Abstract
Long-lived mosquitoes maximize the chances of Plasmodium transmission. Yet, in spite of decades of research, the effect of Plasmodium parasites on mosquito longevity remains highly controversial. On the one hand, many studies report shorter lifespans in infected mosquitoes. On the other hand, parallel (but separate) studies show that Plasmodium reduces fecundity and imply that this is an adaptive strategy of the parasite aimed at redirecting resources towards longevity. No study till date has, however, investigated fecundity and longevity in the same individuals to see whether this prediction holds. In this study, we follow for both fecundity and longevity in Plasmodium-infected and uninfected mosquitoes using a novel, albeit natural, experimental system. We also explore whether the genetic variations that arise through the evolution of insecticide resistance modulate the effect of Plasmodium on these two life-history traits. We show that (i) a reduction in fecundity in Plasmodium-infected mosquitoes is accompanied by an increase in longevity; (ii) this increase in longevity arises through a trade-off between reproduction and survival; and (iii) in insecticide-resistant mosquitoes, the slope of this trade-off is steeper when the mosquito is infected by Plasmodium (cost of insecticide resistance).
Keywords: Culex, Anopheles, Plasmodium relictum, oviposition, parasite manipulation, pleiotropy
1. Introduction
The pattern and intensity of transmission of malaria parasites within a population depends critically on the fitness of their vectors. For this reason, the effect of Plasmodium on factors such as the fecundity and longevity of mosquitoes has traditionally received a lot of attention [1–3].
Mosquito survival is important because it affects Plasmodium transmission in two ways [4]. First, it allows the parasite to complete its extrinsic incubation period within the mosquito. This period (or sporogonic cycle) lasts 10–14 days, depending on the species and on environmental conditions [5,6]. Second, because it increases the potential for infective bites to hosts. Mosquitoes need to take a minimum of two blood meals in order to transmit the parasite, and blood meals are paced by lengthy gonotrophic cycles (the process of host seeking, blood feeding, egg production and oviposition), which last 2–4 days [6]. One would therefore not expect Plasmodium to decrease the survival of their vectors [1,3,7]. And yet, in a meta-analysis on Plasmodium-infected mosquito survival conducted in 2002, Ferguson & Read [3] showed that although half of the studies reported no effect of Plasmodium on survival, the other half reported shorter lifespans in infected than in uninfected mosquitoes. Although the reasons for these contradictory results are probably multifactorial, the negative effects of Plasmodium on survival were more likely to appear in non-natural mosquito–Plasmodium combinations, which lead Ferguson & Read [3] to conclude that Plasmodium may be harmful only in novel vector species. Unfortunately, there are few experimental (non-human) malaria models that combine mosquito and Plasmodium species with a common evolutionary history, so the question of the effect of Plasmodium on mosquito longevity is still largely unresolved.
As Plasmodium only transmits horizontally, mosquito fecundity is, in contrast, of no direct consequence for the parasite's fitness. Yet, a consistent observation from most mosquito–Plasmodium studies is that the parasite has a strong detrimental effect on mosquito fecundity. Indeed, several species of malaria have been shown to significantly reduce the fecundity (number of eggs) and fertility (number of hatched larvae) of different species of mosquitoes (reviewed in Hurd [1]). Hurd [1] has convincingly argued that this parasite-mediated life-history shift cannot be explained by differences in the quantity or quality of the blood meal taken by infected and uninfected mosquitoes. Rather, the ovaries of Plasmodium-infected females undergo a series of physiological changes (reduction in oocyte vitellogenin content, apoptosis of follicular epithelial cells) that culminate in the resorption of a considerable proportion of the developing oocytes (reviewed in Hurd [1,2]). Although no molecule of Plasmodium origin has been identified that would justify talking about parasite manipulation, it has been widely assumed that reproductive curtailment is an adaptive strategy of the parasite to increase mosquito survival through a trade-off in energy allocation between reproduction and survival [1,7,8]. To our knowledge, however, the existence of a physiological trade-off between fecundity and longevity in mosquitoes has never been formally demonstrated. Indeed, none of the longevity studies reviewed by Ferguson & Read [3] or published since [9–11] has measured the effects of Plasmodium on fecundity and longevity in the same individual.
Here, we revisit the survival and fecundity effects of Plasmodium on mosquitoes, taking advantage of the recent development of the avian malaria system [12], the only currently available non-human experimental model that uses a natural mosquito–Plasmodium combination. This new animal model associates the avian malaria parasite Plasmodium relictum (SGS1 lineage) and its natural vector, the mosquito Culex pipiens [13,14]. We carried out two different experiments. The first of these was performed in much the same way as previous longevity experiments: mosquitoes were given either an infected or an uninfected blood meal, and their mortality was recorded daily in cages until all mosquitoes died. In the second experiment, the protocol was repeated except that this time mosquitoes were followed individually to quantify first their fecundity (defined here as the number of eggs laid during the first gonotrophic cycle) and then their (post-reproductive) survival.
In both of these experiments, we explored whether genetically distinct mosquito lines respond differently to the same Plasmodium infection. There is, indeed, substantial evidence that host genetic factors play a major role in determining the outcome of Plasmodium infections [15–17]. In the field, a key source of mosquito genetic variation is associated with the evolution of insecticide resistance. Following intensive insecticide use, many mosquito populations have evolved several genetically distinct mechanisms of insecticide resistance. These mechanisms can be broadly classified into two types: metabolic resistance (the detoxification of the insecticide through the overproduction of specific enzymes) and target-site resistance (point mutations that insensitize the molecular targets of the insecticide [18]). Culex pipiens has a well-deserved reputation for being one of the mosquito species where the molecular and genetic bases of these two mechanisms of insecticide resistance are best understood [19–21]. In addition, the evolution of these two mechanisms of insecticide resistance in Cx. pipiens has been shown to entail a battery of correlated life-history changes in the insect, which have been widely interpreted as being the result of pleiotropic effects of the insecticide-resistant genes [22]. Yet the role of insecticide resistance in determining the outcome of mosquito-Plasmodium interactions has been largely unexplored ([22], but see [23]).
The specific aims of the present study are thus to determine: (i) whether Plasmodium alters the survival and/or fecundity of mosquitoes using an experimentally novel, albeit natural, mosquito–Plasmodium combination; (ii) whether there is a negative correlation (trade-off) between fecundity and survival; and (iii) whether the genetic variations that arise through the evolution of insecticide resistance modulate the effect of Plasmodium on these life-history traits.
