Abstract
Background
Extra-cardiac conduit (ECC) and Lateral atrial tunnel (LAT) total cavopulmonary connection are both widely used in management of functionally univentricular hearts. The impact of the type of connection on early outcomes after Fontan operation remains unclear. We evaluated the impact of Fontan type on early outcome in a large clinical database.
Methods
Patients in the Society of Thoracic Surgeons Congenital Heart Surgery Database undergoing the Fontan operation (2000–2009) were included. In multivariable analysis, we evaluated the impact of Fontan type (ECC vs. LAT) on in-hospital mortality, Fontan takedown/revision, Fontan failure (in-hospital mortality or Fontan takedown/revision), post-operative length of stay (LOS), and complications, adjusting for patient, procedural, and center factors.
Results
A total of 2747 patients from 68 centers were included: 61% were male and 45% had a right dominant ventricle. ECC Fontan (vs. LAT) was performed in 63%; in all 65% were fenestrated. In multivariable analysis with adjustment for patient, procedural (including fenestration), and center factors (including Fontan volume), ECC Fontan was associated with significantly higher Fontan takedown/revision (OR 2.73, 95%CI 1.09–6.87) and Fontan failure (OR 2.28, 95%CI 1.13–4.59), and longer post-operative length of stay (adjusted estimated difference in LOS: +1.4 days).
Conclusions
These multicenter data suggest that of the two prevalent forms of Fontan connection in current use, the LAT Fontan may be associated with superior early outcomes.
Keywords: Congenital Heart Disease, Outcomes, Fontan
Introduction
By the late 1980s, the atriopulmonary connection pioneered by Fontan and Kreutzer [1,2] gave way to the concept of total cavopulmonary connection (TCPC) [3,4]. The potential benefit of “modified Fontan procedures” was extended to patients with a wide spectrum of cardiac anomalies [5]. It became apparent that flow disturbances and energy losses within the surgical pathways could be minimized by eliminating the atrial reservoir. A staged approach, with preliminary superior cavopulmonary connection followed by Fontan completion by either lateral atrial tunnel or extracardiac conduit type of TCPC is now by far the most prevalent approach to surgery for functionally univentricular hearts [6].
Advocacy for either lateral atrial tunnel or extracardiac conduit Fontan procedures has been based largely on theoretical advantages over the long-term. Advantages claimed for the lateral atrial tunnel include the ability to complete the Fontan circulation in young, very small patients, with potential for growth of the entire cavopulmonary pathway [7]. Advantages claimed for the extracardiac conduit include ease of construction without aortic cross-clamping and fewer atrial incisions and suture lines, with potential for reduction of arrhythmias [8]. Many surgeons prefer one technique over the other but continue nonetheless to utilize both, reserving one technique for selective indications [9]. Some institutions have gravitated toward exclusive use of one or the other type of operation.
Interestingly, little emphasis has been placed on analysis of the potential impact of type of connection on early outcomes. This study examines contemporary patterns of usage of the two most prevalent types of total cavopulmonary connection across institutions and evaluates the impact of type of operation on post-operative morbidity and mortality.
Patients and Methods
Data Source
The Society of Thoracic Surgeons Congenital Heart Surgery Database (STSCHSD) contains de-identified operative, peri-operative, and outcomes data on more than 190,000 patients, representing more than three quarters of US centers performing pediatric heart surgery [10]. Data quality and reliability are assured through intrinsic verification of data and site visit data audits [11]. The Duke Clinical Research Institute serves as the data warehouse for STS Databases. This study was approved by the Duke University Medical Center institutional review board with waiver of informed consent.
Patient Population
Details regarding the study population have been published previously [12]. Briefly, to maximize data integrity, analysis was restricted to 68 STS centers with >85% complete data for all study variables. Patients who underwent primary Fontan operation from 2000–2009 were eligible for inclusion. Patients undergoing Fontan conversion or repeat Fontan operation as the index operation were excluded. Patients >6 years of age were excluded as it was felt that these cases represented atypical presentation and/or management, and it is less likely that the patient was undergoing a primary Fontan operation. Patients with missing or invalid data for key variables of age and weight were excluded, along with those undergoing types of Fontan operation other than lateral tunnel or extracardiac conduit.
