Abstract
Coral bleaching has caused catastrophic changes to coral reef ecosystems around the world with profound ecological, social and economic repercussions. While its occurrence is predicted to increase in the future, we have little understanding of mechanisms that underlie changes in the fish community associated with coral degradation. The present study uses a field-based experiment to examine how the intensity of interference competition between juveniles of two species of damselfish changes as healthy corals degrade through thermal bleaching. The mortality of a damselfish that is a live coral specialist (Pomacentrus moluccensis) increased on bleached and dead coral in the presence of the habitat generalist (Pomacentrus amboinensis). Increased mortality of the specialist was indirectly owing to enhanced aggression by the generalist forcing the specialist higher up and further away from shelter on bleached and dead coral. Evidence from this study stresses the importance of changing interspecific interactions to community dynamics as habitats change.
Keywords: habitat degradation, coral bleaching, climate change, fish community, interference competition, interspecific competition
1. Introduction
Habitat degradation is one of the key issues for environmental managers, conservationists and biologists alike owing to its impact on global biodiversity [1,2]. Habitat loss may be acute and can result in dramatic loss of species [3]. Alternatively, there may be a slow degradation of habitats that results in both lethal and sublethal effects, such as reductions in growth and fecundity [4,5]. How an organism responds to a change in its habitat depends on its plasticity in resource use, its propensity to move on scales appropriate to reduce the impact of the local habitat change, and its interactions with other organism within the habitat that overlap in resource requirements.
The production and maintenance of habitats is strongly related to the environmental characteristics that promote the growth of habitat-forming organisms—for instance, prairie grasses, kelp or corals. When environmental conditions change, organisms are influenced directly by the change in environmental characteristics and also by the change in the quality or quantity of the resources that the habitat provided [6]. The effects of habitat degradation and of change on species persistence can be profound, and are well documented for a broad range of ecosystems [7–9]; however, the underlying mechanisms of change are often unknown.
The interactions between organisms that share key resources can strongly influence growth, life-history characteristics, mortality and therefore species abundance patterns [10,11]. While interspecific competition has been shown to be a fundamental process in influencing growth and distributions of species [12], it is largely unknown how the balance of interspecific interactions change as the quality of a habitat degrades. Species and life stages differ in their strength of association with particular habitats, and how they respond to changes in the characteristics of these habitats will be determined by the ability of the changed habitat to meet the inhabitant's resource requirements [13,14]. As the services provided by a habitat change, so will the interactions between species that live within the habitat. Understanding how community processes change as the quality of the environment changes is central to predicting how communities will alter with habitat degradation [15].
Coral reefs are one of the most biologically diverse ecosystems on Earth, but are also one of the most susceptible to climate-induced changes and anthropogenic habitat modification. Coral cover has generally declined globally over the past few decades [16]. Predictions of warming oceans, modified ocean chemistry and the increases in the frequency and severity of storms suggest a further degradation of the live hard corals that provide essential resources for the organisms that live on coral reefs [17]. Coral bleaching owing to ocean warming and run-off has caused catastrophic reductions in hard coral throughout the tropics [9,18] and is predicted to increase in frequency and severity [19,20]. While major changes in fish communities associated with coral bleaching have been documented, the mechanisms underlying these changes are poorly understood [21].
The present study examines the effect of coral bleaching on the interspecific competition between juvenile life stages of two members of the same guild of planktonic damselfishes. As adults, one of the study species, Pomacentrus amboinensis, is a habitat generalist, while the other species, Pomacentrus moluccensis, is found only on live healthy coral. However, at the end of the larval phase, both display a preference for living in live hard coral [22]. Because of the extremely high mortality that occurs immediately after settlement, any alteration to the processes that affect survival can have a disproportionate influence on resulting abundance patterns. Models predict that as live coral dies, competitive asymmetry [23,24] will favour the species with the lowest preference for live coral [13,25,26]; however, there is currently little empirical support for this prediction.
2. Material and methods
Newly settled ambon damselfish (P. amboinensis) and lemon damselfish (P. moluccensis) compete for shelter in the same live coral living space when they first settle from the plankton [27]. Both are planktivores and show similar preferences for live, healthy coral at settlement [22]. Interaction with the other species at the reef edge reduces the growth of both species compared with where either is absent [27]. Within two months on shallow reefs, the two species display a disjunct distribution pattern with P. amboinensis associated with the base of the reef and P. moluccensis with live coral at the top of the reef [27].
Settlement-stage larvae of P. amboinensis and P. moluccensis were collected overnight using light traps (for design, see [28]) moored in open water on the western side of Lizard Island (14′40° S, 145′28° E), in the northern Great Barrier Reef, Australia. Fishes were sorted to species and kept for 24 h in aquaria where they were fed Artemia naupili. All fishes used in the experiments were placed into individually labelled 1 l clip-seal bags and measured with callipers. To reduce transport and handling stress, fishes in bags were transported to the field site in a 60 l bin of seawater (to reduce temperature fluctuations) under subdued light conditions.
