Abstract
While investment in territory defence is expected to be influenced by its benefits, the additional role that costs may play is rarely considered. Here, we quantify both benefits and costs of repelling prospecting males in cooperative meerkats, and demonstrate that both are required to explain the substantial variation in individual contributions to the defence observed. Males benefit more from repelling prospectors than females, as males may lose dominance and be expelled during intrusions. Accordingly, males invest the most in repelling prospectors. We also show that males experience an associated cost in the form of reduced weight gain and, as such, heavier males contribute more to chasing prospectors. Finally, we show evidence of a cost not restricted to individuals engaged in chasing: both males and females reduce their contributions to feeding dependent pups when prospectors are present, resulting in a reduction in pup weight gain in this context. Males appear to adjust their contributions to chasing in light of this cost, chasing at lower rates when their group contains dependent young. Our findings support the view that investment in cooperative behaviours can be attributed to benefits and costs, and highlight the additional importance of considering trade-offs in investment between cooperative behaviours.
Keywords: cooperative breeding, cooperative care, prospecting, territorial behaviour, meerkat, Suricata suricatta
1. Introduction
Group-living species regularly defend territories and the resources within them from intrusions by conspecific rivals [1–3], with individual group members often differing markedly in their contributions [4,5]. Differential benefits may play a key role in mediating these differences in contributions to territory defence: territorial intruders are often in search of breeding opportunities [6], and, consequently, aggression by resident individuals towards intruders is typically sex-specific [7] and can be influenced by social status, which may determine access to breeding opportunities [8,9]. Few studies, however, have considered the likely additional importance of the costs of territorial defence for understanding individual differences in investment [10]. Repelling intruders is expected to entail not only risk to self and energetic costs [5,11,12], but may also entail more complex costs through trade-offs with other key behaviours (e.g. parental care [13]). Measuring these costs along with the benefits should help to advance our understanding of the causes of individual differences in cooperative contributions to territory defence [10,14].
While the costs of repelling intruders may be determined primarily by the level of investment in high-energy or high-risk territorial behaviours (e.g. chasing and fighting [11,15]), the negative effects of territorial intrusions may be widespread across the group. The presence of territorial intruders can be disruptive to normal group activities, with resident individuals engaging in low-risk territorial behaviours at times when they might otherwise have been foraging [3,16]. In addition, as many territorial intrusions occur during the breeding season [6], investment in territorial behaviours may come at the expense of investment in activities related to the rearing of young [13], particularly in species that produce multiple litters within an extended breeding season. Measuring these potential effects of the presence of territorial intruders, in terms of individual changes in body mass and contributions to the care of dependent young, is essential for understanding the overall costs of repelling intruders. Our limited understanding of costs of this kind is doubtless due in part to the difficulties in observing and identifying individual group members during interactions with intruders in the wild [7,17], and simultaneously monitoring changes in state and contributions to care.
In this study, we investigate individual variation in contributions to territorial defence, quantify both its benefits and costs in the cooperatively breeding meerkat, (Suricata suricatta), and consider the extent to which these benefits and costs appear to have shaped the patterns of contributions observed. Meerkats live in groups of up to 50 individuals, where a single, typically unrelated, dominant pair largely monopolizes within-group reproduction, and close inbreeding is avoided [18,19]. Dispersal is delayed beyond the age of sexual maturity in both males and females, who remain in their natal groups as subordinate helpers [20,21], but subordinate males conduct extraterritorial prospecting forays throughout the breeding season [22]. Prospecting males regularly approach foreign groups, and attempt to mate with dominant and subordinate females, which can lead to appreciable levels of extra-group paternity [19,22]. These events may not only reduce dominant male reproductive success [19], but also increase reproductive conflict between dominant and subordinate females, as subordinates typically lack unrelated breeding partners in their natal group [18]. Prospecting males have also been reported to take over established breeding groups [19,23], and previous studies suggest that resident males respond aggressively to intrusions by prospectors [23–25]. However, the factors that affect individual contributions to prospector repulsion have yet to be investigated, the benefits of such behaviour remain poorly understood and its costs are entirely unexplored.
Given that prospectors typically approach groups that are actively foraging, territorial behaviours by residents may be expected to affect energy expenditure and reduce the time individuals are able to spend foraging. However, if the presence of prospecting males is disruptive to a group's overall investment in foraging, reductions in time spent foraging (and, consequently, lower weight gain rates) may extend to the whole group. Meerkat pups start foraging with the group when they are about 30 days old, but remain nutritionally dependent on food provisioned by older group members until approximately 90 days of age [26]. Whether investment in the repulsion of prospectors generates additional costs by trading off against contributions to care in cooperatively breeding species, as has been suggested to occur in birds with biparental care [13], is as yet unknown.
Here, we first investigate the patterns of individual contributions to prospector repulsion through the leading of chases of intruding males. Second, we investigate the benefits of investing in prospector repulsion, focusing specifically on the benefits for residents of averting prospector immigrations (takeovers) in terms of the maintenance of group membership and social status. Third, we explore the potential short-term costs of repelling prospectors, by measuring the effect of prospector presence on individual rates of weight gain and contributions to feeding dependent young. We then consider the extent to which these benefits and costs appear to have shaped the observed patterns of contributions to prospector repulsion.
