Endocrine regulation of inflammatory potential in mast cells identifies insulin as a driving force for lipid body biogenesis and eicosanoid secretion.
Keywords: hormone-sensitive lipase, 5-lipoxygenase, leukotriene, inflammation, lipogenesis, obesity, ectopic lipid formation, leukotrienes
Abstract
Lipid bodies are most studied in adipocytes, where the lipogenic action of insulin initiates their formation. Here, we test the hypothesis that insulin may regulate lipid body content in mast cells and hence, modify their proinflammatory potential. Our data show that insulin causes lipid body accumulation in RBL2H3 and BMMCs. Lipid body accumulation in mast cells is associated with enhanced levels of leukotriene-synthesizing enzymes (LTC4S and 5-LO). Increased basal and antigen-stimulated release of LTC4 is observed in insulin-treated mast cells. Concomitantly, the insulin-containing lipogenic stimulus induces a phenotypic change in mast cells, where this enhancement in leukotriene levels is accompanied by a marked down-regulation in secretory granule content and release in response to stimulus. Mast cells exposed to insulin exhibit altered scatter and fluorescence properties, accumulating in a SSCloFSChi population that exhibits decreased BS staining and degranulation responses and is enriched in NR-positive lipid bodies and eicosanoid synthesis enzymes. Lipid body accumulation in mast cells is mechanistically distinct from the process in adipocytes; for example, it is independent of PPARγ up-regulation and does not involve significant accumulation of conjugated glycerides. Thus, chronic exposure to metabolic stimuli, such as insulin, may be a determinant of the proinflammatory potential of the mast cell.
Introduction
Mast cells are critical to the initiation and maintenance of inflammatory responses, releasing proinflammatory mediators, including histamine, serotonin, matrix-active proteases, eicosanoids and prostanoids, and cytokines and chemokines [1, 2]. Dysregulated inflammation is a feature of the metabolic syndrome and other metabolic disorders [3–5], and mast cells may contribute causally to metabolic pathology. For example, mast cell-deficient mice or animals exposed to mast cell-stabilizing drugs have markedly different outcomes in obesity and type II diabetic models [6]. These data suggest that mast cell-derived signals impact metabolic processes, but the converse potential for metabolic stimuli to modulate mast cell responses has not been widely explored. In vivo data suggest that metabolic status alters the outcome of mast cell-derived inflammation. Rats with streptozoicin- or alloxan-induced type I diabetes exhibit severely compromised anaphylactic responses to antigen or secretagogue, as well as diminished airway sensitivity [7–10]. Indeed, reconstitution of mast cell responses reverses the refractoriness to allergen challenge that is seen in alloxan-induced diabetic rats [10, 11]. Here, we test the premise that insulin can affect one or more aspects of mast cell responses to challenge.
Various endocrine hormones have been investigated for their ability to regulate mast cell histamine release and cytokine production [12]. The ability of insulin to act as an acute primary stimulator of mast cell activation does not seem to be generally supported by published studies. A number of studies have suggested previously that mast cells are largely refractory to insulin over acute time courses [13–15], although chronic insulin does affect BMMC proliferation [15]. A recent revisiting of this idea suggested that insulin, added in acute prestimulation or concurrently with antigen, activates tyrosine kinase pathways in mouse BMMCs and somewhat potentiates FcεRI signaling [16]. Our present study addresses the idea that insulin fluctuations over longer, chronic time courses may modulate mast cell phenotype, and in this fashion, insulin levels could be a determinant of the intensity and duration of subsequent mast cell responses to challenge.
Mast cells contain two specialized proinflammatory organelles [2, 17, 18]. Secretory granules bear histamine, serotonin, and matrix active proteases within a proteoglycan matrix. Far less studied are the cytoplasmic lipid bodies that are found in mast cells, neutrophils, macrophages, and eosinophils [18–21]. These lipid bodies may play a role in the immunocyte that is distinct from the contribution to neutral triglyceride storage and cellular metabolism made by the analogous organelle in adipocytes, although a comparative analysis of the lipid and protein complement of mast cell and adipocyte lipid bodies has not been undertaken [22–28]. In mast cells and other leukocytes, lipid bodies are reservoirs of eicosanoid precursors and sites for eicosanoid synthesis [19–21, 29–32]. Thus, the potential for a mast cell to generate mediators, such as LTC4, may be related to the size of the pool of precursors represented by these lipid bodies.
Lipid bodies are most studied in adipocytes, where the lipogenic action of insulin drives their formation [22, 27, 28]. Here, we test the hypothesis that insulin may similarly regulate lipid body content in mast cells and thereby, modify their proinflammatory potential. Our data show that insulin causes marked lipid body accumulation in RBL2H3 and BMMCs. We find that lipid body accumulation in mast cells is mechanistically distinct from the process in adipocytes; for example, it is independent of up-regulation in PPARγ [33, 34]. Lipid body accumulation in mast cells is associated with enhanced levels of leukotriene-synthesizing enzymes. Moreover, basal and stimulated release of LTC4 is increased in lipogenically treated mast cells. Concomitantly, the insulin-containing lipogenic stimulus induces a phenotypic change in mast cells, where this enhancement in leukotriene levels is accompanied by a marked down-regulation in secretory granule release in response to stimulus. These data support the idea that chronic exposure to metabolic stimuli, such as insulin, may be physiological determinants of the proinflammatory potential of mast cells. Moreover, our data suggest that mast cell lipid body levels and function may be impacted under conditions of metabolic dysregulation, where ectopic lipid accumulation at supraphysiological levels has been reported in cells, such as hepatocytes, myocytes, adipocytes, and foam cells.
MATERIALS AND METHODS
Cell culture
RBL2H3 [35] were grown at 37°C, 5% CO2, in 95% humidity in DMEM (Mediatech, Herndon, VA, USA) with 10% heat-inactivated FBS (Mediatech) and 2 mM glutamine. C57.1 murine mast cells were grown in RPMI, 10% FBS, 2 mM l-Gln, 2 mM nonessential amino acids, 1 mM sodium pyruvate, 50 μM 2-ME, and 5 ng/ml IL-3 at 37°C, 5% CO2, in 95% humidity.
Chemicals, reagents, and stimulations
General chemicals were from VWR International (Radnor, PA, USA) and Sigma-Aldrich (St. Louis, MO, USA). PMA and ionomycin were from Calbiochem (Gibbstown, NJ, USA). IgE anti-DNP and BS were from Sigma and KLH-DNP was from Calbiochem. Antibodies were from the following suppliers: anti-COX1, anti-COX2, anti-perilipin A and B, anti-PPARγ, anti-HSL, anti-ATL (Abcam, Cambridge, MA, USA); anti-5-LO (Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-TIP47 (Novus, Littleton, CO, USA); anti-Grb2 (Cell Signaling Technology, Danvers, MA, USA); anti-ADFP (Biovision, Mountain View, CA, USA); anti-LTC4S (Santa Cruz Biotechnology); anti-ABHD5 (Abcam); NR and ORO(EMD Chemicals, Gibbstown, NJ, USA); and hematoxylin (ScyTek Laboratories, Logan, UT, USA). Alexa- and HRP-conjugated secondary antibodies were from Invitrogen (Temecula, CA, USA) and Amersham (Piscataway, NJ, USA), respectively. FcεRI stimulation used 0.1 μg/ml IgE anti-DNP for 16 h at 37°C, followed by three washes and the addition of 250 ng/ml KLH-DNP for the indicated times. PMA and ionomycin were used at 500 nM and 500 nM, along with IFDI. Lipogenesis/adipogenesis was induced by incubating 3T3L1 adipocytes and RBL2H3 mast cells in the lipogenic cocktail mentioned above for 6 days, beginning 24 h after seeding. Cells were then harvested for analysis on Day 7.
Cell lysis and Western blot
Cells were pelleted (2000 g, 2 min) and washed once in ice-cold PBS. Approximately 107 cells were lysed (ice/30 min) in 350 μl lysis buffer [50 mM Hepes, pH 7.4, 250 mM NaCl, 20 mM NaF, 10 mM iodoacetamide, 0.5% (w/v) Triton X-100, 1 mM PMSF, 500 mg/ml aprotinin, 1.0 mg/ml leupeptin, and 2.0 mg/ml chymostatin]. Lysates were clarified (17,000 g, 20 min). For preparation of total protein, lysates were acetone-precipitated (1.4 vol acetone for 1 h at −20°C, followed by centrifugation at 10,000 g for 5 min). Protein samples were resolved by 10% SDS-PAGE under reducing conditions in a modified Laemmli buffer. Resolved proteins were electrotransferred to PVDF membrane in 192 mM glycine and 25 mM Tris (pH 8.8). For Western blotting, membranes were blocked using 5% nonfat milk in PBS for 1 h at room temperature. Primary antibodies were dissolved in PBS/0.05% Tween-20/0.05% NaN3 and incubated with membranes for 16 h at 4°C. Developing antibodies comprised anti-rabbit or anti-mouse IgGs conjugated to HRP (Amersham). These were diluted to 0.1 μg/ml in PBS/0.05% Tween-20 and incubated with membranes for 45 min at room temperature. A standard washing protocol (four washes of 5 min in 50 ml PBS/0.1% Tween-20 at room temperature) was used between primary and secondary antibodies and following secondary antibody. Signal was visualized using ECL (Amersham) and exposure to Kodak BioMax film. Films were scanned at >600 dpi, and quantification was performed using Image J (U.S. National Institutes of Health, Bethesda, MD, USA).
