Abstract
Timing of first reproduction is a key life-history variable with important implications for global economic development and health. Life-history theory predicts that human reproductive strategies are shaped by mortality regimes. This study provides the first test of the relationship between population-level adolescent fertility (AF) and extrinsic risk at two time points. Data are from United Nations database and were analysed using mediation and moderation techniques. The goals were to determine whether (i) early risk has a stronger impact on fertility than current risk; (ii) current risk mediates the relationship between early risk and fertility outcomes; and (iii) different levels of early risk influence the relationship between current risk and fertility. Results indicated that current risk partially mediated the relationship between early risk and fertility, with early risk having the strongest impact on reproduction. Measures for early and current mortality did not show significant interaction effects. However, a series of separate regression analyses using a quantile split of early risk indicated that high levels of early risk strengthened the relationship between current risk and AF. Overall, these findings demonstrate that reproductive strategies are significantly influenced by fluctuations of early mortality as well as current environmental cues of harshness.
Keywords: life-history theory, reproductive effort, environmental risk
1. Introduction
Timing of first reproduction is a key life-history variable with important implications for global economic development and health. Life-history theory predicts that human reproductive strategies are shaped by mortality regimes. Extrinsic mortality is the local risk of death that is not conditional on an organism's reproductive behaviour [1]. Statistically, extrinsic mortality can be defined as variance in the probability of death that is not accounted for by mating effort or parenting effort (or by extension trade-offs between reproductive and somatic effort). In other words, an organism cannot escape extrinsic mortality by changing its behaviour: it is the age-specific risk of death that is equally shared by all members of a population. Recent empirical advances have demonstrated that extrinsic mortality has profound influences on adaptive behaviour, such as reproductive effort [2–5]. Here, we examine cross-national effects of mortality on timing of reproductive onset indicated by adolescent fertility rates (AFRs). We examine the interaction of population mortality levels around birth and in early life and compare those effects with mortality levels at age of maturity to identify canalization effects from those of facultative reproductive adjustments later in life.
Extrinsic mortality plays a key role in the evolution of life histories and reproductive strategies [1,6–10]. When extrinsic mortality is high, then organisms should reproduce relatively early in life to reduce mortality exposure over time and extend the length of the reproductive span, which should maximize fertility to ‘beat the odds’ that some offspring will die. Conversely, when extrinsic mortality is low, then differential reproductive success is contingent on resources invested in growth, development and parental effort rather than luck. Hence, in low extrinsic risk environments individuals may enhance fitness by delaying reproduction to accrue additional resources (including knowledge and skills), and by reducing fertility and increasing investment per offspring. These predicted relationships hold among mammals: juvenile mortality is negatively correlated with age at maturity, age at weaning, maternal investment and positively correlated with litter size, and pace of reproduction [9].
Empirical evidence suggests that the relationship between mortality and life-history strategies is complex. Environmental effects often show nonlinear relations with parental investment and reproductive effort [3,4,5,11]. Data from a rural Dominican village, for example, demonstrated that moderate levels of extrinsic mortality at birth predicted higher rates of fertility, but low environmental risk and very high environmental risk both resulted in phenotypically similar alterations in reproductive effort [5]. It appears likely that females shut down reproductive development in order to preserve somatic resources either for self-preservation or to accrue resources in highly stressful environments [11,12].
There is long-standing debate regarding the role of development in shaping human reproductive strategies, and questions remain concerning sensitive periods for development. Early childhood, from 1 to about 7 years, has been suggested as a sensitive period that has the strongest effects on adult outcomes [5,8,13–17]. Recent adoption studies show that conditions in the first 42 months are important in shaping development, with a dose–response effect for duration and severity of environmental conditions [18]. The psychological and physiological mechanisms linking environmental risk to reproductive behaviour are unclear, but psychosocial stress, attachment and associated hormones have been implicated [8,10,19,20]. Recently, Coall & Chisholm [20] demonstrated that the relationship between menarche and parental investment is influenced by fluctuations of early psychosocial stress, such that high levels of psychosocial stress led to lower levels of parental investment among mothers.