Data collection
Patient characteristics included age, sex, weight, weight-for-age z-score, cardiac diagnosis (categorized as right dominant lesions, left dominant lesions, and undifferentiated), non-cardiac/genetic abnormality (including the presence of heterotaxy syndrome), number of prior cardiothoracic surgeries, preoperative length of stay, and other pre-operative factors including pre-operative neurologic deficit/seizures, arrhythmia, and complete heart block requiring pacemaker (these pre-operative factors represent those captured by the STS Database which occurred in >0.5% of patients undergoing the Fontan operation). Center characteristics included annual Fontan operation volume. Operative characteristics included Fontan type (extracardiac conduit vs. lateral atrial tunnel) and fenestration status as coded by the surgeon at the time of the operation, and secondary procedures performed at the time of Fontan operation.
Outcomes
The primary outcome was in-hospital mortality. Secondary outcomes included Fontan takedown or revision during the hospitalization, Fontan failure (defined as occurrence of in-hospital mortality, Fontan takedown/revision, or heart transplant), postoperative length of stay, and post-operative complications. Deaths after discharge were not included in the analyses. These are not consistently captured in the STSCHSD currently. Post-operative complications included any of those defined in the STSCHSD [13].
Statistical Analysis
Patient, center, and operative characteristics were described overall and in those undergoing lateral atrial tunnel vs. extracardiac conduit Fontan using standard summary statistics including frequencies and percentages for categorical variables, and median and interquartile range for continuous variables. Unadjusted outcomes were compared between lateral atrial tunnel and extracardiac conduit Fontan groups using the Chi-square and Kruskal-Wallis tests.
The association of Fontan type with outcome was then evaluated in multivariable analysis utilizing logistic and linear regression, with hospital specific intercepts to account for within center clustering. All models were adjusted for age, weight z-score, sex, pre-operative risk factors as described above, non-cardiac/genetic abnormality (including heterotaxy syndrome), pre-operative length of stay, number of previous cardiothoracic surgeries, cardiac diagnosis, presence of fenestration, other secondary surgical procedures performed at the time of fontan, and center Fontan volume. Length of stay was not normally distributed and was therefore log transformed for analysis. Results from logistic regression models are displayed as odds ratios (OR) and 95% confidence intervals and results from linear regression models as parameter estimates and 95% confidence intervals. Finally, a sensitivity analysis was performed repeating the multivariable analysis, but using conditional logistic regression stratified by center (for dichotomous variables) and linear regression with center modeled as a fixed effect (for continuous variables). The initial models used provide averaged estimates across centers (and adjust the confidence intervals for clustering or correlation between patients at the same center), but do not exclude the possibility that any difference found relating to Fontan type may be related to other unaccounted for differences in treatment or outcomes between centers who use one type of Fontan more frequently. The latter models used in the sensitivity analysis, which condition on center, measure the association between treatment and outcome within each center and then combine the results across centers, thus mitigating potential center effects. Missing data were rare (< 0.5% for all variables). Patients with missing data for an endpoint were excluded from analysis involving that endpoint. All analyses were performed using STATA version 11.2 (StataCorp, College Station, TX). A p-value <0.05 was considered statistically significant.
Results
Patient and Operative Characteristics
A total of 2747 patients from 68 centers were included. Patient characteristics for those undergoing extracardiac conduit and lateral atrial tunnel operations were similar in most respects. Extracardiac conduit patients tended to be slightly older (Table 1). An extracardiac conduit Fontan (vs. lateral tunnel) was performed in 63% of cases, and 65% of all Fontans were fenestrated. Thirty-nine percent (n=1058) of patients underwent a total of 1466 secondary procedures at the time of the Fontan operation. The most common secondary procedure was pulmonary arterioplasty in 10.8%; 4.8% underwent atrioventricular valve repair/replacement and 0.8% underwent arch surgery.
Table 1.