Histology staining for lipid content
Cells were plated on coverslips and fixed with 0.4% (w/v) paraformaldehyde (1 h at room temperature), washed twice with dH2O, and stained with 200 μl hematoxylin-Gill #2 (5 min at room temperature). Coverslips were washed twice with tap water before staining with ORO (0.35% in 6:4 EtOH:water, 15 min at room temperature, followed by two dH2O washes) [36, 37]. BS was used at 0.025%, pH 4.0, 30 min at room temperature [38, 39]. Coverslips were mounted in Crystal-Mount (Electron Microscopy Sciences, Hatfield, PA, USA) for imaging. Bright-field and fluorescence imaging was performed on a Nikon Eclipse Ti fluorescence microscopy system and analyzed in NIS-Elements (Nikon Instruments, Melville, NY, USA).
Calcium assay
RBL2H3 were washed and incubated with 1 μM Fluo-4 for 30 min at 37°C in a standard, modified Ringer's solution of the following composition (in mM): NaCl 145, KCl 2.8, CsCl 10, CaCl2 10, MgCl2 2, glucose 10, Hepes · NaOH 10, pH 7.4, 330 mOsm. Cells were transferred to 96-well plates at 50,000 cells/well and stimulated as indicated. Calcium signals were acquired using a Flexstation 3 (Molecular Devices, Sunnydale, CA, USA). Data were analyzed using SoftMax Pro 5 (Molecular Devices).
LTC4 assay
RBL2H3 were treated as indicated and stimulated via the FcεRI or using PMA/ionomycin. After a 1-h incubation, supernatants were assayed for the concentration of LTC4 using a specific enzyme immunoassay kit (Cayman Chemicals, Ann Arbor, MI, USA) and in reference to a standard curve. Color development proceeded for 45 min, and absorbance was read at 405 nm.
β-Hexosaminidase assay
RBL2H3 were plated in cluster plates at 5 × 104 cells/well. Monolayers were washed and incubated in 200 μl Tyrode's buffer before stimulating as described. After 45 min at 37°C, 25 μl supernatant was removed, clarified by microcentrifugation, and transferred to a 96-well plate containing 100 μl/well and 1 mM p-N-acetyl glucosamine (Sigma-Aldrich) in 0.05 M citrate buffer, pH 4.5. After 1 h at 37°C, reactions were quenched by the addition of 100 μl/well 0.2 M glycine, pH 9.0. β-Hexosaminidase levels were read as OD at 405 nm. Results are shown as the mean ± sd.
Flow cytometry
Cells were fixed in 0.4% paraformaldehyde (45 min at room temperature) and resuspended at 1 × 106 cells/ml in FACS buffer (HBSS, 0.5% BSA, 0.05% NaN3). For lipid body analysis, cells were stained with NR (0.1 μg/ml, 30 min at room temperature). For BS staining, cells were incubated with 0.025% BS, pH 4.0 (20 min at room temperature) [39]. For immunocytochemistry analysis, cells were harvested as above and resuspended in FACS buffer. Appropriate flow cytometry controls were performed to block nonspecific binding of primary and secondary antibodies to surface FcγRs on mast cells. Unpermeabilized cells were blocked for 1 h with BSA, followed by rabbit anti-mouse mixed IgGs. Permeabilization was with 0.1% Triton X-100 for 4 min, followed by washing. Primary antibodies were incubated for 30 min at room temperature at a concentration of 0.1–1 μg/ml. After three washes, cells were incubated with 0.05 μg/ml of the indicated Alexa-coupled secondary antibody (Invitrogen). Fluorescence was assessed on a FACSAria flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) at the John A. Burns School of Medicine Flow Cytometry Facility, University of Hawaii (Honolulu, HI, USA). Flow cytometry data were analyzed in FlowJo, Version 9.02.
Triglyceride assay
A triglyceride quantification kit [40] from BioVision was used as follows: cells were harvested, washed in PBS, and homogenized in 5% Triton X-100. The homogenate was twice heated slowly to 100°C and then cooled slowly to room temperature. After centrifugation, the supernatant was incubated with lipase for 20 min at room temperature, followed by incubation with triglyceride reaction mix for 1 h at room temperature. The OD was read at 570 nm.
Real-time qPCR analysis
cDNA was synthesized from 600 ng total RNA using the High-Capacity cDNA transcriptase kit (Applied Biosystems, Foster City, CA, USA). TaqMan Gene Expression Assays were used (Applied Biosystems) for IL-6 (Assay ID: Rn01410330_m1) and β-actin (Assay ID: Rn00667869_m1). The amplifications were carried out in 10 μl containing 1× Taqman Fast Universal Master Mix, 1× Taqman Gene Expression Assay, and purified target cDNA. The cycling parameters were 30 s at 94°C, 40 cycles at 94°C for 3 s, and 60°C for 30 s using the StepOnePlus (Applied Biosystems). Amplifications were performed in triplicate. Signals were normalized to expression of β-actin. Analysis used the method of Pfaffl [41].
Analysis
Results are shown as the mean ± sd. Statistical significance was determined based on a two-way ANOVA (Student's t test). Adjacent to data points in the respective graphs, significant differences were recorded as follows: *P < 0.05; **P < 0.01; ***P < 0.001; no symbol, P > 0.05. Experiments are all n of at least 3.
RESULTS
Chronic insulin induces lipogenesis in mast cells
Over an acute timecourse, we can reproduce published experiments showing only moderate insulin activation of kinase pathways, such as AKT phosphorylation and ERK1/2 activation, and the refractoriness of histamine release to acute insulin (data not shown) [13, 15, 16] in mast cells. In the current study, we are testing the hypothesis that insulin could nevertheless have chronic effects on mast cell proinflammatory responses. By analogy with adipocytes, we hypothesized that chronic insulin exposure induces lipogenesis.
Fig. 1A shows that RBL2H3 mast cells express the Ins-R [13, 15, 16]. Insulin drives the accumulation of mast cell lipid bodies, which stain positively with neutral lipid dyes (ORO and NR; Fig. 1B). In the adipocyte literature, optimal lipogenesis is routinely achieved in vitro through addition of insulin, in combination with an inhibitor of autocrine TNF-α production and stabilization of cAMP levels (Fig. 1C; [42–45]). Here, insulin is driving the lipogenic process. In contrast, the addition of dexamethasone acts to oppose constitutive lipolysis. Dexamethasone, a corticosteroid, opposes production of endogenous TNF-α, a lipolytic cytokine produced by adipocytes and mast cells, and inhibits expression of the HSL. Thus, the prediction arising from the adipocyte literature is that the effects of insulin and dexamethasone would be independently able to induce lipogenesis and act in an additive manner, with one promoting lipogenesis and the other opposing lipolysis. In mast cells, we note a similar additive effect, where insulin is sufficient to drive lipid body accumulation, but its effect is enhanced by the antilipolytic dexamethasone. Fig. 1C and D shows that exposure to this combinatorial stimulus (IFDI) dramatically enhances the lipid content of RBL2H3 and primary C57.1 BMMC. Fig. 1E and F examines the components of the stimulus. Fig. 1F presents quantification of the mean number of lipid bodies observable as discrete structures averaged from 100 cells (left panel) and the area of apparent ORO-positive staining averaged across 10 cells (right panel), showing that IFDI and insulin alone can act as primary drivers of lipogenesis and that the effects of the lipogenic insulin and antilipolytic dexamethasone are, as expected, additive. In contrast with the adipocyte, we find that the cAMP-elevating reagent IBMX is not a major factor in the effect of the IFDI lipogenic stimulus. Fig. 1F shows that in isolation, IBMX does not induce marked lipogenesis, and it does not have an additive or synergistic effect when combined with insulin. Taken together, these data reflect similar dissections of the composite IFDI stimulus published in adipocytes; i.e., insulin is necessary and sufficient to cause lipid body accumulation in the presence of a lipid-rich medium.