2. Material and methods
Three isogenic Cx. pipiens mosquito strains were used in the experiments: one insecticide susceptible strain (SLAB), one insecticide-resistant strain through the overproduction of carboxylesterases (SA4B4) and one insecticide-resistant strain through the modification of the acetylcholinesterase (SR). These lines were obtained by repeated backcrossing of field-collected insecticide-resistant strains into a common (SLAB) susceptible background (see Berticat et al. [24] for details). Since their creation, these lines have been kept under identical rearing conditions. To avoid genetic drift, and owing to the occasional contamination of the lines, these lines are regularly backcrossed into the SLAB background. Larvae were reared as previously described [12]. Larval trays (n = 300 larvae per tray, n = 8 trays per strain in each experiment) were placed inside emergence cages (27 × 40 × 35 cm) with an ad libitum source of a 10 per cent sugar solution for the emerged adults.
Plasmodium relictum (lineage SGS1) is the aetiological agent of the most prevalent form of avian malaria in Europe [13]. This generalist Plasmodium parasite lineage was originally isolated from wild sparrows caught in the region of Dijon (France) in 2009 and subsequently passaged to naive canaries (Serinus canaria) by intraperitoneal injection. Bird experimental infections took place by intraperitoneal injection of ca 50–100 µl of blood from our infected bird stock. Mosquito blood feeding took place 10 days after the infection, to coincide with the acute phase of the bird's parasitaemia (J. Vézilier 2007, unpublished results).
(a). Experiment 1: survival
To explore the effect of Plasmodium on mosquito survival, we first adopted the most consensual protocol followed in longevity studies published to date (see electronic supplementary material, table S1): longevity was quantified in the absence of oviposition, and food was provided ad libitum. For this purpose, 70 female mosquitoes from each of the three strains (SLAB, SA4B4, SR) were haphazardly chosen from the different emergence cages and placed inside an experimental cage (n = 10 experimental cages). Half of these cages were then provided overnight with an infected canary and the other half with a non-infected (control) canary (see Vézilier et al. [12] for details). The following day, unfed and dead female mosquitoes were removed from the experimental cages. One of the infected cages had less than 50 per cent of blood fed mosquitoes and was therefore discarded from the study (see electronic supplementary material, table S2).
To obtain an estimate of blood feeding, infection success and mosquito size, on day 1 post blood meal (pbm), 15 mosquitoes were haphazardly sampled from each of the cages and placed individually in 30 ml plastic tubes covered with a mesh. Food was provided in the form of a cotton pad soaked in a 10 per cent glucose solution placed on top of each tube and replaced daily. Four days later (day 5 pbm), the mosquito was taken out of the tube, and the amount of haematin excreted at the bottom of each tube was quantified as an estimate of the blood meal size [12]. One wing was also removed from each female and measured under a binocular microscope along its longest axis as an index of body size [25,26]. In addition, mosquitoes that had been exposed to the infected canaries were dissected, and the number of oocysts in their midgut counted using a binocular microscope [12]. The rest of the mosquitoes (ca 195 mosquitoes per cage) were kept in the cages and provided with ad libitum food in the form of a 10 per cent sugar solution. Survival of these mosquitoes was assessed every ca 12 h by counting dead individuals lying at the bottom of each cage until all females died. Dead mosquitoes were kept at −20°C and subsequently allocated to one of the three insecticide resistance strains using a RFLP analysis as described in [27].
(b). Experiment 2: fecundity and survival
In the second experiment, where we aimed to quantify fecundity and longevity simultaneously, 70 female mosquitoes from each of the three strains (SLAB, SA4B4, SR) were haphazardly chosen from the different emergence cages and placed together to feed overnight inside an experimental cage (n = 5 infected cages, n = 5 control cages). To simplify the identification of the strains, however, 4 days before the blood meal, the mosquitoes were marked using a small amount (1 µg per female) of either pink, blue or yellow fluorescent powder (RadGlo JST) applied as a dust storm [28]. Preliminary trials have shown that at this concentration the dust has no effect on mosquito survival or oocyst count (Vézilier 2010, unpublished data), and is detectable only by using a binocular microscope. The three colours were used in rotation to mark the three strains so that the strain-colour code was switched from cage to cage.
On day 1 pbm, all engorged females were placed individually in numbered plastic tubes (30 ml) covered with a mesh (haematin tubes). Food was provided in the form of a cotton pad soaked in a 10 per cent glucose solution (as in experiment 1). Four days later (day 5 pbm), all mosquitoes were transferred to a new tube containing 4 ml of mineral water to allow the females to lay their eggs (oviposition tube). The oviposition tubes were provided daily with a cotton pad soaked in mineral water placed on top of each tube. In these conditions, 90 per cent of the females lay their eggs in a single day in the form of a single raft (Vézilier 2010, unpublished results). To obtain an estimate of the infection success, on day 7 pbm, 10 females from each of the infected cages were haphazardly sampled, taken out of the oviposition tubes, dissected and the number of oocysts in their midguts counted with the aid of a binocular microscope.
The rest of the oviposition tubes were checked daily for the presence of eggs. Once oviposition took place, the females were transferred to a new tube to measure their survival (longevity tube), and the egg rafts were photographed using a binocular microscope equipped with a numeric camera, after which they were put back in the insectary where they were checked daily until the emergence of the larvae. Eggs in the photographs were counted using the Mesurim Pro freeware (Academie d'Amiens, France). Larvae were killed by adding 5 ml of 100 per cent ethanol to the tube and counted using a binocular microscope. The longevity tubes were provided with a cotton pad soaked in water (as described earlier) and were monitored daily until the death of the female. On the day of death, the females were measured (wing length) and allocated to one of the three insecticide-resistant strains by examining their colour under a binocular microscope.
(c). Statistical analysis
Analyses were carried out using the r statistical package (v. 2.12.0). The different statistical models built to analyse the data are described in the electronic supplementary material, table S4. The general procedure for building the statistical models was as follows. Models were built by including mosquito strain (SLAB, SA4B4 and SR), parasite treatment (exposed to an infected or a control bird) and mosquito wing size (experiment 2 only) as fixed explanatory variables, and experimental cage as a random explanatory variable. Maximal models, including all higher-order interactions, were simplified by sequentially eliminating non-significant terms and interactions to establish a minimal model [29]. The significance of the explanatory variables was established using a likelihood ratio test (LRT), which is approximately distributed as a chi-square distribution [30] and using p = 0.05 as a cut-off p-value. The significant chi-square values given in the text are for the minimal model, whereas non-significant values correspond to those obtained before the deletion of the variable from the model. A posteriori contrasts were carried out by aggregating factor levels together and by testing the fit of the simplified model using an LRT [29].
Survival data were analysed using Cox proportional hazards mixed effect models (coxme, kinship package). Hazard ratios (HRs) were obtained from these models as an estimate of the ratio between the instantaneous risk of dying between two given factor levels. Two additional standard measurements of survival were obtained from Kaplan–Maier estimates of the survival distribution in each cage: the median survival (the time at which 50% of the population is still alive) and the proportion of mosquitoes that survived till day 14 (the average time at which Plasmodium completes its sporogonic cycle and the mosquito becomes infective [5]).