Patient, Operative, and Center Characteristics
| Overall (n=2747) | Fontan type | ||
|---|---|---|---|
| Lateral tunnel (n=1017) | Extracardiac conduit (n=1730) | ||
| Patient Characteristics | |||
| Age, years | 3.0 (2.3, 3.6) | 2.4 (2.0, 3.1) | 3.2 (2.6, 3.9) |
| Male | 1664 (60.6%) | 617 (60.7%) | 1047 (60.5%) |
| Diagnosis | |||
| Right dominant lesions | 1239 (45.1%) | 497 (48.9%) | 742 (42.9%) |
| Left dominant lesions | 1031 (37.5%) | 389 (38.3%) | 642 (37.1%) |
| Undifferentiated | 477 (17.4%) | 131 (12.9%) | 346 (20.0%) |
| Weight, kg | 13.2 (11.7, 14.8) | 12.2 (10.9, 13.7) | 13.8 (12.4, 15.1) |
| Weight-for-age z-score | −0.7 (−1.5, 0.1) | −0.8 (−1.6, −0.03) | −0.5 (−1.4, 0.2) |
| Pre-operative factors | |||
| Neurologic deficit/seizures | 82 (3.0%) | 21 (2.1%) | 61 (3.5%) |
| Arrhythmia | 50 (1.8%) | 13 (1.3%) | 37 (2.1%) |
| Complete heart block/pacemaker | 16 (0.6%) | 7 (0.7%) | 9 (0.5%) |
| Any non-cardiac/genetic abnormality | 536 (19.5%) | 146 (14.4%) | 390 (22.5%) |
| Heterotaxy syndrome | 249 (9.1%) | 60 (5.9%) | 189 (10.9%) |
| Pre-operative LOS >2 days | 58 (2.1%) | 21 (2.1%) | 37 (2.1%) |
| Number of prior CT operations | 2 (2, 3) | 2 (2, 3) | 2 (2, 3) |
| Operative Characteristics | |||
| Fenestrated Fontan | 1788 (65%) | 881 (87%) | 907 (52%) |
| Secondary procedure at Fontan, n (%) | 1058 (38.5%) | 409 (40.2%) | 649 (37.5%) |
| Center Characteristics | |||
| Center annual Fontan volume, median operations/year | 10.8 (7.7, 31.4) | 14.0 (9.4, 35.6) | 10.3 (7.2, 28.0) |
Data for continuous variables are displayed as median (interquartile range).
LOS=length of stay, CT = cardiothoracic
Overall, 2706 of 2747 (98.5%) procedures were coded as cardiopulmonary bypass (cpb) cases, including 1015 of 1017 (99.8%) lateral tunnels and 1691 of 1730 (97.8%) extracardiac conduit procedures. For those with documented cpb time, the median and interquartile range (minutes) were - overall: 92 (68–120), lateral tunnel: 99 (77–125), and extracardiac conduit: 88 (60–116).
Overall 1149 of 2747 (41.8%) patients had a documented cross-clamp time, including 713 of 1017 (70.1%) for lateral tunnel and 436 of 1730 (25.2%) for extracardiac conduit. Of those with cross-clamp data entered, the times (minutes) were - overall: 43 (29–60), lateral tunnel: 46 (35–63), and extracardiac conduit: 36 (22–55).
Center Variation
The proportion of all Fontan operations accounted for by extracardiac Fontans at a center level is shown in Figure 1. In 42 of 68 centers (62%), 80% or more of patients underwent extracardiac conduit Fontans. Eighteen centers did exclusively extracardiac conduit Fontans. At roughly one third of centers the extracardiac conduit procedure accounted for fewer that half of all Fontans.
Figure 1.
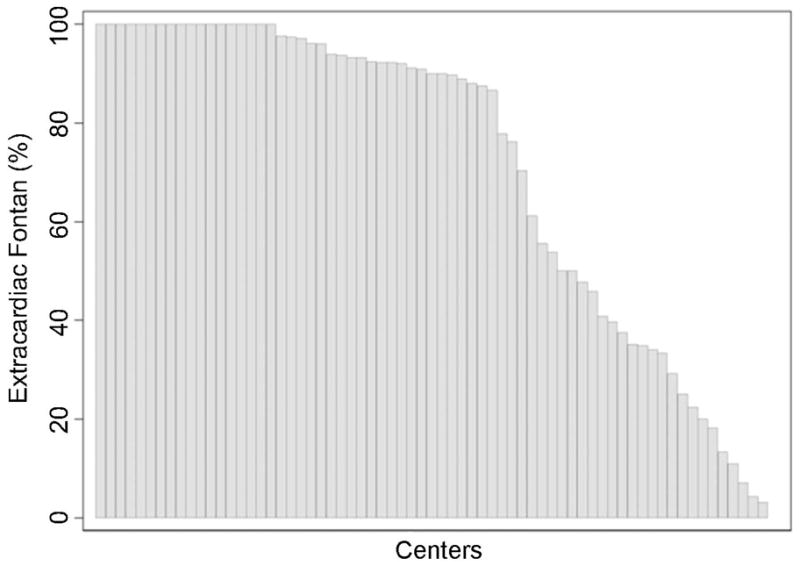
Proportion of Patients Undergoing Extracardiac Conduit Fontan by Center. Each vertical bar represents data from an individual center. Height of each bar indicates percentage of patients undergoing extracardiac conduit Fontan operation (vs. lateral tunnel Fontan) at the center.
Outcomes
Unadjusted outcomes by type of Fontan are displayed in Table 2. As there were no in-hospital transplants performed following the Fontan operation, the data on inhospital Fontan failure includes only mortality or Fontan revision/takedown. The extracardiac conduit Fontan was associated with higher rates of each of the outcomes measured and longer post-operative length of stay in unadjusted analysis. Unadjusted outcomes by type of Fontan and fenestration are displayed in Table 3.
Table 2.
Unadjusted Outcomes Associated with Type of Fontan
| Post-operative Outcomes | Overall (n=2747) | Fontan type | p-value | |
|---|---|---|---|---|
| Lateral tunnel (n=1017) | Extracardiac conduit (n=1730) | |||
| In-hospital mortality | 45 (1.6%) | 9 (0.9%) | 36 (2.1%) | 0.02 |
| Fontan takedown/revision | 37 (1.4%) | 7 (0.7%) | 30 (1.7%) | 0.02 |
| In-hospital mortality or Fontan takedown/revision | 73 (2.7%) | 14 (1.4%) | 59 (3.4%) | 0.001 |
| Post-operative complications | 1111 (40.4%) | 328 (32.3%) | 783 (45.3%) | <0.001 |
| Post-operative LOS | 9 (7, 14) | 9 (7, 13) | 10 (7, 16) | <0.0001 |
LOS = length of stay (days)
Table 3.
Unadjusted Outcomes by Fontan Type +/− Fenestration
| Fontan Type | ||||||
|---|---|---|---|---|---|---|
| Overall (n=2747) | Fenestrated Lateral tunnel (n= 881) | Non-fenestrated Lateral tunnel (n= 136) | Fenestrated Extracardiac (n= 907) | Non-fenestrated Extracardiac (n= 823) | p-value | |
| In-hospital mortality | 45 (1.6%) | 8 (0.9%) | 1 (0.7%) | 23 (2.5%) | 13 (1.6%) | 0.04 |
| Fontan takedown/revision | 37 (1.4%) | 6 (0.7%) | 1 (0.7%) | 16 (1.8%) | 14 (1.7%) | 0.15 |
| In-hospital mortality or Fontan takedown/revision | 73 (2.7%) | 12 (1.4%) | 2 (1.5%) | 35 (3.9%) | 24 (2.9%) | <0.01 |
| Post-operative complications | 1111(40.4%) | 290 (32.9%) | 38 (27.9%) | 450 (49.6%) | 333 (40.5%) | <0.001 |
| Post-operative LOS | 9 (7, 14) | 9 (7, 13) | 8 (5, 12) | 10 (7, 17) | 9 (7, 14) | <0.001 |
In multivariable analysis (Table 4), outcomes for extracardiac conduit Fontan were compared to those for lateral tunnel Fontan. Models were adjusted for patient characteristics, procedural factors including fenestration, and center volume. The extracardiac conduit Fontan was associated with significantly higher odds of Fontan takedown/revision, and Fontan failure, as well as longer postoperative length of stay.
Table 4.
Adjusted Outcomes Associated with Type of Fontan: Extracardiac Conduit vs. Lateral Tunnel Fontan
| Post-operative Outcomes | Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| In-hospital mortality | 2.30 (1.00, 5.26) | 0.049 | 2.00 (0.84, 4.73) | 0.12 |
| Fontan takedown/revision | 2.57 (1.09, 6.06) | 0.03 | 2.73 (1.09, 6.87) | 0.03 |
| In-hospital mortality or Fontan takedown/revision | 2.33 (1.19, 4.55) | 0.01 | 2.28 (1.13, 4.59) | 0.02 |
| Post-operative complications | 1.22 (0.96, 1.55) | 0.10 | 1.24 (0.97, 1.59) | 0.09 |
| Unadjusted Estimate* (95% CI) | p-value | Adjusted Estimate* (95% CI) | p-value | |
|
|
||||
| Post-operative LOS | 0.12 (0.06, 0.18) | <0.001 | 0.13 (0.06, 0.19) | <0.001 |
Lateral Tunnel Fontan serves as the reference group for all data presented in the table.
