Abstract
Ectopic adrenocortical neoplasms arising in the nervous system are very rare. We encountered an intradural, extramedullary case of an adrenocortical neoplasm of indeterminate malignant potential affecting a spinal nerve root in the distal lumbar region of a 5-month-old girl. The lesion recurred 6 months after the initial gross total resection. The tumor in both resections had increased mitotic activity (5/10 high power fields) and MIB-1 labeling indices of 23% and 33% at initial resection and recurrence, respectively. Both tumors demonstrated gains of chromosomes 5 and 12 by interphase cytogenetics, whereas insulin growth factor 2 was identified in the recurrent tumor by immunohistochemistry. This report demonstrates that ectopic adrenocortical tumors in the nervous system may exhibit clinicopathologic and cytogenetic features suggestive of adrenocortical carcinoma.
Keywords: nervous system, spinal cord, adrenocortical tumor, ectopic adrenal, immunohistochemistry, FISH
The finding of ectopic adrenal tissue, including cortex and medulla or cortex alone, is a well-recognized phenomenon occurring at a variety of anatomic locations. They are usually encountered along the gonadal descent paths, but have been described to occur at intracranial locations,9,20 as well in placenta, kidney, pancreas, and gallbladder.8 Furthermore, adrenocortical tumors, both benign and malignant, may also arise at ectopic sites,11,19 presumably from ectopic adrenocortical rests.
A unique anatomic region in which ectopic adrenal cortical neoplasia has been reported is the lower spinal region.1,5,6,10 We report the unusual case of an ectopic adrenocortical neoplasm with indeterminate malignant potential, but with clinical, pathologic, and molecular genetic features suggesting aggressive behavior. The pertinent literature is reviewed.
CASE REPORT
A 5-month-old girl was noted by the parents to be unwilling to support weight on her lower extremities, along with irritability and increased crying at night. The child was the result of a full-term unremarkable pregnancy and exhibited normal development until that point. Neurologic examination disclosed decreased movement of her lower extremities, more pronounced on the left leg, as compared with the upper extremities. A magnetic resonance imaging (MRI) scan demonstrated enlargement of the spinal cord at levels T10 to L2 by an heterogeneously enhancing mass measuring approximately 6 × 1.5 cm (Fig. 1A). The brain, upper spinal cord, abdomen, and specifically, the adrenal glands were unremarkable on MRI. There was no clinical evidence of endocrine dysfunction. She was taken to surgery. At laminectomy, a soft gray-purple, unencapsulated mass was evident upon opening the arachnoid membrane. The tumor was entirely intradural and extramedullary. Although it markedly compressed the spinal cord, a distinct plane of separation was found. Several nerve roots were identified within the substance of the tumor. A gross total resection was performed. No further treatment was given, but recurrent symptoms were noted approximately 6 months later. A repeat MRI demonstrated an enhancing 1.2-cm nodule and a cystic area with a 0.5 cm enhancing nodule in its inferior aspect. A second gross total resection was performed. The patient recovered well after the second surgery and is currently undergoing chemotherapy 5 months postoperatively with a protocol involving cisplatin, doxorubicin, etoposide, and mitotane.
