Review of the role for cytokines and cytokine-responsive STAT transcription factors in dendritic cell development.
Keywords: Cytokines, transcription factors, immune cell development
Abstract
DCs have a vital role in the immune system by recognizing exogenous or self-antigens and eliciting appropriate stimulatory or tolerogenic adaptive immune responses. DCs also contribute to human autoimmune disease and, when depleted, to immunodeficiency. Moreover, DCs are being explored for potential use in clinical therapies including cancer treatment. Thus, understanding the molecular mechanisms that regulate DCs is crucial to improving treatments for human immune disease and cancer. DCs constitute a heterogeneous population including plasmacytoid (pDC) and classic (cDC) subsets; however, the majority of DCs residing in lymphoid organs and peripheral tissues in steady state share common progenitor populations, originating with hematopoietic stem cells. Like other hematopoietic lineages, DCs require extracellular factors including cytokines, as well as intrinsic transcription factors, to control lineage specification, commitment, and maturation. Here, we review recent findings on the roles for cytokines and cytokine-activated STAT transcription factors in DC subset development. We also discuss how cytokines and STATs intersect with lineage-regulatory transcription factors and how insight into the molecular basis of human disease has revealed transcriptional regulators of DCs. Whereas this is an emerging area with much work remaining, we anticipate that knowledge gained by delineating cytokine and transcription factor mechanisms will enable a better understanding of DC subset diversity, and the potential to manipulate these important immune cells for human benefit.
Introduction
Since the discovery of DCs in the early 1970s by the 2011 Nobel Prize winner, Dr. Ralph Steinman, and colleagues [1, 2], significant progress has been achieved in understanding the origin and molecular control of DC lineage differentiation as well as the function of DCs in immunity. For instance, it is now recognized that DCs represent a heterogeneous population that comprises two major categories: plasmacytoid DCs (pDCs) and classic DCs (cDCs), with unique functions for individual subsets in host immune responses and immunological tolerance. Here, we describe recent findings in understanding the molecular regulation of murine and human DC development, with a particular focus on cytokines, STATs, and transcription factors associated with human disease. We reference specific pDC and cDC populations by cell surface marker expression, anatomical location, and/or immunological activity. It should be noted that several excellent reviews on pDC and cDC origin and function have been published recently, providing significant additional information outside the scope of our review [3–15].
MURINE DC SUBSETS AND DEVELOPMENTAL ORIGINS
pDCs were first identified in humans and mice by their ability to produce massive quantities of type I IFNs on viral infection or TLR stimulation [16–18]. Murine pDCs are defined by the surface phenotype CD11cloCD11b−B220+PDCA-1+SiglecH+ as well as their type I IFN-producing capability; however, recent results indicate that certain pDC populations have suppressed type I IFN secretion responses (e.g., Peyer's patch pDCs) [19]. In both species, pDCs are localized in bone marrow, blood, and lymphoid organs in steady state and can accumulate in tissues in disease or during physiological stress [20–24]. PDCs play important roles in antiviral immunity, T lymphocyte activation, and immune tolerance [6, 25–29].
In contrast, cDCs are efficient phagocytic cells that reside within lymphoid and nonlymphoid organs in mice. This broad distribution provides the immune system with the ability to sample antigens across distinct tissue sites. The cDC lineages are typically delineated as being lymphoid-resident or tissue-resident “migratory” DCs, with the latter category having the ability to migrate to lymphoid organs upon activation by cytokines or TLR agonists. Activation of cDCs is accompanied by induction of MHC class II (MHCII) and costimulatory molecule expression, cytokine secretion, and acquisition of potent antigen presentation activity required to stimulate adaptive immune responses [30–32]. Murine cDC subsets are distinguished by cell surface marker proteins, sites of tissue residence, immunological activity, and distinct developmental pathways, with the latter being judged by dependence on overlapping yet divergent molecular signals (Table 1 and references within). It should be mentioned that there is some overlap between cDC and macrophage marker protein expression and function, and some controversy exists in the distinction of these cell types [4, 33–35]. Nonetheless, certain cDC populations such as lymphoid organ CD11chiCD11bloMHCII+CD4−CD8α+ DCs (CD8α+ DCs) and tissue-resident CD11chiCD11bloMHCII+CD103+ DCs (CD103+ DCs) demonstrate efficient antigen cross-presentation ability by using MHC class I to present exogenously derived antigens [36–41], which is not a typical macrophage feature. Accordingly, CD8α+ DCs and CD103+ DCs have important roles in antiviral and antitumor immune responses [5, 42]. Because of their effective antigen presentation properties, human equivalents to murine cDCs are being used for anticancer therapy and future progress in this area is greatly anticipated [43].
Table 1. Surface marker, tissue distribution, and transcriptional regulators of murine DC subsets.
| DC subset | Surface markers | Tissue distribution | Transcription factor(s) requireda | References |
|---|---|---|---|---|
| pDC | CD11cloCD11b−B220+PDCA-1+ SiglecH+ | Lymphoid/nonlymphoid | E2-2, Gfi1, Ikaros, IRF4, IRF8, PU.1, STAT3 | [99, 100, 102–104, 106, 108, 238] |
| cDC | ||||
| CD8α+ | CD11chiMHCII+CD11bloCD4− CD8α+ | Lymphoid | Batf3, Gfi1, Id2, Ikaros, IRF8, PU.1 | [100, 102, 107, 108, 113, 215, 239, 240] |
| CD8α− | CD11chiMHCII+CD11bhiCD8α− CD4+ or CD4− | Lymphoid | Gfi1, IRF2, IRF4, PU.1, RelB, RBP-J | [100, 108, 112, 119–122, 147] |
| CD103+ | CD11c+MHCII+CD11bloCD103+ | Nonlymphoid; lymph nodes; lamina propriab | Batf3, IRF8, Id2 | [116, 118] |
| CD103− | CD11c+MHCII+CD11bhiCD103− | Nonlymphoid; lamina propriab | Unknown | [117] |
| LC | Langerin+MHCIIloCD11b+ CD207hiCD103− | Epidermis | Id2, IRF2, Runx3 | [113, 123, 147] |
While PU.1 function has not been determined in all DC subsets listed, it is expected to be required due to its role in mediating macrophage and DC development.
In mouse lamina propria, the CD103+ and CD103− DCs are further characterized based on their expression of M-CSFR and CX3CR1, including MHCIIhiCD11chiCD103+CD11b+ CX3CR1−M-CSFRlo, MHCIIhiCD11chiCD103−CD11b+CX3CR1+M-CSFRhi, MHCIIhiCD11chiCD103+CD11b−CX3CR1−M-CSFRlo, and MHCIIhiCD11cloCD103−CD11b+CX3CR1+M-CSFRhi [117].
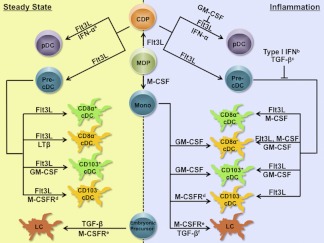
All murine DC subsets, with the exception of epidermal Langerhans cells (LCs), are derived from Flt3+ multipotent hematopoietic progenitors that reside in the bone marrow via an apparently shared pathway, which proceeds through a macrophage-DC progenitor (MDP) (lin−c-kithiCD115+CX3CR1+Flt3+) and a common DC progenitor (CDP) (lin−c-kitloCD115+Flt3+) [44–48] (Fig. 1). The pDC and cDC populations appear to diverge at the CDP, with pDCs developing within bone marrow and cDCs undergoing dispersion to tissues via circulating cDC precursors (precDCs), followed by terminal differentiation in lymphoid or nonlymphoid organs [49–51]. Under inflammatory conditions, cDCs, but not pDCs, can derive from monocytes in tissues [52–54]. Epidermal LCs can also be generated from circulating monocytes that migrate to inflamed skin; for example, after UV exposure. In contrast, in steady state, LCs repopulate locally through self-renewal or from skin-residing, radioresistant precursors seeded during embryogenesis [55–57].