2. Methods
(a). Study population
The study was conducted at the Kuruman River Reserve (26°59′ S, 21°50′ E) and its surrounding ranch land in the southern Kalahari Desert, South Africa. Details on climate and habitat at the study site are described elsewhere [27]. The meerkats in our study population were habituated to close observation (within 2 m) and individually identifiable by unique dye marks on their fur. Groups were visited at least once every three days from 1998 to 2009 as part of a long-term study, and life-history events such as birth, emigration, immigration and changes in dominance were known almost to the day. The presence of prospecting males at a given group was easily noted owing to their conspicuous behaviour (standing for long periods at the edge of a group, facing them and occasionally slinking towards resident individuals) and the ease with which individuals could be identified as foreign to the focal group through their unique dye marks.
(b). Statistical analyses
Analyses were conducted using R (v. 2.13.1 [28]) with lme4 (v. 0.999375-40 [29]) and glmmADMB (v. 0.6.4 [30]) for building linear mixed models (LMMs) and generalized linear mixed models (GLMMs). Details on the model selection method using Akaike's information crietrion (AIC) [31] and on the input variables fitted in each model (i.e. measures of body mass and rainfall) are included in the electronic supplementary material. Here, we report effect size estimates with 95 per cent confidence intervals (CIs) for predictor variables included in the best-fitting model of those considered in each analysis. For all our analyses, individuals were assigned into age categories [20] as follows: pup (less than 91 days of age), juvenile (91–180 days of age), subadult (181–360 days of age), yearling (361–720 days of age) and adult (more than 720 days of age). Adults were either dominant or subordinate [18,19], with all other age categories including only subordinate individuals (rare cases when a yearling was dominant were excluded from our analyses).
(c). Contributions to repelling prospectors
To determine the levels of investment in repelling prospecting males by resident individuals, we conducted behavioural observations of groups when prospectors were present, during the 2008 and 2009 breeding seasons (September–February). We conducted a total of 62 observation sessions (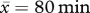 of observation time, s.d. = 27) across 11 groups and recorded ad libitum each time a prospector was chased (i.e. charged at for more than 2 m) by residents, noting the identity of individuals that led chases. Chases led by individuals less than six months old were extremely rare (1% of chases) and were excluded from our analyses.
of observation time, s.d. = 27) across 11 groups and recorded ad libitum each time a prospector was chased (i.e. charged at for more than 2 m) by residents, noting the identity of individuals that led chases. Chases led by individuals less than six months old were extremely rare (1% of chases) and were excluded from our analyses.
We first measured the overall difference between resident males and females in proportions of chases led. The total number of chases led by individuals of each sex (numerator) and the total number of chases recorded per observation session (denominator) were fitted as the proportional response variable in a candidate set of binomial GLMMs with logit link function (for details of terms fitted in each model, see electronic supplementary material, table S1). Chases led by each sex were entered separately into the model, as a single chase could be led by both female and male residents.
A second set of GLMMs was used to determine individual differences among males in the rate at which they led chases (females were excluded given the few chases they led; see §3). We fitted the number of chase leads as the response variable in negative binomial GLMMs (see electronic supplementary material, table S2) that accounted for the duration of each observation session as an offset (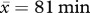 of observation time, s.d. = 26).
of observation time, s.d. = 26).
(d). Benefits of averting prospector takeovers
To determine the effects of prospecting male immigrations on resident individuals' social status and group membership, we measured changes in group composition a week after an immigration event. We considered all cases when foreign males immigrated into groups (n = 27 groups) with a breeding hierarchy of adult females and one or more adult males (regardless of social status) from 12 years of field observations. We restricted our analysis to the fates of individuals over six months old, based on minimum ages for breeding attempts of both males (observed prospecting or mating) and females (observed mating or pregnant). If resident dominants (n = 27 females and 10 males) remained in their group after the immigration event, we assessed whether they maintained their social status up to three months after the event.
(e). Costs of repelling prospectors
(i). Effect of prospectors on the weight gain rates of residents
We compared the rates of weight gained by residents on days when foreign males were prospecting at the group with the rates on days when there were no prospectors (within ± 14 days of the prospecting event), to assess the potential effect of the presence of prospectors. Rates of weight gain (g h−1) were estimated using body mass measurements taken in the mornings before individuals started foraging and again 2–4 h later (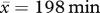 ), collected between 2000 and 2009 from 15 groups. These rates (difference in mass divided by time elapsed between measurements) were fitted as the response variable in two separate candidate sets of LMMs for males and females (see electronic supplementary material, table S3).
), collected between 2000 and 2009 from 15 groups. These rates (difference in mass divided by time elapsed between measurements) were fitted as the response variable in two separate candidate sets of LMMs for males and females (see electronic supplementary material, table S3).
(ii). Effect of prospectors on the pup feeding rates of residents
We compared individual rates of pup feeds on mornings when foreign males were prospecting at the group with the rates on mornings when there were no prospectors (within ± 14 days of the prospecting event), to assess the potential effect of the presence of prospectors. Pup feeds [26] per individual were collected ad libitum while groups were foraging with pups (n = 14 groups). The number of feeds was fitted as the response variable in two separate candidate sets of negative binomial GLMMs for males and females (see electronic supplementary material, table S4) that accounted for the duration of each observation session as an offset (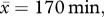 s.d. = 22.9).
s.d. = 22.9).
