MARCKS is involved in directed migration of macrophages via a process involving its phosphorylation, cytoplasmic translocation, and interaction with actin.
Keywords: cytoplasmic translocation, J774A.1 cells, monocyte chemotactic protein 1, chemotaxis
Abstract
A role for MARCKS protein in directed migration of macrophages toward a chemoattractant was investigated. A peptide identical to the N-terminus of MARCKS (the MANS peptide), shown previously to inhibit the function of MARCKS in various cell types, was used. We investigated whether this MARCKS-related peptide could affect migration of macrophages, using the mouse macrophage-like J774A.1 cell line and primary murine macrophages. Both of these cell types migrated in response to the chemoattractants macrophage/MCPs, MCP-1 (25–100 ng/ml) or C5a (5–20 ng/ml). Cells were preincubated (15 min) with MANS or a mis-sense control peptide (RNS), both at 50 μM, and effects on migration determined 3 h after addition of chemoattractants. The movement and interactions of MARCKS and actin also were followed visually via confocal microscopy using a fluorescently labeled antibody to MARCKS and fluorescently tagged phalloidin to identify actin. MANS, but not RNS, attenuated migration of J774A.1 cells and primary macrophages in response to MCP-1 or C5a, implicating MARCKS in the cellular mechanism of directed migration. Exposure of cells to MCP-1 resulted in rapid phosphorylation and translocation of MARCKS from plasma membrane to cytosol, whereas actin appeared to spread through the cell and into cell protrusions; there was visual and biochemical evidence of a transient interaction between MARCKS and actin during the process of migration. These results suggest that MARCKS is involved in directed migration of macrophages via a process involving its phosphorylation, cytoplasmic translocation, and interaction with actin.
Introduction
In airway diseases, such as chronic bronchitis, asthma, and cystic fibrosis, inflammation contributes to airway obstruction and impaired lung function. During an inflammatory response, macrophages migrate to infected or injured areas in response to signaling molecules, such as cytokines and chemokines; however, uncontrolled recruitment can lead to enhanced inflammation. Macrophages are the predominant cells in the alveolar spaces in the lung, and enhanced recruitment of these cells into areas of injury can add to the severity of inflammation [1].
MARCKS, an important substrate for PKC, is a highly conserved, 87-kDa protein involved in several important cellular processes, including cell movement [2–4]. {A related-protein, MacMARCKS (also known as MRP, MARCKS-like protein, or F52), is found in macrophages and other cell types and shares the same evolutionarily conserved functional domains as MARCKS, although it is smaller in size (∼20 kDa) [2, 5, 6]}. Recently, MARCKS was shown to play a prominent role in the regulation of neutrophil migration in vitro [7] and in vivo [8, 9], as well as directed migration of mouse and human stem cells in vitro [10]. These studies used a peptide identical to the amino terminus of human MARCKS, called the MANS peptide, to inhibit MARCKS function, as described in numerous publications from our and other laboratories [7, 8, 11–13].
The purpose of the studies reported here was to determine whether MARCKS is also involved in regulation of directed migration of macrophages. The chemoattractant proteins MCP-1 and C5a were used to stimulate directed migration of the J774A.1 mouse macrophage cell line and isolated primary mouse macrophages, and the MANS peptide was used to determine whether MARCKS was involved in the response. Furthermore, given the reported role of PKC and actin in migration of various cell types and the fact that MARCKS is activated by PKC and is known to bind to and cross-link actin filaments [14, 15], we also investigated a possible link between MARCKS and actin in the response to chemoattractants by assessing, via confocal microscopy, the distribution and interactions of MARCKS and actin in these cells.
The results indicate that MANS, but not the mis-sense control RNS peptide, attenuates chemoattractant-induced migration of J774A.1 cells and primary mouse macrophages. In addition, MARCKS is phosphorylated rapidly and translocates from plasma membrane to cytoplasm after exposure of these cells to MCP-1, further implicating MARCKS in the response. Concurrently, actin appeared to redistribute throughout the migrating cells and especially into cell processes and protrusions; a transient association between MARCKS and actin during this process was observed visually and biochemically (immunoprecipitation). These results suggest that MARCKS is involved integrally in directed migration of macrophages via a mechanism that may involve cytoskeletal association(s).
MATERIALS AND METHODS
Materials
The mouse macrophage cell line J774A.1 was obtained from American Type Culture Collection, (Manassas, VA, USA). DMEM and RPMI 1640 were from Mediatech (Herndon, VA, USA). FBS and penicillin-streptomycin were from Fisher Scientific (Pittsburgh, PA, USA) and amphotericin B from Sigma-Aldrich (St. Louis, MO, USA). BSA was from Gemini Bio-Products (West Sacramento, CA, USA). Transwell inserts were from Corning (Corning, NY, USA), and rat tail collagen type I was from BD Biosciences (San Jose, CA, USA). Mouse rMCP-1 and C5a were from R&D Systems (Minneapolis, MN, USA). The Diff-Quick stain set was purchased from Dade Behring (Newark, DE, USA). The Bradford assay reagent and transblot nitrocellulose membranes were from Bio-Rad Laboratories (Hercules, CA, USA). For Western blot analysis of MARCKS expression in cells, the MARCKS antibody was purchased from Upstate Biotechnology (Lake Placid, NY, USA). Antibodies for phosphorylated MARCKS, HRP-conjugated α-rabbit IgG, and GAPDH were purchased from Cell Signaling Technology (Danvers, MA, USA). The ECL development kit was from GE Life Science Products (Piscataway, NJ, USA). Alexa Fluro 488 and TO-PRO-3 iodine were from Molecular Probes (Eugene, OR, USA). Dako mounting medium was from Dako North America (Carpenteria, CA, USA). All other reagents were purchased from Sigma-Aldrich.