Figure 1. Control of DC subset development by cytokines under steady state and inflammatory conditions.
The ability of cytokines to positively regulate specific DC lineages is indicated by →; negative effects are indicated by ⊢. aIFN-α-IFNAR signals were shown to control the accrual of pDCs in Peyer's patches [106]. b,cType I IFN suppresses the development of CD8α+ and CD8α− DCs, whereas TGF-β inhibits CD11c+B220− DC production [119, 141, 147, 155, 156]. dThe role of M-CSFR ligands (M-CSF or IL-34) was demonstrated in the development of CD103−CD11b+CX3CR1+ DCs in lamina propria [117]. eMice that lack M-CSFR show a more severe deficiency in LCs than those lacking M-CSF [57]. fTGF-β drives development of LC-like cells in human monocyte cultures, in the presence of GM-CSF and IL-4 [52].
HUMAN DC SUBSETS AND THEIR ORIGINS
Characterizing DC populations in humans has been challenging because of the limited availability of hematopoietic, lymphoid, and nonlymphoid tissues. Here we provide a snapshot of the knowledge to date, which we anticipate will continue to evolve rapidly. Excellent reviews of human DC subset diversity, ontogeny, and function are available and contain more in-depth discussion of these topics than we are able to provide [14, 58–61].
In blood and lymphoid tissues, human DCs are broadly classified into HLA-DR+ CD123+pDCs and HLA-DR+ CD11c+ “myeloid DCs,” which include the major CD1c+ and CD141+ subsets (also known as BDCA-1+ and BDCA-3+ subsets, respectively) and which are considered counterparts to murine cDCs (Table 2) [14, 17, 61–65]. Nonetheless, the use of the term myeloid to describe the non-pDC populations may be somewhat imprecise because the developmental origin of human DCs is not fully resolved and lymphoid progenitors contribute to DC generation [14, 60, 66]. CD16+ monocyte-like DCs have also been identified in blood [63]; however, like mice, the relationship of these cells to macrophage and/or DC lineages remains ambiguous [14, 34, 61]. Also analogous to mice, DCs found in human lymphoid organs comprise both resident and migratory populations [65, 67]. HLA-DR+ CD11c+ DCs are found in T-cell, B-cell, and marginal zones of the spleen as well as cortical and T-cell-rich areas of the lymph nodes, with individual subsets showing unique as well as overlapping localization [65, 67, 68]. DCs within splenic T-cell zones exhibit increased expression of the CD86 costimulatory molecule, evidence of activation [68]. Moreover, discrete DC subsets appear to have functional specialization, with blood CD141+ DCs showing analogy to murine CD8α+ DCs in their ability to cross-present exogenous antigens [65, 69–74].
Table 2. Surface marker, tissue distribution, and transcriptional regulators of human DC subsets.
| DC subset | Surface markers | Tissue distribution | Transcription factor(s) | Referencesa |
|---|---|---|---|---|
| pDC | HLA-DR+CD11c−BDCA-2+ BDCA-4+CD123+ | Blood, tonsil, lymph nodes | E2-2, GATA-2, IRF8, SpiB, PU.1 | [99, 216, 221–223, 237] |
| DC | ||||
| CD1c+ (BDCA-1+) | HLA-DR+CD11c+CD11b+ CD1c+ | Blood, tonsil, lymph nodes | GATA-2, IRF8 | [216, 221–223] |
| CD141+ (BDCA-3+) | HLA-DR+CD11c+CD11b− CD141+XCR1+CLEC9A+ | Blood, tonsil, lymph nodes | GATA-2, IRF8 | [216, 221–223] |
| CD103+ | DC-LAMP+CD103+ | Mesenteric lymph nodes | Unknown | NA |
| CD1a+ | CD11c+CD1a+CD14− | Skin, lymph nodes | GATA-2, STAT5 | [157, 221–223] |
| CD14+ | CD11c+ CD14+ CD1a− | Skin, lymph nodes | GATA-2 | [221–223] |
| LC | CD11c+EpCAM+ | Skin, skin-draining lymph nodes | Unknown | NA |
| Mono/DC | ||||
| CD16+ | CD11c+CD16+HLA-DR+ | Blood | GATA-2 | [221–223] |
References for transcriptional regulators are provided. References for DC subset identification and localization can be found in the text.
In nonlymphoid tissues, DC populations are best characterized in skin; these comprise LCs, CD1a+, and CD14+ DCs (Table 2) [75]. Dermal DCs also show phenotypic changes associated with activation and/or maturation, including up-regulation of CD83 [76], suggesting a previous encounter with ligands that activate pattern recognition receptors. In the gut, CD103+ DCs are present in human mesenteric lymph nodes, which may imply a tissue-derived migratory population with homology to murine CD103+ DCs [77]. Despite the identification of numerous human DC populations, major challenges remain in understanding which subsets comprise discrete lineages, the precursor-product relationships between distinct DC subsets (e.g., DCs resident in tissues that migrate to lymph nodes), possible analogy to murine DC populations, and mechanisms that control lineage development.
Hematopoietic stem cell and organ transplants have enabled a glimpse into human DC regenerative mechanisms. The investigation of dermal DC subsets after hematopoietic stem cell transplantation established that human DCs and LCs are derived from donor bone marrow precursors [78, 79]. In human hand allografts, however, LCs remain of graft origin for up to 10 years [80]. The human LC phenotype is consistent with regeneration from bone marrow precursors after systemic hematopoietic disruption, or local renewal in steady state or nonsystemic insults, similar to murine LCs [55–57]. In addition, the hematopoietic stem cell origin of DCs is shared between humans and mice.
OVERVIEW OF REGULATORY FACTORS INVOLVED IN MURINE DC SUBSET GENERATION
Accumulating evidence suggests that the commitment and development of pDC and cDC subsets are controlled by a complex network formed by cytokine-cytokine receptor signals and transcription factors, similar to that for other hematopoietic lineages [7, 81]. The majority of pDC and cDC subsets depend on fms-like tyrosine kinase 3 ligand (Flt3L) and its receptor Flt3 for their development in vivo and respond to elevated circulating Flt3L amounts by expanding their numbers [54, 82–88]. The tumor necrosis family member lymphotoxin-β (LTβ), TGF-β and the cytokines GM-CSF (also known as CSF-2) and M-CSF (also known as CSF-1) also have important roles in directing development of specific pDC and/or cDC subsets [45, 57, 82, 86, 89–98]. STAT transcription factors, activated downstream of cytokine receptor engagement, and an array of lineage-restricted transcriptional regulators, are critical for pDC and cDC differentiation [89, 99–113] (Table 1). Nonetheless, it remains unclear whether and how these signaling cascades interact with each other to switch “on” or “off” the developmental cues for pDC and cDC differentiation from common progenitor populations.
PDCs develop in the bone marrow in response to Flt3L-Flt3 signals, with dependence on numerous transcription factors including STAT3 [89, 105, 106], PU.1 (Sfpi1) [100, 109, 111, 112], Gfi1 [108], Ikaros [110], IRF4 [103], IRF8 [102–104] and E2-2 (Tcf4) [99, 114]. Notably, a unique subset of pDCs in Peyer's patches is also generated from CDPs [106]; however, this population lacks the capability to secrete type I IFNs and requires type I IFN signals for their accrual in steady state [19, 106]. Lymphoid organ CD8α+ DCs and tissue-resident CD103+ DCs are thought to be developmentally related because they derive from a precDC precursor and share dependence on Flt3L-Flt3, IRF8, Batf3, and Id2 [4, 107, 115–118]. In addition, recent studies have revealed that GM-CSF controls the differentiation of MHCII+ DCs in lymphoid organs as well as CD103+ DCs in dermis and lamina propria [82, 92, 117] and potentially other nonlymphoid organs, highlighting a previously unrecognized role for GM-CSF in tissue DC homeostasis. In contrast, the molecular control of lymphoid organ CD11b+CD8α− DCs (including CD4+ and CD4− populations) is less investigated; however, the transcription factors PU.1, IRF2, IRF4, RelB (an NF-κB subunit), and RBP-J (a component of the Notch1 signaling cascade) have been implicated [112, 119–122]. Epidermal LCs appear to use a unique molecular program that involves TGF-β1, M-CSF receptor (M-CSFR, also known as CSF-1R or CD115), Id2, and Runx3 for their development [57, 94, 113, 123, 124].
