Abstract
Memory is a complex and dynamic process that is composed of different phases. Its evolution under natural selection probably depends on a balance between fitness benefits and costs. In Drosophila, two separate forms of consolidated memory phases can be generated experimentally: anaesthesia-resistant memory (ARM) and long-term memory (LTM). In recent years, several studies have focused on the differences between these long-lasting memory types and have found that, at the functional level, ARM and LTM are antagonistic. How this functional relationship will affect their evolutionary dynamics remains unknown. We selected for flies with either improved ARM or improved LTM over several generations, and found that flies selected specifically for improvement of one consolidated memory phase show reduced performance in the other memory phase. We also found that improved LTM was linked to decreased longevity in male flies but not in females. Conversely, males with improved ARM had increased longevity. We found no correlation between either improved ARM or LTM and other phenotypic traits. This is, to our knowledge, the first evidence of a symmetrical evolutionary trade-off between two memory phases for the same learning task. Such trade-offs may have an important impact on the evolution of cognitive capacities. On a neural level, these results support the hypothesis that mechanisms underlying these forms of consolidated memory are, to some degree, antagonistic.
Keywords: memory phases, trade-offs, life history, Drosophila, artificial selection
1. Introduction
Studies of both vertebrates and invertebrates have shown that memory is composed of different phases that differ in duration and time of onset. Two distinct forms of memory—labile, short-term memory and robust, long-term memory—were defined in original studies of memory. These two forms appear to be highly conserved from invertebrates to vertebrates [1]. Long-term memory is typically defined as memory that is resistant to anaesthesia and that depends on new protein synthesis. However, genetic dissection of the memory phases has revealed that, at least in Drosophila [2] and some parasitoid species [3], these two criteria may define two different forms of memory. In Drosophila, anaesthesia-resistant memory (ARM) and long-term memory (LTM) can be independently formed using Pavlovian aversive olfactory conditioning [4]. ARM forms when repeated conditioning sessions immediately follow one another; LTM forms when repeated conditioning sessions are separated by a time interval. These two memory phases differ in their durability and by whether they depend on new protein synthesis. LTM lasts longer than ARM and its formation requires de novo protein synthesis [3,5]. These findings raise questions about the pattern of genetic correlations between different memory phases, and about how these correlations could influence cognitive evolution.
Previous studies have focused on the functional differences between these long-lasting memory phases [2,3,6–8]. Only recently has research begun to address how they are specifically adapted to the needs of an animal behaving in its natural environment and how they respond to natural selection [3,9–11]. The capacity for learning and memory is known to trade-off with other fitness-related traits [12–15]. At the functional level, a recent study established that the formation of ARM gates the formation of LTM via specific oscillations of two pairs of dopaminergic neurons that project to the mushroom body [16]. This study confirmed that the spacing between conditioning events determines whether ARM or LRM will form [17] and that there is a functional trade-off between these consolidated memory phases. Less is known about the evolutionary significance of this functional trade-off and whether evolution acts on ARM and LTM independently.
Although the existence of evolutionary trade-offs is widely assumed, it can be difficult to demonstrate them. Several approaches have been used, including comparative studies on different taxa, phenotypic manipulation, analysis of genetic correlations and selection experiments; however, most of these have interpretive limitations [18,19]. In the present study, we directly addressed how a functional trade-off may affect the evolutionary relationship between ARM and LTM. We artificially selected populations of Drosophila either for improved ARM or LTM, and determined the extent to which selection on one memory phase affects the formation of the other memory phase. Unlike ARM, LTM formation is known to be costly and linked to decreased longevity in conditioned flies [20]. Knowing that learning ability trades off with other traits [13], we quantified longevity, fecundity, stress resistance and development time to demonstrate the ultimate constitutive cost of improved LTM.
2. Material and methods
(a). Fly stock and maintenance
Our base stock population was derived from a wild-type Drosophila melanogaster population collected in the centre of France (Chavroches) in 2006 and maintained in the laboratory under a 12 L : 12 D cycle. All flies used were 3- to 5-day-old adults.
(b). Conditioning assay with mechanical shocks
For all experiments, we used a classical aversive olfactory conditioning regime [20]. Groups of 50 flies were conditioned to associate one of two odours (3-octanol, OCT or 4-methylcyclohexanol, MCH) with mechanical shocks. These two odours are commonly used in Drosophila olfactory conditioning; flies can perceive both odours equally well and can differentiate between them. Half of the fly groups were conditioned with OCT as conditioned stimulus associated with shock (CS+) and the other half with MCH as CS+. The training protocol consisted of five cycles that were either separated by a 20 min rest period (spaced protocol) to induce the formation of LTM or were continuous with no rest period (massed protocol) to induce the formation of ARM. During each cycle, flies were exposed to an odour for 1 min (CS+ = OCT or MCH) accompanied by mechanical shocks as an unconditioned stimulus (US; 2000 r.p.m. vibration pulses of 1 s, delivered every 5 s by a test tube shaker). After a 1 min rest period, during which flies received humid airflow (no shock), flies were exposed to the second odour for 1 min (CS− = MCH or OCT) without shock, followed by another 1-min rest period. This pattern of CS+/rest/CS−/rest made up a single conditioning cycle.
Immediately after conditioning, flies were transferred onto standard food for 24 h. They were then transferred into the central point of a T-maze, in which they were exposed to two divergent currents of air (one carrying the CS+ odour, one carrying the CS−) [20]. Flies were free to make their choice for 1 min, after which they were trapped in their chosen arm of the maze and counted.
