Induction of TLR4/LPS tolerance by acute binge alcohol introduces a new aspect of the complex biological effects of this commonly used substance.
Keywords: monocytes, TNF-α, NF-κB, p50
Abstract
Acute alcohol binge results in immunosuppression and impaired production of proinflammatory cytokines, including TNF-α. TNF-α production is induced by LPS, a TLR4 ligand, and is tightly regulated at various levels of the signaling cascade, including the NF-κB transcription factor. Here, we hypothesized that acute alcohol induces TLR4/LPS tolerance via Bcl-3, a nuclear protein and member of the NF-κB family. We found that acute alcohol pretreatment resulted in the same attenuating effect as LPS pretreatment on TLR4-induced TNF-α production in human monocytes and murine RAW 264.7 macrophages. Acute alcohol-induced Bcl-3 expression and IP studies revealed increased association of Bcl-3 with NF-κB p50 homodimers in alcohol-treated macrophages and in mice. ChIP assays revealed increased occupancy of Bcl-3 and p50 at the promoter region of TNF-α in alcohol-pretreated cells. To confirm that the Bcl-3–p50 complex regulates transcription/production of TNF-α during acute alcohol exposure, we inhibited Bcl-3 expression using a targeted siRNA. Bcl-3 knockdown prevented the alcohol-induced inhibition of TNF-α mRNA and protein production. In a mouse model of binge alcohol, an increase in Bcl-3 and a concomitant decrease in TNF-α but no change in IL-10 production were found in mice that received alcohol followed by LPS challenge. In summary, our novel data suggest that acute alcohol treatment in vitro and in vivo induces molecular signatures of TLR4/LPS tolerance through the induction of Bcl-3, a negative regulator of TNF-α transcription via its association with NF-κB p50/p50 dimers.
Introduction
Alcohol consumed acutely in excessive amounts, also referred to as binge drinking, is the most common form of alcohol abuse, and it results in impaired immune responses (http://www.niaaa.nih.gov/Resources/DatabaseResources/QuickFacts/Youth/Pages/ythdrk6b.aspx) [1]. Previous studies from in vitro and in vivo models demonstrated that acute alcohol down-regulates LPS-induced production of proinflammatory cytokines (TNF-α, IL-6, and IL-1β) and up-regulates anti-inflammatory cytokines, such as IL-10 [2, 3]. These cytokines play a central role in antimicrobial defense; hence, the imbalance of pro- and anti-inflammatory cytokines after acute alcohol exposure contributes to increased susceptibility to infections [4, 5].
LPS is a TLR4 ligand, and activation of TLR4 induces a wide array of downstream signaling cascades. TLR4 activation is elemental for the development of immunity; however, repeated activation of TLR4 results in hyporesponsiveness to subsequent stimulation with TLR4 or other TLR ligands (homo- or heterostimulation), a phenomenon called “LPS-homo or hetero-tolerance” [6, 7]. The transcription factor NF-κB is central to TLR-mediated signaling pathways; therefore, regulation of NF-κB is imperative to TLRs responses [8]. NF-κB is composed of five members, c-Rel, RelA (p65), RelB, p50, and p52 [8], which dimerize further into homo- and heterodimeric complexes. The dimer exchange at the promoter region plays a crucial role in the transcriptional control of target genes, including TNF-α [8].
Bcl-3, a member of the IκB family of proteins, is confined mainly to the nucleus and is not degraded upon NF-κB activation [9]. It interacts specifically with NF-κB p50 and p52 homodimers and enhances p50 homodimer DNA binding [10]. The p50 subunit lacks a DNA transactivation domain, and it negatively regulates NF-κB-mediated gene expression [9, 11, 12]. In recent years, a role of Bcl-3 has been revealed in LPS tolerance via its ability to stabilize the p50 homodimer and thus, has been identified as a negative regulator of TLR4 signaling [13].
Previous studies from our laboratory have shown that acute alcohol treatment inhibits LPS-induced TNF-α production [14]. However, the underlying molecular mechanisms of alcohol-induced inhibition of TLR4/LPS signaling are poorly understood; therefore, in this study, we tested the hypothesis that acute alcohol induces TLR4/LPS tolerance and evaluated the role of Bcl-3 in acute alcohol-induced LPS tolerance. Here, we report that acute alcohol pretreatment increases Bcl-3 expression and promotes Bcl-3 and p50 interactions and the binding of these two proteins to the NF-κB consensus motifs within the TNF-α promoter. Our novel data indicate that acute alcohol activates molecular signatures of TLR4/LPS tolerance through induction of Bcl-3 and its interaction with p50 homodimers.
MATERIALS AND METHODS
Cell culture and reagents
Murine macrophage RAW 264.7 cells were grown in DMEM medium supplemented with antibiotics and 10% FBS (HyClone, Logan, UT, USA) at 37°C with 5% CO2. Human monocytes were isolated as described previously [15]. For induction of tolerance, cells were treated with 25 mM (human monocytes) or 50 mM alcohol (RAW 264.7 cells) or 100 ng/ml LPS, as indicated in figure legends. LPS- or alcohol-tolerant and LPS or alcohol-responsive (normal) cells were washed twice with HBSS and then challenged with 100 ng/ml LPS for the indicated times. The anti-p65, p50, Bcl-3, and IgG antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The ChIP kit was from Active Motif (Carlsbad, CA, USA). The TrueBlot beads were from eBioscience (San Diego, CA, USA). Bcl-3 siRNA, scramble siRNA, and transfection reagents were purchased from Applied Biosystems (Carlsbad, CA, USA).
Animal studies
Eight-week-old C57BL/6 female mice (n=4–6) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The mice were housed and cared for in the animal facility in accordance with the protocol approved by the Institutional Animal Use and Care Committee of the University of Massachusetts Medical School (Worcester, MA, USA). The mice were kept on a chow diet and were orally gavaged with alcohol at 5 g/kg (20% v/v in saline) every 20–24 h for 3 days. The amount of alcohol used in the study was in accordance with previous reports [16]. The control animals received an equal amount of calories, which were substituted with sucrose. After 3 days, some mice were i.p. injected with LPS (0.5 mg/kg), 3 h prior to sacrifice. Blood was collected in serum collection tubes (BD Biosciences, San Jose, CA, USA), processed within 1 h of collection, and stored at −80°C. At the conclusion of experiment, mice were euthanized and sections of livers were collected in RNA later for RNA analysis and snap-frozen in liquid nitrogen for protein analysis.