A second, though not necessarily mutually exclusive, line of thought suggests that it makes little adaptive sense to lock humans into an adult phenotype early in the life course because such canalization could lead to significant mismatch between adult phenotypes and the extant environment [5,21,22]. Hence, humans should be open to environmental influences throughout the life course and conditions at the age of maturity should be particularly salient in shaping behaviour. Mechanisms for later developmental effects are also unknown, but physiological pathways [12] and conscious decision-making are likely important. Cross-national empirical studies have not compared extrinsic mortality at birth and later in life to determine fertility outcomes. Here, we test the following questions: (i) Does early risk have a larger impact on fertility than current risk? (ii) Does current risk mediate the relationship between early risk and fertility? And (iii) Do different levels of early risk influence the relationship between current risk of death and reproductive outcomes? We use mediation and moderation analyses to test our hypotheses. Mediation analysis, or path analysis, is a tool to determine mechanisms that influence the relationship between a predictor and outcome variable. In this case, we use mediation analysis to determine whether current environmental risk mediates the relationship between early risk of death and AFRs. Moderation analysis is then used to test if different levels of early extrinsic risk influence the relationship between current risk and later reproduction.
2. Methods
Data are from the United Nations database, which includes nation-level statistics on human development, health and multiple other indicators [23]. The original sample size included 176 countries. Data for variables are available in the electronic supplementary material. Techniques were not used to account for missing data during analyses; thus, the final sample size included 163 countries. ‘Adolescent fertility rates (AFRs) in 2009’ was selected as the outcome measure for fertility, and is defined as births per 1000 women ages 15–19 AFR. Although this measure does not directly address the timing of reproductive onset, it captures the fast/slow dimension of fertility outcomes; i.e. populations have higher rates of adolescent fertility when environmental conditions are harsh. Extrinsic risk around the year of birth for the 15–19 cohort was measured by ‘female infant mortality rates (FIMRs) from 1995 to 2000’ and ‘infant mortality rates (IMRs) in 1995’. FIMR is measured as the ‘probability of dying between birth and age 1’ [24], whereas IMR is a direct measure of the rate of deaths between birth and age 1 [25]. Data for both FIMR and IMR are expressed as deaths per 1000 births. Extrinsic risk in current environment was measured with ‘adult mortality rates (AMRs) in 2007’ and ‘male mortality (MM) rates in 2007’. AMR and MM are defined as the ‘probability of dying between 15 and 60 years per 1000 population’ [26]. It should be noted that AMR does include deaths owing to maternal causes, which is considered a form intrinsic risk. However, research indicates that this value accounts for less than 0.5 per cent of adult mortality rates in 2005 and 2008, and the value has been decreasing over time [27,28]. Thus, it is unlikely that this small percentage of intrinsic risk will mask the larger effects of extrinsic mortality on reproduction. Inclusion of male mortality rates provides an additional test of extrinsic risk to determine whether the small percentage of maternal deaths changes the outcome of AFRs. Furthermore, infant mortality measures for the current environment could not be used as a measure for risk owing to a high correlation to infant mortality rates in the early environment. Socioeconomic variables were included as control measures. Variables included human development index (HDI) in 2005 [29], GNI per capita (GNI) in 2009 [30], enrolment of females in primary education (PRIM) in 2005 [31], enrolment of females in secondary education (SEC) in 2005 [32] and employment-to-population ratio of males (EMP) in 2006, ages 15 and older [31].
3. Results
Table 1 provides the descriptive statistics for the variables. Data were analysed using Mplus v. 6.1 [32] and Stata/IC v. 10.0 for Macintosh. Mplus is a statistical software program that can be used for structural equation modelling and provides tools to test for mediated relationships among variables. Multiple linear regression and path analysis were used to test the hypotheses. The correlation matrix (table 2) displays the degree of relationships among the variables. The relationships among variables displayed in bold (r > 0.85) were too highly correlated and not simultaneously included in further analyses due to the possibility of statistically unstable solutions [33].
Table 1.
