Abstract
Strong latitudinal patterns in leaf form are well documented in floristic comparisons and palaeobotanical studies. However, there is little agreement about their functional significance; in fact, it is still unknown to what degree these patterns were generated by repeated evolutionary adaptation. We analysed leaf form in the woody angiosperm clade Viburnum (Adoxaceae) and document evolutionarily correlated shifts in leafing habit, leaf margin morphology, leaf shape and climate. Multiple independent shifts between tropical and temperate forest habitats have repeatedly been accompanied by a change between evergreen, elliptical leaves with entire margins and deciduous, more rounded leaves with toothed or lobed margins. These consistent shifts in Viburnum support repeated evolutionary adaptation as a major determinant of the global correlation between leaf form and mean annual temperature. Our results provide a new theoretical grounding for the inference of past climates using fossil leaf assemblages.
Keywords: leaf margin analysis, leaf teeth, Viburnum, leaf shape, elliptical Fourier analysis, biogeography
1. Introduction
The occurrence of similar plant forms in particular environments is often considered strong evidence of evolutionary adaptation. Phylogenies have increasingly been incorporated into ecological studies to test these sorts of adaptive hypotheses and, in most cases, have revealed a more complex situation. For example, Ackerly [1] found that many of the plant lineages now occupying the California chaparral already possessed small thick leaves when they colonized this Mediterranean-type climate. That is, the sclerophyll leaf form did not evolve in response to Mediterranean conditions, but instead was preferentially filtered into these habitats. In another example, the evolution of C4 photosynthesis in grasses was not directly correlated with movements into warmer climates, even though there is complete turnover between C3 and C4 grasses along temperature gradients in grasslands around the world [2,3]. These studies do not disprove the adaptive value of commonly observed traits within particular environments, but they do cast doubt on an initial assumption that these environments were the primary drivers of adaptive evolutionary change. They also highlight the complexity of community assembly and the importance of the ecological sorting of species in generating global patterns in organismal traits. It is from this vantage point that we have examined the correlation between temperature and leaf form in a lineage of woody flowering plants.
It has long been recognized that tropical mesic forests are dominated by evergreen trees that have leaves with entire margins, whereas trees of mesic temperate forests tend to have deciduous leaves with toothed or lobed margins [4]. The relationship between leaf margins and mean annual temperature (MAT) has been quantified in floristic comparisons and in palaeobotanical studies at various scales, and used to infer past climates from fossil leaf assemblages [5–8]. However, the events that have generated this striking global pattern remain obscure. One possibility is that repeated evolutionary change has occurred, with multiple transitions between tropical and temperate zones being accompanied by changes in leaf habit and margin type. Several studies that have documented correlations between MAT, leaf shape and margin dentition across populations of a given species suggest that these traits may be evolutionarily labile [9,10]. Alternatively, there may have been few evolutionary transitions in leaf form, and these may have seldom (if ever) been directly correlated with tropical–temperate transitions in various lineages. In that case, the pattern could be explained by the sorting of lineages with different leaf forms into different habitats, and/or the differential diversification of certain lineages in certain regions.
Partly for these reasons, Little et al. [11] attempted to account for phylogeny when analysing a set of 17 forest plots along a gradient in MAT. Their findings suggested that there were important phylogenetic influences on the nature and significance of leaf form–climate relationships, and they cast doubt on the idea that, for example, cooler climates have directly and repeatedly selected for toothed leaves. However, their study did not identify any specific evolutionary shifts in habitat or in the relevant leaf characters, as their community-based phylogenetic approach did not allow that level of detail. To address the evolutionary question more directly, it is necessary to focus on a reasonably well-sampled clade, where it is clear that there have been multiple transitions both in habitat and in leaf characters.
Our study is centred on the woody angiosperm clade Viburnum (Adoxaceae), which contains approximately 170 species that are found mainly in temperate forests throughout the Northern Hemisphere, but with extensions into tropical regions in southeast Asia and in South America. A series of recent molecular phylogenetic analyses have expanded the sampling of species [12,13], including many more species from tropical forests in Vietnam, Malaysia and Indonesia [13,14]. The emerging picture from these studies is that Viburnum may have originated and initially diversified in Old World montane tropical forests, after which several lineages moved into colder forests at higher latitudes in Asia. Subsequently, four lineages extended into Europe, and five lineages moved into the New World, probably through Beringia beginning in the Eocene. One of these New World lineages diversified into the mountains of Latin America, and eventually entered cloud forests in the Andes [15].
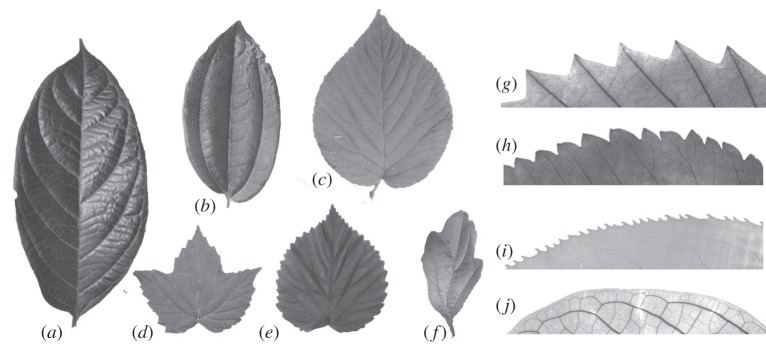
Viburnum is also appropriate for this analysis because of its great diversity in leafing habit, leaf margin type and leaf shape (figure 1). The lineage includes seasonally deciduous, semi-deciduous and fully evergreen species. Viburnum also exhibits a wide range of margin types—of tooth types, sizes, densities and shapes. The geographical distribution of leaves with toothed margins in Viburnum mirrors that observed in broad floristic studies (see the electronic supplementary material, figure S1). In addition to investigating the well-established leaf margin–temperature relationships in Viburnum, we also wanted to look at leaf shape directly. Leaf shape is remarkably diverse in Viburnum and covers a significant proportion of the entire phenotypic landscape for simple-leaved angiosperms. Lobed leaves, associated with temperate climates in many floristic studies, are present in several Viburnum species (e.g. V. trilobum, V. acerifolium, V. foetidum). Unlobed leaf forms vary widely, demanding a quantitative analysis of leaf shape. Leaf boundary layer models predict leaf shape as a key element of plant thermoregulation (reviewed in Nicotra et al. [16]), but leaf shape has largely been neglected in global leaf-climate analyses (but see [8,17]). Jones et al. [18] recently provided the first explicitly phylogenetic analysis of leaf shape evolution, in South African Pelargonium. They found that leaf size and degree of dissection were evolutionary labile traits, but surprisingly, the overall outline of the leaf was fairly conserved.