Nuclear and whole cell protein extraction, EMSA, and Western blot analysis
RAW 264.7 macrophages were stimulated with alcohol (50 mM) or LPS (100 ng/ml) or pretreated with alcohol and then challenged with LPS, as indicated in figure legends. Whole cell, nuclear, and cytoplasmic proteins were extracted as described [15]. Protein concentration was measured using Bio-Rad dye reagent (Bio-Rad Laboratories, Hercules, CA, USA). Nuclear protein (5 μg) was used to carry out EMSA [15]. For Western blot analysis, 20–30 μg nuclear or whole cell protein was subjected to electrophoresis on 10% SDS-polyacrylamide gel and blotted onto nitrocellulose membrane. After blocking, the blot was probed with primary antibody followed by HRP-conjugated secondary antibody. The immunoreactive proteins were detected using an ECL detection kit from Cell Signaling Technology (Danvers, MA, USA). The same blot was stripped and later probed with loading control antibody.
RNA extraction and real-time PCR analysis
RNA was extracted using the RNeasy mini kit (Qiagen, Valencia, CA, USA). The concentration and purity were determined by Biophotometer (Eppendorf, Hauppauge, NY, USA). RNA was converted to cDNA using RT kit (Promega, Madison, WI, USA) and then subjected to real-time PCR (Eppendorf). The primers were synthesized from Integrated DNA Technologies (Coralville, IA, USA), and the specificity of amplification for each primer set was determined by melt curve analysis. The primer sequences were as follows: 18S, forward 5′-GTAACCCGTTGAACCCCATT-3′ and reverse 5′-CCATCCAATCGGTAGTAGCG-3′; mouse Bcl-3, forward 5′-TATGAAGGGCTCACTGCCCTGCA-3′ and reverse 5′-ACTGCATCGATGTCCGCGCC-3′; human Bcl-3, forward 5′-CTATACCCCATGATGTGCCC-3′ and reverse 5′-AGCAATATGGAGAGGCGTGT-3′. Mouse TNF-α and IL-10 primers used in the study were same as described [17]. The data were normalized to internal control 18S, and fold change was calculated compared with unstimulated cells or saline-treated control group.
IP
For IP, equal amounts (200–300 μg) of nuclear or whole cell protein were used and precleared with 50 μl TrueBlot anti-rabbit IgG IP beads (eBioscience). The precleared lysates were incubated with 5 μg anti-p50 antibody, 5 μg anti-Bcl-3 antibody, or IgG as a negative control, overnight at 4°C. The next day, 50 μl TrueBlot anti-rabbit IgG IP beads were added to each sample for 1 h to capture immune complexes. The beads were washed three times with lysis buffer, eluted with sample buffer, and then subjected to Western blotting.
ChIP analysis
RAW 264.7 macrophages were stimulated with LPS (100 ng/ml) or 50 mM alcohol or pretreated with alcohol, followed by LPS stimulation for 30 min, and the ChIP assay was performed with the kit from Active Motif, according to the manufacturer's specifications. Briefly, cells were harvested and fixed with 1% formaldehyde for 10 min at room temperature. Cells were washed with cold PBS and lysed in 1% SDS. The lysates were sonicated to generate DNA fragments ranging from 200 to 1000 bp using a Branson 250 sonicator. The chromatin solution was precleared with protein G agarose beads for 1 h at 4°C and one-tenth of the chromatin was kept as an “input” sample. The remaining chromatin was immunoprecipitated overnight at 4°C with 5 μg anti-Bcl-3 or anti-p50 antibody or IgG (Santa Cruz Biotechnology). The chromatin/antibody complexes captured on the beads were washed and finally eluted with 50 μl elution buffer. The immunoprecipitated and input samples were cross-reversed at 65°C overnight. After treatment with proteinase K and RNase A, the reaction was stopped, and DNA was purified with Qiagen columns (DNA isolation kit) and stored at −20°C until analyzed by semiquantitative PCR, as described below.
Semiquantitative PCR
PCR was performed in a 20-μl vol containing 1 μl ChIP DNA, 1 μM each primer, and 1× buffer containing dNTPs, MgCl2, and FastStart Taq DNA polymerase (Roche Diagnostics, Indianapolis, IN, USA). The PCR conditions were as follows: 1 cycle at 94°C for 5 min, 30 cycles at 94°C, 60°C, and 72°C for 30 s each, and a final cycle at 72°C for 5 min. Equal amounts of PCR products were run on 2% ethidium bromide-stained agarose gel, and images were captured using Quantity One Imager (Bio-Rad Laboratories). The primers used in PCR were designed to amplify NF-κB consensus sequences in the mouse TNF-α promoter region. The primer sequences were: for κ1 site, forward 5′-CGAGAGACCCAAAGGATGAG-3′ and reverse 5′-CTCCCAATCCGTATGACTCC-3′; κ2 site, forward 5′-GAGGCTCCGTGGAAAACTCACTTG-3′ and reverse 5′-GCAGAGCAGCTTGAGAGTTGGGAA-3′; κ3 site, forward 5′-CTTCAGCCACTTCCTCCAAG-3′ and reverse 5′-CCATGCCTGTGTCTATTTCCT-3′; and κ4 site, forward 5′-CAGTTCTCAGGGTCCTATACAACACA-3′ and reverse 5′-GGTAGTGGCCCTACACCTCTGTC-3′, respectively.
RNA interference
RAW 264.7 macrophages were transfected with Bcl-3 siRNA or scrambled siRNA (Applied Biosystems) using siPORT NeoFX transfection agent (Applied Biosysytems), according to the manufacturer's instructions. Transfection efficacy was determined by transfecting the cells with GAPDH siRNA. The cells were stimulated and harvested for mRNA and protein analysis, as indicated in figure legends after 48 h and 72 h of transfection, respectively.
ELISA
The amount of TNF-α in culture supernatants was determined by the ELISA kit, according to the manufacturer's instructions (BD PharMingen, San Diego, CA, USA). Serum levels of TNF-α and IL-10 were measured with ELISA kits (BioLegend, San Diego, CA, USA).
Statistical analysis
Statistical significance was evaluated by ANOVA or nonparametric Mann-Whitney test. A P value of <0.05 was considered statistically significant.
RESULTS
Acute alcohol induces TLR4/LPS tolerance in human monocytes and RAW 264.7 macrophages
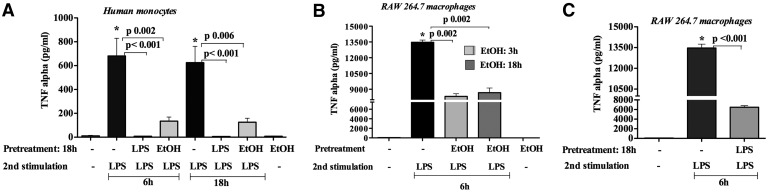
LPS is a potent inducer of inflammatory cytokines, including TNF-α, in vitro and in vivo [15, 18]. LPS is a danger signal for monocytes, and repeated LPS administration results in LPS tolerance, a protective host mechanism to prevent overactivation of inflammatory cytokine responses. Here, we tested the hypothesis that acute alcohol intake could act as a danger signal and result in LPS tolerance in monocytes and macrophages. First, we found that in vitro pretreatment of human monocytes with alcohol or LPS for ∼18 h blunted TNF-α production upon LPS challenge compared with treatment-naïve cells exposed to the same dose or duration of LPS (6 or 18 h; Fig. 1A). Alcohol-alone treatment did not induce TNF-α (Fig. 1A). These findings indicate that acute alcohol pretreatment induces TLR4/LPS tolerance in human monocytes.