Peptides
MANS and RNS peptides were synthesized by Genemed Synthesis (San Antonio, TX, USA). MANS peptide is identical to the first 24 aa of MARCKS: MA-GAQFSKTAAKGEAAAERPGEAAVA. The RNS peptide contains the same amino acids as MANS but in random sequence: MA-GTAPAAEGAGAEVKRASAEAKQAF. Both peptides have been described in detail previously [2].
Cell culture
J774A.1 cells were cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg amphotericin B. Cells were seeded in T75 tissue-culture flasks and maintained at 37°C in an atmosphere containing 5% CO2 until cells reached ∼80% confluency. Cell viability was >90%, as assessed using a Cellometer and trypan blue dye exclusion.
Isolation of primary mouse lung macrophages
The use of animals in this study was approved by the Institutional Animal Care and Use Committee of North Carolina State University (Raleigh, NC, USA). CD-1 mice were purchased from Charles River (Wilmington, MA, USA) at 6–10 weeks old. The animals were heavily sedated with ketamine/xylazine (100 mg/kg and 10 mg/kg body weight, respectively), administered i.p. and exsanguinated by cutting the left renal artery and abdominal aorta. The abdomen was exposed carefully by cutting through the ribcage, avoiding the lungs. Red blood cells were flushed using a 10-ml syringe, and a 21-gauge needle was used to inject 5–10 ml 0.9% sterile NaCl through the right ventricle of the heart. The trachea was exposed surgically and a 20-gauge catheter inserted. The lungs then were filled with 3 ml dispase through the catheter, followed immediately by instillation of 0.45 ml 1% low-melt agarose, and then the thorax was covered with crushed ice for 2 min to solidify the agarose. The trachea was sealed with surgical silk and the heart and lungs removed and rinsed with 5 ml sterile PBS. The lungs were transferred to 2 ml dispase in a sterile culture tube and then placed in a 37°C water bath for 30 min, after which, they were placed in 7 ml freshly prepared DMEM with 100 μl 0.7% DNase I type II, put in a 60-mm Petri dish, and teased apart carefully and gently swirled for 5–10 min to release cells. The mixture was then filtered through a 100-μ sterile cell filter into a 50-ml Falcon tube prior to gentle centrifugation at 200 g for 12 min at 4°C. The supernatant was aspirated and the cell pellet resuspended in 10 ml DMEM + 10% FBS and incubated for 30 min at 37°C in 5% CO2. Macrophages stuck to the plastic dish, whereas unattached cells were washed away with sterile PBS, and fresh medium was then added to the cells. To ensure that the cell suspension preparation was enriched with lung macrophages, differential staining with Diff-Quick, followed by counting under a light microscope, was performed. Viability of isolated lung macrophages was assessed using a Cellometer with trypan blue dye exclusion; macrophage viability was >90%.
Transwell migration assay
Migration assays were performed in 6.5 mm Transwell plates with 8 μm pore inserts. The upper side of one insert was thinly coated for 1 h with rat tail type I collagen. J774A.1 cells or primary murine macrophages (1×105 cells) were resuspended in 100 μl chemotaxis buffer (RMPI 1640 plus 0.02% BSA). Cells were added to the upper chamber and 600 μl migration medium, with or without chemotactic factors: MCP-1 (25, 50, or 100 ng/ml), PMA (10, 50, or 100 nM), or C5a (5, 10, or 20 ng/ml) added to the lower chamber. Cells were allowed to migrate through the insert membrane for 3 h at 37°C under a 5% CO2 atmosphere. In some experiments, cells were first pretreated with MANS or RNS peptide (25–100 μM) or PBS for 15 min at 37°C. The inserts were then washed with PBS, and nonmigrating cells remaining on the upper surface of the insert were removed with a cotton swab. The migrated cells on the insert were fixed, stained with Diff-Quick, and mounted on glass slides. Migration was measured visually by counting using a light microscope at 40× magnification. The mean number of cells in 10 randomly chosen fields was calculated for each treatment. A migration index was calculated by dividing the number of cells that migrated in response to the chemokine by the number of cells that migrated randomly (RPMI/0.02% BSA) with a reference index >1, indicating chemotaxis.
Western blotting
Expression of MARCKS and phosphorylated MARCKS was measured via Western blot. Unstimulated or MCP-1 (100 ng/ml)-, PMA (100 nM)-, or C5a (10 ng/ml)-exposed cells were washed with PBS, scraped, lysed, sonicated, and then centrifuged at 14,000 g for 15 min at 4°C. The protein concentrations of total cell lysates were quantified by a Bradford assay (Bio-Rad Laboratories). Proteins were denatured by boiling in 2× SDS sample buffer for 5 min. The sample lysates (30 μg) were loaded on 4–15% SDS polyacrylamide gels and then transferred to nitrocellulose membranes, which were blocked with 5% nonfat milk and incubated in primary antibodies to MARCKS or to phospho-MARCKS. Specific bands were visualized after incubation with secondary antibodies, mouse or rabbit anti-mouse IgG conjugated to HRP, by ECL, followed by exposure to film.