Figure 1.
A sample of leaf shapes and margins in Viburnum. (a–f) Leaf shapes, not at the same scale. (a) V. sambucinum, southeast Asia. (b) V. davidii, China. (c) V. lantanoides, eastern North America. (d) V. acerifolium, eastern North America. (e) V. molle, eastern US. (f) V. foetidum, southeast Asia, southern China, Taiwan. (g–j) Leaf margins from cleared leaves, not at the same scale. (g) V. dentatum, eastern North America. (h) V. plicatum, China, Taiwan, Japan. (i) V. lentago, eastern North America. (j) V. sambucinum, southeast Asia.
2. Material and methods
(a). Viburnum phylogeny
We analysed 101 Viburnum accessions, adding 11 previously unsampled species or subspecies to the 90 species dataset of Clement & Donoghue [13]. Following the methods described in [13], we sequenced nine chloroplast regions (matK, ndhF, petB-petD, rbcL, rpl32-trnL(UAG), trnC-ycf6, trnG-trnS, trnH-psbA and trnK) and the nuclear ribosomal internal transcribed spacer region (ITS) for each new accession. The newly generated sequences have been deposited in Genbank (see the electronic supplementary material, appendix S1).
Phylogenetic analyses were conducted in MrBayes v. 3.1.2 [19]. The data were divided among three partitions corresponding to (i) chloroplast coding sequences (4104 bp), (ii) chloroplast non-coding and intergenic spacer regions (4982 bp) and (iii) ITS (645 bp). Each data partition were analysed under a GTR + G + I model of sequence evolution. We ran six chains for 40 million generations, sampling the posterior distribution every 1000 generations and allowing model parameters to be unlinked among the data partitions. We assessed stationarity by examining the distribution of model parameters and likelihoods using Tracer v. 1.5 [20]. A majority-rule consensus tree was generated after removing the first 10 per cent of the trees sampled from the posterior distribution. Thirteen accessions for which sufficient leaf character data were lacking were pruned from the 101-tip tree and we used the resulting 88-tip tree in further analyses. For character evolution analyses, we smoothed the branch lengths of our Bayesian consensus tree, using penalized likelihood as implemented in the software program r8s [21] with the root age set to one.
(b). Character coding
(i). Habitat
We categorized each species as belonging to one of four major habitat types: (i) tropical, including montane subtropical forests, with limited temperature seasonality; (ii) warm temperate, generally broad-leaved evergreen forests; (iii) cloud forest, which are high elevation forests in tropical regions that experience cooler temperatures, but not significant temperature seasonality; or (iv) cold temperate, including deciduous temperate and boreal forests. These categories and scorings correspond to those in Weber et al. [22], with the addition of a warm temperate category to accommodate several Viburnum species living in broad-leaved evergreen forest that occurs in mid-latitude regions (e.g. the oak–laurel forest of southern Japan [23]).
We also used available online data from the Global Biodiversity Information Facility (www.gbif.org) to extract quantitative climate data from collection localities for as many species as possible. After removing duplicate records and observational data, we obtained 10 167 georeferenced Viburnum localities for 62 taxa. The distribution of sampling across taxa was highly uneven; 10 taxa were represented by only one location, and 39 of the taxa were represented by at least five different locations. We extracted 19 bioclimatic variables from each location using the WorldClim dataset [24] and calculated species means for each variable.
(ii). Leafing habit
We characterized leafing strategies in Viburnum as (i) evergreen, in which leaves are retained all year long; (ii) semi-deciduous ‘leaf-exchangers’, which generally maintain a leafy canopy year round but may have a brief period of leaflessness between flushes; or (iii) seasonally deciduous, in which leaves are lost synchronously each year and the plant is in a leafless state for a significant period of time. These categories match those used by Weber et al. [22].
(iii). Leaf margins
We built both a categorical and a quantitative dataset for leaf margins. For the categorical dataset, we scored each taxon as (i) having smooth, entire margins; (ii) mostly smooth margins, with occasional, small and irregularly placed teeth; or (iii) regularly toothed margins. We also selected 24 leaves for each taxon and quantified the degree of dentition (Dteeth) by counting the number of teeth per leaf internal perimeter (perimeter of leaf after teeth have been removed; teeth per centimetre as in Royer et al. [17]).
(c). Quantifying leaf shape
We digitally photographed leaves from 88 taxa, using both living collections and herbarium specimens. The resulting dataset included 24–60 individual leaf images for each taxon. We quantified the leaf outline directly using elliptical Fourier analysis [25], and used phylogenetic principal component analysis (PCA) [26] to reduce the large set of potentially correlated Fourier descriptors to a smaller set of uncorrelated principal components, which we treated as continuous morphological characters. Full methods are explained in the electronic supplementary material, appendix S2.
(d). Phylogenetic comparative analyses
In our analyses of character evolution, we reduced our multi-state categories to binary states. For each factor, we consistently had two categories that were clearly delineated and described the majority of our species, and one or more ‘intermediate’ categories that described a smaller number. To create binary characters, we pooled these intermediate taxa into one or the other of the primary character states. For instance, in the case of habitat type, we ran three analyses: (i) cold temperate versus all others; (ii) warm and cold temperate versus all others; and (iii) tropical versus all others.