ROLES FOR CYTOKINES IN MURINE DC DEVELOPMENT
Flt3L
Flt3L exists as both membrane-bound and soluble isoforms and is produced by tissue stromal cells and activated T cells [125]. The effects of Flt3L in hematopoiesis appear to be restricted by the expression of the receptor Flt3, which is found primarily on early hematopoietic progenitors with short-lived repopulating activity, DC precursors, and differentiated pDCs and cDCs [44, 45, 50, 125]. Flt3L is capable of driving the generation of pDCs and the equivalent of lymphoid organ CD8α+ and CD8α− DCs (e.g., CD24hiSIRP-αloCD11blo and CD24loSIRP-αhiCD11bhi DCs, respectively) from mouse bone marrow progenitor cells in vitro [84, 98, 126, 127]. Flt3L also stimulates pDC, CD11c+CD11b+ and CD11c+CD11b− DC generation from fetal liver cultures [89, 128]. Consistently, enforced expression of Flt3L in vivo by injection of recombinant cytokine or inducible transgene causes a massive expansion of DCs, particularly pDCs and CD8α+ DCs in spleen, as well as tissue CD103+ DCs, whereas deficiency in either Flt3L or Flt3 leads to a significant reduction of pDCs and cDCs [50, 54, 82, 83, 85–88, 117, 118, 129–132]. Furthermore, MDPs are reduced approximately 40% in Flt3-deficient mice but not in Flt3L-deficient mice, indicating a potential for other ligands to activate Flt3 as well as a partial impact of Flt3 function on the DC progenitor compartment [50]. Collectively, these data demonstrate a critical and nonredundant role of Flt3L-Flt3 signaling in murine pDC and cDC homeostasis (Fig. 1). In contrast, DC progenitor commitment appears to be under the control of Flt3L-Flt3 as well as other, as yet undescribed, signals in vivo.
GM-CSF
GM-CSF was the original cytokine used to derive cDCs in vitro from mouse and human bone marrow progenitors or human monocytes, often in conjunction with IL-4, for use in the laboratory [90, 133, 134]. More recently, GM-CSF has been used to generate and/or stimulate human DCs in clinical applications, including cancer immunotherapy [135–139]. Recombinant GM-CSF possesses a relatively short half-life in vivo. Thus, approaches to overexpress this cytokine have involved generation of GM-CSF transgenic mice, GM-CSF-secreting cells (e.g., melanoma), protein modification to extend half-life (e.g., polyethylene glycol or pegylation), or hydrodynamic gene transfer with GM-CSF-encoding plasmids [86, 91, 106, 139, 140]. Systemic GM-CSF overexpression results in substantial increases in CD11c+CD11b+ DCs and CD8α− DCs, which are a major subset of the CD11c+CD11b+ DC population, in bone marrow, spleen, lymph nodes, and thymus [86, 91, 106, 140] (Fig. 1). However, the significance of GM-CSF in DC homeostasis was thought to be limited, because mice that lack GM-CSF or GM-CSF receptor β chain show relatively minor defects in lymphoid organ DC abundance compared with Flt3 or Flt3L deficiency [91]. This dogma was challenged by recent studies, which demonstrated that GM-CSF is indispensable for the development of CD103+ DCs in lamina propria, skin, and skin-draining lymph nodes [82, 92, 117], indicating a major role in regulating tissue-resident DCs. Moreover, GM-CSF was shown to induce the expression of the CD103 marker in DCs in vitro [115], which further suggests that GM-CSF mediates the differentiation of tissue resident CD103+ DCs (Fig. 1). However, these results also introduce a potentially confounding issue in evaluating CD103+ DC responses to GM-CSF in vivo, because alternative methods to verify the GM-CSF-dependent regulation of this subset must be used.
IFNs
Type I IFNs, such as IFN-α and IFN-β, have been recently recognized to control pDC and cDC amounts after systemic infection or cytokine delivery, in addition to their well-known enhancing effects on MHC and costimulatory molecule expression and their immunomodulatory activity [81, 106, 141–146]. Recombinant IFN-β, or type I IFN-inducing measles (MV) or lymphocytic choriomeningitis (LCMV) viral infection, inhibits Flt3L- or GM-CSF-mediated CD11c+MHCII+ DC development in vitro and Flt3L-responsive expansion of splenic CD8α+ DCs in vivo; with viral infection, effects were shown to be due to type I IFN-dependent mechanisms [141]. Similarly, in Irf2−/− mice, excessive type I IFN signaling causes a severe reduction in splenic CD11b+CD8α− and CD4+CD8α− DCs, as well as epidermal CD4+LCs [119, 147]. Collectively, these results indicate that type I IFN acts as a negative regulator of the CD8α+ and CD8α− DC subsets (Fig. 1).
In contrast, infection with attenuated vaccinia Ankara (MVA) virus in neonatal mice enhances serum levels of Flt3L and increases splenic pDC and CD8α+ DC amounts [148]. MVA-induced Flt3L production and DC expansion are dependent on intact type I and type II IFN signaling cascades [148], suggesting that IFNs can initiate a feedback mechanism to enhance Flt3L-dependent DC production. Furthermore, IFN-α overexpression by hydrodynamic gene transfer promotes the accumulation of lin−Flt3+ bone marrow progenitor cells, precursors to pDCs and cDCs, as well as pDCs numbers in vivo [106] (Fig. 1). Recombinant IFN-α also induces pDC generation and inhibits CD11c+CD11b+ DC production when added to Flt3L or GM-CSF cultures in vitro [106]. Moreover, type I IFN and STAT1 signals mediate the accrual of the pDC population within intestinal Peyer's patches in homeostasis [106]; however, the mechanisms involved in this unique IFN function are not yet clear. In contrast, a recent report demonstrated that systemic viral infection causes apoptosis of pDCs via a type I IFN-dependent mechanism, leading to a significant reduction of pDC numbers in spleen [143]. Whether this pathway affects the amounts of DC progenitors and/or pDCs in bone marrow and the role for viral-mediated TLR signals in splenic pDC cell death remains to be determined. Separately, LCMV-elicited IFNs were shown to drive conversion of pDCs to a cDC-like phenotype by inducing CD11b and suppressing B220 expression in pDCs in Flt3L cultures or in vivo [142]. Hence, type I IFNs have been reported to have either enhancing or suppressive effects on pDCs, whereas a more consistent picture of their ability to decrease cDC numbers emerges. The discrepancy in pDC responses may result from the duration and amounts of type I IFNs, the timing between IFN exposure and pDC analysis, as well as the effects of other regulators (e.g., TLR signals) in the scenario.