Figure 1. Acute alcohol induces TLR4/LPS tolerance in monocytes/macrophages.
(A) Human monocytes (n=4) were pretreated with 25 mM ethanol (EtOH) or 100 ng/ml LPS for 18 h, cells were washed two times with PBS, and fresh medium was added and restimulated with 100 ng/ml LPS for 6 or 18 h. (B) Murine RAW 264.7 macrophages were pretreated with 50 mM ethanol for 3 or 18 h or (C) 100 ng/ml LPS for 18 h, cells were washed two times with PBS, and fresh medium was added and restimulated with 100 ng/ml LPS for 6 h. Cell-free supernatants were collected and analyzed for TNF-α levels by ELISA. Data represent the mean value (sd as error bars) of four independent experiments (*P<0.05 compared with unstimulated cells). ANOVA was used for statistical analysis.
To further evaluate the effect of acute alcohol on macrophages, we next tested RAW 264.7 macrophages that are representatives of hepatic macrophages (Kupffer cells) [17]. Alcohol pretreatment for 18 h followed by LPS challenge for 6 h resulted in decreased, LPS-induced TNF-α production compared with alcohol-naïve cells (Fig. 1B). This observation implies that acute alcohol pretreatment induces LPS tolerance in human monocytes and RAW 264.7 macrophages (Fig. 1A and B). When RAW 264.7 macrophages received an initial LPS stimulation for 18 h followed by a second LPS challenge for 6 h, TNF-α production was dampened significantly compared with the LPS response of unstimulated macrophages (Fig. 1C). As this observation was consistent with the classical “LPS tolerance”, and the alcohol-pretreated cells also showed a similar response in human monocytes and RAW 264.7 macrophages, we were interested to further investigate the molecular mechanism of acute alcohol-induced TLR4 tolerance.
Acute alcohol induces Bcl-3, a molecular signature of LPS tolerance
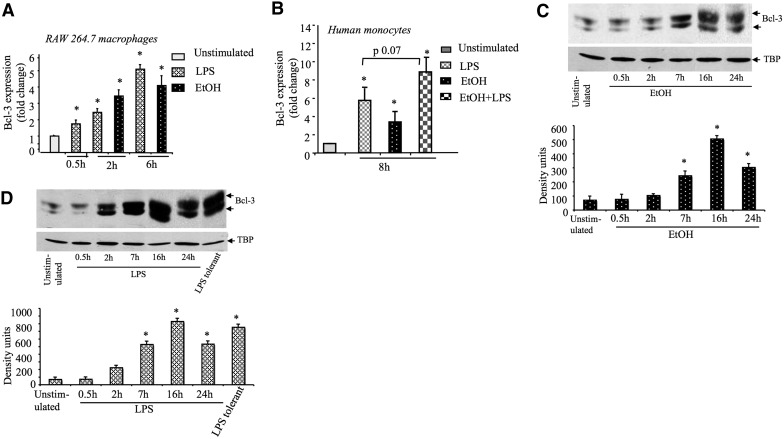
The molecular signature of LPS tolerance in macrophages is a result of a coordinated expression of intracellular proteins that characterize TLR4/LPS tolerance [19]. Bcl-3 has been identified recently as a key regulator of LPS tolerance by stabilizing NF-κB p50 homodimers [13]. To dissect the mechanisms by which acute alcohol induces LPS tolerance, we first evaluated Bcl-3 expression in alcohol-treated RAW 264.7 cells. We found that acute alcohol treatment rapidly increased the Bcl-3 expression, and maximum induction was seen at 6 h of stimulation in alcohol- and LPS- treated RAW 264.7 cells at the mRNA level (Fig. 2A). We also evaluated Bcl-3 expression in human monocytes and indeed, found maximum induction with ∼8 h of stimulation, after treatment with alcohol, LPS, or their combination (Fig. 2B). This observation suggests that acute alcohol induces Bcl-3 in human monocytes as well as in murine macrophages.
Figure 2. Acute alcohol induces Bcl-3 expression.
(A) RAW 264.7 macrophages or (B) human monocytes (n=4) were stimulated with 100 ng/ml LPS or 25 mM (monocytes) or 50 mM (RAW 264.7 cells) ethanol or their combination (EtOH+LPS), and total RNA was extracted and analyzed for Bcl-3 mRNA expression by real-time PCR. Data were normalized to 18S and represent the mean value (sd as error bars) of at least three independent experiments (*P<0.05 vs. unstimulated cells). (C and D) RAW 264.7 macrophages were stimulated with 50 mM ethanol or 100 ng/ml LPS for indicated times, nuclear proteins were extracted and subjected to Western blot analysis with anti-Bcl-3 or loading control antibody [tata-binding protein (TBP)]. To induce LPS tolerance, cells were pretreated with 100 ng/ml LPS for 18 h, washed with PBS two times, and restimulated with LPS for 2 h. The mean density units (sd as error bars in the bar graph) from three experiments are shown (*P<0.05 vs. unstimulated cells). ANOVA (B) or nonparametric Mann Whitney test (A,C–D) was used for statistical analysis.
Next, we assessed the kinetics of nuclear Bcl-3 protein expression in alcohol or LPS-treated RAW 264.7 cells. Baseline Bcl-3 expression in unstimulated RAW 264.7 cells was amplified by alcohol or LPS treatment with similar kinetics (Fig. 2C and D). Alcohol and LPS, respectively, increased Bcl-3 levels starting at 2 h with a maximum increase observed at 16 h. Furthermore, we detected multiple forms of Bcl-3 in alcohol or LPS-treated cells; this observation is consistent with earlier reports in LPS-treated cells and represents different extents of Bcl-3 phosphorylation [10]. In LPS-tolerant cells, Bcl-3 expression was augmented further compared with unstimulated cells in response to LPS challenge (Fig. 2D), indicating the involvement of Bcl-3 in LPS tolerance [13]. We also found increased nuclear p50 after alcohol pretreatment (data not shown).
