Abstract
Maternal effects can be adaptive and because of their intrinsic time delays may have important effects on population dynamics. In vertebrates, and increasingly invertebrates, it is well established that offspring defence is in part determined by maternal parasite exposure. It has also been suggested that there may be indirect maternal effects on immunity mediated by other components of the maternal environment, including density and resource availability. Here, we examine the effect maternal resource availability has on the immunity of offspring in an insect—virus system. We use five different maternal resource levels and examine immunity in the offspring both directly, by challenge with a virus, and by measuring a major component of the immune system, across three offspring environments. Both the direct infection assay and the measure of immunocompetence show clearly that offspring from mothers in poor environments are more resistant to parasites. This may result from life-history optimization of mothers in poor environments, or because the poor environment acts as a cue for higher disease risk in the next generation. This emphasizes the importance of maternal effects on disease resistance, mediated through indirect environmental factors that will have important implications to both the ecological and evolutionary dynamics of host–parasite interactions.
Keywords: immunity, resource, trans-generational, Plodia interpunctella, phenoloxidase
1. Introduction
Maternal effects generally occur when an individual's phenotype is affected not only by the environment it experiences, but also the environment experienced by its mother [1–4]. It is increasingly apparent that these maternal effects are widespread in nature, having been particularly well studied in plants [5] and invertebrates [4,6–8]. Maternal effects are recognized as an important mechanism for a rapid multi-generational response to changes in the environment [8–10], and are central to the broader field of indirect genetic effects [11,12]. In addition, there are likely to be important ecological effects of the delays intrinsic to maternal effects, since they tend to act to destabilize populations [9–15]. As such, understanding the role of maternal effects is an important challenge for both ecologists and evolutionary biologists.
Parasites are ubiquitous and may have important impacts on the population ecology [14], community ecology [15] and evolutionary dynamics of their hosts [16]. It is now well established that offspring defence is often in part determined by maternal exposure to parasites [17–25]. For example, in vertebrates, the immune capacity of offspring is often dependent on the level of immune stimulation of their parents [19], via maternal antibody transmission either through the milk and placenta of mammals or through the eggs of birds, reptiles and fishes [17,19]. Recent research has also highlighted the possibility of maternal transfer of immunity in invertebrate systems [20–24], although the mechanisms underlying these effects are less well understood. Thus, it is clear that maternal effects caused by the presence, composition and intensity of parasites in the maternal environment are likely to play a key role in offspring immunity.
Less intuitively, there is also some evidence that maternal environmental conditions may affect the level of investment in disease defence in offspring [26,27]. For example, increased levels of antibodies and androgens are transferred into the eggs from kittiwake mothers in poor nutritional conditions [28], and Daphnia magna reared in crowded low resource conditions produced offspring with less than half the susceptibility to bacterial infection [2]. It is unclear however, how widespread these indirect effects are, and given their potential importance to both the evolution and population ecologies of hosts and parasites, it is important that we examine them in more detail. In particular, it is unclear whether they result from the stress of a low resource environment or vary across a range of maternal environments. Here, we examine in detail, the effect of maternal resource quality on offspring immune investment across a range of environments. We manipulate maternal food quality in the Indian meal moth, Plodia interpunctella, and measure both the immunocompetence of offspring and their direct susceptibility to a natural virus. In addition, we also assayed the offspring under different food qualities in order to examine how any maternal effects might be mediated by offspring environment.
2. Methods
(a). Establishment of maternal generation
The Indian meal moth (P. interpunctella) and its granulosis virus (PiGV) were used as the experimental system. Maternal populations of five different resource qualities were established using adult moths from a large out-bred stock population. Resource quality can be precisely altered in the system by replacing a percentage of the carbohydrate source in the standard food with methylcellulose, an indigestible bulking agent [29,30]. The methylcellulose maintains the consistency of the food media and as an indigestible bulking agent effectively dilutes the quality of the food, while maintaining the quantity. We have shown previously that this reduction in food quality reduces growth rate, pupal size and resistance to infection with PiGV [30]. Five nutrition levels were used: the highest quality, standard food media, containing no methylcellulose, then media with 10, 20, 30 or 40 per cent replacement of carbohydrate with methylcellulose. Fifty adult P. interpunctella, of mixed age (to average out age effects on quality of eggs), from the stock populations were placed on 100 g of one of each of the five respective food levels, in individual clear plastic Nalgene pots. Adults were left for 24 h in order to mate and lay eggs, after which all the adults were removed. Pots were then maintained in incubators at 27°C, 16 L : 8 D cycle, until the emergence of adult moths (parental generation). This experimental set-up was then repeated for all five resource qualities in the maternal environment over four consecutive days, in order to produce four replicate sets of the parental generation. Throughout the experiment, all the experimental pots were cycled within and between incubators in order to control for effects of incubator and position within.