We reconstructed the ancestral states at internal nodes using our own implementation of the likelihood-based methods described by Pagel [27], which allows these models to be fit on trees containing unresolved nodes. We also tested for correlated evolution between sets of discrete traits by computing the likelihood that the probability of a state change in one trait was dependent on the state of the other. The Akaike information criterion was calculated from this likelihood to compare the fit of three models that made different evolutionary assumptions. The first model assumed a single transition rate between character states and among characters (ER, or equal rates model). The second assumed that the transition rates for any given trait were directionally symmetrical (e.g. 0 → 1 = 1 → 0), but that different traits could have different transition rates (symmetrical model). The third model assumed that all transition rates were different (all rates different model). We also tested the fit of a model that assumed that the two traits evolved independently of one another. We conducted all analyses in R using custom scripts (available from J.M.B. upon request).
We also tested for significant relationships between variables with a series of phylogenetic regression analyses, using the gls function in R. For each set of variables, we derived a phylogenetic covariance matrix from our phylogenetic branch lengths, incorporating several different branch length transformations that mimic alternative models of character evolution: a pure Brownian motion model, which assumes that a character has evolved under neutral drift; an Ornstein–Uhlenbeck (OU) model, which assumes that a character has evolved under stabilizing selection; and Pagel's Lambda, which employs a Brownian motion model but optimizes the covariance matrix according to an estimate of phylogenetic signal [28,29].
3. Results
(a). Correlated evolution of leafing habit and margin type with habitat in Viburnum
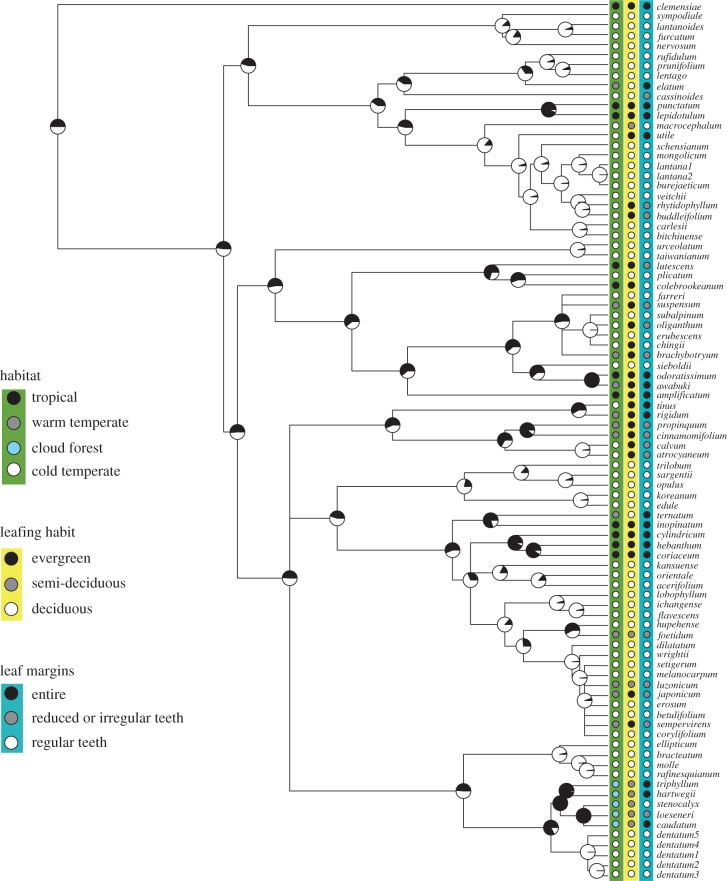
Mapping habitat onto our Viburnum phylogeny revealed a complex history of repeated shifts between tropical and temperate regions that were consistently correlated with corresponding shifts in leafing habit and leaf margin type (figure 2). Regardless of how we formed binary variables from our multi-state characters, we recovered significant support for correlated evolution of habitat, leafing habit and leaf margin type (see the electronic supplementary material, table S1). In Viburnum, species living in tropical environments tend to have evergreen leaves with entire margins, while species living in the temperate zone tend to have deciduous leaves with toothed margins. This pattern has arisen via multiple transitions between the tropical and temperate zone that were associated with corresponding shifts between these two distinct leaf syndromes.
Figure 2.
The phylogenetic distribution of habitat, leafing habit and leaf margins on a majority-rule consensus tree for 88 Viburnum accessions obtained from a Bayesian analysis of DNA sequences (see text). The tree was rooted using V. clemensiae based on previous studies; branch lengths were obtained using r8s. Pie charts at internal nodes represent maximum likelihood reconstructions of habitat type, using a binary coding. Black, tropical, warm temperate or cloud forest; white, cold temperate.
The robust support for correlated evolution emerged in spite of uncertainty in the exact phylogenetic placement and directionality of many of these transitions (figure 2). To help place bounds on this uncertainty, we examined ancestral state reconstructions within the 95% confidence intervals surrounding the maximum-likelihood estimates for the transitions in two binary characters: (i) tropical versus all others, and (ii) cold temperate versus all others (for general method, see [30]). All scenarios resulted in roughly 8–14 habitat transitions, with shifts occurring in both directions (e.g. tropical to temperate and temperate to tropical). We also examined the correlation between the uncertainties in our ancestral state reconstructions, and found that these were tightly linked across traits (see the electronic supplementary material, figure S2); an internal node with a 60 per cent probability of being tropical also has a roughly 60 per cent probability of being evergreen with entire leaf margins. This provided additional evidence that character changes clustered tightly together in particular regions of the tree.
(b). Elliptical Fourier analyses of leaf shape
The first principal component (PC1) accounted for more than 85.3 per cent of the total variation in leaf shape (figure 3). PC1 represented leaf length/width ratio; leaves with a low PC1 score are orbicular (round), whereas leaves with a high PC1 score are elliptical (elongate). The second principal component (PC2) captured a shift between an obovate and ovate shape (whether the leaf is wider above or below the midpoint), and also distinguished lobed from non-lobed species. PC1 is similar to the Feret's diameter ratio from Royer et al. [17], though an important difference is that our PC1 axis contains positional information about the major and minor axes, with the longest axis always running from leaf base to leaf tip.