(c). Selection regimes for artificial selection on anaesthesia-resistant memory and long-term memory formation
The experiment consisted of two selection regimes: ARM and LTM (figure 1). For each selection regime, eight replicate lines were selected for specific memory improvement and eight other lines were kept as control lines, for a total of four groups and 32 lines (eight ARM lines, eight ARM control lines, eight LTM lines, eight LTM control lines). Every generation, 100 flies (males and females mixed) from each selected line and each control line were randomly selected and divided into two groups. One group of 50 flies was conditioned to avoid OCT and the other to avoid MCH. For the selected lines, only flies that moved towards the CS− odour were kept for breeding the next generation. For the control lines, all flies were allowed to breed the next generation, whether they chose the CS− or the CS+. To avoid differential inbreeding between control and selected lines, we randomly selected flies from the control line to ensure that we had the same number of flies for control line X (1 ≤ X ≤ 8), as the number of flies that made the ‘correct choice’ for the corresponding selected line X. For each line, after testing and counting, flies were grouped and kept for 3 days on standard food medium. On day four, they were allowed to oviposit on standard food, and the eggs were kept to form the next generation. The number of offspring obtained at each generation for each line varied between 200 and 300 flies.
Figure 1.
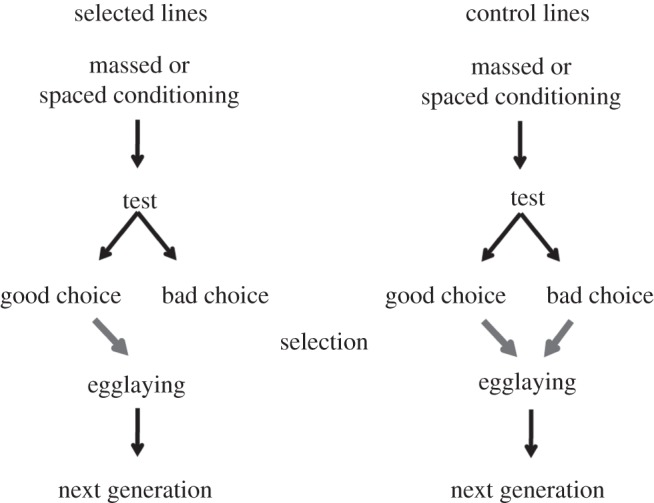
Experimental selection design. From a natural population, 16 lines were conditioned in the Pavlovian olfactory paradigm for each memory type (eight ARM with eight control lines and eight LTM with eight control lines); then 24 h later, memory retention was tested in a T-maze. Only flies that made the correct choice were kept to breed the next generation. A random selection of control flies, corresponding to the number of selected flies that made the correct choice were kept to breed.
After 23 and 28 generations of selection, we measured ARM and LTM for all control and selected lines in order to test how selection on a specific memory phase impacts other memory phases and whether a potential evolutionary trade-off between the two memory phases can be observed.
We calculated the memory score as the difference in the proportion of flies from the sample conditioned to avoid MCH that chose OCT and the proportion of flies from the sample conditioned to avoid OCT that chose OCT. Memory scores vary between −1 and 1. A memory score of 0 suggests no response to the initial conditioning procedure; a memory score of 1 suggests that all flies avoided the odour they had been trained to avoid. To compare memory scores, we angularly transformed all proportions before statistical analyses [21]. Prior to analysis, we checked that errors were normally distributed. Unless noted otherwise, all statistical analyses were conducted using SPSS software. We used ANOVA to compare memory scores for each selection regime by including selection type (control versus selection) as the fixed factor and replicate line as a random factor nested within-selection type.
(d). Behavioural assay with electric shock
ARM and LTM memory phases were first described in an aversive olfactory conditioning protocol using electric shock and not mechanical shock as the US [4]. We tested selected and control lines from both regimes using electric shock as the US, in order to check whether the mechanical shock paradigm produced the same results as the traditional electrical shock paradigm. We conducted these tests after 30 generations.
(e). Correlated responses to other phenotypic traits
(i). Longevity
We measured longevity of the selected and control lines after 30 generations of selection. After emergence, we transferred groups of 50 virgin males or females into 120 × 50 × 90 mm plastic cages that contained one Petri dish filled with standard food medium. We changed food once per week and counted the number of dead flies twice per week. We measured longevity on the two selection regimes (eight control and eight selected lines for each regime) with two cages per sex and per line (32 lines, two sexes, two replicates = 128 cages).
For each sex and selection regime, we compared the longevity of control versus selected lines by applying a linear mixed model on median longevity per cage that included a random effect of replicate line nested within-selection type.
To get additional information, we also analysed the effect of selection regime on longevity by comparing the mortality rate of each group (selected versus control) using a package designed specifically for mortality rate analysis in the software R derived from Winmodest [22]. For each sex, we first used Akaike's information criterion to select the most parsimonious model and found the logistic model that best suited our data. Then, for each sex, we fitted this model:
where μ is the instantaneous mortality rate at age x and a, b and s are the parameters of the model: a is the initial mortality rate, b is the rate at which mortality increases with age and s is the deceleration of the mortality rate. The observed mortality rates were estimated by μx = −ln(px)/Δx, where px is the proportion of flies surviving from age x to age x + Δx.
(ii). Stress resistance
We measured oxidative stress resistance of the selected and control lines after 40 generations of selection. To assay resistance to paraquat—a powerful agent known to cause oxidative damage that mimics the damage that occurs during ageing [23]—we isolated groups of 20 mixed sex flies and kept them in vials with fresh food. One day later, we transferred groups of flies to new vials that contained no food but that contained filter paper impregnated with paraquat solution (33 mM) diluted in 5 per cent sucrose solution. After 30 h of treatment, we removed flies from the vial and counted the dead ones. We measured mortality rate for five replicates each of selected and control lines (five replicates, two selection regimes each with eight selected lines and eight corresponding control lines = 160 vials). We analysed paraquat resistance using a generalized linear model, with treatment as the fixed effect and line nested within each treatment as the random effect.