Immunofluorescence
J774A.1 cells were seeded on glass coverslips that had been placed in six-well tissue-culture plates. The cells were treated with vehicle or MCP-1 (100 ng/ml) for 1.5, 3, 5, or 10 min, then washed with PBS, and fixed in 4% paraformaldehyde for 20 min prior to blocking in 10% normal donkey blocking serum with 1.0% Triton X in PBS for 1 h at room temperature. For MARCKS staining, the cells were incubated in primary MARCKS antibody overnight at 4°C, washed with PBS, and then incubated in secondary donkey anti-mouse fluorescent (red) antibody for 1 h at room temperature. For actin staining, cells were incubated in phalloidin with Alexa 488 (green) for 30 min, and for nuclear staining, cells were incubated with TO-PRO-3 iodine (blue) for 15 min and then washed with PBS. Stained cells were mounted on glass slides using Dako mounting medium. Confocal imaging was performed using a C1 Nikon confocal microscope at the Laboratory for Advanced Electron and Light Optical Methods at North Carolina State University, College of Veterinary Medicine.
Statistical analysis
Results from at least three experiments are presented as mean ± sem. Statistical significance of the data was determined using one-way ANOVA; P < 0.05 was considered significant.
RESULTS
J774A.1 cells
MCP-1, PMA, or C5a are chemoattractants for J774A.1 cells.
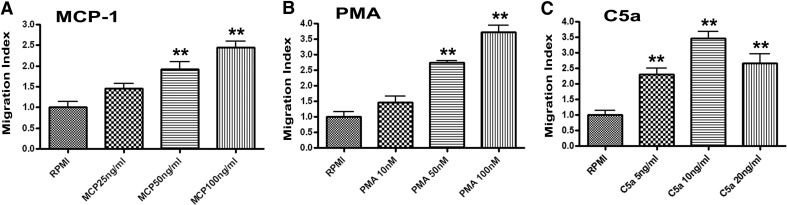
A range of concentrations of MCP-1 (25, 50, or 100 ng/ml), PMA (10, 50, or 100 nM), or C5a (5, 10, or 20 ng/ml) was tested for effects on J774A.1 cell migration after 3 h using an in vitro Transwell migration assay. A concentration- dependent stimulation of J774A.1 cell migration in response to MCP-1 is shown in Fig. 1A. MCP-1 at 50 or 100 ng/ml significantly increased migration of J774A.1 cells compared with medium alone toward the lower surface of the polycarbonate membrane through 8 μm pores (P<0.01). PMA also stimulated a concentration-dependent migration of J774A.1 cells, whiles exposure to C5a elicited a peak response at10 ng/ml (Fig. 1B and C). Thus, each of these chemoattractants, MCP-1, PMA, and C5a, enhanced directed migration of J774A.1 cells above random migration (control) levels.
Figure 1. J774A.1 cells migrate in response to: (A) MCP-1 (25, 50, or 100 ng/ml), (B) PMA (10, 50, or 100 nM), and (C) C5a (5, 10, or 20 ng/ml).
Cells were exposed for 3 h, at which time, migrating cells were quantified. Data represent mean ± sem; n = 3; **significantly different from media control, P < 0.01.
Effects of MARCKS-related peptides on MCP-1- or C5a-induced J774A.1 cell migration.
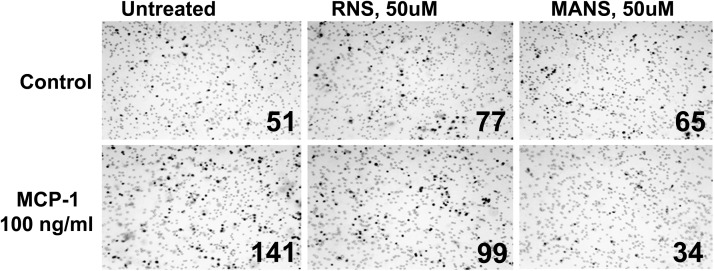
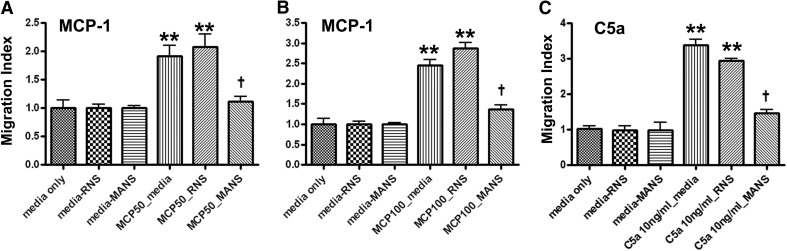
Effects of pretreatment for 15 min with the MANS or RNS peptide (each at 50 μM) on MCP-1- or C5a-induced cell migration were assessed. Fig. 2 shows micrographs of representative fields of cells that migrated in response to MCP-1. Pretreatment with MANS, but not the RNS control peptide, attenuated MCP-1- and C5a-induced migration (Fig. 3).