Descriptive statistics for criterion and predictor variables.
| variable | obs. | mean | s.d. | min | max | skewness | kurtosis |
|---|---|---|---|---|---|---|---|
| AFR 2009 | 174 | 51.85 | 42.64 | 2.92 | 201.7 | 1.11 | 3.81 |
| AMR 2007 | 176 | 217.52 | 135.45 | 58 | 725 | 1.21 | 4.39 |
| MM 2007 | 176 | 254.74 | 140.70 | 68 | 795 | 0.97 | 3.86 |
| IMR 1995 | 176 | 47.70 | 40.63 | 4 | 169 | 0.92 | 2.92 |
| FIMR 95-00 | 176 | 42.54 | 36.96 | 3 | 148.9 | 0.92 | 2.87 |
| HDI 2005 | 165 | 0.634 | 0.179 | 0.27 | 0.94 | −0.32 | 2.02 |
| GNI 2009 | 166 | 11 242.47 | 16 308.03 | 150 | 86 440 | 2.02 | 6.83 |
| PRIM 2005 | 147 | 1 904 169 | 7 128 098 | 1134 | 6.6e + 07 | 7.57 | 63.17 |
| SEC 2005 | 139 | 1469 725 | 5 330 815 | 806 | 4.78e + 07 | 7.26 | 58.58 |
| EMP 2006 | 163 | 71.24 | 9.46 | 46.1 | 91.1 | −0.37 | 2.47 |
Table 2.
Correlation matrix for all variables. Values displayed in bold are above the 0.85 cut-off as proposed by Kline [33].
| AFR 2009 | AMR 2007 | MM 2007 | IMR 1995 | FIMR 95-00 | HDI 2005 | GNI 2005 | PRIM 2005 | SEC 2005 | EMP 2006 | |
|---|---|---|---|---|---|---|---|---|---|---|
| AFR 2009 | 1.000 | |||||||||
| AMR 2007 | 0.68 | 1.000 | ||||||||
| MM 2007 | 0.64 | 0.98 | 1.000 | |||||||
| IMR 1995 | 0.75 | 0.79 | 0.74 | 1.000 | ||||||
| FIMR 95-00 | 0.76 | 0.81 | 0.75 | 0.99 | 1.000 | |||||
| HDI 2005 | −0.78 | −0.81 | −0.77 | −0.94 | −0.94 | 1.000 | ||||
| GNI 2009 | −0.57 | −0.60 | −0.62 | −0.61 | −0.60 | 0.75 | 1.000 | |||
| PRIM 2005 | −0.00 | −0.06 | −0.06 | 0.04 | 0.05 | −0.01 | −0.06 | 1.000 | ||
| SEC 2005 | −0.07 | −0.09 | −0.09 | −0.01 | −0.02 | −0.01 | −0.06 | 0.08 | 1.000 | |
| EMP 2006 | 0.47 | 0.22 | 0.17 | 0.49 | 0.49 | −0.53 | −0.29 | 0.06 | 0.11 | 1.000 |
In the first phase of analysis, AFR was regressed on variable combinations of extrinsic risk (FIMR and AMR, IMR and AMR, FIMR and MM, IMR and MM). Next, control variables measuring education and socioeconomic factors were added sequentially to each model. Variables GNI, PRIM and SEC did not converge due to different measurement scales (see table 1 for minimum and maximum values). They were divided by 1000, but did not demonstrate statistically significant results. EMP was the only variable that resulted in statistically significant results with measures for current risk and early risk, and was retained in further analyses. Finally, quadratic effects were tested, but did not yield significant results.
In the second step, path analysis (mediation analysis) was conducted using Mplus v. 6.1 to address the following questions: (i) Does extrinsic risk in early childhood have a stronger impact on fertility than current extrinsic risk? And (ii) Does current extrinsic risk mediate the relationship between early extrinsic risk and fertility outcomes? The model was tested using maximum-likelihood robust estimation to correct for non-normal data (see table 1 for skewness and kurtosis values.1 In order for current risk (CR) to mediate the relationship between early risk of death (ER) and AFR, results must display the following characteristics: (i) ER must be significantly related to AFR; (ii) ER must be significantly associated with CR; and (iii) CR must be significantly related to AFR [34]. Results from the path analysis across all variable combinations satisfied these requirements.