(iii). Effect of prospectors on the weight gain rates of pups
Using the method described for our analysis on weight gain rates in (i) and body mass records from the foraging sessions in the pup feed analysis (ii), we determined whether pups also gained less weight per hour when prospectors were present than when absent (see electronic supplementary material, table S5).
3. Results
(a). Contributions to repelling prospectors
Chases were typically led by a single resident individual (range 1–3 leaders; n = 344 chases, of which 234 had a single leader), with males leading a higher proportion of chases than females (figure 1a). Within males, age-corrected body mass had a positive effect on the rate of chase leads (electronic supplementary material, table S7; GLMM estimate 0.49, 95% CI 0.05–0.93), the presence of pups had a negative effect (−0.46, 95% CI −0.84 to −0.07) and adults, regardless of social status, led chases at higher rates than subadults (figure 1b), after controlling for the effects of group size and the number of prospectors present (for estimates including interaction term, see electronic supplementary material, table S7).
Figure 1.
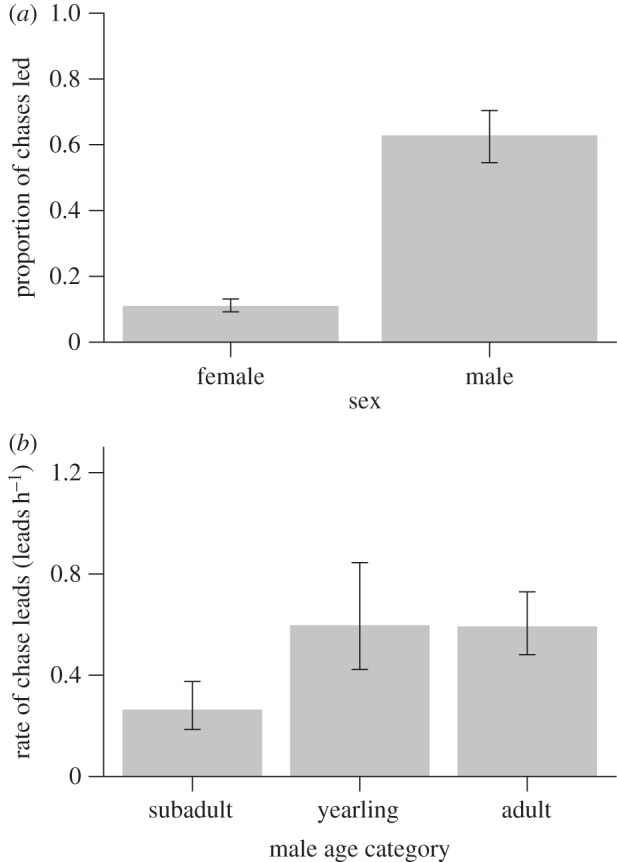
Differences (a) between resident females and males in the proportion of chases of prospectors led (see electronic supplementary material, table S6; GLMM estimate, sex = 2.62, 95% CI 1.71–3.52); and (b) between resident males of different age category in their rates of chase leads (electronic supplementary material, table S7; GLMM estimate, subadult = −5.43, 95% CI −5.78 to −5.08, yearling = −4.61, 95% CI −4.98 to −4.24, adult = −4.62, 95% CI −5.03 to −4.21). Bars present predicted means ± s.e. from the GLMMs, estimated using means of predictor variables not graphed. Proportions in (a) do not sum to one because leaders were not identified in all of the chases recorded.
(b). Benefits of averting prospector takeovers
Resident males were more likely than females to be affected by the immigration of a prospecting male within the first week of the event (out of 27 groups there were 18 wherein males lost dominance or emigrated, and only 2 wherein females disappeared; Fisher's exact test: p < 0.001). Nine males lost dominance and 34 subordinate males left their groups permanently within a week after a prospector immigration event, with all dominant males ultimately leaving over the next three months (n = 10 dominant and 89 subordinate males). Only two subordinate females disappeared from their groups within the first week of the arrival of a new male and one female lost dominance to a subordinate in her group during the next three months (n = 27 dominant and 118 subordinate females). Within males, subadults were the least likely to be affected by prospector immigrations (figure 2).
Figure 2.
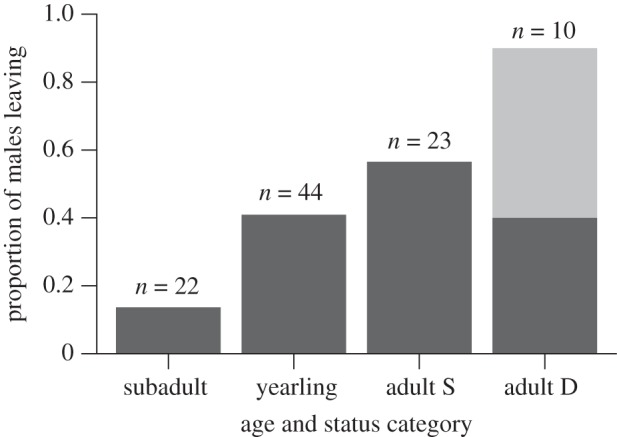
Proportion of males in each age and social status (S, subordinate; D, dominant) category that permanently left their group (dark grey) or stayed but lost dominance (light grey), within the first week after a prospecting male immigrated into their group (n = 27 immigration events).