M-CSF and TGF-β
In addition to the cytokines mentioned above, M-CSF and TGF-β have reported roles in pDC and cDC production. M-CSF is constitutively synthesized by a variety of cell types, including tissue stromal cells, endothelial cells, macrophages, and osteoblasts [7, 149]. The M-CSFR is expressed in MDPs, CDPs, and precDCs [45, 48, 150]. M-CSF induces differentiation of pDCs and CD11c+B220− DCs from total bone marrow in vitro, albeit at low frequency, enhances Flt3L-responsive pDC generation, and augments splenic pDC and CD11c+B220− DC numbers upon injection [45, 93] (Fig. 1). However, these DC populations appear relatively normal in mice lacking functional M-CSF or M-CSFR [95, 96], suggesting redundancy of the M-CSF-M-CSFR signaling cascade with other pathways. In contrast, M-CSFR-mediated signals are uniquely required for LC maintenance in homeostatic conditions and for generation of CD103−CD11b+CX3CR1+ DCs from monocytes in lamina propria [57, 117]. M-CSF has been shown to activate STAT3 in DCs and other cell types [151–153], suggesting a potential mechanism by which DC differentiation is regulated; however, this hypothesis remains to be tested.
TGF-β is uniquely required for steady-state LC generation in mice, operating via an autocrine pathway [94, 154] (Fig. 1). TGF-β controls expression of the transcription factor Id2, which is necessary for LC development [113]. In contrast, the transcription factor Runx3, which is also required for LC generation, does not appear to be under direct regulation by TGF-β. Rather, Runx3 mediates the effects of TGF-β on LC development as well as TGF-β-dependent inhibition of mature CD11c+CD11b+ DCs in lung and CD11c+ DCs in spleen [123]. In fact, TGF-β has a well-established role in suppressing cDC development and function, including antigen presentation [155, 156] (Fig. 1). TGF-β has been shown to inhibit STAT5 phosphorylation while enhancing PU.1 amounts in DCs derived from human CD34+ progenitor cultures [157], suggesting additional mechanisms by which it may regulate DCs.
Effects of cytokine production during physiological stress on DCs
Although Flt3L, GM-CSF, M-CSF, type I IFNs, and TGF-β are critical factors for homeostatic DC development, an important aspect of their role involves their expression during infection, inflammation, and other physiological stresses (e.g., irradiation and cancer), and subsequent effects on the abundance and function of DC populations. For example, circulating GM-CSF is normally below the detectable threshold in the steady state but is readily induced in stromal cells, activated T cells, and NK cells during bacterial infection [158, 159]. In response to Listeria monocytogenes infection, GM-CSF drives the accumulation of inflammatory DCs, i.e., TNF-α/iNOS-producing DCs in the infected mouse spleen [159]. Similarly, enhanced secretion of type I IFN occurs during viral infections, such as vesicular stomatitis virus, MV, or LCMV and, as indicated previously, influences pDC and cDC abundance in vivo [106, 141–143]. In vitro, IFN-α exposure during pDC development leads to the generation of pDCs that preferentially elicit IL-17-producing CD4+ T-cell responses upon TLR stimulation versus T helper 1 responses that are induced by Flt3L-derived pDCs [106]. These results and others suggest that IFNs alter pDC function [106, 142]. In contrast, TGF-β is frequently expressed in the tumor microenvironment, where it is thought to suppress the immunostimulatory activity of tumor-associated antigen-presenting cells [155]. The mechanisms by which TGF-β regulates DC function are poorly understood and require additional investigation; these studies are important to pursue as a means to determine potential methods to overcome TGF-β-mediated DC suppression. Thus, physiological stress can affect cytokine production with consequent results on DC subset amounts and DC function, suggesting that the DC lineages respond to altered physiologic conditions to regulate necessary immune responses. This area of DC biology remains understudied; however, because cytokines are well-established mediators of physiologic stress responses, we anticipate that there will be a significant role for alterations in local and systemic cytokine amounts in fine-tuning DC subset abundance and DC functional responses.
CYTOKINE REGULATION OF HUMAN DCs
Clinical cytokine administration and ex vivo cultures with purified growth factors have provided information about the mechanisms that control human DC production. For example, administration of recombinant Flt3L stimulates expansion of human pDCs and DCs, indicating that the essential role for Flt3L in DC development is conserved in humans and mice [160]. In agreement, human pDCs and DCs can be produced in vitro from CD34+ hematopoietic progenitor cells in Flt3L cultures [126, 161–163]. Furthermore, expression of the Flt3 internal tandem duplication mutant (Flt3-ITD) in acute myeloid leukemia is associated with enhanced pDC and DC frequencies in peripheral blood [164]. Interestingly, Flt3-ITD is linked to increased STAT5-stimulating activity relative to that of wild-type Flt3 [165–168]. The nature of STAT signal transduction has been reported to influence DC function [169], although it is not yet clear whether DCs from Flt3-ITD-positive individuals have distinct activity. This is important to understand, because effects on DCs could potentially participate in Flt3-ITD-mediated leukemic progression. In contrast, GM-CSF is used to generate large amounts of DCs ex vivo from human peripheral blood monocytes or CD34+ progenitors for clinical therapy, or to enhance DC function in vaccine-based treatments [170–178]; however, GM-CSF has been reported to have disparate effects on DC generation in vivo, and thus its role remains unresolved [179, 180].
CYTOKINE-RESPONSIVE STAT PATHWAYS IN MURINE DC GROWTH AND DIFFERENTIATION
STAT3
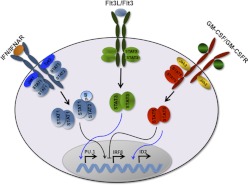
Engagement of Flt3L with Flt3 induces the intrinsic tyrosine kinase function of the receptor, which is reported to activate numerous intracellular signaling intermediates including the serine and threonine kinases Erk1/2, pAkt, mammalian target of rapamycin (mTOR), as well as the transcription factors STAT3 and STAT5a [181–183]. Much of the initial Flt3 signaling data derived, however, from work in hematopoietic tissue culture systems; thus, the relationship of Flt3-responsive pathways to Flt3-mediated DC development was not clear. Subsequently, it was shown that Flt3L rapidly stimulates STAT3 tyrosine phosphorylation in lin−Flt3+ bone marrow progenitor cells (e.g., within 30 min) [89]. This evidence suggests that STAT3 is directly activated by the Flt3 receptor in DC progenitors in response to Flt3L (Fig. 2). Flt3-dependent autocrine pathways have also been suggested [127]; however, these remain to be verified in DC progenitors. Collectively, the results point to a functional role for STAT3 in DC development.
Figure 2. Regulation of DC-related transcription factors by cytokine-responsive STATs.
Black arrows indicate pathways defined in DCs or DC progenitors; blue arrows indicate pathways identified elsewhere, yet require evaluation in DCs and their progenitors.
To examine STAT3 function in vivo, mice with conditional Stat3 deletion have been used because germ-line Stat3 removal causes early lethality [184]. The initial report using hematopoietic Stat3-deficient mice described a substantial reduction in the total CD11c+ DC compartment in lymphoid organs in steady state, impaired CD8α+ DC generation in response to Flt3L treatment in vivo, and reduced production of CD11c+CD11b− DCs, which comprise both pDC and CD8α+ DCs, in Flt3L cultures in vitro [105]. In contrast, an independent study using a similar hematopoietic Stat3-deficient mouse model was the first to show that pDC amounts in vivo are dependent on STAT3 function, whereas nearly normal numbers of bone marrow and splenic CD11c+CD11b+ DCs were detected in this study [106]. In addition, STAT3 was shown to be essential for Flt3L-dependent proliferation of purified lin−Flt3+ progenitors as well as Flt3L-driven pDC and CD11c+CD11b+ DC production in vitro, but not GM-CSF-dependent expansion of CD11c+CD11b+ DCs in bone marrow cultures [106]. Mice with DC-restricted Stat3 deletion also show relatively normal abundance of cDCs in lymphoid organs [185]. Collectively, therefore, the data indicate that STAT3 mediates Flt3L-responsive DC progenitor growth and is necessary for producing the full complement of pDCs in vivo. The role for STAT3 in cDC generation remains unclear; cDCs appear to be dependent on STAT3 when Flt3L is the sole growth factor in vitro; however, discrepancies exist between reports of STAT3 function in vivo. To resolve this, further work needs to be done to assess the amounts of defined DC progenitor populations (e.g., MDPs and CDPs) as well as distinct lymphoid and tissue-resident cDC subsets, along with confirming cDC distinction from macrophage populations that can resemble cDCs [33–35]. These studies should be pursued in hematopoietic or DC-restricted Stat3-deficient mice under homeostatic and Flt3L-driven hematopoiesis.