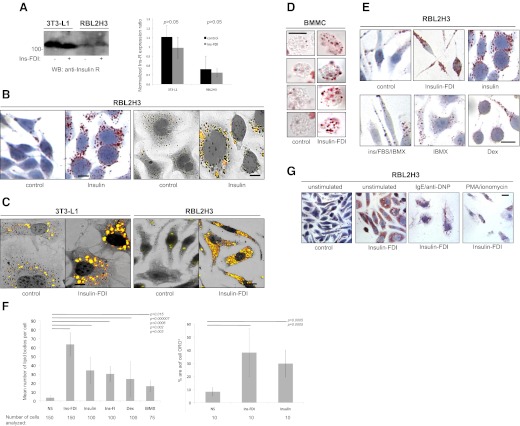
Figure 1. Insulin-containing lipogenic stimuli induce lipid bodies in mast cells.
(A) Ins-R expression in mast cells. 3T3-L1 and RBL2H3 cells (5×106 cells/lane) were lysed as described in Materials and Methods. Total protein was acetone-precipitated and resolved using 10% SDS-PAGE. After electrotransfer, resolved proteins were probed for the presence of the Ins-R (left panel) using 0.01 μg/ml rabbit anti-Ins-R and a HRP-conjugated secondary. MW is shown in kDa. Expected MW of the Ins-R is 121 kDa. (Right panel) Quantification of the left panel. Ins-FDI, IFDI; WB, Western blot. (B) Insulin induces mast cell lipogenesis. RBL2H3 mast cells were plated on glass coverslips and incubated with insulin (0.1 μg/ml) for 6 days at 37°C. After PFA fixation, cells were stained with ORO and hematoxylin and then visualized using bright field (two left panels) or FITC emission overlaid on phase images (two right panels). (C and D) Multicomponent lipogenic treatment induced marked lipid body accumulation in mast cells. RBL2H3 and 3T3L1 cells were plated on glass coverslips (C), whereas C57.1 BMMCs (D) were grown in suspension, in the presence or absence of a stimulus comprising 10% FBS, 0.01 mg/ml insulin, 0.25 μM dexamethasone, and 2.5 μM IBMX (IFDI) for 6 days at 37°C. After PFA fixation, cells were stained with ORO and hematoxylin and then imaged as above. (E) Dissection of IFDI stimulus. As in B and C, RBL2H3 were stimulated for 6 days with components of the IFDI stimulus, at the concentrations indicated above, and stained with ORO for lipid bodies. Dex, Dexamethasone. (F) Quantification of lipid body abundance. Cells were stimulated as described above, and mounted coverslips were stained with ORO. (Left panel) Number of lipid bodies/cell, averaged from the indicated number of cells. Lipid bodies were counted in a sample-blinded fashion, as observably discrete structures using a 100× objective. (Right panel) Averaged area of ORO staining/cell, assessed by IMAGE J analysis. (G) Intracellular lipid bodies are mobilized in response to antigenic and pharmacological stimulation in mast cells. RBL2H3 cells were incubated with IFDI for 6 days at 37°C and then stimulated via the FcεRI (IgE 1 μg/ml for 16 h, followed by 250 ng/ml KLH-DNP for 60 min) or PMA/ionomycin (1 μM each for 60 min). Poststimulation, cells were fixed immediately in PFA and stained with ORO and hematoxylin. n > 3.
Lipid bodies in macrophages, eosinophils, and neutrophils are dynamically regulated in response to challenge [32, 46–48]. Moreover, Dvorak et al. [31] have shown by electron microscopy that lipid bodies in basophils disperse their contents into degranulation channels. FcεRI and PMA/ionomycin stimulation cause depletion of the IFDI-induced lipid bodies in mast cells (Fig. 1G). Taken together, these data indicate that lipid body accumulation in mast cells can be induced by insulin and an insulin-containing lipogenic stimulus that initiates similar pathways in adipocytes. Moreover, the large number of IFDI-induced lipid bodies in mast cells is mobilized by antigen receptor stimulation.
Phenotypic biasing of mast cells in response to insulin-containing lipogenic stimuli
We sought to characterize the phenotype of mast cells that had been exposed to IFDI and have assumed the highly lipid body-rich state shown above. With the use of flow cytometry, we compared IFDI-treated cells with controls. Fig. 2A shows that control mast cells divide into SSChiFSClo and SSCloFSChi subpopulations. With IFDI treatment or insulin alone, there is a marked shift of cells into the SSCloFSChi population (Fig. 2A). The secretory granule load of mast cells is reflected in their scatter properties assayed by flow cytometry [49], with high granularity conferring high SSC properties. As a population, IFDI-treated mast cells decrease in SSC and increase in FSC in comparison with controls (Fig. 2A). We hypothesized that this shift in scatter properties could reflect alterations in functional capability of mast cells.
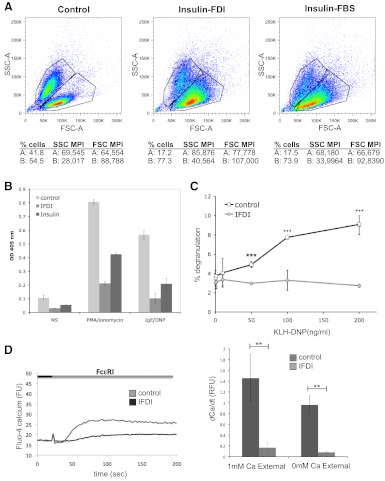
Figure 2. Insulin-containing lipogenic stimuli induce phenotypic change in mast cell granule content.
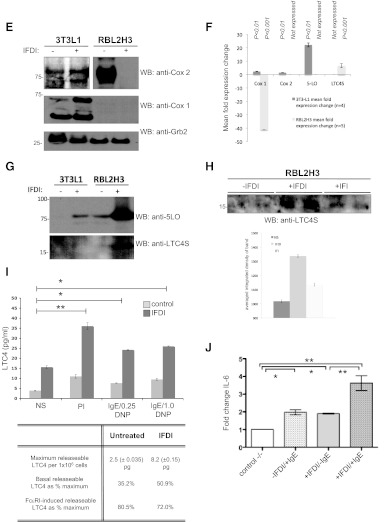
(A) Scatter profile of control and IFDI-treated mast cells. RBL2H3 cells were incubated with IFDI treatment, insulin-FBS, or left untreated for 6 days at 37°C. Cells (5×105)/sample were harvested and fixed in PFA before assessed for cell size (FSC) and granularity/membrane complexity (SSC) using a FACSAria (BD Biosciences). Based on FSC and SSC, two distinct subpopulations (A and B) can be distinguished. FSC/SSC-A, FSC/SSC-area. (B and C) Insulin-containing lipogenic stimuli suppress degranulation of mast cells in response to antigenic and pharmacological activation. RBL2H3 cells were incubated with IFDI treatment, insulin alone, or left untreated for 6 days at 37°C. Cells were exposed to PMA and ionomycin (B) for 60 min or primed overnight with 1 μg/ml IgE and stimulated with the indicated doses of KLH-DNP for 20 min (B and C). Degranulation was quantified using a β-hexosaminidase assay. (D) Calcium signaling is suppressed in IFDI-treated RBL2H3. (Left panel) FcεRI-induced calcium fluxes in control and 6-day IFDI-treated RBL2H3. Experiment was performed in 1 mM external calcium. FU, Fluorescence units. (Right panel) Analysis of initial rates of calcium mobilization in control and IFDI-treated RBL2H3. Values are shown as mean of three experiments ± sd. Experiments were performed in 1 mM external or nominally calcium-free (0 mM CaCl2+1 mM EGTA) Ringer solution. RFU, Relative fluorescence units; dCa/dt, rate of change in calcium signal over time. (E) COX regulation in mast cells and adipocytes following IFDI exposure. 3T3L1 and RBL2H3 (5×106 cells/lane) were stimulated with IFDI for 6 days, and protein lysates were prepared as described. Western blot analysis for the indicated proteins was performed as described (anti-COX1 2.5 μg/ml; anti-COX2 0.17 mg/ml). (F) Quantification of Western blot analyses for fold change in expression of Cox 1, Cox 2, 5-LO, and LTC4S. (G and H) LTC4S and 5-LO regulation in 3T3L1 and RBL2H3. Cells (1×107 cells/lane) were treated with IFDI or IFI for 6 days, and lysates were Western blotted as described using anti-LTC4S (2.0 μg/ml) and anti-5-LO (0.4 μg/ml). (H, lower panel) Quantification of LTC4S expression changes in response to IFDI and IFI. (I) LTC4 assay in control and IFDI-treated mast cells. RBL2H3 were exposed to IFDI or vehicle for 6 days. After 15 min stimulation at 37°C with vehicle or PMA/ionomycin (PI; 1 μM/1 μM) or via FcεRI (IgE 1 μg/ml for 16 h, followed by 0.25 or 1.0 μg/ml KLH-DNP), LTC4 levels were assayed. LTC4 concentrations were calculated by reference to a standard curve and are expressed as mean ± sd of triplicate samples. (J) IL-6 transcript levels in mast cells exposed to lipogenic stimuli. mRNA was isolated from control and IFDI-treated cells that were unstimulated or activated via FcεRI. qPCR analysis of IL-6 transcript levels was performed as described in Materials and Methods. Average of IL-6 fold change was made of two independent experiments. n > 3; *P < 0.05; **P < 0.01; ***P < 0.001; no symbol, P > 0.05.