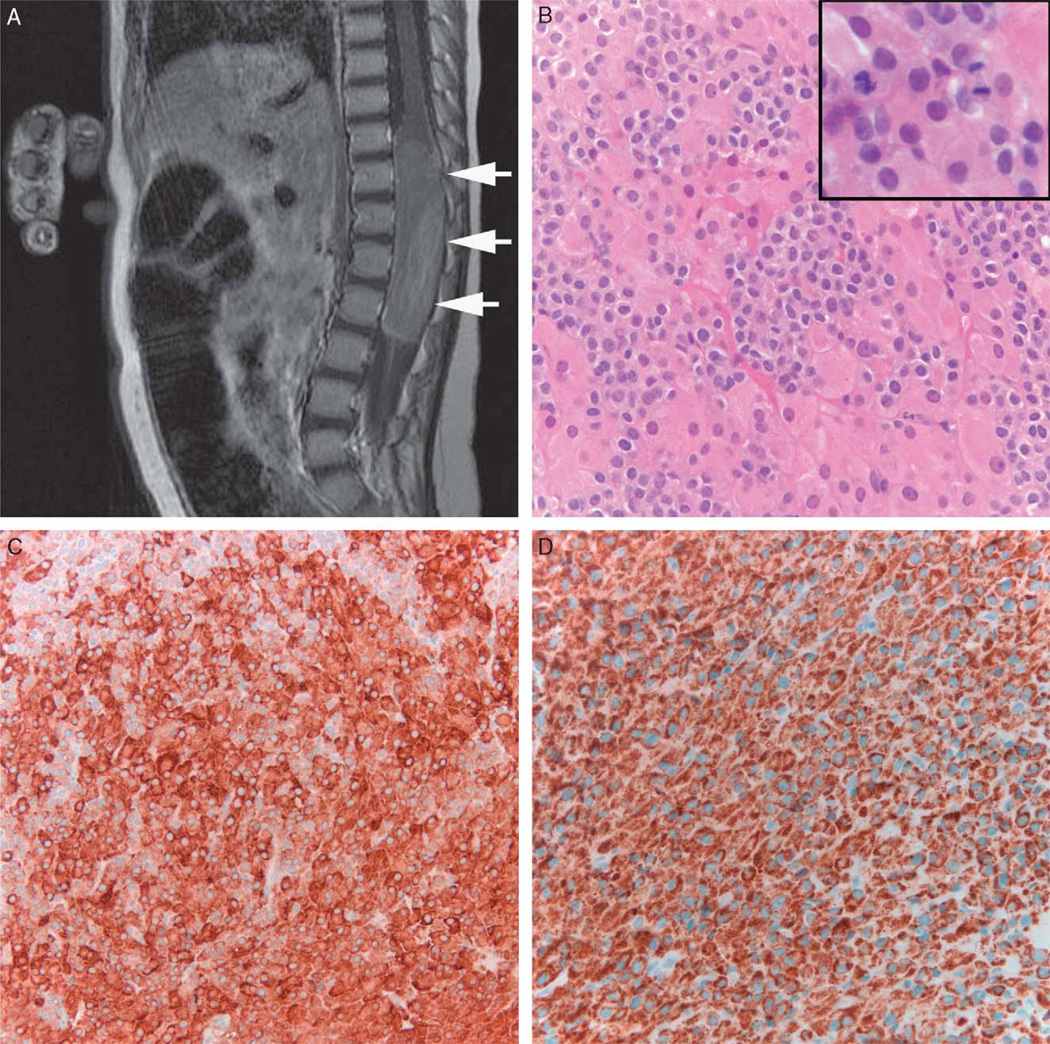
FIGURE 1.
Ectopic adrenocortical tumor. Sagittal T1 weighted MRI postcontrast demonstrates a large neoplasm in the lower spinal cord with mild to moderate heterogeneous enhancement (arrows) (A). The tumor histologically showed a biphasic cell population with large eosinophilic and small clear cells (B). Mitotic activity was brisk (inset). Immunohistochemical stains demonstrated strong reactivity for inhibin (C) and melan-A (D). MRI indicates magnetic resonance imaging.
