dunnocks (ca 1.5) recorded was unchanged (figure 5b,c, respectively). The mean maximum count for the greenfinch has repeated seasonal variation, with peak values during the winter and the lowest values during the summer months. For the chaffinch, there was a drop in the peak count from 2005 to 2008, although this was not marked in comparison with the extent of variation amongst recent years, and the peak count showed evidence of recovery in 2009 (figure 5b). For the dunnock, the mean maximum count has remained stable since the emergence of finch trichomonosis, 2005–2009 (figure 5c).
Figure 5.
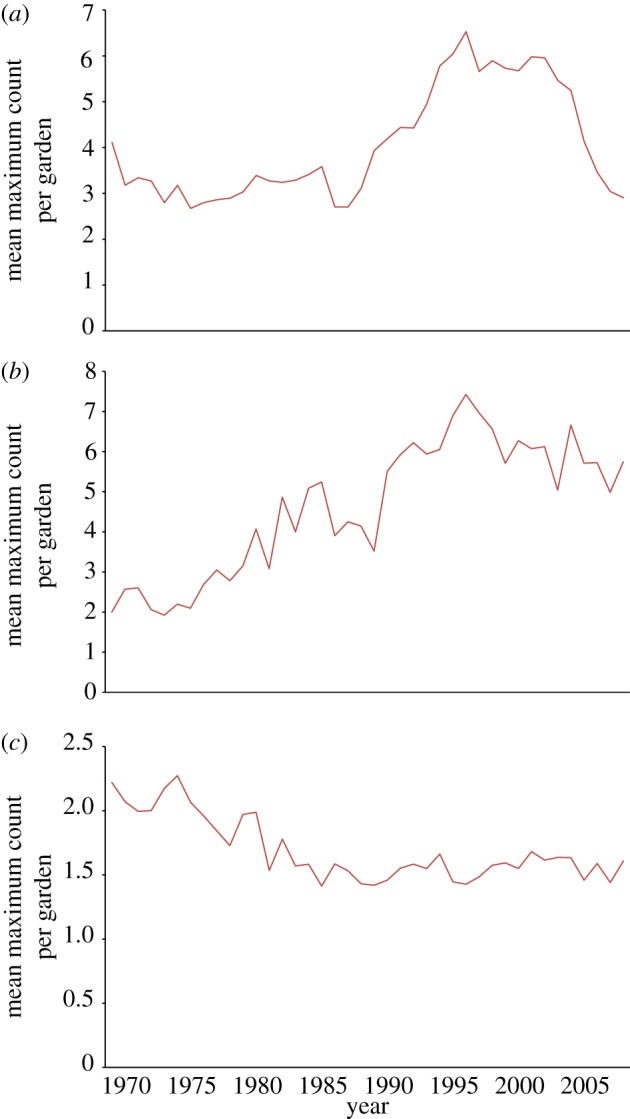
GBFS mean maximum count per garden for UK for (a) greenfinch, (b) chaffinch and (c) dunnock.
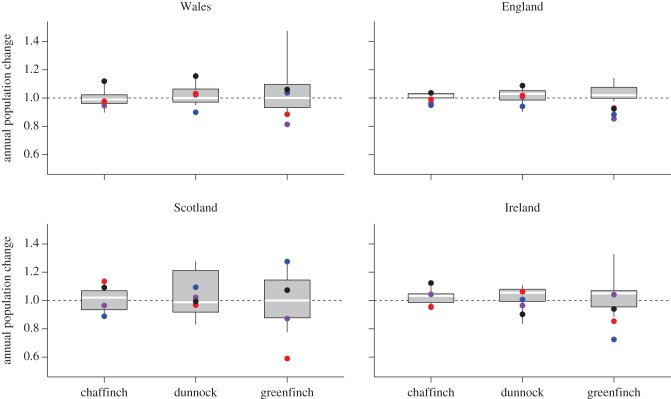
The BBS and CBS data indicate variation in the timing and extent of the greenfinch population decline across the British Isles (BTO & BirdWatch Ireland, unpublished data). In England, continued significant year-on-year declines occurred following the initial trichomonosis epidemic in 2006; the population decline recorded was 14.7 per cent in 2006/2007, 11.7 per cent in 2007/2008, 7.1 per cent in 2008/2009 and 7.6 per cent in 2009/2010 (figure 6). In Wales, a marked greenfinch decline was observed following the first year of epidemic mortality in 2006 (18.7% in 2006/2007) only, although this was non-significant; no further decline was observed in subsequent years. In Ireland, there was no decline in 2006/2007, but a significant decline in greenfinch numbers occurred following mortality in 2007 (27.5% in 2007/2008) with further, non-significant, decline in 2008 (14.6% in 2008/2009). Finally, in Scotland, a highly significant and marked decline occurred following the epidemic mortality of 2008 alone (41% in 2008/2009). There was little evidence for chaffinch population declines that could be directly attributed to finch trichomonosis; the annual rate of population change remained within the range for the pre-finch trichomonosis period, 1994–2006. Similarly, there was no evidence of any dunnock population decline that might be attributable to the disease, although there was a non-significant reduction of 10 per cent in Wales for 2007/2008 (figure 6). These data relate to an overall national decline in the greenfinch breeding population in the order of 1.5 million birds (ca 35%; figure 7).