When the response variable was a proportion (e.g. hatching rate), the data were analysed using a linear mixed effect model with a binomial error distribution (lmer, lme4 package), otherwise mixed effect models with a normal error distribution were used (lme, nlme package). The differences in wing length between mosquito strains (experiment 1) were analysed using standard general linear models (glm, with the associated F-statistics addressing a given factor significance).
(d). Ethics statement
Animal experiments were carried out in strict accordance with the ‘National Charter on the Ethics of Animal Experimentation’ of the French Government, and all efforts were made to minimize suffering. Experiments were approved by the Ethical Committee for Animal Experimentation established by the authors’ institution (CNRS) under the auspices of the French Ministry of Education and Research (permit no. CEEA-LR-1051).
3. Results
(a). Experiment 1: survival
Midgut dissections revealed that most of the mosquitoes fed on an infected canary contained at least one oocyst (83% on average, see electronic supplementary material, table S2). This high-infection rate agrees with previous studies carried out in this system [12]. For this reason, and to simplify the reminder of the text, we refer to mosquitoes fed on an infected canary as being infected.
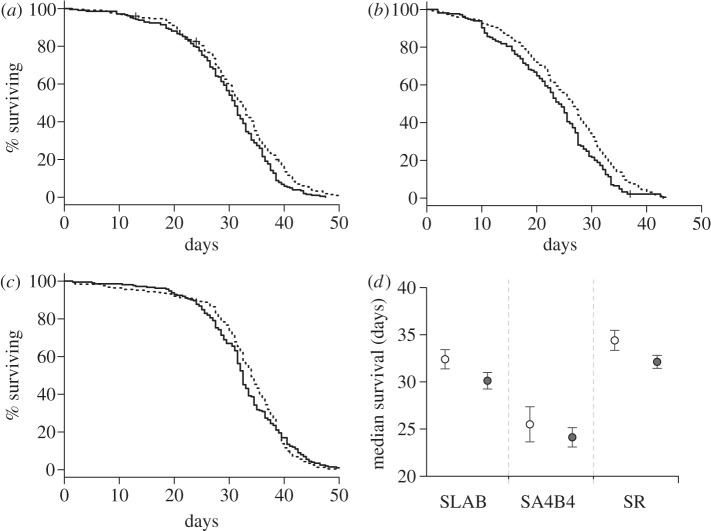
The Cox proportional hazards model revealed that P. relictum had no effect on mosquito survival (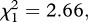 p = 0.103, see electronic supplementary material, table S4 model 1). There was, however, a strong insecticide resistance effect (model 1,
p = 0.103, see electronic supplementary material, table S4 model 1). There was, however, a strong insecticide resistance effect (model 1, 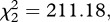 p < 0.001, figure 1a–d): the instantaneous risk of death was twice as high for esterase-resistant (SA4B4) than for susceptible (SLAB) mosquitoes (model 1, HR ± s.e. = 2.23 ± 0.07), and somewhat lower for acetylcholinesterase-resistant (SR) mosquitoes (model 1, HR ± s.e.: 0.82 ± 0.07). The analysis of the median survival and of the survival to day 14 gave identical results: a strong strain effect (median survival: model 2,
p < 0.001, figure 1a–d): the instantaneous risk of death was twice as high for esterase-resistant (SA4B4) than for susceptible (SLAB) mosquitoes (model 1, HR ± s.e. = 2.23 ± 0.07), and somewhat lower for acetylcholinesterase-resistant (SR) mosquitoes (model 1, HR ± s.e.: 0.82 ± 0.07). The analysis of the median survival and of the survival to day 14 gave identical results: a strong strain effect (median survival: model 2, 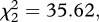 p < 0.001; survival to day 14: model 3,
p < 0.001; survival to day 14: model 3, 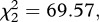 p < 0.001) but no effect of Plasmodium infection (model 2,
p < 0.001) but no effect of Plasmodium infection (model 2, 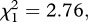 p = 0.096 and model 3,
p = 0.096 and model 3, 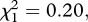 p = 0.656, respectively). This insecticide resistance effect on mosquito survival does not stem from differences in mosquito size, as the three mosquito stains used in the experiment had similar wing length (model 4, F114,2 = 2.38, p = 0.099).
p = 0.656, respectively). This insecticide resistance effect on mosquito survival does not stem from differences in mosquito size, as the three mosquito stains used in the experiment had similar wing length (model 4, F114,2 = 2.38, p = 0.099).
Figure 1.
Mosquito survivorship in experiment 1. Kaplan–Meier survival curve for the insecticide susceptible strain (a) SLAB and two insecticide-resistant strains: (b) SA4B4 and (c) SR after feeding on control uninfected (dashed line) or infected (full line) canaries. (d) Mean ± s.e. of the median survival of mosquitoes (i.e. time at which 50% of the females were still alive) for each strain for each treatment (empty circles: mosquitoes exposed to control uninfected canaries, grey circles: females that fed on infected birds).
(b). Experiment 2: fecundity and survival
On average, 87 per cent of the mosquitoes exposed to a control bird and 61 per cent of mosquitoes exposed to an infected bird took a blood meal (model 5, 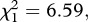 p = 0.010). Midgut dissections revealed that almost 90 per cent of the mosquitoes fed on an infected canary were infected (see electronic supplementary material, table S2).
p = 0.010). Midgut dissections revealed that almost 90 per cent of the mosquitoes fed on an infected canary were infected (see electronic supplementary material, table S2).
(i). Fecundity and hatching success
As expected, haematin, its quadratic term haematin2 and mosquito size were found to be strong predictors of the amount of eggs laid in both control (model 7, 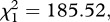 p < 0.001,
p < 0.001, 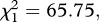 p < 0.001 and
p < 0.001 and 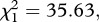 p < 0.001, respectively) and infected mosquitoes (model 8,
p < 0.001, respectively) and infected mosquitoes (model 8, 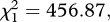 p < 0.001,
p < 0.001, 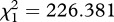 and
and 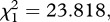 p < 0.001, respectively). The number of eggs laid by females (henceforth fecundity) was strongly dependent on whether the females were infected or not (model 6,
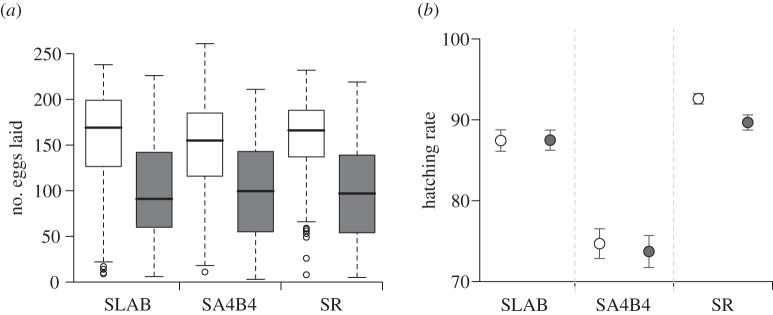
p < 0.001, respectively). The number of eggs laid by females (henceforth fecundity) was strongly dependent on whether the females were infected or not (model 6, 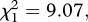 p = 0.003; figure 2a). Egg rafts of infected females contained on average 55 ± 4 eggs less than rafts from their uninfected counterparts. Insecticide resistance, however, had no effect on fecundity (model 6,
p = 0.003; figure 2a). Egg rafts of infected females contained on average 55 ± 4 eggs less than rafts from their uninfected counterparts. Insecticide resistance, however, had no effect on fecundity (model 6, 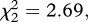 p = 0.261; figure 2a).
p = 0.261; figure 2a).