(iii). Fecundity
We measured fecundity of the selected and control lines after 42 generations of selection. Virgin females were isolated from all selected and control lines, and kept in vials having fresh food until they were 3 days old. Then, groups of five females were separated and placed with three wild-type males for 2 days. After mating, flies were transferred to cages, where females were allowed to lay eggs on a Petri dish filled with a solution of agar and sugar for 24 h. We performed four replicates of each line (eight selected for ARM, eight selected for LTM and 16 control).
We analysed fecundity using a generalized linear model on the number of eggs laid for both selection regimes, with treatment as the fixed effect and replicate lines nested within the treatment (selected or control) as the random effect.
(iv). Egg-to-adult survival
We measured egg-to-adult survival of the selected and control lines after 42 generations of selection. To do this, we transferred groups of about 100 flies from each selected and control line to a box where females were allowed to lay eggs in one Petri dish filled with agar for 12 h. From each Petri dish, we isolated five groups of 30 larvae and transferred them to vials containing standard food. We then measured the total number of emerging adult flies in each vial.
(v). Unconditioned response to odours
To confirm that differences in learning and memory between selection regimes were not confounded with differences in odour perception, we gave groups of naïve flies a choice between one of the two odours (OCT or MCH at the same concentration used in the conditioning experiments) in the solvent (mineral oil) and tested them in a T-maze. We performed this experiment after 22 generations of selection on all control and selected lines (two replicates per line per odour).
3. Results
(a). Artificial selection on anaesthesia-resistant memory and long-term memory formation
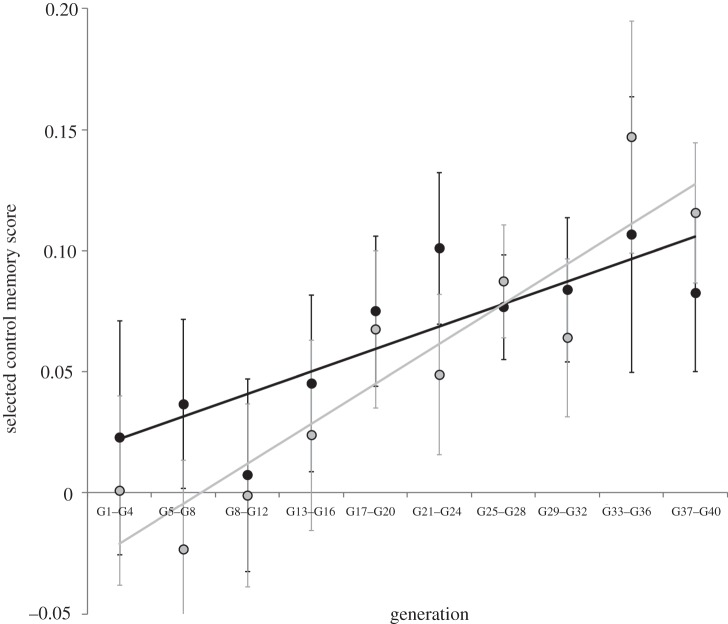
Over generations, the consolidated memory phases of the selected lines increased compared with control lines (figure 2). Lines selected for improved LTM showed progressive memory increase compared with their respective control lines when tested 24 h after spaced conditioning (repeated measures ANOVA within-subject effects: generation: F9,126 = 12.54, p < 0.001; control versus selected × generation: F9,126 = 8.9, p = 0.02). Lines selected for improved ARM showed a similar pattern 24 h after massed conditioning (repeated measures ANOVA within-subject effects: generation: F9,126 = 7.97, p < 0.001; control versus selected × generation: F9,126 = 2.6, p = 0.007). Over generations, no difference in the rate of memory score increase could be observed between the two selected regimes (F9,126 = 1.7, p = 0.08).
Figure 2.
Evolution of the difference in memory scores (mean ± s.e.m. based on variation among lines) between selected and control lines over the course of the selection regime. Memory scores have been grouped into sets of four generations for graphical purposes. Black circles, selection regime LTM; grey circles, selection regime ARM.
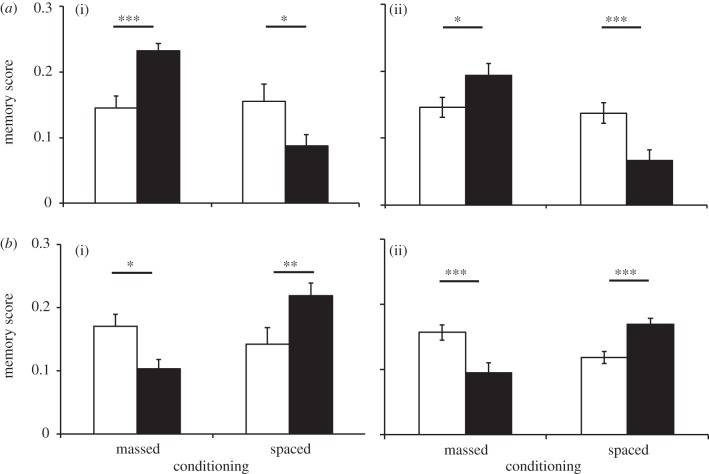
When tested after 23 or 28 generations (figure 3), lines selected for improved ARM had higher memory scores than their respective control lines 24 h after massed conditioning (figure 3a; generation 23: F1,14 = 16.6, p = 0.001; generation 28: F1,14 = 5.3, p = 0.04). Lines selected for improved LTM had higher memory scores than control lines 24 h after spaced conditioning (generation 23: F1,14 = 7.6, p = 0.01; generation 28: F1,14 = 15.6, p = 0.001). Interestingly, and in addition to the previously described functional antagonism between ARM and LTM, lines selected for improved ARM had lower 24 h memory scores than controls when subjected to spaced conditioning (figure 3b; generation 23: F1,14 = 5.6, p = 0.023; generation 28: F1,14 = 10.05, p = 0.007), and lines selected for improved LTM had lower 24 h memory scores than controls when subjected to massed conditioning (generation 23: F1,14 = 6.4, p = 0.016; generation 28: F1,14 = 11.4, p = 0.004).