Mast cells drive inflammation in a multifaceted manner. Antigen or secretagogue stimulation results in degranulation of preformed mediators (serotonin and histamine), de novo synthesis of bioactive lipids (LTC4 and PGD2), and induction of cytokine gene transcription. Many of the events are critically dependent on a biphasic intracellular calcium response, comprising release from InsP3-gated calcium stores [1, 4, 5], followed by activation of the calcium release-activated calcium current/Orai1 influx pathway. We asked whether lipogenic stimuli had any effect on the degranulation response and secretory granule population in mast cells. Fig. 2B and C shows that degranulation responses are suppressed in IFDI-treated mast cells in response to pharmacological and antigenic stimulation of mast cells. As secretory responses are dependent on mobilization of intracellular free calcium [31], we asked if calcium signals were intact in IFDI-treated cells. Fig. 2D (left panel) shows that the initial rate and intensity of FcεRI-induced calcium fluxes are compromised in IFDI-treated cells. Analysis of initial rates in the absence and presence of extracellular calcium indicates (Fig. 2D, right panel) that the progress of initial InsP3-dependent calcium signals (store release) is suppressed dramatically in lipid body-enriched cells.
We asked whether the transition to the SSCloFSChi phenotype was associated with any gain of function in the RBL2H3 mast cells. There is a significant literature in other leukocytes (neutrophils, eosinophils, and macrophages) suggesting that lipid bodies are reservoirs of the arachidonic acid precursors of eicosanoid lipids, such as leukotrienes [18, 20, 21, 31, 32, 50, 51]. Moreover, there is some evidence that the proximal synthetic enzymes for leukotrienes and PGs are actually located within the lipid body, leading to the proposal that lipid bodies represent a possible secondary site for eicosanoid synthesis in addition to the recognized cytosolic PLA2-dependent pathway. Indeed, phospholipid-derived and triglyceride pools of arachidonic acid have been described in macrophages [52], and lipid bodies are thought to contain a unilamellar layer of phospholipid that may be a PLA2 substrate. In mast cells, we noted that density-gradient isolated lipid bodies in mast cells contain eicosanoid metabolizing enzymes and that lipid bodies colocalize with anti-LTC4S immunofluorescence (data not shown). Moreover, mast cell levels of COX and LO enzymes are regulated during lipogenesis. IFDI induces down-regulation of COX2 (Fig. 2E) and a striking up-regulation of 5-LO and LTC4S (Fig. 2G), indicating a transition toward the eicosanoid synthesis program. Quantifications of these data are presented in Fig. 2F. The COX2 down-regulation is highly dexamethasone-dependent [53] (data not shown), whereas LTC4S up-regulation is accomplished by insulin in the absence of dexamethasone (Fig. 2H). Mast cells incubated with lipogenic stimuli for 6 days exhibit elevated basal LTC4 levels and when stimulated via the FcεRI receptor, release significantly more LTC4 than untreated cells (Fig. 2I). These data suggest that the up-regulation in leukotriene synthesis enzymes during lipogenesis is functionally significant for mast cells.
Another important aspect of the mast cell phenotype is the production of cytokines and chemokines in response to stimulation. In the context of metabolic disease, mast cell- derived IL-6 and IFN-γ have been shown to promote directly adipose inflammation and angiogenesis, promoting obesity [6]. A panel of cytokines assessed by microarray revealed few alterations in response to IFDI exposure, with the exception being a marked down-regulation in IL-3 transcripts [54] and some up-regulation in the Th2 cytokines IL-2, IL-6, and IL-10 (data not shown). Mast cell-derived IL-6 has been suggested to play a causative role in diet-induced obesity and glucose intolerance [6], and our data suggest that basal IL-6 levels are up-regulated by chronic exposure to lipogenic stimuli in mast cells (Fig. 2J), and the degree of IL-6 transcriptional activation in response to subsequent antigen stimulation is also enhanced significantly in IFDI-exposed mast cells.
Lipid body-enriched mast cells have discrete properties from those in adipocytes
The data presented above suggest that there are marked distinctions between IFDI-treated cells and untreated RBL2H3. We further explored the properties of the SSCloFSChi population, in which the IFDI-treated cells accumulate. Mast cell secretory granules are autofluorescent as a result of their serotonin content [55]. We examined the intrinsic autofluorescent properties of both subpopulations and noted that the SSChiFSClo population (referred to as Population A) is characterized by higher autofluorescence, reflecting higher content of secretory granules (data not shown). Moreover, this population loads more intensely with the granule-staining dye BS (Fig. 3A and B) [38]. Moreover, using FITC autofluorescence as a measurement of granule content, we noted that the stimulus-induced loss in this parameter as well as MPI were more pronounced in the SSChiFSClo population than in the SSCloFSChi cells (Fig. 3C and D).
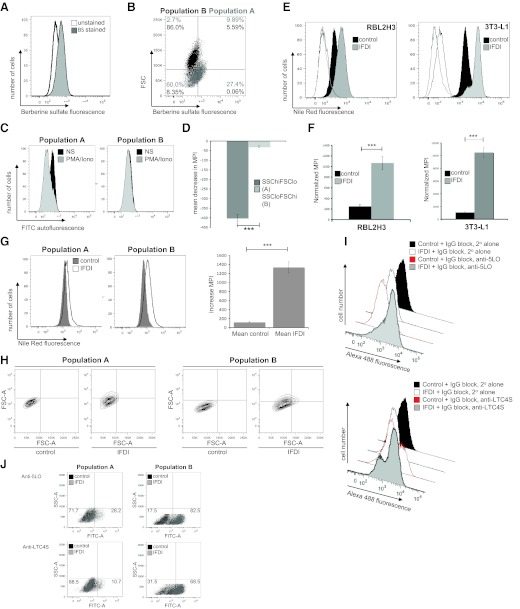
Figure 3. Characterization of IFDI-induced subpopulations in mast cells.
(A and B) BS staining is enhanced in the IFDI-induced SSCloFSChi subpopulation. RBL2H3 were treated with IFDI for 6 days, as described. Staining with BS was performed as described in Materials and Methods (B, left panel). Subpopulations of IFDI-treated cells were assessed for levels of BS accumulation (B, right panel). (C) SSChiFSClo cell subpopulation is diminished and loses autofluorescence after stimulation of mast cell degranulation. RBL2H3 cells were treated with IFDI for 6 days as described. Cells were exposed to vehicle or PMA/ionomycin for 20 min. Poststimulation, cells (5×105) were harvested, fixed immediately in PFA, and analyzed using a FACSAria (BD Biosciences) cell sorter for autofluorescence levels. (D) Quantification of fluorescence loss. Normalized decrease in MPI in response to PMA/ionomycin stimulation of control and lipid body-enriched mast cells (data averaged from three experiments). (E) Flow cytometric quantification of lipid body accumulation in adipocytes and mast cells. RBL2H3 (left panel) and 3T3L1 (right panel) were incubated with IFDI for 6 days at 37°C. After PFA fixation, cells were stained with NR (1 h at room temperature). Flow cytometry was used to establish quantitative values of intracellular lipid body accumulation by evaluating MPIs of the NR emission in the Texas Red channel. Light- and dark-gray, unfilled traces represent autofluorescence of IFDI-treated and control cells, respectively. (F) Quantification of fluorescence gain. Normalized increase in MPI in response to IFDI treatment of RBL2H3 (left panel) and 3T3-L1 (right panel; data averaged from three experiments). (G and H) NR accumulation is preferentially localized to SSCloFSChi mast cell subpopulation. RB2H3 were analyzed for NR accumulation, as described after 6 days of exposure to vehicle control or IFDI. The increase in NR fluorescence intensity (G, left panel) and number of NR+ cells in response to IFDI (H) is greater in Population B than A. Fluorescence gain (normalized increase in MPI) in response to IFDI treatment of RBL2H3 in Populations A and B was averaged from three experiments (G, right panel). (I and J) Up-regulation in eicosanoid-metabolizing enzymes are preferentially localized to a SSCloFSChi mast cell subpopulation. Up-regulation in 5-LO and LTC4S levels following IFDI treatment of RBL2H3 was measured using flow cytometry. (I) Anti-5-LO and LTC4S staining in a whole population of RBL2H3. (J) Increase in positively staining cells in populations A and B, respectively, following IFDI treatment. n > 3; ***P < 0.001; no symbol, P > 0.05.