Patch reefs used in the field experiment were composed of one of the three states of the bushy hard coral, Pocillopora damicornis: live healthy coral, thermally bleached coral, or dead-algal-covered coral. Bleached coral was either thermally bleached over 10 days using the protocol of McCormick et al. [22] or naturally bleached and detached from the main reef. Dead coral was covered by algae and some sessile invertebrates, but still had an architecture similar to that of live healthy coral.
Both fish species naturally settle on patch reef environments near the continuous reef. In this habitat, juveniles are exposed to a diverse range of predators that use a variety of feeding modes from ambush (lizardfish Synodus dermatogenys and small cods Cephalopholis microprion) to pursuit (dottybacks Pseudochromis fuscus and wrasse Thalassoma lunare). These fishes can be observed to capitalize on juveniles that venture too far from shelter.
(a). Experimental protocol
Length-matched interspecific pairs of P. amboinensis and P. moluccensis were placed on patch reefs composed of one of three health states of coral. Fish length was standardized as it has been found to be important in determining the outcome of interaction between newly settled fishes and their survival [29,30]. In addition, solitary individuals of P. moluccensis were placed on each of the three patch types. Availability of fish only allowed solitary individuals of P. amboinensis to be placed on healthy coral patches. These solitary individuals controlled for the effects of interspecific interactions. Reefs (approx. 18 × 15 × 18 cm) were placed on a 20 × 20 cm concrete tile (to prevent death through smothering) on a sandflat, arranged 4–5 m apart and 3–4 m away from continuous reef. Patches were cleared of any fishes or invertebrates using a hand net, prior to release. Captured fishes were released on natural reefs away from the study area. A small wire cage (approx. 30 × 30 × 30 cm, 12 mm mesh size) was placed over the patch to allow fishes to acclimatize to their new surroundings while being protected from predators. Cages were removed 40–60 min after release of the fishes between 09.00 and 11.00 h. Fish presence was monitored two to three times per day (i.e. after the initial acclimation period, the evening after release and the following morning) for approximately 72 h. Water temperature during the census periods averaged 28.6°C. Our previous studies that have tagged fishes have shown that settlement-stage damselfish do not migrate from these isolated reefs and that any loss can most parsimoniously be attributed to predation [31].
(b). Behavioural assessment
The behaviour of each fish placed on the patch reefs was monitored 40–60 min after placement on the reef following the protocol of McCormick [21]. Briefly, behaviour of the focal fish was assessed over a 3 min period by a scuba diver positioned 1.5 m away from the patch. Six aspects of activity and behaviour were assessed: (i) maximum distance ventured from the habitat patch; (ii) height above substratum (categorized as percentage of the time spent within the bottom, middle or third of the patch); (iii) number of fin displays; (iv) the number of chases or bites; and (v) number of avoidance episodes in response to a conspecific. Two additional variables were devised to summarize the information and reduce the number of variables that were required in analyses. Relative height on the patch was summarized as a cumulative proportion of the time spent at varying heights over the 3 min observation period, with the top of the patch taken as height of 1, mid-patch a height of 0.5 and bottom a height of 0.
(c). Statistical analyses
Survival (up to 72 h) of fishes among the four habitats by three species combinations was compared using multiple-sample survival analysis that uses a Cox's proportional hazard model (Statistica v. 9.0). Survival curves of each fish size and substrata were calculated and plotted using the Kaplan–Meier product-limit method. The Kaplan–Meier method is a non-parametric estimator of survival that incorporates incomplete (censored) observations, such as those cases where censuses had to be terminated on trials prior to their completion owing to time limitations of a field trip. Projected survival trajectories were compared between the three substrata using a Chi-square statistic, whereas differences in survival between particular pairs of treatment fishes were compared using a Cox-F statistic.
Two behavioural variables (maximum distance ventured and relative height) were compared between species, habitat and context (solitary or paired) combinations with a one-factor ANOVA (Type III SS) followed by Tukey's honestly significant difference (HSD) tests. An aggression index was produced using the first principal component of the correlation matrix of the number of displays, chases or bites and avoidance events. This aggression index (for fishes placed in pairs) was compared with a two-factor ANOVA (factors: species, habitat). Residual analysis was used to examine assumptions of ANOVA.