(c). Costs of repelling prospectors
(i). Effect of prospectors on the weight gain rates of residents
Rates of weight gain dropped more than 60 per cent and 20 per cent for dominant and adult subordinate males, respectively, when prospectors were present at the group (figure 3), after controlling for the effect of total rainfall in the past month (see electronic supplementary material, table S8a; LMM estimate 3.43, 95% CI 2.28–4.59, quadratic term = −1.18, 95% CI −1.72 to −0.63). The rates of weight gain of females, by contrast, were not affected by the presence of prospectors in any age or social status category (see electronic supplementary material, table S8b).
Figure 3.
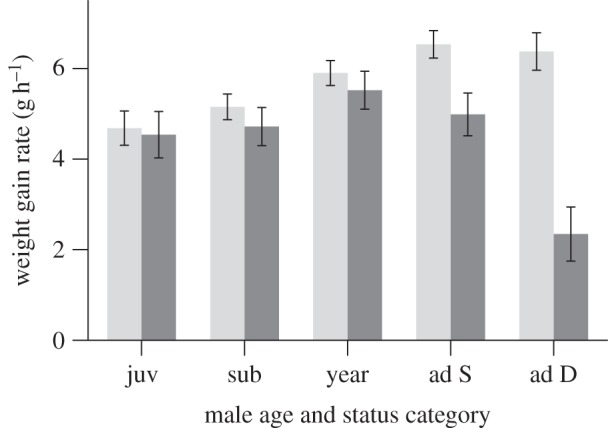
Weight gain rates (g/h) of resident males across different age and social status categories when prospectors were absent (light grey) and present (dark grey) at their group (see electronic supplementary material, table S8a). LMM estimates with 95% CI of interaction with the presence of a prospector: juvenile = −0.14 (−1.35 to 1.06), subadult = −0.44 (−1.39 to 0.51), yearling = −0.38 (−1.32 to 0.56), adult subordinate = −1.55 (−2.60 to −0.49), adult dominant = −4.03 (−5.36 to −2.71). Bars present predicted means ± s.e. from the LMM, estimated using means of predictor variables not graphed.
(ii). Effect of prospectors on pup feeding rates of residents
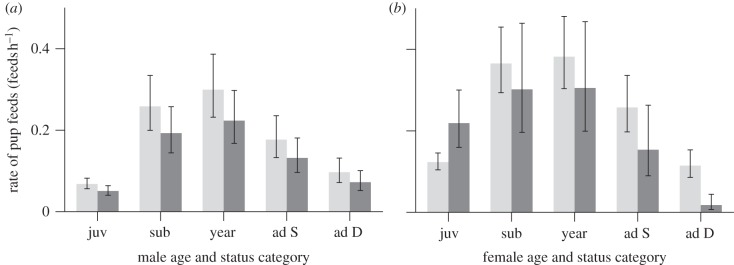
Males across all age and status categories fed pups at lower rates when prospecting males were present at the group than when there were no prospectors (figure 4a), after controlling for the effects of number of pups foraging with the group (see electronic supplementary material, table S9a; GLMM estimate 0.23, 95% CI 0.03–0.43), modal pup age (−0.55, 95% CI −0.76 to −0.35, quadratic term = −1.69, 95% CI −2.12 to −1.26), group size (−0.87, 95% CI −1.11 to −0.63) and total rainfall in the past month (0.53, 95% CI 0.34–0.73). Adult females also reduced their rates of pup feeds in the presence of prospectors, with dominants lowering their rates by 50 per cent (figure 4b) when controlling for the effects of modal pup age (see electronic supplementary material, table S9b; GLMM −0.61, 95% CI −0.81 to −0.40, quadratic term = −1.77, 95% CI −2.21 to −1.34) and group size (−0.75, 95% CI −0.98 to −0.52). By contrast, juvenile females showed a considerable increase in pup feeding rate when prospectors were present (figure 4b).
Figure 4.
Pup feed rates (feeds h−1) of (a) males and (b) females across different age and social status categories when prospectors were absent (light grey) and present (dark grey) at their group. See electronic supplementary material, table S9a for males; GLMM estimate with 95% CI: prospector present = −0.29 (−0.52 to −0.06). See electronic supplementary material, table S9b for females; GLMM estimates with 95% CI of interaction with the presence of a prospector: juvenile = 0.57 (0.07–1.07), subadult = −0.19 (−0.47 to 0.09), yearling = −0.22 (−0.49 to 0.04), adult subordinate = −0.52 (−1.02 to −0.02), adult dominant = −1.86 (−3.05 to −0.66). Bars present predicted means ± s.e. from the GLMMs, estimated using means of predictor variables not graphed.
(iii). Effect of prospectors on the weight gain rates of pups
Pups gained considerably less weight per hour of foraging when prospectors were present than when they were not (figure 5), after controlling for the effects of pup age (see electronic supplementary material, table S10; LMM estimate 1.30, 95% CI 0.83–1.76), number of pups (−0.20, 95% CI −0.34 to −0.05) and total rainfall in the past month (1.29, 95% CI 0.80–1.78).
Figure 5.