Figure 3.
(i) Generation 23 and (ii) generation 28. Memory scores 24 h after massed or spaced conditioning for control (open bars) and selected (filled bars) (a) ARM or (b) LTM lines using mechanical shock as the US (mean ± s.e.m. based on variation among lines, n = 5–9 per replicate line). *p < 0.05; **p < 0.01; ***p < 0.001.
We also tested our selected and control lines using electric shock rather than using mechanical shock as the US (figure 4), and obtained the same pattern of results. After 30 generations of selection, flies selected for improved ARM had higher memory scores when tested after massed conditioning (F1,14 = 7.9, p = 0.014) but lower scores when they were tested after spaced conditioning (F1,14 = 4.1, p = 0.039). Flies selected for improved LTM had higher memory scores when tested after spaced conditioning (F1,14 = 6.8, p = 0.021) but lower scores when tested after massed conditioning (F1,14 = 17.4, p = 0.01). This indicates that the evolution of improved consolidated memory was not specific to an association with mechanical shocks.
Figure 4.
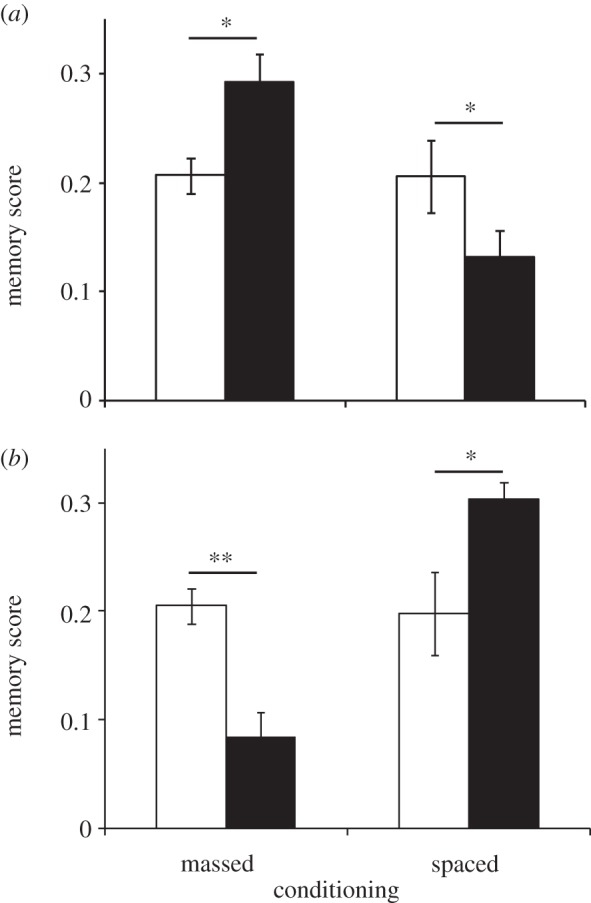
Generation 30. Memory scores 24 h after massed or spaced conditioning for (a) control (open bars) and selected ARM (filled bars) or (b) control (open bars) and selected LTM (filled bars) lines using electric shock as the unconditioned stimulus (mean ± s.e.m. based on variation among lines, n = 5–9 per replicate line). *p < 0.05; **p < 0.01; ***p < 0.001.
(b). Correlated response in other phenotypic traits
(i). Longevity
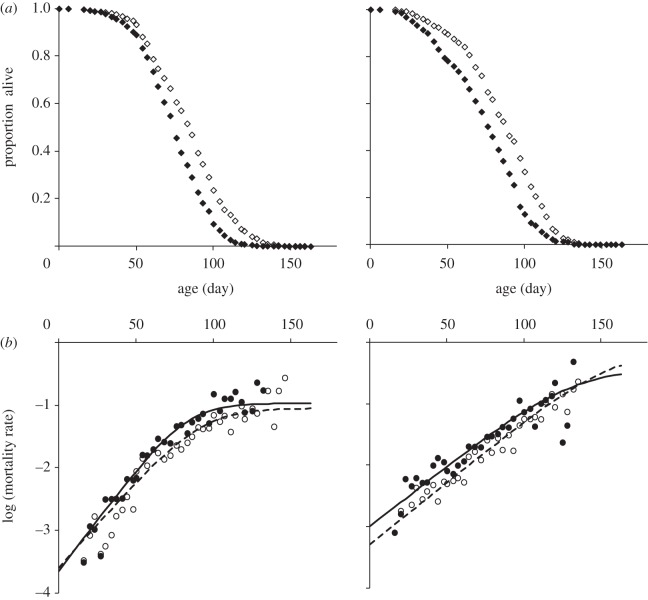
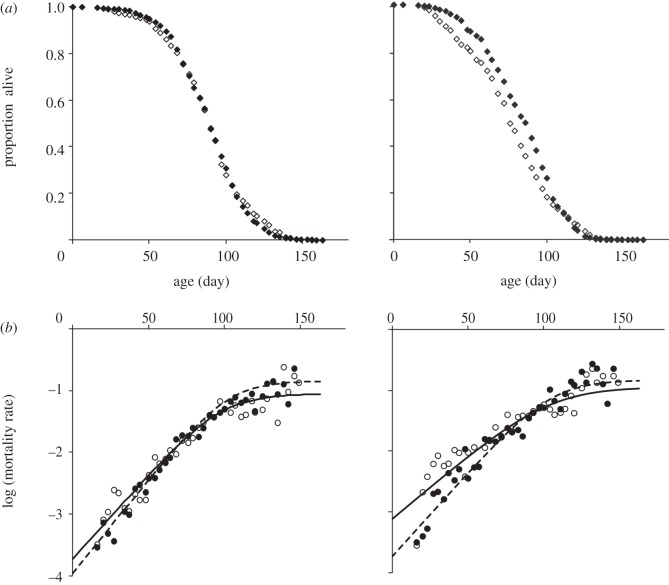
To investigate the effect of the selection regime on lifespan, we measured the longevity of groups of males and groups of females drawn from each of the selected and control lines. Males from lines selected for improved LTM died sooner than males from control lines (see the electronic supplementary material, figure S1 and figure 5; F1,14 = 5.45, p = 0.034), whereas males selected for improved ARM lived significantly longer than males from control lines (electronic supplementary material, figure S1 and figure 6; F1,14 = 4.84, p = 0.042). Despite a similar general trend, there was no difference in longevity between females selected for either regime and their respective control (ARM: F1,14 = 0.92, p = 0.76; LTM: F1,14 = 2.53, p = 0.13). In males, the effect on longevity for both selection regimes was primarily the result of a difference in the initial mortality rate (parameter a). We also found an increase in the rate at which mortality increased with age (parameter b) in lines selected for improved ARM. In females, mortality rate analyses did not reveal any effect of selection for either regime.
Figure 5.
Generation 30. Correlated response in longevity to selection for improved LTM. (a) Age-specific survival for virgin females (left panel) and males (right panel) of control and selected lines is expressed by the mean proportion of flies alive ± s.e.m. (open diamonds, control; filled diamonds, selected). (b) Age-specific mortality rate for both selected and control lines for males and females (open circles, control; filled circles, selected). Regression lines represent the most parsimonious logistic model for each sex.
Figure 6.
Generation 30. Correlated response in longevity to selection for improved ARM. (a) Age-specific survival for virgin females (left panel) and males (right panel) of control and selected lines is expressed as the mean proportion of living flies ± s.e.m. (open diamonds, control; filled diamonds, selected). (b) Age-specific mortality rate for both selected and control lines for males and females (open circles, control; filled circles, selected). Regression lines represent the most parsimonious logistic model for each sex.
(ii). Stress resistance
There was no effect of selection regime on mortality after 30 h of paraquat treatment; selected flies did not differ from controls (LTM selection: F1,14 = 0.03, p = 0.8; ARM selection: F1,14 = 0.1, p = 0.76).