3. Results
The mortality of P. amboinensis did not differ between habitats, even when P. moluccensis was absent from the habitat patch (χ22 = 7.03, p = 0.071, figure 1a). However, the mortality trajectories of newly settled P. moluccensis were affected by habitat and the presence of P. amboinensis (χ32 = 27.36, p < 0.0001, figure 1b). Survival of P. moluccensis when alone did not differ among the three habitats (χ22 = 1.38, p = 0.501, figure 1b). However, survival of P. moluccensis did differ among the three habitats when present with P. amboinensis (χ22 = 8.896, p = 0.012). The survival of P. moluccensis on bleached and dead coral in the presence of P. amboinensis did not differ from one another (Cox F-test, F98,40 = 1.410, p = 0.111), suggesting that P. moluccensis died fastest when on bleached and dead coral in the presence of P. amboinensis (figure 1b).
Figure 1.
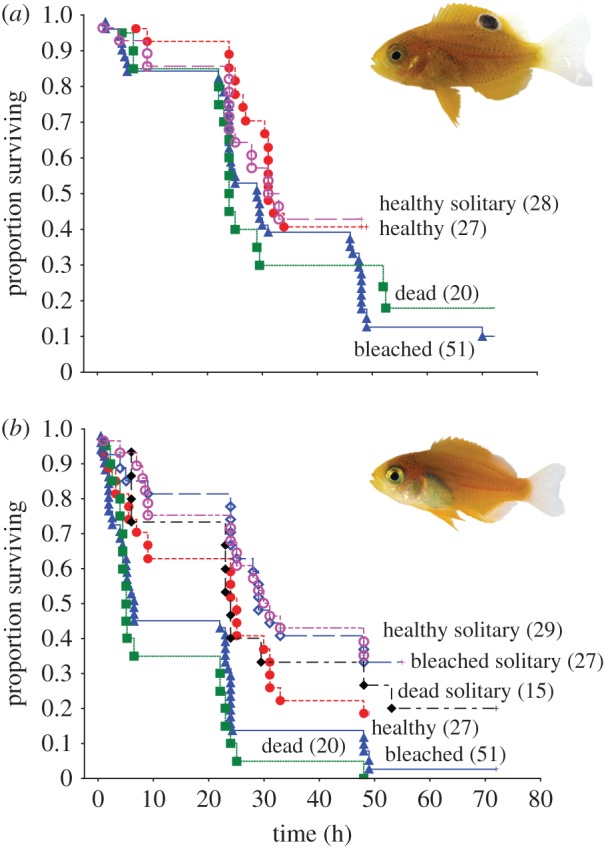
Effects of interspecific competition on the survival of (a) Pomacentrus amboinensis, and (b) Pomacentrus moluccensis on three hard coral habitats. Kapalin–Meier survival trajectories illustrate the different survivals on patches of live healthy, live bleached and dead (algal-covered) Pocillopora damicornis hard coral. Individuals are either placed in size-matched pairs on the habitats or placed as solitary individuals. Numbers in brackets represent the total number of trials undertaken for each treatment.
The survival of P. moluccensis on healthy coral was substantially reduced when a similar sized P. amboinensis also inhabited the coral patch (Cox F-test, F36,44 = 1.770, p = 0.035; electronic supplementary material, figure S1a). Pomacentrus moluccensis and P. amboinensis alone on healthy coral had similar survival (approx. 42% after 36 h) to P. amboinensis when it was present with P. moluccensis (χ22 = 1.04, p = 0.594; electronic supplementary material, figure S1a). Survival of P. moluccensis on bleached coral patches was markedly lower when P. amboinensis was present (Cox F-test, F90,98 = 2.174, p < 0.0001; electronic supplementary material, figure S1b). Survival of P. moluccensis when alone on bleached coral was similar to that of P. amboinensis when P. moluccensis was present (Cox F-test, F36,90 = 1.552, p = 0.051). Survival on dead coral was similar to survival on bleached coral for both species. Survival of P. moluccensis was markedly lower on dead coral in the presence of P. amboinensis compared to when alone (Cox F-test, F24,40 = 3.149, p < 0.0007; electronic supplementary material, figure S1c).
The position of fishes on the habitat patch differed between species and with habitat type (max distance ventured: F7,154 = 7.32, p < 0.001; relative height: F7,154 = 128.9, p < 0.001; figure 2). Pomacentrus amboinensis stayed low and close to shelter regardless of habitat type. By contrast, the position of P. moluccensis on the reef was influenced by whether it was on the patch alone or together with P. amboinensis, and with habitat type (Tukey's tests; figure 2). Pomacentrus moluccensis adopted a similar position on the reef to P. amboinensis when alone, but the fish was higher up the reef when together with P. amboinensis. Pomacentrus moluccensis ventured twice as far from the reef when on bleached or dead coral habitats (figure 2a) and also stayed significantly higher up the reef on bleached and dead coral (figure 2b).
Figure 2.