All models were adjusted for the pre-operative and operative variables noted in the Methods section, including fenestration status.
log days
Estimated difference in length of stay (LOS) in actual days, extracardiac conduit vs. lateral tunnel= +1.4 days
Heterotaxy
Heterotaxy was diagnosed in 249 patients (9%). Of these, 189 underwent extracardiac conduit and 60 underwent lateral atrial tunnel Fontan operations. Discharge mortality was higher among heterotaxy patients (4.8% vs 1.3%, p<0.001), as was Fontan failure (6.0% vs 2.3%, p=0.001). However, within the heterotaxy subgroup, extracardiac conduit and lateral atrial tunnel Fontan procedures were associated with similar rates of mortality (4.8% vs 5.0%, p=0.94) and similar rates of Fontan failure (5.8% vs 6.7%, p=0.81). In addition, as noted above, results of the main analysis are not attributable to the relative proportion of patients with heterotaxy in the different treatment groups, as this variable was included in and adjusted for in the multivariable models.
Center Effects
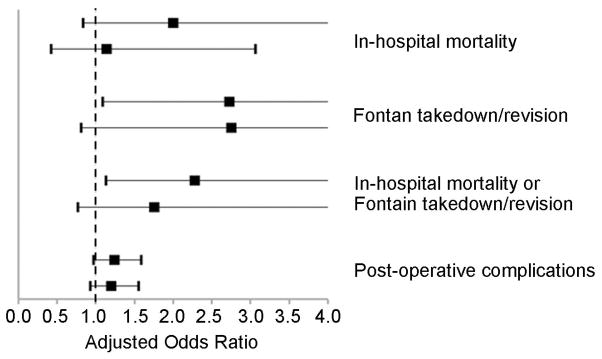
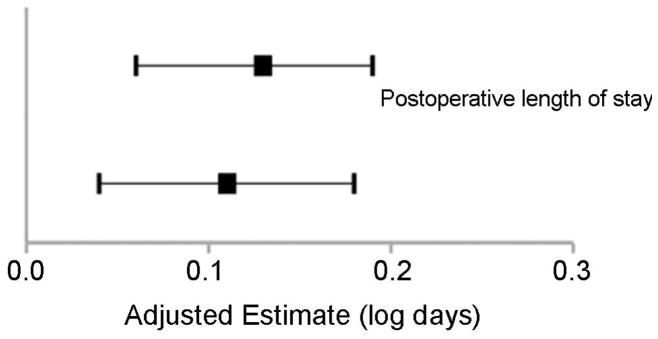
We performed a sensitivity analysis to examine the potential influence of center effects. The models used in the main analysis presented above do not exclude the possibility that differences found relating to Fontan type may be related to other unaccounted for differences in treatment or outcomes across centers. In the sensitivity analysis, we utilized models which condition on center, or measure the association between treatment and outcome within each center and then combine these results, thus mitigating potential center effects. This analysis yeilded results that were generally similar (no change in direction of point estimates) in comparison with the main models (Figure 2).
Figure 2.
Results of Sensitivity Analysis (extracardiac conduit vs. lateral atrial tunnel Fontan).
A. Dichotomous Outcome Variables. Adjusted odds ratios (black box), and 95% confidence intervals (line) are shown. The line of unity (odds ratio=1.0) is depicted by the dotted line. B. Continuous Outcome Variables. Adjusted estimates (black box; log days), and 95% confidence intervals (line) are shown. The line of unity in this case is represented by the solid line where the adjusted estimate = zero. Lateral atrial tunnel Fontan serves as the reference for all data shown. In both A. and B., two point estimates are shown for each outcome variable. In all cases, the top estimate represents that obtained from the main models, while the bottom estimate represents that obtained from the conditional models used to address potential center effects. In all cases, results were generally similar between the main model results and the conditional model results.
Comment
Variation in practices between centers is prevalent in the management of congenital heart disease [12,14,15]. In this large multi-institutional cohort, we found substantial center-to-center variation regarding the use of the two most common types of modified Fontan operations. The extracardiac conduit Fontan was more prevalent overall, with several institutions using it exclusively.
We found important differences in early outcomes based on the type of Fontan connection. In multivariable analysis, the extracardiac conduit Fontan was associated with significantly higher rates of Fontan takedown/revision, and Fontan failure, and with longer postoperative length of stay, despite adjustment for patient characteristics, procedural factors including fenestration, and center Fontan volume. While contemporary single center series report excellent outcomes using one or the other technique either exclusively or predominantly [8,16,17], few previous studies have compared outcomes across techniques in large cohorts. Evaluation of a large multi-institutional population allows adequate statistical power to make such comparisons. A previous study estimated the mortality risk associated with 148 individual congenital heart operations and assigned procedures to 5 categories based on mortality risk (category 1 = lowest mortality; category 5 = highest mortality) [18]. That combined dataset from the STSCHSD and the European Association for Cardiothoracic Surgery Congenital Heart Surgery Database included data from nearly 80,000 operations conducted in 2002–2006. In that study, fenestrated lateral atrial tunnel Fontan was found to be associated with low mortality and assigned to category 1, while all other types of Fontan procedures were assigned to higher categories [18]. The present analysis builds on that previous analysis by specifically comparing outcomes between the two most prevalent forms of Fontan connection in current use, and using multivariable analysis to adjust for patient and center factors which may also impact outcome. We found similar results in that the lateral atrial tunnel Fontan appeared to be associated with superior early outcome.
Just as each type of operation has unique features that may determine performance over the long-term, the design of each operation also can impact hemodynamics during the critical period of early postoperative recovery. M. de Leval and associates compared flow dynamics of dfferent types of Fontan connections using computational fluid dynamic methods [19]. With Bove, they demonstrated that the lateral tunnel Fontan as performed after the hemi-Fontan had lower power losses than the extracardiac conduit Fontan. Inferior vena cava flow into the pulmonary arteries was evenly distributed with the lateral tunnel. The extracardiac conduit Fontan exhibited preferential perfusion to the left lung. Bove suggested that geometric designs with minimal enegy losses and even flow distribution are likely to translate into improved clinical outcomes [20,21]. In vitro studies of extracardiac conduit Fontan circuits suggest that variations in caval offset influence energy losses and distribution of overall flow and hepatic flow to right and left lungs [22]. While the extracardiac conduit Fontan is a technically straightforward operation, variability in geometry may affect circulatory efficiency in the critical postoperative period.
Hemodynamic perfomance of the extracardiac conduit Fontan circulation depends upon the relationship of conduit size to patient size. Itatani and associates explored the impact of conduit size on energy loss, and found that larger conduits are associated with inefficient flow due to turbulence or stagnation [23]. They modeled the circulation based on angiographic data from 17 Fontan patients (mean body surface area, 0.53 m2) and observed significant backward flow during expiration with larger conduits. Stagnation volume increased with increased conduit size (14 mm, 9.20% vs 22 mm, 33.9% conduit volume at rest). They concluded that conduits of 16–18 mm diameter were optimal for patients in the size range they studied. Implanting larger conduits may be at odds with the goal of optimizing Fontan circulation during the critical early postoperative period.
Some investigators suggest that fenestration may mitigate early postoperative morbidity frequently observed in earlier eras [24,25]. Others challenge routine use of fenestration, particularly in the setting of the extracardiac conduit Fontan [26,27]. In this study we observed that roughly two-thirds of operations included fenestration. Fenestration was one of the procedural factors adjusted for in the multivariable analysis. As such, the observed differences in outcomes between groups are independent of any effect of fenestration. The study does not, however, address the question of whether fenestration may be more reliably achieved in the context of one or the other type of Fontan connection. Data available for analysis reflect only the decision to fenestrate at the time of surgery; not whether the fenestration(s) stayed open and affected hemodynamics.
Limitations
Limitations of this study are related to the limitations of the STS Database and the observational nature of the analysis. Although this is the largest study evaluating the association between early outcomes and type of cavopulmonary connection, data from the STS Database may not be generalizable to all patients at all centers. In our analysis, we accounted for several factors which have been identified in previous Fontan studies as potentially important variables impacting outcome. These include diagnosis, presence of genetic/non-cardiac abnormalities, previous interventions, other pre-operative comorbidities, as well as hemodynamic/anatomic abnormalities (atrioventricular valve regurgitation, coarctation, etc) requiring intervention at the time of the Fontan. Other variables, not currently captured by the database, may be important. Pre-operative pulmonary artery pressure and pulmonary vascular resistance and echocardiographic variables are not captured in the STS Database. Therefore we could not account for these factors in our analysis. The database does not enable us to know with certainty whether all patients underwent a prior superior cavopulmonary anastomosis. Finally, the size of the conduit used for extracardiac Fontans is not captured. Due to these limitations, we are not able to draw inferences regarding the exact underlying mechanisms accounting for the observed associations between outcomes and type of Fontan connection.
Despite these limitations, we feel that the distribution of the data regarding proportion of extracardiac conduit vs. lateral tunnel Fontan use across centers does not support a hypothesis that patients who underwent extracardiac conduit Fontan were sicker to begin with. Rather, it appears that Fontan type is related more to center preference, given the fact that many centers did extracardiac conduit Fontans exclusively, while others used it rarely and did primarily lateral tunnel type Fontans.
Conclusion
This large multi-institutional analysis suggests that of the two prevalent forms of Fontan connection in current use, the lateral tunnel Fontan may be associated with superior early outcomes in terms of freedom from takedown or revision of the Fontan connection, Fontan failure, and postoperative length of stay. Fortunately, all adverse outcome measures occurred with relatively low frequency with both types of operations. While evidence for the potential long-term benefits of one or the other operative strategy continues to accumulate, treatment decisions should also include consideration of the association between early postoperative outcomes and operative strategy.
Acknowledgments
Dr. Pasquali received grant support from the National Heart, Lung, and Blood Institute (1K08HL103631-01).
Footnotes
Presented at the 58th Annual Meeting of the Southern Thoracic Surgical Association, San Antonio, TX, November 9–12, 2011
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreutzer G, Galindez E, Bono H, de Palma C, Laura JP. An operation for the correction of tricuspid atresia. J Thorac Cardiovasc Surg. 1973;66:613. [PubMed] [Google Scholar]
- 3.de Leval MR, Kilner P, Gewillig M, Bull C, McGoon DC. Total cavopulmonary connection. A logical alternative to atriopulmonary connection for complex Fontan operation. J Thorac Cardiovasc Surg. 1988;96:682. [PubMed] [Google Scholar]
- 4.Jonas RA, Castaneda AR. Modified Fontan procedure: atrial baffle and systemic venous to pulmonary artery anastomotic techniques. J Cardiac Surg. 1988;3:91–96. doi: 10.1111/j.1540-8191.1988.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 5.Mayer JE, Jr, Helgason H, Jonas RA, et al. Extending the limits for modified Fontan procedures. J Thorac Cardiovasc Surg. 1986;92:1021–8. [PubMed] [Google Scholar]
- 6.Norwood WI, Jacobs ML. Fontan’s procedure in two stages. Am J Surg. 1993;166:548–55. doi: 10.1016/s0002-9610(05)81151-1. [DOI] [PubMed] [Google Scholar]
- 7.Pizarro C, Mroczek T, Gidding SS, Murphy JD, Norwood WI. Fontan completion in infants. Ann Thorac Surg. 2006 Jun;81(6):2243–48. doi: 10.1016/j.athoracsur.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Petrossian E, Reddy VM, Collins KK, et al. The extracardiac conduit Fontan operation using minimal approach extracorporeal circulation: early and midterm outcomes. J Thorac Cardiovasc Surg. 2006;132:1054–63. doi: 10.1016/j.jtcvs.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs ML, Pelletier GJ, Pourmoghadam KK, et al. Protocols associated with no mortality in 100 consecutive Fontan procedures. Eur J Cardiothorac Surg. 2008;33:626–32. doi: 10.1016/j.ejcts.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs ML, Daniel M, Mavroudis C, et al. Report of the 2010 Society of Thoracic Surgeons Congenital Heart Surgery Practice and Manpower Survey. Ann Thorac Surg. 2011;92:762–768. doi: 10.1016/j.athoracsur.2011.03.133. [DOI] [PubMed] [Google Scholar]
- 11.Clarke DR, Breen LS, Jacobs ML, et al. Verification of data in congenital cardiac surgery. Cardiol Young. 2008;18 (Suppl2):177–187. doi: 10.1017/S1047951108002862. [DOI] [PubMed] [Google Scholar]
- 12.Wallace MC, Jaggers J, Li JS, et al. Center Variation in Patient Age and Weight at Fontan Operation and Impact on Postoperative Outcomes. Ann Thorac Surg. 2011;91:1445–52. doi: 10.1016/j.athoracsur.2010.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. [Accessed October 25, 2011];STS Congenital Database Full Specifications. http://www.sts.org/documents/pdf/Congenital_DataSpecs_250.pdf.
- 14.Johnson JN, Jaggers J, Li S, et al. Center variation and outcomes associated with delayed sternal closure after stage 1 palliation for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2010;139:1205–10. doi: 10.1016/j.jtcvs.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wernovsky G, Ghanayem N, Ohye RG, et al. Hypoplastic left heart syndrome: consensus and controversies in 2007. Cardiol Young. 2007;17 (Suppl 2):75–86. doi: 10.1017/S1047951107001187. [DOI] [PubMed] [Google Scholar]
- 16.Backer CL, Deal BJ, Kaushal S, et al. Extracardiac versus intra-atrial lateral tunnel fontan: extracardiac is better. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2011;14:4–10. doi: 10.1053/j.pcsu.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch JC, Goldberg C, Bove EL, et al. Fontan operation in the current era: a 15-year single institution experience. Ann Surg. 2008;248:402–10. doi: 10.1097/SLA.0b013e3181858286. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien SM, Clarke DR, Jacobs JP, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–53. doi: 10.1016/j.jtcvs.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 19.de Leval MR, Dubini G, Migliavacca F, et al. Use of computational fluid dynamics in the design of surgical procedures: application to the study of competitive flows in cavo-pulmonary connections. J Thorac Cardiovasc Surg. 1996;111:502–13. doi: 10.1016/s0022-5223(96)70302-1. [DOI] [PubMed] [Google Scholar]
- 20.Bove EL, de Leval MR, Migliavacca F, Guadagni G, Dubini G. Computational fluid dynamics in the evaluation of hemodynamic performance of cavopulmonary connections after the Norwood procedure for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2003;126:1040–7. doi: 10.1016/s0022-5223(03)00698-6. [DOI] [PubMed] [Google Scholar]
- 21.Bove EL, de Leval MR, Migliavacca F, Balassio G, Dubini G. Toward optimal hemodynamics: computer modeling the Fontan circuit. Pediatr Cardiol. 2007;28:477–481. doi: 10.1007/s00246-007-9009-y. [DOI] [PubMed] [Google Scholar]
- 22.Ensley AE, Lynch P, Chatzimavroudis GP, Lucas C, Sharma S, Yoganathan AP. Toward designing the optimal total cavopulmonary connection: an in vitro study. Ann Thorac Surg. 1999;68:1384–90. doi: 10.1016/s0003-4975(99)00560-3. [DOI] [PubMed] [Google Scholar]
- 23.Itatani K, Miyaji K, Tomoyasu T, et al. Optimal conduit size of the extracardiac Fontan operation based on energy loss and flow stagnation. Ann Thorac Surg. 2009;88:565–72. doi: 10.1016/j.athoracsur.2009.04.109. [DOI] [PubMed] [Google Scholar]
- 24.Bridges ND, Lock JE, Castaneda AR. Baffle fenestration with subsequent transcatheter closure. Modification of the Fontan operation for patients at increased risk. Circulation. 1990;82:1681–9. doi: 10.1161/01.cir.82.5.1681. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs ML, Norwood WI., Jr Fontan operation: influence of modifications on morbidity and mortality. Ann Thorac Surg. 1994;58:945–51. doi: 10.1016/0003-4975(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 26.Thompson LD, Petrossian E, McElhinney DB, et al. Is it necessary to routinely fenestrate an extracardiac fontan? J Am Coll Cardiol. 1999;34:539–44. doi: 10.1016/s0735-1097(99)00228-4. [DOI] [PubMed] [Google Scholar]
- 27.Schreiber C, Horer J, Vogt M, et al. Nonfenestrated extracardiac total cavopulmonary connection in 132 consecutive patients. Ann Thorac Surg. 2007;84:894–899. doi: 10.1016/j.athoracsur.2007.04.034. [DOI] [PubMed] [Google Scholar]