(iii). Fecundity
There was no effect of the selection regime on the number of eggs laid by young flies; selected flies did not differ from controls (LTM selection: F1,14 = 0.51, p = 0.47; ARM selection: F1,14 = 2.14, p = 0.16).
(iv). Egg-to-adult survival
There was no effect of the selection regime on egg-to-adult survival; selected flies did not differ from controls (LTM selection: F1,14 = 0.93, p = 0.35; ARM selection: F1,14 = 1.36, p = 0.26).
(v). Unconditioned response to odours
In contrast to the effect of the selection regime on consolidated memory, there was no effect of selection on the unconditioned response to odours. On average, 93% of the flies from the control and selected lines avoided both odourants (LTM selection: F1,14 = 0.41, p = 0.53; ARM selection: F1,14 = 0.32, p = 0.58).
4. Discussion
In this study, we report that, for a single associative learning task, selection for improvement of one specific consolidated memory phase resulted in an increase in performance for that memory phase but a decrease in performance for the other memory phase. Moreover, selection for improved LTM decreased male longevity, while selection for improved ARM increased male longevity.
(a). Evolutionary trade-off between anaesthesia-resistant memory and long-term memory
Studies on variation in memory capacities between closely related species or between populations of the same species have mostly compared global performances in cognitive tasks without focusing on the memory dynamics. An exception is work by Smid et al. [24], who recently demonstrated species-specific memory dynamics by comparing two closely related parasitoid species. In a classical conditioning set-up, Cotesia glomerata formed LTM only, whereas Cotesia rubecula formed both ARM and LTM. The present study goes further into investigating memory dynamics, and suggests that there is strong genetic diversity for each of the two memory phases and the possibility that natural selection may act on the memory dynamics.
In particular, we observed a symmetrical evolutionary trade-off between two memory phases for the same cognitive task. The correlated response to selection may be owing to pleiotropic effects of genes targeted by selection or to genetic hitchhiking of alleles at loci that are closely linked to the target genes [25]. We believe the second hypothesis to be unlikely. Because of the large base population, it is not likely that strong linkage disequilibrium could have arisen by drift, unless one of the alleles involved had been very rare—that is, present only in a few copies in the gene pool. But, it is unlikely that such an allele would be represented in all replicate lines, and therefore we would not have seen a consistent response.
Functional trade-offs are known to place strong constraints on the evolution of animal performance [26,27]. Although at a different level, our results are consistent with recent findings that ARM and LTM are functionally mutually antagonistic [17], and that ARM interferes with LTM formation [16]. Thus, activation of the ARM pathway after either a single conditioning or massed conditioning prevents the formation of LTM. During the rest interval of the spaced conditioning, the ARM pathway is inhibited by dopaminergic neurons, allowing LTM formation [16]. ARM and LTM are therefore functionally exclusive. This functional trade-off may have shaped the evolutionary trajectory of the memory phases and prevented a global increase of memory capacities. We do not yet know what are the neurobiological and genetic targets of our selection regimes, or whether all of our replicate lines followed the same evolutionary trajectory. The activity of dopaminergic neurons is known to affect LTM formation in mammals [28] and Drosophila [16], and could be a good candidate for future studies.