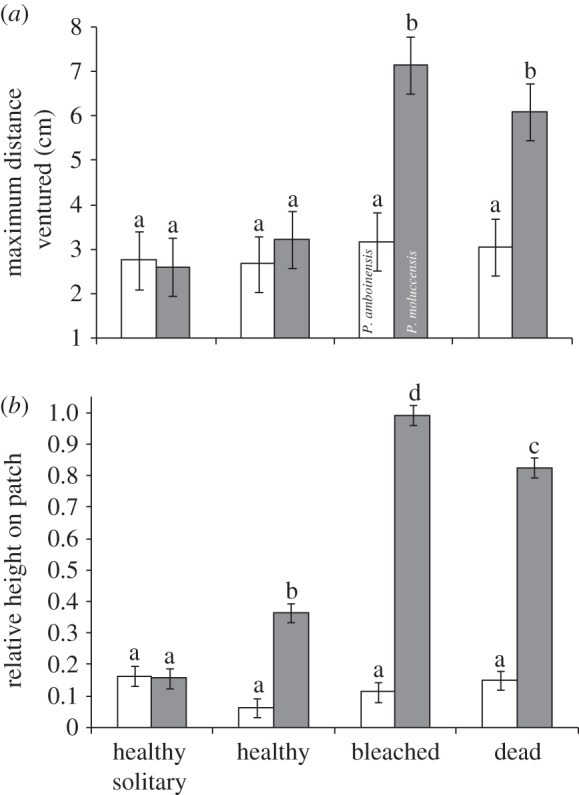
Influence of coral degradation on fish vulnerability. Spatial patterns of juvenile Pomacentrus amboinensis (white bars) and P. moluccensis (grey bars) placed in pairs or as solitary individuals onto one of three hard coral habitat types (healthy live, bleached live, dead Pocillopora damicornis). (a) Mean maximum distance ventured from the habitat recorded 40–60 min after release. (b) Mean relative height on the patch reef (a value of 1 represents 100% of time spent on top of patch; 0, 100% time at the base). Letters above the bars represent Tukey's HSD groups. Errors are standard errors.
The distribution pattern of P. moluccensis appeared to be driven in part by changes to the aggression level of P. amboinensis (species × treatment interaction: F2,116 = 10.471, p = 0.0001; figure 3). The first principal component that comprised the aggression index represented the negative relationship between avoidances and displays/chases or bites (factor loadings −0.63, 0.53, 0.56, respectively) and accounted for 50.7 per cent of the variability. Pomacentrus amboinensis was consistently more aggressive than P. moluccensis, which was almost always the subordinate species. The significant interaction between species and habitat suggests that P. amboinensis was more aggressive on bleached coral than healthy coral.
Figure 3.
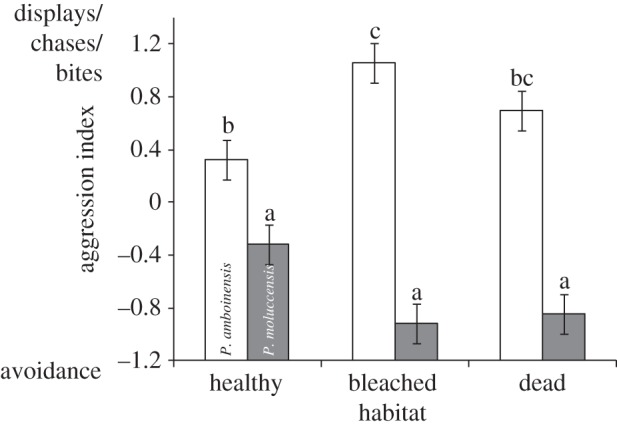
Level of aggression displayed by juvenile Pomacentrus amboinensis (white) and Pomacentrus moluccensis (grey) 40–60 min after release in size-matched pairs onto a patch of habitat (healthy live, bleached live, dead Pocillopora damicornis). Aggression recorded as the first principal component of displays, chases, bites and avoidance events between pairs. Letters above the bars represent Tukey's HSD groups. Errors are standard errors (n = 20–21).
4. Discussion
Coral reef fishes live in strongly structured communities and the dynamics that regulate these communities are closely tied to the characteristics of their habitat [13]. Dramatic shifts in fish community composition are associated with coral disturbance [9,32,33]. The ultimate cause of these changes is often ascribed to a loss of topography or shelter [34], but there is little understanding of the processes that instigate change [35]. The present study illustrates that as habitats alter, the intensity of the processes that regulate the communities change. Interspecific competition was important in determining the initial distribution of the two damselfishes studied, and the intensity was found to alter as the habitat degraded from live healthy coral through to dead-algal-covered coral. Mortality intensified for the species that specialized on the healthy coral habitat. Behavioural observations showed that this was an indirect effect of the heightened aggression by the generalist towards the specialist, forcing it into a position of higher risk.