Figure 3.
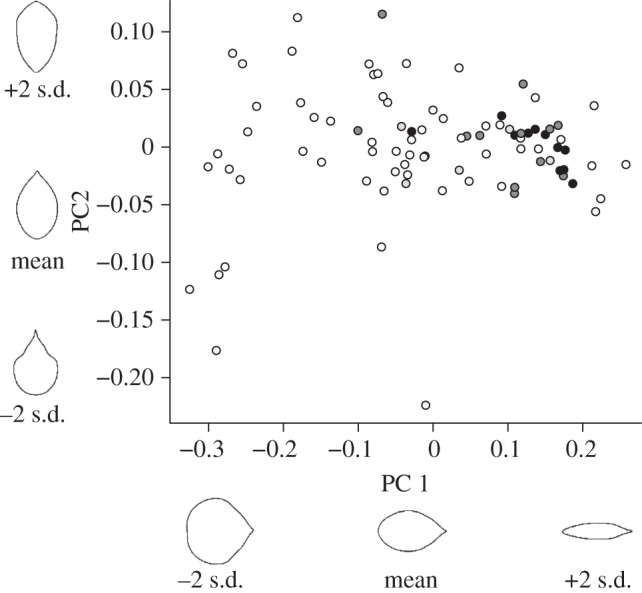
Principal components analysis of elliptical Fourier descriptors of leaf shape. Species coded by habitat type (black circles, tropical; dark grey circles, warm temperate; light grey circles, cloud forest; white circles, cold temperate). Leaf shapes represent back transformations of Fourier descriptors using mean PC values ±2 s.d.
(c). Leaf shape and tropical/temperate transitions
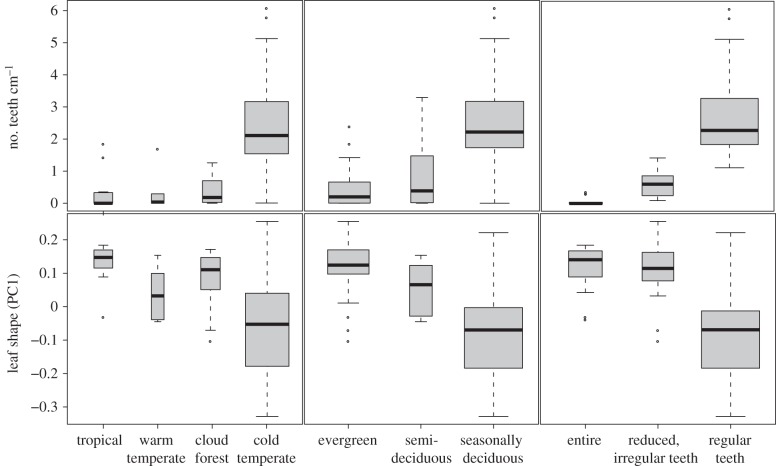
Our PC1 axis contributes an additional dimension to the previously recognized tropical/temperate patterns in leaf form (p < 0.001; electronic supplementary material, table S2; figure 4). Orbicular leaves were strongly correlated with a cold temperate environment, a deciduous habit and toothed margins. Elliptical leaves were strongly correlated with a tropical/subtropical environment, an evergreen habit and entire margins. While Brownian, OU and Lambda models all produced significant results, the OU and Lambda models were consistently a better fit. Overall, our results strongly supported the circumscription of two primary leaf syndromes in Viburnum: in tropical and montane/subtropical environments, Viburnum species tend to be evergreen and bear leaves that are elliptical with entire margins; in the temperate zone, they tend to be deciduous and bear leaves that are more round shaped, with regularly toothed margins (figure 5).
Figure 4.
Box plots of teeth density and leaf shape (PC1) in relation to habitat, leafing habit and leaf margin type. Width of box is proportional to sample size. Lines indicate medians, boxes represent the interquartile range and whiskers extend to the most extreme values within 1.5 times the interquartile range.
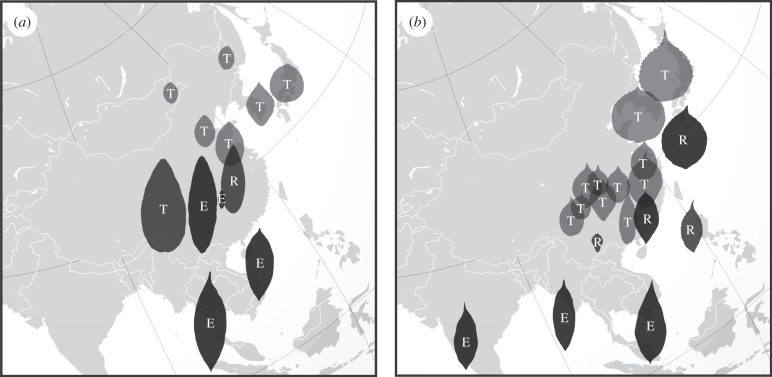
Figure 5.
Biogeographical patterns of Viburnum leaf syndromes in Asia. This region is home to most of the tropical/temperate transitions in Viburnum. E, entire margins; R, reduced or irregular teeth; T, regularly toothed; black, evergreen; dark grey, semi-deciduous; light grey, seasonally deciduous. (a) Leaf patterns in the Valvatotinus clade. (b) Leaf patterns in the Succodontotinus clade.
(d). Correlations between leaf form and quantitative climate data
The limited quantitative climate dataset we generated provided an independent line of evidence to support our discrete habitat categories. Both the PC1 shape axis and Dteeth were strongly correlated with MAT (p < 0.01; electronic supplementary material, table S3). Furthermore, quantitative climate data allowed us to put a finer point on the habitat-leaf form correlations; PC1 and Dteeth were more strongly associated with temperature seasonality than with MAT, and, in particular, the mean temperature of the coldest quarter. There was no relationship between either of the variables and mean temperature of the warmest quarter, nor with mean annual precipitation. We found no significant relationship between leaf size and any other leaf trait or climate variable (see the electronic supplementary material, tables S2 and S3).
