Intact vascular perfusion done while intravitally visualizing the intestine: mucosal DCs and T cells display activity similar to that found in secondary lymphoid organs.
Keywords: T cells, dendritic cells, intestine
Abstract
FIVM has provided many insights into the regulation of immunity. We report the validation of an approach for visualizing murine small bowel via single- and multiphoton FIVM. Tissue damage is limited to ∼200 μm, immediately adjacent to the incision, as confirmed by intravital PI staining. Treatment with 10 KDa dextran-FITC and 70 KDa dextran-TR confirms that perfusion is intact. Selective filtration of 10 KDa but not 70 KDa dextran from the blood indicated that kidney function is also intact. Interestingly, lamina propria vasculature is semipermeable to 10 KDa dextran. Next, reporter mice expressing egfp from the CX3CR1 locus, egfp from the FoxP3 locus, or RFP from the IL-17F locus were used to track DC subsets, FoxP3+ Tregs, or Th17f cells, respectively. Resident cx3cr1+/egfp cells were sessile but actively probed the surrounding microenvironment. Both T cell populations patrol the lamina propria, but the Th17f cells migrate more rapidly than Tregs. Together, these data demonstrate intact vascular perfusion, while intravitally visualizing the mucosal surface of the small bowel. Lastly, the cx3cr1+ DCs and T cells display activity similar to that found in steady-state, secondary lymphoid organs.
Introduction
The technology of FIVM has provided significant advances in our understanding of immunobiology. Much has been learned about the regulation of immunity in secondary lymphoid organs [1]. Fewer studies have examined orchestration and regulation of immunity at effector sites, and studies that have carefully applied FIVM to understand regulation of immunity in the intestine are limited [2–5].
The intestine is unique in that it hosts ∼1014 microbes while maintaining relative immune quiescence [6]. The enteric flora has long been known to play an important role in the development of the gut-associated lymphoid tissue, driving peristalsis, protecting from infectious disease, and assisting in food digestion [7–10]. It is further unique in that it contains immunologic priming and effector sites, such as organized lymphoid follicles and lamina propria, respectively. As FIVM studies have provided valuable insight into the microanatomic organization and orchestration of immunity in LN and spleen, we aimed to establish a system that allows for intravital examination of the intestine while maintaining tissue viability and vascular perfusion.
We report here application of single- and multiphoton FIVM as a technique to study the regulation of immunity in the intestinal mucosa. Following surgical exposure of the mucosal surface of murine distal ileum, the tissue is mechanically stabilized and imaged. Using a combination of vital dyes and genetic reporters, the integrity of epithelial cells and underlying lamina propria cells was confirmed to be intact. Fluorescent-conjugated dextrans, 70 KDa and 10 KDa, were used to confirm blood perfusion. These dyes were cleared from the vasculature with predicted kinetics, indicating perfusion was intact. Surprisingly, cx3cr1+/egfp DCs in lamina propria were sessile despite the constant exposure of this tissue to enteric microbes. However, they actively extend and retract dendrities, a process called probing. The sessile, while probing behavior, is surprisingly akin to DC activity in a steady-state, secondary lymphoid organ. Th17 Teff and FoxP3+ Treg were motile, similar to T cell migration, reported at other organ sites [11–14]. Together, these data validate the ability to stably visualize the intestinal mucosal effector site while maintaining tissue health and demonstrate DC and T cell behavior surprisingly akin to that in secondary lymphoid organs at steady-state, despite the presence of enteric microbes.
MATERIALS AND METHODS
Mice
WT and cx3cr1egfp/egfp C57BL/6 animal breeders were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and maintained at the Freimann Life Science Center at the University of Notre Dame (Notre Dame, IN, USA). In some experiments, cx3cr1egfp/egfp animals were derived and maintained in the Skirball Institute for Biomolecular Medicine (New York, NY, USA) [15]. WT or cx3cr1+/egfp F1 animals, 8–12 weeks of age, were used in all experiments. FoxP3-GFP knock-in mice were kindly provided by Drs. Vijay Kuchroo and Mohamed Oukka and have been described [16]. Animals were age- and sex-matched as appropriate. All experiments were conducted as approved by the Institutional Animal Care and Use Committee.