Figure 6.
Annual rate of population change as measured by the BBS by country. Boxes show mean and quartiles of annual changes and whiskers minimum and maximum annual change observed in the period 1994–2006; points, the population change recorded in 2006/2007 (purple), 2007/2008 (blue), 2008/2009 (red), 2009/2010 (black). Dotted line indicates no population change.
Figure 7.
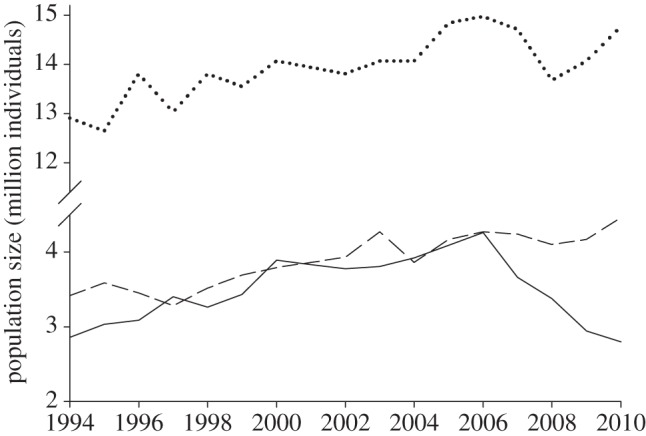
Annual population size of chaffinch (dotted line), dunnock (dashed line) and greenfinch (solid line) in the United Kingdom 1994–2010. The population size in 2000 for each species was taken from Newson et al. [20] and scaled by the annual BBS index in each year [22].
4. Discussion
Our surveillance and PME data identified annually recurring seasonal (late summer) epidemic mortality of passerines (predominantly greenfinches and chaffinches) in Britain due to finch trichomonosis since this was first observed in 2006 [12]. By using independent datasets, we identified substantial declines not only in the number of gardens that greenfinches visit (GBW data) but also in the number of individual greenfinches visiting each garden (GBFS data), declines that carry through into the wider breeding population (BBS/CBS data). Large numbers of dead finches were found in a high proportion of the finch trichomonosis incidents. Because we would predict considerable under-reporting of passerine carcasses due to their small body size [23,24], these data indicate large-scale site mortality; consequently, attributing the observed BBS/CBS greenfinch declines to epidemic mortality seems entirely plausible.
While mortality continued to occur in the chaffinch population beyond that reported in 2006 [12], this was at a much lower level than in the greenfinch and was not associated with detectable population declines, either at the level of individual countries or across the British Isles as a whole. Populations of dunnock, a species in which finch trichomonosis was rarely detected, were stable throughout the study period. These results are consistent with the greenfinch population decline being a direct consequence of finch trichomonosis and not due to other possible factors that might affect the frequency with which birds visit gardens, such as severe weather or changes in natural food abundance.
It is not clear why the apparent incidence of the disease peaked in the late summer/early autumn of each year. Although overall greenfinch and chaffinch population sizes are at their highest at this time of year, population densities are comparatively low (figure 4) as the birds disperse to feed on natural foods in the late summer/early autumn. The seasonal increase in disease incidence, therefore, is unlikely to be a simple function of population density. It might, however, be due to an influx of naive juvenile birds in the population following the relatively synchronous breeding season.