Terrestrial theory suggests that specialists should be superior competitors in the habitat on which they specialize, and may also be more prone to extinction owing to restrictions in their resource requirements [36]. However, this has seldom been examined in marine environments. One study of coral dwelling gobies found that species which only inhabited one species of coral became locally extinct when corals bleached and died [37]. A study of monitored populations of damselfish from the Great Barrier Reef found that niche breadth explained 74 per cent of the variation in species' mean response to coral decline, with coral specialists being more susceptible than generalists [38]. The same study also suggested that because juveniles were more closely associated with specific coral types than adults, coral degradation could impact populations through its impact on the vulnerable juvenile stages. The current study adds additional complexity to this trend by examining changes in the competitive dynamics when both species are in the early juvenile life phase and still have a strong association with live coral. The species that is a generalist as an adult, P. amboinensis, preferentially settles to live coral and its distribution among habitats slowly diversifies over the next two months after settlement [27]. This fish is the dominant competitor at the life stage when live coral is its preferred habitat. It is presently unknown whether the competitive outcome on live coral differs between the two species as adults. As live coral declines, it is expected that the intensity of competition (inter- and intraspecific) will increase, and the interactions between juveniles described in the present study will become more important in determining the development of assemblage composition [34].
The effects of habitat loss on the resident fish community depends on the established interspecific dominance hierarchy and whether the interaction network adapts to the new resource base. Here, the habitat generalist, P. amboinensis, was the superior competitor even when in the habitat of the coral specialist. Mortality trajectories were similar for both species when alone, even when the coral specialist was on dead coral. These results support a longitudinal field study of the impact of coral bleaching on fishes that found little impact of bleaching on the abundance of tagged P. moluccensis on a reef in Papua New Guinea [24]. It appears that the avoidance of aggression from the dominant P. amboinensis either forces the subordinate P. moluccensis into a more vulnerable location, or distracts them and thereby lowers their vigilance to nearby predators. Competitive dominance can shift among species depending on habitat characteristics in damselfishes [25], but the present study highlights that this will not always be the case. It also highlights that interference competition can have a major indirect effect on mortality through habitat-related behavioural modification.
While aggression from the dominant species was more pronounced on bleached and dead coral, both fish also modified their spatial position when alone. On healthy coral, solitary individuals of both species stayed close to shelter. By contrast, on bleached and dead coral, P. moluccensis moved further away from the patch that increased their vulnerability to piscivores in the area. A similar shift in spatial distribution was found for P. amboinensis in a previous study [21]. The reasons for this are open to speculation, but may involve avoidance of necrotic or novel odours from the live bleached and dead-algal-covered corals, or an attempt to improve camouflage away from the bleached coral [21,39].
It is unclear how intraspecific competition would modify the effects of interspecific competition. Intraspecific competition is often stronger than interspecific competition [40,41]. The present study dealt with a simple system of two recruits on a habitat patch, which is a common situation during the recruitment season [22]. A previous study showed that intraspecific interactions between P. amboinensis recruits indirectly influenced mortality and the intensity of interactions between individuals were affected by habitat [21]. Previous tests between ecologically similar reef fishes have found effects on growth rather than on mortality (reviewed by [15,42,43]). Intraspecific [21] and interspecific interactions involving P. amboinensis both indirectly lead to enhanced mortality through aggression of dominant individuals, forcing subordinates into areas of greater risk. It is unclear whether the detrimental effects of interspecific competition would be ameliorated or compounded by intraspecific competition. Both forms of competition are density-dependent but the detrimental effects, and the extent to which negative effects need to be weighed against the positive effects of group dynamics (e.g. distraction leading to lower per capita mortality; [44]), are likely to be context-dependent [45]. While coexistence of the two species on small habitat units appears unlikely at this vulnerable life stage, coexistence at broader spatial scales will be achieved by spatial and temporal patchiness in recruitment and longer term priority effects that will offset the competitive superiority of P. amboinensis through size advantages [46].
While the present experiments were conducted over very short time-periods, they are on a time scale relevant to the processes that regulate juvenile populations and the replenishment of reef fishes and other organisms with complex life histories [47,48]. Previous studies have shown that mortality can be extreme during the first few days after settlement, and that processes within this transition period can greatly affect cohort strength [49]; proportionately more than similar processes at later life stages. Interactions changed as live coral degraded and this indirectly enhanced mortality by altering the spatial risk of the subordinate species to predation. Thus, it was the interactions between competing species and the presence of appropriate predators that led to mortality of the specialist on bleached and dead coral, rather than the change in habitat itself, at least over the short-term. Prediction of the trajectory that a community on a changing habitat patch will follow is contingent on understanding the links between species and how these alter as habitat characteristics change.
Acknowledgments
This research was conducted under animal ethics approval from JCU and a research permit from the Great Barrier Reef Marine Park Authority.