Generation of Th17f-RFP FoxP3-EGFP mice
Screening of rBAC and resolved BAC constructs has been described [17, 18]. Briefly, the IL-17f gene carrying BAC p4E16 was purchased from Life Technologies (Carlsbad, CA, USA). Overlapping PCR was performed to amplify mRFP (with stop codon) [19], flanked with IL-17f genomic sequences. The overlapping PCR product was cloned into shuttle vector [18] and transformed into competent p4E16 cells. After integration and resolution, the rBAC carrying IL-17f-mRFP was cut with NotI to remove the vector backbone. The linearized rBAC DNA was purified by pulse-field gel electrophoresis and injected into fertilized C57BL/6 oocytes. DNA injection was done in the transgenic facility of the Memorial Sloan Kettering Cancer Center (New York, NY, USA), and pups were screened by PCR. BAC transgenic mice (p4E16-mRFP) were continually bred with C57BL/6 mice (purchased from Taconic, Hudson, NY, USA) for colony expansion. Animals were crossed to Foxp3-ires-eGFP mice. All mice were bred and used in our specific pathogen-free animal facility, according to the New York University School of Medicine Institutional Animal Care and Use Committee (New York, NY, USA). See Supplemental Data for further details about the Th-17 phenotype.
Surgery and anesthesia
Animals were anesthetized and maintained under anesthesia, as described previously [20]. Animals were anesthetized with a rodent cocktail of ketamine (50 mg/Kg), xylazine (10 mg/Kg), and acepromazine (1.7 mg/Kg), injected into the peritoneum. Anesthesia was maintained during image acquisition with one-half dose s.c. boosting every 45 min for up to 2.5 h. Animals can be maintained under anesthesia for up to 4 h. However, in some animals, respiration is affected at later time-points (>3 h), as indicated by labored, slowed, and deep breathing. For surgery, the anesthetized animal was placed on a heating pad set at 37°C, and abdominal fur was trimmed. To access the peritoneal cavity, a 1- to 1.5-cm incision was made through the skin along the abdominal midline to expose the peritoneal wall. An additional incision was made through the peritoneal wall to expose the abdominal cavity. Next, a 3- to 4-cm loop of the distal ileum was externalized. A segment of the mucosal surface was exposed by making two partial transverse incisions along the gut wall, ∼0.5 cm apart. A longitudinal incision was next made to expose the mucosal surface, and blood loss was limited by cauterizing at the edges of the incisions. The intestinal contents at the exposed area and up to 1 cm of the flanking region were removed with cotton swabs. Care is taken to avoid disrupting the mucosal layer. At no point was mesentery or mesenteric vasculature disrupted. Upon completion of surgery, the entire animal was then placed on an inverted microscope stage insert, with the mucosal surface of the intestine resting on the coverslip, which itself has glued to it single, 1.5 × 1.5-cm nonmoisturized, 20-lb printing paper with a 0.5 × 0.5-cm slit, on center, through which the intestine is aligned with the objective. Consequently, the externalized intestine is sandwiched between the paper glued to the coverslip and sterile gauze placed on the abdomen, upon which, the animal is now resting. Therefore, the entire exposed tissue is covered by the animal, which protects the tissue from dehydration. The animal is maintained at 37°C in a black-out environmental chamber with supplemental medical-grade oxygen supplied via a nose cone.
Rodent chow
Animals were maintained on Harlan Teklad Irradiated Global 19% Protein Extruded Rodent Diet 2919 (Indianapolis, IN, USA). Rodent chow did not cause any significant autofluorescence in the intestinal mucosa.