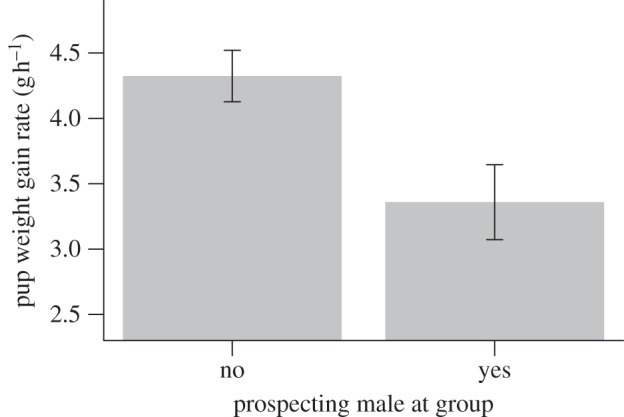
Weight gain rates (g h−1) of pups when prospectors were absent and present at their group (see electronic supplementary material, table S10; LMM estimate, prospector present = −0.96, 95% CI −1.50 to −0.43). Bars present predicted means ± s.e. from the LMM, estimated using means of predictor variables not graphed.
4. Discussion
Our findings suggest that patterns of individual contributions to cooperative territory defence can be attributed to variation in both the benefits and costs of territorial behaviours, and highlight the potential importance of considering trade-offs in investment between cooperative behaviours. Males invested substantially more than females in repelling prospectors, reflecting the benefits of keeping prospectors at bay: males lost dominance and were likely to be expelled from their groups following prospector immigrations, whereas females were not affected by prospector takeovers. However, territorial defence is likely to be costly, as suggested by the reduction in weight gain for males, but not for females, in the presence of prospectors. Accordingly, males that were heavier for their age were able to invest more in leading chases of prospectors. We also show evidence of an additional cost of conflict with extra-group individuals, which is not restricted to those individuals engaged in chasing: both males and females reduced their contributions to pup feeding when prospectors were present, resulting in a marked reduction in pup weight gain in this context. This finding suggests a trade-off between investment in cooperative territorial defence and cooperative care of young. Indeed, males appeared to adjust their contributions to chasing in light of this cost, leading chases at lower rates when their group contained dependent young.
Dominant male meerkats may lose substantial fitness to prospecting males through reductions in not only current [19,22] but also future reproductive success when prospector intrusions and immigrations are successful. Although we did not directly observe males being expelled from their groups during takeovers, nine of the resident males that lost dominance (including those that permanently left) were unrelated to the dominant female, and therefore unlikely to have left voluntarily [19,22,32]. Extra-group male takeovers leading to evictions or even death of resident breeding males have been reported in a number of species where males fiercely defend their territory and mates (e.g. lions, Panthera leo [11]; golden lion tamarins, Leontopithecus rosalia [2]). Given that the probability of becoming a dominant male breeder is low and that reproductive success as a dominant is dependent on tenure length [19,33], repelling prospectors as a way of defending the dominant position may be as important as its function in preventing female extra-group mating. These benefits of investing in territorial behaviours are likely to explain the high rates of chases led by dominant males.
Yearling and adult subordinate males were also expelled from their groups within the first week after the successful immigration of a prospecting male. The threat of losing the safe haven from which to conduct forays in search of mating and dispersal opportunities [34] may explain why older subordinate males led chases of prospectors at rates similar to those of dominants. Subordinate individuals of both sexes are also expected to gain greater indirect fitness benefits from helping to raise offspring fathered by the dominant male rather than by the extra-group males, as he is typically their own father [19,32]. By chasing prospectors, subordinates may therefore be exhibiting an aggressive form of ‘mother guarding’ [35], while also contributing to secure their father's tenure as the dominant breeder.
By contrast, the residency and social status of females were rarely affected by male takeovers, suggesting that, unlike males, females stand to gain little direct benefit from repelling prospectors. Indeed, subordinate females may gain direct benefits from tolerating the presence of prospectors by obtaining access to breeding opportunities [19,32] and, in the case of prospector takeovers, by having an unrelated resident male to partner them if they were to inherit dominance. These direct fitness benefits, coupled with the absence of a clear direct cost arising from male takeovers, probably explain why females contributed substantially less than males to the repulsion of prospecting males. Furthermore, the energetic costs of territorial behaviours may outweigh any potential indirect fitness gain for subordinate females, as females in better condition are more likely to breed [36]. Given that subordinate females are capable of breeding, it is perhaps surprising that the arrival of a new male did not lead to changes in dominance, as has been reported in other species where close inbreeding is avoided (e.g. Damaraland mole-rats, Cryptomys damarensis [37]). Sharp & Clutton-Brock [38] suggest that in meerkats the probability of subordinate females successfully challenging the dominant female is extremely low and the cost of failure is high. This lack of a threat to the dominant female's tenure may also explain why dominant females invested little in chasing prospectors.
The cost of repelling prospectors was reflected in the changes in weight gain rates of residents in the presence of prospectors: males invested highly in chasing prospectors and suffered reduced weight gain rates, whereas females, who invested little in chasing, were unaffected by the presence of prospectors. These results are in line with what was found in stitchbirds (Notiomystis cincta), where male weight loss during the fertile period of their mate is associated with the effort invested in chasing intruding males [15]. Although dominant males led chases at rates comparable with those of adult subordinates, they suffered substantially greater reductions in weight gain than subordinates, suggesting that they may engage in additional behaviours that detract from foraging during prospector intrusions. Probable increases in other previously reported territorial behaviours (e.g. scent marking [16,23,25]) are likely to have contributed to the 60 per cent reduction in weight gain rate observed in dominant males. Given that prospectors typically follow groups for entire days and visit the same groups repeatedly [39], these short-term reductions in weight gain among resident males could lead to significant weight loss over the entire breeding season, as has been shown in stitchbirds [15] and several ungulate species [40]. Indeed, our findings suggest that males adjust their contributions to chasing so as to mitigate this potential weight loss cost, as males that were heavier for their age contributed more to chasing than those that were lighter.