The main protocol used in our selection experiment involved training groups of flies, not individual flies. Chabaud et al. [7] found that ARM retrieval was facilitated by group size, whereas LTM retrieval was inhibited. In particular, after massed training individual flies seem to show a memory retrieval deficit that can be compensated by social interaction within a group. Future studies should investigate whether our selection regimes had different effects on social interaction among flies.
(b). Long-term memory and lifespan: antagonistic pleiotropic interaction
In order to identify the ultimate constitutive costs of the evolutionary trade-off, we evaluated various fitness-related phenotypes. We showed that males selected for improved LTM had reduced longevity and males selected for improved ARM (and reduced LTM) had increased longevity. These results confirmed the previously described trade-off between learning ability and longevity [13], and also point to a specific pleiotropic effect between the ability to form LTM and lifespan. Such an evolutionary trade-off could be explained by the energetic cost of the development of the central nervous system.
This constitutive cost was mainly observed in males. Other studies have obtained similar sex-specific costs associated with improved learning and memory performance. Burger et al. [13] evaluated longevity of flies selected for an improved ability to associate the flavour of an oviposition substrate with an aversive bitter taste, and in this study, female longevity decreased in selected lines. However, this protocol involved a female-specific behaviour that could explain this sex-specific cost. This specificity is consistent with the idea that there are different memory modules that govern behaviour in response to different environments. Interestingly, amine neurotransmitters are known to regulate the longevity of animals [29]. In Drosophila, it has been shown that a quantitative trait locus for variation in longevity maps onto the aromatic l-amino acid decarboxylase gene, which is required for dopamine and serotonin synthesis [30]. Although the exact physiological processes involved in the selection procedure have not yet been elucidated, the present study highlights the complex interaction of different memory functions and the potential impact of these functional relationships on evolutionary trajectories.
No specific effects of the selection regime were observed on fecundity, stress resistance or larval development. Interestingly, studies have consistently demonstrated correlations between vertebrate brain size- and fecundity-related traits [31]. Recently, more direct evidence has been found in a Pieris rapae study, in which a comparison of butterflies from full-sibling families revealed a negative correlation between learning ability and fecundity [32]. Whether trade-offs between traits are fixed or highly plastic remains an open question; our experimental approach compared only early fecundity over a relatively short-time.
(c). Ecological and evolutionary relevance
The evolutionary trade-off between ARM and LTM revealed in the present study raises questions about the adaptive value of each of these two memory phases under more natural conditions. ARM and LTM have been clearly distinguished only in Drosophila so far, although strong evidence suggests that they form in some parasitoid species. However, the effect of the spacing between conditioning trials on LTM formation is well documented in invertebrates and vertebrates, suggesting conserved properties of memory dynamics. A study of learning and memory in the grapsid crab Chasmagnathus has shown that spaced conditioning induces LTM that lasts at least a week, whereas massed conditioning induces protein synthesis-independent retention that lasts 3 days and that cannot be attributed to a short-term memory [33]. Similarly, in a motor-learning task, mice have been shown to form a protein synthesis-independent memory that lasts for 1 day when massed-trained, but a protein synthesis-dependent memory that lasts several days when spaced-trained [34]. It is thus likely that genetically and functionally independent forms of long-term memory also exist in other organisms but have yet to be identified. The spacing effect on the formation of ARM-like memory or LTM clearly has an adaptive value. It may ensure that only information acquired over several independent events—that should thus have a high predictive value—is stored in a costly but long-lasting protein synthesis-dependent memory phase. Environmental variability is therefore likely to play a role in ARM versus LTM formation and evolution. In a previous study, Mery et al. [35] showed that variation in foraging behaviour was related to variation in LTM capacity. In D. melanogaster, the foraging gene encodes a cGMP-dependent protein kinase (PKG) known to contribute to behavioural plasticity in larvae and adults [36]. Flies with high PKG activity tend to forage more frequently between food patches—and thus potentially experience increased environmental heterogeneity—and they show lower LTM than flies with low PKG activity. However, there were no differences in ARM formation. Similarly, the two parasitoid species cited earlier also differ in their foraging strategies and may experience different environmental heterogeneity. Adult C. rubecula's host lays single eggs on diverse host plants, whereas C. glomerata's host lays clutches of eggs on clustered plants of the same species [24]. Information about the host plants is thus different between these two parasitoid species. Cotesia rubecula's information has low predictability, and preferentially leads to the formation of ARM compared with C. glomerata, which forms LTM.
Understanding the level of interdependence among the different memory phases is a fundamental step, allowing us to better understand the evolution of cognition. The symmetrical evolutionary trade-off between two consolidated memory phases observed in our selected lines may open new perspectives on the study of the evolution of memory and of its constraints. It may also explain the natural variation in the different memory phases observed between closely related species [3,37,38]. Moreover, these experiments provide important insights into the genetic bases of life-history trait evolution and their pleiotropic effects. Linking neurobiology, ecology and evolutionary biology will open new perspectives on how natural selection can shape animal cognition.
Acknowledgements
We thank S. Wardrop for useful manuscript corrections. This work was supported by the European Research Council under the European Community's Seventh Framework Programme (FP7/2007–2013)/ERC grant (agreement no. 209540) to F.M. and by the Agence Nationale pour la Recherche to T.P. F.L. was supported by the Fondation pour la Recherche Medicale.