Intravital imaging
Animals were placed with the abdominal side down on a custom-made stage insert (Ludl Electronic Produces, Hawthorne, NY, USA), which contains a hole with coverslip (No. 1.5: 0.16–0.19 mm thick) to allow for access of an inverted objective. The stage insert with the animal is then placed on an inverted microscope stage of an Olympus FV1000 (Center Valley, PA, USA), equipped with a fempto-second-pulsed Ti:Sapphire laser with dispersion compensation (Newport Mai Tai DeepSee HP, Irvine, CA, USA) and 25×/1.05NA XL PlanN objective with 37°C objective heater. The microscope is equipped with customized filters mounted on four external nondescanned detectors. This microscope stage and head unit are enclosed in a blacked-out environmental chamber, which is maintained at a constant 37°C. In some studies, image acquisition was conducted on a Zeiss LSM 510 confocal with Plan-Apochromat 20×/0.75NA objective (Zeiss, Thornwood, NY, USA). Animals were euthanized at the termination of each experiment. For time-lapse image acquisition, 1 vol was collected every 30 s, with z-slices acquired every 5 μm. The maximum depth acquired over a 30-s interval by multiphoton was 75 μm with a 640 × 640 pixel size and 2 μs dwell time. The maximum depth acquired with a single-photon light source was 20 μm. The appearance of labeled blood vessels or cx3cr1+/egfp cells indicated that the focal plane was past the epithelium and just penetrating into the lamina propria. Therefore, the zero point was defined at 10 μm lower (on an inverted platform) than the plane containing the first signals on blood vessels or cx3cr1+/egfp cells. An area of autofluorescence was observed 15–20 μm lower than the first signals of blood vessels, which was interpreted as autofluorescence in the mucosal layer. Although autofluorescence was present, it was limited to the mucosal layer and did not pose a challenge to imaging epithelia or lampina propria. The depth routinely chosen for multiphoton image acquisition was 0–75 μm. Resolution at depths as much as 120 μm can be achieved with multiphoton excitation.
Treatment with vital dyes
Immediately prior to image acquisition, animals were retro-orbitally injected with fluorescently conjugated dyes, as indicated, in a 50- to 100-μl vol in saline at the following doses: 20 μg propidium iodide (PI) (Sigma-Aldrich, St. Louis, MO, USA), 20 pmol Qtracker 655 (Life Technologies), 2 mg 10 kD-FITC (TdB Consultancy, Uppsala, Sweden), 0.5 mg 70 kD TR (Life Technologies), and 0.25 mg Hoechst 33342 (Life Technologies).
Analysis of perfusion and permeability
All images were analyzed using Imaris software (Bitplane Scientific Software, South Windsor, CT, USA). Blood vessels were delineated by nonpermeable Qtracker 655. For image analysis at select time intervals, 10 vol of interest (5×5×1 μm) was chosen inside and outside, immediately adjacent to the blood vessel. The relative fluorescence intensities of each volume of 10 KDa-FITC and 70 KDa-TR dextran were determined. A total of three mice was examined. Therefore, each time-point is represented by 30 measurements for each dye. To quantify perfusion, the intravascular relative fluorescence intensity of 10 kD-FITC and 70 kD-TR was plotted over time. Percent fluorescence was calculated by normalizing the relative fluorescence intensity at 1 min postinjection. To quantify vascular permeability, the relative fluorescence intensities of each dye were calculated ratio-metrically and plotted as extra- versus intravascular, at 1 min postinjection.
Adoptive transfer of primed CD4 T cells in small intestine
Splenocytes from C57BL/6 mice were sorted for CD4+CD62 ligand+ cells using a MACS separator kit (Miltenyi Biotec, Auburn, CA, USA). The cells were activated in 96-well polystyrene flat-bottom plates (Costar, Corning, NY, USA) in the presence of 3 μg/ml immobilized anti-CD3, 1 μg/ml soluble anti-CD28, 50 U/ml IL-2, and 1 μM retinoic acid for 4 days and then allowed to rest in the presence of IL-2 only, for an additional 2 days. Subsequently, the cells were labeled with 1 μM CFSE and retro-orbitally injected into syngeneic recipient C57BL/6 mice. Recipient animals were imaged intravitally using multiphoton microscopy at 1 day post-transfer.
Leukocyte migration analysis
Leukocyte migration was quantified using Volocity (Improvision, now PerkinElmer, Waltham, MA, USA) and Imaris (Bitplane Scientific Software). Each cell was tracked semiautomatically and confirmed visually during each frame collected. For each analysis, every cell is examined during each acquisition volume (once every 30 s).
Statistics
Statistical analysis was conducted using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). All data sets were examined for Gaussian distribution via a D'Agostino and Pearson normality test. For determination of significance of differences between two groups, a two-tailed nonparametric t-test (Gaussian) or Mann-Whitney test (non-Gaussian), as appropriate, with a 95% confidence interval, was conducted. Significance is defined as P ≤ 0.05.