In addition, it is important to consider whether and how other Flt3-responsive signaling cascades affect DC development. A recent report demonstrated that Flt3L rapidly activates mTOR signaling in DC progenitors [181]. Flt3L-driven development of pDCs and CD8α+ DCs in vitro is suppressed by the mTOR inhibitor rapamycin or by the PI3K-mTOR inhibitor phosphatase and tensin homolog (PTEN), whereas DC-restricted Pten deletion enhances CD8α+ DCs and CD103+ DCs in vivo, indicating that the PI3K-mTOR pathway mediates Flt3L growth-promoting effects on pDCs, CD8α+ DCs, and CD103+ DCs [181]. These results establish an important STAT3-independent pathway by which Flt3 regulates DCs.
STAT5
Here, we refer to the two separate gene products, STAT5a and STAT5b, as STAT5a/b because their roles in DCs have not been examined individually in detail to our knowledge. STAT5a/b is rapidly activated in purified lin−Flt3+ bone marrow progenitors by GM-CSF and serves as its major STAT signal, whereas Flt3L and type I IFNs do not induce detectable amounts of tyrosine-phosphorylated STAT5a/b [89, 106] (Fig. 2). These data indicate that DC progenitors respond directly to GM-CSF stimulation. This point is significant because GM-CSF has been most commonly used to derive cDCs in vitro from heterogeneous bone marrow samples or monocyte populations, and the precise expression pattern of the functional GM-CSF receptor (α and β subunits) during DC development from multipotent progenitor cells is poorly understood.
Two distinct mouse models have been generated to examine the function of STAT5a and STAT5b in vivo [186, 187]. Mice that have complete deletion of Stat5a and Stat5b in the germline have perinatal lethality [187]. Thus, it was necessary to investigate DC development in fetal liver cultures or chimeric animals transplanted with Stat5a−/− Stat5b−/− fetal liver cells. These studies showed that STAT5a/b has an inhibitory role in pDC development by mediating the suppressive effects of GM-CSF in in vitro cultures [89, 98]. The effects of Stat5a/b deficiency on cDCs are less well understood. This is due in part to the need for a more complete analysis of distinct cDC subsets in chimeric Stat5a−/−Stat5b−/− mice as well as to the fact that these chimeras show a general suppression of total cell numbers in spleen, most likely related to the requirement for STAT5 in T-lymphocyte development [188], which may confound organ development and indirectly affect cDCs. Nonetheless, the results to date indicate that STAT5a/b is not critical for Flt3L-driven CD11c+CD11b+ DC production in vitro or for controlling the abundance of splenic CD11c+CD11b− and CD11c+CD11b+ DCs after bone marrow reconstitution. However, STAT5a/b appears to be required for GM-CSF-dependent generation of CD11c+CD11b+ DCs in vitro [89]. Thus, STAT5a/b may have specific roles in one or more cDC subsets, while being dispensable in other cDC populations. Further studies are needed to understand the role for STAT5 in regulating specific cDC subsets in homeostatic and physiological stress conditions.
STAT1 and STAT2
STAT1 is activated by numerous cytokines; however its major role is to mediate the effects of type I and type II IFNs in vivo [189, 190]. Type I IFNs are secreted by both innate and adaptive immune cells, including pDCs, leukocytes, NK cells and T cells [25, 191, 192]. All type I IFNs are recognized by a common receptor formed by two subunits, IFNAR1 and IFNAR2, which are coupled to the JAK kinases, Tyk2 and Jak1. Upon engagement with the ligand, the receptor subunits undergo a conformational rearrangement, resulting in Jak activation and phosphorylation of STAT1 and STAT2 as well as additional intracellular signaling proteins [193–195]. The STATs subsequently form the IFN-stimulated gene factor 3 (ISGF3) complex with p48/IRF9; STAT1 homodimers are also detected [193]. Thus, type I IFN signal transduction involves both ISGF3 as well as STAT1 homodimers (Fig. 2).
Genetic deletion of STAT2, but not STAT1, abrogates virus- or IFN-β-induced inhibition of CD8α+ DC production in vivo and CD11c+MHCII+ DC generation in bone marrow cultures supplemented with Flt3L [141], suggesting a specific requirement for STAT2 and possibly ISGF3 during this process. On the other hand, STAT1 is necessary for IFN-α-stimulated induction of pDCs in bone marrow or spleen of mice receiving IFN-α hydrodynamic gene transfer [106]. pDCs and CD11c+CD11b+ DCs are found in normal numbers in animals lacking IFNAR, STAT1, or STAT2 [106, 141], indicating that type I IFN signals are dispensable for pDC and CD11c+CD11b+ DC homeostasis. Strikingly, however, Peyer's patch pDCs were significantly reduced in Ifnar−/− and Stat1−/− mice [106]. The Peyer's patch pDCs are a unique population that express the pDC cell surface marker phenotype (e.g., CD11c+CD11b−B220+PDCA-1+SiglecH+), yet show decreased amounts of mRNA for the transcription factor E2-2, reduced expression of the gut homing receptor CCR9, and impaired ability to produce type I IFNs in response to TLR triggering [106]. These results indicate that type I IFN signaling is uniquely required for pDC accumulation in the intestinal Peyer's patches, suggesting important roles in mediating gut immune responses.
CROSS-TALK BETWEEN STATS AND DC LINEAGE-REGULATORY TRANSCRIPTION FACTORS
One of the outstanding questions in hematopoiesis, including DC development, is the extent to which instructive and stochastic mechanisms contribute to lineage specification, commitment, and differentiation. Cytokines have the potential to elicit instructive signals in responsive cell types by virtue of their ability to directly regulate gene transcription. In fact, cytokines are essential for providing instructive messages to developing CD4+ T lymphocytes [196, 197]; however, whether this activity is shared among other lympho-hematopoietic lineages is unclear. In contrast, lineage-restricted transcription factors have well-established roles in determining cell fate decisions [198–203]. Even subtle differences (∼2-fold) in the expression of these transcriptional regulators can have a profound effect on hematopoiesis [201–204]. Yet, in most cases, we lack a clear understanding of the mechanisms that control the expression of lineage-restricted transcription factors, and, thus, our knowledge of initiating and reinforcing events that influence hematopoietic lineage decisions is limited.
Recent evidence suggests that STATs regulate certain DC transcription factors and affect developmental decisions in DC progenitors. For example, ectopic overexpression and activation of STAT3 in Flt3− hematopoietic progenitors, which normally lack DC developmental potential, stimulates Flt3 and PU.1 expression and permits Flt3− progenitors to differentiate into DCs [205]. Additional work has shown that cytokines and STATs influence the expression of other DC-related transcription factors, which participate in the regulation of DC lineage differentiation. The progress on this topic is described below, although much work remains to be done to achieve a thorough understanding of the interactions between cytokine-STAT pathways and DC lineage transcription factors.