We are grateful to the many people who assisted with various aspects of field and laboratory logistics: M. Meekan, J. Moore, J. Maddams, C. Weaver, D. Feros, J. Scannell, and G. Winstanley. Logistic support was provided by staff at the Lizard Island Research Station (Australian Museum). O. Lönnstedt and two anonymous reviewers provided comments on a version of the manuscript. Funding was provided through the ARC Centre of Excellence for Coral Reef Studies (CE0561432).
References
- 1.Pimm S. L., et al. 2001. Environment: can we defy nature's end? Science 293, 2207–2208 10.1126/science.1061626 (doi:10.1126/science.1061626) [DOI] [PubMed] [Google Scholar]
- 2.Fischer J., Lindenmayer D. B. 2007. Landscape modification and habitat fragmentation: a synthesis. Glob. Ecol. Biogeogr. 16, 265–280 10.1111/j.1466-8238.2007.00287.x (doi:10.1111/j.1466-8238.2007.00287.x) [DOI] [Google Scholar]
- 3.Tilman D. 1994. Competition and biodiversity in spatially structured habitats. Ecology 75, 2–16 10.2307/1939377 (doi:10.2307/1939377) [DOI] [Google Scholar]
- 4.Newcomb-Homan R., Regosin J. V., Rodrigues D. M., Reed J. M., Windmiller B. S., Romero L. M. 2003. Impacts of varying habitat quality on the physiological stress of spotted salamanders (Ambystoma maculatum). Anim. Conserv. 6, 11–18 10.1017/S1367943003003032 (doi:10.1017/S1367943003003032) [DOI] [Google Scholar]
- 5.Pratchett M. S., Wilson S. K., Berumen M. L., McCormick M. I. 2004. Sub-lethal effects of coral bleaching on an obligate coral feeding butterflyfish. Coral Reefs 23, 352–356 10.1007/s00338-004-0394-x (doi:10.1007/s00338-004-0394-x) [DOI] [Google Scholar]
- 6.Shima J. S., Osenberg C. W. 2003. Cryptic density dependence: effects of covariation between density and site quality in reef fish. Ecology 84, 46–52 10.1890/0012-9658(2003)084[0046:CDDEOC]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[0046:CDDEOC]2.0.CO;2) [DOI] [Google Scholar]
- 7.Myers N., Mittermeier R. A., Mittermeier C. G., da Fonseca G. A. B., Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 10.1038/35002501 (doi:10.1038/35002501) [DOI] [PubMed] [Google Scholar]
- 8.Brooks T. M., et al. 2002. Habitat loss and extinction in the hotspots of biodiversity. Conserv. Biol. 16, 909–923 10.1046/j.1523-1739.2002.00530.x (doi:10.1046/j.1523-1739.2002.00530.x) [DOI] [Google Scholar]
- 9.Jones G. P., McCormick M. I., Srinivasan M., Eagle J. V. 2004. Coral decline threatens fish biodiversity in marine reserves. Proc. Natl Acad. Sci. USA 101, 8251–8253 10.1073/pnas.0401277101 (doi:10.1073/pnas.0401277101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tilman D. 1982. Resource competition and community structure. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- 11.Schoener T. W. 1983. Field experiments on interspecific competition. Am. Nat. 122, 240–285 10.1086/284133 (doi:10.1086/284133) [DOI] [Google Scholar]
- 12.Hixon M. A., Jones G. P. 2005. Competition, predation, and density dependent mortality in demersal marine fishes. Ecology 86, 2847–2859 10.1890/04-1455 (doi:10.1890/04-1455) [DOI] [Google Scholar]
- 13.Connell J. H. 1978. Diversity in tropical rain forests and coral reefs. Science 199, 1302–1310 10.1126/science.199.4335.1302 (doi:10.1126/science.199.4335.1302) [DOI] [PubMed] [Google Scholar]
- 14.MacNally R. C. 1995. Ecological versatility and community ecology. Cambridge, UK: Cambridge University Press [Google Scholar]
- 15.Carr M. H., Anderson T. W., Hixon M. A. 2002. Biodiversity, population regulation, and the stability of coral-reef fish communities. Proc. Natl Acad. Sci. USA 99, 11 241–11 245 10.1073/pnas.162653499 (doi:10.1073/pnas.162653499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkinson C. R. 2004. Status of coral reefs of the world 2004, vols 1 and 2. Townsville, Australia: Australian Institute of Marine Science [Google Scholar]
- 17.Pandolfi J. M., Connolly S. R., Marshall D. J., Cohen A. L. 2011. Projecting coral reef futures under global warming and ocean acidification. Science 333, 418–422 10.1126/science.1204794 (doi:10.1126/science.1204794) [DOI] [PubMed] [Google Scholar]