We examine a range of resources in order to increase the chances of seeing a gradual response to variable nutrition. A priori, in experiments such as this, it is unclear how strong an effect we will see and thus arbitrarily high and low levels can be problematic. If the difference is too low, a real effect may not be picked up, and if too high, the effect may be owing to extreme conditions.
(b). Establishment of offspring generation
Offspring populations were established by splitting 150 adults from each of the maternal populations into three groups of 50. As previously, each group was allowed to lay eggs onto 100 g of one of three offspring food levels; high-quality (0% methylcellulose), medium-quality (20% methylcellulose) and low-quality (40% methylcellulose) food. This experimental set-up was carried out for each of the 20 maternal populations, (five maternal environments, each with four blocked repeats), producing each quality of offspring food from each maternal population (producing a total of 60 offspring populations). Populations were maintained under identical conditions to the maternal generations, until the larvae reached third instar [30] (see the electronic supplementary material).
(c). Infection assay
Third instar individuals from each population were removed and left to starve for 2 h before being orally dosed with a freshly prepared 7.5 per cent virus solution made up from frozen virus stocks, mixed with 0.1 per cent Coomassie Brilliant Blue R dye and 2 per cent sucrose (to encourage feeding). Larvae were dosed using a droplet method, using a microlitre syringe [30]. The bioassays were carried out four times in order to get a large sample size. In total, 100 individuals that took up visible virus solution in at least half of their gut were placed in 25-well Petri dishes with an abundant supply of their respective offspring food quality. The virus infects post-ingestion by crossing the gut wall and replicating systemically to produce a mass of occluded virus that takes over the insect's entire body and usually lyses and finally kills the host. All individuals are dosed as early third instar larvae (1 day after eclosion) that are easily determined by their head and relative body size [30]. Infection risk in this system is highly stage-dependent, and therefore, this allows us to standardize the assay across the different food resources. Larvae were kept in incubators as described earlier and checked for signs of infection; infected larvae are clearly visible because of their opaque white colour and failure to pupate. The assay was repeated four times for each population over four blocked days (see the electronic supplementary material).
Phenoloxidase (PO) is an established assay of an invertebrate's ability to launch a generalized immune response. It is involved in the production of melanin, encapsulation and a number of cytotoxic substances that kill parasites on infection [23,31,32]. As such we use it as an assay of resistance to pathogens other than viruses. Using the method of Barnes & Siva-Jothy [31], 12 fifth instar larvae from each offspring population were bled to produce a haemolymph sample. Samples were taken by flushing larval body cavities through, using 0.1 ml of ice-cold dH2O, which were then frozen at −80°C, before being assayed. To assay the PO activity of each sample, samples were thawed on ice and centrifuged for 5 min at 6500 r.p.m., at 4°C. A 96 well plate was prepared over ice, with 20 µl of sample supernatant, 160 µl dH2O and 20 µl of 15 mM L-dopa, per well. We block all treatments and populations across plates to control for between plate variation. The plates were then run in a heated (30°C) spectrophotometer, for 1 h, taking a reading every 15 s, and using Softmax software to calculate the maximal rate of reaction, the Vmax. The Vmax is used as the measure of PO activity, and is calculated from the slope of the absorbance over time (see the electronic supplementary material).
A summary of the design of the experiment is given in the electronic supplementary material. To determine whether maternal environment had an effect on offspring virus infection and PO activity, and whether this depended on the offspring environment (maternal × offspring interaction), we compared each maternal treatment across all offspring food levels. We fit the environments as ordered factors rather than a continuous variable because the variation in resource level is not linear (fitting the environment as a continuous variable gives the same insights). The experiment was blocked over 4 days, and we therefore included block in the model as a fixed main effect but do not consider interactions. For both the virus infection and PO experiments, we have replicate populations of each treatment that we fit as a random term (a more conservative analysis where we analyse the means of the virus assays gives the same qualitative results). We transformed the infection data using logits and square root transformed the PO data in order to normalize it and then fit linear mixed models using the R statistical package v. 2.4.1 [33]. We present the results of a model where offspring environment is nested within maternal environment but the less conservative model where they are not nested provides stronger evidence for the patterns we describe. An analysis where we fit a second random term to take into account the initial blocking design also gives the same qualitative results.
3. Results
There were no significant interactions between the maternal and offspring environments (table 1). We found that individuals in poor maternal environments (more methylcellulose) produce offspring that are significantly less likely to be infected with virus (table 1 and figure 1). Furthermore, their offspring have significantly higher PO activity (table 1 and figure 2) and therefore potentially better immunity to a wide range of non-viral pathogens and parasites. When we look at the pattern in the virus infectivity data, there is some suggestion that the effects of maternal environment become more apparent in offspring that have been raised on poorer quality food. This could be the result of individuals raised on the highest quality food-type having a superabundance of resources that mask any effects. This pattern is not seen in the PO data where there is some suggestion that individuals on the poorest food with mothers also on the poorest food may be immune depressed, perhaps owing to an overall level of poor health. However, the overall pattern is clear: poor maternal environment leads to more resistant offspring with higher overall immune activity.