PU.1
The ETS family member PU.1 (encoded by the gene Sfpi1) is required for the development of multiple hematopoietic lineages including DCs. Mice with germline deletion of Sfpi1 die in late gestation or shortly after birth because of a profound deficiency in fetal lymphomyelopoiesis [109, 206]. Conditional inactivation of Sfpi1 or reduced PU.1 expression leads to a significant reduction in pDCs, CD8α+, and CD8α− DCs in adult mice, as well as enhanced granulopoiesis and a preleukemic or leukemic state depending on PU.1 amounts [100, 207, 208]. In addition, PU.1-deficient lin− bone marrow progenitors give rise to Gr-1+ granulocytes, but not pDCs and cDCs, upon adoptive transfer into lethally irradiated recipients [100]. In contrast, overexpression of PU.1 in bone marrow progenitors or committed pre-T precursors induces DC or macrophage differentiation, suggesting that PU.1 can deliver an instructive message for DC differentiation [100, 209, 210].
PU.1 is highly expressed in hematopoietic progenitors including CDPs; its expression is maintained during cDC differentiation, while undergoing down-regulation during the development of pDCs [100]. As mentioned previously, STAT3 overexpression promotes Sfpi1 expression in Flt3− bone marrow progenitors [205]. Chromatin immunoprecipitation (ChIP) assays from our laboratory showed that STAT3 interacts with a region 5′ of the Sfpi1 transcriptional initiation site in myeloid cells treated with G-CSF (D. Yoon and S. Watowich, data not shown), in agreement with G-CSF- and STAT3-responsive induction of Sfpi1 mRNA [211, 212], suggesting that Sfpi1 (PU.1) may be a direct target of STAT3. Furthermore, Sfpi1 mRNA amounts are induced in lin−Flt3+ bone marrow progenitor cells in response to Flt3L [89]. These results suggest that Flt3-STAT3 signaling may promote DC development in part through the control of Sfpi1 transcription (Fig. 2). Both copies of Sfpi1 are required for maintaining Flt3 expression in hematopoietic progenitors, and PU.1 interacts with the Flt3 promoter region to induce Flt3 mRNA [100], identifying Flt3 as a critical target of PU.1. Collectively, these data suggest that STAT3, PU.1, and Flt3 form a signaling network to regulate DC development.
IRF8
IRF8 is a key transcription factor in DC development, regulating the production of pDCs, lymphoid CD8α+ DCs, tissue resident CD103+ DCs, and LCs [101, 104, 116, 118]. Similar to animals with reduced (but not an absence of) PU.1 expression, Irf8−/− mice have excessive granulocyte production and a myeloproliferative-like condition [213]. In fact, IRF8 was recently found to inhibit granulopoiesis and induce DC lineage commitment in hematopoietic progenitor cells [214]. Interestingly, a spontaneous point mutation (R294C) within a domain of IRF8 that mediates cofactor association selectively impairs the development of CD8α+ and CD103+ DCs, without affecting pDC generation [116, 118, 215]. The IRF8 R294C mutation disables the interaction of IRF8 with the partner proteins IRF2, PU.1, and SpiB [215], thus abolishing the recruitment of IRF8:protein complexes to the promoters of target genes. These results imply that IRF8 target genes in pDCs and cDCs require distinct IRF8 partner proteins or that pDC genes are regulated by IRF8 independent of cofactor association. IRF8 mutations in the DNA binding domain (i.e., K108E and T80A) consistently inhibit monocyte and/or DC production in humans [216], as discussed in greater detail below, and are accompanied by a myeloproliferative disorder characterized by excessive granulocyte production.
Irf8 mRNA is inducible by Flt3L and repressed by GM-CSF in lin−Flt3+ bone marrow progenitor cells [89], suggesting regulation by cytokine-responsive STATs. Analysis by electrophoretic mobility shift assay, ChIP, and reporter assays revealed that GM-CSF-activated STAT5 binds directly to a consensus STAT site within the Irf8 proximal promoter (approximately −180 bp) [89], which was previously shown to mediate IFN-γ-STAT1-dependent induction [217]. In contrast, however, GM-CSF-activated STAT5 recruitment to the Irf8 promoter inhibits Irf8 transcription, potentially contributing to suppression of pDC development by GM-CSF [89, 98, 106] (Fig. 2). Flt3L-activated STAT3 does not detectably interact with the STAT consensus site in the Irf8 promoter (H. Li and S. Watowich, unpublished data), suggesting that STAT3 is either not involved in Irf8 regulation or uses a binding site different from that used by STAT1 and STAT5. IFN-α also has the ability to up-regulate Irf8 expression in murine lin−Flt3+ bone marrow progenitor cells [106] as well as human hematopoietic cells, including samples from patients with chronic myelogenous leukemia, a disease that is treated with IFN-α [218, 219]. IFN-α-activated STAT1 binds the STAT site within the Irf8 proximal promoter and directly induces IFN-α-dependent Irf8 transcription [106]. These data indicate that Irf8 is regulated in opposite fashion by GM-CSF-activated STAT5 (inhibitory) and IFN-α-activated STAT1 (stimulatory), suggesting potential competition between cytokine-responsive STATs at the Irf8 promoter that may influence Irf8 expression and DC lineage decisions.
E2-2 and Id2
The helix-loop-helix proteins E2-2 and Id2 have emerged as key transcription factors that play nearly opposite roles in DC lineage development. E2-2 is highly expressed in pDCs but not cDCs [99]. E2-2 controls pDC specification and/or commitment and regulates the expression of pDC-related genes (e.g., Tlr7, Tlr9, and Irf7) [99, 114]. Sustained E2-2 expression is important for preserving pDC cell identity because conditional ablation in pDCs causes cells to acquire a cDC-like phenotype [114]. In contrast, the E protein antagonist Id2 is required for the development of CD8α+ DCs, CD103+ DCs, and LCs [113, 118]. Deletion of Id2 leads to enhanced pDC amounts and increased type I IFN production from pDCs, indicating that Id2 suppresses pDC development and function [113]. Mice that are heterozygous for Tcf4 contain reduced numbers of pDCs [99], indicating that both alleles of Tcf4 are required for pDC homeostasis. Collectively, these results suggest that the expression level of E2-2 and Id2 must be maintained precisely for specifying proper numbers of pDCs and cDCs in vivo.
The genes encoding E2-2 and Id2 each contain STAT consensus sites within their proximal promoter regions (H. Li and S. Watowich, unpublished results); nonetheless, it remains to be determined whether or how STATs are involved in regulating their expression in DC progenitors. Using ChIP assays, Yang et al. [220] demonstrated that cytokine-responsive STAT5 was recruited to the Id2 promoter in activated CD8+ T cells, suggesting that Id2 is directly regulated by STAT5 (Fig. 2). Thus, future studies to investigate STAT-mediated regulation of Id2, as well as E2-2, in developing DCs will provide important information on the roles for cytokines in directing DC lineage fate decisions and terminal differentiation.
TRANSCRIPTIONAL CONTROL OF HUMAN DCs
Insight into key transcriptional regulators of human DCs has emerged from elegant studies of human genetic disorders or immunodeficiency, as well as classic laboratory approaches using gene-specific overexpression and knockdown in cell culture systems. In fact, human studies have led the field in identifying new DC transcriptional regulators. For example, investigation of the molecular basis of a multilineage human immunodeficiency, characterized by loss of monocytes, B cells, NK cells, peripheral blood HLA-DR+ DCs, and dermal CD14+ and CD1a+ DCs, revealed GATA-2 as an important factor for DCs [221–223]. GATA-2 has a critical role in murine hematopoietic stem cell maintenance [224–226]; yet, to our knowledge, GATA-2 function in murine DCs remains to be established. Examining this will require methods to bypass the early developmental block induced by gene deletion. Consistent with murine GATA-2 activity, however, individuals that carry GATA-2 mutations associated with DC deficiency (termed DCML or MonoMac syndrome) show evidence of disrupted hematopoiesis in bone marrow including hypocellularity and reduced progenitor amounts [14, 221]. DC deficiency-associated GATA-2 mutations comprise loss or amino acid substitution in the C-terminal zinc finger domain, which is required for DNA binding and are therefore predicted to affect DC development by haploinsufficiency or dominant inhibitory activity [223]. Presumably, these mutations are less severe than complete loss of GATA-2 function would be in humans and may impair GATA-2-driven expression of key stem cell regulators (e.g., Scl/TAL1 complex components) [227, 228], as well as factors that are necessary for DC specification and/or development [229].