The greenfinch is a highly gregarious species, exhibiting flock-feeding behaviour, particularly during the late autumn and early winter months [25]. The effect that the decline in flock size may have on the species ecology (e.g. social behaviour, feeding, predator avoidance, breeding) is currently unknown. Similarly, the impact of reduced host population density on the epidemiology of finch trichomonosis is unclear. Further research is required to determine the consequences of the observed declines in flock size and population density on finch behaviour and parasite transmission dynamics.
We first identified trichomonosis in wild passerines in 2005. Our retrospective analysis of passerine morbidity and/or mortality incident reports, however, identified a small number that met our criteria for suspected finch trichomonosis in each year, 2001–2004. Across incidents, only non-specific signs of ill health were reported in the affected finches. No PME findings were available from these incidents, and it is plausible that they may have been due to other infectious diseases. For example, colibacillosis has been reported as a cause of multiple mortality and morbidity incidents in finch species from Scotland, with 94 per cent of confirmed cases in the months from March to May during the period 1997–2000 [26]. This disease, caused by Escherichia albertii (Escherichia coli serotype O86), has also been found in finches in England (B. Lawson, K. M. Colvile, A. A. Cunningham 2011, unpublished data) and it seems likely that colibacillosis may account for a number of the historical ‘possible finch trichomonosis’ incidents. In addition, while salmonellosis incidence peaks during the winter months, six of 118 incidents confirmed in Britain from 1993 to 2003 inclusive occurred during the period from 1 April to 30 September [16]. It is possible, therefore, that some of these incidents were due to salmonellosis.
Our retrospective analysis of post-mortem reports detected a greenfinch mortality incident in 1993 that was almost certainly due to trichomonosis. It is possible that this incident represented an isolated spillover event of T. gallinae from Columbiformes to Passeriformes that did not perpetuate within the new host order. The species of parasite involved was not definitively identified, however, and no specimens exist to investigate this further. Other incidents of possible finch trichomonosis were also identified retrospectively on PME, based on the presence of lesions consistent with finch trichomonosis and an inability to culture Salmonella sp., and for which the results would be consistent with our definition of ‘suspected trichomonosis’. None of these incidents involved species other than the greenfinch and chaffinch. One such incident occurred in Cornwall in October 2004 and it is tempting to speculate that this might have been a precursor to the current finch trichomonosis epidemic; however, because samples were not available for further diagnostic testing, it is not possible to confirm whether or not this was the case. This incident may be significant, however, as the author making the observation had examined ca 400 wild birds from SW England between 1979 and 2001, and in that period had not previously seen necrotic ingluvitis in a finch from which Salmonella sp. could not be isolated (V. R. Simpson, unpublished data).
It is noteworthy that we found no evidence of a single spatial cluster, or subsequent geographical radiation from a focus of the disease within the British Isles in 2005, as has occurred following some EID events, e.g. mycoplasmal conjunctivitis [27]; West Nile virus [28]; white-nose syndrome of bats [29]. There was, however, an indication of spread within England and Wales (see also Lawson et al. [15]), with apparent subsequent spread to Ireland and within Scotland.
The systematic and opportunistic surveillance data demonstrate spatial and temporal variation in the regions of peak finch trichomonosis mortality across the period 2006–2008, which is mirrored by the staggered onset of greenfinch population declines in countries within the British Isles. The level of finch trichomonosis mortality reports was sustained within England from 2006 to 2009, with declines in the breeding population detected in each year since 2007, whereas the opportunistic disease reports peaked in Wales during 2006 and no breeding population declines were detected subsequent to 2007. The systematic surveillance data for Wales, however, showed finch trichomonosis occurring at an increased percentage of monitored sites in 2008. This might be a reflection of a lower observer effort in 2008 compared with previous years owing to a restricted period of data collection and a smaller number of participants (table 3). Large numbers of reports of finch trichomonosis incidents were not received from Scotland until 2007, with additional spread in 2008. Notable declines of the greenfinch breeding population were not detected in Scotland until 2009.
While initial reports of finch trichomonosis had been received in Great Britain in 2005, finch trichomonosis consistent mortality was not widely reported in Ireland until 2007, suggesting spread from the British mainland to this island. This delay might have been due to a lower level of surveillance in Ireland, but the BBS and CBS data, showing marked declines in the Irish greenfinch population in 2007/2008 and in 2008/2009, provide additional support for this ‘later spread’ hypothesis. We have recently documented the apparent spread of finch trichomonosis from Great Britain to continental Europe [15], which also indicates that the initial emergence of the disease occurred on the British mainland.
Although most columbiform and finch species do not generally undertake long-distance migratory movements, many species show some seasonal dispersive movement within the British Isles [30]. In particular, many species move south and west in the autumn to avoid periods of regionally low temperature and reduced food availability; in the spring, they reverse these movements. Thus, a late autumn or a winter outbreak that infected birds sub-lethally could be spread the following spring by bird movements. It is likely that movements such as these acted as the mechanism by which the disease spread within the British Isles and facilitated spread into Europe [15].
The reason why finch trichomonosis emerged as a widespread cause of mortality in 2005, with subsequent epidemic spread, is unknown, but might relate to host, parasite or environmental factors. Previously, we hypothesized sympatric columbiforms to be the most likely source of the T. gallinae parasite in British finches [12]. Although the frequency of visits to gardens by feral pigeons (Columba livia) and collared doves (Streptopelia decaocto) has remained more or less constant over the past 15 years (BTO, unpublished data), the presence of wood pigeons (Columba palumbus) in gardens has increased markedly in recent years (http://blx1.bto.org/gbw-dailyresults/results/gbwr270-20.html). Concurrently, both the UK populations of chaffinch and greenfinch and the presence of these species in gardens also increased ([22]; figures 5 and 7). Taken together, these factors might have increased opportunities for parasite spillover to new host species, particularly at shared feeding stations, and for an increased likelihood of sustained transmission amongst finches.
The historic, 1993, single incident of apparent finch trichomonosis that was concurrent and sympatric with an outbreak of likely trichomonosis in doves supports the hypothesis that columbiforms were the source of the parasite. This incident, and our findings of other possible historical finch trichomonosis incidents, indicates that isolated spillover events might have happened in the past. If so, these outbreaks appear to have been localized and to have quickly died out without propagation to the epidemic proportions seen in recent years. Sequence data and platform-based multi-locus typing tools have shown that a single clonal strain of T. gallinae is the causative agent of the finch trichomonosis epidemic [13]. Although this is most consistent with a single spill-over event, further work is required to determine the extent of parasite diversity within British columbiform populations and to ascertain if there is any evidence that genetic change in the parasite increased its transmissibility/virulence to novel host species.
The UK breeding greenfinch population has declined by ca 1.5 million birds since the onset of seasonal epidemic mortality caused by finch trichomonosis; our data strongly support the hypothesis that this decline can be attributed to finch trichomonosis. Indeed, the overall mortality due to this disease is likely to far exceed this figure. Each breeding pair typically rears four or five young birds in a season [22], many of which may also have succumbed to the disease before being recruited into the breeding population. The observed population decline in greenfinches directly followed a 20-year period (1985–2005) of sustained population increase of ca 60 per cent [22]. The number of greenfinches (and chaffinches) visiting gardens also increased through the 1980s and 1990s, overlapping with a period of wider population growth as revealed by breeding season surveys. It is noteworthy that finch trichomonosis appears to have negated this increase, with the number of greenfinches visiting gardens now similar to those seen in the 1970s (figure 5).
Within England, seasonal epidemic mortality has continued for 4 years with significant annual population declines. To date, there is no evidence of a year-on-year reduction in the annual rate of decline of the breeding greenfinch population within England that might indicate developing immunity, and the continued impact of the disease on this species should be monitored. Although a regional decline of ca 20 per cent was observed in the breeding chaffinch population in the region of England with the greatest disease incidence in 2006 [12], no significant annual declines were observed nationally for this species by either the BBS or the CBS during the period, 2006–2010. Both greenfinches and chaffinches share a flocking nature, use similar habitats and frequently occur together in mixed flocks [25]. It seems plausible, therefore, that each species has similar exposure rates to the parasite. There may be some innate differential susceptibility to infection with, or disease caused by, T. gallinae between these host species that could explain the differential impacts observed. Immunological studies are required to investigate the mechanisms of host resistance to T. gallinae.