Table 1.
Effects of maternal and offspring environment on virus infection and PO activity of offspring generation larvae.
| dependent variable | PO levels |
virus infectivity |
||||
|---|---|---|---|---|---|---|
| d.f. | F | p-value | d.f. | F | p-value | |
| block | 2,403 | 2.35 | 0.09 | 3,42 | 14.51 | <0.001 |
| maternal environment | 4,39 | 2.70 | 0.04 | 4,42 | 8.71 | <0.001 |
| maternal by offspring environment | 10,403 | 1.57 | 0.11 | 10,42 | 1.19 | 0.32 |
Figure 1.
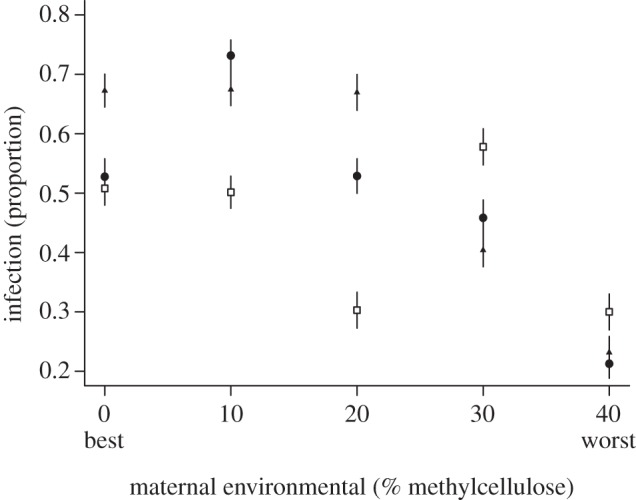
The proportion of infection at the five different maternal environments across the three offspring environments (open squares, high quality; filled circles, medium quality; filled triangles, low quality). Mean proportions and s.e. are given.
Figure 2.
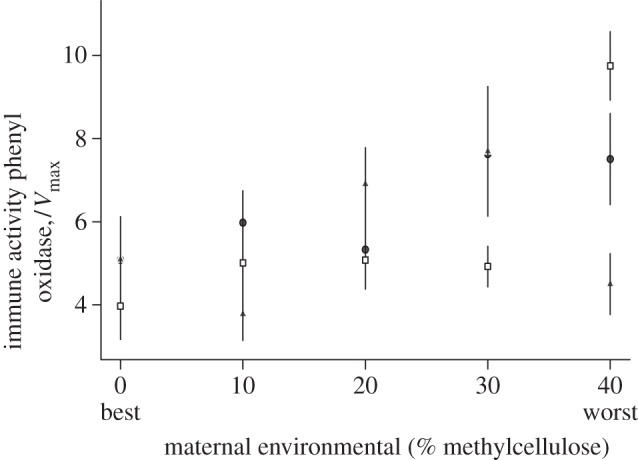
PO activity at the five different maternal environments across the three offspring environments (open squares, high quality; filled circles, medium quality; filled triangles, low quality). Mean Vmax activity and s.e. are given.
4. Discussion
We show that poor maternal environment significantly increases offspring defence against parasites. Mothers from the lowest quality maternal environments produce offspring that are less susceptible to direct challenge with a specific natural viral infection and have a higher PO activity, which suggests that they are more immunocompetent. These results emphasize that maternal effects can be mediated through a complex set of environmental cues, including indirect ones. Here, we find an increased offspring investment in resistance to parasites in response to low maternal resource quality, rather than direct exposure of parents to parasites themselves. Given that resource quality may have a number of other maternally mediated effects, there are important implications of these results to the evolution of host defence and host–parasite population dynamics.
It is not intuitive that low maternal environment should lead to higher investment in offspring resistance to disease. Indeed, good quality maternal environments might allow mothers to allocate more provisioning to offspring and therefore have higher resistance. It is well established that the activation and deployment of the immune system may be costly [34], and there is good evidence that there are evolutionary costs to the maintenance of defence in the absence of parasites and pathogens [35,36]. Fundamentally, when individuals have fewer resources to invest in total, there is a conflict between the optimal allocation of resource put into defence and an overall lower level of resources, potentially leading to lower immunity [30,37]. However, under poor environments each offspring may be relatively more valuable to the mother and she may therefore invest more overall into each offspring [2,6,7,38]. We did not count the number of offspring produced in our experiment, but poorer quality environments may lead to fewer, larger offspring, with altered growth rates [30,39,40]. Overall, our results are consistent with this idea that poor environments make offspring more valuable, and suggest that this also includes investing more in immunity [2].