Figure 2. MCP-1 acts as a chemoattractant for J774A.1 cells.
The MANS, but not the RNS, peptide inhibits cell migration. Cells were untreated or treated with 50 μM peptide prior to adding MCP-1 in a Transwell migration assay. Cells that migrated through the pores to the lower side of the insert membrane, were fixed and counted at 40× magnification. That number is given on each panel. Contrast was enhanced for this figure after the panels were assembled to clearly define the migrated cells from the visible membrane pores.
Figure 3. Inhibitory effect of MANS peptide on MCP-1 induced migration.
J774A.1 cells were pretreated with 50 μM MANS or RNS. Migration was measured in response to: (A) MCP-1 (50 ng/ml); (B) MCP-1 (100 ng/ml); or (C) C5a (10 ng/ml). Cells were exposed for 3 h, at which time, migrating cells were quantified. Data represent mean ± sem; n = 3; **significantly different from media control; †significantly different from stimulated/media control; P < 0.05.
Effects of MCP-1, PMA, and C5a on phosphorylation of MARCKS in J774A.1 cells.
As a MARCKS-related peptide attenuated migration of J774A.1 cells, as shown above, the effects of the chemoattractants on MARCKS phosphorylation were assessed. Stimulation of J774A.1 cells with 100 ng/ml MCP-1, PMA (100 nm), or C5a (10 ng/ml) for 10–180 s resulted in rapid phosphorylation of MARCKS, followed by quick dephosphorylation (Fig. 4).
Figure 4. Western blot analysis of expression of phosphorylated MARCKS and total MARCKS protein.
J774A.1 cells were exposed to: medium only; PMA (100 nM); MCP-1 (100 ng/ml); or C5a (10 ng/ml). Cells were phosphorylated (P) rapidly in response to all three stimuli compared with medium control. Blots are representative of three replicate experiments.
Effects of MCP-1 on intracellular translocation of MARCKS in J774A.1 cells.
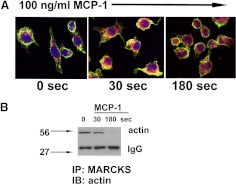
Phosphorylation of MARCKS can result in its translocation from the plasma membrane to the cytosol in various cell types [16]. Here, the intracellular movement of MARCKS and its interaction with the cytoskeletal protein, actin, were analyzed visually via confocal microscopy after exposure of J774A.1 cells to MCP-1, which was shown above to enhance phosphorylation of MARCKS and provoke directed migration in these cells. MARCKS appeared to translocate from plasma membrane to cytoplasm at time-points of 10 and 180 s after exposure of the cells (Fig. 5A). Actin also appeared to respond rapidly to MCP-1 in these cells; it was observed to spread throughout the cell and extend to the cell periphery and into cellular protrusions. As is apparent from Fig. 5A, MARCKS and actin appeared to colocalize near membranes and in the cytosol of stimulated cells 30 s after MCP-1 exposure. Additional immunoprecipitation studies of MARCKS and actin bore this out: as illustrated in Fig. 5B, actin appeared to associate with MARCKS at 30 s after MCP-1 exposure, similar to the confocal microscopic analysis. This association seemed transient, however, as interactions between actin and MARCKS were not apparent at later time-points.
Figure 5. MARCKS and actin localization and intracellular movement in response to MCP-1 (100 ng/ml) in J774A.1 cells.
(A) Cells were stained for MARCKS (red), actin (green), or nucleus (blue) and examined by confocal microscopy as described. Regions in which MARCKS and actin overlap appear yellow. In response to chemokine stimulation, MARCKS translocates to the cytoplasm within the cells, where actin appears as well at 30 s. Representative fields are shown. Original magnification, 600×. (B) Western blot probed for actin after immunoprecipitation (IP) with MARCKS antibody. The time-points correspond to those in A and show a decrease in interaction between the two proteins at 180 s. IB, Immunoblot.
Primary mouse macrophages
MANS peptide inhibits migration of primary mouse macrophages in response to MCP-1.
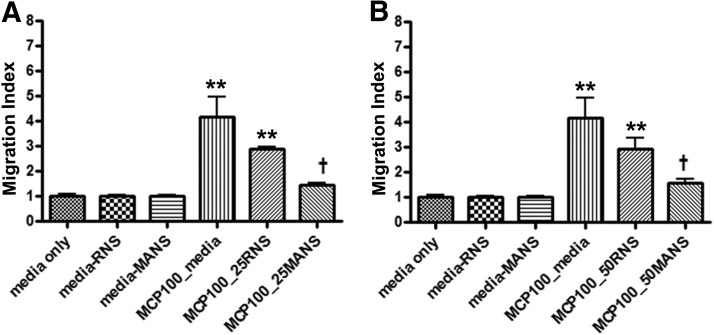
The optimal concentration of MCP-1 to effectively induce migration of isolated primary mouse macrophages was determined in preliminary experiments to be between 25 and 100 ng/ml (data not shown). A role for MARCKS protein in this process was investigated by exposing the cells to the MANS or RNS peptide (25 and 50 μM) for 15 min) prior to exposure to 100 ng/ml MCP-1 for 3 h. As illustrated in Fig. 6, MANS significantly (P<0.05) inhibited the ability of the cells to migrate into the lower wells toward MCP-1, whereas the RNS control peptide had a slight but statistically insignificant effect. These results suggest that MARCKS protein is involved in directed migration of primary macrophages in vitro.