4. Discussion
A global pattern in leaf form has long been evident: woody plants living in warm wet climates tend to have leaves with entire margins, whereas those in colder climates more often have leaves with toothed margins. This has been documented mainly through comparisons of the percentage of toothed leaves in forest plots spread along a latitudinal gradient. The pattern demands an explanation, but it has not been clear what sort of explanation is most appropriate.
One possibility is that the pattern has resulted from many separate evolutionary shifts from one leaf syndrome to the other. For example, as plants have moved from warmer into colder habitats, there may have been repeated evolutionary shifts in leaf form. Another possibility is that clades with particular leaf types have differentially diversified in different regions. For example, a small set of lineages that had evolved toothed leaves (possibly even under warm conditions) may have successfully transitioned into colder climates (possibly for reasons unrelated to leaf form) and then radiated throughout the temperate regions. Some have argued that shifts between biomes are uncommon [31,32], and Donoghue [31] suggested that plant movements into colder seasonal climates in particular has been a relatively rare event, leaving northern temperate floras both biased and depauperate with respect to lineage representation.
Of course, these two extreme forms of explanation are not mutually exclusive. However, the relative contributions of each scenario do bear strongly on how we might interpret the adaptive significance of global patterns in leaf form. The greater the number of independent correlated shifts between leaf syndromes and habitat, the stronger the evidence for an evolutionary advantage to having a ‘temperate’ and a ‘tropical’ leaf form. This is a question that can best be answered in the context of well-sampled and phylogenetically well-understood lineages. In such a context, we can identify the relevant movements and character shifts, whereas this is generally not possible in analysing floristic datasets [11].
In Viburnum, we have now documented a strong evolutionary correlation between tropical–temperate transitions and leaf syndromes. Given our current species sampling and level of phylogenetic resolution within Viburnum, we cannot yet confidently establish the exact phylogenetic location and direction of every shift between tropical and temperate habitats. However, it is abundantly clear, even now, that multiple shifts have occurred, and that these have very likely taken place in both directions (figure 2). Where temperate species appear to have been derived from more tropical ancestors (e.g. V. plicatum and V. sieboldii), we see the correlated evolution of deciduous, toothed leaves. Similarly, where species inhabiting warm temperate forests appear to have been derived from more cold-adapted ancestors (e.g. V. foetidum and V. japonicum), we see a reversion to an evergreen habit along with irregular toothing or entire margins. The re-emergence of an elliptical leaf is perhaps most evident in the Latin American cloud forest species (e.g. V. triphyllum and relatives). Overall, these findings strongly justify an adaptive explanation.
Several authors have offered hypotheses for the functional significance of toothed leaf margins, which mostly consider the association of leaf teeth with a deciduous habit [33]. Royer & Wilf ([34], see also [35]) suggested that leaf teeth provide early-season boosts to plant growth because leaves mature first at their margins. They documented higher rates of gas exchange in the toothed area during early spring, with elevated rates quickly declining over the first several weeks. A total leaf carbon budget was not calculated in that study, and so it is not clear how substantially this phenomenon contributes to the overall carbon gain. Indeed, Feild et al. [36] reported non-significant photosynthetic contributions of teeth in Chloranthus japonica, and instead suggested that guttating leaf teeth provide for positive xylem pressure a safe outlet that prevents flooding of leaf mesophyll. Associations between deciduousness, leaf teeth and positive root pressure have been casually noted in other plant groups [37], but many plants with positive pressure do not have teeth (e.g. Cornus), and many toothed species do not exhibit positive pressure [38]. Alternatively, Givnish [39] provided a mechanical argument based on observations that toothed leaves tend to be thinner and thus might use their venation network for substantial structural support, in addition to vascular transport. Modelling each secondary vein as a solitary longitudinal scaffold, the optimal shape of tissue supported by the vein should be wedge-shaped owing to the acropetal tapering of the vein. In a pinnately veined leaf, this would result in a series of parallel ‘wedges’ of leaf tissue that, together, would effectively form a toothed margin. This is an interesting idea, but relies on a series of assumptions that have never been tested.
The relationship we recovered between leaf shape (PC1) and climate in Viburnum was also found in recent floristic work that included additional leaf physiognomic variables to better predict MAT from leaf form [8,17]. This suggests that the shape element of our Viburnum tropical and temperate leaf syndromes may also be a more general plant response to movement between these biomes. A shift between elliptical and orbicular leaf shapes could significantly impact leaf energy balance, as rounder leaves are also wider, which will increase still air boundary layer thickness and decrease the potential for convective cooling under slow wind speeds [39,40]. Perhaps, leaves in hotter environments are relatively narrower to allow for more efficient convective heat exchange. It has recently been demonstrated that the majority of carbon fixation in plants occurs at approximately 22°C in both temperate and tropical biomes [41], and altering leaf shape may be one way to maintain an optimal temperature for photosynthesis.
We suspect that this argument is not important here, because our MAT gradient is driven largely by differences in temperatures of the coldest months; air temperatures during the growing season are not significantly different between habitats, or between species with differing leaf syndromes (see the electronic supplementary material, table S3). In other words, the growing season temperatures that Viburnum species experience have been largely maintained during temperate/tropical transitions, and the primary environmental changes that Viburnum has repeatedly confronted are the length and severity of a cold season. It is also important to emphasize that there are both trait–environment and inter-trait correlations to consider. Leaf shape has clearly evolved in consistent directions along an environmental gradient, but so have other aspects of leaf form (habit, teeth), and all of these characters are tightly integrated in Viburnum. Perhaps, each trait is responding independently to an external selective pressure that varies along this gradient. Alternatively, a toothed margin may be selected for, and the genetic/developmental changes that result in teeth could have pleiotropic effects on leaf shape (or vice versa) [42]. Another alternative is that both a toothed margin and a rounder leaf are downstream consequences of an additional, currently unidentified plant response to a cooler and more seasonal environment, or perhaps even to biotic factors, such as herbivory ([43], but see [44]).