Poor resource may be a cue to the risk of challenge by disease; high population densities often lead to poor individual resource levels, and may also be correlated with higher individual disease risk. Given that immune defence is costly [41,42], we would expect natural selection to favour individuals that invest more when there is the greatest threat of disease. If transmission is positively density-dependent [43–45], the risk of infection may increase at high density, leading to the idea that it may be optimal to invest more in defence in crowded conditions [31,46,47]. There is experimental evidence of this ‘density-dependent prophylaxis’ (DDP) within a generation in a number of systems and further evidence from comparative studies of social and solitary species [31,46–50]. Our results are consistent with the existence of trans-generational DDP in response to increased disease risk, if low resources act as a cue. Mitchell & Read [2] varied density and resource quality and showed that there was more investment in resistance in poor high-density maternal environments. Here, we find the same effect only by varying resource quality, which suggests that quality per se may be a cue of disease risk. Recently, Ben-Ami et al. [51] found consistent effects to Mitchell & Read [2] in the same system only by varying food quality, a result that along with our results suggests that resource levels are a sufficient cue [2]. It would be interesting to examine the maternal effects on immunity of density, independently of resource quality.
Maternal effects have also been shown to have important effects on population dynamics [13]. In particular, cyclic fluctuations in population density may be caused when maternal effects produce a lag in density dependence [10]. There has been some theoretical examination of the effect of within generation DDP on host–parasite population dynamics. White & Wilson [52] use a discrete-time model, representing non-overlapping insect generations and found that DDP stabilizes the dynamics, while Reilly & Hajek [53] using a continuous-time model within the season and a discrete-time map between seasons reported that DDP has a destabilizing effect on the population. Given the intrinsic delays involved in maternal effects, the population dynamical implications are likely to be even more complex. The link between density, resource and maternal investment in offspring resistance leads to a complex set of density-dependent delays that requires detailed modelling to understand its implications to host–parasite population dynamics.
In addition to our direct test of defence through challenge with a viral pathogen, we also found equivalent maternal effects mediated through PO activity. Again, individuals from poorer quality maternal environments have higher PO levels and therefore better immune defence. PO is part of a generalized immune response, involved in the encapsulation of infecting parasites, including bacteria and fungi, and in the production of cytotoxic substances [54]. It also has an important role in wound healing and bacterial and fungal defence. PO production is known to be costly [46], and these costs of PO production may be the explanation of why individuals on the poorest food quality, with mothers also on the poorest food, have the lowest level of PO. Recent work into the little understood defence of virus by invertebrates has linked the PO enzyme cascade to viral defence [55,56]. Galleria mellonella mount immune defences of baculoviral infection, not only by apoptosis and sloughing off of infected cells, but also by encapsulation of virus-infected cells and nodule formation, both of which involve PO activity. Plasma PO was thought to be directly responsible for the anti-viral activity of the larval Lepidoptera, as PO inhibitors removed the effect [56]. However, there is also evidence that PO is much less important to viral resistance than to other parasites [57,58]. As such we would argue that PO is unlikely to be involved in immunity to the virus [58] and should be seen as an indicator of immunity to other classes of pathogen, including bacteria and fungi. Our results are therefore best interpreted as providing evidence that offspring resistance, both in general and to a wide range of parasites, increases with poor maternal environments.
A link between maternal food quality and offspring resistance has also been reported in D. magna [2], with a recent study showing a genetic component of maternal effects [40]. It was suggested that the short generation times of organisms such as D. magna mean that the offspring are likely to encounter a similar environment to that of their mother. Mothers therefore, invest in resistance in their offspring since the maternal low-quality environment may be a cue for higher disease risk. Plodia interpunctella also has short generation times; populations in optimum laboratory conditions have a generation time of approximately 40 days. Since mothers are likely to mate and lay eggs in close proximity, offspring are likely to be in similar environments to their mothers [59,60]. It would therefore be interesting to see how investment in offspring immunity compares in organisms with much longer generation times. Transfer of acquired immunity across generations has been found in invertebrate systems [21–23], and maternal transfer of immunity in vertebrates is well known to be via antibodies [18], however the mechanisms are unknown in invertebrates. It has been proposed that a cue which may drive the expression of maternal effects has been suggested in worker bumble-bees Bombus terrestris, which show maternal immune priming of offspring [61], and lasting specific enhanced resistance to secondary exposure to pathogens [62]. Similar cues may be involved in maternal effects owing to the environment, however as discussed earlier, it may also be owing to differential investment in the offspring. More detailed research is required on the mechanisms that lead to maternal effects in invertebrates. In our experiments, we use an isolate of the virus that contains many genotypes. Although we have no evidence of specificity in our system, this may have important implications to distinguishing between maternal and genetic effects, as recently discussed by Luijckx et al. [63].