Our analyses also revealed evidence of a second cost associated with prospector repulsion, which was not restricted solely to those individuals engaged in chasing. In the presence of prospectors, both male and female group members fed dependent pups at lower rates, resulting in reduced overall rates of weight gain among the pups. Among males, this pattern could reflect an energetic trade-off between chasing and pup feeding, as contributions to pup feeding are state-dependent [20]. Similarly, elevations in testosterone levels could occur among resident males engaged in chasing intruders, and this too could account for their reduced rates of pup feeding [13]. Given that females chased prospectors less frequently than males, it is perhaps surprising that they too showed a reduction in pup feeding rates comparable with that observed in males. The presence of prospectors and the chasing that ensues may disrupt normal foraging and care activities even for residents that do not take an active role in repelling prospectors. Females, particularly those with greater chances of breeding, may prioritize maintaining their own condition rather than feeding pups under these circumstances. Indeed, adult females greatly reduced their contributions to feeding, whereas juvenile (i.e. reproductively immature) females increased their contributions in the presence of prospectors.
While young females appeared to partially compensate for the reductions in pup feeding rates of others when prospectors were present by increasing their own rates, pups still experienced an overall reduction in their rates of weight gain when prospectors were present. Although we have measured only the short-term effects on pup body mass, these reductions in weight gain could have long-term effects if they occur frequently over the breeding season, as pups in better body condition have a higher probability of gaining reproductive success as adults [21]. To our knowledge, this is the first study to demonstrate effects of intrusions on contributions to cooperative care, findings that are suggestive of a trade-off in investment between cooperative behaviours. Indeed, our findings suggest that cooperative contributions to chasing are adjusted so as to minimize this net cost of chasing, as males were less likely to lead chases when dependent pups were foraging with their group.
In conclusion, dominant and older subordinate male meerkats seem to cooperate in territorial defence by chasing intruders. By repelling prospectors, dominant males may secure their top breeding position and reproductive success; males, in general, secure their group membership; and subordinates gain indirect fitness benefits from assisting in this regard. However, territorial defence appears to be costly, as shown by the reduction in weight gain among males, but not among females, in the presence of prospectors. The negative effects of male territorial intrusions also extend to pup weight gain, as feeding rates across individuals of both sexes were lower in the presence of prospectors. Both benefits and costs of prospector repulsion appear to have shaped the patterns of cooperative contributions to territorial defence, as males contributed substantially more than females, did so in a condition-dependent manner and tempered their chasing when simultaneously feeding dependent young. Together, our findings support the view that variation in individual contributions to cooperative behaviour can be attributed to variation in both its benefits and costs, and highlight the additional importance of considering trade-offs in investment between different cooperative behaviours.
Acknowledgments
We thank the management teams and the many volunteers at the Kalahari Meerkat Project (KMP) over the years for data collection; M. Manser for her role in maintaining the KMP; the Kotze family for allowing us to work on their land; and the Northern Cape Conservation Authority for granting us permission to conduct research in the Kalahari. We also thank D. Levesque and N. Harrison for their invaluable help in collecting data; D. Lukas for stimulating discussions; A. Bateman and X. Harrison for statistical advice; and S. English, I. Cuthill and two anonymous reviewers for comments that greatly improved the original manuscript. The long-term project was funded by research grants of NERC (UK) and Earthwatch Institute (USA). R.M. was supported by the Instituto para la Formación y Aprovechamiento de Recursos Humanos (Panama) and the Secretaría Nacional de Ciencia, Tecnología e Innovación (Panama) scholarship programme.