A benefit of using maximum-likelihood estimation is that it provides information for ‘goodness of fit’, or the extent to which the proposed model fits the data. Model fit indices included Chi-square statistic (χ2; study criterion of p > 0.010), comparative fit index (CFI; study criterion of greater than 0.95), root mean square error (RMSEA; study criterion of 0.08 or lower) and standardized root mean residual (SRMR; study criterion of 0.08 or lower).
Separate models were analysed using each combination of early and current extrinsic risk to determine the best fitting model. For the purpose of simplicity, the model demonstrating the best global fit will be presented here, and includes variables IMR, AMR, and EMP. Global fit for all four models is presented in table 3. Overall, the models displayed good global fit, except for the RMSEA values. These high values often occur owing to small sample size and few degrees of freedom, but do not necessarily indicate that the model is ill fit [33]. At first, we tested whether IMR had a stronger impact than AMR on AFR. Results indicated that IMR had a stronger direct effect on AFR than AMR (IMR: b = 0.47, p < 0.001; AMR: b = 0.18, p < 0.01). Table 4 presents the direct effects for all models of early and current extrinsic risk, and shows support that all measures for early risk had a stronger impact on fertility than current risk.
Table 3.
Global fit of mediated models. Each model controls for the direct effect of EMP.
| χ2 (d.f.) | p-value | CFI | RMSEA | SRMR | |
|---|---|---|---|---|---|
| model: IMR and AMR | |||||
| partially mediated model | χ2(1) = 6.10 | 0.014 | 0.97 | 0.177 | 0.040 |
| model: FIMR and AMR | |||||
| partially mediated model | χ2(1) = 7.55 | 0.006 | 0.96 | 0.200 | 0.043 |
| model: IMR and MM | |||||
| partially mediated model | χ2(1) = 9.41 | 0.002 | 0.95 | 0.227 | 0.050 |
| model: FIMR and MM | |||||
| partially mediated model | χ2(1) = 11.37 | 0.007 | 0.95 | 0.252 | 0.052 |
Table 4.
Direct, indirect and total effects of all variables presented in completely standardized regression coefficients across all variable combinations. The 90% CIs are included in parentheses.
| causal variable | endogenous variables (90% CI) |
model statistics | ||
|---|---|---|---|---|
| AFR | IMR | AMR | EMP | r2 = 0.60 |
| direct | 0.468b (0.32–0.61) | 0.235a (0.11–0.36) | 0.188b (0.10–0.28) | p < 0.001 |
| total ind. | 0.178a (0.08–0.28) | — | — | n = 163 |
| total effect | 0.646 | 0.235 | 0.188 | |
| AFR | FIMR | AMR | EMP | r2 = 0.61 |
| direct | 0.527b (0.37–0.68) | 0.182a (0.05–0.31) | 0.172b (0.08–0.26) | p < 0.001 |
| total ind. | 0.142a (0.04–0.25) | — | — | n = 163 |
| total effect | 0.669 | 0.182 | 0.172 | |
| AFR | IMR | MM | EMP | r2 = 0.60 |
| direct | 0.477b (0.34–0.61) | 0.227b (0.12–0.34) | 0.197b (0.11–0.29) | p < 0.001 |
| total ind. | 0.164a (0.08–0.25) | — | — | n = 163 |
| total effect | 0.641 | 0.227 | 0.197 | |
| AFR | FIMR | MM | EMP | r2 = 0.61 |
| direct | 0.530b (0.39–0.67) | 0.182a (0.07–0.30) | 0.180b (0.09–0.27) | p < 0.001 |
| total ind. | 0.135a (0.05–0.22) | — | — | n = 163 |
| total effect | 0.665 | 0.182 | 0.180 | |
ap < 0.01; bp < 0.001.
