Abstract
Usually studied as pairwise interactions, mutualisms often involve networks of interacting species. Numerous tropical arboreal ants are specialist inhabitants of myrmecophytes (plants bearing domatia, i.e. hollow structures specialized to host ants) and are thought to rely almost exclusively on resources derived from the host plant. Recent studies, following up on century-old reports, have shown that fungi of the ascomycete order Chaetothyriales live in symbiosis with plant-ants within domatia. We tested the hypothesis that ants use domatia-inhabiting fungi as food in three ant–plant symbioses: Petalomyrmex phylax/Leonardoxa africana, Tetraponera aethiops/Barteria fistulosa and Pseudomyrmex penetrator/Tachigali sp. Labelling domatia fungal patches in the field with either a fluorescent dye or 15N showed that larvae ingested domatia fungi. Furthermore, when the natural fungal patch was replaced with a piece of a 15N-labelled pure culture of either of two Chaetothyriales strains isolated from T. aethiops colonies, these fungi were also consumed. These two fungi often co-occur in the same ant colony. Interestingly, T. aethiops workers and larvae ingested preferentially one of the two strains. Our results add a new piece in the puzzle of the nutritional ecology of plant-ants.
Keywords: symbiosis, nutritional ecology, ant–plant–fungus interaction, myrmecophyte
1. Introduction
Ants and plants are among the most dominant taxa in tropical ecosystems, and the ecological importance of their mutualistic interactions is brought to light by two considerations: these interactions structure food webs [1] and the mutualistic benefits exchanged can convey ecological advantages to the partners [2]. Yet the nutritional ecology of these interactions is still poorly understood [3]. In particular, trophic relationships with micro-organisms are just beginning to be investigated [4].
Opportunistic interactions between ants and plants are often mutualistic, and have repeatedly given rise to specialized symbiotic mutualisms between the so-called myrmecophytes (also called ‘ant-plants’), which provide symbiotic ants with nesting cavities (specialized hollow structures, called domatia) in addition to food rewards, and specialist ‘plant-ants’ [5]. Plant-ants often not only protect their hosts against herbivores and pathogens, as well as against competing plants [6–8], they also confer nutritional benefits to their host plants (reviewed by Rico-Gray & Oliveira [9]).
A new, potential source of complexity in the trophic structure of ant–myrmecophyte associations is added by the recent finding that many ant–plant symbioses include long-ignored fungal partners growing within domatia [10]. Patches of fungi growing in domatia were reported as early as the beginning of the twentieth century [11–13], but have attracted little attention, being considered as pests or as commensals [11,13,14]. The difficulty of identifying fungi accurately long discouraged their study. Recent molecular investigations showed that the fungi occupying domatia in a very diverse set of myrmecophytes from throughout the tropics all belong to the ascomycete order Chaetothyriales, and that each seems to be somewhat specific to a particular ant–plant symbiosis [10,15]. Moreover, recent characterization of trophic fluxes among the three partners showed that plant-ants provide their symbiotic fungi with nutrients, probably through the accumulation of waste products [16]. However, the role of fungi in these tripartite symbioses is not yet clear. The trophic relationships between plant-ants and their symbiotic fungi may prove particularly important in helping us better understand the nutritional ecology of ant–plant symbioses.
The symbiotic nature of the relationship between domatia-inhabiting fungi and plant-ants, and the peculiar behaviour of ants towards fungal patches [10,16], led us to consider that these interactions could be new cases of agriculture by insects. In this context, we tested the hypothesis that plant-ants consume the symbiotic fungi growing within domatia of their host plants. We experimentally labelled the fungal patch directly in the field either with calcofluor white stain, a fluorescent dye that binds to fungal cell walls, or with 15N, a stable isotope of nitrogen that is very rare in nature. We then looked for the label in ants.
2. Material and methods
(a). Study species and sites
To assess the generality of our findings, we conducted the same experiments on three phylogenetically independent ant–plant symbioses on two continents (see electronic supplementary material, figure S1). In all of these, ants nest in swollen, hollow stems of the host tree.
Petalomyrmex phylax Snelling (Formicinae) is a small ant (worker body length of 2–3 mm) that obligatorily inhabits the understorey myrmecophytic tree Leonardoxa africana (Baill.) Aubrév. subsp. africana (Fabaceae, Caesalpinioideae). The distribution range of this system is restricted to coastal rainforests of Cameroon [17]. Tetraponera aethiops Smith (Pseudomyrmecinae) is a large ant (worker body length approx. 1 cm) that obligatorily inhabits the pioneering tree Barteria fistulosa Mast. (Passifloraceae). This system is widespread in central Africa [18]. Fieldwork on these two ant–plant systems was conducted in Cameroon, near the village of Nkolo (3°13.278′ N, 10°14.888′ E). Pseudomyrmex penetrator Smith (Pseudomyrmecinae) is a medium-sized ant (worker body length approx. 0.6 cm) that obligatorily inhabits myrmecophytic species of the genus Tachigali Aubl. (Fabaceae, Caesalpinioideae). This ant and its host trees are widely distributed in the eastern and central Amazon basin and adjacent Guianas [19]. Fieldwork on this species was conducted in French Guiana, near Kourou (Montagne des Singes; 5°04.443′ N, 52°41.958′ W). The species of Tachigali in this site could not be identified with certainty, because we found no flowering individuals. Identifying species of Tachigali is difficult in the absence of fertile specimens [20].