MATERIALS AND METHODS
Both specimens were formalin-fixed and routinely processed for light microscopy. All hematoxylin-eosin stained sections were reviewed. Immunohistochemical stains were performed in a Dako autostainer (Dako North America Inc, Carpinteria, CA) using the Dual Link Envision + or ADVANCE (Dako) detection systems with antibodies directed against glial fibrillary acidic protein (Dako, Carpinteria, CA, polyclonal; 1:4000), S100 protein (Dako, polyclonal; 1:1600), cytokeratin CAM 5.2 (Becton Dickinson, 1:50), cytokeratin AE1/AE3 (Zymed, South San Francisco, CA; 1:200), chromogranin (Chemicon; LK2H10; 1:500), synaptophysin (ICN, clone SY38; 1:40), neurofilament protein(Dako, clone 2F11, 1:75), melan A (Dako, clone A103, 1:500), inhibin (Serotec, clone R1, 1:60), p16 (NeoMarkers, clone 16P07; 1:400), p53 (Dako, clone DO7;1:2000), and epidermal growth factor receptor (Dako, 2-18C9; pre-diluted). Immunohistochemistry for insulin growth factor 2 (IGF2) (Upstate Biotechnology, clone S2F2; 1:100) was performed upon the initial specimen and the recurrent tumor specimen using the avidin-biotin-peroxidase method and a monoclonal antibody in duplicate, as previously described.3 MIB-1 labeling indices were quantified using Hamamatsu NanoZoomer Digital Pathology for scanning images and IHCScore software for computer-assisted analyses (Bacus Laboratories Inc). Ultrastructural studies were performed upon glutaraldehyde-fixed tissues routinely processed, Epon embedded, and stained with uranyl acetate and lead citrate on a Tecnai 12 transmission electron microscope (FEI Corp, Eindhoven, Netherlands).
Dual color fluorescent in situ hybridization (FISH) studies were also performed using the following locus-specific identifier probes (Abbott Molecular/Vysis, Des Plaines, IL) targeting chromosome arms and regions previously found to be aberrant in adrenocortical neoplasms7,14,16: 1p36, EGR1(5q31), p16 (9p21), CCND1 (11q13), TP53(17p13.1), PDGFRA (4q, custom made) with the corresponding reference probes 1q25, D5S23, D5S721(5p15.2), CEP 9, CEP 11, CEP 17, CEP 4, and CEP 12. A total of 100 nonoverlapping nuclei were enumerated by a single observer (F.J.R.).
RESULTS
Pathology
The original tumor was characterized by a biphasic pattern of medium sized cells with round nuclei and clear cytoplasm, and larger cells with relatively abundant eosinophilic cytoplasm, larger nuclei, and prominent nucleoli (Fig. 1B). The mitotic index was 5/10 high power (× 40) fields, but no atypical mitoses were noted. Necrosis was not present. Immunohistochemical stains demonstrated strong and diffuse expression of inhibin and Melan-A (Figs. 1C, D). Stains for synaptophysin and cytokeratin CAM 5.2 showed focal staining (not shown). Neurofilament protein labeled only rare tumor cells. The neoplastic cells were negative for S100 protein, glial fibrillary acidic protein, cytokeratin AE1/AE3, and chromogranin. Given the unusual location and young age, a descriptive diagnosis of “adrenocortical tumor of indeterminate malignant potential” was chosen, rather than of adrenocortical carcinoma, despite the considerable mitotic activity.
The recurrent tumor was histologically identical to the first resection, aside from the finding of occasional cells with bright eosinophilic granules. The mitotic index was again 5/10 high power fields. The MIB-1 labeling index was 23% and 33% in the initial resection and recurrent tumor, respectively.