Across all four models, current risk partially mediated the relationship between early risk and AFR, controlling for the effect of EMP (table 4). Figure 1 displays the path model for IMR, AMR, EMP and AFR. The values in the model should be interpreted as standardized regression coefficients. For instance, a one standard deviation increase in EMP predicted a 0.24 unit increase in AFR (p = 0.002). The mediated effect was calculated by multiplying the main direct effect of IMR → AMR and AMR → AFR. Thus, a one standard deviation increase in IMR predicted a 0.18 unit increase in AFR, through the prior impact of AMR and controlling for the effect of EMP (p = 0.003). Overall, this model explains 60 per cent of the variance in AFR. These findings lend support that the effects of early extrinsic risk on subsequent reproduction are mediated by current environmental cues; however, early extrinsic risk has the strongest total effect on reproductive behaviour (table 4).
Figure 1.
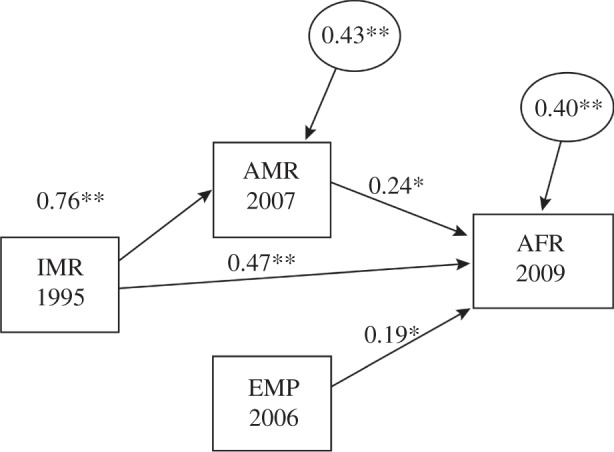
Path model for infant mortality 1995, adult mortality rates in 2007, employment–population ratio for males 2006 and adolescent fertility rates 2009. Values should be interpreted as standardized regression coefficients. The values in squares represent manifest variables and circles include residual values. Overall, this model explains 60% of the variance in AFR. *p < 0.01; **p < 0.001.
Finally, we were interested in testing whether different levels of early extrinsic risk influenced the relationship between current risk of death and AF outcomes. Moderation analysis was conducted in Stata/IC v. 10.0 to test this hypothesis among all variable combinations measuring early and current extrinsic risk. First, the continuous × continuous interaction effect between current risk and early risk was included in regression analyses that initially measured the main effects for each variable combination and controlled for the effect of EMP. Results did not yield statistically significant interactions for any of the models. Interaction effects were also tested for EMP and both measures for extrinsic risk. Results were not significant. New measures for early risk were then created using three quantiles for low, moderate and high risk in Stata/IC v. 10. A series of regression analyses were conducted at each level of early risk for all variable combinations while controlling for EMP. Across models, results were significant at all 3 levels of risk in the early environment. Figure 2 displays the relationship between AMR and AFR at three levels of IMR. Findings indicate that at low levels of early extrinsic risk, fertility rates remain low in the presence of current risk. This pattern suggests that current risk of death does not cause populations to shift to a fast life-history strategy if extrinsic risk was low in the sensitive period. Perhaps these populations did not acquire the cognitive/behavioural ‘tools’ in the early environment that lead to increased mating effort in response high risk of death. Conversely, populations that experience high levels of early extrinsic risk display a fast life-history strategy in the presence of current extrinsic risk (figure 2). In this case, current risk may serve as a recognizable cue for populations to increase mating effort.
Figure 2.
The estimated effects of adolescent fertility rates in 2009 regressed on adult mortality in 2007 during low, moderate and high levels of infant mortality in 1995. The graph includes 95% CIs and plotted data points at three quantiles of IMR. AFR 2009 is expressed as ‘births per 1000 women’ and AMR 2007 is expressed as ‘probability of dying between 15 and 60 years per 1000 population’.
This relationship between early and current risk remained stable across all variable combinations and shed additional light on the mediated relationship between early extrinsic risk and AFR by suggesting that early environmental cues canalize reproductive behaviour (see electronic supplementary material for results).