References
- 1.Packer C., Scheel D., Pusey A. E. 1990. Why lions form groups: food is not enough. Am. Nat. 136, 1–19 10.1086/285079 (doi:10.1086/285079) [DOI] [Google Scholar]
- 2.Baker A. J., Dietz J. M. 1996. Immigration in wild groups of golden lion tamarins (Leontopithecus rosalia). Am. J. Primatol. 38, 47–56 (doi:10.1002/(SICI)1098-2345(1996)38:1<47::AID-AJP5>3.0.CO;2-T) [DOI] [PubMed] [Google Scholar]
- 3.Boydston E. E., Morelli T. L., Holekamp K. E. 2001. Sex differences in territorial behavior exhibited by the spotted hyena (Hyaenidae, Crocuta crocuta). Ethology 107, 369–385 10.1046/j.1439-0310.2001.00672.x (doi:10.1046/j.1439-0310.2001.00672.x) [DOI] [Google Scholar]
- 4.Heinsohn R., Packer C., Pusey A. E. 1996. Development of cooperative territoriality in juvenile lions. Proc. R. Soc. Lond. B 263, 475–479 10.1098/rspb.1996.0071 (doi:10.1098/rspb.1996.0071) [DOI] [PubMed] [Google Scholar]
- 5.Kitchen D. M., Horwich R. H., James R. A. 2004. Subordinate male black howler monkey (Alouatta pigra) responses to loud calls: experimental evidence for the effects of intra-group male relationships and age. Behaviour 141, 703–723 10.1163/1568539042245196 (doi:10.1163/1568539042245196) [DOI] [Google Scholar]
- 6.Møller A. P. 1987. Intruders and defenders on avian breeding territories: the effect of sperm competition. Oikos 48, 47–54 10.2307/3565687 (doi:10.2307/3565687) [DOI] [Google Scholar]
- 7.Cant M. A., Otali E., Mwanguhya F. 2002. Fighting and mating between groups in a cooperatively breeding mammal, the banded mongoose. Ethology 108, 541–555 10.1046/j.1439-0310.2002.00795.x (doi:10.1046/j.1439-0310.2002.00795.x) [DOI] [Google Scholar]
- 8.O'Riain M. J., Jarvis J. U. M. 1997. Colony member recognition and xenophobia in the naked mole-rat. Anim. Behav. 53, 487–498 10.1006/anbe.1996.0299 (doi:10.1006/anbe.1996.0299) [DOI] [Google Scholar]
- 9.Cooney R. 2002. Colony defense in Damaraland mole-rats, Cryptomys damarensis. Behav. Ecol. 13, 160–162 10.1093/beheco/13.2.160 (doi:10.1093/beheco/13.2.160) [DOI] [Google Scholar]
- 10.Nunn C. L. 2000. Collective benefits, free-riders, and male extra-group conflict. In Primate males: causes and consequences of variation in group composition (ed. Kappeler P. M.), pp. 192–204 Cambridge, UK: Cambridge University Press [Google Scholar]
- 11.Grinnell J., Packer C., Pusey A. E. 1995. Cooperation in male lions: kinship, reciprocity or mutualism? Anim. Behav. 49, 95–105 10.1016/0003-3472(95)80157-X (doi:10.1016/0003-3472(95)80157-X) [DOI] [Google Scholar]
- 12.Lazaro-Perea C. 2001. Intergroup interactions in wild common marmosets, Callithrix jacchus: territorial defence and assessment of neighbours. Anim. Behav. 62, 11–21 10.1006/anbe.2000.1726 (doi:10.1006/anbe.2000.1726) [DOI] [Google Scholar]
- 13.Wingfield J. C., Hegner R. E., Dufty A. M., Ball G. F. 1990. The challenge hypothesis: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846 10.1086/285134 (doi:10.1086/285134) [DOI] [Google Scholar]
- 14.Heinsohn R., Packer C. 1995. Complex cooperative strategies in group-territorial African lions. Science 269, 1260–1262 10.1126/science.7652573 (doi:10.1126/science.7652573) [DOI] [PubMed] [Google Scholar]
- 15.Low M. 2006. The energetic cost of mate guarding is correlated with territorial intrusions in the New Zealand stitchbird. Behav. Ecol. 17, 270–276 10.1093/beheco/arj025 (doi:10.1093/beheco/arj025) [DOI] [Google Scholar]
- 16.Jordan N. R., Cherry M. I., Manser M. B. 2007. Latrine distribution and patterns of use by wild meerkats: implications for territory and mate defence. Anim. Behav. 73, 613–622 10.1016/j.anbehav.2006.06.010 (doi:10.1016/j.anbehav.2006.06.010) [DOI] [Google Scholar]
- 17.Wingfield J. C., Lewis D. M. 1993. Hormonal and behavioral responses to simulated territorial intrusion in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. Anim. Behav. 45, 1–11 10.1006/anbe.1993.1001 (doi:10.1006/anbe.1993.1001) [DOI] [Google Scholar]
- 18.O'Riain M. J., Bennett N. C., Brotherton P. N. M., McIlrath G., Clutton-Brock T. H. 2000. Reproductive suppression and inbreeding avoidance in wild populations of co-operatively breeding meerkats (Suricata suricatta). Behav. Ecol. Sociobiol. 48, 471–477 10.1007/s002650000249 (doi:10.1007/s002650000249) [DOI] [Google Scholar]
- 19.Spong G. F., Hodge S. J., Young A. J., Clutton-Brock T. H. 2008. Factors affecting the reproductive success of dominant male meerkats. Mol. Ecol. 17, 2287–2299 10.1111/j.1365-294X.2008.03734.x (doi:10.1111/j.1365-294X.2008.03734.x) [DOI] [PubMed] [Google Scholar]
- 20.Clutton-Brock T. H., Russell A. F., Sharpe L. L., Young A. J., Balmforth Z., McIlrath G. M. 2002. Evolution and development of sex differences in cooperative behavior in meerkats. Science 297, 253–256 10.1126/science.1071412 (doi:10.1126/science.1071412) [DOI] [PubMed] [Google Scholar]