In principle, if there was strong selection with significantly higher mortality in the poorer resource environments, we may have selected for robust mothers that also had higher overall resistance. However, we know that there is not significant mortality in the maternal environment for a number of reasons. The number of adults produced over the first 5 days was recorded in this experiment and in others [30], and there is no evidence of resource-dependent mortality. Second, individuals raised on the different qualities of food do not have higher mortalities, they develop for longer and are smaller [30]. Third, the major source of mortality in the ad libitum group pots is cannibalism by later instars on early instars, but in one generation experiments such as ours this is negligible.
We have emphasized the role of the maternal environment in insect resistance. Our work highlights the importance of including maternal effects in the understanding of host–pathogen systems, and may have wide reaching impacts when trying to understand disease outbreaks, dynamics and control. The trans-generational effects are produced from changes in resource quality rather than from challenge by parasites. Furthermore, Ben-Ami et al. [51] have recently shown that maternal environment may influence the variance in immunity as well as the mean. Together these results emphasize that there are indirect maternal effects that may be critical to the host–parasite interaction.
Acknowledgements
We thank the NERC and the Leverhulme Trust for funding; Rebecca Finlay and Hannah Tidbury for technical support and Diane Bowler, Britt Koskella and Sophie Evison for comments on the manuscript.
References
- 1.Fox C., Czesak M., Mousseau T., Roff D. 1999. The evolutionary genetics of an adaptive maternal effect: egg size plasticity in a seed beetle. Evolution 53, 552–560 10.2307/2640791 (doi:10.2307/2640791) [DOI] [PubMed] [Google Scholar]
- 2.Mitchell S. E., Read A. F. 2005. Poor maternal environment enhances offspring disease resistance in an invertebrate. Proc. R. Soc. B 272, 2601–2607 10.1098/rspb.2005.3253 (doi:10.1098/rspb.2005.3253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mousseau T. A., Dingle H. 1991. Maternal effects in insects life histories. Annu. Rev. Entomol. 36, 511–534 10.1146/annurev.en.36.010191.002455 (doi:10.1146/annurev.en.36.010191.002455) [DOI] [Google Scholar]
- 4.Rossiter M. C. 1996. Incidence and consequences of inherited environmental effects. Annu. Rev. Ecol. Syst. 27, 451–476 10.1146/annurev.ecolsys.27.1.451 (doi:10.1146/annurev.ecolsys.27.1.451) [DOI] [Google Scholar]
- 5.Hereford J., Moriuchi K. S. 2005. Variation among populations of Diodia teres (Rubiaceae) in environmental maternal effects. J. Evol. Biol. 18, 124–131 10.1111/j.1420-9101.2004.00797.x (doi:10.1111/j.1420-9101.2004.00797.x) [DOI] [PubMed] [Google Scholar]
- 6.Beckerman A. P., Benton T. G., Lapsley C. T., Koesters N. 2006. How effective are maternal effects at having effects? Proc. R. Soc. B 273, 485–493 10.1098/rspb.2005.3315 (doi:10.1098/rspb.2005.3315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernardo J. 1996. Maternal effects in animal ecology. Am. Zool. 36, 83–105 10.1093/icb/36.2.83 (doi:10.1093/icb/36.2.83) [DOI] [Google Scholar]
- 8.Mousseau T., Fox C. 1998. Maternal effects as adaptations for transgenerational phenotypic plasticity in insects. In Maternal effects as adaptations (eds Mousseau T. A., Fox C. W.), pp. 159–177 Oxford, UK: Oxford University Press [Google Scholar]
- 9.Benton T. G., Ranta E., Kaitala V., Beckerman A. P. 2001. Maternal effects and the stability of population dynamics in noisy environments. J. Anim. Ecol. 70, 590–599 10.1046/j.1365-2656.2001.00527.x (doi:10.1046/j.1365-2656.2001.00527.x) [DOI] [Google Scholar]
- 10.Mousseau T., Fox C. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407 10.1016/S0169-5347(98)01472-4 (doi:10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 11.Agrawal A., Brodie E., III, Wade M. 2001. On indirect genetic effects in structured populations. Am. Nat. 158, 308–323 10.1086/321324 (doi:10.1086/321324) [DOI] [PubMed] [Google Scholar]