Acute alcohol down-regulates LPS-induced p65 subunits of NF-κB
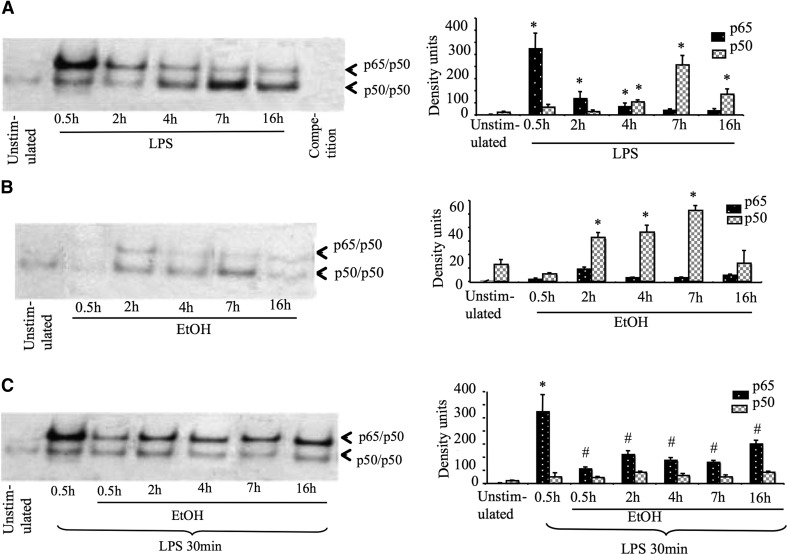
As Bcl-3 was shown to interact with NF-κB, specifically with p50 homodimers in inducing LPS tolerance, next, we evaluated the role of NF-κB activation in alcohol-exposed cells. NF-κB plays a central role in TLR4/LPS signaling and subsequently regulates TNF-α gene transcription. Using EMSA, we determined that LPS stimulation resulted in rapid induction of p65/p50 heterodimers (30 min—2 h), followed by an increase in the p50 homodimers at later stages (maximum induction at 7 h, which remained up to 16h; Fig. 3A). The increase in p50/p50 homodimers is a marker of LPS-tolerant macrophages [13]. We found that acute alcohol treatment (50 mM) alone slightly induced p50/p50 homodimers. In contrast to LPS, there was no significant induction of p65/p50 heterodimers in acute alcohol alone-treated cells (Fig. 3B). Furthermore, when cells were pretreated with alcohol (0.5–16 h) and later challenged with LPS for 30 min, LPS-induced p65/p50 heterodimer induction was blunted significantly compared with LPS alone-treated cells (Fig. 3C), suggesting alcohol induces LPS tolerance at the transcription level.
Figure 3. Acute alcohol down-regulates LPS-induced p65 subunits of NF-κB.
RAW 264.7 macrophages were stimulated with (A) 100 ng/ml LPS or (B) 50 mM ethanol or (C) pretreated with alcohol and then challenged with LPS for 30 min. Equal amount of nuclear proteins were subjected to EMSA. On the left, the mean density units (sd as error bars in the bar graph) from three experiments are shown (*P<0.05 vs. unstimulated cells; #P<0.05 compared with LPS alone-treated cells). Nonparametric Mann Whitney test was used for statistical analysis.
Association of Bcl-3 and p50 after alcohol treatment
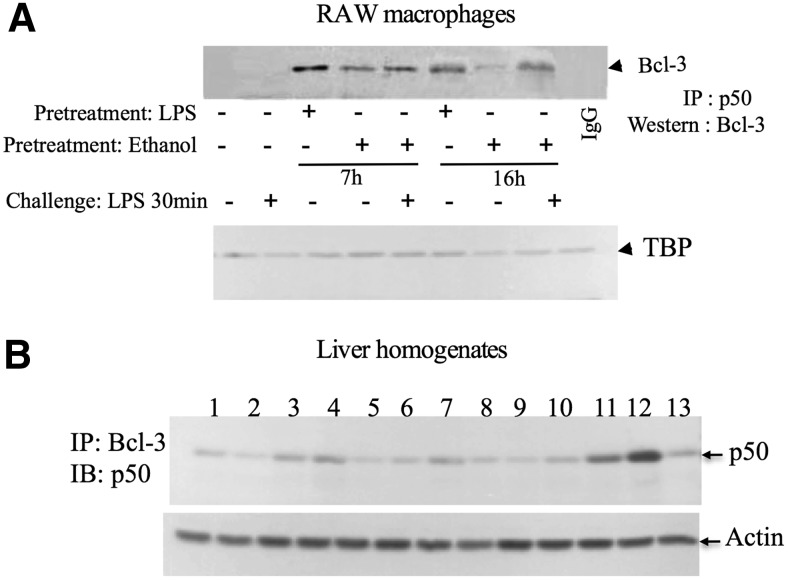
Based on the observation that acute alcohol induces Bcl-3 and p50, we next sought to evaluate whether alcohol treatment promoted the association of Bcl-3 with NF-κB p50. Nuclear extracts from alcohol or LPS or alcohol-pretreated RAW 264.7 cells were immunoprecipitated with anti-p50 antibody or IgG as a negative control and analyzed by Western blot analysis using anti Bcl-3 antibody. LPS stimulation alone for 7 or 16 h but not for 30 min induced a robust induction of Bcl-3–p50 complexes. We found that alcohol treatment alone also resulted in a significant increase in Bcl-3–p50 association compared with untreated cells (Fig. 4A). Furthermore, when the cells were pretreated with alcohol for 7 h followed by LPS stimulation for 30 min, we found even greater association of Bcl-3 with p50 compared with alcohol alone-treated cells (Fig. 4A). Next, we performed IP from liver homogenates of mice that received LPS or alcohol binge. Alcohol treatment resulted in a slight increase in Bcl3–p50 complexes, which was increased further when these mice were challenged with LPS (Fig. 4B). LPS (0.5 mg/kg) alone for 3 h did not induce the Bcl3–p50 complex formation (Fig. 4B). These data indicate that NF-κB p50 is in complex with Bcl-3 and might have a functional role in alcohol-induced LPS tolerance.
Figure 4. Acute alcohol increases Bcl-3– p50 complex formation.
(A) RAW 264.7 macrophages were stimulated with 100 ng/ml LPS or 50 mM ethanol or pretreated with ethanol as indicated and later challenged or not with LPS for 30 min. Equal amounts of nuclear proteins (∼300 μg) were immunoprecipitated with anti-p50 antibody or IgG (a negative control), and immune complexes were captured with anti-Bcl-3 antibody. The results are representative of two independent experiments. (B) Equal amount of whole cell proteins (300 μg) isolated from the livers of saline-treated mice (lanes 1–3), alcohol binge-treated mice (lanes 4–6), LPS-treated mice (lanes 7–9), and alcohol binge-treated mice challenged with LPS (lanes 10–12) were immunoprecipitated with anti-Bcl3 antibody or IgG control (lane 13) and immunoblotted (IB) with anti-p50 antibody. For loading control, equal amount of nuclear (A) or whole cell proteins (B) were loaded on separate gels and probed with anti-tata-binding protein (A) or actin (B).