RESULTS AND DISCUSSION
The technology of multiphoton FIVM has provided many exciting and sometimes unexpected insights in immunology [1]. The application of this technology to mucosal immunobiology has the potential to provide many novel insights into the fundamental cellular and molecular regulation of homeostasis in this unique organ. A major challenge to microscopically visualizing internal organs via FIVM is to develop surgical procedures that minimize the impact on tissue health and movement. This study demonstrates a careful evaluation of tissue health and viability, while overcoming the additional challenge of peristaltic movement. Additionally, we describe homeostatic leukocyte behavior, surprisingly similar to that found in steady-state, secondary lymphoid organs.
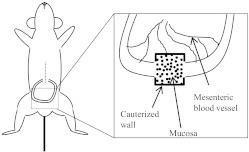
Following anesthesia, a distal loop of the small bowel is externalized by making an incision along the midline to minimize rupturing vasculature along the parietal peritoneum. A loop of the distal ileum is then externalized and placed on sterile gauze, which is resting on the abdomen of the animal. This prevents contact of the intestinal wall with abdominal fur. Following externalization of the distal ileum, an incision was made along the intestinal wall to access the mucosal surface for visualization. The incision was cauterized to prevent blood loss (Fig. 1). The entire animal was then placed on an inverted microscope stage insert as described in Materials and Methods.
Figure 1. Schematic of surgery.
A 1-cm cut is made along the abdominal midline (dashed line) of anesthetized animals following hair removal. A loop of the distal small bowel is then externalized. A small, 0.5-cm incision is made along the intestinal wall, and luminal contents are removed carefully to expose the mucosal surface (inset, with dots). Bleeding is stopped by cauterizing the incision (inset, bold lines).
Visualization on inverted and upright microscope platforms was attempted, but maximal success with mechanical tissue stabilization was observed on an inverted platform. We believe the combination of the weight of the animal and tactile resistance provided by paper and gauze are what provide for image stability. Nonetheless, there is variability of peristaltic movement along the intestine and even within a viewing field.
We varied on the use of confocal (single-photon) versus multiphoton application in this study as a result of availability of the technology. Early in the study, we aimed to established parameters for acquiring stable images. As described above, minimizing peristaltic movement, while visualizing the intestinal mucosa, was best achieved on an inverted microscopy stage. However, in the early phase of this study, a multiphoton light source was not available on an inverted stage. Although, we later re-examined T cell trafficking of endogenous Th17-RFP animals (described below), the signal-to-noise ratio was not sufficient to visualize these cells via multiphoton.
To evaluate the effect of surgery on tissue viability, animals were treated with 20 μg PI, and 250 μg Hoechst counterstain via i.v. injection. PI-positive cells were limited to <200 μm from the incision (Fig. 2). Therefore, cell death as a result of the surgical technique was limited to the region immediately adjacent to the incision. For all subsequent experiments, images were acquired in regions of normal tissue health.
Figure 2. Tissue damage limited to incision region.
Animals were treated i.v. with 20 μg PI (yellow/orange) in saline, while intravitally imaging distal ileum via multiphoton. In some experiment, animals were coinjected with 250 μg Hoechst 33342 (green; n=4).
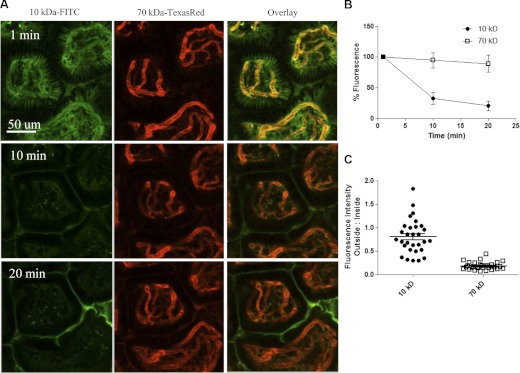
To evaluate tissue perfusion, 10 KDa-FITC and 70 KDa-TR were injected i.v. into WT C57BL/6 mice while imaging. Both dyes were observed in the lamina propria blood vessels in <30 s, indicating that perfusion is intact (Fig. 3 and Supplemental Video 1). The loss of intravascular fluorescence intensity over time was more readily apparent with 10 KDa-FITC than 70 KDa-TR dextran (Fig. 3A). Quantitative analysis of intravascular fluorescence intensity revealed that >50% of the intravascular 10 KDa-FITC dextran is lost within 10 min. Conversely, >90% of the intravascular 70 -Da dextran is retained as late as 20 min postinjection (Fig. 3B). These finding are consistent with the observation that kidneys filter 3–10 KDa dextrans within minutes, whereas larger molecular weight dextrans, >40 KDa, persist for an extended period in blood vessels [21]. The presence of dextrans in the bladder was confirmed grossly. Therefore, whereas it is likely that anesthesia has effects on the cardiovascular system, the intestinal mucosa and kidneys remain perfused using this surgical procedure.