The lineage-based approach that we have taken in this study provides the means to examine each of these adaptive hypotheses more carefully. Phylogenies are especially useful for identifying species with transitional character states that provide unique insights into the initial selection pressures acting on a complex trait or syndrome, and unpacking the order of evolutionary events [45–47]. We have identified relevant transitional taxa in several Viburnum clades. For example, V. luzonicum lives mostly in broad-leaved evergreen, warm–temperate forests in eastern Asia. These plants are semi-deciduous, and individuals in the field produce both ‘preformed’ leaves (developed initially inside of a resting bud) and ‘neoformed’ leaves (developed directly along an expanding shoot) along the same branch (M.J.D. 2011, personal observation). Remarkably, the preformed leaves exhibit a ‘temperate’ morphology (rounder, toothed) while the neoformed leaves are more ‘tropical’ (more elongate, entire). Differences in leaf shape between preformed and neoformed leaves have been observed before [48] and hint at a very different type of adaptive explanation that could simultaneously account for a number of the relevant variables. It is possible that simple shifts in the timing of growth, and the formation of leaves either inside or outside of resting buds, might influence both leaf shape and margins. Until now, attention has mainly focused on the functioning of mature leaves, but the potential relationship between leaf form and the efficient packing of young leaves as they develop within resting buds deserves further study [49,50].
5. Conclusions
Working with a well-sampled phylogeny for a single clade with multiple tropical–temperate transitions and extraordinary leaf diversity, we documented correlated shifts in leaf habit and margins with climate. We have discovered that leaf shape is also tightly correlated with these variables. Thus, in Viburnum, we conclude that multiple evolutionary shifts have played a significant role in generating the overall latitudinal gradient in leaf form. Although we certainly agree with Little et al. [11] that global patterns in leaf form are difficult to interpret in the absence of phylogenetic knowledge, here we show how phylogenies can be positively insightful, as opposed to simply diminishing the strength of an ahistorical correlation. These analyses must be extended to other relevant clades to see whether our results are general. In the meantime, our study motivates a very close look at the events surrounding individual tropical–temperate transitions. We predict that identifying and understanding each of the evolutionary stages along the tropical–temperate habitat gradient will be essential to uncovering the real functional significance of this global pattern. In this sense, phylogeny is not something to be ‘factored out’ of studies of leaf form. Instead, when fully embraced, phylogenetic trees present an unparalleled potential to identify the correct drivers of phenotypic evolution.
Acknowledgments
We thank the staff of the Arnold Arboretum of Harvard University for access to living plants, and their assistance throughout this project. We also thank Harvard University Herbaria and the Yale University Herbarium for access to their specimens and allowing us to photograph them. We are very grateful to Tetsukazu Yahara, Jer-Ming Hu, Kuo-Fang Chung, Ching-I Peng, Deby Arifiani and Patrick Sweeney for facilitating our recent field studies in Japan, Taiwan, Vietnam, Malaysia and Indonesia. Finally, we thank Monica Arakaki, Radika Bhaskar, Alejandro Brambila, Pascal-Antoine Christin, Laura Garrison, Matt Ogburn, Anastasia Rahlin and Elizabeth Spriggs for helpful discussion, and Stephen Smith for his advice with analyses. This work was supported in part by NSF grant IOS-0843231 to E.J.E., IOS-0842800 to M.J.D. and IOS-0842771 to L.S.
S.B.S., L.S., M.J.D. and E.J.E. conceptualized the project; S.B.S., W.L.C., D.S.C., M.J.D. and E.J.E. collected data; S.B.S., W.L.C., J.M.B. and E.J.E. performed analyses; S.B.S., M.J.D. and E.J.E. wrote the first draft of the manuscript, and all authors contributed substantially to revisions.
References
- 1.Ackerly D. D. 2004. Adaptation, niche conservatism, and convergence: comparative studies of leaf evolution in the California chaparral. Am. Nat. 163, 654–671 10.1086/383062 (doi:10.1086/383062) [DOI] [PubMed] [Google Scholar]
- 2.Teeri J. A., Stowe L. G. 1976. Climatic patterns and distribution of C4 grasses in North America. Oecologia 23, 1–12 [DOI] [PubMed] [Google Scholar]
- 3.Edwards E. J., Smith S. A. 2010. Phylogenetic analyses reveal the shady history of C4 grasses. Proc. Natl Acad. Sci. USA 107, 2532–2537 10.1073/pnas.0909672107 (doi:10.1073/pnas.0909672107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey I. W., Sinnott E. W. 1916. The climatic distribution of certain types of angiosperm leaves. Am. J. Bot. 3, 24–39 10.2307/2435109 (doi:10.2307/2435109) [DOI] [Google Scholar]