Figure 6. Inhibitory effect of MANS peptide on MCP-1-induced migration in primary mouse macrophages.
Isolated cells were pretreated with: (A) 25 μM or (B) 50 μM MANS or RNS peptide for 15 min. Migration was measured in response to MCP-1 (100 ng/ml). Cells were exposed for 3 h, at which time, migrating cells were quantified. Data represent mean ± sem; n = 3; **significantly different from media control; †significantly different from MCP-1/media control; P < 0.05.
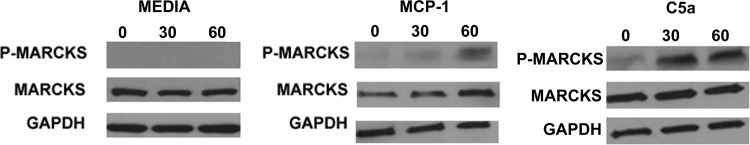
Effect of MCP-1 and C5a on MARCKS phosphorylation in primary mouse macrophages.
Protein expression of phosphorylated MARCKS after exposure to MCP-1 or C5a was investigated by Western blotting. Unstimulated primary macrophages did not express detectable amounts of phosphorylated MARCKS. Cells exposed to MCP-1 (100 ng/ml) or C5a (10 ng/ml), concentrations that provoked directed migration of the cells as described above, also enhanced rapid phosphorylation of MARCKS, which was apparent within 1 min after exposure (Fig. 7).
Figure 7. Western blots of phosphorylated MARCKS protein expression.
Primary murine macrophages were exposed to medium only, MCP-1 (100 ng/ml), or C5a (10 ng/ml). The cells were phosphorylated rapidly (within 30–60 s) upon exposure to either chemmoattractant. Blots are representative of five separate experiments.
Cytotoxicity.
All reagents at the concentrations used were tested for cytotoxicity using a Promega Cytotox 96 assay kit, according to the manufacturer's instructions. The data were expressed as the ratio of released LDH to total LDH. There was little to no cytotoxic effect observed for any of the reagents tested at the concentrations used in the studies (data not shown).
DISCUSSION
Chemoattraction of inflammatory leukocytes, such as macrophages and neutrophils, leading them to injured or inflamed tissues, is an essential step in the host response to infection, but exacerbated inflammation can cause severe local cell and tissue damage. The actual movement of macrophages in response to chemoattractant stimulation involves a series of steps, including protrusion of filopodia and lamellipodia at the leading front toward the chemoattractant gradient, adhesion of the protruding edge to the substratum via focal adhesion complexes, contraction of the cytoplamic actomyosin complex, and finally, release from contact sites at the tail of the cell [17, 18]. This type of movement clearly requires integral involvement of the cytoskeleton, and thus, MARCKS, which binds to actin and myosin and cross-links actin filaments [11, 19], appeared to be a reasonable molecule to investigate as potential importance in the migratory process. This hypothesis was reinforced by published studies showing MARCKS to be involved integrally in migration of various cells types. For example, MARCKS plays a major role in regulating directed migration of vascular endothelium [20], motility and metastatic potential of melanoma [21], and cholangiocarcinoma [22] cell lines and myoblast migration during myogenesis [23]. In many of these studies, the phosphorylation of MARCKS and its translocation to the cytoplasm were shown to be involved in the response, and inhibiting MARCKS function via, for example, gene silencing [22, 23] also attenuated directed migration and motility. Additionally, it has been shown that down-regulation of expression of MRP in the macrophage-like RAW 264.7 cell line resulted in marked reduction in chemotaxis toward MCP-1 or ECM proteins [24].
In recent studies from our laboratory, MARCKS was shown to be involved in directed migration of isolated human neutrophils [7], as well as murine bone marrow-derived mesenchymal stem cells [10]. In these studies, the role of MARCKS in the migration process was shown via use of a peptide identical to the conserved amino terminus of MARCKS, the MANS peptide, which attenuated migration of both cell types in a concentration-dependent manner when the cells were stimulated to migrate in response to fMLF, IL-8, or leukotriene B4 in the case of neutrophils, or C5a, stromal cell-derived factor-1α, or MCP-1 in the stem cell studies, concomitant with enhanced and rapid MARCKS phosphorylation.
The chemokines, MCP-1 and C5a, were used in the present study to induce migration of J774A.1 cells and primary mouse macrophages. MCP-1 is typically expressed in tissues during inflammation and is induced in a variety of cell types by proinflammatory mediators, such as TNF-α, IL-1, or endotoxin [25, 26]. C5a exerts its chemotactic effect on various immune cells, including monocytes, as well as on endothelial cells [27, 28]. Interestingly, MCP-1 and C5a exert their effects through GPCRs, specifically, MCP-1/CCR2 and C5a/C5aR. These receptors, once activated, trigger a set of cellular reactions that result in inositol triphosphate formation, intracellular calcium release, and PKC activation [29, 30]. This signaling pathway could be the link between MCP-1 and C5a binding to receptors on the cell surface and resultant PKC-triggered phosphorylation of MARCKS, which may promote changes in the cytoskeleton and facilitate or drive cell spreading and migration [31].