- 12.Wolf J. B., Brodie E. D., III, Cheverud J. M., Moore A. J., Wade M. J. 1998. Evolutionary consequences of indirect genetic effects. Trends Ecol. Evol. 13, 64–69 10.1016/S0169-5347(97)01233-0 (doi:10.1016/S0169-5347(97)01233-0) [DOI] [PubMed] [Google Scholar]
- 13.Ginzburg L. R., Taneyhill D. E. 1994. Population cycles of forest Lepidoptera: a maternal effect hypothesis. J. Anim. Ecol. 63, 79–92 10.2307/5585 (doi:10.2307/5585) [DOI] [Google Scholar]
- 14.Hudson P., Dobson A., Newborn D. 1998. Prevention of population cycles by parasite removal. Science 282, 2256. 10.1126/science.282.5397.2256 (doi:10.1126/science.282.5397.2256) [DOI] [PubMed] [Google Scholar]
- 15.Lafferty K. D., Sammond D. T., Kuris A. M. 1994. Analysis of larval Trematode communities. Ecology 75, 2275–2285 10.2307/1940883 (doi:10.2307/1940883) [DOI] [Google Scholar]
- 16.Boots M., Mealor M. 2007. Local interactions select for lower pathogen infectivity. Science 315, 1284. 10.1126/science.1137126 (doi:10.1126/science.1137126) [DOI] [PubMed] [Google Scholar]
- 17.Badyaev A. V., Hamstra T. L., Oh K. P., Seaman D. A. A. 2006. Sex-biased maternal effects reduce ectoparasite-induced mortality in a passerine bird. Proc. Natl Acad. Sci. USA 103, 14 406–14 411 10.1073/pnas.0602452103 (doi:10.1073/pnas.0602452103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badyaev A. V., Uller T. 2009. Parental effects in ecology and evolution: mechanisms, processes and implications. Phil. Trans. R. Soc. B 364, 1169–1177 10.1098/rstb.2008.0302 (doi:10.1098/rstb.2008.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brinkhof M., Heeb P. 1999. Immunocompetence of nestling great tits in relation to rearing environment and parentage. Proc. R. Soc. Lond. B 266, 2315–2322 10.1098/rspb.1999.0925 (doi:10.1098/rspb.1999.0925) [DOI] [Google Scholar]
- 20.Kurtz J., Franz K. 2003. Evidence for memory in invertebrate immunity. Nature 425, 37–38 10.1038/425037a (doi:10.1038/425037a) [DOI] [PubMed] [Google Scholar]
- 21.Little T. J., O'Connor B., Colegrave N., Watt K., Read A. F. 2003. Maternal transfer of strain-specific immunity in an invertebrate. Curr. Biol. 13, 489–492 10.1016/S0960-9822(03)00163-5 (doi:10.1016/S0960-9822(03)00163-5) [DOI] [PubMed] [Google Scholar]
- 22.Moret Y. 2006. ‘Trans-generational immune priming’: specific enhancement of the antimicrobial immune response in the mealworm beetle, Tenebrio molitor. Proc. R. Soc. B 273, 1399–1405 10.1098/rspb.2006.3465 (doi:10.1098/rspb.2006.3465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadd B., Kleinlogel Y., Schmid-Hempel R., Schmid-Hempel P. 2005. Trans-generational immune priming in a social insect. Biol. Lett. 1, 386–388 10.1098/rsbl.2005.0369 (doi:10.1098/rsbl.2005.0369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadd B., Schmid-Hempel P. 2007. Facultative but persistent trans-generational immunity via the mother's eggs in bumblebees. Curr. Biol. 17, R1046–R1047 10.1016/j.cub.2007.11.007 (doi:10.1016/j.cub.2007.11.007) [DOI] [PubMed] [Google Scholar]
- 25.Tidbury H., Pedersen A. B., Boots M. 2010. Within and transgenerational immune priming in an insect to a DNA virus. Proc. R. Soc. B 278, 871–876 10.1098/rspb.2010.1517 (doi:10.1098/rspb.2010.1517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulinier T., Staszewski V. 2008. Maternal transfer of antibodies: raising immuno-ecology issues. Trends Ecol. Evol. 23, 282–288 10.1016/j.tree.2007.12.006 (doi:10.1016/j.tree.2007.12.006) [DOI] [PubMed] [Google Scholar]
- 27.Grindstaff J. L., Brodie E. D., Ketterson E. D. 2003. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc. R. Soc. Lond. B 270, 2309–2319 10.1098/rspb.2003.2485 (doi:10.1098/rspb.2003.2485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gasparini J., Boulinier T., Gill V. A., Gil D., Hatch S. A., Roulin A. 2007. Food availability affects the maternal transfer of androgens and antibodies into eggs of a colonial seabird. J. Evol. Biol. 20, 874–880 10.1111/j.1420-9101.2007.01315.x (doi:10.1111/j.1420-9101.2007.01315.x) [DOI] [PubMed] [Google Scholar]
- 29.Boots M. 2000. Density-independent resource limitation and the transmission of an insect pathogen. Oecologia 124, 172–175 10.1007/s004420050004 (doi:10.1007/s004420050004) [DOI] [PubMed] [Google Scholar]