Figure 3. Perfusion and vascular permeability.
(A) The lamina propria of distal ileum was visualized via multiphoton, immediately following i.v. coinjection of 2 mg 10 kDa dextran-FITC (green) and 0.5 mg 70 kDa dextran-TR (orange) in saline. Time-lapse images were acquired every 30 s for up to 20 min following treatment of dextran dyes (Supplemental Video 1). (B) Quantitative kinetic analysis of the relative intravascular fluorescence up to 20 min, normalized to total fluorescence of each dye at 1 min postinjection. (C) Ratiometric analysis of relative fluorescence intensity of extra- versus intravascular dextran at 1 min postinjection; n = 3.
To examine vascular permeability, a ratiometric analysis of extra- versus intravascular dextran was conducted. At 1 min postinjection, the relative fluorescence of 10 KDa dextran outside of the blood vessel is almost equal to that inside of the blood vessel, with a ratio of 0.80 ± 0.02 (Fig. 3C). Conversely, 70 KDa dextran is mostly intravascular, with a ratio of 0.18 ± 0.02 (Fig. 3C). Therefore, 10 KDa dextran is 4.4-fold more permeable than 70 KDa dextran in distal ileum. This technique allows for the intravital analysis of the mucosal vascular integrity in almost real time. Such measurements are important for target validation and delivery of therapeutics aimed at targeting the intestine.
Animals expressing EGFP under control of a single allele of the endogenous cx3cr1 promoter, cx3cr1+/egfp, allow for the visualization of DCs and were examined by IVM [15, 22]. Immediately prior to image acquisition, animals were i.v.-treated with 10 KDa dextran-TR to ensure perfusion. Analysis of DC activity in lamina propria reveals a sessile but probing activity of endogenous CX3CR1 cells (Fig. 4 and Supplemental Video 2). Interestingly, this activity is akin to that of DCs in secondary lymphoid organs [11]. cx3cr1+/egfp cell migration was not observed in a total of three animals, six villi/animal, and 10–40 cells/villus, examined over a period of 20 min each. Although the current paradigm is that a subset of cx3cr1+/egfp DCs sample mucosal antigen and traffic to the mesenteric LN to present antigen to T cells [2, 23, 24], the relative frequency of this behavior may be rare during homeostasis. Previous studies have demonstrated that cx3cr1+/egfp cells include a subset of DCs, which reach through epithelial cell tight junctions and play an important role in protection from salmonella [22]. Whereas these cells may play a sentinel role, their mobilization may require a pathogenic signal, such as that derived from Salmonella. The probing action of cx3cr1+/egfp cells indicated that the relative lack of cell migration in this study is not secondary to a lack of cellular metabolic activity.
Figure 4. Monocyte and DC activity in lamina propria.
cx3cr1+/egfp mice were imaged via intravital laser-scanning confocal microscopy over a 20-min period, with a frame rate of 1/30 s. Each mucosal villus is abundant with DCs, which are extending and retracting dendrites, but remain sessile over time (highlighted by white circles); n = 3.