The results of the studies reported here indicate that MARCKS protein is an important regulator of directed migration of mouse macrophages in vitro. Firstly, exposure of J774A.1 cells or primary murine macrophages to physiologically relevant chemoattractants, MCP-1 or C5a, provoked rapid phosphorylation of MARCKS within the cells. Secondly, preincubation of either cell type with a peptide identical to the amino terminus of MARCKS (the MANS peptide), shown previously to block function of MARCKS in various cell types [7, 8, 11–13], also attenuated directed migration of the cells in response to either chemoattractant, providing additional evidence that MARCKS (and probably phosphorylation of MARCKS) plays a role in regulating macrophage movement along a chemoattractant gradient. As MARCKS is known to bind to and cross-link actin, movement of actin in J774A.1 cells in response to MCP-1 also was investigated; actin appeared to respond to MCP-1 by moving out into the cell periphery and into cell processes and protrusions, most probably playing a role in cell movement. MARCKS also appeared to translocate to the cytoplasm from sites near the plasma membrane in response to MCP-1, and associations between MARCKS and actin in the migrating cells were visually apparent (as observed by confocal microscopy). Additional coimmunoprecipitation studies using antibodies to MARCKS and actin supported this, as MARCKS-actin associations were obvious at 30 s after stimulation by MCP-1 in these cells and appeared to dissipate after 3 min. Thus, it would appear that MARCKS phosphorylation and reorganization of actin seem to be temporally, biochemically, and visually connected, and these two proteins appear to colocalize as a first step in migrating macrophages.
Given the findings described above, an obvious question to address is the actual mechanism(s) whereby MARCKS regulates macrophage migration. Relatedly, why would inhibition of MARCKS function with the MANS peptide, which as it is identical to the MARCKS N-terminus possibly affects MARCKS membrane binding [12], reduce migration? Whereas the exact role played by MARCKS in regulating arrangement of the actin cytoskeleton is not clear, there are several potential mechanisms, whereby disruption of MARCKS binding to membranes could affect cytoskeletal structure and function. MARCKS has been suggested as an important factor in anchoring the actin cytoskeleton to the plasma membrane; therefore, dissociation of MARCKS from the plasma membrane or disruption of MARCKS membrane-binding function can have the downstream effect of detaching actin microfilaments from membranes and in turn, disrupt or negatively affect actin distribution and function [19]. Relatedly, MARCKS phosphorylation and translocation from membrane to cytosol have been shown to regulate the dynamics of actin cytoskeletal organization [11], as well as local actin filament structure and integrin-mediated muscle cell spreading [3, 4]. Another possibility is that MARCKS is involved in the movement of actin within cells by assisting in the association of actin filaments to other cytoskeletal proteins: MARCKS has been shown to colocalize with membrane-associated cytoskeletal proteins, such as tetraspanins, proteins that form membrane complexes specifically with integrins and can play major roles in integrin-mediated cellular migration [32, 33], as well as with the cytoskeletal proteins, vinculin and talin [34]. One actin-dependent action of these cells that was not affected by the MANS or RNS peptide was phagocytosis, as pretreatment with either peptide did not affect phagocytosis by these cells in a preliminary study (data not shown).
Another potential mechanism that may be relevant to this response, could be the reported MARCKS-related sequestering of PI(4,5)P2 in membrane lipid rafts; when MARCKS is phosphorylated and translocates to the cytoplasm, there is increased availability of PI(4,5)P2, which can lead to reactivation of cortical actin microfilaments and consequent reorganization of cellular actin [21, 35–37]. Thus, any or all of these mechanisms might come into play in assessing how MARCKS can regulate cytoskeletal activity and consequent migration of macrophages.
In conclusion, the results of these studies indicate that a peptide identical to the highly conserved N-terminus of MARCKS protein that has been shown to attenuate MARCKS function, inhibits directed migration of J774A.1 cells and primary murine macrophages in response to PMA, MCP-1, or C5a. A pathway involving phosphorylation of MARCKS in response to these chemoattractants, followed by its translocation to the cytoplasm, leading, in turn, to reorganization of cellular actin and resultant regulation of macrophage migration, is indicated. Based on the results, one could hypothesize that the MANS peptide interferes with the ability of the N-terminal region of cellular MARCKS to bind to the inner face of the plasma membrane in these cells and to thereby affect its normal function in regulating cytoskeletal organization. The precise mechanism by which MARCKS is involved in macrophage migration remains to be elucidated.
ACKNOWLEDGMENTS
This work was supported by grant R37 HL36982 from U.S. National Institutes of Health. We acknowledge Drs. Troy Ghashghaei and Ben Jaquart for providing training on the C1 Nikon confocal microscope at the Laboratory for Advanced Electron and Light Optical Methods at North Carolina State University, College of Veterinary Medicine.
Footnotes
- MA
- N-terminal myristate acid
- MANS
- myristoylated N-terminal sequence
- MARCKS
- myristoylated alanine-rich C kinase substrate
- MRP
- myristoylated alanine-rich C kinase substrate-related protein
- PI(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- RNS
- random N-terminal sequence
AUTHORSHIP
T.D.G. developed the study design, collected and analyzed the data, and prepared the manuscript. J.P. assisted with confocal imaging experiments. Q.Y. assisted with isolation of murine lung macrophages. All authors contributed significantly to the design of the study, collection and assessment of data, and development of this paper. All authors approved the final manuscript.