- 21.Russell A. F., Young A. J., Spong G., Jordan N. R., Clutton-Brock T. H. 2007. Helpers increase the reproductive potential of offspring in cooperative meerkats. Proc. R. Soc. B 274, 513–520 10.1098/rspb.2006.3698 (doi:10.1098/rspb.2006.3698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young A. J., Spong G., Clutton-Brock T. H. 2007. Subordinate male meerkats prospect for extra-group paternity: alternative reproductive tactics in a cooperative mammal. Proc. R. Soc. B 274, 1603–1609 10.1098/rspb.2007.0316 (doi:10.1098/rspb.2007.0316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doolan S. P., Macdonald D. W. 1996. Dispersal and extra-territorial prospecting by slender-tailed meerkats (Suricata suricatta) in the south-western Kalahari. J. Zool. 240, 59–73 10.1111/j.1469-7998.1996.tb05486.x (doi:10.1111/j.1469-7998.1996.tb05486.x) [DOI] [Google Scholar]
- 24.Young A. J., Carlson A. A., Clutton-Brock T. H. 2005. Trade-offs between extraterritorial prospecting and helping in a cooperative mammal. Anim. Behav. 70, 829–837 10.1016/j.anbehav.2005.01.019 (doi:10.1016/j.anbehav.2005.01.019) [DOI] [Google Scholar]
- 25.Mares R., Young A. J., Levesque D. L., Harrison N., Clutton-Brock T. H. 2011. Responses to intruder scents in the cooperatively breeding meerkat: sex and social status differences and temporal variation. Behav. Ecol. 22, 594–600 10.1093/beheco/arr021 (doi:10.1093/beheco/arr021) [DOI] [Google Scholar]
- 26.Brotherton P. N. M., Clutton-Brock T. H., O'Riain M. J., Gaynor D., Sharpe L., Kansky R., McIlrath G. M. 2001. Offspring food allocation by parents and helpers in a cooperative mammal. Behav. Ecol. 12, 590–599 10.1093/beheco/12.5.590 (doi:10.1093/beheco/12.5.590) [DOI] [Google Scholar]
- 27.Russell A. F., Clutton-Brock T. H., Brotherton P. N. M., Sharpe L. L., Mcilrath G. M., Dalerum F. D., Cameron E. Z., Barnard J. A. 2002. Factors affecting pup growth and survival in co-operatively breeding meerkats Suricata suricatta. J. Anim. Ecol. 71, 700–709 10.1046/j.1365-2656.2002.00636.x (doi:10.1046/j.1365-2656.2002.00636.x) [DOI] [Google Scholar]
- 28.R Development Core Team. 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 29.Bates D., Maechler M., Bolker B. 2011. lme4: linear mixed-effects models using S4 classes. R package; See http:/cran.r-project.org/web/packages/lme4//index.html [Google Scholar]
- 30.Skaug H., Fournier D., Nielsen A., Magnusson A., Bolker B. 2011. glmmADMB: generalized linear mixed models using AD Model Builder. R package; See http://glmmadmb.r-forge.r-project.org [Google Scholar]
- 31.Burnham K. P., Anderson D. R. 2002. Model selection and multimodel inference, 2nd edn New York, NY: Springer [Google Scholar]
- 32.Griffin A. S., Pemberton J. M., Brotherton P. N. M., McIlrath G., Gaynor D., Kansky R., O'Riain J., Clutton-Brock T. H. 2003. A genetic analysis of breeding success in the cooperative meerkat (Suricata suricatta). Behav. Ecol. 14, 472–480 10.1093/beheco/arg040 (doi:10.1093/beheco/arg040) [DOI] [Google Scholar]
- 33.Clutton-Brock T. H., Hodge S. J., Spong G., Russell A. F., Jordan N. R., Bennett N. C., Sharpe L. L., Manser M. B. 2006. Intrasexual competition and sexual selection in cooperative mammals. Nature 444, 1065–1068 10.1038/Nature05386 (doi:10.1038/Nature05386) [DOI] [PubMed] [Google Scholar]
- 34.Kokko H., Ekman J. 2002. Delayed dispersal as a route to breeding: territorial inheritance, safe havens, and ecological constraints. Am. Nat. 160, 468–484 10.1086/342074 (doi:10.1086/342074) [DOI] [PubMed] [Google Scholar]
- 35.Welbergen J. A., Quader S. 2006. Mother guarding: how offspring may influence the extra-pair behaviour of their parents. Proc. R. Soc. B 273, 2363–2368 10.1098/rspb.2006.3591 (doi:10.1098/rspb.2006.3591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clutton-Brock T. H., Hodge S. J., Flower T. P. 2008. Group size and the suppression of subordinate reproduction in Kalahari meerkats. Anim. Behav. 76, 689–700 10.1016/j.anbehav.2008.03.015 (doi:10.1016/j.anbehav.2008.03.015) [DOI] [Google Scholar]
- 37.Cooney R., Bennett N. C. 2000. Inbreeding avoidance and reproductive skew in a cooperative mammal. Proc. R. Soc. Lond. B 267, 801–806 10.1098/rspb.2000.1074 (doi:10.1098/rspb.2000.1074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharp S. P., Clutton-Brock T. H. 2011. Reluctant challengers: why do subordinate female meerkats rarely displace their dominant mothers? Behav. Ecol. 22, 1337–1343 10.1093/beheco/arr138 (doi:10.1093/beheco/arr138) [DOI] [Google Scholar]
- 39.Drewe J. A., Madden J. R., Pearce G. P. 2009. The social network structure of a wild meerkat population: 1. inter-group interactions. Behav. Ecol. Sociobiol. 63, 1295–1306 10.1007/s00265-009-0782-x (doi:10.1007/s00265-009-0782-x) [DOI] [Google Scholar]
- 40.Mysterud A., Langvatn R., Stenseth N. C. 2004. Patterns of reproductive effort in male ungulates. J. Zool. 264, 209–215 10.1017/S0952836904005618 (doi:10.1017/S0952836904005618) [DOI] [Google Scholar]