- 30.Boots M., Begon M. 1994. Resource limitation and the lethal and sublethal effects of a viral pathogen in the Indian meal moth. Ecol. Entom. 19, 319–326 10.1111/j.1365-2311.1994.tb00248.x (doi:10.1111/j.1365-2311.1994.tb00248.x) [DOI] [Google Scholar]
- 31.Barnes A., Siva-Jothy M. 2000. Density-dependent prophylaxis in the mealworm beetle Tenebrio molitor L. (Coleoptera: Tenebrionidae): cuticular melanization is an indicator of investment in immunity. Proc. R. Soc. Lond. B 267, 177. 10.1098/rspb.2000.0984 (doi:10.1098/rspb.2000.0984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolff J., Siva-Jothy M. T. 2003. Invertebrate ecological immunology. Science 301, 472–475 10.1126/science.1080623 (doi:10.1126/science.1080623) [DOI] [PubMed] [Google Scholar]
- 33.R Development Core Team 2005. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 34.Moret Y., Schmid-Hempel P. 2000. Survival for immunity: the price of immune system activation for bumblebee workers. Science 290, 1166–1168 10.1126/science.290.5494.1166 (doi:10.1126/science.290.5494.1166) [DOI] [PubMed] [Google Scholar]
- 35.Boots M., Begon M. 1993. Trade-offs with resistance to a granulosis virus in the Indian meal moth, examined by a laboratory evolution experiment. Funct. Ecol. 7, 528–534 10.2307/2390128 (doi:10.2307/2390128) [DOI] [Google Scholar]
- 36.Kraaijeveld A. R., Godfray H. C. J. 1997. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature 389, 278–280 10.1038/38483 (doi:10.1038/38483) [DOI] [PubMed] [Google Scholar]
- 37.Steinhaus M. 1958. Stress as a factor in insect disease. Pro. Tenth Int. Conf. Entomology 4, 725–730 [Google Scholar]
- 38.Frost P. C., Ebert D., Larson J. H., Marcus M. A., Wagner N. D., Zalewski A. 2010. Transgenerational effects of poor elemental food quality on Daphnia magna. Oecologia 162, 865–872 10.1007/s00442-009-1517-4 (doi:10.1007/s00442-009-1517-4) [DOI] [PubMed] [Google Scholar]
- 39.Ebert D. 1994. Fractional resource allocation into few eggs: Daphnia as an example. Ecology 75, 568–571 10.2307/1939560 (doi:10.2307/1939560) [DOI] [Google Scholar]
- 40.Stjernman M., Little T. J. 2011. Genetic variation for maternal effects on parasite susceptibility. J. Evol. Biol. 24, 2357–2363 10.1111/j.1420-9101.2011.02363.x (doi:10.1111/j.1420-9101.2011.02363.x) [DOI] [PubMed] [Google Scholar]
- 41.McKean K. A., Yourth C. P., Lazzaro B. P., Clark A. G. 2008. The evolutionary costs of immunological maintenance and deployment. BMC Evol. Biol. 8, 76. 10.1186/1471-2148-8-76 (doi:10.1186/1471-2148-8-76) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmid-Hempel P. 2003. Variation in immune defence as a question of evolutionary ecology. Proc. R. Soc. Lond. B 270, 357–366 10.1098/rspb.2002.2265 (doi:10.1098/rspb.2002.2265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson R. M., May R. M. 1979. Population biology of infectious diseases I. Nature 280, 361–367 10.1038/280361a0 (doi:10.1038/280361a0) [DOI] [PubMed] [Google Scholar]
- 44.Ryder J. J., Miller M. R., White A., Knell R. J., Boots M. 2007. Host–parasite population dynamics under combined frequency- and density-dependent transmission. Oikos 116, 2017–2026 10.1111/j.2007.0030-1299.15863.x (doi:10.1111/j.2007.0030-1299.15863.x) [DOI] [Google Scholar]
- 45.Ryder J. J., Webberley K. M., Boots M., Knell R. J. 2005. Measuring the transmission dynamics of a sexually transmitted disease. Proc. Natl Acad. Sci. USA 102, 15 140–15 143 10.1073/pnas.0505139102 (doi:10.1073/pnas.0505139102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson K., Reeson A. F. 1998. Density-dependent prophylaxis: evidence from Lepidoptera and baculovirus interactions? Ecol. Entomol. 23, 100–101 10.1046/j.1365-2311.1998.00107.x (doi:10.1046/j.1365-2311.1998.00107.x) [DOI] [Google Scholar]
- 47.Wilson K., Thomas M. B., Blanford S., Doggett M., Simpson S. J., Moore S. L. 2002. Coping with crowds: density-dependent disease resistance in desert locusts. Proc. Natl Acad. Sci. USA 99, 5471–5475 10.1073/pnas.082461999 (doi:10.1073/pnas.082461999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cotter S. C., Hails R. S., Cory J. S., Wilson K. 2004. Density-dependent prophylaxis and condition-dependent immune function in Lepidopteran larvae: a multivariate approach. J. Anim. Ecol. 73, 283–293 10.1111/j.0021-8790.2004.00806.x (doi:10.1111/j.0021-8790.2004.00806.x) [DOI] [Google Scholar]