DISCLOSURES
K.B.A. holds 150,000 founders' shares of a start-up biotech company, BioMarck, and serves as a scientific consultant and member of the scientific advisory board without monetary compensation. He receives over $100,000 yearly in research grants from the U.S. National Institutes of Health and U.S. Environmental Protection Agency. He is editor-in-chief of the American Journal of Respiratory Cell and Molecular Biology and receives a stipend of <$100,000/year from the American Thoracic Society for this. All other authors declare that they have no competing interests.
REFERENCES
- 1. Sergejeva S., Ivanov S., Lotvall J., Linden A. (2005) Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 33, 248–253 [DOI] [PubMed] [Google Scholar]
- 2. Spizz G., Blackshear P. J. (2001) Overexpression of the myristoylated alanine-rich C-kinase substrate inhibits cell adhesion to extracellular matrix components. J. Biol. Chem. 276, 32264–32273 [DOI] [PubMed] [Google Scholar]
- 3. Disatnik M. H., Boutet S. C., Lee C. H., Mochly-Rosen D., Rando T. A. (2002) Sequential activation of individual PKC isozymes in integrin-mediated muscle cell spreading: a role for MARCKS in an integrin signaling pathway. J. Cell Sci. 115, 2151–2163 [DOI] [PubMed] [Google Scholar]
- 4. Disatnik M. H., Boutet S. C., Pacio W., Chan A. Y., Ross L. B., Lee C. H., Rando T. A. (2004) The bi-directional translocation of MARCKS between membrane and cytosol regulates integrin-mediated muscle cell spreading. J. Cell Sci. 117, 4469–4479 [DOI] [PubMed] [Google Scholar]
- 5. Aderem A. (1992) Signal transduction and the actin cytoskeleton: the roles of MARCKS and profilin.Trends Biochem. Sci. 17, 438–443 [DOI] [PubMed] [Google Scholar]
- 6. Blackshear P. J., Verghese G. M., Johnson J. D., Haupt D. M., Stumpo D. J. (1992) Characteristics of the F52 protein, a MARCKS homologue. J. Biol. Chem. 267, 13540–13546 [PubMed] [Google Scholar]
- 7. Eckert R. E., Neuder L. E., Park J., Adler K. B., Jones S. L. (2010) Myristoylated alanine-rich C-kinase substrate (MARCKS) protein regulation of human neutrophil migration. Am. J. Respir. Cell Mol. Biol. 42, 586–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foster W. M., Adler K. B., Crews A. L., Potts E. N., Fischer B. M., Voynow J. A. (2010) MARCKS-related peptide modulates in vivo secretion of airway Muc5ac. Am. J. Physiol. Lung Cell. Mol. Physiol. 299, L345–L352 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Damera G., Jester W. F., Jiang M., Zhao H., Fogle H. W., Mittelman M., Haczku A., Murphy E., Parikh I., Panettieri R. A., Jr., (2010) Inhibition of myristoylated alanine-rich C kinase substrate (MARCKS) protein inhibits ozone-induced airway neutrophilia and inflammation. Exp. Lung Res. 36, 75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller J. F., Lankford S. M., Adler K. B., Brody A. R. (2010) (2010) Mesenchymal stem cells require MARCKS protein for directed chemotaxis in vitro. Am. J. Respir. Cell Mol. Biol. 43, 253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y., Martin L. D., Spizz G., Adler K. B. (2001) MARCKS protein is a key molecule regulating mucin secretion by human airway epithelial cells in vitro. J. Biol. Chem. 276, 40982–40990 [DOI] [PubMed] [Google Scholar]
- 12. Singer M., Martin L. D., Vargaftig B. B., Park J., Gruber A. D., Li Y., Adler K. B. (2004) A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat. Med. 10, 193–196 [DOI] [PubMed] [Google Scholar]
- 13. Agrawal A., Rengarajan S., Adler K. B., Ram A., Ghosh B., Fahim M., Dickey B. F. (2007) Inhibition of mucin secretion with MARCKS-related peptide improves airflow obstruction in a mouse model of asthma. J. Appl. Physiol. 102, 399–405 [DOI] [PubMed] [Google Scholar]
- 14. Myat M. M., Anderson S., Allen L. A., Aderem A. (1997) MARCKS regulates membrane ruffling and cell spreading. Curr. Biol. 7, 611–614 [DOI] [PubMed] [Google Scholar]
- 15. Yue L., Lu S., Garces J., Jin T., Li J. (2000) Protein kinase C-regulated dynamitin-macrophage-enriched myristoylated alanine-rich C kinase substrate interaction is involved in macrophage cell spreading. J. Biol. Chem. 275, 23948–23956 [DOI] [PubMed] [Google Scholar]
- 16. Thelen M., Rosen A., Nairn A. C., Aderem A. (1991) Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature 351, 320–322 [DOI] [PubMed] [Google Scholar]
- 17. Jones G. E. (2000) Cellular signaling in macrophage migration and chemotaxis. J. Leukoc. Biol. 68, 593–602 [PubMed] [Google Scholar]