4. Discussion
Results were significant for main effects of extrinsic mortality in infancy and at maturity on fertility rates. Early extrinsic risk had a larger impact on fertility than current risk; however, conditions in the current environment partially mediated the relationship between early extrinsic risk and subsequent fertility. Results from the moderation analysis were not significant, but a quantile split at three levels suggested that different levels of early risk influence the relationship between current extrinsic risk and reproduction. Harsh conditions in the early environment resulted in a stronger relationship between current risk and fertility outcomes. This result is consistent with a sensitive period in early childhood (from 1 to about 7 years) [5,8,13–15,17], and is similar to a suggestion made by Low et al. [3] regarding fertility patterns in Zimbabwe. These researchers found high-population-level mortality rates in the current environment in association with delayed reproduction. Low et al. [3] proposed that this pattern was due to the recent and rapid spread of HIV. Findings such as these may benefit from a closer examination of how conditions across the lifespan influence timing and pace of reproduction. By using findings from the current study, it may be predicted that those populations delaying reproduction in the presence of current risk faced low levels of early extrinsic risk and may not have developed behavioural strategies to respond to harsh environmental cues.
This study has several limitations that need to be addressed. First, we did not use life expectancy as a measure for early extrinsic mortality, which diverges from previous research on life-history outcomes [3,35]. However, early principals of life-history theory indicate that local mortality rates are a primary predictor of fertility [7], and cross-disciplinary research shows that mortality rates are a reliable indicator of extrinsic risk in shaping life-history strategies [5,36,37]. Furthermore, other studies demonstrate that life expectancy and infant mortality rates are strongly correlated [38,39]. Thus, our measures for extrinsic risk may not be a serious limitation, but future research should focus on other indicators for extrinsic risk in shaping reproductive behaviour.
Use of nation-level data is also problematic, as methodological inconsistencies sometimes lead to less-than ideal data. We compensated for this by using the most up-to-date statistics available from the UN database (see the electronic supplementary material). Furthermore, multiple historical, ecological, psychological and social factors shape reproductive outcomes. Our models accounted for approximately 60 per cent of AFRs, which suggests that other factors are influencing reproduction. Unfortunately, the UN database does not include these measures. This limitation provides another avenue of future research that focuses on specific cultures to test the interaction between early and current extrinsic risk. Despite these potential concerns, our significant findings support both theoretical and empirical research that examines developmental and environmental factors that shape life-history strategies, and provides the first population-level examination of the interaction between early and current extrinsic risk in shaping fertility outcomes.
Life-history theory provides a framework to investigate environmental cues that shape timing of reproduction. Empirical research is beginning to demonstrate a nonlinear, complex relationship between extrinsic risk and fertility outcomes [3,5]. Our results draw attention to the complex relationship between early and current environmental risk at the population-level. Although extrinsic mortality at birth strongly canalizes adult reproductive effort, current risk of mortality also appears to influence fertility patterns across populations.
Acknowledgements
We thank Stoney Brooks, Leonard Burns, Mark Caudell, Courtney Helfrecht, Craig Parks, Casey Roulette and reviewers for helpful comments and suggestions.
Endnote
Skewness and kurtosis were calculated in Stata/IC v. 10. The acceptable value for skewness is 0 and kurtosis is 3.