In conclusion, we present evidence for a marked and continued decline of the breeding greenfinch population across the British Isles, with concomitant reduction in flock size, as a result of the EID finch trichomonosis. This further strengthens the conclusions of Robinson et al. [12]: the population impact of this EID is unprecedented in British wild birds subjected to infectious disease. It represents the first epidemic mortality caused by T. gallinae in a non-columbiform host, and the greatest estimate of wild bird deaths recorded due to this parasite worldwide [10]. Because finch trichomonosis has continued in the British Isles in 2010 and 2011 (K. M. Colvile, A. A. Cunningham, B. Lawson, T. W. Pennycott & V. R. Simpson, unpublished data) and has now spread to continental Europe [15], where both greenfinch and chaffinch populations are common and widespread [25], there is potential for further mortality to occur due to this EID. Appraisal of population census and disease surveillance data is required to determine whether population declines of passerines occur in other European countries subsequent to the spread of finch trichomonosis. Also, potential impacts of the disease on predatory or scavenging birds, due to possible increased frequency of transmission from passerine prey, should be evaluated.
In addition to the situation in Europe, trichomonosis emerged as a disease of passerines, principally of purple finch (Carpodacus purpureus) and American goldfinch (Carduelis tristis), in the Canadian Maritime Provinces in 2007 [31]. Research is required to determine whether the apparent contemporaneous emergence of finch trichomonosis on either side of the Atlantic is linked, perhaps through emergence of the same clonal strain of T. gallinae. Addressing the question of how this EID emerged may be key to identifying whether other protozoal diseases could similarly jump hosts, with potentially equally severe consequences.
Acknowledgements
We thank the BTO Garden BirdWatch participants and members of the public for participating in the surveillance schemes and for submitting cases for examination. We also thank Malcolm Bennett and Laura Hughes (University of Liverpool), Matthew Perkins, Shaheed Macgregor and Shinto John (IoZ), James Kirkwood (Universities Federation for Animal Welfare) for assistance with pathological investigations. We are grateful to Olivia Crowe and Niall Hatch (BirdWatch Ireland) for supplying the CBS data and information on trichomonosis reports in Ireland, respectively, to Tony Patterson (Agri-Food and Biosciences Institute) for providing finch trichomonosis PME data for Northern Ireland, and to Paul Holmes for providing 2005 PME data for Shropshire. The BBS is organized by the BTO, and jointly funded by the BTO, the JNCC (on behalf of the Council for Nature Conservation and the Countryside, the Countryside Council for Wales, Natural England and Scottish Natural Heritage) and the RSPB. The GBFS is organized and funded by the BTO and its supporters. The CBS is a joint scheme of the National Parks and Wildlife Service and BirdWatch Ireland. This is a publication of the Garden Bird Health Initiative that received financial support from the following organizations: Birdcare Standards Association; British Trust for Ornithology; British Veterinary Association Animal Welfare Foundation; CJ Wildbird Foods; Cranswick Pet Products; Defra; Gardman Ltd.; RCVS Trust Small Grants Programme Reference 000443; RSPB; John and Pamela Salter Trust (R16982); Tom Chambers Ltd., and the Universities Federation for Animal Welfare. A.A.C. is supported by a Royal Society Wolfson Research Merit Award.