We developed a flow cytometric assay for lipogenesis in mast cells. In adipocytes and mast cells, NR, which stains lipid bodies similarly to ORO, is detectable above autofluorescence levels in the FITC (data not shown) and Texas Red channels and increases even further in response to the lipogenic IFDI treatment (Fig. 3E and F). The affects of lipogenesis on the adiopocyte and the mast cell can also be visualized by the significant increase in the MPI of NR (Fig. 3F). We used this assay to compare the NR (lipid body) accumulation in the SSChiFSClo and SSCloFSChi cells (Fig. 3G and H). Fig. 3G shows that the IFDI-induced increase in NR accumulation (reflecting lipid body load) is more pronounced in the SSCloFSChi cells, whereas more cells become NR+ after IFDI treatment in Population B than Population A (Fig. 3H).
The large increases in 5-LO and LTC4S levels (Fig. 3I and J) are more pronounced in Population B than in Population A (Fig. 3J). These data are consistent with observations from other cell systems, such as neutrophils, which suggest that the lipid bodies that we see preferentially accumulating in Population B, are reservoirs of eicosanoid-metabolizing enzymes [21, 32]. Together, these data indicate that the insulin-exposed mast cell population becomes biased toward a functional phenotype, where bioactive lipid production is enhanced, but degranulation responses are compromised.
Lipid body biogenesis is mechanistically different in mast cells and adipocytes
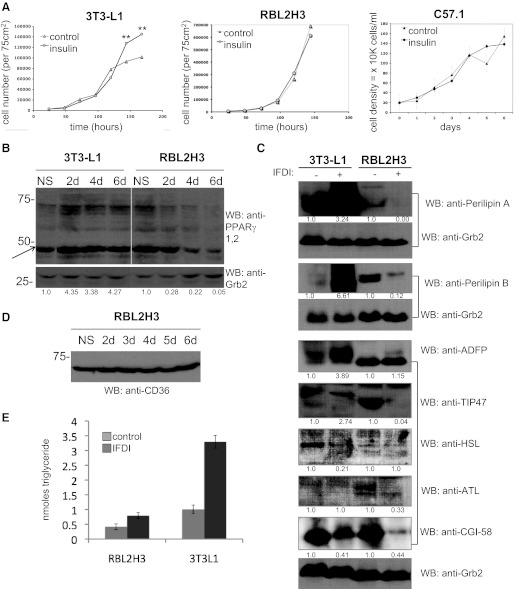
Lipid body biogenesis in adipocytes is a highly ordered process that is accompanied by hyperproliferation and dependent on PPARγ [22, 23, 25, 26, 34, 56]. Mature lipid bodies have a specific protein content that reflects their function as accessible stores of NTGs. We assessed several features of lipogenesis in mast cells in comparison with adipocytes. Fig. 4A shows that chronic insulin exposure does not alter proliferation patterns in the mast cell lines used here. Our data show that insulin is having the hyperproliferative effect on adipocytes that is suggested by the literature. However, the effect of insulin on RBL2H3 and C57.1 mast cells is that it does not enhance proliferation significantly. Thus, in these transformed mast cell lines, we do not see evidence for insulin acting as a growth factor per se, which does not preclude such an effect in primary, untransformed cells. Moreover, it should be noted that the IFDI treatment used in our experiments to drive high levels of lipogenesis essentially causes both mast cell lines to cease proliferating after 48 h. Mast cells treated with IFDI or insulin remain viable. In RBL2H3 treated with insulin or IFDI, 90% of cells are trypan blue-negative at 6 days compared with ∼92% in controls (n=>10) up to at least 144 h after addition, but if IFDI-treated, they show cell-cycle arrest and accumulation in G0 and G1 (not shown). Dissection of the IFDI treatment suggested that IBMX (but not dexamethasone) contributes to this proliferation defect.
Figure 4. Lipid body biogenesis is mechanistically different in mast cells and adipocytes.
(A) Effects of insulin on proliferation. 3T3L1, RBL2H3, and C57.1 BMMCs were incubated with insulin alone or untreated for 6 days at 37°C. Live cell numbers were established by trypan blue exclusion assay. (B) PPARγ regulation by IFDI in adipocytes and mast cells. 3T3L1 and RBL2H3 were incubated for the indicated time [in days (d)] with IFDI as described. Cell lysates were prepared and Western blotted for the presence of PPARγ using rabbit polyclonal anti-PPARγ (0.1 μg/ml for 1 h), followed by anti-rabbit IgG secondary. The pan-PPARγ antibody here recognized a strong band corresponding to PPARγ 1 but not 2. Arrow indicates PPARγ1. Expression ratios derived from Image J quantification of the Western blot are shown below the blot panel. (C) Western blot analysis of lipid body-associated proteins in adipocytes and mast cells. 3T3L1 and RBL2H3 (5×106 cells/lane) were stimulated with IFDI for 6 days, and protein lysates were prepared as described. Western blot analysis was performed as described in Materials and Methods with the following antibodies: anti-perilipin A (5.0 μg/ml); anti-perilipin B (1.0 μg/ml); anti-ADFP (0.67 μg/ml); anti-TIP-47 (1.2 μg/ml); anti-HSL (2.0 μg/ml); anti-ATL (0.67 μg/ml); and anti-ABHD5 (CGI-58; 1.0–2.0 μg/ml). Expression ratios derived from Image J quantification of the Western blot are shown below the blot panel. Anti-Grb2 Western blots are presented as loading controls. (D) Time course of CD36 regulation in response to IFDI. RBL2H3 (5×106 cells/lane) were stimulated with IFDI for 6 days, and protein lysates were prepared as described. Western blot analysis was performed as described in Materials and Methods using anti-CD36. (E) Mono-, di-, and triglyceride levels in mast cells are not altered by exposure to lipogenic stimuli. RBL2H3 or 3T3-L1 cells were treated with IFDI for 6 days. Cells (5.1×106/sample) were washed and permeabilized, and triglycerides were harvested as described in Materials and Methods. OD at 570 nm was converted to nmole-conjugated glycerides using a standard curve. Values are expressed as mean of triplicates ± sd. n > 3; **P < 0.01; no symbol, P > 0.05.
In adipocytes, up-regulation of PPARγ is an essential step in lipogenesis [34, 56]. In mast cells, PPARγ1 is down-regulated during lipid body biogenesis (Fig. 4B), but PPARγ2 was not reproducibly detected. In adipocytes, lipid body formation involves multiprotein complexes formed from the PAT family and the up-regulation of proteins, such as HSL and CD36, which are involved in NTG metabolism [25, 26, 51, 57]. In Fig. 4C, we examined lipid body-associated protein expression in response to lipogenic stimulation. Expression of PAT family proteins and markers of adipocyte differentiation, in resting and IFDI-stimulated mast cells, have a distinct profile from that of the adipocyte [23]. Levels of Perilipins A and B increase in adipocytes exposed to IFDI but are poorly expressed in mast cells and down-regulated further by IFDI. The fatty acid carrier protein TIP47 is expressed predominantly in mast cells in its higher mobility, dephospho form and is not up-regulated in response to IFDI, in contrast to its up-regulation in IFDI-treated 3T3-L1. ADFP and ATL are down-regulated in mast cells in response to IFDI, as opposed to marked up-regulation or unchanged expression in the adipocyte. HSL is down-regulated as adipocytes enter a lipogenic state, consistent with a decrease in their lipolytic potential. HSL is poorly expressed in mast cells and is not altered by IFDI exposure. CGI-58 levels are decreased slightly after 6-day IFDI treatment in adipocytes and mast cells. In adipocytes, the tetraspan fatty acid receptor protein CD36 is up-regulated by IFDI treatment. Conversely, CD36 is expressed consistently in resting or IFDI-stimulated mast cells (Fig. 4D). Overall, it appears that proteins involved in the biogenesis of NTG-containing lipid bodies in adipocytes are less critical for the induction of lipid body synthesis that we see in IFDI-treated mast cells.
Finally, we assessed the bulk neutral lipid content (using an assay that measures mono-, di-, and triglycerides) of RBL2H3 and 3T3-L1 after 6 days of exposure to IFDI. Here, we observe that lipid body biogenesis in 3T3-L1 is associated with a large increase in the cellular pool of conjugated glycerides, consistent with published observations that the major component of the adipocyte lipid body is a neutral triglyceride (Fig. 4E). In contrast, RBL2H3 show only a moderate and not statistically significant increase in storage of lipids measured by this assay (Fig. 4E). These data suggest, first that the new pool of lipid induced by IFDI in mast cells is distinct from that in adipocytes and second, that the primary components of the induced lipid bodies are lipids that are not detected by an assay for conjugated glycerides, such as free fatty acids, sterols, etc.