Figure 2.
Plasmodium infection effect on (a) fecundity and (b) egg hatching rate of Culex pipiens females. (a) Box and whisker plots of the number of eggs laid by the insecticide susceptible strain SLAB and the two insecticide-resistant strains SA4B4 and SR after mosquitoes have fed on control uninfected (empty boxes) or Plasmodium-infected canaries (grey boxes). Bold horizontal black bars show the median number of eggs. Boxes above and below the median show the first and third quartiles respectively. Dashed lines delimit 1.5 times the inter-quartile range on both side of the box, above which individual counts are considered outliers and marked as empty circles. (b) Mean hatching rate (± s.e.) for uninfected (empty circles) or infected mosquitoes (grey circles) of the three strains. Only mosquitoes whose eggs were productive (i.e. from which at least one larva emerged) were included in the analysis.
On average, 92 per cent of the egg rafts laid by Cx. pipiens females produced at least one larva (see electronic supplementary material, table S3). The proportion of larvae hatched in each raft (hatching rate) was dependent on the interaction between the strain and infection status of the female (model 9, 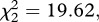 p < 0.001). Hatching rate was significantly lower for SA4B4 mosquitoes, but the effect of Plasmodium was apparent only in SR females (figure 2b).
p < 0.001). Hatching rate was significantly lower for SA4B4 mosquitoes, but the effect of Plasmodium was apparent only in SR females (figure 2b).
(ii). Survival
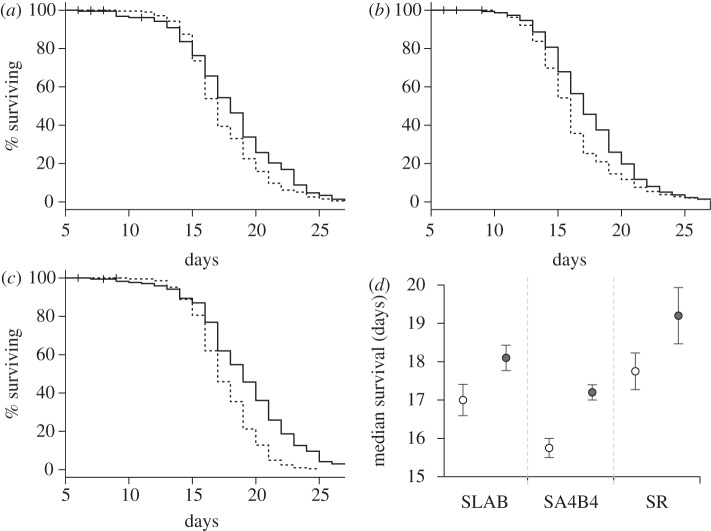
Post-egg-laying survival was significantly higher for infected mosquitoes than for control mosquitoes (model 10, 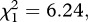 p = 0.012). This result was consistent across the three strains (model 10, strain × infection:
p = 0.012). This result was consistent across the three strains (model 10, strain × infection: 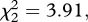 p = 0.141; figure 3a–d). The Cox proportional hazards model revealed that the instantaneous risk of death of infected mosquitoes was 35 per cent lower than that of control ones (model 10, HR ± s.e.: 1.51 ± 0.14). In addition, infection significantly increased the median survival by 1.3 days (model 11,
p = 0.141; figure 3a–d). The Cox proportional hazards model revealed that the instantaneous risk of death of infected mosquitoes was 35 per cent lower than that of control ones (model 10, HR ± s.e.: 1.51 ± 0.14). In addition, infection significantly increased the median survival by 1.3 days (model 11, 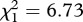 , p = 0.010). The proportion of mosquitoes that survived till day 14 also increased following Plasmodium infection, although only for SA4B4 mosquitoes (model 12, strain × infection interaction,
, p = 0.010). The proportion of mosquitoes that survived till day 14 also increased following Plasmodium infection, although only for SA4B4 mosquitoes (model 12, strain × infection interaction, 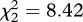 , p = 0.015). As expected, mosquito wing size was strongly correlated to their survival (model 10,
, p = 0.015). As expected, mosquito wing size was strongly correlated to their survival (model 10, 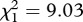 , p = 0.003). Adding the number of eggs laid by female mosquitoes as a covariate into the Cox proportional hazards model improved the model fit (model 13,
, p = 0.003). Adding the number of eggs laid by female mosquitoes as a covariate into the Cox proportional hazards model improved the model fit (model 13, 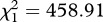 , p < 0.001) and removed the significance of the main infection effect (model 13,
, p < 0.001) and removed the significance of the main infection effect (model 13, 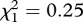 , p = 0.620), which suggests that the effect of Plasmodium on mosquito survival is mediated through a reduction in female fecundity.
, p = 0.620), which suggests that the effect of Plasmodium on mosquito survival is mediated through a reduction in female fecundity.
Figure 3.
Mosquito survivorship in experiment 2. Kaplan–Meier survival curve for the insecticide susceptible strain (a) SLAB and two insecticide-resistant strains (b) SA4B4 and (c) SR after feeding on control uninfected (dashed line) or infected (full line) canaries. Mosquito survival was recorded daily from their entry in the oviposition tube, 5 days after the blood meal. (d) Mean ± s.e. of the median survival of mosquitoes (i.e. time at which 50% of the females were still alive) for each strain for each treatment (empty circles: mosquitoes exposed to control uninfected canaries, grey circles: females that fed on infected birds).
Mosquito strain was also found to have a strong effect on survival (model 10, 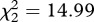 , p = 0.001): the instantaneous risk of death of metabolic-resistant SA4B4 mosquitoes was found to be 30 per cent higher than for susceptible SLAB and target-site-resistant SR mosquitoes (model 10, HR ± s.e. = 0.77 ± 0.07), while the latter two strains a similar survivorship (model 10,
, p = 0.001): the instantaneous risk of death of metabolic-resistant SA4B4 mosquitoes was found to be 30 per cent higher than for susceptible SLAB and target-site-resistant SR mosquitoes (model 10, HR ± s.e. = 0.77 ± 0.07), while the latter two strains a similar survivorship (model 10, 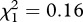 , p = 0.689). Similar results were obtained when analysing the median survival (model 11,
, p = 0.689). Similar results were obtained when analysing the median survival (model 11, 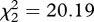 , p < 0.001), although in this case SLAB mosquitoes lived significantly shorter than SR mosquitoes (model 11,
, p < 0.001), although in this case SLAB mosquitoes lived significantly shorter than SR mosquitoes (model 11, 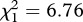 , p = 0.009; figure 3d).