- 49.Hochberg M. E. 1991. Viruses as costs to gregarious feeding-behavior in the Lepidoptera. Oikos 61, 291–296 10.2307/3545236 (doi:10.2307/3545236) [DOI] [Google Scholar]
- 50.Reeson A. F., Wilson K., Gunn A., Hails R. S., Goulson D. 1998. Baculovirus resistance in the noctuid Spodoptera exempta is phenotypically plastic and responds to population density. Proc. R. Soc. Lond. B 265, 1787–1791 10.1098/rspb.1998.0503 (doi:10.1098/rspb.1998.0503) [DOI] [Google Scholar]
- 51.Ben-Ami F., Ebert D., Regoes R. R. 2010. Pathogen dose infectivity curves as a method to analyze the distribution of host susceptibility: a quantitative assessment of maternal effects after food stress and pathogen exposure. Am. Nat. 175, 106–115 10.1086/648672 (doi:10.1086/648672) [DOI] [PubMed] [Google Scholar]
- 52.White K. A. J., Wilson K. 1999. Modelling density-dependent resistance in insect–pathogen interactions. Theor. Popul. Biol. 56, 163–181 10.1006/tpbi.1999.1425 (doi:10.1006/tpbi.1999.1425) [DOI] [PubMed] [Google Scholar]
- 53.Reilly J. R., Hajek A. E. 2008. Density-dependent resistance of the gypsy moth Lymantria dispar to its nucleopolyhedrovirus, and the consequences for population dynamics. Oecologia 154, 691–701 10.1007/s00442-007-0871-3 (doi:10.1007/s00442-007-0871-3) [DOI] [PubMed] [Google Scholar]
- 54.Hultmark D. 1993. Immune reactions in Drosophila and other insects: a model for innate immunity. Trends Genet. 9, 178–183 10.1111/j.1420-9101.2011.02363.x (doi:10.1111/j.1420-9101.2011.02363.x) [DOI] [PubMed] [Google Scholar]
- 55.Büyükgüzel E., Tunaz H., Stanley D., Büyükgüzel K. 2007. Eicosanoids mediate Galleria mellonella cellular immune response to viral infection. J. Insect. Physiol. 53, 99–105 10.1016/j.jinsphys.2006.10.012 (doi:10.1016/j.jinsphys.2006.10.012) [DOI] [PubMed] [Google Scholar]
- 56.Popham H. J. R., Shelby K. S., Brandt S. L., Coudron T. A. 2004. Potent virucidal activity in larval Heliothis virescens plasma against Helicoverpa zea single capsid nucleopolyhedrovirus. J. Gen. Virol. 85, 2255–2261 10.1099/vir.0.79965-0 (doi:10.1099/vir.0.79965-0) [DOI] [PubMed] [Google Scholar]
- 57.Clem R. 2005. The role of apoptosis in defense against baculovirus infection in insects. Curr. Topics Microbiol. Immunol. 289, 113–129 10.1007/3-540-27320-4_5 (doi:10.1007/3-540-27320-4_5) [DOI] [PubMed] [Google Scholar]
- 58.Saejeng A., Tidbury H., Siva-Jothy M. T., Boots M. 2010. Examining the relationship between hemolymph phenoloxidase and resistance to a DNA virus, Plodia interpunctella granulosis virus (PiGV). J. Insect Physiol. 57, 246–250 10.1016/j.jinsphys.2010.03.025 (doi:10.1016/j.jinsphys.2010.03.025) [DOI] [PubMed] [Google Scholar]
- 59.Boots M., Childs D., Reuman D. C., Mealor M. 2009. Local interactions lead to pathogen-driven change to host population dynamics. Curr. Biol. 19, 1660–1664 10.1016/j.cub.2009.07.070, (doi:10.1016/j.cub.2009.07.070,) [DOI] [PubMed] [Google Scholar]
- 60.Briggs C., Sait S., Begon M., Thompson D., Godfray H. 2000. What causes generation cycles in populations of stored-product moths? J. Anim. Ecol. 69, 352–366 10.1046/j.1365-2656.2000.00398.x (doi:10.1046/j.1365-2656.2000.00398.x) [DOI] [Google Scholar]
- 61.Moret Y., Schmid-Hempel P. 2001. Entomology: immune defence in bumble-bee offspring. Nature 414, 506. 10.1038/35107138 (doi:10.1038/35107138) [DOI] [PubMed] [Google Scholar]
- 62.Sadd B. M., Schmid-Hempel P. 2006. Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr. Biol. 16, 1206–1210 10.1016/j.cub.2006.04.047 (doi:10.1016/j.cub.2006.04.047) [DOI] [PubMed] [Google Scholar]
- 63.Luijckx P., Ben-Ami F., Mouton L., Du Pasquier L., Ebert D. 2011. Cloning of the unculturable parasite Pasteuria ramosa and its Daphnia host reveals extreme genotype–genotype interactions. Ecol. Lett. 14, 125–131 10.1111/j.1461-0248.2010.01561.x (doi:10.1111/j.1461-0248.2010.01561.x) [DOI] [PubMed] [Google Scholar]