DISCUSSION
This paper presents data suggesting that chronic exposure to insulin has marked consequences for mast cell function in vitro. These data support and extend previous in vivo studies showing that mast cell-driven responses are attenuated in the absence of systemic insulin [7–9, 11, 58]. Insulin drives the formation of lipid bodies in mast cells, and we propose that this process has a distinct mechanism and endpoint from that observed in adipocytes. Mast cell lipid body formation is not associated with a large increase in neutral triglyceride storage and is not associated with an up-regulation in PPARγ or its downstream transcriptional targets, although there may be residual expression of PPARγ in IFDI-treated mast cells. Rather, supporting observations from previous electron microscopy studies [21, 31, 32, 59], these lipid bodies are associated with an up-regulation in eicosanoid synthesis enzymes and a concomitant increase in the intensity of leukotriene release following antigen receptor stimulation. The supraphysiological levels of lipid body formation that are induced in this study are accompanied by a marked suppression of secretory responses. At least under the experimental conditions used here, these data suggest that insulin can bias the outcome of mast cell activation by promoting a poorly secretory but pro-eicosanoid phenotype. It is interesting to consider this idea in light of observations that mast cells from different tissue locations can be differentiated on the basis of functional phenotypes. Initial studies about mast cell subsets focused on receptor heterogeneity, protease expression differences, and cytokine profile, but there is also evidence of differential ability to produce histamine secretion and bioactive lipids [60, 61]. Our data suggest that these phenotypes may be plastic, in the sense that they can be regulated dynamically in nonproliferating cells, and that signals such as insulin may play a role in establishing the local phenotype of mast cells [9, 58].
Lipid bodies in mast cells have been less well-studied than their counterparts in adipocytes or the mast cell secretory granule. Several key studies performed using EM suggest that mast cell lipid bodies are reservoirs of arachidonic acid and synthetic enzymes for eicosanoid proinflammatory mediators and that the contents of lipid bodies are discharged into exosomal structures upon antigen receptor stimulation [21, 31, 32]. Our data support and extend these findings. Even the large numbers of lipid bodies induced by the IFDI stimulus can be subsequently depleted in response to FcεRI activation. Moreover, our data support findings that eicosanoid synthesis may be localized to lipid bodies in mast cells [21] and other immunocytes [19, 30, 50, 62]. However, lipid bodies are also sites of protein synthesis and MHC class I trafficking, extending the possible implications for mast cells of the lipid body up-regulation that we observe [18, 20, 51, 63, 64]. The content of the IFDI-induced lipid bodies in mast cells is a clear focus for future study. Our data suggest that whereas adipocytes store conjugated glycerides in their lipid bodies after IFDI exposure, the induced pools of mast cell lipid do not have the same composition. Our data suggest that the major components of the induced mast cell lipid bodies must be lipids that are not mono-, di-, or triglycerides. Our ongoing lipidomic analyses may show these to be free fatty acids, sterols, and other lipid species, whose role in mast cell functional responses remains to be elucidated.
A role for insulin may suggest that other stimuli (such as leptin, ghrelin, and glucagon) may regulate lipid body load in mast cells. A direct stimulus that controls the number of lipid bodies in mast cells has not been identified previously. In other immunocytes (e.g., macrophages), immunological challenge results in an increase in lipid body load [19, 46, 47], but here, we show that insulin may play a key role in determining mast cell lipid body numbers in the absence of challenge. It is not clear what constitutes a “normal” lipid body load in mast cells or whether under physiological or pathophysiological conditions, their number ever increases dramatically. Based on a limited number of published electronmicroscopy studies, it appears that the ratio of secretory granules to lipid bodies in primary mast cells is high, with <10 lipid bodies typically observed/cell [17, 64]. There are numerous precedents for supraphysiological accumulation of lipid bodies under conditions of pathological metabolic dysregulation. For example, ectopic accumulation of lipids is reported in the hepatic steatosis that accompanies nonalcoholic fatty liver disease, and in adipocytes and myocytes from obese individuals. Within the immune system, the accumulation of macrophage lipid to generate foam cells is well-studied in atherosclerotic plaques. Our data suggest that high insulin levels over chronic time courses could create ectopic accumulation in mast cells with concomitant effects on the proinflammatory and immunoregulatory function of these cells.
The possibility of insulin being the driving stimulus that establishes lipid body content in mast cells has interesting implications. Building on our in vitro data, there are important in vivo tests of this hypothesis that need to be performed, primarily in genetic and chemically induced models of type I diabetes, where we would predict a decrease in the lipid body (and hence, eicosanoid content) of mast cells in certain tissue locations. In addition, an unbiased screen for other determinants of lipid body content, especially in a model of mast cell differentiation, is important. Our in vitro findings have potentially interesting implications under normal physiological conditions, prompting investigation of whether daily fluctuations in insulin levels are able to regulate mast cell lipid body load. Perhaps more likely is the idea that hyperinsulinaemia, associated with the insulin-resistant stage of the progression through obesity and type II diabetes [4], could be altering mast cell status. This brings to mind the systemic elevation in cytokine IL-6 and an increase in asthma-like symptoms and in atopy that associate with this aspect of metabolic syndrome[3, 5, 65]. Moreover, there is compelling in vivo evidence from the laboratory of Shi and coworkers [6], suggesting that altered mast cell abundance, in turn, dramatically alters the outcome of obesity induction via the high-fat diet in mice. Our data provide an interesting counterpoint to these studies. The Shi study [6] suggests that mast cell-derived factors alter outcomes in metabolic syndrome and obesity, and our data provide a direct mechanism by which the metabolic/endocrine environment can alter mast cell production of various mediators that may impact both immunological tissues, the mast cell microenvironment in dermis and mucosa, and (intriguingly) the environment of mast cells that reside in adipose. Finally, we note with interest the implications of insulin regulation of mast cell proinflammatory potentials in type I diabetes. The “mutual exclusion” between atopic spectrum disorders and type I diabetes has been widely noted in epidemiological studies [3, 5, 65]. Immunologically, the idea of atopy as a Th2 disease and type I diabetes as a Th1 disease provides for an explanation of the apparent inverse correlation between the incidences of these diseases [66]. If, however, insulin deficiency has an implication for mast cell function, then the role of these cells as sources of the Th2 cytokines IL-4, -6, and -10 and in the production of leukotrienes that are causative in pathologies such as atopic asthma, may be worthy of further study.
ACKNOWLEDGMENTS
This work was funded by the National Science Foundation Experimental Program to Stimulate Competitive Research (EPSCoR; EPS-0903833; to H.T.); Hawaii Community Foundation (Victoria S. and Bradley L. Geist Foundation) Award 45,408 (to H.T.); and an award from the General Atlantic Corp. through Mr. J. Michael Windsor (to H.T.). Ms. Ashley Hirashima provided technical assistance, and Dr. Alexandra Gurary provided guidance with flow cytometry [John A. Burns School of Medicine Core Facility supported by Centers of Biomedical Research Excellence (COBRE); U. S. National Institutes of Health P20RR018727]. The U.S. National Institute on Minority Health and Health Disparities (P20MD06084) provided scientific infrastructure support at Chaminade University. The authors thank Dr. Alexander Stokes for discussion of the manuscript.
Footnotes
- 5-LO
- 5-lipoxygenase
- ABHD5
- 1-acylglycerol-3-phosphate O-acyltransferase
- ADFP
- adipophilin
- ATL
- adipose tissue lipase
- BS
- berberine sulfate
- CGI-58
- comparative gene identification 58
- FSC
- forward-scatter
- Grb2
- growth factor receptor-bound protein 2
- HSL
- hormone-sensitive lipase
- IBMX
- isobutylmethylxanthine
- IFDI
- insulin, FBS, dexamethasone, and IBMX at at 0.01 mg/ml, 10% (w/v), 0.25 μM, and 2.5 μM, respectively
- Ins-R
- insulin receptor
- InsP3
- inositol P3
- KLH
- keyhole limpet hemocyanin
- LTC4S
- leukotriene C4 synthase
- MPI
- mean peak fluorescence intensity
- NR
- Nile Red
- NTG
- neutral triacylglycerol
- ORO
- Oil Red O
- PAT
- perilipin, adipophilin, tail-interacting protein of 47 kDa
- PPAR
- peroxisome proliferator-activated receptor
- qPCR
- quantitative PCR
- SSC
- side-scatter
- TIP47
- tail-interacting protein of 47 kDa
AUTHORSHIP
W.E.G. carried out lipid body protocol development, histology and fluorescence microscopy, flow cytometry, and Western blots in Figs. 2 and 4. L.M.N.S. carried out calcium assays, secretion assays, and triglyceride assays and collaborated on Western blots. K.M-U. performed RT-PCR analysis. H.T. directed the project, assisted on flow cytometry and microscopy experiments, and was responsible for data analysis and manuscript preparation. All authors participated in manuscript writing and development.