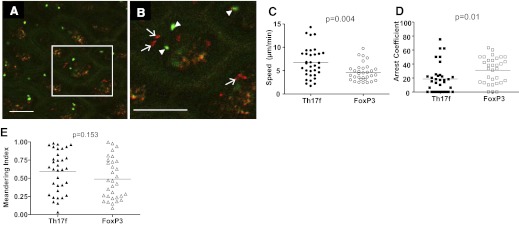
To further ensure that the lack of cx3cr1+/egfp cell migration was not a result of a lack of tissue health, endogenous T cell migration was examined. Migration of Teffs and Tregs in tissue is a highly controlled process, dependent on vascular perfusion, gas exchange (normoxia), and temperature regulation [12, 13, 25]. We examined the behavior of endogenous Th17 Teffs and FoxP3 Tregs in vivo via intravital confocal laser-scanning fluorescence microscopy, using a BAC transgenic-expressing RFP under the IL-17f promoter (Th17-RFP), crossed to transgenic animals expressing EGFP under the FoxP3 promoter (FoxP3-EGFP). Visualization of Th17-RFP required confocal (single-photon) microscopy as a result of a relatively weak signal of RFP in Th17 cells. We carefully discriminated between autofluorescence and Th17f-RFP cells by excluding autofluorescent signals found in multiple detectors (Fig. 5A and B, and Supplemental Video 3). FoxP3+ T cells (green) can be seen migrating in situ, in and out of the focal plane, and can be readily identified by dynamic, morphologic changes, as they traffic in villous lamina propria. Th17f cells (red) can also be identified as a result of their dynamic behavior, including a discernable leading edge, and they migrate in situ (Fig. 5A and B, and Supplemental Video 3). Th17f T cells migrated at a mean speed of 7 μm/min, whereas FoxP3 T cells migrated at a mean speed of 5 μm/min; P = 0.004 (Fig. 5B). The slower speed of FoxP3 T cells compared with Th17 T cells was a result of a significant increase in arrest coefficient, 31% and 19%, respectively; P = 0.01 (Fig. 5C). These differences were not a result of global variations in T cell migration of each subset, as no differences were seen in meandering index; P = 0.15 (Fig. 5E). The mean speed of Th17 Teffs is slightly slower to that of CD4 T cells reported previously in situ [11–14]. Nonetheless, the combination of perfusion and the dynamic behavior of T cells and DCs together indicate that cell and tissue health are intact in this system. The significance of the distinct behavior of Teff and Treg subsets during homeostasis is unclear. However, the increased arrest of FoxP T cells suggests that Treg interactions with resident DCs and/or other cells are more prolonged compared with that of Teffs. These data further suggest that Th17 Teffs survey more tissue/unit time and therefore, more APCs than FoxP T cells during homeostasis.
Figure 5. Migration of endogenous T cells in intestinal mucosa.
(A) Th17f Teff (red) and FoxP3 Treg (green) in lamina propria were visualized intravitally via laser-scanning confocal microscopy. (B) Inset of A. Th17f Teff (arrows) and FoxP3 Tregs (arrowheads). Original scale bars = 100 μm; n = 3. (C) FoxP Tregs (open circles) migrate slower than Th17f Teffs (closed circles); P = 0.004. Each dot represents the average 2D speed of an individual cell. (D) FoxP cells (open squares) show greater arrest coefficient than Th17f cells (closed squares); P = 0.01. (E) No difference is observed in meandering between Th17f (closed triangles) and FoxP3 cells (open triangles); P = 0.15. The horizontal line on each graph indicates the mean of all of the cells depicted, which are pooled together from three mice.
As the studies with endogenously primed T cells were limited to confocal microscopy, it is possible that the slower speed observed was a result of the lack of 3D resolution. Therefore, we used an adoptive transfer system, where naïve donor T cells were differentiated into gut homing T cells, loaded with a CFSE tracer, and visualized in recipient animals via multiphoton FIVM (Fig. 6, Supplemental Video 4). A comparative analysis of 2D versus 3D migratory behavior revealed that 2D analysis shows a slower speed compared with 3D analysis (10 μm/min vs. 12 μm/min, respectively) of the same cells (Fig. 6B). Cells that move along the z-plane are not quantified in a 2D analysis, and thus, speeds are likely biased compared with 3D analysis. No significant difference in arrest coefficient or meandering index was observed (Fig. 6C and D).
Figure 6. Migration of primed T cells.