- 5.Wolfe J. A. 1971. Tertiary climatic fluctuations and methods of analysis of tertiary floras. Palaeogeogr. Palaeoclimatol. Palaeoecol. 9, 27–57 10.1016/0031-0182(71)90016-2 (doi:10.1016/0031-0182(71)90016-2) [DOI] [Google Scholar]
- 6.Wilf P. 1997. When are leaves good thermometers? A new case for leaf margin analysis. Paleobiology 23, 373–390 [Google Scholar]
- 7.Gregory-Wodzicki K. 2000. Relationships between leaf morphology and climate, Bolivia: implications for estimating paleoclimate from fossil floras. Paleobiology 26, 668–688 (doi:10.1666/0094-8373(2000)026<0668:RBLMAC>2.0.CO;2) [DOI] [Google Scholar]
- 8.Peppe D., et al. 2011. Sensitivity of leaf size and shape to climate: global patterns and paleoclimatic applications. New Phytol. 190, 724–739 10.1111/j.1469-8137.2010.03615.x (doi:10.1111/j.1469-8137.2010.03615.x) [DOI] [PubMed] [Google Scholar]
- 9.Royer D. L., Meyerson L. A., Robertson K. M., Adams J. M. 2009. Phenotypic plasticity of leaf shape along a temperature gradient in Acer rubrum. PLoS ONE 4, e7653 (doi:10.1371/journal.pone.0007653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Royer D. L., McElwain J. C., Adams J. M., Wilf P. 2008. Sensitivity of leaf size and shape to climate within Acer rubrum and Quercus kelloggii. New Phytol. 179, 808–817 10.1111/j.1469-8137.2008.02496.x (doi:10.1111/j.1469-8137.2008.02496.x) [DOI] [PubMed] [Google Scholar]
- 11.Little S. A., Kembel S. W., Wilf P. 2010. Paleotemperature proxies from leaf fossils reinterpreted in light of evolutionary history. PLoS ONE 5, e15161. 10.1371/journal.pone.0015161 (doi:10.1371/journal.pone.0015161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winkworth R. C., Donoghue M. J. 2005. Viburnum phylogeny based on combined molecular data: implications for taxonomy and biogeography. Am. J. Bot. 92, 653–666 10.3732/ajb.92.4.653 (doi:10.3732/ajb.92.4.653) [DOI] [PubMed] [Google Scholar]
- 13.Clement W. L., Donoghue M. J. 2011. Dissolution of Viburnum section Megalotinus (Adoxaceae) of southeast Asia and its implications for morphological evolution and biogeography. Int. J. Plant Sci. 172, 559–573 10.1086/658927 (doi:10.1086/658927) [DOI] [Google Scholar]
- 14.Clement W. L., Donoghue M. J. 2012. Barcoding success as a function of phylogenetic relatedness in Viburnum, a clade of woody angiosperms. BMC Evol. Biol. 12, 73. 10.1186/1471-2148-12-73 (doi:10.1186/1471-2148-12-73) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore B. R., Donoghue M. J. 2007. Correlates of diversification in the plant clade Dipsacales: geographic movement and evolutionary innovations. Am. Nat. 170, S28–S55 10.1086/519460 (doi:10.1086/519460) [DOI] [PubMed] [Google Scholar]
- 16.Nicotra A. B., Leigh A., Boyce C. K., Jones C. S., Niklas K. J., Royer D. L., Tsukaya H. 2011. The evolution and functional significance of leaf shape in the angiosperms. Funct. Plant Biol. 38, 535. 10.1071/FP11057 (doi:10.1071/FP11057) [DOI] [PubMed] [Google Scholar]
- 17.Royer D., Wilf P., Janesko D., Kowalski E., Dilcher D. 2005. Correlations of climate and plant ecology to leaf size and shape: potential proxies for the fossil record. Am. J. Bot. 92, 1141–1151 10.3732/ajb.92.7.1141 (doi:10.3732/ajb.92.7.1141) [DOI] [PubMed] [Google Scholar]
- 18.Jones C. S., Bakker F. T., Schlichting C. D., Nicotra A. B. 2009. Leaf shape evolution in the South African genus Pelargonium L'Her. (Geraniaceae). Evolution 63, 479–497 10.1111/j.1558-5646.2008.00552.x (doi:10.1111/j.1558-5646.2008.00552.x) [DOI] [PubMed] [Google Scholar]
- 19.Huelsenbeck J. P., Ronquist F. 2001. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17, 754–755 10.1093/bioinformatics/17.8.754 (doi:10.1093/bioinformatics/17.8.754) [DOI] [PubMed] [Google Scholar]
- 20.Rambaut A., Drummond A. J. 2007. Tracer v.1.4. See http://beast.bio.ed.ac.uk/Tracer
- 21.Sanderson M. J. 2006. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19, 301–302 10.1093/bioinformatics/19.2.301 (doi:10.1093/bioinformatics/19.2.301) [DOI] [PubMed] [Google Scholar]
- 22.Weber M. G., Clement W. L., Donoghue M. J., Agrawal A. A. In press Phylogenetic and experimental tests of interactions among mutualistic plant defense traits in Viburnum (Adoxaceae). Am. Nat. [DOI] [PubMed] [Google Scholar]
- 23.Hattori T., Nakanishi S. 1985. On the distributional limits of the lucidophyllous forest in the Japanese archipelago. Bot. Mag. Tokyo 98, 317–333 10.1007/BF02488498 (doi:10.1007/BF02488498) [DOI] [Google Scholar]
- 24.Hijmans R., Cameron S., Parra J. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 10.1002/joc.1276 (doi:10.1002/joc.1276) [DOI] [Google Scholar]
- 25.Kuhl F., Giardina C. 1982. Elliptic Fourier features of a closed contour. Comput. Graph. Image Process. 18, 236–258 10.1016/0146-664X(82)90034-X (doi:10.1016/0146-664X(82)90034-X) [DOI] [Google Scholar]
- 26.Revell L. J. 2009. Size-correction and principal components for interspecific comparative studies. Evolution 63, 3258–3268 10.1111/j.1558-5646.2009.00804.x (doi:10.1111/j.1558-5646.2009.00804.x) [DOI] [PubMed] [Google Scholar]
- 27.Pagel M. 1994. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. Lond. B 255, 37–45 10.1098/rspb.1994.0006 (doi:10.1098/rspb.1994.0006) [DOI] [Google Scholar]