References
- 1.Stearns S. C. 1992. The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Anderson K. G. 2010. Life expectancy and the timing of life history events in developing countries. Hum. Nat. 21, 103–123 10.1007/s12110-010-9087-z. (doi:10.1007/s12110-010-9087-z.) [DOI] [Google Scholar]
- 3.Low B. S., Hazel A., Parker N., Welch K. B. 2008. Influences on women's reproductive lives – unexpected ecological underpinnings. Cross-Cult. Res. 42, 201–219 10.1177/1069397108317669 (doi:10.1177/1069397108317669) [DOI] [Google Scholar]
- 4.Quinlan R. 2007. Human parental effort & environmental risk. Proc. R. Soc. B 274, 121–125 10.1098/rspb.2006.3690 (doi:10.1098/rspb.2006.3690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinlan R. 2010. Extrinsic mortality effects on reproductive strategies in a Caribbean community. Hum. Nat. 21, 124–139 10.1007/s12110-010-9085-1 (doi:10.1007/s12110-010-9085-1) [DOI] [Google Scholar]
- 6.Roff D. A. 2002. Life history evolution. Sunderland, MA: Sinauer [Google Scholar]
- 7.Chisholm J. S. 1993. Death, hope, and sex: life history theory and the development of reproductive strategies. Curr. Anthropol. 34, 1–24 10.2277/0521597080 (doi:10.2277/0521597080) [DOI] [Google Scholar]
- 8.Chisholm J. 1999. Death, hope and sex: steps to an evolutionary ecology of mind and morality. New York, NY: Cambridge [Google Scholar]
- 9.Promislow D. E. L., Harvey P. H. 1991. Mortality rates and the evolution of mammal life histories. Acta Oecol. 12, 119–137 [Google Scholar]
- 10.Daly M., Wilson M. 1997. Life expectancy, economic inequality, homicide, and reproductive timing in Chicago neighborhoods. Br. Med. J. 314, 1271–1274 10.1136/bmj.314.7089.1271 (doi:10.1136/bmj.314.7089.1271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis B. J. 2004. Timing of pubertal maturation in girls: an integrated life history approach. Psychol. Bull. 130, 920–958 10.1037/0033-2909.130.6.920 (doi:10.1037/0033-2909.130.6.920) [DOI] [PubMed] [Google Scholar]
- 12.Vitzthum V. J. 2001. Why not so great is still good enough: Flexible responsiveness in human reproductive functioning. In Reproductive ecology and human evolution (ed. Ellison P. T.), pp. 179–202 New York, NY: Aldine de Gruyter [Google Scholar]
- 13.Belsky J., Steinberg L., Draper P. 1991. Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization . Child Dev. 62, 647–670 10.1111/j.1467-8624.1991.tb01558.x (doi:10.1111/j.1467-8624.1991.tb01558.x) [DOI] [PubMed] [Google Scholar]
- 14.Draper P., Harpending H. 1982. Father absence and reproductive strategy: an evolutionary perspective. J. Anthropol. Res. 38, 255–279 [Google Scholar]
- 15.Ellis B., McFadyen-ketchum S., Dodge K., Pettit G., Bates J. 1999. Quality of early family relationships and individual differences in the timing of pubertal maturation in girls: a longitudinal test of an evolutionary model. J. Pers. Soc. Psychol. 77, 387–401 10.1037/0022-3514.77.2.387 (doi:10.1037/0022-3514.77.2.387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nettle D., Coall D. A., Dickins T. E. 2011. Early-life conditions and age at first pregnancy in British women. Proc. R. Soc. B 278, 1721–1727 10.1098/rspb.2010.1726 (doi:10.1098/rspb.2010.1726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinlan R., Quinlan M. 2007. Parenting and cultures of risk: a comparative analysis of infidelity, aggression and witchcraft. Am. Anthropol. 109, 164–179 10.1525/aa.2007.109.1.164 (doi:10.1525/aa.2007.109.1.164) [DOI] [Google Scholar]
- 18.Rutter M., O'Conner T. 2004. Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Dev. Psych. 40, 81–94 10.1037/0012-1649.40.1.81 (doi:10.1037/0012-1649.40.1.81) [DOI] [PubMed] [Google Scholar]
- 19.Flinn M.V., Quinlan R., Coe K., Ward C. 2008. Evolution of the human family. In Family relations: an evolutionary perspective (eds Salmon C., Shackelford T.), pp. 16–38 Oxford, UK: Oxford University Press [Google Scholar]