The role of IRF8 in DC development appears to be conserved between humans and mice, because IRF8 DNA-binding domain mutants have been identified in human immunodeficiency associated with reduced circulating DC amounts and enhanced susceptibility to mycobacterial infections [216]. For instance, the IRF8 K108E mutant is associated with autosomal recessive DC deficiency, accompanied by significant depletion of all peripheral blood DC subsets and monocytes [216]. In contrast, the IRF8 T80A mutant is associated with a less severe, autosomal dominant immunodeficiency characterized by reduction of circulating CD1c+ DCs [216]. The severity of the phenotype associated with homozygous IRF8 K108E status required hematopoietic stem cell transplantation to correct the immunodeficiency [216].
Mutations in other DC-related transcription factors, for example, E2-2, STAT3, and Id2, are also present in humans with congenital disease [99, 230, 231]. In Pitt-Hopkins syndrome, heterozygous mutation of TCF4, encoding E2-2, leads to reduced expression of BDCA-2 and ILT7 on pDCs and impaired pDC IFN-α secretion responses upon CpGA stimulation [99]. These results suggest that the requirement for both copies of the gene encoding E2-2 in pDCs (or pDC progenitors) is conserved between humans and mice [99]. In addition, E2-2 demonstrates instructive function because its overexpression in CD34+ thymic progenitors promotes pDC generation in the presence of Flt3L and IL-7 [232]. Individuals with STAT3 mutations that abrogate transcriptional activity (e.g., mutations in the DNA binding, SH2, or transactivation domains) have an impaired ability to produce tolerogenic DCs in response to IL-10, consistent with the role for STAT3 in IL-10 signal transduction [233–235]. In contrast, congenital Id2 mutations have not been associated with DC deficiency to date, although overexpression of Id2 or the related factor Id3 in CD34+ progenitors inhibits pDC development in agreement with the negative role for Id2 in murine pDCs [231, 236].
The transcription factors STAT5, Spi-B, and PU.1 have also been implicated in human DC development by inhibition or overexpression assays. Enhancing STAT5a activity by introducing wild-type STAT5a or the constitutively active mutant STAT5a1*6 into CD34+ hematopoietic progenitors abolishes the generation of preinterstitial DCs and pre-LCs, yet promotes the development of interstitial DCs and LCs from their precursors, suggesting that STAT5a mediates distinct effects at different stages of human DC development [157]. In contrast, suppression of Spi-B or PU.1 significantly inhibits pDC development in vitro, and after transplantation of manipulated CD34+ cells into irradiated neonatal Rag2−/−Il2rg−/− (RAG-2- and γc-deficient) mice [237]. Although Spi-B does not appear to be required for pDC development in mice, the function of PU.1 may be conserved [100, 113, 237].
CONCLUDING REMARKS
As our understanding of DC heterogeneity and relevant developmental events improves, the extent to which intrinsic transcription factors, extrinsic cytokine signals, and tissue-specific microenvironmental signals cross-talk to drive DC subset specification must be determined for a full elucidation of the molecular pathways that regulate these crucial immune cells. Growing evidence suggests that STAT family members play an essential role in DC development; in part, STAT function appears to involve direct control of certain DC lineage-regulatory transcription factors. STATs are also expected to control a broad array of genes needed for proper growth, survival and maturation of DC progenitors. Nevertheless, it remains unclear how STATs intersect with DC lineage-regulatory transcription factors and microenvironmental factors to instruct the differentiation of one DC subset versus another from common progenitor/precursor cells in vivo. Another open question is how epigenetic mechanisms are integrated into the already complicated molecular circuitry that controls DC development and whether STATs can function as epigenetic regulators in addition to their role as transcription factors. Moreover, because of the phenotypic differences between mouse and human DCs, translation of knowledge gained from mouse studies into clinical settings remains a major challenge that must be addressed.
ACKNOWLEDGMENTS
This work was supported by grants to S.S.W. from the U.S. National Institutes of Health (AI073587 and AI098099) and the M. D. Anderson Center for Cancer Epigenetics. H.S.L. is supported in part by the R.E. Bob Smith Education Fund.
Footnotes
- Batf3
- basic leucine zipper transcription factor
- ATF-like 3; cDC
- classic DC
- CDP
- common DC progenitor
- ChIP
- chromatin immunoprecipitation
- Flt3L
- fms-like tyrosine kinase 3 ligand
- Gfi1
- growth factor independent 1 transcription repressor
- Id2
- inhibitor of DNA binding 2
- IRF4
- interferon regulatory factor 4
- IRF8
- interferon regulatory factor 8
- ISGF3
- IFN-stimulated gene factor 3
- ITD
- internal tandem duplication
- LC
- Langerhans cell
- LCMV
- lymphocytic choriomeningitis virus
- LTβ
- lymphotoxin-β
- M-CSFR
- M-CSF receptor
- MDP
- macrophage-DC progenitor
- MHCII
- major histocompatibility class II protein
- mTOR
- mammalian target of rapamycin.
- MV
- measles virus
- MVA
- attenuated vaccinia Ankara
- pDC
- plasmacytoid DC
- PI3K
- phosphoinositol 3-kinase
- precDC
- cDC precursor
- PTEN
- phosphatase and tensin homolog
AUTHORSHIP
H.S.L. wrote the manuscript; H.S.L and S.S.W. revised and edited the manuscript.