In adult ants, a sizeable subspherical pouch called the infrabuccal pocket is located just beneath the tongue. This pouch filters out and compacts the solid debris resulting from cleaning and feeding activities (adult ants can swallow only liquids) in the form of an ovoid pellet that is from time to time regurgitated and discarded. However, workers in the subfamily Pseudomyrmecinae (to which belong T. aethiops and Ps. penetrator) routinely feed their larvae with infrabuccal pellets. The larvae in this subfamily have a special pocket-like structure below the head, called the trophothylax, that the workers fill with infrabuccal pellets [21,22]. Petalomyrmex phylax larvae (subfamily Formicinae) do not have a trophothylax.
Two strains of fungi were previously identified in domatia of B. fistulosa occupied by T. aethiops [10]. Both strains are classified as members of the Chaetothyriales based on morphological and molecular evidence, but they are too divergent from named species to be confidently assigned to a described genus. These two strains were recently made available as pure cultures [15]. They will be called Y1 and Y9 hereafter and correspond, respectively, to the same species as strains CTeY6 and CTeY7 in the study of Voglmayr et al. [15]. Strains detected in fungal patches from Pe. phylax/L. africana and Ps. penetrator/Tachigali ant–plant symbioses were different [15]. In this study, we used only pure cultures of the two strains from domatia of B. fistulosa occupied by T. aethiops.
(b). Labelling the natural fungal patch with calcofluor
Experimental labelling of the fungal patch with calcofluor was performed on ten colonies of Pe. phylax, ten of T. aethiops and three of Ps. penetrator. For each colony, a single occupied domatium was cut off the tree. The domatium was carefully cut longitudinally, and the ants (adults and brood) placed in a separate plastic container. The fungal patch received 30–70 µl of pure calcofluor (Fluka, Buchs, Switzerland), depending on its size, so that the whole patch was in contact with the dyeing liquid. After 20 min (1 h for colonies of Ps. penetrator, which were tested first; we later established that 20 min was a sufficiently long period) the fungal patch was rinsed five times with water, and the excess of water absorbed with paper. The domatium was reassembled using tape and placed in the plastic container with the ants. The ants moved spontaneously into their domatium within 24 h and were provided ad libitum with diluted multiflora honey from a commercial source. Domatia were frozen once they had been transported back to the laboratory (i.e. 6 days after labelling for Pe. phylax and T. aethiops, and 11 days for Ps. penetrator). For each domatium, we investigated fluorescence under UV light in hyphae of the fungal patch, plant cells of the inner surface of the domatium, gut of larvae, hyphae in workers' infrabuccal pellets and hyphae in the content of larval trophothylaces (the last two categories were not investigated for Pe. phylax, whose larvae have no trophothylax and are not fed with infrabuccal pellets). The fungal patch and domatium inner surface were scraped gently with the tip of a scalpel blade, and the collected material was placed in a drop of distilled water between microscope slides. Gut of larvae, workers’ infrabuccal pellets and contents of larval trophothylaces were dissected in distilled water and squashed between slides. A microspectrofluorometer (Jobin-Yvon, Longjumeau, France) equipped with an Olympus BX 60 microscope (Olympus, Tokyo, Japan) was used to obtain the emission fluorescence spectrum of a selected area of 4 µm diameter from each item. This surface is smaller than the section of a hypha of the focal fungi. Using a xenon lamp and monochromators, UV light of wavelength of 365.5–368.5 nm was produced to excite the sample. The resultant fluorescence was detected with a charge-coupled device (CCD) camera, and the fluorescence emission spectra were produced by the SpectraMax software package (Jobin-Yvon). In one of the ten Pe. phylax colonies, fluorescence was investigated visually, but no measure was performed, which explains why the sample size is ten when accounting for the presence/absence of fluorescence, and nine when measure of wavelength is considered. In this colony, no fluorescence similar to that of the fungal patch was detected in larvae.
As a negative control, we collected a single occupied domatium from each of ten colonies of Pe. phylax, ten of T. aethiops and one of Ps. penetrator. Ants were provided ad libitum with diluted multiflora honey from a commercial source. Domatia were frozen once transported back to the laboratory, and fluorescence under UV light was investigated following the same procedure as described earlier.