Electron Microscopy
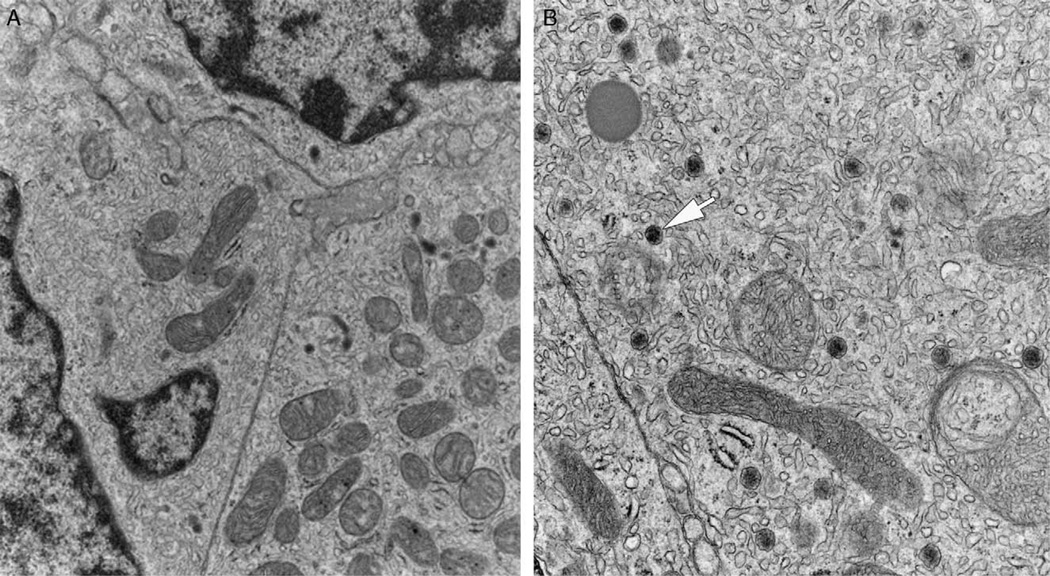
Ultrastructurally, most tumor cells were polygonal in shape with irregularly shaped nuclei and dispersed chromatin. Their cytoplasm contained abundant smooth endoplasmic reticulum and mitochondria with tubulovesicular cristae. Occasional dense core secretory granules were present. Intercellular junctions were scant and poorly formed (Fig. 2).
FIGURE 2.
Electron microscopy of ectopic adrenocortical tumor. Large polygonal cells with abundant smooth endoplasmic reticulum and numerous mitochondria were the predominant finding (A). Some mitochondria demonstrated tubulovesicular cristae characteristic of steroid producing cells. Occasional dense core secretory granules were also present (arrow) (B).
Molecular Genetic and Prognostic Markers
Staining for IGF2 was negative in the initial resection specimen but diffuse and weak to moderate in degree in the recurrent tumor (Figs. 3A, B). Focal circumferential and membranous epidermal growth factor receptor expression was noted in the recurrent tumor alone. Both specimens showed patchy p16 immunoreactivity in a cytoplasmic and nuclear distribution, and moderate to diffuse nuclear p53 reactivity.
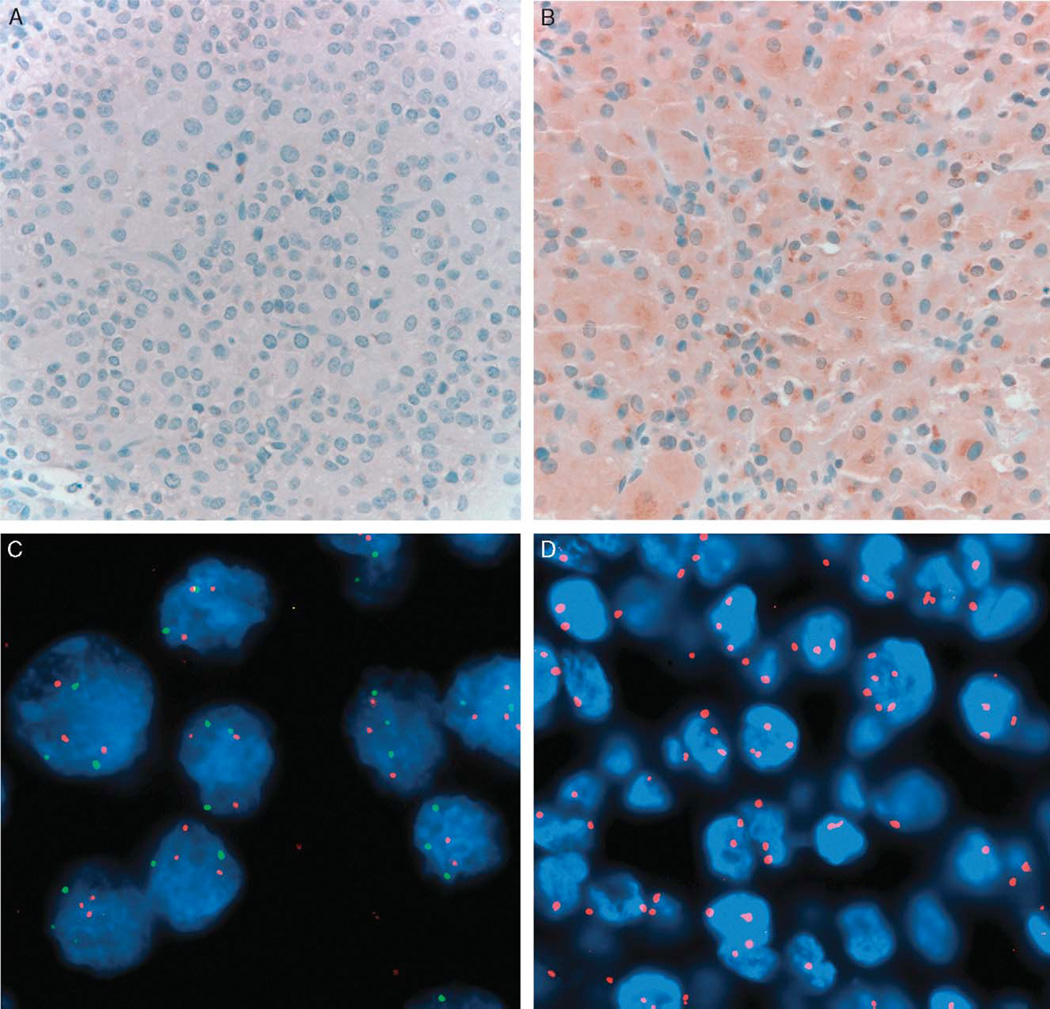
FIGURE 3.