References
- 1.Daszak P., Cunningham A. A., Hyatt A. D. 2000. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science 287, 443–449 10.1126/science.287.5452.443 (doi:10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- 2.Jones K. E., Patel N. G., Levy M. A., Storeygard A., Balk D., Gittleman J. L., Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990–993 10.1038/nature06536 (doi:10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams E. S., Yuill T., Artois M., Fischer J., Haigh S. A. 2002. Emerging infectious diseases in wildlife. Rev. Sci. Tech. off Int. Epiz. 21, 139–157 [DOI] [PubMed] [Google Scholar]
- 4.Hochachka W. M., Dhondt A. A. 2000. Density-dependent decline of host abundance resulting from a new infectious disease. Proc. Natl Acad. Sci. USA 97, 5303–5306 10.1073/pnas.080551197 (doi:10.1073/pnas.080551197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaDeau S. L., Kilpatrick A. M., Marra P. P. 2007. West Nile virus emergence and large-scale declines of North American bird populations. Nature 447, 710–713 10.1038/nature05829 (doi:10.1038/nature05829) [DOI] [PubMed] [Google Scholar]
- 6.Chvala S., Kolodziejek J., Nowotny N., Weissenböck H. 2004. Pathology and viral distribution in fatal Usutu virus infections of birds from the 2001 and 2002 outbreaks in Austria. J. Comp. Pathol. 131, 176–185 10.1016/j.jcpa.2004.03.004 (doi:10.1016/j.jcpa.2004.03.004) [DOI] [PubMed] [Google Scholar]
- 7.Chvala S., Bakonyi T., Bukovsky C., Meistera T., Bruggerd K., Rubel F., Nowotny N., Weissenböck H. 2007. Monitoring of Usutu virus activity and spread by using dead bird surveillance in Austria, 2003–2005. Vet. Microbiol. 122, 237–245 10.1016/j.vetmic.2007.01.029 (doi:10.1016/j.vetmic.2007.01.029) [DOI] [PubMed] [Google Scholar]
- 8.Altizer S., Hochachka W. M., Dhondt A. A. 2004. Seasonal dynamics of mycoplasmal conjunctivitis in eastern North American house finches. J. Anim. Ecol. 73, 309–322 10.1111/j.0021-8790.2004.00807.x (doi:10.1111/j.0021-8790.2004.00807.x) [DOI] [Google Scholar]
- 9.Dhondt A. A., et al. 2005. Dynamics of a novel pathogen in an avian host: mycoplasmal conjunctivitis in house finches. Acta Tropica 94, 77–93 10.1016/j.actatropica.2005.01.009 (doi:10.1016/j.actatropica.2005.01.009) [DOI] [PubMed] [Google Scholar]
- 10.Forrester D. J., Foster G. W. 2008. Trichomonosis In Parasitic diseases of wild birds (eds Atkinson C. T., Thomas N. J., Hunter D. B.), pp. 120–153 Ames, Iowa: Wiley-Blackwell [Google Scholar]
- 11.Pennycott T. W., Lawson B., Cunningham A. A., Simpson V., Chantrey J. 2005. Necrotic ingluvitis in wild finches. Vet. Rec. 157, 360. [DOI] [PubMed] [Google Scholar]
- 12.Robinson R. A., et al. 2010. Emerging infectious disease leads to rapid population declines of common British birds. PLoS ONE 55, e12215. 10.1371/journal.pone.0012215 (doi:10.1371/journal.pone.0012215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawson B., Cunningham A., Chantrey J., Hughes L. A., John S. K., Bunbury N., Bell D. J., Tyler K. M. 2011. A clonal strain of Trichomonas gallinae is the aetiologic agent of an emerging avian epidemic disease. Infect. Genet. Evol. 11, 1638–1645 10.1016/j.meegid.2011.06.007 (doi:10.1016/j.meegid.2011.06.007) [DOI] [PubMed] [Google Scholar]
- 14.Cannon A. R., Chamberlain D. E., Toms M. P., Hatchwell B. J., Gaston K. J. 2005. Trends in the use of private gardens by wild birds in Great Britain 1995–2002. J. Appl. Ecol. 42, 659–671 10.1111/j.1365-2664.2005.01050.x (doi:10.1111/j.1365-2664.2005.01050.x) [DOI] [Google Scholar]
- 15.Lawson B., et al. 2011. Evidence of spread of the emerging infectious disease finch trichomonosis, by migrating birds. Ecohealth 42, 143–153 10.1007/s10393-011-0696-8 (doi:10.1007/s10393-011-0696-8). [DOI] [PubMed] [Google Scholar]