, p = 0.009; figure 3d).
(iii). Fecundity–survival trade-off
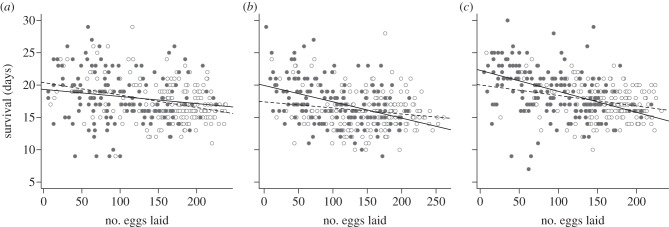
Mosquito survival and fecundity were strongly negatively correlated: the higher the number of eggs laid by the females, the lower their subsequent survival (model 14, 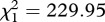 , p < 0.001). This result was consistent across the three strains (figure 4a–c). The effect of infection, however, differed between the strains (model 15, strain × infection × fecundity:
, p < 0.001). This result was consistent across the three strains (figure 4a–c). The effect of infection, however, differed between the strains (model 15, strain × infection × fecundity: 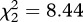 , p = 0.015). Analysing each mosquito strain separately unravelled that in SA4B4 females and, to a lesser extent, in SR females the slope of the fecundity–survival relationship was significantly steeper for infected females (infection × fecundity: model 17, SA4B4:
, p = 0.015). Analysing each mosquito strain separately unravelled that in SA4B4 females and, to a lesser extent, in SR females the slope of the fecundity–survival relationship was significantly steeper for infected females (infection × fecundity: model 17, SA4B4: 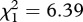 , p = 0.012, SR: model 18,
, p = 0.012, SR: model 18, 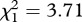 , p = 0.054; figure 4b,c). No such effect was apparent in SLAB mosquitoes (model 16,
, p = 0.054; figure 4b,c). No such effect was apparent in SLAB mosquitoes (model 16, 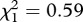 , p = 0.444; figure 4a).
, p = 0.444; figure 4a).
Figure 4.
Longevity–fecundity physiological trade-off in Culex pipiens mosquitoes. Raw plots of the number of eggs that the insecticide susceptible strain (a) SLAB and the two insecticide-resistant strains (b) SA4B4 and (c) SR laid against their survival after oviposition. Empty circles show uninfected mosquitoes plots, filled circles represent infected females. The linear regression of mosquito survival against the number of eggs each female laid was plotted using a dashed line for uninfected mosquitoes and a full line for infected females.
4. Discussion
(a). Longevity and fecundity
In this study, we revisited the question of the effect of Plasmodium on mosquito survival and fecundity using a natural vector–parasite combination (the mosquito Cx. pipiens and the avian malaria parasite P. relictum). In the first experiment, we compared the survival of infected and uninfected mosquitoes in much the same way as in most longevity studies published to date (see electronic supplementary material, table S1): female mosquitoes were not provided an oviposition substrate, food was provided ad libitum and mosquito longevity was recorded daily within their experimental cages. In these conditions, we found no effect of Plasmodium on mosquito survival. This result was consistent across the three mosquito strains and agrees with the studies reported by Ferguson & Read [3] where none of the natural mosquito–parasite associations showed any significant effect on survival. In the second experiment, however, both oviposition and subsequent (post-reproductive) survival were individually monitored. In these conditions, we found a drastic decrease of fecundity with infection, which was consistent across the three mosquito lines. Infected females laid, on average 30 per cent less eggs than uninfected ones, a reduction equivalent to that previously reported in other mosquito–Plasmodium combinations [8,31–33]. The widespread effect of Plasmodium on mosquito fecundity has been interpreted in terms of energy reallocation between reproduction and survival [1,3,7,8]. The results from our second experiment show, for the first time, that a reduction in fecundity in infected mosquitoes is indeed associated to an increase in survival. Infected mosquitoes had significantly longer lifespans than their uninfected counterparts, an effect that was consistent across the different mosquito lines and lifespan measurements used. Two lines of statistical evidence suggest that there is a causal relationship between the decrease in fecundity and the increase in survival observed. First, the number of eggs laid by each individual female was negatively correlated to their subsequent survival (figure 4a–c). Second, adding the number of eggs laid as a covariate in our Cox proportional hazard model removed the significance of infection on survival, implying that parasite-induced increase in survival was mediated through an alteration of mosquito reproductive output.
Why was this increase in longevity not found in our first experiment or in any of the survival experiments carried to date using natural mosquito–Plasmodium combinations? One possibility is that the discrepancy is due to minor, but potentially important differences in the experimental protocols. In our first experiment, longevity was measured in cages, whereas in the second experiment, mosquitoes were isolated in tubes. In the first experiment, mosquitoes were provided sugar ad libitum for the duration of the experiment, whereas in the second experiment, for practical reasons, sugar was provided only until the fourth day pbm. We can think of no reason why sugar restriction could explain why infected mosquitoes lived longer, unless by restricting sugar we forced the trade-off between fecundity and longevity. Previous studies show that, if anything, sugar restrictions tend to reduce the effect of Plasmodium on longevity [9]. We believe, however, that there is an alternative and potentially more likely explanation. Something that our first experiment and all previous longevity experiments have in common is that mosquitoes were not allowed to oviposit (or were allowed to oviposit for a very short, and possibly insufficient, amount of time, electronic supplementary material, table S1). Oviposition deprivation can have two potential effects on longevity. On the one hand, there is ample evidence that egg resorption kicks in when insects are forced to retain their eggs [34,35], which seems to be an adaptive strategy to redirect resources against other physiological processes, including longevity [36]. It is thus possible that in oviposition-deprived females the surplus nutrients resulting from egg resorption are redirected towards maintenance, thereby obscuring any eventual differences in longevity between infected and uninfected mosquitoes (note that as uninfected females have a higher fecundity, the net gain in surplus nutrients obtained from resorption would be also higher, thereby compensating for their lower longevity). On the other hand, oviposition-deprived females do not incur the costs of egg laying. Oviposition has indeed been reported to have a negative effect on insect fitness [37,38], suggesting that egg laying per se is indeed costly.