(c). Labelling the natural fungal patch with 15N
Experimental enrichment of the fungal patch with 15N was performed on ten colonies of Pe. phylax, nine of T. aethiops and nine of Ps. penetrator. For each colony, two occupied domatia were cut off the tree. One of the two received no treatment (negative control). The other was carefully cut longitudinally and the ants (adults and brood) placed in a separate plastic container. The fungal patch received 30–70 µl of an aqueous solution containing 3 mg ml−1 glycine enriched in 15N (98% of molecules marked). After 1 h, the fungal patch was rinsed five times with water, and the excess of water absorbed with paper. The domatium was reassembled using tape and placed in the plastic container with the ants. The ants moved spontaneously into their domatium within 24 h and were provided ad libitum with diluted multiflora honey from a commercial source. Workers, larvae and the fungal patch were collected 4 days later from each domatium and immediately dried under silica gel. Isotopic abundances were measured with an elemental analyser (N) connected to an isotopic mass spectrometer (Finnigan Delta S, Finnigan MAT, Bremen, Germany) at the Service Central d'Analyse of the CNRS (SCA, Solaize, France).
(d). Replacement of the natural fungal patch with pure cultures of the domatia fungi enriched in 15N
For the purpose of the experiment, pure cultures of fungal strains Y1 and Y9 were grown in Petri dishes on a 2 per cent malt extract agar medium containing 0.75 mg ml−1 glycine enriched in 15N (98% of molecules marked). Both strains can occur in either black yeast or hyphal stage. At the time of the experiment, strains were in the black yeast stage and were thus in the form of a thin crust on top of the culture medium. The experiment was conducted on ten colonies of Pe. phylax and ten of T. aethiops. However, it could not be performed on Ps. penetrator, because fieldwork in French Guiana occurred before pure cultures were obtained. For each colony, two occupied domatia were cut off the tree. Each domatium was carefully cut longitudinally, and the ants (adults and brood) were placed in a separate plastic container. The fungal patch was removed with a scalpel and replaced by an equivalent surface of pure culture of one strain. Only a thin, superficial layer of the fungal culture was used. We are confident that culture medium was not introduced into domatia, because we did not alter the basal part of the fungal culture (which is in contact with culture medium). Each colony received both strains, one in each domatium (these were kept separate). Each domatium was reassembled using tape and placed in the plastic container with the corresponding ants. The ants moved spontaneously into the domatium within 24 h and were provided ad libitum with diluted multiflora honey from a commercial source. Workers and brood were collected 4 days later from each domatium and immediately dried under silica gel. For Pe. phylax, all workers from each colony were pooled and treated as a single sample (one measure of δ15N per colony), and larvae were treated the same way, but for T. aethiops, we measured δ15N in three individual workers and larvae in each colony. These values were averaged, and the means used as a single value for each colony. For T. aethiops, the head of each worker was removed before measurement of δ15N in order to exclude any contribution from hyphae in infrabuccal pellets, and test more specifically for ingestion of fungal material into the gut of workers in this species.
Negative controls were the same as in the previous experiment, so we used tests for independent samples to compare experimental and control values. Isotopic abundances were measured as in the previous experiment.
Statistical analyses were performed using R v. 2.13.1 [23].
3. Results
(a). Ant larvae ingest hyphae from domatia fungal patches: evidence from calcofluor staining
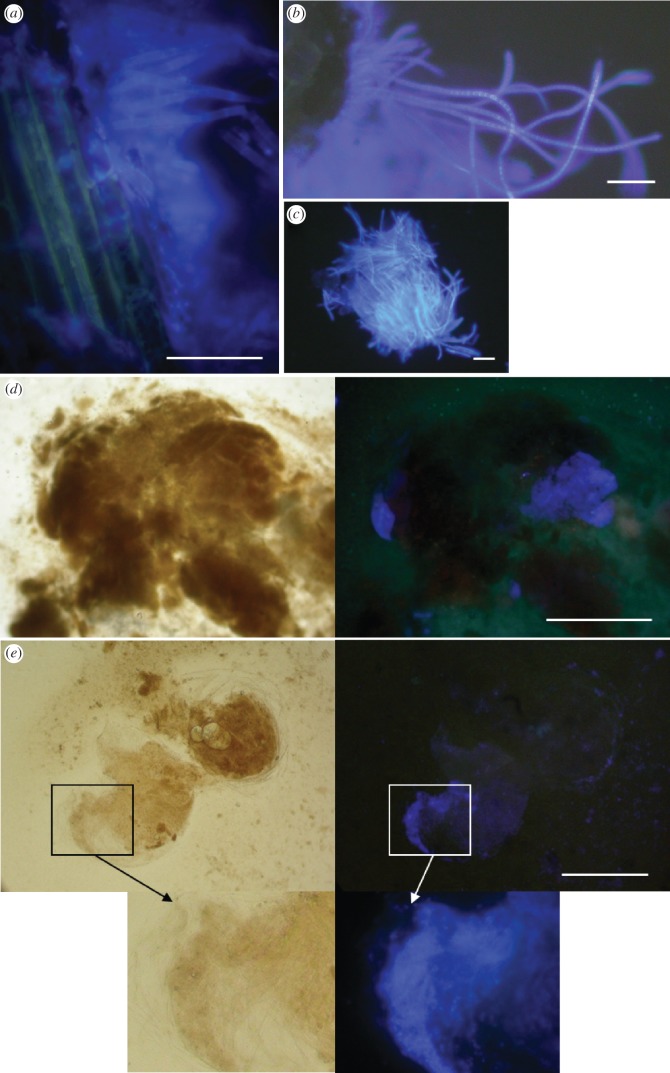
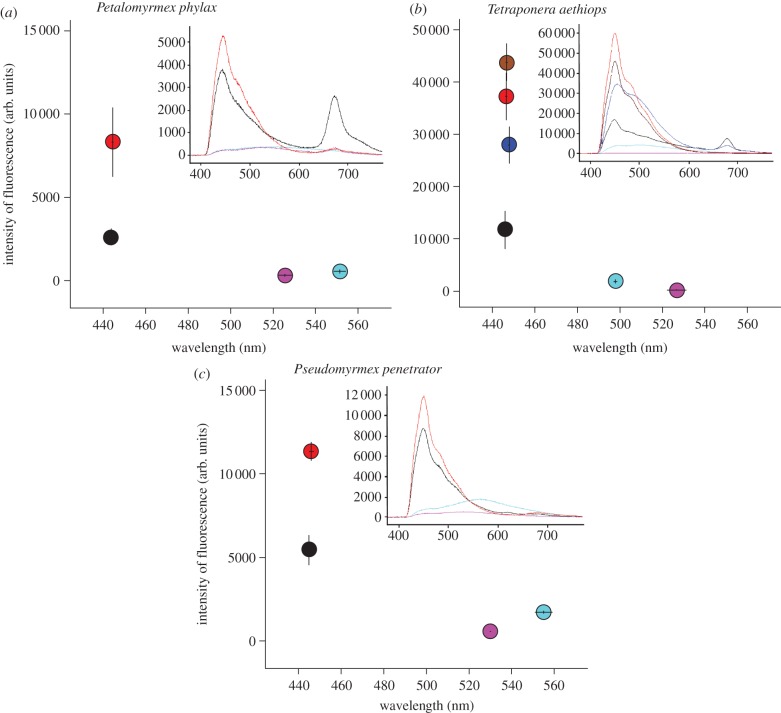
Fungal hyphae exposed to calcofluor within domatia showed a strong bluish fluorescence under UV light, whereas fungal hyphae from control domatia emitted no fluorescence (figure 1a,b). Both wavelength (λmax) and intensity of the most intense peak measured on each spectrum differed significantly between the labelled and control hyphae (figure 2; Mann–Whitney tests, Pe. phylax, for both λmax and intensity: z = 3.7, p = 0.00024; T. aethiops, for both λmax and intensity: z = 3.8, p = 0.00016; no test performed for Ps. penetrator because of small sample size). In fact, spectra for control hyphae were similar to those obtained with only water between slides (data not shown). This shows that calcofluor readily stained hyphae in blue, and that the fungal patch did not emit any spontaneous fluorescence that could interfere with the detection of experimental labelling.
Figure 1.
Microscopic observation of fluorescence under UV light after labelling of fungal patches with calcofluor. (a) Bluish hyphae from fungal patch in a domatium of Tachigali sp. occupied by the ant Pseudomyrmex penetrator. Note that the plant cells emit a faint yellowish fluorescence distinct from that emitted by the fungal patch. (b) Same as panel (a), for the ant–plant system Tetraponera aethiops/Barteria fistulosa. (c) Cluster of bluish hyphae from an infrabuccal pellet of a Tetraponera aethiops worker. (d) Part of the gut of a large Tetraponera aethiops larva emitting, under UV light (right panel), a bluish fluorescence similar to that of the fungal patch; left panel shows same under white light. (e) Same as panel (d), for a small Pseudomyrmex penetrator larva. Scale bars: (a–c) 50 µm, (d,e) 0.5 mm.
Figure 2.
Wavelength and intensity of the most intense fluorescence obtained by microspectrofluorometry under excitation at 365.5–368.5 nm for hyphae of the fungal patch labelled with calcofluor (red), hyphae of the fungal patch in control domatia (pink), plant cell from the inner wall of a domatium in which the fungal patch was labelled with calcofluor (light blue), part of larval gut showing bluish fluorescence (black), hyphae in infrabuccal pellet of workers (and one winged female; dark blue) and hyphae in larval trophothylax (brown), for the three study systems. Means ± s.e. The inserts within each graph show examples of fluorescence emission spectra for each of the above items.
Plant cells from the inner surface of domatia showed a very faint yellowish fluorescence under UV light, and were not labelled bluish by calcofluor (figure 1a,b). Both wavelength and intensity of the most intense peak measured on each spectrum differed significantly between the cells from the inner surface and hyphae in labelled domatia (figure 2; Wilcoxon tests, Pe. phylax, for both λmax and intensity: z = 2.7, p = 0.0077; T. aethiops, for both λmax and intensity: z = 2.8, p = 0.0051; no test performed for Ps. penetrator because of small sample size). This shows that the faint fluorescence of plant cells did not interfere with the detection of experimental labelling.
A bluish fluorescence similar to that emitted by labelled fungal patches was detected under UV light in the gut of a few larvae from six of ten colonies of Pe. phylax, five of ten colonies of T. aethiops and two of three colonies of Ps. penetrator (see electronic supplementary material, table S1; figures 1 and 2). Fluorescent parts of larval gut were less conspicuous for Pe. phylax than for the two other species, and labelling was thus much more difficult to detect in this species. The nature of the fluorescent material was impossible to determine (figure 1d,e). Wavelength was not significantly different between labelled fungal hyphae in the patch and fluorescent parts of the gut of larvae (figure 2; each larva was considered an independent sample; Mann–Whitney tests, Pe. phylax, z = 1.6, p = 0.10; for T. aethiops, z = 1.7, p = 0.09; no test performed for Ps. penetrator because of small sample size; for sample sizes, see electronic supplementary material, table S1). For T. aethiops, hyphae with fluorescence similar to that emitted by labelled fungal patches were observed in the content of trophothylaces from three of six larvae investigated (three colonies), and in all the infrabuccal pellets investigated (24 from 10 colonies; figures 1c and 2). One of those infrabuccal pellets was from a winged female, the others from workers. Fluorescent hyphae in infrabuccal pellets and trophothylaces were found to be either isolated or in clusters (as in figure 1c).
For control colonies, no bluish fluorescence was detected in any of the investigated larvae: 104 larvae from ten colonies of Pe. phylax (8–16 larvae per colony), 140 larvae from ten colonies of T. aethiops (10–35 larvae per colony) and 10 larvae from one colony of Ps. penetrator. Similarly, no bluish fluorescence was detected in the infrabuccal pellets of 23 workers or in the content of trophothylaces of 29 larvae from ten colonies of T. aethiops (two to three infrabuccal pellets, and two to six trophothylaces per colony).
Raw data are available as electronic supplementary material, table S2.
(b). Ants ingest hyphae from domatia fungal patches: evidence from the 15N pulse-chase experiment
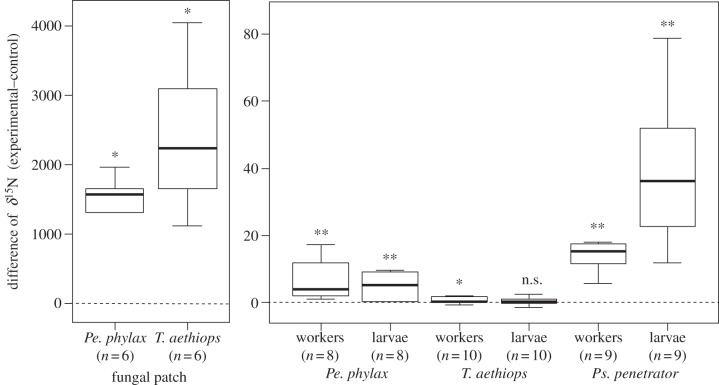
Fungal patches exposed to 15N-glycine had much higher δ15N values than those from control domatia for both Pe. phylax and T. aethiops colonies (figure 3; Wilcoxon tests for paired samples, v = 0, p = 0.031). Regarding Ps. penetrator, fungal patches from only two experimental domatia were analysed, and showed δ15N values as high as in the two other systems. This confirms that the labelling procedure was efficient in the three systems.
Figure 3.
Difference of δ15N between colonies that had their fungal patch exposed to 15N-glycine and control colonies. Horizontal lines represent median, boxes represent first and third quartiles, and whiskers represent first and ninth deciles. *p < 0.05, **p < 0.01, n.s. not significant.
For Pe. phylax and Ps. penetrator, values of δ15N were significantly higher for workers and larvae from colonies that had their fungal patch labelled than for control colonies (figure 3; Wilcoxon tests for paired samples, v = 0, p < 0.01). For T. aethiops, the difference was low but significant for workers (v = 5, p = 0.039), whereas it was not significant for larvae (v = 12, p = 0.25).
Raw data are available as electronic supplementary material, table S3.
(c). Ants ingest hyphae from pure strains of domatia fungi: evidence from the fungal patch replacement experiment
Petalomyrmex phylax workers and larvae from colonies that received pieces of labelled pure cultures of either fungal strain had δ15N values higher than control colonies (see electronic supplementary material, figure S2; Wilcoxon tests for independent samples, for workers, strain Y1: w = 10, p = 0.0062, strain Y9: w = 12, p = 0.012; for larvae, strain Y1: w = 15, p = 0.055, strain Y9: w = 0, p = 0.00067). By contrast, T. aethiops workers and larvae had δ15N values higher than control colonies only when they received strain Y1 (see electronic supplementary material, figure S2; for workers, strain Y1: w = 0, p = 0.000022, strain Y9: w = 23, p = 0.079; for larvae, strain Y1: w = 17, p = 0.022, strain Y9: w = 34, p = 0.40).
Raw data are available as electronic supplementary material, table S4.
4. Discussion
Our results clearly demonstrate that the three plant-ants we studied ingest fungi from the patches growing within domatia. After labelling the fungal patch with a fluorescent dye, we found the same fluorescence in the gut of larvae of the three species as in hyphae of the fungal patch. This strongly suggests that the larvae consumed hyphae from domatia fungi, because we could not detect such fluorescence in any part of the ant-plant except the labelled fungal patch. However, a low proportion of larvae showed the fluorescence: 10 per cent for Pe. phylax and T. aethiops, and 50 per cent for Ps. penetrator (see electronic supplementary material, table S1). Experimental colonies of this last species were exposed for 11 days to the labelled fungal patch instead of 6 days as in the two other species, strongly suggesting that duration of the experiment was too short for an accurate assessment of fungal ingestion by larvae. As pseudomyrmecine ants feed their larvae with the content of their infrabuccal pocket [22], which is used to store debris from cleaning and feeding activities, we also checked the content of infrabuccal pellets and of trophothylaces (infrabuccal pellets packed in a special pouch of the larva) for labelled hyphae in one of our study models (T. aethiops). The presence of labelled hyphae in both structures confirmed that hyphae from domatia fungal patches were transferred to larvae by workers. Although all T. aethiops workers examined had infrabuccal pellets containing labelled hyphae, most larvae had none in their trophothylax, showing that they were not fed on a daily basis. This suggests again that the labelling experiment should have been conducted over a longer period of time to estimate accurately the extent to which larvae are fed with domatia fungi.
Labelling domatia fungal patches with 15N also showed that larvae of Pe. phylax and Ps. penetrator consume domatia fungi. We could not detect any increase in δ15N in larvae of T. aethiops. As discussed earlier, larvae of this species are not fed on a daily basis, and it is possible that the larvae we used for δ15N had not been fed in the course of the experiment, especially as exposure to labelled fungi lasted only for 4 days. Workers of T. aethiops are much larger than those of the two other species; in consequence, fewer larvae were used for isotopic analysis. We thus had a higher chance of including larvae fed with labelled fungi in the two other species, which would explain why we detected an increase in δ15N in larvae of these species over such a short exposure time. The 15N-labelling experiment also showed that workers of the three species had collected domatia fungi. However, worker heads were included in 15N analyses, so that an increase in δ15N may be explained by the presence of hyphae from fungal patches in the infrabuccal pocket as the result of cleaning activities.
Replacing the natural fungal patch with a piece of pure culture of domatia fungal strains labelled with 15N showed that larvae and workers ingested the introduced fungus. Unlike in the previous experiment, heads of T. aethiops were removed before isotopic analysis, so we are confident that the increase in 15N in workers resulted from true ingestion of the fungus, rather than from the simple presence in the infrabuccal pocket, owing to cleaning activities, of fungal material that would eventually be discarded. The two fungal strains introduced were isolated from patches in B. fistulosa domatia occupied by T. aethiops, and tested on both P. phylax and T. aethiops. Petalomyrmex phylax apparently did not show a consistent difference in its consumption of the two strains. However, consumption by larvae and workers of T. aethiops was detected only with strain Y1. The two strains were initially isolated from a single fungal patch, meaning that they co-occurred in a single host plant and colony. Our results suggest that T. aethiops workers are able to discriminate between the two strains and prefer one to the other, a phenomenon that should be further investigated.
Pseudomyrmecine ants feed their larvae with the contents of the infrabuccal pocket [22], which contains debris from cleaning activities. Ingestion of hyphae from domatia fungal patches could thus be a mere by-product of such activities. However, although Pe. phylax workers do not feed their larvae with infrabuccal pellets, larvae nevertheless ingested fungal hyphae, suggesting active feeding on domatia fungi in this species at least. Behavioural observations [10], specificity of fungal strains with plant-ant species [15] and trophic flow from ants to domatia fungi [16] also suggest tight interaction between domatia fungi and plant-ants. The use of these fungi as a food source thus probably explains why ants maintain them within domatia. Whatever the mechanism behind ingestion of domatia fungi (either passive or active), our results demonstrate that they are part of the diet of plant-ants. Whether the ants depend on this resource still remains to be determined. Defossez et al. [16] fed Pe. phylax ants with 15N and showed that N was transferred to the fungal patch and the host plant within 10 days. After almost 2 years, δ15N values were still very high in the system and similar for the three partners, indicating nitrogen recycling. Our results add a new piece of information about the trophic web of this symbiosis. Not only do the ants feed the fungal patch, they also feed on it.
Our study clearly shows that plant-ants have access to food sources that were unsuspected prior to this study. The geographical and taxonomic diversity of the three systems studied, and the fact that fungi seem to be associated with most ant–plant symbioses [11–13,15,24–26], suggest that ingestion of domatia-inhabiting fungi by plant-ants may be a widespread phenomenon. The main obstacle to understanding the nutritional ecology of ant–plant interactions is the lack of knowledge on the qualitative and quantitative aspects of the food sources available to ants. The composition of extrafloral nectar and food bodies is known only in a relatively small subset of plants, and the role of other potential food sources, such as the one evidenced in this study, remains virtually unexplored. Systematic survey of inputs in ant nutrition and detailed characterization of the role of microsymbionts are challenges for future research that must be surmounted if we are to understand the evolutionary diversification of ants and how they contribute to the functioning of tropical ecosystems.
Acknowledgements
This study was funded by grants to R.B. and D.M. from the ‘Young scientists’ (research agreement no. ANR-06-JCJC-0127) and ‘Sixth extinction’ (C3A project) programmes of the French Agence Nationale de la Recherche. We thank the Ministry of Scientific Research and Innovation of the Republic of Cameroon for permitting field experimentation and sample collection. We thank Xavier Garde and the IRD in Yaoundé for providing logistic help in Cameroon. We thank Big John, his family and the traditional chief for their hospitality at the village of Nkolo, Cameroon.
References
- 1.McKey D., Gaume L., Brouat C., Di Giusto B., Pascal L., Debout G., Dalecky A., Heil M. 2005. The trophic structure of tropical ant–plant–herbivore interactions: community consequences and coevolutionary dynamics. In Biotic interactions in the tropics: their role in the maintenance of species diversity (eds Burslem D., Pinard M., Hartley S.), pp. 386–413 Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Heil M., McKey D. 2003. Protective ant–plant interactions as model systems in ecological and evolutionary research. Annu. Rev. Ecol. Evol. Syst. 34, 425–453 10.1146/annurev.ecolsys.34.011802.132410 (doi:10.1146/annurev.ecolsys.34.011802.132410) [DOI] [Google Scholar]
- 3.Davidson D. W., Cook S. C., Snelling R. R., Chua T. H. 2003. Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300, 969–972 10.1126/science.1082074 (doi:10.1126/science.1082074) [DOI] [PubMed] [Google Scholar]
- 4.Cook S. C., Davidson D. W. 2006. Nutritional and functional biology of exudate-feeding ants. Entomol. Exp. Appl. 118, 1–10 10.1111/j.1570-7458.2006.00374.x (doi:10.1111/j.1570-7458.2006.00374.x) [DOI] [Google Scholar]
- 5.Davidson D. W., McKey D. 1993. The evolutionary ecology of symbiotic ant–plant relationships. J. Hymenopt. Res. 2, 13–83 [Google Scholar]
- 6.Frederickson M. E., Greene M. J., Gordon D. M. 2005. ‘Devil's gardens’ bedevilled by ants. Nature 437, 495–496 10.1038/437495a (doi:10.1038/437495a) [DOI] [PubMed] [Google Scholar]
- 7.Letourneau D. K. 1998. Ants, stem-borers, and fungal pathogens: experimental tests of a fitness advantage in Piper ant-plants. Ecology 79, 593–603 10.2307/176956 (doi:10.2307/176956) [DOI] [Google Scholar]
- 8.Rosumek F. B., Silveira F. A. O., Neves F. D., Barbosa N. P. D., Diniz L., Oki Y., Pezzini F., Fernandes G. W., Cornelissen T. 2009. Ants on plants: a meta-analysis of the role of ants as plant biotic defenses. Oecologia 160, 537–549 10.1007/s00442-009-1309-x (doi:10.1007/s00442-009-1309-x) [DOI] [PubMed] [Google Scholar]
- 9.Rico-Gray V., Oliveira P. S. 2007. The ecology and evolution of ant–plant interactions. Chicago, IL: The University of Chicago Press [Google Scholar]
- 10.Defossez E., Selosse M. A., Dubois M. P., Mondolot L., Faccio A., Djieto-Lordon C., McKey D., Blatrix R. 2009. Ant-plants and fungi: a new threeway symbiosis. New Phytol. 182, 942–949 10.1111/j.1469-8137.2009.02793.x (doi:10.1111/j.1469-8137.2009.02793.x) [DOI] [PubMed] [Google Scholar]
- 11.Bailey I. W. 1920. Some relations between ants and fungi. Ecology 1, 174–189 10.2307/1929134 (doi:10.2307/1929134) [DOI] [Google Scholar]
- 12.Docters van Leeuwen W. 1929. Einige Beobachtungen ueber das Zusammenleben von Camponotus quadriceps F. Smith mit dem Ameisenbaum Endospermum formicarum Becc. aus New Guinea. Treubia 10, 431–437 [Google Scholar]
- 13.Miehe H. 1911. Untersuchungen über die javanische Myrmecodia. Abhandl. Math. Phys. Kl. K. Säch. Gesell. Wiss. 32, 312–361 [Google Scholar]
- 14.Bequaert J. 1922. Ants in their diverse relations to the plant world. Bull. Am. Mus. Nat. Hist. 45, 333–583 [Google Scholar]
- 15.Voglmayr H., Mayer V., Maschwitz U., Moog J., Djieto-Lordon C., Blatrix R. 2011. The diversity of ant-associated black yeasts: insights into a newly discovered world of symbiotic interactions. Fungal Biol. 115, 1077–1091 10.1016/j.funbio.2010.11.006 (doi:10.1016/j.funbio.2010.11.006) [DOI] [PubMed] [Google Scholar]
- 16.Defossez E., Djieto-Lordon C., McKey D., Selosse M. A., Blatrix R. 2011. Plant-ants feed their host plant, but above all a fungal symbiont to recycle nitrogen. Proc. R. Soc. B 278, 1419–1426 10.1098/rspb.2010.1884 (doi:10.1098/rspb.2010.1884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKey D. 2000. Leonardoxa africana (Leguminosae: Caesalpinioideae): a complex of mostly allopatric subspecies. Adansonia 22, 71–109 [Google Scholar]
- 18.Breteler F. J. 1999. Barteria Hook. f. (Passifloraceae) revised. Adansonia 21, 306–318 [Google Scholar]
- 19.Ward P. S. 1999. Systematics, biogeography and host plant associations of the Pseudomyrmex viduus group (Hymenoptera: Formicidae), Triplaris- and Tachigali-inhabiting ants. Zool. J. Linn. Soc. 126, 451–540 10.1111/j.1096-3642.1999.tb00157.x (doi:10.1111/j.1096-3642.1999.tb00157.x) [DOI] [Google Scholar]
- 20.Van der Werff H. 2008. A synopsis of the genus Tachigali (Leguminosae: Caesalpinioideae) in northern South America. Ann. Missouri Bot. Gard. 95, 618–661 10.3417/2007159 (doi:10.3417/2007159) [DOI] [Google Scholar]
- 21.Wheeler W. M. 1918. A study of some ant larvae, with a consideration of the origin and meaning of the social habit among insects. Proc. Am. Phil. Soc. 57, 293–343 [Google Scholar]
- 22.Wheeler W. M., Bailey I. W. 1920. The feeding habits of pseudomyrmine and other ants. Trans. Am. Phil. Soc. 22, 235–279 10.2307/1005485 (doi:10.2307/1005485) [DOI] [Google Scholar]
- 23.R Development Core Team 2011. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 24.Blatrix R., Bouamer S., Morand S., Selosse M. A. 2009. Ant–plant mutualisms should be viewed as symbiotic communities. Plant Signal. Behav. 4, 554–556 10.4161/psb.4.6.8733 (doi:10.4161/psb.4.6.8733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janzen D. H. 1972. Protection of Barteria (Passifloraceae) by Pachysima ants (Pseudomyrmecinae) in a Nigerian rain-forest. Ecology 53, 885–892 10.2307/1934304 (doi:10.2307/1934304) [DOI] [Google Scholar]
- 26.Schremmer V. F. 1984. Untersuchungen und Beobachtungen zur Ökoethologie der Pflanzenameise Pseudomyrmex triplarinus, welche die Ameisenbäume der Gattung Triplaris bewohnt. Zool. Jahrb. Abt. Syst. Oekol. Geogr. Tiere 111, 385–410 [Google Scholar]