References
- 1.Dudai Y. 2004. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 55, 51–86 10.1146/annurev.psych.55.090902.142050 (doi:10.1146/annurev.psych.55.090902.142050) [DOI] [PubMed] [Google Scholar]
- 2.Margulies C., Tully T., Dubnau J. 2005. Deconstructing memory in Drosophila. Curr. Biol. 15, R700–R713 10.1016/j.cub.2005.08.024 (doi:10.1016/j.cub.2005.08.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Berg M., Duivenvoorde L., Wang G. H., Tribuhl S., Bukovinszky T., Vet L. E. M., Dicke M., Smid H. M. 2011. Natural variation in learning and memory dynamics studied by artificial selection on learning rate in parasitic wasps. Anim. Behav. 81, 325–333 10.1016/j.anbehav.2010.11.002 (doi:10.1016/j.anbehav.2010.11.002) [DOI] [Google Scholar]
- 4.Tully T., Preat T., Boynton S. C., Delvecchio M. 1994. Genetic dissection of consolidated memory in Drosophila. Cell 79, 35–47 10.1016/0092-8674(94)90398-0 (doi:10.1016/0092-8674(94)90398-0) [DOI] [PubMed] [Google Scholar]
- 5.Tully T., Boynton S., Brandes C., Dura J. M., Mihalek R., Preat T., Villella A. 1990. Genetic dissection of memory formation in Drosophila melanogaster. Cold Spring Harbor Symp. Quant. Biol. 55, 203–211 10.1101/SQB.1990.055.01.022 (doi:10.1101/SQB.1990.055.01.022) [DOI] [PubMed] [Google Scholar]
- 6.Isabel G., Comas D., Preat T. 2007. From molecule to memory system: genetic analyses in Drosophila. In Memories: molecules and circuits (eds Bontempi B., Silva A. J., Christen Y.), pp. 41–57 Berlin, Germany: Springer. [Google Scholar]
- 7.Chabaud M. A., Isabel G., Kaiser L., Preat T. 2009. Social facilitation of long-lasting memory retrieval in Drosophila. Curr. Biol. 19, 1654–1659 10.1016/j.cub.2009.08.017 (doi:10.1016/j.cub.2009.08.017) [DOI] [PubMed] [Google Scholar]
- 8.Chen C. C., Wu J. K., Lin H. W., Pai T. P., Fu T. F., Wu C. L., Tully T., Chiang A. S. 2012. Visualizing long-term memory formation in two neurons of the Drosophila brain. Science 335, 678–685 10.1126/science.1212735 (doi:10.1126/science.1212735) [DOI] [PubMed] [Google Scholar]
- 9.Bleeker M. A. K., Smid H. M., Steidle J. L. M., Kruidhof H. M., Van Loon J. J. A., Vet L. E. M. 2006. Differences in memory dynamics between two closely related parasitoid wasp species. Anim. Behav. 71, 1343–1350 10.1016/j.anbehav.2005.09.016 (doi:10.1016/j.anbehav.2005.09.016) [DOI] [Google Scholar]
- 10.Mery F., Pont J., Preat T., Kawecki T. J. 2007. Experimental evolution of olfactory memory in Drosophila melanogaster. Physiol. Biochem. Zool. 80, 399–405 10.1086/518014 (doi:10.1086/518014) [DOI] [PubMed] [Google Scholar]
- 11.Raine N. E., Chittka L. 2008. The correlation of learning speed and natural foraging success in bumble-bees. Proc. R. Soc. B 275, 803–808 10.1098/rspb.2007.1652 (doi:10.1098/rspb.2007.1652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mery F., Kawecki T. J. 2003. A fitness cost of learning ability in Drosophila melanogaster. Proc. R. Soc. Lond. B 270, 2465–2469 10.1098/rspb.2003.2548 (doi:10.1098/rspb.2003.2548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger J. M. S., Kolss M., Pont J., Kawecki T. J. 2008. Learning ability and longevity: a symmetrical evolutionary trade-off in Drosophila. Evolution 62, 1294–1304 10.1111/j.1558-5646.2008.00376.x (doi:10.1111/j.1558-5646.2008.00376.x) [DOI] [PubMed] [Google Scholar]
- 14.Kolss M., Kawecki T. J. 2008. Reduced learning ability as a consequence of evolutionary adaptation to nutritional stress in Drosophila melanogaster. Ecol. Entomol. 33, 583–588 10.1111/j.1365-2311.2008.01007.x (doi:10.1111/j.1365-2311.2008.01007.x) [DOI] [Google Scholar]
- 15.Burns J. G., Foucaud J., Mery F. 2011. Costs of memory: lessons from ‘mini’ brains. Proc. R. Soc. B 278, 923–929 10.1098/rspb.2010.2488 (doi:10.1098/rspb.2010.2488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Placais P.-Y., et al. 2012. Slow oscillations in two pairs of dopaminergic neurons gate long-term memory formation in Drosophila. Nat. Neurosci. 15, 592–599 10.1038/nn.3055 (doi:10.1038/nn.3055) [DOI] [PubMed] [Google Scholar]
- 17.Isabel G., Pascual A., Preat T. 2004. Exclusive consolidated memory phases in Drosophila. Science 304, 1024–1027 10.1126/science.1094932 (doi:10.1126/science.1094932) [DOI] [PubMed] [Google Scholar]
- 18.Reznick D. 1985. Costs of reproduction: an evaluation of the empirical evidence. Oikos 44, 257–267 10.2307/3544698 (doi:10.2307/3544698) [DOI] [Google Scholar]
- 19.Futuyma D. J., Moreno G. 1988. The evolution of ecological specialization. Annu. Rev. Ecol. Syst. 19, 207–233 10.1146/annurev.ecolsys.19.1.207 (doi:10.1146/annurev.ecolsys.19.1.207) [DOI] [Google Scholar]