REFERENCES
- 1. Abraham S. N., St John A. L. (2010) Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 10, 440–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galli S. J., Tsai M. (2010) Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur. J. Immunol. 40, 1843–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Husemoen L. L., Glumer C., Lau C., Pisinger C., Morch L. S., Linneberg A. (2008) Association of obesity and insulin resistance with asthma and aeroallergen sensitization. Allergy 63, 575–582 [DOI] [PubMed] [Google Scholar]
- 4. Ioannidis I. (2008) The road from obesity to type 2 diabetes. Angiology 59, 39S–43S [DOI] [PubMed] [Google Scholar]
- 5. Lee E. J., In K. H., Ha E. S., Lee K. J., Hur G. Y., Kang E. H., Jung K. H., Lee S. Y., Kim J. H., Shin C., Shim J. J., Kang K. H., Yoo S. H. (2009) Asthma-like symptoms are increased in the metabolic syndrome. J. Asthma. 46, 339–342 [DOI] [PubMed] [Google Scholar]
- 6. Liu J., Divoux A., Sun J., Zhang J., Clement K., Glickman J. N., Sukhova G. K., Wolters P. J., Du J., Gorgun C. Z., Doria A., Libby P., Blumberg R. S., Kahn B. B., Hotamisligil G. S., Shi G. P. (2009) Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 15, 940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carvalho V. F., Barreto E. O., Cordeiro R. S., Lagente V., Martins M. A., e Silva P. M. (2005) Mast cell changes in experimental diabetes: focus on attenuation of allergic events. Mem. Inst. Oswaldo Cruz 100 (Suppl. 1), 121–125 [DOI] [PubMed] [Google Scholar]
- 8. Casaco A., Carvajal D., Tolon Z. (1991) Diabetes-induced rat hyposensitivity to compound 48/80. Can. J. Physiol. Pharmacol. 69, 886–888 [DOI] [PubMed] [Google Scholar]
- 9. Cavalher-Machado S. C., de Lima W. T., Damazo A.S., de Frias Carvalho V., Martins M. A., e Silva P. M., Sannomiya P. (2004) Down-regulation of mast cell activation and airway reactivity in diabetic rats: role of insulin. Eur. Respir. J. 24, 552–558 [DOI] [PubMed] [Google Scholar]
- 10. Diaz B. L., Serra M. F., Alves A. C., Pires A. L., Correa F. M., Cordeiro R. S., Martins M. A., e Silva P. M. (1996) Alloxan diabetes reduces pleural mast cell numbers and the subsequent eosinophil influx induced by allergen in sensitized rats. Int. Arch. Allergy Immunol. 111, 36–43 [DOI] [PubMed] [Google Scholar]
- 11. de Oliveira Barreto E., de Frias Carvalho V., Diaz B. L., Balduino A., Cordeiro R. S., Martins M. A., Rodrigues e Silva P. M. (2003) Adoptive transfer of mast cells abolishes the inflammatory refractoriness to allergen in diabetic rats. Int. Arch. Allergy Immunol. 131, 212–220 [DOI] [PubMed] [Google Scholar]
- 12. Csaba G., Pallinger E. (2007) In vitro effect of hormones on the hormone content of rat peritoneal and thymic cells. Is there an endocrine network inside the immune system? Inflamm. Res. 56, 447–451 [DOI] [PubMed] [Google Scholar]
- 13. Hirai K., Miyamasu M., Yamaguchi M., Nakajima K., Ohtoshi T., Koshino T., Takaishi T., Morita Y., Ito K. (1993) Modulation of human basophil histamine release by insulin-like growth factors. J. Immunol. 150, 1503–1508 [PubMed] [Google Scholar]
- 14. Kuna P., Reddigari S. R., Schall T. J., Rucinski D., Sadick M., Kaplan A. P. (1993) Characterization of the human basophil response to cytokines, growth factors, and histamine releasing factors of the intercrine/chemokine family. J. Immunol. 150, 1932–1943 [PubMed] [Google Scholar]
- 15. Lessmann E., Grochowy G., Weingarten L., Giesemann T., Aktories K., Leitges M., Krystal G., Huber M. (2006) Insulin and insulin-like growth factor-1 promote mast cell survival via activation of the phosphatidylinositol-3-kinase pathway. Exp. Hematol. 34, 1532–1541 [DOI] [PubMed] [Google Scholar]
- 16. Kettner A., Di Matteo M., Santoni A. (2010) Insulin potentiates FcεRI-mediated signaling in mouse bone marrow-derived mast cells. Mol. Immunol. 47, 1039–1046 [DOI] [PubMed] [Google Scholar]
- 17. Hammel I., Dvorak A. M., Peters S. P., Schulman E. S., Dvorak H. F., Lichtenstein L. M., Galli S. J. (1985) Differences in the volume distributions of human lung mast cell granules and lipid bodies: evidence that the size of these organelles is regulated by distinct mechanisms. J. Cell Biol. 100, 1488–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Melo R. C., D'Avila H., Wan H. C., Bozza P. T., Dvorak A. M., Weller P. F. (2011) Lipid bodies in inflammatory cells: structure, function, and current imaging techniques. J. Histochem. Cytochem. 59, 540–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bandeira-Melo C., Phoofolo M., Weller P. F. (2001) Extranuclear lipid bodies, elicited by CCR3-mediated signaling pathways, are the sites of chemokine-enhanced leukotriene C4 production in eosinophils and basophils. J. Biol. Chem. 276, 22779–22787 [DOI] [PubMed] [Google Scholar]
- 20. Bozza P. T., Melo R. C., Bandeira-Melo C. (2007) Leukocyte lipid bodies regulation and function: contribution to allergy and host defense. Pharmacol. Ther. 113, 30–49 [DOI] [PubMed] [Google Scholar]
- 21. Dvorak A. M., Dvorak H. F., Peters S. P., Shulman E. S., MacGlashan D. W., Jr., Pyne K., Harvey V. S., Galli S. J., Lichtenstein L. M. (1983) Lipid bodies: cytoplasmic organelles important to arachidonate metabolism in macrophages and mast cells. J. Immunol. 131, 2965–2976 [PubMed] [Google Scholar]
- 22. Beller M., Thiel K., Thul P. J., Jackle H. (2010) Lipid droplets: a dynamic organelle moves into focus. FEBS Lett. 584, 2176–2182 [DOI] [PubMed] [Google Scholar]
- 23. Brasaemle D. L., Dolios G., Shapiro L., Wang R. (2004) Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 279, 46835–46842 [DOI] [PubMed] [Google Scholar]
- 24. Cermelli S., Guo Y., Gross S. P., Welte M. A. (2006) The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr. Biol. 16, 1783–1795 [DOI] [PubMed] [Google Scholar]
- 25. Robenek H., Buers I., Hofnagel O., Robenek M. J., Troyer D., Severs N. J. (2009) Compartmentalization of proteins in lipid droplet biogenesis. Biochim. Biophys. Acta 1791, 408–418 [DOI] [PubMed] [Google Scholar]
- 26. Robenek H., Robenek M. J., Troyer D. (2005) PAT family proteins pervade lipid droplet cores. J. Lipid Res. 46, 1331–1338 [DOI] [PubMed] [Google Scholar]
- 27. Walther T. C., Farese R. V., Jr., (2009) The life of lipid droplets. Biochim. Biophys. Acta 1791, 459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wolins N. E., Brasaemle D. L., Bickel P. E. (2006) A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 580, 5484–5491 [DOI] [PubMed] [Google Scholar]
- 29. Accioly M. T., Pacheco P., Maya-Monteiro C. M., Carrossini N., Robbs B. K., Oliveira S. S., Kaufmann C., Morgado-Diaz J. A., Bozza P. T., Viola J. P. (2008) Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res. 68, 1732–1740 [DOI] [PubMed] [Google Scholar]
- 30. Bozza P. T., Yu W., Penrose J. F., Morgan E. S., Dvorak A. M., Weller P. F. (1997) Eosinophil lipid bodies: specific, inducible intracellular sites for enhanced eicosanoid formation. J. Exp. Med. 186, 909–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dvorak A. M., Hammel I., Schulman E. S., Peters S. P., MacGlashan D. W., Jr., Schleimer R. P., Newball H. H., Pyne K., Dvorak H. F., Lichtenstein L. M.., et al. (1984) Differences in the behavior of cytoplasmic granules and lipid bodies during human lung mast cell degranulation. J. Cell Biol. 99, 1678–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dvorak A. M., Morgan E., Schleimer R. P., Ryeom S. W., Lichtenstein L. M., Weller P. F. (1992) Ultrastructural immunogold localization of prostaglandin endoperoxide synthase (cyclooxygenase) to non-membrane-bound cytoplasmic lipid bodies in human lung mast cells, alveolar macrophages, type II pneumocytes, and neutrophils. J. Histochem. Cytochem. 40, 759–769 [DOI] [PubMed] [Google Scholar]
- 33. White U. A., Stephens J. M. (2010) Transcriptional factors that promote formation of white adipose tissue. Mol. Cell. Endocrinol. 318, 10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tontonoz P., Spiegelman B. M. (2008) Fat and beyond: the diverse biology of PPARγ. Annu. Rev. Biochem. 77, 289–312 [DOI] [PubMed] [Google Scholar]
- 35. Passante E., Frankish N. (2009) The RBL-2H3 cell line: its provenance and suitability as a model for the mast cell. Inflamm. Res. 58, 737–745 [DOI] [PubMed] [Google Scholar]
- 36. DiDonato D., Brasaemle D. L. (2003) Fixation methods for the study of lipid droplets by immunofluorescence microscopy. J. Histochem. Cytochem. 51, 773–780 [DOI] [PubMed] [Google Scholar]
- 37. Koopman R., Schaart G., Hesselink M. K. (2001) Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem. Cell Biol. 116, 63–68 [DOI] [PubMed] [Google Scholar]
- 38. Berlin G., Enerback L. (1983) Fluorescent berberine binding as a marker of secretory activity in mast cells. Int. Arch. Allergy Appl. Immunol. 71, 332–339 [DOI] [PubMed] [Google Scholar]
- 39. Enerback L. (1974) Berberine sulphate binding to mast cell polyanions: a cytofluorometric method for the quantification of heparin. Histochemistry 42, 301–313 [DOI] [PubMed] [Google Scholar]
- 40. Zou C., Shen Z. (2007) One-step intracellular triglycerides extraction and quantitative measurement in vitro. J. Pharmacol. Toxicol. Methods 56, 63–66 [DOI] [PubMed] [Google Scholar]
- 41. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Green H., Kehinde O. (1975) An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5, 19–27 [DOI] [PubMed] [Google Scholar]
- 43. Green H., Kehinde O. (1979) Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J. Cell. Physiol. 101, 169–171 [DOI] [PubMed] [Google Scholar]
- 44. Kim S.J., Nian C., McIntosh C. H. (2011) Adipocyte expression of the glucose-dependent insulinotropic polypeptide receptor involves gene regulation by PPARγ and histone acetylation. J. Lipid Res. 52, 759–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Russell T. R., Ho R. (1976) Conversion of 3T3 fibroblasts into adipose cells: triggering of differentiation by prostaglandin F2α and 1-methyl-3-isobutyl xanthine. Proc. Natl. Acad. Sci. USA 73, 4516–4520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Almeida P. E., Silva A. R., Maya-Monteiro C. M., Torocsik D., D'Avila H., Dezso B., Magalhaes K. G., Castro-Faria-Neto H. C., Nagy L., Bozza P. T. (2009) Mycobacterium bovis bacillus Calmette-Guerin infection induces TLR2-dependent peroxisome proliferator-activated receptor γ expression and activation: functions in inflammation, lipid metabolism, and pathogenesis. J. Immunol. 183, 1337–1345 [DOI] [PubMed] [Google Scholar]
- 47. D'Avila H., Melo R. C., Parreira G. G., Werneck-Barroso E., Castro-Faria-Neto H. C., Bozza P. T. (2006) Mycobacterium bovis bacillus Calmette-Guerin induces TLR2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J. Immunol. 176, 3087–3097 [DOI] [PubMed] [Google Scholar]
- 48. D'Avila H., Roque N. R., Cardoso R. M., Castro-Faria-Neto H. C., Melo R. C., Bozza P. T. (2008) Neutrophils recruited to the site of Mycobacterium bovis BCG infection undergo apoptosis and modulate lipid body biogenesis and prostaglandin E production by macrophages. Cell Microbiol. 10, 2589–2604 [DOI] [PubMed] [Google Scholar]
- 49. Perretti M., Nuti S., Parente L. (1990) Investigation of rat mast cell degranulation using flow cytometry. J. Pharmacol. Methods 23, 187–194 [DOI] [PubMed] [Google Scholar]
- 50. Johnson M. M., Vaughn B., Triggiani M., Swan D. D., Fonteh A. N., Chilton F. H. (1999) Role of arachidonyl triglycerides within lipid bodies in eicosanoid formation by human polymorphonuclear cells. Am. J. Respir. Cell Mol. Biol. 21, 253–258 [DOI] [PubMed] [Google Scholar]
- 51. Wan H. C., Melo R. C., Jin Z., Dvorak A. M., Weller P. F. (2007) Roles and origins of leukocyte lipid bodies: proteomic and ultrastructural studies. FASEB J. 21, 167–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Triggiani M., Oriente A., de Crescenzo G., Rossi G., Marone G. (1995) Biochemical functions of a pool of arachidonic acid associated with triglycerides in human inflammatory cells. Int. Arch. Allergy Immunol. 107, 261–263 [DOI] [PubMed] [Google Scholar]
- 53. Ristimaki A., Narko K., Hla T. (1996) Down-regulation of cytokine-induced cyclo-oxygenase-2 transcript isoforms by dexamethasone: evidence for post-transcriptional regulation. Biochem. J. 318, 325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carvalho Vde F., Barreto Ede O., Farias-Filho F. A., Gomes L. H., Mendonca Lde L., Cordeiro R. S., Martins M. A., Rodrigues e Silva P. M. (2009) Reduced expression of IL-3 mediates intestinal mast cell depletion in diabetic rats: role of insulin and glucocorticoid hormones. Int. J. Exp. Pathol. 90, 148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Williams R. M., Shear J. B., Zipfel W. R., Maiti S., Webb W. W. (1999) Mucosal mast cell secretion processes imaged using three-photon microscopy of 5-hydroxytryptamine autofluorescence. Biophys. J. 76, 1835–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sugii S., Olson P., Sears D. D., Saberi M., Atkins A. R., Barish G. D., Hong S. H., Castro G. L., Yin Y. Q., Nelson M. C., Hsiao G., Greaves D. R., Downes M., Yu R. T., Olefsky J. M., Evans R. M. (2009) PPARγ activation in adipocytes is sufficient for systemic insulin sensitization. Proc. Natl. Acad. Sci. USA 106, 22504–22509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hodges B. D., Wu C. C. (2010) Proteomic insights into an expanded cellular role for cytoplasmic lipid droplets. J. Lipid Res. 51, 262–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vianna E. O., Garcia-Leme J. (1995) Allergen-induced airway inflammation in rats. Role of insulin. Am. J. Respir. Crit. Care Med. 151, 809–814 [DOI] [PubMed] [Google Scholar]
- 59. Dvorak A. M., Dvorak H. F., Galli S. J. (1983) Ultrastructural criteria for identification of mast cells and basophils in humans, guinea pigs, and mice. Am. Rev. Respir. Dis. 128, S49–S52 [DOI] [PubMed] [Google Scholar]
- 60. Galli S. J. (1990) New insights into “the riddle of the mast cells”: microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab. Invest. 62, 5–33 [PubMed] [Google Scholar]
- 61. Triggiani M., Casolaro V., Genovese A., Spadaro G., Marone G. (1993) Heterogeneity of human Fc ε RI-bearing cells. Ann. Allergy 71, 133–138 [PubMed] [Google Scholar]
- 62. Silva A. R., Pacheco P., Vieira-de-Abreu A., Maya-Monteiro C. M., D'Alegria B., Magalhaes K. G., de Assis E. F., Bandeira-Melo C., Castro-Faria-Neto H. C., Bozza P. T. (2009) Lipid bodies in oxidized LDL-induced foam cells are leukotriene-synthesizing organelles: a MCP-1/CCL2 regulated phenomenon. Biochim. Biophys. Acta 1791, 1066–1075 [DOI] [PubMed] [Google Scholar]
- 63. Bougneres L., Helft J., Tiwari S., Vargas P., Chang B. H., Chan L., Campisi L., Lauvau G., Hugues S., Kumar P., Kamphorst A. O., Dumenil A. M., Nussenzweig M., MacMicking J. D., Amigorena S., Guermonprez P. (2009) A role for lipid bodies in the cross-presentation of phagocytosed antigens by MHC class I in dendritic cells. Immunity 31, 232–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dvorak A. M. (2005) Mast cell secretory granules and lipid bodies contain the necessary machinery important for the in situ synthesis of proteins. Chem. Immunol. Allergy 85, 252–315 [DOI] [PubMed] [Google Scholar]
- 65. Vieira V. J., Ronan A. M., Windt M. R., Tagliaferro A. R. (2005) Elevated atopy in healthy obese women. Am. J. Clin. Nutr. 82, 504–509 [DOI] [PubMed] [Google Scholar]
- 66. Dahlquist e.a. (2000) Decreased prevalence of atopic diseases in children with diabetes. The EURODIAB Substudy 2 Study Group. J. Pediatr. 137, 470–474 [DOI] [PubMed] [Google Scholar]