- 18.Eakin C. M., et al. 2010. Caribbean corals in crisis: record thermal stress, bleaching, and mortality in 2005. PLoS ONE 5, e13969. 10.1371/journal.pone.0013969 (doi:10.1371/journal.pone.0013969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donner S. D., Skirving W. J., Little C. M., Oppenheimer M., Hoegh-Guldberg O. 2005. Global assessment of coral bleaching and required rates of adaptation under climate change. Glob. Change Biol. 11, 1–15 10.1111/j.1529-8817.2003.00895.x (doi:10.1111/j.1529-8817.2003.00895.x) [DOI] [PubMed] [Google Scholar]
- 20.Anthony K. R. N., Maynard J. A., Diaz-Puildo G., Mumby P. J., Marshall P. A., Cao L., Hoegh-Guldberg O. 2011. Ocean acidification and warming will lower coral reef resilience. Glob. Change Biol. 17, 1798–1808 10.1111/j.1365-2486.2010.02364.x (doi:10.1111/j.1365-2486.2010.02364.x) [DOI] [Google Scholar]
- 21.McCormick M. I. 2009. Behaviourally mediated phenotypic selection in a disturbed coral reef environment. PLoS ONE 4, e7096. 10.1371/journal.pone.0007096 (doi:10.1371/journal.pone.0007096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormick M. I., Moore J. A. Y., Munday P. L. 2010. Influence of habitat degradation on fish replenishment. Coral Reefs 29, 537–546 10.1007/s00338-010-0620-7 (doi:10.1007/s00338-010-0620-7) [DOI] [Google Scholar]
- 23.Munday P. L., Jones G. P., Caley M. J. 2001. Interspecific competition and coexistence in a guild of coral-dwelling fishes. Ecology 82, 2177–2189 10.1890/0012-9658(2001)082[2177:ICACIA]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[2177:ICACIA]2.0.CO;2) [DOI] [Google Scholar]
- 24.Bonin M. C., Srinivasan M., Almany G. R., Jones G. P. 2009. Interactive effects of interspecific competition and microhabitat on early post-settlement survival in a coral reef fish. Coral Reefs 28, 265–274 10.1007/s00338-008-0451-y (doi:10.1007/s00338-008-0451-y) [DOI] [Google Scholar]
- 25.Ebersole J. P. 1985. Niche separation of two damselfish species by aggression and differential microhabitat utilization. Ecology 66, 14–20 10.2307/1941302 (doi:10.2307/1941302) [DOI] [Google Scholar]
- 26.Cantrell R. S., Cosner C., Fagan W. F. 1998. Competitive reversals inside ecological reserves: the role of external habitat degradation. J. Math. Biol. 37, 491–533 10.1007/s002850050139 (doi:10.1007/s002850050139) [DOI] [PubMed] [Google Scholar]
- 27.McCormick M. I., Weaver C. In press It pays to be pushy: intracohort interference competition between two reef fishes. PLoS ONE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meekan M. G., Wilson S. G., Halford A., Retzel A. 2001. A comparison of catches of fishes and invertebrates by two light trap designs, in tropical NW Australia. Mar. Biol. 139, 373–381 10.1007/s002270100577 (doi:10.1007/s002270100577) [DOI] [Google Scholar]
- 29.Holmes T. H., McCormick M. I. 2009. Influence of prey body characteristics and performance on predator selection. Oecologia 159, 401–413 10.1007/s00442-008-1220-x (doi:10.1007/s00442-008-1220-x) [DOI] [PubMed] [Google Scholar]
- 30.Perez-Dominguez R., Munch S. B. 2010. Extreme selection on size in the early lives of fishes. Evolution 64, 2450–2457 [DOI] [PubMed] [Google Scholar]
- 31.Hoey A., McCormick M. I. 2004. Selective predation for low body condition at the larval-juvenile transition of a coral reef fish. Oecologia 139, 23–29 10.1007/s00442-004-1489-3 (doi:10.1007/s00442-004-1489-3) [DOI] [PubMed] [Google Scholar]
- 32.Graham N. A. J., Wilson S. K., Jennings S., Polunin N. V. C., Bijoux J. P., Robinson J. 2006. Dynamic fragility of oceanic coral reef ecosystems. Proc. Natl Acad. Sci. USA 103, 8425–8429 10.1073/pnas.0600693103 (doi:10.1073/pnas.0600693103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson S. K., Graham N. A. J., Pratchett M. S., Jones G. P., Polunin N. V. 2006. Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Glob. Change Biol. 12, 2220–2234 10.1111/j.1365-2486.2006.01252.x (doi:10.1111/j.1365-2486.2006.01252.x) [DOI] [Google Scholar]