REFERENCES
- 1. Steinman R. M., Cohn Z. A. (1974) Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J. Exp. Med. 139, 380–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steinman R. M., Cohn Z. A. (1973) Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 137, 1142–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu K., Nussenzweig M. C. (2010) Origin and development of dendritic cells. Immunol. Rev. 234, 45–54 [DOI] [PubMed] [Google Scholar]
- 4. Hashimoto D., Miller J., Merad M. (2011) Dendritic cell and macrophage heterogeneity in vivo. Immunity 35, 323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shortman K., Heath W. R. (2010) The CD8+ dendritic cell subset. Immunol. Rev. 234, 18–31 [DOI] [PubMed] [Google Scholar]
- 6. Reizis B., Bunin A., Ghosh H. S., Lewis K. L., Sisirak V. (2011) Plasmacytoid dendritic cells: recent progress and open questions. Annu. Rev. Immunol. 29, 163–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmid M. A., Kingston D., Boddupalli S., Manz M. G. (2010) Instructive cytokine signals in dendritic cell lineage commitment. Immunol. Rev. 234, 32–44 [DOI] [PubMed] [Google Scholar]
- 8. Merad M., Manz M. G. (2009) Dendritic cell homeostasis. Blood 113, 3418–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Helft J., Ginhoux F., Bogunovic M., Merad M. (2010) Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol. Rev. 234, 55–75 [DOI] [PubMed] [Google Scholar]
- 10. Cheng P., Zhou J., Gabrilovich D. (2010) Regulation of dendritic cell differentiation and function by Notch and Wnt pathways. Immunol. Rev. 234, 105–119 [DOI] [PubMed] [Google Scholar]
- 11. Romani N., Clausen B. E., Stoitzner P. (2010) Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol. Rev. 234, 120–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swiecki M., Colonna M. (2010) Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol. Rev. 234, 142–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dominguez P. M., Ardavin C. (2010) Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol. Rev. 234, 90–104 [DOI] [PubMed] [Google Scholar]
- 14. Collin M., Bigley V., Haniffa M., Hambleton S. (2011) Human dendritic cell deficiency: the missing ID? Nat. Rev. Immunol. 11, 575–583 [DOI] [PubMed] [Google Scholar]
- 15. Belz G. T., Nutt S. L. (2012) Transcriptional programming of the dendritic cell network. Nat. Rev. Immunol. 12, 101–113 [DOI] [PubMed] [Google Scholar]
- 16. Cella M., Jarrossay D., Facchetti F., Alebardi O., Nakajima H., Lanzavecchia A., Colonna M. (1999) Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5, 919–923 [DOI] [PubMed] [Google Scholar]
- 17. Siegal F. P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P. A., Shah K., Ho S., Antonenko S., Liu Y. J. (1999) The nature of the principal type 1 interferon-producing cells in human blood. Science 284, 1835–1837 [DOI] [PubMed] [Google Scholar]
- 18. Asselin-Paturel C., Boonstra A., Dalod M., Durand I., Yessaad N., Dezutter-Dambuyant C., Vicari A., O'Garra A., Biron C., Briere F., Trinchieri G. (2001) Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2, 1144–1150 [DOI] [PubMed] [Google Scholar]
- 19. Contractor N., Louten J., Kim L., Biron C. A., Kelsall B. L. (2007) Cutting edge: Peyer's patch plasmacytoid dendritic cells (pDCs) produce low levels of type I interferons: possible role for IL-10, TGFβ, and prostaglandin E2 in conditioning a unique mucosal pDC phenotype. J. Immunol. 179, 2690–2694 [DOI] [PubMed] [Google Scholar]
- 20. Nestle F. O., Conrad C., Tun-Kyi A., Homey B., Gombert M., Boyman O., Burg G., Liu Y. J., Gilliet M. (2005) Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 202, 135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farkas L., Beiske K., Lund-Johansen F., Brandtzaeg P., Jahnsen F. L. (2001) Plasmacytoid dendritic cells (natural interferon-α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 159, 237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jahnsen F. L., Lund-Johansen F., Dunne J. F., Farkas L., Haye R., Brandtzaeg P. (2000) Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J. Immunol. 165, 4062–4068 [DOI] [PubMed] [Google Scholar]
- 23. Wollenberg A., Wagner M., Gunther S., Towarowski A., Tuma E., Moderer M., Rothenfusser S., Wetzel S., Endres S., Hartmann G. (2002) Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J. Invest. Dermatol. 119, 1096–1102 [DOI] [PubMed] [Google Scholar]
- 24. Bangert C., Friedl J., Stary G., Stingl G., Kopp T. (2003) Immunopathologic features of allergic contact dermatitis in humans: participation of plasmacytoid dendritic cells in the pathogenesis of the disease? J. Invest. Dermatol. 121, 1409–1418 [DOI] [PubMed] [Google Scholar]
- 25. Liu Y. J. (2005) IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23, 275–306 [DOI] [PubMed] [Google Scholar]
- 26. Reizis B., Colonna M., Trinchieri G., Barrat F., Gilliet M. (2011) Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nat. Rev. Immunol. 11, 558–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lande R., Gilliet M. (2010) Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Ann. N. Y. Acad. Sci. 1183, 89–103 [DOI] [PubMed] [Google Scholar]
- 28. Kadowaki N., Antonenko S., Lau J. Y., Liu Y. J. (2000) Natural interferon α/β-producing cells link innate and adaptive immunity. J. Exp. Med. 192, 219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takagi H., Fukaya T., Eizumi K., Sato Y., Sato K., Shibazaki A., Otsuka H., Hijikata A., Watanabe T., Ohara O., Kaisho T., Malissen B. (2011) Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity 35, 958–971 [DOI] [PubMed] [Google Scholar]
- 30. Lanzavecchia A., Sallusto F. (2001) Regulation of T cell immunity by dendritic cells. Cell 106, 263–266 [DOI] [PubMed] [Google Scholar]
- 31. Villadangos J. A., Schnorrer P. (2007) Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat. Rev. Immunol. 7, 543–555 [DOI] [PubMed] [Google Scholar]
- 32. Pulendran B., Tang H., Denning T. L. (2008) Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr. Opin. Immunol. 20, 61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rivollier A., He J., Kole A., Valatas V., Kelsall B. L. (2012) Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 209, 139–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hume D. A. (2008) Macrophages as APC and the dendritic cell myth. J. Immunol. 181, 5829–5835 [DOI] [PubMed] [Google Scholar]
- 35. Hume D. A. (2011) Applications of myeloid-specific promoters in transgenic mice support in vivo imaging and functional genomics but do not support the concept of distinct macrophage and dendritic cell lineages or roles in immunity. J. Leukoc. Biol. 89, 525–538 [DOI] [PubMed] [Google Scholar]
- 36. Den Haan J. M., Bevan M. J. (2002) Constitutive versus activation-dependent cross-presentation of immune complexes by CD8+ and CD8− dendritic cells in vivo. J. Exp. Med. 196, 817–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Allan R. S., Smith C. M., Belz G. T., van Lint A. L., Wakim L. M., Heath W. R., Carbone F. R. (2003) Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science 301, 1925–1928 [DOI] [PubMed] [Google Scholar]
- 38. Belz G. T., Smith C. M., Kleinert L., Reading P., Brooks A., Shortman K., Carbone F. R., Heath W. R. (2004) Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci. U. S. A. 101, 8670–8675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim T. S., Braciale T. J. (2009) Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One 4, e4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beauchamp N. M., Busick R. Y., Alexander-Miller M. A. (2010) Functional divergence among CD103+ dendritic cell subpopulations following pulmonary poxvirus infection. J. Virol. 84, 10191–10199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bedoui S., Whitney P. G., Waithman J., Eidsmo L., Wakim L., Caminschi I., Allan R. S., Wojtasiak M., Shortman K., Carbone F. R., Brooks A. G., Heath W. R. (2009) Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10, 488–495 [DOI] [PubMed] [Google Scholar]
- 42. Del Rio M. L., Bernhardt G., Rodriguez-Barbosa J. I., Forster R. (2010) Development and functional specialization of CD103+ dendritic cells. Immunol. Rev. 234, 268–281 [DOI] [PubMed] [Google Scholar]
- 43. Koski G. K., Cohen P. A., Roses R. E., Xu S., Czerniecki B. J. (2008) Reengineering dendritic cell-based anti-cancer vaccines. Immunol. Rev. 222, 256–276 [DOI] [PubMed] [Google Scholar]
- 44. D'Amico A., Wu L. (2003) The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J. Exp. Med. 198, 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Onai N., Obata-Onai A., Schmid M. A., Ohteki T., Jarrossay D., Manz M. G. (2007) Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat. Immunol. 8, 1207–1216 [DOI] [PubMed] [Google Scholar]
- 46. Fogg D. K., Sibon C., Miled C., Jung S., Aucouturier P., Littman D. R., Cumano A., Geissmann F. (2006) A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311, 83–87 [DOI] [PubMed] [Google Scholar]
- 47. Naik S. H., Sathe P., Park H. Y., Metcalf D., Proietto A. I., Dakic A., Carotta S., O'Keeffe M., Bahlo M., Papenfuss A., Kwak J. Y., Wu L., Shortman K. (2007) Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat. Immunol. 8, 1217–1226 [DOI] [PubMed] [Google Scholar]
- 48. Liu K., Victora G. D., Schwickert T. A., Guermonprez P., Meredith M. M., Yao K., Chu F. F., Randolph G. J., Rudensky A. Y., Nussenzweig M. (2009) In vivo analysis of dendritic cell development and homeostasis. Science 324, 392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Naik S. H., Metcalf D., van Nieuwenhuijze A., Wicks I., Wu L., O'Keeffe M., Shortman K. (2006) Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 7, 663–671 [DOI] [PubMed] [Google Scholar]
- 50. Waskow C., Liu K., Darrasse-Jeze G., Guermonprez P., Ginhoux F., Merad M., