Abstract
Predicting future species extinctions from patterns of past extinctions or current threat status relies on the assumption that the taxonomic and biological selectivity of extinction is consistent through time. If the driving forces of extinction change through time, this assumption may be unrealistic. Testing the consistency of extinction patterns between the past and the present has been difficult, because the phylogenetically explicit methods used to model present-day extinction risk typically cannot be applied to the data from the fossil record. However, the detailed historical and fossil records of the New Zealand avifauna provide a unique opportunity to reconstruct a complete, large faunal assemblage for different periods in the past. Using the first complete phylogeny of all known native New Zealand bird species, both extant and extinct, we show how the taxonomic and phylogenetic selectivity of extinction, and biological correlates of extinction, change from the pre-human period through Polynesian and European occupation, to the present. These changes can be explained both by changes in primary threatening processes, and by the operation of extinction filter effects. The variable patterns of extinction through time may confound attempts to identify risk factors that apply across time periods, and to infer future species declines from past extinction patterns and current threat status.
Keywords: extinction filter, extinction risk, macroecology, comparative method, New Zealand, birds
1. Introduction
The patterns and drivers of extinction are of considerable interest to conservation biologists who seek to limit the irreversible loss of biodiversity. Extinction is also of interest to evolutionary biologists and macroecologists who wish to understand the role of extinction in shaping species assemblages. These two perspectives—ecological and evolutionary—have come together in comparative studies of extinction risk. One of the aims of such studies is to identify features of species that tend to place them at greater risk of extinction [1,2]. Extinction can be influenced by a range of ecological processes, many of which may be unique to a particular species or a habitat, but a growing number of studies have also identified traits that are significantly associated with extinction risk across many species. For example, body size is associated with extinction risk in birds and mammals, with larger-bodied species more likely to be currently threatened with extinction [3–5]. As well as biological selectivity, there is phylogenetic and taxonomic selectivity in extinction, with some clades or taxa more likely to contain species that have gone extinct or become threatened [2,6,7]. Here, we ask whether the selectivity of extinction changes over time: are the kinds of species that are most threatened now the same as those that went extinct in the past, or that will go extinct in the future?
The biological correlates and lineage selectivity of current extinction risk have been used to predict the patterns and causes of extinction in other time periods. In particular, current correlates of extinction risk have been used to predict potential future species declines and extinctions in, for example, birds [3,6,8–10], mammals [11,12], fish [13,14], invertebrates [15,16] and plants [17]. Increasingly, correlates of current extinction risk are being used to predict the loss of species owing to climate change [18–20]. These predictions rely on being able to extrapolate present-day patterns of extinction risk to future species extinctions. However, little is known about the consistency of extinction patterns over time. It is reasonable to expect that patterns of decline and extinction will change over time, owing to the changing nature of threatening processes.
Furthermore, correlates of extinction may change through time as the result of extinction filter effects, where past patterns of extinctions make assemblages appear resilient to threats they have faced before [21]. In this way, selective extinctions in one period of history may alter the structure of the assemblage, influencing the taxonomic and phylogenetic selectivity of extinction, and the biological correlates of extinction risk, in subsequent periods. For these reasons, studies of extinction risk in contemporary assemblages may not be sufficient to capture the way that extinction patterns and processes change through time [16,22].
To investigate temporal changes in extinction patterns, it is necessary to consider patterns of species loss from assemblages in the past. Many studies have been conducted on patterns of species loss from the fossil record over geological time [23–25]. These studies have the advantage of being able to include a large number of extinctions over a broad time period, but often have coarse temporal resolution and are generally restricted to a broad taxonomic canvas. Increasingly, studies of extinction patterns are using patterns of historic extinctions and recent species declines, typically using data on estimated risk of extinction from the IUCN database [3,4,20,26]. While such studies benefit from a finer species-level resolution, they are usually restricted to a narrow time period, typically encompassing a period of dramatic anthropogenic environmental change. Using information only from extant assemblages limits the ability to uncover changing patterns of extinction risk over time [16,22].
Both types of studies, geological and recent, are commonly limited by the availability of information on the phylogenetic relationships among species in past assemblages. Phylogenetic information is needed in studies of extinction risk in order to account for the fact that many of the factors that make species vulnerable to extinction, such as low reproductive rate or lack of predator avoidance behaviour, are likely to be similar among closely related species [27]. This confounds the search for biological traits that cause taxonomic selectivity of extinction. For example, one of the most striking avian groups from New Zealand (NZ) was the giant moas, an endemic radiation of flightless ratites [28,29]. All species of moa went extinct soon after human colonization [30]. Because all moas share many traits in common, simply considering a tally of extinct species does not allow us to determine whether moas were vulnerable to extinction because they were, for example, among the largest animals in the assemblage, or because they tasted particularly good or whether there was some other aspect of moa biology that made them extinction prone. Only by controlling for the confounding effect of relatedness using an analysis that explicitly allows for phylogenetic history can we tease out different correlates of extinction [22,27].
The reliance on phylogenetic information has meant that many studies are clade-based, focusing on a particular well-studied group, regardless of their ecological roles or their geographical distribution. Alternatively, studies focusing on species assemblages have typically either failed to correct for non-independence of species data [10,19] or relied on higher taxonomic categories as a proxy for phylogeny [31,32]. Ideally, studies of changing patterns of extinction risk through time should be based on geographically well-defined assemblages for which a complete phylogeny is known, for both extinct and extant taxa, and for which extinctions can be identified in different time periods, or associated with particular impacts or threats.
In this study, we track the patterns of extinction through time for an assemblage consisting of all known native bird species in New Zealand. We chose the NZ avifauna because of the uniquely detailed information available for a large assemblage on the timing of many extinctions, including both recent extinctions following human arrival and natural extinctions spanning tens of millions of years. The NZ avifauna is a diverse assemblage, with hundreds of species filling a wide variety of ecological roles, yet within a well-defined geographical area with a distinct geological and evolutionary history [31]. A wealth of fossil material of NZ birds has been described [33], particularly from a number of distinct sites, such as the Miocene St Bathan's fauna [34] and the Holocene Lake Poukawa fauna [35]. For species that went extinct before historical records began, there is insufficient temporal resolution in the fossil record to allow the timing of species extinctions to be delineated [31], however the rich fossil fauna does allow us to add extinct species to our phylogeny, and to infer species present in each of four distinct phases of human impact.
This rich palaeontological record has allowed us to construct an assemblage-level phylogeny for all described NZ native bird species, including 87 extinct species [36]. Species were included in the phylogeny using one of three methods: (i) using DNA sequences for 11 mitochondrial and two nuclear genes, including ancient DNA, where available [28,29]; (ii) using DNA sequences from close relatives to place the lineage in the assemblage phylogeny; (iii) the use of conservative taxonomic constraints during the phylogenetic reconstruction (see Lanfear & Bromham [36] for details). The use of taxonomic constraints to place species for which no sequence data were available precludes the estimation of a single phylogenetic tree of all NZ birds, because taxonomy usually provides limited resolution of phylogenetic placement. We used Bayesian phylogenetic inference to produce a set of 1000 phylogenetic trees that provides a description of our knowledge of the relationships between all species in the assemblage. This permits the use of rigorous phylogenetic comparative methods to account for statistical non-independence owing to relatedness, while simultaneously accounting for phylogenetic uncertainty, so we can determine whether the identification of correlates of extinction risk holds for a range of reasonable phylogenetic hypotheses, rather than being specific to a particular tree [37].
The inclusion of extinct species in our analysis allows us to reconstruct the assemblage for different periods in the past and so take a temporal view of the patterns and correlates of extinction. Rather than defining equal periods along a timescale, we divide the extinctions by their occurrence in four distinct phases of the ecological history of New Zealand: (i) before human arrival, (ii) after the arrival of Polynesians but before the arrival of Europeans; (iii) after European arrival and (iv) species currently threatened with extinction. This allows us to ask whether the kinds of species most vulnerable to extinction (with respect to taxonomy, phylogeny and biological traits) have remained constant or changed across these distinctly different periods.
2. Material and methods
We compiled a list of all known native birds of NZ, based primarily on ‘The Checklist of the Birds of New Zealand’ [38], comprising 274 species and subspecies, of which 187 are extant and 87 are extinct (see electronic supplementary material, table S1).
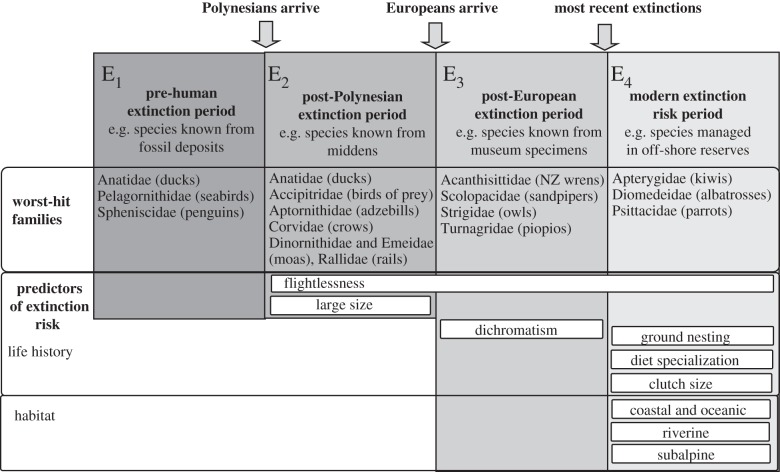
We identified species that went extinct in each of four distinct phases (figure 1), as follows:
— pre-human extinction (E1): species known only from pre-human fossil or subfossil deposits (29 species);
— post-Polynesian extinction (E2): species extant at the time of humans arrival in NZ but extinct before the arrival of European settlers, for example species known from middens (35 species);
— post-European extinction (E3): species extant at the time of arrival of European settlers but now extinct (23 species); and
— currently threatened (E4): extant species categorized by the IUCN [39] as threatened with extinction, i.e. categories near threatened (NT), vulnerable (VU), endangered (EN) and critically endangered (CR; 90 species).
Figure 1.
Historical extinction periods for New Zealand birds. This diagram shows the families identified as suffering a higher number of extinctions in each period than expected, and traits that showed significant correlation with extinction risk in over 80% of phylogenies (see electronic supplementary material, table S4 and figures S1 and S2 for details). In the pre-human (E1) and post-Polynesian (E2) periods, the only life-history variables analysed were body size, flightlessness and sexual size dimorphism. Habitat variables and diet preferences were not analysed for E1 and E2.
The remaining species are considered not to be at immediate risk of extinction, being extant species classified as least concern (LC) by the IUCN (97 species).
We make assumption that a species known only from deposits dated to before human arrival went extinct in the pre-human period. The oldest described fossil species in our dataset are penguins from the Early Palaeocene; however, the majority of fossil taxa are from the Holocene. While we include all extinct species that have been formally described in the literature, there must also be unknown or undescribed species, particularly from periods poorly represented in the NZ fossil record. Furthermore, the taxonomic identity and systematic placement of fossil taxa may be uncertain: for example, DNA analysis of moa bones suggests one-third of specimens or more may be misidentified [28]. The pre-human period of the assemblage history is therefore poorly known, so we make few inferences about ancient extinction patterns. Nonetheless, there are sufficient described species to allow us to ask if there is any pattern to the species known from the pre-human fossil record but absent from the record after the arrival of humans.
Humans arrived relatively recently in NZ, with recent estimates of the timing of colonization by Polynesian people at around AD 1280 [40]. The impact on native flora and fauna was striking, with wide-scale habitat change, hunting pressure and the introduction of the nest predator, the Pacific rat (Rattus exulans) [31]. Europeans first reached NZ in the 1600s, but did not set up permanent settlements until the early nineteenth century. In addition to broad-scale habitat modification and novel hunting pressure (e.g. hunting for specimens), Europeans introduced a wide range of mammalian predators to NZ, including cats, rats and mustelids. We distinguish extinctions after European colonization (E3) from species currently threatened with extinction (E4), because these two categories represent non-equivalent species responses (complete extinction versus population decline) and because they represent different phases of extinction risk in NZ. Since 1968, no avian species has gone completely extinct in NZ, and the period after the last extinction is also marked by concerted conservation effort to save threatened species from extinction, for example by maintaining populations in predator-free reserves.
(a). Life-history data
A wide range of species characteristics could contribute to species decline, but it is neither practical nor desirable to include the maximum number of measurable traits. Instead, we targeted traits that have been found to be associated with extinction risk in previous studies of decline in bird species. All analyses included only those species for which estimates of the relevant variables were available in the literature (see electronic supplementary material, table S1).
Body size has been found to be positively correlated with extinction risk in birds [3,8,41–45], so we included mean female adult body mass as a factor in our analysis. Body size estimates for extinct species are typically based on extrapolation from limb bone length (see references in electronic supplementary material, table S1). Clearly, these are imperfect measures but should serve as an approximate guide to the relationship between size and extinction risk. Where only a range of values was available, we used the geometric mean of the maximum and minimum. We also included average clutch size, where available, because higher extinction risk in birds has been associated with low fecundity [8,42,44]. We include only estimates based on observation of contemporary or recently extinct species.
Introduced predators are a critical factor in the decline of many birds species in NZ [46] and island avifaunas generally [47]. In particular, nest predation can cause population declines in birds [48–50], and ground-nesting birds may be particularly vulnerable [43,51,52]. Therefore, we categorized NZ birds according to whether they are known to nest primarily on the ground or not. Flightlessness has also been identified as a risk factor in NZ bird extinctions [10,32,45], so we categorized species as either flight able (including weak fliers) or flightless.
The relationship between sexual selection and extinction risk in birds is unclear, with some studies finding that species that show evidence of marked sexual selection have a greater risk of local extinction [53,54], others showing that species with more marked plumage differences between the sexes have a lower extinction risk [8], and others finding no link between sexual size dimorphism or plumage dichromatism and population trends [55]. We analysed whether sexually dimorphic species have a greater extinction risk using two measures of sexual dimorphism. Firstly, we recorded as a binary variable whether species are known to be sexually dichromatic, with consistent differences in plumage coloration between males and females. This is likely to be an underestimate, as some differences occur in parts of the colour spectrum not obvious to human observers [56]. We do not analyse dichromatism for species extinct before historical records began. For all species, where available, we include sexual size dimorphism as a continuous variable, calculated as the ratio of mean female mass to mean male mass.
Diet has been shown to be associated with extinction risk in birds [10,43,52]. We assigned each species to one or more of the following diet categories: (i) aquatic and marine invertebrates and vertebrates; (ii) aquatic and marine vegetation; (iii) terrestrial vegetation including fruits and seeds, (iv) terrestrial invertebrates, (v) terrestrial vertebrates. To represent diet specialization, we counted the number of categories a species was recorded as utilizing, so for example a species that is known to eat terrestrial and marine invertebrates and fruit in season scored 3, while a species that only preys on terrestrial vertebrates scored 1. Specific habitat types have been correlated with declines in bird populations, as well as habitat specialization in general [42,52]. Each species was assigned to one of the following categories: (i) forest and scrub; (ii) coastal and oceanic; (iii) lakes, swamps and wetlands, (iv) grassland; (v) riverine; (vi) subalpine. We did not analyse diet or habitat preferences for species extinct before European settlement owing to the uncertainty of inferring these variables for fossil species.
(b). Phylogeny of all New Zealand birds
Reconstructions of phylogenetic history, like any biological estimates, cannot be treated as absolute point estimates, but must allow for uncertainty. We used a Bayesian phylogenetic analysis of DNA sequence data, plus information from taxonomy, to estimate the evolutionary history of all known native NZ bird species, both extant and extinct [36]. We performed our analyses on a sample of 1000 phylogenetic trees drawn from the posterior distribution, in order to account for phylogenetic uncertainty in our analysis (for details, see Lanfear & Bromham [36]).
(c). Taxonomic and phylogenetic selectivity of extinction
Although biological correlates of extinction have been identified for many groups, it is possible that extinction in any given assemblage is random with respect to relatedness, so that no group contains more extinct species than expected by chance. This could arise because extinction acts like a ‘field of bullets’ [57] where chance of persistence is essentially independent of species traits, or because extrinsic factors overwhelm any intrinsic risk factors [26], or because the intrinsic risk factors themselves do not have strong phylogenetic signal [37]. So our first step is to ask whether extinction of NZ birds has been non-random with respect to taxonomy and phylogeny.
If extinction is random, then the number of extinct or threatened species per higher taxon should be approximately proportional to the number of species in that taxon [44,58,59]. In order to ask whether the extinctions that occurred in each extinction period were a random subset of the species assemblage in that period, we conducted a series of randomization tests. We compared the distributions of extinct species across families with expected distributions generated by randomly sampling the number of extinct species per period from the list of all species present in the assemblage in that period, generating 10 000 such random samples per period. We then compared the value of the Poisson parameter of the observed distribution to the set of 10 000 random Poisson parameter values. We repeated this procedure for each of the historical periods, adjusting the membership of each family to reflect the extinctions that occurred in the previous period and the addition of any new arrivals. For the contemporary assemblage, the comparison was made using the number of species listed as threatened by the IUCN. We used the null distributions to identify families that suffered significantly more or less extinctions than expected in each historical period.
Many studies have demonstrated that current extinction risk has phylogenetic signal, that is, close relatives tend to be more similar in threat status than expected if extinction risk was phylogenetically random [2]. One way of measuring the phylogenetic signal of extinction risk is to ask whether extinct (or threatened) species are phylogenetically clustered or overdispersed within the tree of all species in the assemblage. We used two measures of phylogenetic clustering: mean pairwise distance (MPD) and mean nearest taxon distance (MNTD). MPD is the mean path length between all possible pairs of extinct species in a given time period, whereas MNTD is the mean path length between each extinct species and its nearest extinct relative in a given time period [60]. We explicitly modelled phylogenetic uncertainty using a Bayesian framework. In addition, we repeated the analyses on two types of phylogenetic representations. Because the phylogeny contains species with varying amounts of sequence data, and some taxa are placed on taxonomic information alone, our ability to estimate branch lengths accurately varies over the tree. Therefore, we repeated analyses on both phylograms (with branch lengths estimated by Bayesian inference) and cladograms (with unitary branch lengths). For each extinction period, we compared observed MPD and MNTD values with a null distribution of 999 values, calculated by randomizing the tip labels of the phylogeny of the assemblage for that time period (see electronic supplementary material, table S3 for details of analysis).
(d). Correlates of extinction risk
We then asked whether the species characteristics we recorded correlate with probability of extinction in each historical period. For each of the historical faunas, we fitted univariate models predicting extinction probability from species biological traits, using regression on phylogenetically independent contrasts implemented with the crunch algorithm in the R library caper (http://cran.r-project.org/web/packages/caper). For the contemporary period (E4), we analysed extinction risk both as a binary variable (non-threatened versus threatened, pooling all species listed as NT, VU, EN or CR) and as an ordinal variable (LC = 0, NT = 1, VU = 2, EN = 3, CR = 4). We also conducted multivariate regressions to test for the effect of covariation between traits. We identified intercorrelated predictors and used Akaike information criterion (AIC) to compare models constructed from different combinations of these predictors. One model was considered a better fit to the data than another where the difference in AIC (ΔAIC) was more than 2. This set of tests and model comparisons were conducted using each of the 1000 trees in the posterior sample to calculate independent contrasts. This gave us distributions of 1000 slope estimates and p-values for each univariate test, and 1000 ΔAIC values for each model comparison.
3. Results
(a). Taxonomic and phylogenetic selectivity of extinction
Extinct species in every historical extinction period are a non-random sample of the species known to have existed in that time period (figure 1). The overall distributions of extinct species across families are significantly different from those expected if extinction was random with respect to taxonomy, indicating that some families suffer disproportionately more extinctions than others. Although we expect these tests to lack power for many families with low species richness, we identified a number of families as having a disproportionately high number of extinctions, and we found that the extinction-prone families vary across time periods (see electronic supplementary material, table S2).
The phylogenetic analyses also suggest that extinctions in each historical period were not random with respect to phylogeny, and that some lineages suffered a greater proportion of species lost to extinction than others (electronic supplementary material, table S3). Both MPD and MNTD values suggest significant clustering of extinct species in cladograms for categories E1, E2 and E3. Currently threatened species (E4) are significantly clustered in the phylograms.
(b). Correlates of extinction risk
Because extinction risk analyses were performed on a posterior sample of 1000 alternative phylogenies, we cannot report a single parameter estimate and p-value for each test. Instead, we present the distributions of p-values for each test, the median value of the slopes (B50) and the percentage of the 1000 tests that have a p-value below 0.05 (see electronic supplementary material, table S4 and figures S1 and S2).
The regressions reveal that the significant correlates of extinction vary across the four time periods. Only body size, flightlessness and sexual dimorphism were analysed for E1, none of which showed significant correlations with extinction risk. The lack of correlates of extinction risk in the pre-human period may be due to low power (few species with data), or because relevant factors were not able to be included in the analysis, or because extinctions were effectively random with respect to life history. These results should be interpreted with caution, but they suggest that prior to human occupation, large flightless birds were not at significantly greater risk of extinction.
Although flightless species did not suffer higher extinction rates in the pre-human period, they experienced a disproportionately high rate of extinction in all periods of human occupation (see electronic supplementary material, table S4). Other life-history correlates vary in their association with extinction risk over the different periods of human impact (figure 1). Body size was only associated with extinction risk in the post-Polynesian period (E2), when large-bodied species were more likely to go extinct. Dichromatic species had a higher extinction rate following European settlement (E3), but are not currently at higher risk of extinction. Smaller average clutch sizes and ground-nesting are associated with current extinction threat, but neither of these factors was associated with extinction likelihood in the recent past. When analysed as an ordinal variable, only flightlessness and ground-nesting were significant correlates of extinction risk in contemporary fauna (see electronic supplementary material, table S4). We did not analyse diet categories separately, but we show that diet breadth is negatively associated with current extinction risk. Species in riverine, subalpine and coastal or oceanic habitats are more likely to be currently threatened with extinction.
We found that body size, ground-nesting and flightedness covaried significantly, so we compared different multivariate combinations of these predictors. Whether extinction risk is treated as a binary or an ordinal variable, the model that includes ground-nesting and flightedness is most often preferred over other models, suggesting that both of these traits have a significant independent impact on contemporary extinction risk (see electronic supplementary material, figure S2).
4. Discussion
We show that extinction of birds in NZ has not been random, but has been biased towards particular lineages. The phylogenetic analysis confirms that extinct species are clustered on the phylogeny, reflecting the fact that traits that increase extinction risk, such as body size, low fecundity or particular habitat preferences, tend to be more similar among related taxa. This non-independence of extinction risk among species requires that any analysis of correlates of extinction, whether focusing on the present or over longer time periods, must take phylogenetic relatedness into account.
Reconstructing the NZ avifauna for different historical periods offers a unique opportunity to track patterns of extinction over time in a large assemblage. Adding an historical dimension to the analysis of extinction has revealed that both the taxonomic and phylogenetic selectivity of extinction, and the biological and ecological correlates of extinction, have varied across time periods representing key stages in the ecological history of NZ. The families with the greatest proportion of threatened species today did not have significantly more extinctions in the past, and, with the exception of flightlessness, the traits that predispose species to high extinction risk today are not the same as those associated with extinction in the past. There are two likely explanations for the changing patterns of extinction over time: changes in the predominating threatening processes, and modification of the structure of the assemblage through an extinction filter effect.
Caution must be exercised in interpreting the results from the pre-human period as the fossil record is unavoidably biased. Most studies of bird extinction in NZ and other Pacific islands have not examined the pre-human extinctions as a separate phase of extinction patterns [10,31,32,61], possibly because the data are imperfect. The NZ fossil fauna from the pre-human period is dominated by several well-studied deposits, for example the Miocene St Bathan's fauna from the South Island of NZ that was deposited in a freshwater lake and is dominated by waterfowl fossils [34,62]. Biases in preservation and discovery have likely resulted in better sampling of species associated with, for example, aquatic environments than with high-altitude forest. More generally, large-bodied species are more likely to be described from the fossil record than smaller and less robust specimens. These unavoidable sampling biases may account for the non-random pattern of extinctions in the pre-human period, with more ducks, penguins and seabirds known to have gone extinct that expected from a random sample of taxa inferred to be present at the time. It is therefore interesting to note that even though described fossil taxa are likely to be biased towards large species, there is still no detectable bias in extinctions towards larger, flightless species. The data availability for these variables is sufficient to allow us to detect a strong bias if one were present, which suggests that in the pre-human period, when NZ was free of mammalian predators, large-bodied or flightless species were no more likely to go extinct than other species.
The arrival of humans in NZ brought extensive modification of natural habitat, and saw the introduction of new predators, most notably the Pacific rat (R. exulans) [31,40]. In addition, birds were hunted for both food and ornament by human settlers. Hunting had a significant impact on the decline and extinction of endemic bird species, and those species that were hunted were at a greater risk of extinction than others [63]. We see the impact of Polynesian hunting pressure reflected in the increased extinction risk of large species, as well as flightless birds [64]. This resulted in a disproportionate loss of species from families with many large or flightless species such as the moas (Dinorthinidae and Emeidae) and rails (Rallidae).
The arrival of Europeans brought further environmental modification, and a range of new mammalian predators, so flightless species remained particularly prone to extinction. Hunting birds for food declined, which may account for the lack of size bias in extinctions after the arrival of Europeans. However, Europeans brought new kinds of hunting pressure, such as providing specimens for natural history collections [65,66]. It is tempting to speculate that this is why dichromatic species were at a greater extinction risk in this period, though it is difficult to separate out the effect of collecting from other causes of species decline. For example, the decline and eventual extinction of the huia (Heteralocha acutirostris), which had striking sexual dimorphism in bill shape, may have been exacerbated by excessive collecting to supply the collector's market and for fashion accessories [65,67], but clearance of lowland forest and the introduction of mammalian predators are also likely to have contributed significantly to the species' decline. Dichromatism has also been associated with establishment and survival of introduced birds in NZ, with more strongly dichromatic bird species less likely to maintain successful populations [68], potentially because it is costly and increases mortality [54]. However, while we find that dichromatic species were more prone to extinction in the recent past, we find no evidence that dichromatism is linked to current extinction risk.
We found that species that nest on the ground are more likely to be currently threatened with extinction, which is likely to reflect the impact of introduced predators such as cats, dogs, rats and stoats [31,32]. Low clutch size is also correlated with threat status, as it may limit the capacity of a population decimated by predators to return to sustainable levels [44]. The success of both native and introduced bird species in NZ has been greater for species with larger clutch sizes [45]. Species with a more specialized diet are also more likely to be currently threatened, possibly reflecting the difficulty of maintaining large populations on specialized food sources in a greatly reduced habitat area [44]. As a result of extensive habitat loss, some populations of native NZ birds with specialized diets can only sustain recruitment when reintroduced to the mainland if their diets are supplemented [69,70].
Temporal patterns of extinction risk may alter the composition of an assemblage significantly, an effect known as an extinction filter. Extinction filters can alter patterns of extinction in subsequent periods, making species with particular characteristics appear resilient to a threat they have faced before [21]. Large body size is commonly found to be associated with high extinction risk in mammals [4,71,72] and birds [3,41,44], and there are a range of plausible mechanisms by which large size may confer elevated risk, including increased hunting pressure and lower rates of population increase [4]. However, large body size is not a predictor of current extinction risk in NZ birds. Extinctions in the post-Polynesian period (E2) were significantly skewed towards larger species, to the extent that all of the largest bird species (greater than 9 kg) became extinct, clearly visible as a truncation of the right hand tail of the body size distribution between E2 and E3 (figure 2). After the largest species disappear from the assemblage, there is no longer any detectable influence of body size on extinction risk. Because there is also a change in the predominating threatening processes between periods, we cannot attribute with certainty the changing effect of body size to an extinction filter effect. But the lack of a current association between body size and extinction risk may be partly the result of the extinction of large species during the period of heavy hunting pressure after human arrival in NZ. Body size extinction filters have also been suggested to operate in other faunas where large size may have predisposed species to extinction in the past [4,58,73,74].
Figure 2.
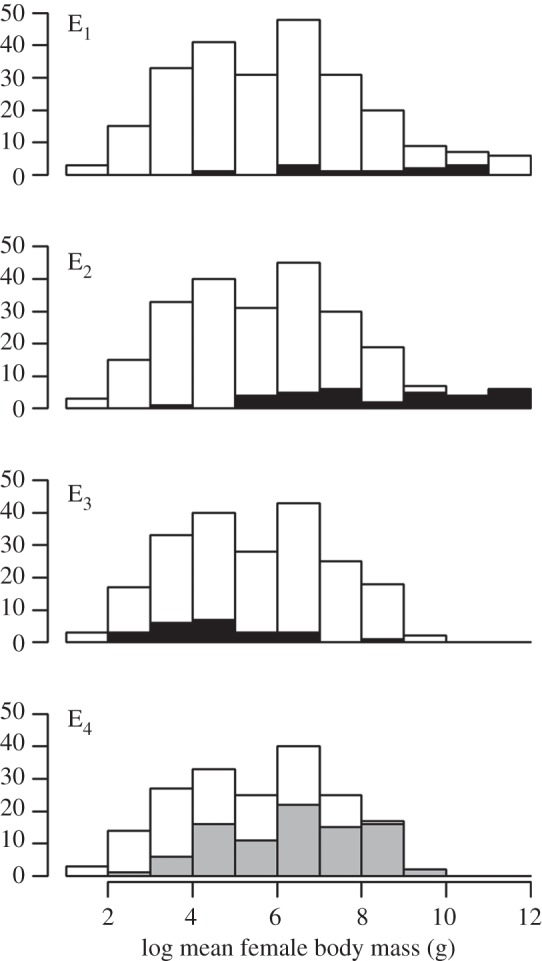
Frequency distributions of female body mass for New Zealand birds in different extinction risk periods. White bars are all species in each size category inferred to be present in each period, black bars are the species that went extinct in each period, and grey bars are species currently threatened with extinction.
An additional explanation for the finding that current correlates of extinction risk do not correspond to patterns of past extinctions is that measures of contemporary extinction risk, typically based on range restriction and population decline, may not accurately reflect patterns of absolute extinction. For example, ground-nesting species, which are the most vulnerable to introduced predators, are more likely to be currently threatened (E4). But there have not been significantly more ground-nesting species driven to extinction in the period since these predators were introduced (E3). One possible explanation is that predators significantly reduce the populations of ground-nesting species but many vulnerable species have escaped absolute extinction by persisting in small numbers in protected areas, such as offshore islands [75].
5. Conclusions
The study of correlates of extinction risk has gained much momentum in the past decade, and is increasingly being applied to prediction of future population declines. In particular, it is hoped that by illuminating patterns of current threatened species it will be possible to identify species at risk of future declines so that pre-emptive action can be taken [3,11,12]. However, we have shown that both the selectivity and the biological correlates of extinction in the NZ avifauna have varied across different periods, characterized by changing human impact. This result is important because changing drivers of extinction are often assumed, but have rarely been demonstrated to be statistically significant and beyond the effect of taxonomic bias or phylogenetic non-independence. Extinction filters may have also contributed to variation in extinction correlates over time by removing vulnerable species from the assemblage, in particular the extinction of all of the largest species following human arrival in NZ. These findings demonstrate that patterns of current extinction risk in a contemporary fauna do not necessarily reflect patterns of extinction in the past. This finding has important implications for the growing number of studies that explain past extinction patterns or predict future species losses based on current species declines.
References
- 1.Fisher D. O., Owens I. P. F. 2004. The comparative method in conservation biology. Trends Ecol. Evol. 19, 391–398 10.1016/j.tree.2004.05.004 (doi:10.1016/j.tree.2004.05.004) [DOI] [PubMed] [Google Scholar]
- 2.Purvis A., Cardillo M., Grenyer R., Collen B. 2005. Correlates of extinction risk: phylogeny, biology, threat and scale. In Phylogeny and conservation (eds Purvis A., Gittleman J. L., Brooks T.), pp. 295–316 Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Lee T. M., Jetz W. 2010. Unravelling the structure of species extinction risk for predictive conservation science. Proc. R. Soc. B 278, 1329–1338 10.1098/rspb.2010.1877 (doi:10.1098/rspb.2010.1877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardillo M., Mace G. M., Jones K. E., Bielby J., Bininda-Emonds O. R. P., Sechrest W., Orme C. D. L., Purvis A. 2005. Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241 10.1126/science.1116030 (doi:10.1126/science.1116030) [DOI] [PubMed] [Google Scholar]
- 5.Bennett P. M., Owens I. P. F. 1997. Variation in extinction risk among birds: chance or evolutionary predisposition? Proc. R. Soc. Lond. B 264, 401–408 10.1098/rspb.1997.0057 (doi:10.1098/rspb.1997.0057) [DOI] [Google Scholar]
- 6.Russell G. J., Brooks T. M., McKinney M. M., Anderson C. G. 1998. Present and future taxonomic selectivity in bird and mammal extinctions. Conserv. Biol. 12, 1365–1376 10.1046/j.1523-1739.1998.96332.x (doi:10.1046/j.1523-1739.1998.96332.x) [DOI] [Google Scholar]
- 7.McKinney M. M. 1997. Extinction vulnerability and selectivity: combining ecological and paleontological views. Annu. Rev. Ecol. Syst. 28, 495–516 10.1146/annurev.ecolsys.28.1.495 (doi:10.1146/annurev.ecolsys.28.1.495) [DOI] [Google Scholar]
- 8.Krüger O., Radford A. N. 2008. Doomed to die? Predicting extinction risk in the true hawks Accipitridae. Anim. Conserv. 11, 83–91 10.1111/j.1469-1795.2007.00155.x (doi:10.1111/j.1469-1795.2007.00155.x) [DOI] [Google Scholar]
- 9.Norris K., Harper N. 2004. Extinction processes in hot spots of avian biodiversity and the targeting of pre-emptive conservation action. Proc. R. Soc. Lond. B 271, 123–130 10.1098/rspb.2003.2576 (doi:10.1098/rspb.2003.2576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyer A. G. 2009. Consistent ecological selectivity through time in Pacific island avian extinctions. Conserv. Biol. 24, 511–519 10.1111/j.1523-1739.2009.01341.x (doi:10.1111/j.1523-1739.2009.01341.x) [DOI] [PubMed] [Google Scholar]
- 11.Cardillo M., Purvis A., Sechrest W., Gittleman J. L., Bielby J., Mace G. M. 2004. Human population density and extinction risk in the world's carnivores. PLoS Biol. 2, e197. 10.1371/journal.pbio.0020197 (doi:10.1371/journal.pbio.0020197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardillo M., Mace G. M., Gittleman J. L., Purvis A. 2006. Latent extinction risk and the future battlegrounds of mammal conservation. Proc. Natl Acad. Sci. USA 103, 4157–4161 10.1073/pnas.0510541103 (doi:10.1073/pnas.0510541103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olden J. D., Poff N. L., Bestgen K. R. 2008. Trait synergisms and the rarity, extirpation, and extinction risk of desert fishes. Ecology 89, 847–856 10.1890/06-1864.1 (doi:10.1890/06-1864.1) [DOI] [PubMed] [Google Scholar]
- 14.Dulvy N. K., Reynolds J. D. 2002. Predicting extinction vulnerability in skates. Conserv. Biol. 16, 440–450 10.1046/j.1523-1739.2002.00416.x (doi:10.1046/j.1523-1739.2002.00416.x) [DOI] [Google Scholar]
- 15.Larson E. R., Olden J. D. 2010. Latent extinction and invasion risk of crayfishes in the Southeastern United States. Conserv. Biol. 24, 1099–1110 10.1111/j.1523-1739.2010.01462.x (doi:10.1111/j.1523-1739.2010.01462.x) [DOI] [PubMed] [Google Scholar]
- 16.Chiba S., Roy K. 2011. Selectivity of terrestrial gastropod extinctions on an oceanic archipelago and insights into the anthropogenic extinction process. Proc. Natl Acad. Sci. USA 108, 9496–9501 10.1073/pnas.1100085108 (doi:10.1073/pnas.1100085108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saar L., Takkis K., Pärtel M., Helm A. 2012. Which plant traits predict species loss in calcareous grasslands with extinction debt? Divers. Distrib. 18, 808–817 10.1111/j.1472-4642.2012.00885.x (doi:10.1111/j.1472-4642.2012.00885.x) [DOI] [Google Scholar]
- 18.Graham N. A. J., et al. 2011. Extinction vulnerability of coral reef fishes. Ecol. Lett. 14, 341–348 10.1111/j.1461-0248.2011.01592.x (doi:10.1111/j.1461-0248.2011.01592.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidson A. D., Boyer A. G., Kim H., Pompa-Mansilla S., Hamilton M. J., Costa D. P., Ceballos G., Brown J. H. 2012. Drivers and hotspots of extinction risk in marine mammals. Proc. Natl Acad. Sci. USA 109, 3395–3400 10.1073/pnas.1121469109 (doi:10.1073/pnas.1121469109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray K. A., Rosauer D., McCallum H., Skerratt L. F. 2011. Integrating species traits with extrinsic threats: closing the gap between predicting and preventing species declines. Proc. R. Soc. B 278, 1515–1523 10.1098/rspb.2010.1872 (doi:10.1098/rspb.2010.1872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balmford A. 1996. Extinction filters and current resilience: the significance of past selection pressures for conservation biology. Trends Ecol. Evol. 11, 193–196 10.1016/0169-5347(96)10026-4 (doi:10.1016/0169-5347(96)10026-4) [DOI] [PubMed] [Google Scholar]
- 22.Ezard T. H. G., Aze T., Pearson P. N., Purvis A. 2011. Interplay between changing climate and species’ ecology drives macroevolutionary dynamics. Science 332, 349–351 10.1126/science.1203060 (doi:10.1126/science.1203060) [DOI] [PubMed] [Google Scholar]
- 23.Harnik P. G. 2011. Direct and indirect effects of biological factors on extinction risk in fossil bivalves. Proc. Natl Acad. Sci. USA 108, 13 594–13 599 10.1073/pnas.1100572108 (doi:10.1073/pnas.1100572108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unwin D. M. 1988. Extinction and survival in birds. In Extinction and survival in the fossil record (ed. Larwood G. P.), pp. 295–318 Oxford, UK: Clarendon Press [Google Scholar]
- 25.Jablonski D. H., Raup D. M. 1995. Selectivity of end-Cretaceous marine bivalve extinctions. Science 268, 389–391 10.1126/science.11536722 (doi:10.1126/science.11536722) [DOI] [PubMed] [Google Scholar]
- 26.Duncan J. R., Lockwood J. L. 2001. Extinction in a field of bullets: a search for causes in the decline of the world's freshwater fishes. Biol. Conserv. 102, 97–105 10.1016/S0006-3207(01)00077-5 (doi:10.1016/S0006-3207(01)00077-5) [DOI] [Google Scholar]
- 27.Purvis A. 2008. Phylogenetic approaches to the study of extinction. Annu. Rev. Ecol. Syst. 39, 301–319 10.1146/annurev.ecolsys.063008.102010 (doi:10.1146/annurev.ecolsys.063008.102010) [DOI] [Google Scholar]
- 28.Baker A. J., Huynen L. J., Haddrath O., Millar C. D., Lambert D. M. 2005. Reconstructing the tempo and mode of evolution in an extinct clade of birds with ancient DNA: the giant moas of New Zealand. Proc. Natl Acad. Sci. USA 102, 8257–8262 10.1073/pnas.0409435102 (doi:10.1073/pnas.0409435102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bunce M., et al. 2009. The evolutionary history of the extinct ratite moa and New Zealand Neogene paleogeography. Proc. Natl Acad. Sci. USA 49, 20 646–20 651 10.1073/pnas.200223397 (doi:10.1073/pnas.200223397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holdaway R. N., Jacomb C. 2000. Rapid extinction of the moas (Aves: Dinornithiformes): model, test, and implications. Science 287, 2250–2254 10.1126/science.287.5461.2250 (doi:10.1126/science.287.5461.2250) [DOI] [PubMed] [Google Scholar]
- 31.Holdaway R. N. 1999. Introduced predators and avifaunal extinction in New Zealand. In Extinctions in near time: causes, contexts, and consequences (ed. MacPhee R. D. E.), pp. 189–238 New York, NY: Kluwer Academic [Google Scholar]
- 32.Duncan R. P., Blackburn T. M. 2004. Extinction and endemism in the New Zealand avifauna. Glob. Ecol. Biogeogr. 13, 509–517 10.1111/j.1466-822X.2004.00132.x (doi:10.1111/j.1466-822X.2004.00132.x) [DOI] [Google Scholar]
- 33.Worthy T. H., Holdaway R. N. 2002. The lost world of the Moa: prehistoric life of New Zealand. Indiana, IN: Indiana University Press [Google Scholar]
- 34.Worthy T. H., Tennyson A. J. D., Jones C., McNamara J. A., Douglas B. J. 2007. Miocene waterfowl and other birds from Central Otago, New Zealand. J. Syst. Palaeontol. 5, 1–39 10.1017/S1477201906001957 (doi:10.1017/S1477201906001957) [DOI] [Google Scholar]
- 35.Worthy T. H. 2004. The Holocene fossil waterfowl fauna of Lake Poukawa, North Island, New Zealand. Tuhinga 15, 77–120 [Google Scholar]
- 36.Lanfear R., Bromham L. 2011. Estimating phylogenies for species assemblages: a complete phylogeny for the past and present native birds of New Zealand. Mol. Phylogenet. Evol. 61, 958–963 10.1016/j.ympev.2011.07.018 (doi:10.1016/j.ympev.2011.07.018) [DOI] [PubMed] [Google Scholar]
- 37.Thomas G. H. 2008. Phylogenetic distributions of British birds of conservation concern. Proc. R. Soc. B 275, 2077–2083 10.1098/rspb.2008.0549 (doi:10.1098/rspb.2008.0549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gill B. 2010. Checklist of the birds of new zealand. New Zealand: Ornithological Society of New Zealand [Google Scholar]
- 39.IUCN 2010. IUCN red list of threatened species. See http://www.iucnredlist.org [Google Scholar]
- 40.Wilmshurst J. M., Anderson A. J., Higham T. F. G., Worthy T. H. 2008. Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proc. Natl Acad. Sci. USA 105, 7676–7680 10.1073/pnas.0801507105 (doi:10.1073/pnas.0801507105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaston K. J., Blackburn T. M. 1995. Birds, body size and the threat of extinction. Phil. Trans. R. Soc. Lond. B 347, 205–212 10.1098/rstb.1995.0022 (doi:10.1098/rstb.1995.0022) [DOI] [Google Scholar]
- 42.Sodhi N. S., et al. 2010. Deforestation and avian extinction on tropical landbridge islands. Conserv. Biol. 24, 1290–1298 10.1111/j.1523-1739.2010.01495.x (doi:10.1111/j.1523-1739.2010.01495.x) [DOI] [PubMed] [Google Scholar]
- 43.Van Turnhout C. A. M., Foppen R. P. B., Leuven R. S. E. W., Van Strien A., Siepel H. 2010. Life-history and ecological correlates of population change in Dutch breeding birds. Biol. Control 143, 173–181 10.1016/j.biocon.2009.09.023 (doi:10.1016/j.biocon.2009.09.023) [DOI] [Google Scholar]
- 44.Owens I. P. F., Bennett P. M. 2000. Ecological basis of extinction risk in birds: habitat loss versus human persecution and introduced predators. Proc. Natl Acad. Sci. USA 97, 12 144–12 148 10.1073/pnas.200223397 (doi:10.1073/pnas.200223397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cassey P. 2001. Determining variation in the success of New Zealand land birds. Glob. Ecol. Biogeogr. 10, 161–172 10.1046/j.1466-822x.2001.00224.x (doi:10.1046/j.1466-822x.2001.00224.x) [DOI] [Google Scholar]
- 46.Whitehead A. L., Elliott G. P., McIntosh A. R. 2010. Large-scale predator control increases population viability of a rare New Zealand riverine duck. Austral Ecol. 35, 722–730 10.1111/j.1442-9993.2009.02079.x (doi:10.1111/j.1442-9993.2009.02079.x) [DOI] [Google Scholar]
- 47.Blackburn T. M., Cassey P., Duncan R. P., Evans K. L., Gaston K. J. 2004. Avian extinction and mammalian introductions on oceanic islands. Science 305, 1955–1958 10.1126/science.1101617 (doi:10.1126/science.1101617) [DOI] [PubMed] [Google Scholar]
- 48.Chalfoun A. D., Thompson F. R., Ratnaswamy M. J. 2002. Nest predators and fragmentation: a review and meta-analysis. Conserv. Biol. 16, 306–318 10.1046/j.1523-1739.2002.00308.x (doi:10.1046/j.1523-1739.2002.00308.x) [DOI] [Google Scholar]
- 49.Martin T. E. 1995. Avian life history evolution in relation to nest sites, nest predation, and food. Ecol. Monogr. 65, 101–127 10.2307/2937160 (doi:10.2307/2937160) [DOI] [Google Scholar]
- 50.Remes V., Matysiokova B., Cockburn A. 2012. Nest predation in New Zealand songbirds: exotic predators, introduced prey and long-term changes in predation risk. Biol. Conserv. 148, 54–60 10.1016/j.biocon.2012.01.063 (doi:10.1016/j.biocon.2012.01.063) [DOI] [Google Scholar]
- 51.Thaxter C. B., Joys A. C., Gregory R. D., Baillie S. R., Noble D. G. 2010. Hypotheses to explain patterns of population change among breeding bird species in England. Biol. Conserv. 143, 2006–2019 10.1016/j.biocon.2010.05.004 (doi:10.1016/j.biocon.2010.05.004) [DOI] [Google Scholar]
- 52.Gregory R. D., et al. 2007. Population trends of widespread woodland birds in Europe. Ibis 149, 78–97 10.1111/j.1474-919X.2007.00698.x (doi:10.1111/j.1474-919X.2007.00698.x) [DOI] [Google Scholar]
- 53.McLain D. K., Moulton M. P., Redfearn T. P. 1995. Sexual selection and the risk of extinction of introduced birds on oceanic island. Oikos 74, 27–34 10.2307/3545671 (doi:10.2307/3545671) [DOI] [Google Scholar]
- 54.Doherty P. F., Sorci G., Royle J. A., Hines J. E., Nichols J. D., Boulinier T. 2003. Sexual selection affects local extinction and turnover in bird communities. Proc. Natl Acad. Sci. USA 100, 5858–5862 10.1073/pnas.0836953100 (doi:10.1073/pnas.0836953100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prinzing A., Brandle M., Pfeifer R., Brandl R. 2002. Does sexual selection influence population trends in European birds? Evol. Ecol. Res. 4, 49–60 [Google Scholar]
- 56.Igic B., Leuschner N., Parker K. A., Ismar S. M., Gill B. J., Lovegrove T. G., Millar C. D., Hauber M. E. 2010. Size dimorphism and avian-perceived sexual dichromatism in a New Zealand endemic bird, the whitehead Mohoua albicilla. J. Morphol. 271, 697–704 [DOI] [PubMed] [Google Scholar]
- 57.Raup D. M. 1992. Extinction: bad genes or bad luck? New York, NY: W.W. Norton & Company; [PubMed] [Google Scholar]
- 58.Cardillo M., Bromham L. 2001. Body size and risk of extinction in Australian mammals. Conserv. Biol. 15, 1435–1440 10.1046/j.1523-1739.2001.00286.x (doi:10.1046/j.1523-1739.2001.00286.x) [DOI] [Google Scholar]
- 59.Matthews L. J., Arnold C., Machanda Z., Nunn C. L. 2011. Primate extinction risk and historical patterns of speciation and extinction in relation to body mass. Proc. R. Soc. B 278, 1256–1263 10.1098/rspb.2010.1489 (doi:10.1098/rspb.2010.1489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Webb C. O. 2000. Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am. Nat. 156, 145–155 10.1086/303378 (doi:10.1086/303378) [DOI] [PubMed] [Google Scholar]
- 61.Steadman D. W. 1995. Prehistoric extinctions of Pacific island birds: biodiversity meets zooarchaeology. Science 267, 1123–1131 10.1126/science.267.5201.1123 (doi:10.1126/science.267.5201.1123) [DOI] [PubMed] [Google Scholar]
- 62.Worthy T. H., Tennyson A. J. D., Hand S. J., Scofield R. P. 2008. A new species of the diving duck Manuherikia and evidence for geese (Aves: Anatidae: Anserinae) in the St Bathans Fauna (Early Miocene), New Zealand. J. R. Soc. NZ 38, 97–114 10.1080/03014220809510549 (doi:10.1080/03014220809510549) [DOI] [Google Scholar]
- 63.Duncan R. P., Blackburn T. M., Worthy T. H. 2002. Prehistoric bird extinctions and human hunting. Proc. R. Soc. Lond. B 269, 517–521 10.1098/rspb.2001.1918 (doi:10.1098/rspb.2001.1918) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyer A. G. 2008. Extinction patterns in the avifauna of the Hawaiian islands. Divers. Distrib. 14, 509–517 10.1111/j.1472-4642.2007.00459.x (doi:10.1111/j.1472-4642.2007.00459.x) [DOI] [Google Scholar]
- 65.Tennyson A., Martinson P. 2006. Extinct birds of New Zealand. Wellington, NZ: Te Papa Press [Google Scholar]
- 66.Myers J. G. 1923. The present position of the endemic birds of New Zealand. N Z J. Sci. Technol. 6, 65–99 [Google Scholar]
- 67.Moore-Colyer R. J. 2000. Feathered women and persecuted birds: the struggle against the plumage trade c. 1860–1922. Rural History 11, 57–73 10.1017/S0956793300001904 (doi:10.1017/S0956793300001904) [DOI] [Google Scholar]
- 68.Sorci G., Møller A. P., Clobert J. 1998. Plumage dichromatism of birds predicts introduction success in New Zealand. J. Anim. Ecol. 67, 263–269 10.1046/j.1365-2656.1998.00199.x (doi:10.1046/j.1365-2656.1998.00199.x) [DOI] [Google Scholar]
- 69.Armstrong D. P., Castro I., Griffiths R. 2007. Using adaptive management to determine requirements of re-introduced populations: the case of the New Zealand hihi. J. Appl. Ecol. 44, 953–962 10.1111/j.1365-2664.2007.01320.x (doi:10.1111/j.1365-2664.2007.01320.x) [DOI] [Google Scholar]
- 70.Houston D., McInnes K., Elliott G., Eason D., Moorhouse R., Cockrem J. 2007. The use of a nutritional supplement to improve egg production in the endangered kakapo. Biol. Conserv. 138, 248–255 10.1016/j.biocon.2007.04.023 (doi:10.1016/j.biocon.2007.04.023) [DOI] [Google Scholar]
- 71.Cardillo M., Gittleman J. L., Purvis A. 2008. Global patterns in the phylogenetic structure of island mammal assemblages. Proc. R. Soc. B 275, 1549–1556 10.1098/rspb.2008.0262 (doi:10.1098/rspb.2008.0262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Purvis A., Agapow P. M., Gittleman J. L., Mace G. M. 2000. Nonrandom extinction and the loss of evolutionary history. Science 288, 328–330 10.1126/science.288.5464.328 (doi:10.1126/science.288.5464.328) [DOI] [PubMed] [Google Scholar]
- 73.Turvey S. T., Fritz S. A. 2011. The ghosts of mammals past: biological and geographical patterns of global mammalian extinction across the Holocene. Phil. Trans. R. Soc. B 366, 2564–2576 10.1098/rstb.2011.0020 (doi:10.1098/rstb.2011.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lyons S. K., Smith F. A., Brown J. H. 2004. Of mice, mastodons and men: human-mediated extinctions on four continents. Evol. Ecol. Res. 6, 339–358 [Google Scholar]
- 75.Towns D. R., Ballantine W. J. 1993. Conservation and restoration of New Zealand island ecosystems. Trends Ecol. Evol. 8, 452–457 10.1016/0169-5347(93)90009-E (doi:10.1016/0169-5347(93)90009-E) [DOI] [PubMed] [Google Scholar]