(A) In vitro-primed CD4 cells were adoptively transferred into syngeneic WT C57BL/6 mice and visualized intravitally via multiphoton microscopy. Qtracker 655 (Life Technologies) was injected i.v. to highlight vasculature (red). CFSE-loaded T cells (green) were tracked (white line) for a 15-min time period at 16 h post-transfer. Original scale bar = 50 μm; n = 3. (B–D) T cell migration was quantified using a 3D volume and 2D slice from a total of three animals, and data were pooled. Each symbol represents an individual cell, and the horizontal line on each graph indicates the mean. (B) Average speed of T cells from a 2D image (open circles) was slower than as the same cells analyzed in 3D (12 μm/min and 10 μm/min, respectively; P=0.03). (C) No significant difference was seen in arrest coefficient in 3D (3 μm/min) versus 2D (10 μm/min). (D) No significant difference in meandering index was observed in 3D versus 2D analysis (0.48 vs. 0.44, respectively).
Together, these data validate procedures to quantitatively evaluate mucosal perfusion, vascular permeability, and leukocyte behavior at single-cell resolution in vivo and provide an important tool for more closely examining the role of DC migration in maintaining homeostasis in the gastrointestinal tract. This system also provides an important tool for understanding the microanatomic locale of T cell-DC interactions and the genetic requirements for T cell-APC interactions and defining the relative contribution of APC subsets in spatial-temporal regulation of T cell activation in vivo.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by U.S. National Institute of Diabetes and Digestive and Kidney Diseases DK078153 (P.V.), Indiana University School of Medicine-South Bend (P.V.), and U.S. NIH R01055037 (M.L.D.). We thank M. Oukka and V. Kuchroo for the FoxP3-ires-eGFP mice. We also thank Skirball Animal Facilities at New York University Langone School of Medicine and Freimann Life Science Center at University of Notre Dame.
SEE CORRESPONDING EDITORIAL ON PAGE 407
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- 2D/3D
- two/three-dimensional
- BAC
- bacterial artificial chromosome
- FIVM
- fluorescence intravital microscopy
- FoxP3
- forkhead box P3
- mRFP
- monomeric RFP
- p4E16
- RP23-4E16
- RFP
- red fluorescence protein
- Teff
- effector T cell
- TR
- Texas red
- Treg
- regulatory T cell
AUTHORSHIP
P.V. and M.L.D. designed experiments. Y.S. and D.R.L. designed and developed BAC transgenic animals. C.X. and P.V. conducted experiments and prepared the manuscript.
REFERENCES
- 1. Germain R. N., Miller M. J., Dustin M. L., Nussenzweig M. C. (2006) Dynamic imaging of the immune system: progress, pitfalls and promise. Nat. Rev. Immunol. 6, 497–507 [DOI] [PubMed] [Google Scholar]
- 2. Chieppa M., Rescigno M., Huang A. Y., Germain R. N. (2006) Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 203, 2841–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kao J. Y., Zhang M., Miller M. J., Mills J. C., Wang B., Liu M., Eaton K. A., Zou W., Berndt B. E., Cole T. S., Takeuchi T., Owyang S. Y., Luther J. (2010) Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology 138, 1046–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zinselmeyer B. H., Dempster J., Gurney A. M., Wokosin D., Miller M., Ho H., Millington O. R., Smith K. M., Rush C. M., Parker I., Cahalan M., Brewer J. M., Garside P. (2005) In situ characterization of CD4+ T cell behavior in mucosal and systemic lymphoid tissues during the induction of oral priming and tolerance. J. Exp. Med. 201, 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adams C. L., Kobets N., Meiklejohn G. R., Millington O. R., Morton A. M., Rush C. M., Smith K. M., Garside P. (2004) Tracking lymphocytes in vivo. Arch. Immunol. Ther. Exp. (Warsz) 52, 173–187 [PubMed] [Google Scholar]
- 6. Backhed F., Ley R. E., Sonnenburg J. L., Peterson D. A., Gordon J. I. (2005) Host-bacterial mutualism in the human intestine. Science 307, 1915–1920 [DOI] [PubMed] [Google Scholar]