- 18. Sheetz M. P., Felsenfeld D., Galbraith C. G., Choquet D. (1999) Cell migration as a five-step cycle. Biochem. Soc. Symp. 65, 233–243 [PubMed] [Google Scholar]
- 19. Hartwig J. H., Thelen M., Rosen A., Janmey P. A., Nairn A. C., Aderem A. (1992) MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature 356, 618–622 [DOI] [PubMed] [Google Scholar]
- 20. Kalwa H., Michel T. (2011) The MARCKS protein plays a critical role in phosphatidyinositol 4,5-biphosphate metabolism and directed cell movement in vascular endothelial cells. J. Biol. Chem. 286, 2320–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen X., Rotenberg S. A. (2010) PhosphoMARCKS drives motility of mouse melanoma cells. Cell. Signal. 22, 1097–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Techasen A., Loilome W., Namwat N., Takahashi E., Sugihara E., Puapairoj A., Miwa M., Saya H., Yongvanit P. (2010) Myristoylated alanine-rich C kinase substrate phosphorylation promotes cholangiocarcinoma cell migration and metastasis via the protein kinase C-dependent pathway. Cancer Sci. 101, 658–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dedieu S., Mazeres G., Poussard S., Brustis J-J., Cottin P. (2003) Myoblast migration is prevented by a calpain-dependent accumulation of MARCKS. Biol. Cell 95, 615–623 [DOI] [PubMed] [Google Scholar]
- 24. Chun K. R., Bae E. M., Kim J. K., Suk K., Lee W. H. (2009) Suppression of the lipopolysaccharide-induced expression of MARCKS-related protein (MRP) affects transmigration in activated RAW264.7 cells. Cell. Immunol. 256, 92–98 [DOI] [PubMed] [Google Scholar]
- 25. Proost P., Wuyts A., Van Damme J. (1996) Human monocyte chemotactic proteins-2 and -3: structural and functional comparison with MCP-1. J. Leukoc. Biol. 59, 67–74 [DOI] [PubMed] [Google Scholar]
- 26. Strieter R. M., Kunkel S. L. (1993) The immunopathology of chemotactic cytokines. Adv. Exp. Med. Biol. 351, 19–28 [DOI] [PubMed] [Google Scholar]
- 27. Kurihara R., Yamaoka K., Sawamukai N., Shimajiri S., Oshita K., Yukawa S., Tokunaga M., Iwata S., Saito K., Chiba K., Tanaka Y. (2010) C5a promotes migration, proliferation, and vessel formation in endothelial cells. Inflamm. Res. 59, 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hartmann K., Henz B. M., Kruger-Krasagakes S., Kohl J., Burger R., Guhl S., Haase I., Lippert U., Zuberbier T. (1997) C3a and C5a stimulate chemotaxis of human mast cells. Blood 89, 2863–2870 [PubMed] [Google Scholar]
- 29. Lodi P. J., Garrett D. S., Kuszewski J., Tsang M. L., Weatherbee J. A., Leonard W. J., Gronenborn A. M., Clore G. M. (1994) High-resolution solution structure of the β chemokine hMIP-1 β by multidimensional NMR. Science 263, 1762–1767 [DOI] [PubMed] [Google Scholar]
- 30. Sallusto F., Baggiolini M. (2008) Chemokines and leukocyte traffic. Nat. Immunol. 9, 949–952 [DOI] [PubMed] [Google Scholar]
- 31. Larsson C. (2006) Protein kinase C and the regulation of the actin cytoskeleton. Cell. Signal. 18, 276–284 [DOI] [PubMed] [Google Scholar]
- 32. Berditchevski F., Odintsova E. (1999) Characterization of integrin-tetraspanin adhesion complexes: role of tetraspanins in integrin signaling. J. Cell Biol. 146, 477–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Estrada-Bernal A., Gatlin J. C., Sunpaweravong S., Pfenninger K. H. (2009) Dynamic adhesions and MARCKS in melanoma cells. J. Cell Sci. 122, 2300–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosen A., Nairn A. C., Greengard P., Cohn Z. A., Aderem A. (1989) Bacterial lipopolysaccharide regulates the phosphorylation of the 68K protein kinase C substrate in macrophages. J. Biol. Chem. 264, 9118–9121 [PubMed] [Google Scholar]
- 35. Glaser M., Wanaski S., Buser C. A., Boguslavsky V., Rashidzada W., Morris A., Rebecchi M., Scarlata S. F., Runnels L. W., Prestwich G. D., Chen J., Aderem A., Ahn J., McLaughlin S. (1996) Myristoylated alanine-rich C kinase substrate (MARCKS) produces reversible inhibition of phospholipase C by sequestering phosphatidylinositol 4,5-bisphosphate in lateral domains. J. Biol. Chem. 271, 26187–26193 [DOI] [PubMed] [Google Scholar]
- 36. Wang J., Arbuzova A., Hangyas-Mihalyne G., McLaughlin S. (2001) The effector domain of myristoylated alanine-rich C kinase substrate binds strongly to phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 276, 5012–5019 [DOI] [PubMed] [Google Scholar]
- 37. Laux T., Fukami K., Thelen M., Golub T., Frey D., Caroni P. (2000) GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J. Cell Biol. 149, 1455–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]