- 16.Lawson B., Howard T., Kirkwood J. K., Macgregor S. K., Perkins M., Robinson R. A., Ward L. W., Cunningham A. A. 2010. The epidemiology of salmonellosis in garden birds in England and Wales, 1993 to 2003. Ecohealth 7, 294–306 10.1007/s10393-010-0349-3 (doi:10.1007/s10393-010-0349-3) [DOI] [PubMed] [Google Scholar]
- 17.Erwin K. G., Kloss C., Lyles J., Felderhoff J., Fedynich A. M., Henke S. E., Roberson J. A. 2000. Survival of Trichomonas gallinae in white-winged dove carcasses. J. Wildl. Dis. 36, 551–554 [DOI] [PubMed] [Google Scholar]
- 18.Census 2001 2002. 2001 Census in England and Wales. See http://www.statistics.gov.uk/census2001/census2001.asp (accessed January 13 2010) [Google Scholar]
- 19.Chamberlain D. E., Vickery J. A., Glue D. E., Robinson R. A., Conway G. J., Woodburn R. J. W., Cannon A. R. 2005. Annual and seasonal trends in the use of garden feeders by birds in winter. Ibis 147, 563–575 10.1111/j.1474-919x.2005.00430.x (doi:10.1111/j.1474-919x.2005.00430.x) [DOI] [Google Scholar]
- 20.Newson S. E., Evans K. L., Noble D. G., Greenwood J. J. D., Gaston K. J. 2008. Use of distance sampling to improve estimates of national population sizes for common and widespread breeding birds in the UK. J. Appl. Ecol. 45, 1330–1338 10.1111/j.1365-2664.2008.01480.x (doi:10.1111/j.1365-2664.2008.01480.x) [DOI] [Google Scholar]
- 21.Coombes R. H., et al. 2009. Countryside Bird Survey Report 1998–2007, pp. 1–21 Wicklow, Ireland: BirdWatch Ireland [Google Scholar]
- 22.Baillie S. R., et al. 2010. Breeding birds in the wider countryside: their conservation status 2010. BTO Research Report No. 565. Thetford, Norfolk, UK: BTO See http://www.bto.org/birdtrends.
- 23.Balcomb R. 1986. Songbird carcasses disappear rapidly from agricultural fields. Auk 103, 817–820 [Google Scholar]
- 24.Wobeser G., Wobeser A. G. 1992. Carcass disappearance and estimation of mortality in a simulated die-off of small birds. J. Wildl. Dis. 28, 548–554 [DOI] [PubMed] [Google Scholar]
- 25.Newton I. 1972. Finches. pp. 1–288 London, UK: Collins [Google Scholar]
- 26.Pennycott T. W., Cinderey R. N., Park A., Mather H. A., Foster G. 2002. Salmonella enterica subspecies enterica serotype Typhimurium and Escherichia coli 086 in wild birds at two garden sites in south-west Scotland. Vet. Rec. 151, 563–567 10.1136/vr.151.19.563 (doi:10.1136/vr.151.19.563) [DOI] [PubMed] [Google Scholar]
- 27.Fischer J. R., Stallknecht D. E., Luttrell M. P., Dhondt A. A., Converse J. A. 1997. Mycoplasmal conjunctivitis in wild songbirds: the spread of a new contagious disease in a mobile host population. Emerg. Infect. Dis. 3, 69–72 10.3201/eid0301.970110 (doi:10.3201/eid0301.970110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayes E. B., Komar N., Nasci R. S., Montgomery S. P., O'Leary D. R., Campbell G. L. 2005. Epidemiology and transmission dynamics of West Nile virus disease. Emerg. Infect. Dis. 11, 1167–1173 10.3201/eid1108.050289a (doi:10.3201/eid1108.050289a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frick W. E., Pollock J. F., Hicks A. C., Langwig K. E., Reynolds D. S., Turner G. G., Butchkoski C. M., Kunz T. H. 2010. An emerging disease causes regional population collapse of a common North American bat species. Science 329, 679–682 10.1126/science.1188594 (doi:10.1126/science.1188594) [DOI] [PubMed] [Google Scholar]
- 30.Wernham C., Toms M., Marchant J., Clark J., Siriwardena G., Baillie S. 2002. The migration atlas: movements of the birds of Britain and Ireland. London, UK: T & A D Poyser [Google Scholar]
- 31.Forzán M. J., Vanderstichel R., Melekhovets Y. F., McBurney S. 2010. Trichomoniasis in finches from the Canadian maritime provinces: an emerging disease. Can. Vet. J. 51, 391–396 [PMC free article] [PubMed] [Google Scholar]