Bcl3–p50 complexes bind to the TNF-α promoter in acute alcohol-treated cells
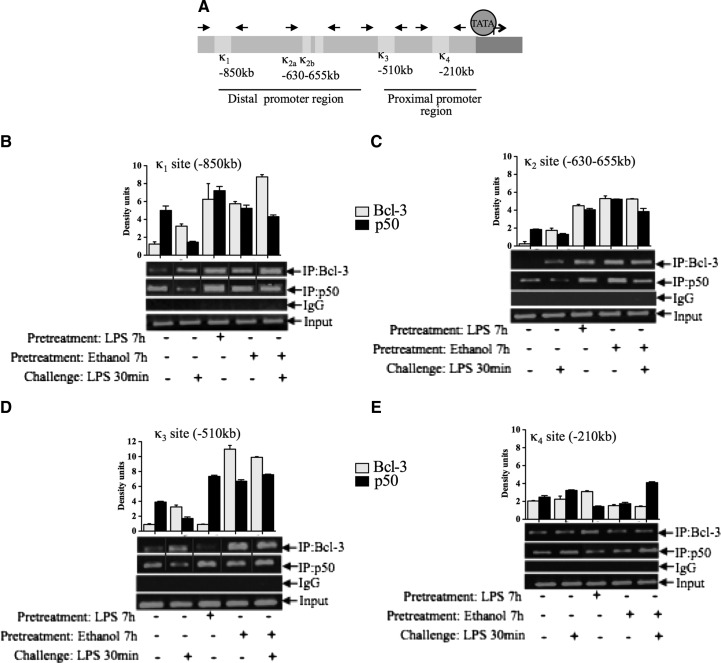
Bcl-3 has been shown to enhance the inhibitory activity of the p50 homodimers and consequently modulate NF-κB transcriptional activity [13]. Previous reports demonstrated that NF-κB binds to four consensus sequences within the murine TNF-α promoter as depicted in Fig. 5A [20, 21]. To assess the effect of alcohol on transcriptional activity of the TNF-α promoter and to elucidate the role of p50 and Bcl-3, we performed ChIP experiments. At κ1–κ3 promoter sites, Bcl-3 was induced by alcohol, LPS, or both, whereas p50, in general, was constitutive in all four κB sites (Fig. 5B–E). At the κ1 (−850 kb) site, alcohol and LPS stimulation (7 h) resulted in increased recruitment of Bcl-3 and p50. Moreover, alcohol pretreatment increased Bcl-3 binding compared with LPS (30 min) or alcohol alone-treated cells (Fig. 5B). Similarly, at the κ2 site (−630 to 655 kb), there was increased Bcl-3 and p50 binding in alcohol or LPS-treated cells (7 h) with no further increase in alcohol-pretreated cells (Fig. 5C). Overall, at the distal region of the TNF-α promoter, an increase in p50 and Bcl-3 binding was seen in alcohol-treated cells. At the proximal promoter region, alcohol or LPS alone (7 h) increased p50 binding compared with unstimulated cells. Furthermore, we found an amplification of p50 binding in alcohol-pretreated cells (Fig. 5D). This observation is in agreement with previous studies, suggesting the involvement of the κ3 site in LPS tolerance [11, 13]. At the κ4 site, which is near the transcription site of TNF-α (−210 kb), Bcl-3 and p50 appear to be constitutive (Fig. 5E). The pretreatment of alcohol further amplified the increase in baseline and as well, LPS (30 min)-induced p50 occupancy, implying a possible role of proximal κB sites in alcohol-induced LPS tolerance (Fig. 5D and E). These results from the ChIP study suggest that acute alcohol-induced TLR4 tolerance is through the up-regulation of the binding of Bcl-3 and p50 at the TNF-α promoter.
Figure 5. Acute alcohol pretreatment increases Bcl-3 and p50 binding at the TNF-α promoter.
(A) Schematic representations of κb sites to the TNF-α promoter. (B–E) RAW 264.7 macrophages were stimulated with 50 mM ethanol or 100 ng/ml LPS or pretreated with alcohol for 7 h and challenged with LPS for 30 min and processed for ChIP analysis. The sonicated chromatin was immunoprecipitated with anti-p50 or anti-Bcl-3 or IgG antibody and reverse cross-linked with NaCl at 65°C, and finally, DNA was eluted with the Qiagen kit. The eluted DNA was analyzed by semiquantitative PCR with the primers specific to κB sites of TNF-α promoter. The densitometery data are shown as a bar graph from two independent experiments. Solid lines (B and D) indicate that samples were run on the same gel, however lanes were broken and pasted to a new position to present the data.
Bcl-3 knockdown prevents acute alcohol-induced inhibition of TNF-α at the mRNA and protein levels
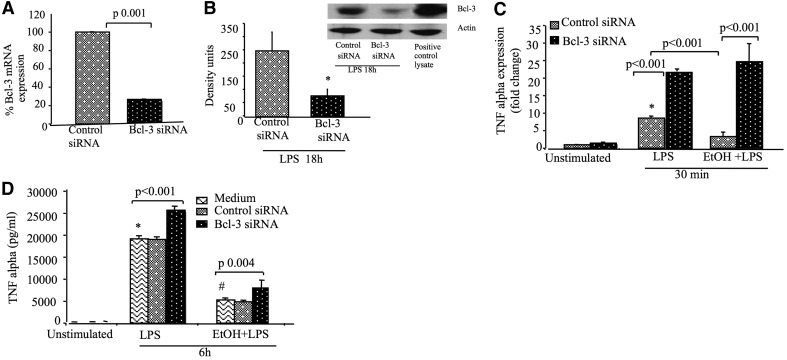
To evaluate the functional role of Bcl-3–p50 interaction in acute alcohol-treated cells, we inhibited Bcl-3 using a siRNA approach. The efficacy of the Bcl-3 siRNA knockdown was >80% at the mRNA level and ∼60% at the protein level (Fig. 6A and B). Bcl-3 knockdown resulted in a significant increase in TNF-α mRNA levels compared with control siRNA-treated cells in response to LPS stimulation (Fig. 6C). Whereas acute alcohol treatment inhibited the LPS-induced TNF-α transcription in control siRNA-treated cells, Bcl-3 siRNA significantly prevented the alcohol-induced inhibition of LPS-induced TNF-α transcription (Fig. 6C). There was also a significant, modest increase in TNF-α production at the protein level after Bcl-3 knockdown in cells treated with LPS or pretreated with alcohol (Fig. 6D). These data demonstrate that Bcl-3 plays a critical role in the regulation of TNF-α during acute alcohol-induced LPS tolerance.
Figure 6. Knockdown of Bcl-3 prevents acute alcohol-induced inhibition of TNF-α.
(A–D) RAW 264.7 macrophages were transfected with Bcl-3 or control siRNA. (A and B) Knockdown efficiency of Bcl-3 siRNA was checked at the mRNA and protein levels by real-time PCR and Western blot analysis, respectively. (C) After 48 h of transfection, cells were stimulated or not with 100 ng/ml LPS or 50 mM ethanol, followed by LPS stimulation for 30 min, and total RNA was harvested and subjected to real-time PCR for TNF-α mRNA levels. The data were normalized to 18S and represent fold change compared with unstimulated cells. (D) For TNF-α production, cells were stimulated or not with LPS or ethanol and LPS for 6 h, and supernatants were collected and analyzed by ELISA after 72 h of the transfection. The data are presented as the mean value (sd as error bars) of at least three independent experiments (*P<0.05 vs. unstimulated cells; #P<0.05 compared with LPS alone-treated cells). ANOVA was used for statistical analysis.
Binge alcohol administration induces Bcl-3 in mice in response to LPS challenge
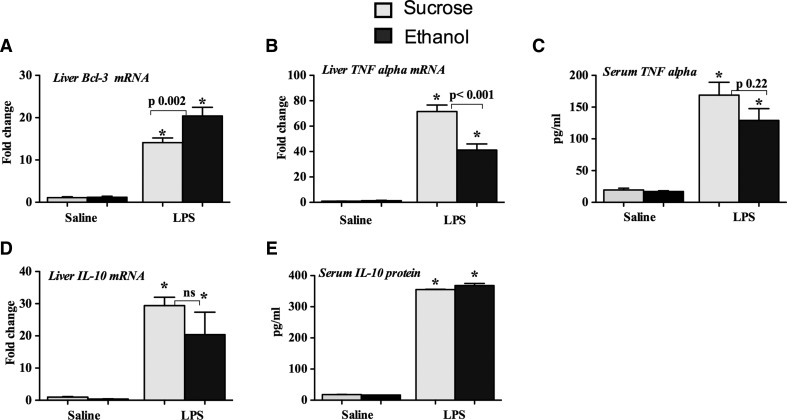
Finally, we extended our in vitro findings to a mouse model of acute alcohol administration. Mice were orally gavaged daily with alcohol for 3 days to mimic alcohol binge conditions. We found induction in Bcl-3 mRNA expression in the livers of mice administered with LPS for 3 h (Fig. 7A). More importantly, alcohol binge significantly augmented LPS-induced Bcl-3 induction (Fig. 7A). This result was consistent with our in vivo findings, where we found increased association between Bcl-3 and p50 in livers of mice after acute alcohol binge followed by a LPS challenge (Fig. 4B). Consistent with the hypothesis of alcohol-induced TLR4/LPS tolerance, alcohol binge inhibited LPS-induced TNF-α mRNA levels in the liver, and serum levels were also decreased (Fig. 7B and C). IL-10, an immunoinhibitory cytokine, was not inhibited by alcohol binge at the mRNA level in the liver (Fig. 7D) or in the serum (Fig. 7E) after LPS challenge Collectively, our in vitro and in vivo results suggest a role of Bcl-3 in alcohol-induced LPS tolerance.
Figure 7. Binge alcohol administration induces Bcl-3 in mice in response to LPS challenge.
Eight-week-old C57BL/6J female mice were orally gavaged with alcohol (n=6; 5 g/kg) or sucrose (n=4), as described in Materials and Methods. Three days later, mice received saline or LPS (0.5 mg/kg) i.p. for 3 h and were euthanized (n=4–6). (A, B, and D) Total RNA was isolated from the livers and processed to analyze the expression of Bcl-3 (A), TNF-α (B), and IL-10 (D) by real-time PCR using gene-specific primers and normalized to 18S. The protein levels of TNF-α (C) and IL-10 (E) were measured in the serum by ELISA. Data represent mean ± sem (*P<0.05 compared with saline-treated mice). ANOVA was used for statistical analysis.
DISCUSSION
Alcohol binge drinking is the most common form of acute alcohol abuse worldwide. Acute alcohol acts as an immunosuppressive agent by inhibiting APC function and decreasing the production of proinflammatory cytokine production in response to various pathogens [1, 2, 22] and thus, interferes with a normal immune response. Here, we report that acute alcohol binge induces Bcl-3 expression, which inhibits TNF-α at the mRNA and protein levels, identifying alcohol-induced Bcl-3 as a mediator of TLR4 tolerance at the molecular/chromatin level. We showed that acute alcohol alone decreases TNF-α production and report for the first time that this is a result of acute alcohol-induced LPS tolerance in human monocytes and murine macrophages.
Recently, Bcl-3 was shown to play a role in inducing TLR4 tolerance through its interaction with NF-κB p50 homodimers [13]. Bcl-3 is an atypical member of the IκB family of proteins that mainly resides in the nucleus and does not undergo degradation upon LPS stimulation [23]. We reasoned that Bcl-3 might play a role in alcohol-induced TLR4/LPS tolerance, and indeed, we found induction of Bcl-3 at the mRNA and protein levels. Previous studies identified various negative regulators of LPS signaling, and the involvement of different kinases has been reported in the anti-inflammatory effects of acute alcohol [15, 24–27].
NF-κB is a key transcription factor that is involved in the regulation of TNF-α in response to TLR activation. Our results suggest that alcohol pretreatment inhibited LPS-induced p65/p50 activation, suggesting alcohol exerts its effect at the transcriptional level. Indeed, previous reports in human monocytes demonstrated preferential induction of p50/p50 NF-κB dimers after acute in vitro alcohol treatment and in vivo alcohol consumption in humans [15, 26].
Bcl-3 is known to bind to the TNF-α promoter via its interaction with p50 homodimers and facilitates the dimer exchange [13]. Indeed, we found that Bcl-3 was present in a complex with p50 in alcohol and LPS-treated cells and in a mouse model of acute alcohol binge. The association of p50 with Bcl-3 plays an important role in TLR4 tolerance [13, 28]. The dimer exchange at the chromatin level determines the fate of gene regulation. The functional role of Bcl-3–p50 association in acute alcohol-pretreated cells in context to TNF-α regulation was confirmed further in a ChIP assay. We showed increased binding of Bcl-3 and p50 in alcohol-treated or LPS-activated cells at the distal promoter region of the TNF-α promoter. We also demonstrated that pretreatment of alcohol further augmented LPS-induced p50 and Bcl-3 binding at the TNF-α promoter, suggesting the involvement of p50–Bcl-3 complexes in alcohol-induced LPS tolerance. Involvement of the κ3 site in LPS tolerance has been reported by different groups [11, 13]. The constitutive binding of p50 and Bcl-3 that we observed near the transcriptional start site of TNF-α could be a mechanism to limit a basal increase in TNF-α transcription. This alcohol-induced inhibition via Bcl3-p50 might be specific for the TNF-α promoter, as we found only a minimal increase in global p50 binding.
We also showed that Bcl-3 knockdown prevented the alcohol-induced inhibition of TNF-α at the mRNA and protein levels, further supporting the functional role of Bcl-3 in alcohol-induced LPS tolerance. Interestingly, the increase of TNF-α at the mRNA level was more robust in Bcl-3 knockdown cells, suggesting the role of Bcl-3 in regulating TNF-α at the transcriptional level. Our observation is supported by the notion that Bcl-3 stabilizes p50 homodimers binding at the promoter region and hence, increases its occupancy at the TNF-α promoter [13]. It is reasonable to argue that as a result of decreased p50 homodimers binding in the absence of Bcl-3, we found a robust increase in TNF-α mRNA level in Bcl-3 knockdown cells.