- 7. Sartor R. B. (2011) Key questions to guide a better understanding of host-commensal microbiota interactions in intestinal inflammation. Mucosal Immunol. 4, 127–132 [DOI] [PubMed] [Google Scholar]
- 8. Ivanov I. I., Littman D. R. (2011) Modulation of immune homeostasis by commensal bacteria. Curr. Opin. Microbiol. 14, 106–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velazquez P., Wei B., Braun J. (2005) Surveillance B lymphocytes and mucosal immunoregulation. Springer Semin. Immunopathol. 26, 453–462 [DOI] [PubMed] [Google Scholar]
- 10. Hooper L. V., Wong M. H., Thelin A., Hansson L., Falk P. G., Gordon J. I. (2001) Molecular analysis of commensal host-microbial relationships in the intestine. Science 291, 881–884 [DOI] [PubMed] [Google Scholar]
- 11. Shakhar G., Lindquist R. L., Skokos D., Dudziak D., Huang J. H., Nussenzweig M. C., Dustin M. L. (2005) Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat. Immunol. 6, 707–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang J. H., Cardenas-Navia L. I., Caldwell C. C., Plumb T. J., Radu C. G., Rocha P. N., Wilder T., Bromberg J. S., Cronstein B. N., Sitkovsky M., Dewhirst M. W., Dustin M. L. (2007) Requirements for T lymphocyte migration in explanted lymph nodes. J. Immunol. 178, 7747–7755 [DOI] [PubMed] [Google Scholar]
- 13. Miller M. J., Wei S. H., Parker I., Cahalan M. D. (2002) Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science 296, 1869–1873 [DOI] [PubMed] [Google Scholar]
- 14. Mempel T. R., Henrickson S. E., Von Andrian U. H. (2004) T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 [DOI] [PubMed] [Google Scholar]
- 15. Jung S., Aliberti J., Graemmel P., Sunshine M. J., Kreutzberg G. W., Sher A., Littman D. R. (2000) Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bettelli E., Carrier Y., Gao W., Korn T., Strom T. B., Oukka M., Weiner H. L., Kuchroo V. K. (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 [DOI] [PubMed] [Google Scholar]
- 17. Sparwasser T., Gong S., Li J. Y., Eberl G. (2004) General method for the modification of different BAC types and the rapid generation of BAC transgenic mice. Genesis 38, 39–50 [DOI] [PubMed] [Google Scholar]
- 18. Gong S., Yang X. W., Li C., Heintz N. (2002) Highly efficient modification of bacterial artificial chromosomes (BACs) using novel shuttle vectors containing the R6Kγ origin of replication. Genome Res. 12, 1992–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell R. E., Tour O., Palmer A. E., Steinbach P. A., Baird G. S., Zacharias D. A., Tsien R. Y. (2002) A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 7877–7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Velazquez P., Cameron T. O., Kinjo Y., Nagarajan N., Kronenberg M., Dustin M. L. (2008) Cutting edge: activation by innate cytokines or microbial antigens can cause arrest of natural killer T cell patrolling of liver sinusoids. J. Immunol. 180, 2024–2028 [DOI] [PubMed] [Google Scholar]
- 21. Dunn K. W., Sandoval R. M., Kelly K. J., Dagher P. C., Tanner G. A., Atkinson S. J., Bacallao R. L., Molitoris B. A. (2002) Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am. J. Physiol. Cell. Physiol. 283, C905–C916 [DOI] [PubMed] [Google Scholar]
- 22. Niess J. H., Brand S., Gu X., Landsman L., Jung S., McCormick B. A., Vyas J. M., Boes M., Ploegh H. L., Fox J. G., Littman D. R., Reinecker H. C. (2005) CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 [DOI] [PubMed] [Google Scholar]
- 23. Vallon-Eberhard A., Landsman L., Yogev N., Verrier B., Jung S. (2006) Transepithelial pathogen uptake into the small intestinal lamina propria. J. Immunol. 176, 2465–2469 [DOI] [PubMed] [Google Scholar]
- 24. Kelsall B. L., Rescigno M. (2004) Mucosal dendritic cells in immunity and inflammation. Nat. Immunol. 5, 1091–1095 [DOI] [PubMed] [Google Scholar]
- 25. Stoll S., Delon J., Brotz T. M., Germain R. N. (2002) Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science 296, 1873–1876 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.