Prognostic and cytogenetic markers of ectopic adrenocortical tumor. The tumor demonstrated absent staining for IGF2 in the initial resection (A), but weak to moderate staining in the recurrent tumor (B). Fluorescence in situ hybridization on the recurrent tumor demonstrating >2 copies of 5p (green) and 5q (red) (C) and CEP 12 (red) (D), reflecting gains of chromosomes 5 and 12. The findings were similar in the tumor at initial resection (not shown). IGF2 indicates insulin growth factor.
FISH studies demonstrated gains of chromosome 5 and 12, mostly in the form of trisomy (46% and 37% of cells in the initial tumor and 47% and 49% in the recurrence, respectively) (Figs. 3C, D). Normal dosage of chromosomes 1, 4, 9, 11, and 17 was also observed (not shown).
DISCUSSION
Ectopic adrenocortical tumors are a rare occurrence in the nervous system with only isolated case reports1,5,6,10 (Table 1). Their clinicopathologic features are summarized in Table 1. Ectopic adrenal tissue has been described at intracranial locations before,9,20 including occurrence as a component of a pituitary choristoma containing adrenocortical cells in a corticotroph adenoma.2,12 Ectopic adrenocortical tumors in the nervous system instead seem to occur exclusively in the lower spinal region at a median patient age of 22 years and with a notable (5:1) female predominance. Of the 6 described tumors, 2 were intramedullary and the remaining 4 (including the case reported herein) intradural and extramedullary (Table 1).
TABLE 1.
Ectopic Adrenal Neoplasms of the Nervous System in the Literature
| Case | Age/Sex | Clinical History | Imaging/Location | Surgery | Pathology | Laboratory | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Kepes et al6 | 8/F | Bilateral leg pain | MRI: circumscribed mass at L2 | GTR: well encapsulated intradural extramedullary mass | Gross: 2.5 × 1.4 × 1.3 cm mottled brown yellow mass | Increased androstenedione in tumor by radioimmunoassay; clinically nonfunctional | NA | Uneventful postoperative course |
| Micro: adrenal cortical adenoma without atypia | ||||||||
| Mitchell et al10 | 16/F | R thigh pain × 3 mo | Myelogram: complete spinal block at L1-2 | GTR: 2 cm well circumscribed intradural extramedullary mass attached to the L2 sensory nerve root | Gross: 1.8 × 1.5 × 1.5 cm encapsulated smooth ovoid mass | Immunohistochemical staining of steroidogenic enzymes in tumor tissue | Observation | NED 11 y postoperatively |
| Micro: adrenal cortical adenoma without atypia | ||||||||
| Mitchell et al10 | 63/F | Pain in lower back radiating to lower extremities and weakness × 10 mo | MRI: enhancing lesion at the conus involving a nerve root in the cauda equina | GTR: red tan intradural extramedullary encapsulated mass | Gross: 1.5 cm spherical mass | Immunohistochemical staining of steroidogenic enzymes in tumor tissue | NA | Improvement in pain, doing well postoperatively |
| Micro: adrenal cortical adenoma without atypia | ||||||||
| Cassarino et al1 | 27/M | Progressive lower extremity weakness and lumbar ache × 1 y | Myelogram: block at the level of the conus | GTR: Intramedullary tumor removed piecemeal | Gross: soft tan-brown aggregate 3.0 × 2.0 × 1.8cm | NA | None | NED 1 y postoperatively |
| Micro: oncocytic adrenocortical adenoma without atypia | ||||||||
| Karikari et al5 | 27/F | Bilateral LE pain, increased urination × 8 wk | MRI: T1 hyperintensity, T2 hypointensity, homogeneous enhancement; conus/L2 location; associated spinal cord lipoma | STR: vascular intramedullary tumor | Gross: 3.6 × 2.3 × 1.1 cm red brown mass | NA | Observation | Surgery for tethered cord at 3 mo |
| Micro: adrenal cortical adenoma without atypia | Stable small focus of residual tumor on MRI 11 mo after first resection | |||||||
| Present case | 5 mo/F | Inability to bear weight in LE, irritability | MRI: 6×1.5 cm heterogeneously enhancing mass situated at T10-L2 | 1-GTR: soft gray-purplish and unencapsulated mass, intradural extramedullary | Gross: multiple fragments | NA | 1-Observation | Recurrent tumor at 6 mo undergoing chemotherapy |
| 2-GTR | Micro: Adrenocortical neoplasm of undetermined malignant potential | 2-After second surgery, chemotherapeutic protocol including cisplatin, doxorubicin, etoposide, and mitotane |
F indicates female; GTR, gross total resection; L, left; LE, lower extremity; M, male; MRI, magnetic resonance imaging; NA, data not available/not performed; NED, no evidence of disease; R, right; STR, subtotal resection.
Our current case is unique in that it exhibited worrisome histopathologic features suggestive of malignancy, including elevated proliferative indices, lack of a capsule, and infiltration of nerve roots, and appreciable size (6 cm compared with a median of 2.5 cm in previously reported cases) and prompt recurrence despite gross total resection. By comparison, no recurrences were reported in previously documented ectopic adrenocortical tumors involving the spinal region.
Ectopic adrenal tissue is most commonly found in the upper abdomen or along the well-known path of gonadal descent, likely because of the close developmental association of the gonadal ridge with the adrenal gland. Why ectopic adrenocortical tumors arise outside this area and in the lower spinal cord region is unclear. The case reported by Karikari and colleagues5 was associated with a low-lying tethered cord and “cord lipoma.” They speculated that premature separation of ectoderm from neuroectoderm before neurulation is completed may permit invasion of the neural groove by mesodermal tissue committed to the formation of adrenocortical tissue. In extramedullary adrenocortical tumors lacking an association with dysraphism, retroperitoneal adrenal rests gaining intradural access by way of exiting nerves or entering vessels has been also postulated.6
Histologically, the differential diagnosis of spinal adrenocortical tumors is broad and includes ependymoma, meningioma, paraganglioma of the filum terminale, and germ cell tumors, including the hepatoid variant of yolk sac tumor, among others. The combination of morphologic features, unique immunophenotype (melan A, inhibin A, and synatophysin +), and ultrastructural findings (abundant smooth endoplasmic reticulum and mitochondria with tubulovesicular cristae) were essential in ruling out other entities. Once the adrenocortical phenotype of these tumors is proven, a metastatic carcinoma originating in the adrenals must be excluded by abdominal imaging, as in our case. However, it should be noted that when adrenocortical carcinoma involves the nervous system, it is in the form of intracranial metastases in the context of systemic spread.18
In recent years, there has been an increase in our understanding of the molecular alterations underlying adrenocortical neoplasia, particularly with the aid of high throughput molecular techniques.4,7,15–17 Comparative genomic hybridization has shown aberrations in chromosomes and chromosomal arms 1p, 4q, 5, 11, 12, and 17 to be common in these neoplasms. The finding of Sidhu and colleagues, specifically that gains in chromosomes 5 and 12 and losses in chromosome regions 1p and 17p are more closely associated with carcinoma than adenoma, is of particular interest.15 Gains of chromosome 5 and 12 were identified in our current case by FISH, although no losses were observed in the other regions tested. We found immunohistochemical overexpression of p53, which suggests the possibility of a TP53 point mutation, a more common mechanism of inactivation in some tumors than large deletions identifiable by FISH.13 Although loss of heterozygosity at 9p21, the locus for the tumor suppressor gene p16, is reported to be more frequent in adrenocortical carcinomas than in adenomas,14 we found patchy immunoreactivity for p16 by immunohistochemistry in our case and no deletions by FISH. IGF2 is a marker that has been found to be overexpressed in malignant adrenocortical tumors compared with adenomas in prior studies at the mRNA and protein level.3,17 It is of note that in our case it was observed by immunohistochemistry in the recurrent tumor, but not the primary.
In summary, we report the unique occurrence of a primary adrenocortical tumor in the spinal region of a young patient. Although frank histologic malignancy was not present, and the more cautious diagnosis of adrenocortical tumor of indeterminate malignant potential was initially made, the pathologic, molecular, and clinical features of this tumor, including its prompt recurrence after a gross total resection, suggest the lesion is a low-grade carcinoma. Although the occurrence of adrenocortical tumors in central nervous system is rare, pathologists should be aware that ectopic adrenocortical tumors both benign and clinically aggressive may arise at this location.
ACKNOWLEDGMENT
The authors thank the Cytogenetic Shared Resource at the Mayo Clinic for technical assistance.
Supported in part by NIH training grant T32 NS07494-04 (F.J.R.).
REFERENCES
- 1.Cassarino DS, Santi M, Arruda A, et al. Spinal adrenal cortical adenoma with oncocytic features: report of the first intramedullary case and review of the literature. Int J Surg Pathol. 2004;12:259–264. doi: 10.1177/106689690401200309. [DOI] [PubMed] [Google Scholar]
- 2.Coire CI, Horvath E, Kovacs K, et al. A composite silent corticotroph pituitary adenoma with interspersed adrenocortical cells: case report. Neurosurgery. 1998;42:650–654. doi: 10.1097/00006123-199803000-00039. [DOI] [PubMed] [Google Scholar]
- 3.Erickson L, Jin L, Sebo T, et al. Pathologic features and expression of insulin-like growth factor-2 in adrenocortical neoplasms. Endocr Pathol. 2001;12:429–435. doi: 10.1385/ep:12:4:429. [DOI] [PubMed] [Google Scholar]
- 4.Gicquel C, Bertagna X, Gaston V, et al. Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. Cancer Res. 2001;61:6762–6767. [PubMed] [Google Scholar]
- 5.Karikari IO, Uschold TD, Selznick LA, et al. Primary spinal intramedullary adrenal cortical adenoma associated with spinal dysraphism: case report. Neurosurgery. 2006;59:E1144. doi: 10.1227/01.NEU.0000245588.31334.83. [DOI] [PubMed] [Google Scholar]
- 6.Kepes JJ, O’Boynick P, Jones S, et al. Adrenal cortical adenoma in the spinal canal of an 8-year-old girl. Am J Surg Pathol. 1990;14:481–484. doi: 10.1097/00000478-199005000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Kjellman M, Kallioniemi OP, Karhu R, et al. Genetic aberrations in adrenocortical tumors detected using comparative genomic hybridization correlate with tumor size and malignancy. Cancer Res. 1996;56:4219–4223. [PubMed] [Google Scholar]
- 8.Lack EE. Tumors of the Adrenal Glands and Extraadrenal Paraganglia. Washington DC: American Registry of Pathology; 2007. pp. 40–42. [Google Scholar]
- 9.Meyer A. A congenital intracranial intradural adrenal. Anat Rec. 1917;12:43–50. [Google Scholar]
- 10.Mitchell A, Scheithauer BW, Sasano H, et al. Symptomatic intradural adrenal adenoma of the spinal nerve root: report of two cases. Neurosurgery. 1993;32:658–661. doi: 10.1227/00006123-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Morimoto Y, Hiwada K, Nanahoshi M, et al. Cushing’s syndrome caused by malignant tumor in the scrotum: clinical, pathologic and biochemical studies. J Clin Endocrinol Metab. 1971;32:201–210. doi: 10.1210/jcem-32-2-201. [DOI] [PubMed] [Google Scholar]
- 12.Oka H, Kameya T, Sasano H, et al. Pituitary choristoma composed of corticotrophs and adrenocortical cells in the sella turcica. Virchows Arch. 1996;427:613–617. doi: 10.1007/BF00202893. [DOI] [PubMed] [Google Scholar]
- 13.Perry A, Anderl K, Borell TJ, et al. Detection of p16, RB, CDK4, and p53 gene deletion and amplification by fluorescence in situ hybridization in 96 gliomas. Am J Clin Pathol. 1999;112:801–809. doi: 10.1093/ajcp/112.6.801. [DOI] [PubMed] [Google Scholar]
- 14.Pilon C, Pistorello M, Moscon A, et al. Inactivation of the p16 tumor suppressor gene in adrenocortical tumors. J Clin Endocrinol Metab. 1999;84:2776–2779. doi: 10.1210/jcem.84.8.5877. [DOI] [PubMed] [Google Scholar]
- 15.Sidhu S, Marsh DJ, Theodosopoulos G, et al. Comparative genomic hybridization analysis of adrenocortical tumors. J Clin Endocrinol Metab. 2002;87:3467–3474. doi: 10.1210/jcem.87.7.8697. [DOI] [PubMed] [Google Scholar]
- 16.Sidhu S, Gicquel C, Bambach CP, et al. Clinical and molecular aspects of adrenocortical tumourigenesis. ANZ J Surg. 2003;73:727–738. doi: 10.1046/j.1445-2197.2003.02746.x. [DOI] [PubMed] [Google Scholar]
- 17.Slater EP, Diehl SM, Langer P, et al. Analysis by cDNA microarrays of gene expression patterns of human adrenocortical tumors. Eur J Endocrinol. 2006;154:587–598. doi: 10.1530/eje.1.02116. [DOI] [PubMed] [Google Scholar]
- 18.Wagner AS, Fleitz JM, Kleinschmidt-Demasters BK. Pediatric adrenal cortical carcinoma: brain metastases and relationship to NF-1, case reports and review of the literature. J Neurooncol. 2005;75:127–133. doi: 10.1007/s11060-005-0376-z. [DOI] [PubMed] [Google Scholar]
- 19.Wallace EZ, Leonidas JR, Stanek AE, et al. Endocrine studies in a patient with functioning adrenal rest tumor of the liver. Am J Med. 1981;70:1122–1125. doi: 10.1016/0002-9343(81)90886-x. [DOI] [PubMed] [Google Scholar]
- 20.Wiener MF, Dallgaard SA. Intracranial adrenal gland: a case report. AMA Arch Pathol. 1959;67:228–233. [PubMed] [Google Scholar]