- 34.Pratchett M. S., Munday P. L., Wilson S. K., Graham N. A. J., Cinner J. E., Bellwood D. R., Jones G. P., Polunin N. V. C., McClanahan T. R. 2008. Effects of climate-induced coral bleaching on coral-reef fishes: ecological and economic consequences. Oceanogr. Mar. Biol. 46, 251–296 10.1201/9781420065756.ch6 (doi:10.1201/9781420065756.ch6) [DOI] [Google Scholar]
- 35.Graham N. A. J., Wilson S. K., Pratchett M. S., Polunin N. V. C., Spalding M. D. 2009. Coral mortality versus structural collapse as drivers of corallivorous butterflyfish decline. Biodivers. Conserv. 18, 3325–3336 10.1007/s10531-009-9633-3 (doi:10.1007/s10531-009-9633-3) [DOI] [Google Scholar]
- 36.McKinney M. L. 1997. Extinction vulnerability and selectivity: combining ecological and paleontological views. Annu. Rev. Ecol. Syst. 28, 495–516 10.1146/annurev.ecolsys.28.1.495 (doi:10.1146/annurev.ecolsys.28.1.495) [DOI] [Google Scholar]
- 37.Munday P. L. 2004. Habitat loss, resource specialization, and extinction on coral reefs. Glob. Change Biol. 10, 1642–1647 10.1111/j.1365-2486.2004.00839.x (doi:10.1111/j.1365-2486.2004.00839.x) [DOI] [Google Scholar]
- 38.Wilson S. K., Burgess S. C., Cheal A. J., Emslie M. J., Fisher R., Miller I., Polunin N. V. C., Sweatman H. P. A. 2008. Habitat utilization by coral reef fish: implications for specialists versus generalists in a changing environment. J. Anim. Ecol. 77, 220–228 10.1111/j.1365-2656.2007.01341.x (doi:10.1111/j.1365-2656.2007.01341.x) [DOI] [PubMed] [Google Scholar]
- 39.Coker D. J., Pratchett M. S., Munday P. L. 2009. Coral bleaching and habitat degradation increase susceptibility to predation for coral-dwelling fishes. Behav. Ecol. 20, 1204–1210 10.1093/beheco/arp113 (doi:10.1093/beheco/arp113) [DOI] [Google Scholar]
- 40.Forrester G. E., Evans B., Steele M. A., Vance R. R. 2006. Assessing the magnitude of intra- and interspecific competition in two coral reef fishes. Oecologia 148, 632–640 10.1007/s00442-006-0397-0 (doi:10.1007/s00442-006-0397-0) [DOI] [PubMed] [Google Scholar]
- 41.Tack A. J. M., Ovaskainen O., Harrison P. J., Roslin T. 2009. Competition as a structuring force in leaf miner communities. Oikos 118, 809–818 10.1111/j.1600-0706.2008.17397.x (doi:10.1111/j.1600-0706.2008.17397.x) [DOI] [Google Scholar]
- 42.Jones G. P. 1991. Post-recruitment processes in the ecology of coral reef fish populations: a multifactorial perspective. In The ecology of fishes on coral reefs (ed. Sale P. F.), pp. 294–328 San Diego, CA: Academic Press [Google Scholar]
- 43.Samhouri J. F., Steele M. A., Forrester G. E. 2009. Inter-cohort competition drives density dependence and selective mortality in a marine fish. Ecology 90, 1009–1020 10.1890/07-1161.1 (doi:10.1890/07-1161.1) [DOI] [PubMed] [Google Scholar]
- 44.Cresswell W., Quinn J. L. 2011. Predicting the optimal prey group size from predator hunting behaviour. J. Anim. Ecol. 80, 310–319 10.1111/j.1365-2656.2010.01775.x (doi:10.1111/j.1365-2656.2010.01775.x) [DOI] [PubMed] [Google Scholar]
- 45.Adam T. C. 2011. High-quality habitat and facilitation ameliorate competitive effects of prior residents on new settlers. Oecologia 166, 121–130 10.1007/s00442-010-1826-7 (doi:10.1007/s00442-010-1826-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geange S. W., Stier A. C. 2009. Order of arrival affects competition in two reef fishes. Ecology 90, 2868–2878 10.1890/08-0630.1 (doi:10.1890/08-0630.1) [DOI] [PubMed] [Google Scholar]
- 47.Gosselin L. A., Qian P. 1997. Juvenile mortality in benthic marine invertebrates. Mar. Ecol. Prog. Ser. 146, 265–282 10.3354/meps146265 (doi:10.3354/meps146265) [DOI] [Google Scholar]
- 48.Almany G. R., Webster M. S. 2006. The predation gauntlet: early post-settlement mortality in coral reef fishes. Coral Reefs 25, 19–22 10.1007/s00338-005-0044-y (doi:10.1007/s00338-005-0044-y) [DOI] [Google Scholar]
- 49.Steele M. A. 1997. The relative importance of processes affecting recruitment of two temperate reef fishes. Ecology 78, 129–145 10.1890/0012-9658(1997)078[0129:TRIOPA]2.0.CO;2 (doi:10.1890/0012-9658(1997)078[0129:TRIOPA]2.0.CO;2) [DOI] [Google Scholar]