We do not know what is the actual mechanism of Plasmodium-associated fecundity reduction in our system. As both infected and uninfected mosquitoes took a blood meal, the reduction in fecundity must be directly associated to the presence of the parasite in the blood of the birds. There are different ways in which this could have happened. Our haematin quantifications suggested that infected females may have taken a smaller blood meal than uninfected ones. However, haematin (a product of the degradation of haemoglobin) does not quantify blood meal volume but the haemoglobin (red blood cells) ingested. As infected birds are strongly anaemic [39], an equal volume of blood automatically renders lower haematin values in infected birds. Although haemoglobin represents approximately 80 per cent of the proteins in the blood, haematin may not provide an accurate estimate of the total contribution of the blood meal to egg production. We cannot, however, totally eliminate the possibility that our mosquitoes took a smaller blood meal when feeding from an infected bird, although to our knowledge this effect has not been reported in studies that quantify mosquito blood meal size using gravimetric methods [40]. In addition, Hurd et al. [1] have convincingly argued that the fecundity reductions they find in their system are not associated to differences in haematin. Differences in blood quantity aside, a second possibility is that P. relictum induces changes in the nutritional value of the bird's blood. Anaemia, for one, inevitably reduces the amount of protein available for egg production. In addition, there is abundant evidence that Plasmodium decreases the nutrient composition of blood [41], either because these nutrients are scavenged by the parasite or as a host's response to the infection. Host blood quality has been found to be crucial for mosquito fecundity, although most of the evidence available comes from studies comparing mosquitoes fed on different host species [42]. The final, and most intriguing, possibility is that fecundity reduction is directly or indirectly associated with the presence of the parasite within the mosquito (most of the mosquitoes fed on an infected bird were infected, and had relatively high parasitaemias). Hurd et al. [1] have shown that the presence of Plasmodium within mosquitoes reduces fecundity through a combination of an impaired intake of yolk protein by the ovaries coupled with an increase in egg resorption mediated by follicular cells apoptosis. The proximate mechanism triggering these changes remains to be established, but both the cost of the immune system activation [43,44] and the consumption of resources by the developing oocysts have been pointed out as likely explanations [45].
Speculations as to whether this decrease in fecundity and correlated increase in longevity is adaptive for the parasite, for the mosquito or whether it is a simple pathological by-product of the infection are beyond the scope of this study. Indeed, the parasite could manipulate the host physiology to its own advantage and it is not difficult to argue why it would be adaptive for a parasite with no vertical transmission to reduce fecundity in order to increase longevity (and thus, as we have argued earlier, transmission). Another possibility that has been invoked [2] is that the fecundity reduction could be an adaptive strategy of sick mosquitoes, aimed at curtailing current reproduction in order to favour the chances of reproducing when conditions get better. We would argue, however, that such a current versus future reproductive trade-off is unlikely to take place in mosquitoes. It is indeed difficult to imagine how, in a mosquito world where every oviposition event needs to be preceded by a highly risky blood feeding event (arguably, the most likely source of mortality of mosquitoes in the wild [7]), it could be advantageous for a mosquito to dispose of ready-to-lay eggs in this way.
For technical reasons (inability to follow mosquitoes individually over several different blood feeding events), our experiment ran through a single gonotrophic cycle (a blood feeding event followed by an oviposition). In Cx. pipiens, the proportion of multiparous females is estimated to be less than 20 per cent [46], but multi-feeding, multiparous females are the most interesting individuals epidemiologically speaking. Extensive work of Hurd and co-workers on this subject in Anopheles mosquitoes seems to suggest that the Plasmodium-induced reproductive curtailment may persist for several gonotrophic cycle [47–49], but the concomitant effects on longevity have, to our knowledge, never been investigated.
(b). Insecticide resistance
In both experiments, esterase-resistant (SA4B4) mosquitoes had significantly shorter lifespans than either susceptible (SLAB) or target-site-resistant (SR) mosquitoes. Lifespan reductions in insecticide-resistant Culex mosquitoes have been reported before, albeit under extreme experimental conditions (non-sugar fed, non-blood fed), where mosquitoes survived a maximum of ca 3 days [25], way below the intrinsic incubation time of malarial parasites. Interestingly, however, our results agree with field estimates of mosquito overwintering survival in the field, where esterase-resistant Cx. pipiens mosquitoes have been shown to fare considerably worse than acetylcholinesterase or susceptible mosquitoes [50]. Our results also show that esterase-overproducing (SA4B4) and, to a lesser extent, acetylcholinesterase (SR) mosquitoes suffered a higher cost of infection than their susceptible (SLAB) counterparts. Indeed, while in the latter strain, the longevity-fecundity trade-off is independent of infection, in SA4B4 and SR strains, each additional egg laid costs more in terms of survival units when the mosquito has a Plasmodium infection. The isogenic strains, we used in this experiment, have a single (SLAB) genetic background. Further work needs to be carried out, ideally using field-collected sympatric insecticide-resistant and susceptible mosquitoes, to establish whether our results are generalizable to other genetic backgrounds.
(c). Conclusion
In conclusion, we provide the first reported account of an increase in longevity associated to a decrease in fecundity in Plasmodium-infected mosquitoes. Whether differences in the experimental protocol can explain the differences obtained between the results of our second experiment and previous results is beyond the scope of this paper and would need further experiments. However, we contend that mosquito longevity and fecundity should, whenever possible, be quantified concomitantly for two reasons: first, because oviposition deprivation is a situation unlikely to be encountered by mosquitoes in the field, whose main reason to blood feed is, after all, to obtain sufficient proteins to mature, and lay, a batch of eggs. Second, because as life-history theory predicts (and our own results show), fecundity and longevity are inextricably linked. Irrespective of what caused the discrepancies between this and previous experiments, the substantial increase in longevity found in Plasmodium-infected mosquitoes deserves further attention for its important implications in disease transmission.
Acknowledgements
The authors thank François Gatchitch, Philippe Perret, Mylène Weill and Flore Zélé for their help and advice during these experiments. Radiant Color NV kindly provided the mosquito pigments. J.V. is funded through an FCT grant attributed by the GABBA programme of the University of Porto, S.G. by an ANR Jeune Chercheur and an ERC Starting Grant EVOLEPID 243054, and A.R. by an ANR SEST grant.
References
- 1.Hurd H. 2003. Manipulation of medically important insect vectors by their parasites. Annu. Rev. Entomol. 48, 141–161 10.1146/annurev.ento.48.091801.112722 (doi:10.1146/annurev.ento.48.091801.112722) [DOI] [PubMed] [Google Scholar]
- 2.Hurd H. 2009. Evolutionary drivers of parasite-induced changes in insect life-history traits: from theory to underlying mechanisms. Adv. Parasitol. 68, 85–110 10.1016/s0065-308x(08)00604-0 (doi:10.1016/s0065-308x(08)00604-0) [DOI] [PubMed] [Google Scholar]
- 3.Ferguson H. M., Read A. F. 2002. Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol. 18, 256–261 10.1016/S1471-4922(02)02281-X (doi:10.1016/S1471-4922(02)02281-X) [DOI] [PubMed] [Google Scholar]
- 4.Smith D. L., McKenzie F. E. 2004. Statics and dynamics of malaria infection in Anopheles mosquitoes. Malar. J. 3 (doi:10.1186/1475–2875-3-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson H. M., Read A. F. 2002. Genetic and environmental determinants of malaria parasite virulence in mosquitoes. Proc. R. Soc. Lond. B 269, 1217–1224 10.1098/rspb.2002.2023 (doi:10.1098/rspb.2002.2023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Read A. F., Lynch P. A., Thomas M. B. 2009. How to make evolution-proof insecticides for malaria control. PLoS Biol. 7, e58. 10.1371/journal.pbio.1000058 (doi:10.1371/journal.pbio.1000058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz A., Koella J. C. 2001. Trade-offs, conflicts of interest and manipulation in Plasmodium-mosquito interactions. Trends Parasitol. 17, 189–194 10.1016/S1471-4922(00)01945-0 (doi:10.1016/S1471-4922(00)01945-0) [DOI] [PubMed] [Google Scholar]
- 8.Ferguson H. M., Rivero A., Read A. F. 2003. The influence of malaria parasite genetic diversity and anaemia on mosquito feeding and fecundity. Parasitology 127, 9–19 10.1017/s0031182003003287 (doi:10.1017/s0031182003003287) [DOI] [PubMed] [Google Scholar]
- 9.Ferguson H. M., MacKinnon M. J., Chan B. H., Read A. F. 2003. Mosquito mortality and the evolution of malaria virulence. Evolution 57, 2792–2804 [DOI] [PubMed] [Google Scholar]
- 10.Dawes E. J., Churcher T. S., Zhuang S., Sinden R. E., Basanez M. G. 2009. Anopheles mortality is both age- and Plasmodium-density dependent: implications for malaria transmission. Malar. J. 8, 228. 10.1186/1475-2875-8-228 (doi:10.1186/1475-2875-8-228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aboagye-Antwi F., Guindo A., Traore A. S., Hurd H., Coulibaly M., Traore S., Tripet F. 2010. Hydric stress-dependent effects of Plasmodium falciparum infection on the survival of wild-caught Anopheles gambiae female mosquitoes. Malar. J. 9, 243. 10.1186/1475-2875-9-243 (doi:10.1186/1475-2875-9-243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vézilier J., Nicot A., Gandon S., Rivero A. 2010. Insecticide resistance and malaria transmission: infection rate and oocyst burden in Culex pipiens mosquitoes infected with Plasmodium relictum. Malar. J. 9, 379. 10.1186/1475-2875-9-379 (doi:10.1186/1475-2875-9-379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valkiunas G. 2005. Avian malaria parasites and other haemosporidia. Boca Raton, FL: CRC Press [Google Scholar]
- 14.Kimura M., Darbro J. M., Harrington L. C. 2010. Avian malaria parasites share congeneric mosquito vectors. J. Parasitol. 96, 144–151 10.1645/ge-2060.1 (doi:10.1645/ge-2060.1) [DOI] [PubMed] [Google Scholar]
- 15.De Roode J. C., Culleton R., Cheesman S. J., Carter R., Read A. F. 2004. Host heterogeneity is a determinant of competitive exclusion or coexistence in genetically diverse malaria infections. Proc. R. Soc. Lond. B 271, 1073–1080 10.1098/rspb.2004.2695 (doi:10.1098/rspb.2004.2695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackinnon M. J., Gaffney D. J., Read A. F. 2002. Virulence in rodent malaria: host genotype by parasite genotype interactions. Infect. Genet. Evol. 1, 287–296 10.1016/S1567-1348(02)00039-4 (doi:10.1016/S1567-1348(02)00039-4) [DOI] [PubMed] [Google Scholar]
- 17.Lambrechts L., Halbert J., Durand P., Gouagna L. C., Koella J. C. 2005. Host genotype by parasite genotype interactions underlying the resistance of anopheline mosquitoes to Plasmodium falciparum. Malar. J. 4 1073. 10.1186/1475-2875-4-3 (doi:10.1186/1475-2875-4-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemingway J., Ranson H. 2000. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 45, 371–391 10.1146/annurev.ento.45.1.371 (doi:10.1146/annurev.ento.45.1.371) [DOI] [PubMed] [Google Scholar]
- 19.Raymond M., Chevillon C., Guillemaud T., Lenormand T., Pasteur N. 1998. An overview of the evolution of overproduced esterases in the mosquito Culex pipiens. Phil. Trans. R. Soc. Lond. B 353, 1707–1711 10.1098/rstb.1998.0322 (doi:10.1098/rstb.1998.0322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weill M., et al. 2003. Insecticide resistance in mosquito vectors. Nature 423, 136–137 10.1038/423136b (doi:10.1038/423136b) [DOI] [PubMed] [Google Scholar]
- 21.Lenormand T., Bourguet D., Guillemaud T., Raymond M. 1999. Tracking the evolution of insecticide resistance in the mosquito Culex pipiens. Nature 400, 861–864 10.1038/23685 (doi:10.1038/23685) [DOI] [PubMed] [Google Scholar]
- 22.Rivero A., Vézilier J., Weill M., Read A. F., Gandon S. 2010. Insecticide control of vector-borne diseases: when is insecticide resistance a problem? PLoS Pathgen 6 e1001000 (doi:10.1371/journal.ppat.1001000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vontas J. G., McCarroll L., Karunaratne S., Louis C., Hurd H., Hemingway J. 2004. Does environmental stress affect insect-vectored parasite transmission? Physiol. Entomol. 29, 210–213 10.1111/j.0307-6962.2004.00410.x (doi:10.1111/j.0307-6962.2004.00410.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berticat C., Boquien G., Raymond M., Chevillon C. 2002. Insecticide resistance genes induce a mating competition cost in Culex pipiens mosquitoes. Genet. Res. 79, 41–47 [DOI] [PubMed] [Google Scholar]
- 25.Agnew P., Berticat C., Bedhomme S., Sidobre C., Michalakis Y. 2004. Parasitism increases and decreases the costs of insecticide resistance in mosquitoes. Evolution 58, 579–586 [PubMed] [Google Scholar]
- 26.Clements A. N. 2000. The biology of mosquitoes development, nutrition and reproduction. Wallingford, UK: CABI Publishing [Google Scholar]
- 27.Berticat C., Dubois M. P., Marquine M., Chevillon C., Raymond M. 2000. A molecular test to identify resistance alleles at the amplified esterase locus in the mosquito Culex pipiens. Pest Manage. Sci. 56, 727–731 (doi:10.1002/1526-4998(200009)56:9<727::AID-PS214>3.0.CO;2-I) [DOI] [Google Scholar]
- 28.Service M. W. 1993. Mosquito ecology: field sampling methods . 2nd edn London, UK: Elsevier. [Google Scholar]
- 29.Crawley M. J. 2007. The R Book. Chichester, UK: John Wiley & Sons, Ltd [Google Scholar]
- 30.Bolker B. M. 2008. Ecological models and data in R. New Jersey, NJ: Princeton University Press [Google Scholar]
- 31.Hogg J. C., Hurd H. 1995. Malaria-induced reduction of fecundity during the first gonotrophic cycle of Anopheles stephensi mosquitos. Med. Vet. Entomol. 9, 176–180 10.1111/j.1365-2915.1995.tb00175.x (doi:10.1111/j.1365-2915.1995.tb00175.x) [DOI] [PubMed] [Google Scholar]
- 32.Hogg J. C., Hurd H. 1997. The effects of natural Plasmodium falciparum infection on the fecundity and mortality of Anopheles gambiae sl in north east Tanzania. Parasitology 114, 325–331 10.1017/S0031182096008542 (doi:10.1017/S0031182096008542) [DOI] [PubMed] [Google Scholar]
- 33.Hacker C. S. 1971. The differential effect of Plasmodium gallinaceum on the fecundity of several strains of Aedes aegypti. J. Invertebr. Pathol. 18, 373–377 10.1016/0022-2011(71)90040-1 (doi:10.1016/0022-2011(71)90040-1) [DOI] [PubMed] [Google Scholar]
- 34.Delobel A. 1989. Effect of groundnut pods (Arachis hypogea) and Imaginal feeding on oogenesis, mating and oviposition in the seed beetle Caryedon serratus. Entomol. Exp. Appl. 52, 281–289 10.1111/j.1570-7458.1989.tb01278.x (doi:10.1111/j.1570-7458.1989.tb01278.x) [DOI] [Google Scholar]
- 35.RiveroLynch A. P., Godfray H. C. J. 1997. The dynamics of egg production, oviposition and resorption in a parasitoid wasp. Funct. Ecol. 11, 184–188 10.1046/j.1365-2435.1997.00076.x (doi:10.1046/j.1365-2435.1997.00076.x) [DOI] [Google Scholar]
- 36.Xue R. D., Ali A., Barnard D. R. 2005. Effects of forced egg-retention in Aedes albopictus on adult survival and reproduction following application of DEET as an oviposition deterrent. J. Vector Ecol. 30, 45–48 [PubMed] [Google Scholar]
- 37.Yanagi S., Miyatake T. 2003. Costs of mating and egg production in female Callosobruchus chinensis. J. Insect Physiol. 49, 823–827 (doi:10.1016/s0022–1910(03)00119-7) [DOI] [PubMed] [Google Scholar]
- 38.Siva-Jothy M. T., Tsubaki Y., Hooper R. E. 1998. Decreased immune response as a proximate cost of copulation and oviposition in a damselfly. Physiol. Entomol. 23, 274–277 10.1046/j.1365-3032.1998.233090.x (doi:10.1046/j.1365-3032.1998.233090.x) [DOI] [Google Scholar]
- 39.Cellier-Holzem E., Esparza-Salas R., Garnier S., Sorci G. 2010. Effect of repeated exposure to Plasmodium relictum (lineage SGS1) on infection dynamics in domestic canaries. Int. J. Parasitol. 40, 1447–1453 10.1016/j.ijpara.2010.04.014 (doi:10.1016/j.ijpara.2010.04.014) [DOI] [PubMed] [Google Scholar]
- 40.Gray E. M., Bradley T. J. 2006. Malarial infection in Aedes aegypti: effects on feeding, fecundity and metabolic rate. Parasitology 132, 169–176 10.1017/s0031182005008966 (doi:10.1017/s0031182005008966) [DOI] [PubMed] [Google Scholar]
- 41.LeRoux M., Lakshmanan V., Daily J. P. 2009. Plasmodium falciparum biology: analysis of in vitro versus in vivo growth conditions. Trends Parasitol. 25, 474–481 10.1016/j.pt.2009.07.005 (doi:10.1016/j.pt.2009.07.005) [DOI] [PubMed] [Google Scholar]
- 42.Takken W., Lindsay S. W. 2003. Factors affecting the vectorial competence of Anopheles gambiae: a question of scale. In Ecological aspects for application of genetically modified mosquitoes 2, 75–90 Dordrecht, The Netherlands: Springer. [Google Scholar]
- 43.Ahmed A. M., Hurd H. 2006. Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiae via follicular apoptosis. Microb. Infect. 8, 308–315 10.1016/j.micinf.2005.06.026 (doi:10.1016/j.micinf.2005.06.026) [DOI] [PubMed] [Google Scholar]
- 44.Araujo R. V., Maciel C., Hartfelder K., Capurro M. L. 2011. Effects of Plasmodium gallinaceum on hemolymph physiology of Aedes aegypti during parasite development. J. Insect Physiol. 57, 265–273 10.1016/j.jinsphys.2010.11.016 (doi:10.1016/j.jinsphys.2010.11.016) [DOI] [PubMed] [Google Scholar]
- 45.Hurd H., Carter V., Nacer A. 2005. Interactions between malaria and mosquitoes: the role of apoptosis in parasite establishment and vector response to infection. Curr. Top. Microbiol. Immunol. 289, 185–217 10.1007/3-540-27320-4-9 (doi:10.1007/3-540-27320-4-9) [DOI] [PubMed] [Google Scholar]
- 46.Mueller G. C., Junnila A., Schlein Y. 2010. Effective control of adult Culex pipiens by spraying an attractive toxic sugar bait solution in the vegetation near larval habitats. J. Med. Entomol. 47, 63–66 10.1603/033.047.0108 (doi:10.1603/033.047.0108) [DOI] [PubMed] [Google Scholar]
- 47.Hogg J. C., Carwardine S., Hurd H. 1997. The effect of Plasmodium yoelii nigeriensis infection on ovarian protein accumulation by Anopheles stephensi. Parasitol. Res. 83, 374–379 10.1007/s004360050265 (doi:10.1007/s004360050265) [DOI] [PubMed] [Google Scholar]
- 48.Hogg J. C., Hurd H. 1995. Plasmodium yoelii nigeriensis: the effect of high and low intensity of infection upon the egg production and bloodmeal size of Anopheles stephensi during three gonotrophic cycles. Parasitology 111, 555–562 10.1017/S0031182000077027 (doi:10.1017/S0031182000077027) [DOI] [PubMed] [Google Scholar]
- 49.Ahmed A. M., Maingon R., Romans P., Hurd H. 2001. Effects of malaria infection on vitellogenesis in Anopheles gambiae during two gonotrophic cycles. Insect Mol. Biol. 10, 347–356 10.1046/j.0962-1075.2001.00273.x (doi:10.1046/j.0962-1075.2001.00273.x) [DOI] [PubMed] [Google Scholar]
- 50.Gazave E., Chevillon C., Lenormand T., Marquine M., Raymond M. 2001. Dissecting the cost of insecticide resistance genes during the overwintering period of the mosquito Culex pipiens. Heredity 87, 441–448 10.1046/j.1365-2540.2001.00926.x (doi:10.1046/j.1365-2540.2001.00926.x) [DOI] [PubMed] [Google Scholar]