- 20.Coall D. A., Chisholm J. S. 2010. Reproductive development and parental investment during pregnancy: moderating influence of mother's early environment. Am. J. Hum. Biol. 20, 143–153 10.1002/ajhb.20965 (doi:10.1002/ajhb.20965) [DOI] [PubMed] [Google Scholar]
- 21.Whiting B. 1980. Culture and social behavior: a model for the development of social behavior. Ethos 8, 95–116 10.1525/eth.1980.8.2.02a00010 (doi:10.1525/eth.1980.8.2.02a00010) [DOI] [Google Scholar]
- 22.Quinlan R. J., Flinn M. V. 2003. Intergenerational transmission of conjugal stability in a Caribbean village. J. Comp. Fam. Stud. 34, 569–583 [Google Scholar]
- 23.United Nations Statistics Division. 2011. See http://data.un.org/Default.aspx (accessed 1 October 2011). [Google Scholar]
- 24.United Nations 2012. Department of Economic and Social Affairs: Population Division, Population Estimates and Projections Section. See http://esa.un.org/unpd/wpp/index.htm (accessed 10 April 2012). [Google Scholar]
- 25.Millennium Development Goals Indicators 2012. The Official United Nations Site for the MDG Indicators. See http://mdgs.un.org/unsd/mdg/ (accessed 10 January 2012). [Google Scholar]
- 26.World Health Organization. 2012. See http://www.who.int/whosis/en/ (accessed 10 January 2012). [Google Scholar]
- 27.Hill K., Thomas K., AbouZahr C., Walker N., Say L., Inoue M., Suzuki E. 2007. Estimates of maternal mortality worldwide between 1990 and 2005: an assessment of available data. Lancet 370, 1311–1319 10.1016/S0140-6736(07)61572-4 (doi:10.1016/S0140-6736(07)61572-4) [DOI] [PubMed] [Google Scholar]
- 28.Hogan M. C., Foreman K. J., Naghavi M., Ahn S. Y., Wang M., Makela S., Lopez A. D., Lozano R., Murray C. J. L. 2010. Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards millennium development goal 5. Lancet 375, 1609–1623 10.1016/S0140-6736(10)60518-1 (doi:10.1016/S0140-6736(10)60518-1) [DOI] [PubMed] [Google Scholar]
- 29.Human Development Reports 2012. See http://hdr.undp.org/en/statistics/indices/ (accessed 10 January 2012). [Google Scholar]
- 30.The State of the World's Children 2011. See http://www.unicef.org/sowc2011/index.php (accessed 1 October 2011). [Google Scholar]
- 31.United Nations Statistics Division: Gender Info 2007. See http://unstats.un.org/unsd/demographic/products/genderinfo/ (accessed 10 January 2011) [Google Scholar]
- 32.Muthén B., Muthén L. 2010. Mplus user's guide (version 6.0). Los Angeles, CA: Muthén & Muthén [Google Scholar]
- 33.Kline R. B. 2010. Principles and practice of structural equation modeling. New York, NY: The Guilford Press [Google Scholar]
- 34.Baron R. M., Kenny D. A. 1986. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 51, 1173–1182 10.1037/0022-3514.51.6.1173 (doi:10.1037/0022-3514.51.6.1173) [DOI] [PubMed] [Google Scholar]
- 35.Chishom J. S., Quinlivan J. A., Peterson R. W., Coall D. A. 2005. Early stress predicts age at menarche and first birth, adult attachment, and expected lifespan. Hum. Nat. 16, 233–265 10.1007/s12110-005-1009-0 (doi:10.1007/s12110-005-1009-0). [DOI] [PubMed] [Google Scholar]
- 36.Bereczkei T. 1993. R-selected reproductive strategies among Hungarian gipsies: a preliminary analysis. Ethol. Sociobiol. 14, 71–88 10.1016/0162-3095(93)90008-6 (doi:10.1016/0162-3095(93)90008-6) [DOI] [Google Scholar]
- 37.Geronimus A. T., Bound J., Colen C. G. 2011. Excess black mortality in the United States and in selected black and white high-poverty areas, 1980–2000. Am. J. Public Health 101, 720–729 10.2105/AJPH.2010.195537 (doi:10.2105/AJPH.2010.195537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hertz E., Herbert J. R., Landon J. 1994. Social and environmental factors and life expectancy, infant mortality, and maternal mortality rates: Results of a cross-national comparison. Soc. Sci. Med. 39, 105–114 10.1016/0277-9536(94)90170-8 (doi:10.1016/0277-9536(94)90170-8) [DOI] [PubMed] [Google Scholar]
- 39.Lee K., Park S., Khoshnood B., Hsieh H. L., Mittendorf R. 1997. Human development index as a predictor of infant and maternal mortality rates. J. Pediatr. 131, 430–433 10.1016/S0022-3476(97)80070-4 (doi:10.1016/S0022-3476(97)80070-4) [DOI] [PubMed] [Google Scholar]