Because of its crucial role in immune responses, TNF-α expression is tightly regulated at transcriptional and post-transcriptional levels, such as mRNA stability, translation initiation, or by transacting proteins [17, 29]. In agreement with this, we found only a modest increase of TNF-α at the protein level in LPS or alcohol-pretreated-Bcl-3 knockdown cells in vitro. Our in vivo studies in mice with binge drinking followed by an in vivo LPS challenge corroborated our in vitro observation. Decreased TNF-α in alcohol-pretreated mice challenged with LPS could be extrapolated as a possible mechanism by which acute alcohol consumption decreases the resistance to infections. Consistent with our findings on the role of Bcl-3 in alcohol binge-induced attenuation of LPS response, Bcl-3 knockout mice were shown to produce more inflammatory cytokines and to be highly susceptible to septic shock compared with WT mice [30]. Our results indicated no significant changes in serum IL-10 levels in the in vivo alcohol binge model in response to TLR4 ligand treatment. Consistent with our finding, a previous study also reported no significant changes in IL-10 levels in the acute alcohol model with TLR3 ligand stimulation [31]. Previously, Carmody et al. [13] reported that bone marrow-derived macrophages deficient in Bcl-3 produced more IL-10 when treated with CpG. We also found increased IL-10 in Bcl-3 knockdown LPS-treated RAW 264.7 cells (data not shown). Given the role of Bcl-3 in inducing the stabilization of p50, it is likely that it might play a role in inducing tolerance to other TLRs.
Although our results suggest that alcohol-induced LPS tolerance is governed partially by Bcl-3, the mechanism underlying Bcl-3 induction by alcohol is not known. Whereas alcohol-induced Bcl-3 mediated tolerance to a TLR4 ligand, the role of Bcl-3 in tolerance to other TLR stimulation remains to be explored. Bcl-3 is induced by IL-10, and in turn, Bcl-3 regulates IL-10 [11]. Kinases such as GSK3 and ERK are suggested to phosphorylate Bcl-3 and modulate its transcriptional activity [32]. Previously, we reported decreased ERK activation in acute alcohol-exposed monocytes [15]. The role of GSK3 in acute alcohol-induced TNF-α inhibition is not known, however inhibition of GSK3 results in decreased production of TNF-α via increased IL-10 [33], and the effect of acute alcohol on GSK3 activity is yet to be explored. Recently, GSK3 has been shown to mediate TNF-α-induced LPS tolerance [34]. In addition, the transcription factor STAT3 is known to increase the transcription of Bcl-3 [35]. Previously, our laboratory demonstrated that acute alcohol induces STAT3 binding and its phosphorylation in monocytes [36]; therefore, we speculate that acute alcohol might induces Bcl-3 via STAT3. Recently, miRNAs have emerged as fine regulators of gene function, and we found that several miRNAs, such as miR-9, miR-19, miR-27, miR-150, miR-145, miR-186, miR-365, and miR-381, have putative binding sites in the 3'UTR of Bcl-3 mRNA (http://www.microrna.org). It is possible that acute alcohol modulates Bcl-3 expression via targeting one of these miRNAs; however, further studies are warranted to confirm this speculation.
Taken together, our data indicate that acute alcohol-induced TLR4/LPS tolerance is governed, at least in part, by Bcl-3 at the TNF-α gene promoter and represents one of the negative mediators of alcohol-induced LPS tolerance. This demonstrates a mechanism by which binge alcohol drinking interferes with innate immune responses.
ACKNOWLEDGMENTS
This work was supported by U. S. National Institute on Alcohol Abuse and Alcoholism grant #AA011576 (G.S.). We thank Shiv Mundkur for his technical help.
Footnotes
- ChIP
- chromatin immunoprecipitation
- IP
- immunoprecipitation
- miRNA
- microRNA
- siRNA
- small interfering RNA
AUTHOR CONTRIBUTIONS
G.S. and S.B. perceived of the idea and outlined the study. S.B., A.T., O.T., D.C., and K.K. carried out the experiments. J.P. carried out animal experiments and performed statistics. G.S. and S.B. analyzed the data and wrote the manuscript, and all authors contributed to the editing.
REFERENCES
- 1. Szabo G., Mandrekar P. (2009) A recent perspective on alcohol, immunity, and host defense. Alcohol. Clin. Exp. Res. 33, 220–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boe D. M., Nelson S., Zhang P., Quinton L., Bagby G. J. (2003) Alcohol-induced suppression of lung chemokine production and the host defense response to Streptococcus pneumoniae. Alcohol. Clin. Exp. Res. 27, 1838–1845 [DOI] [PubMed] [Google Scholar]
- 3. Mandrekar P., Catalano D., Girouard L., Szabo G. (1996) Human monocyte IL-10 production is increased by acute ethanol treatment. Cytokine 8, 567–577 [DOI] [PubMed] [Google Scholar]
- 4. Goral J., Kovacs E. J. (2005) In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J. Immunol. 174, 456–463 [DOI] [PubMed] [Google Scholar]
- 5. Oak S., Mandrekar P., Catalano D., Kodys K., Szabo G. (2006) TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J. Immunol. 176, 7628–7635 [DOI] [PubMed] [Google Scholar]
- 6. Li C. H., Wang J. H., Redmond H. P. (2006) Bacterial lipoprotein-induced self-tolerance and cross-tolerance to LPS are associated with reduced IRAK-1 expression and MyD88-IRAK complex formation. J. Leukoc. Biol. 79, 867–875 [DOI] [PubMed] [Google Scholar]
- 7. De Vos A. F., Pater J. M., van den Pangaart P. S., de Kruif M. D., van 't Veer C., van der Poll T. (2009) In vivo lipopolysaccharide exposure of human blood leukocytes induces cross-tolerance to multiple TLR ligands. J. Immunol. 183, 533–542 [DOI] [PubMed] [Google Scholar]
- 8. Hayden M. S., Ghosh S. (2008) Shared principles in NF-κB signaling. Cell 132, 344–362 [DOI] [PubMed] [Google Scholar]
- 9. Wulczyn F. G., Naumann M., Scheidereit C. (1992) Candidate proto-oncogene bcl-3 encodes a subunit-specific inhibitor of transcription factor NF-κ B. Nature 358, 597–599 [DOI] [PubMed] [Google Scholar]
- 10. Nolan G. P., Fujita T., Bhatia K., Huppi C., Liou H. C., Scott M. L., Baltimore D. (1993) The bcl-3 proto-oncogene encodes a nuclear I κ B-like molecule that preferentially interacts with NF-κ B p50 and p52 in a phosphorylation-dependent manner. Mol. Cell. Biol. 13, 3557–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuwata H., Watanabe Y., Miyoshi H., Yamamoto M., Kaisho T., Takeda K., Akira S. (2003) IL-10-inducible Bcl-3 negatively regulates LPS-induced TNF-α production in macrophages. Blood 102, 4123–4129 [DOI] [PubMed] [Google Scholar]
- 12. Muhlbauer M., Chilton P. M., Mitchell T. C., Jobin C. (2008) Impaired Bcl3 up-regulation leads to enhanced lipopolysaccharide-induced interleukin (IL)-23P19 gene expression in IL-10(−/−) mice. J. Biol. Chem. 283, 14182–14189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carmody R. J., Ruan Q., Palmer S., Hilliard B., Chen Y. H. (2007) Negative regulation of Toll-like receptor signaling by NF-κB p50 ubiquitination blockade. Science 317, 675–678 [DOI] [PubMed] [Google Scholar]
- 14. Mandrekar P., Catalano D., Szabo G. (1997) Alcohol-induced regulation of nuclear regulatory factor-κ β in human monocytes. Alcohol. Clin. Exp. Res. 21, 988. [PubMed] [Google Scholar]
- 15. Mandrekar P., Bala S., Catalano D., Kodys K., Szabo G. (2009) The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J. Immunol. 183, 1320–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beier J. I., Luyendyk J. P., Guo L., von Montfort C., Staunton D. E., Arteel G. E. (2009) Fibrin accumulation plays a critical role in the sensitization to lipopolysaccharide-induced liver injury caused by ethanol in mice. Hepatology 49, 1545–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bala S., Marcos M., Kodys K., Csak T., Catalano D., Mandrekar P., Szabo G. (2011) Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor α (TNFα) production via increased mRNA half-life in alcoholic liver disease. J. Biol. Chem. 286, 1436–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dokladny K., Lobb R., Wharton W., Ma T. Y., Moseley P. L. (2010) LPS-induced cytokine levels are repressed by elevated expression of HSP70 in rats: possible role of NF-κB. Cell Stress Chaperones 15, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Biswas S. K., Lopez-Collazo E. (2009) Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30, 475–487 [DOI] [PubMed] [Google Scholar]
- 20. Collart M. A., Baeuerle P., Vassalli P. (1990) Regulation of tumor necrosis factor α transcription in macrophages: involvement of four κ B-like motifs and of constitutive and inducible forms of NF-κ B. Mol. Cell. Biol. 10, 1498–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ye J., Wang L., Zhang X., Tantishaiyakul V., Rojanasakul Y. (2003) Inhibition of TNF-α gene expression and bioactivity by site-specific transcription factor-binding oligonucleotides. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L386–L394 [DOI] [PubMed] [Google Scholar]
- 22. D'Souza N. B., Bagby G. J., Nelson S., Lang C. H., Spitzer J. J. (1989) Acute alcohol infusion suppresses endotoxin-induced serum tumor necrosis factor. Alcohol. Clin. Exp. Res. 13, 295–298 [DOI] [PubMed] [Google Scholar]
- 23. Kerr L. D., Duckett C. S., Wamsley P., Zhang Q., Chiao P., Nabel G., McKeithan T. W., Baeuerle P. A., Verma I. M. (1992) The proto-oncogene bcl-3 encodes an I κ B protein. Genes Dev. 6, 2352–2363 [DOI] [PubMed] [Google Scholar]
- 24. Mandrekar P., Jeliazkova V., Catalano D., Szabo G. (2007) Acute alcohol exposure exerts anti-inflammatory effects by inhibiting IκB kinase activity and p65 phosphorylation in human monocytes. J. Immunol. 178, 7686–7693 [DOI] [PubMed] [Google Scholar]
- 25. Drechsler Y., Dolganiuc A., Norkina O., Romics L., Li W., Kodys K., Bach F. H., Mandrekar P., Szabo G. (2006) Heme oxygenase-1 mediates the anti-inflammatory effects of acute alcohol on IL-10 induction involving p38 MAPK activation in monocytes. J. Immunol. 177, 2592–2600 [DOI] [PubMed] [Google Scholar]
- 26. Norkina O., Dolganiuc A., Catalano D., Kodys K., Mandrekar P., Syed A., Efros M., Szabo G. (2008) Acute alcohol intake induces SOCS1 and SOCS3 and inhibits cytokine-induced STAT1 and STAT3 signaling in human monocytes. Alcohol. Clin. Exp. Res. 32, 1565–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greenberg S. S., Jie O., Zhao X., Wang J. F. (1998) Role of PKC and tyrosine kinase in ethanol-mediated inhibition of LPS-inducible nitric oxide synthase. Alcohol 16, 167–175 [DOI] [PubMed] [Google Scholar]
- 28. Wessells J., Baer M., Young H. A., Claudio E., Brown K., Siebenlist U., Johnson P. F. (2004) BCL-3 and NF-κB p50 attenuate lipopolysaccharide-induced inflammatory responses in macrophages. J. Biol. Chem. 279, 49995–50003 [DOI] [PubMed] [Google Scholar]
- 29. Falvo J. V., Tsytsykova A. V., Goldfeld A. E. (2010) Transcriptional control of the TNF gene. Curr. Dir. Autoimmun. 11, 27–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruan Q., Zheng S. J., Palmer S., Carmody R. J., Chen Y. H. (2010) Roles of Bcl-3 in the pathogenesis of murine type 1 diabetes. Diabetes 59, 2549–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pruett S. B., Schwab C., Zheng Q., Fan R. (2004) Suppression of innate immunity by acute ethanol administration: a global perspective and a new mechanism beginning with inhibition of signaling through TLR3. J. Immunol. 4, 2715–2724 [DOI] [PubMed] [Google Scholar]
- 32. Viatour P., Dejardin E., Warnier M., Lair F., Claudio E., Bureau F., Marine J. C., Merville M. P., Maurer U., Green D., Piette J., Siebenlist U., Bours V., Chariot A. (2004) GSK3-mediated BCL-3 phosphorylation modulates its degradation and its oncogenicity. Mol. Cell 16, 35–45 [DOI] [PubMed] [Google Scholar]
- 33. Beurel E., Michalek S. M., Jope R. S. (2010) Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 31, 24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park S. H., Park-Min K. H., Chen J., Hu X., Ivashkiv L. B. (2011) Tumor necrosis factor induces GSK3 kinase-mediated cross-tolerance to endotoxin in macrophages. Nat. Immunol. 12, 607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brocke-Heidrich K., Ge B., Cvijic H., Pfeifer G., Löffler D., Henze C., McKeithan T. W., Horn F. (2006) BCL3 is induced by IL-6 via Stat3 binding to intronic enhancer HS4 and represses its own transcription. Oncogene 55, 7297–7304 [DOI] [PubMed] [Google Scholar]
- 36. Norkina O., Dolganiuc A., Shapiro T., Kodys K., Mandrekar P., Szabo G. (2007) Acute alcohol activates STAT3, AP-1, and Sp-1 transcription factors via the family of Src kinases to promote IL-10 production in human monocytes. J. Leukoc. Biol. 3, 752–762 [DOI] [PubMed] [Google Scholar]