- 20.Mery F., Kawecki T. J. 2005. A cost of long-term memory in Drosophila. Science 308, 1148. 10.1126/science.1111331 (doi:10.1126/science.1111331) [DOI] [PubMed] [Google Scholar]
- 21.Sokal R. R., Rohlf F. J. 1995. Biometry: the principles and practice of statistics in biological research. New York, NY: Freeman [Google Scholar]
- 22.Pletcher S. D. 1999. Model fitting and hypothesis testing for age-specific mortality data. J. Evol. Biol. 12, 430– 439 [Google Scholar]
- 23.Smith L. L., Rose M. S., Wyatt I. 2008. The pathology and biochemistry of paraquat. In CIBA Foundation Symp. 65: Oxygen Free Radicals and Tissue Damage. pp. 321–341 Chichester, UK: John Wiley & Sons, Ltd; [DOI] [PubMed] [Google Scholar]
- 24.Smid H. M., Wang G., Bukovinszky T., Steidle J. L. M., Bleeker M. A. K., Van Loon J. J. A., Vet L. E. M. 2007. Species-specific acquisition and consolidation of long-term memory in parasitic wasps. Proc. R. Soc. B 274, 1539–1546 10.1098/rspb.2007.0305 (doi:10.1098/rspb.2007.0305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falconer D. S., Mackay T. F. C. 1996. Introduction to quantitative genetics. Harlow, UK: Longman Green [Google Scholar]
- 26.Hills T., Hertwig R. 2011. Why aren't we smarter already. Curr. Direct. Psychol. Sci. 20, 373–377 10.1177/0963721411418300 (doi:10.1177/0963721411418300) [DOI] [Google Scholar]
- 27.Bennett A. F., Lenski R. E. 2007. An experimental test of evolutionary trade-offs during temperature adaptation. Proc. Natl Acad. Sci. USA 104(Suppl 1), 8649–8654 10.1073/pnas.0702117104 (doi:10.1073/pnas.0702117104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossato J. I., Bevilaqua L. R. M., Izquierdo I., Medina J. H., Cammarota M. 2009. Dopamine controls persistence of long-term memory storage. Science 325, 1017–1020 10.1126/science.1172545 (doi:10.1126/science.1172545) [DOI] [PubMed] [Google Scholar]
- 29.Rollo C. D. 2009. Dopamine and aging: intersecting facets. Neurochem. Res. 34, 601–629 10.1007/s11064-008-9858-7 (doi:10.1007/s11064-008-9858-7) [DOI] [PubMed] [Google Scholar]
- 30.De Luca M., Roshina N. V., Geiger-Thornsberry G. L., Lyman R. F., Pasyukova E. G., Mackay T. F. C. 2003. Dopa decarboxylase (Ddc) affects variation in Drosophila longevity. Nat. Genet. 34, 429–433 10.1038/ng1218 (doi:10.1038/ng1218) [DOI] [PubMed] [Google Scholar]
- 31.Lefebvre L., Sol D. 2008. Brains, lifestyles and cognition: are there general trends? Brain Behav. Evol. 72, 135–144 10.1159/000151473 (doi:10.1159/000151473) [DOI] [PubMed] [Google Scholar]
- 32.Snell-Rood E. C., Davidowitz G., Papaj D. R. 2011. Reproductive tradeoffs of learning in a butterfly. Behav. Ecol. 22, 291–302 10.1093/beheco/arq169 (doi:10.1093/beheco/arq169) [DOI] [Google Scholar]
- 33.Hermitte G., Pedreira M. E., Tomsic D., Maldonado H. 1999. Context shift and protein synthesis inhibition disrupt long-term habituation after spaced, but not massed, training in the crab Chasmagnathus. Neurobiol. Learn. Memory 71, 34–49 10.1006/nlme.1998.3858 (doi:10.1006/nlme.1998.3858) [DOI] [PubMed] [Google Scholar]
- 34.Okamoto T., Endo S., Shirao T., Nagao S. 2011. Role of cerebellar cortical protein synthesis in transfer of memory trace of cerebellum-dependent motor learning. J. Neurosci. 31, 8958–8966 10.1523/jneurosci.1151-11.2011 (doi:10.1523/jneurosci.1151-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mery F., Belay A. T., So A. K. C., Sokolowski M. B., Kawecki T. J. 2007. Natural polymorphism affecting learning and memory in Drosophila. Proc. Natl Acad. Sci. USA 104, 13 051–13 055 10.1073/pnas.0702923104 (doi:10.1073/pnas.0702923104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira H. S., Burgess E., Campos A., Greenspan R., Osborne K., Robichon A., Sokolowski M. B. 1995. Genetic and molecular analysis of foraging behavior in Drosophila melanogaster. Behav. Genet. 25, 300 [Google Scholar]
- 37.Clayton N. S. 1998. Memory and the hippocampus in food-storing birds: a comparative approach. Neuropharmacology 37, 441–452 10.1016/s0028-3908(98)00037-9 (doi:10.1016/s0028-3908(98)00037-9) [DOI] [PubMed] [Google Scholar]
- 38.Hoedjes K. M., Kruidhof H. M., Huigens M. E., Dicke M., Vet L. E. M., Smid H. M. 2011. Natural variation in learning rate and memory dynamics in parasitoid wasps: opportunities for converging ecology and neuroscience. Proc. R. Soc. B 278, 889–897 10.1098/rspb.2010.2199 (doi:10.1098/rspb.2010.2199) [DOI] [PMC free article] [PubMed] [Google Scholar]