- 28.Hansen T., Martins E. 1996. Translating between microevolutionary process and macroevolutionary patterns: the correlation structure of interspecific data. Evolution 50, 1404–1417 10.2307/2410878 (doi:10.2307/2410878) [DOI] [PubMed] [Google Scholar]
- 29.Freckleton R. P., Harvey P. H., Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726 10.1086/343873 (doi:10.1086/343873) [DOI] [PubMed] [Google Scholar]
- 30.Beaulieu J., Jhwueng D.-C., Boettiger C., O'Meara B. C. 2012. Modeling stabilizing selection: expanding the Ornstein–Uhlenbeck model of adaptive evolution. Evolution. 10.1111/j.1558-5646.2012.01619.x (doi:10.1111/j.1558-5646.2012.01619.x) [DOI] [PubMed] [Google Scholar]
- 31.Donoghue M. J. 2008. A phylogenetic perspective on the distribution of plant diversity. Proc. Natl Acad. Sci. USA 105, 11 549–11 555 10.1073/pnas.0801962105 (doi:10.1073/pnas.0801962105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crisp M. D., et al. 2009. Phylogenetic biome conservatism on a global scale. Nature 458, 754–756 10.1038/nature07764 (doi:10.1038/nature07764) [DOI] [PubMed] [Google Scholar]
- 33.Royer D. L., Peppe D. J., Wheeler E. A., Niinemets Ü. 2012. Roles of climate and functional traits in controlling toothed versus untoothed leaf margins. Am. J. Bot. 99, 915–922 10.3732/ajb.1100428 (doi:10.3732/ajb.1100428) [DOI] [PubMed] [Google Scholar]
- 34.Royer D. L., Wilf P. 2006. Why do toothed leaves correlate with cold climates? Gas exchange at leaf margins provides new insights into a classic paleotemperature proxy. Int. J. Plant Sci. 167, 11. 10.1086/497995 (doi:10.1086/497995) [DOI] [Google Scholar]
- 35.Baker-Brosh K. F., Peet R. K. 1997. The ecological significance of lobed and toothed leaves in temperate forest trees. Ecology 78, 1250–1255 10.1890/0012-9658(1997)078 (doi:10.1890/0012-9658(1997)078) [DOI] [Google Scholar]
- 36.Feild T., Sage T., Czerniak C. 2005. Hydathodal leaf teeth of Chloranthus japonicus (Chloranthaceae) prevent guttation-induced flooding of the mesophyll. Plant Cell Environ. 28, 1179–1190 10.1111/j.1365-3040.2005.01354.x (doi:10.1111/j.1365-3040.2005.01354.x) [DOI] [Google Scholar]
- 37.Lechowicz M. 1984. Why do temperate deciduous trees leaf out at different times? adaptation and ecology of forest communities. Am. Nat. 124, 821–842 10.1086/284319 (doi:10.1086/284319) [DOI] [Google Scholar]
- 38.Hacke U., Sauter J. 1996. Xylem dysfunction during winter and recovery of hydraulic conductivity in diffuse-porous and ring-porous trees. Oecologia 105, 435–439 10.1007/BF00330005 (doi:10.1007/BF00330005) [DOI] [PubMed] [Google Scholar]
- 39.Givnish T. 1979. On the adaptive significance of leaf form. In Topics in plant population biology (eds Solbrig O. T., Raven P. H., Jain S., Johnson G.), pp. 375–407 New York, NY: Columbia University Press [Google Scholar]
- 40.Parkhurst D., Loucks O. 1972. Optimal leaf size in relation to environment. J. Ecol. 60, 505–512 10.2307/2258359 (doi:10.2307/2258359) [DOI] [Google Scholar]
- 41.Helliker B. R., Richter S. L. 2008. Subtropical to boreal convergence of tree-leaf temperatures. Nature 454, 511–514 10.1038/nature07031 (doi:10.1038/nature07031) [DOI] [PubMed] [Google Scholar]
- 42.Tsukaya H. 2005. Leaf shape: genetic controls and environmental factors. Int. J. Dev. Biol. 49, 547–555 10.1387/ijdb.041921ht (doi:10.1387/ijdb.041921ht) [DOI] [PubMed] [Google Scholar]
- 43.Lamont A. 1970. Problems of leaf ecology. Scott. J. Sci. 1, 104–143 [Google Scholar]
- 44.Potter D., Kimmerer T. 1988. Do holly leaf spines really deter herbivory? Oecologia 75, 216–221 10.1007/BF00378601 (doi:10.1007/BF00378601) [DOI] [PubMed] [Google Scholar]
- 45.Edwards E. J., Donoghue M. J. 2006. Pereskia and the origin of the cactus life-form. Am. Nat. 167, 777–793 10.1086/504605 (doi:10.1086/504605) [DOI] [PubMed] [Google Scholar]
- 46.Ogburn R. M., Edwards E. J. 2009. Anatomical variation in Cactaceae and relatives: trait lability and evolutionary innovation. Am. J. Bot. 96, 391–408 10.3732/ajb.0800142 (doi:10.3732/ajb.0800142) [DOI] [PubMed] [Google Scholar]
- 47.Christin P., Sage T., Edwards E., Ogburn R., Khoshravesh R., Sage R. 2011. Complex evolutionary transitions and the significance of C3-C4 intermediate forms of photosynthesis in Molluginaceae. Evolution 65, 643–660 10.1111/j.1558-5646.2010.01168.x (doi:10.1111/j.1558-5646.2010.01168.x) [DOI] [PubMed] [Google Scholar]
- 48.Critchfield W. 1960. Leaf dimorphism in Populus trichocarpa. Am. J. Bot. 47, 699–711 10.2307/2439521 (doi:10.2307/2439521) [DOI] [Google Scholar]
- 49.Kobayashi H., Kresling B., Vincent J. F. V. 1998. The geometry of unfolding tree leaves. Proc. R. Soc. Lond. B 265, 147–154 10.1098/rspb.1998.0276 (doi:10.1098/rspb.1998.0276) [DOI] [Google Scholar]
- 50.Couturier E., Courrech du Pont S., Douady S. 2011. The filling law: a general framework for leaf folding and its consequences on leaf shape diversity. J. Theor. Biol. 289, 47–64 10.1016/j.jtbi.2011.08.020 (doi:10.1016/j.jtbi.2011.08.020) [DOI] [PubMed] [Google Scholar]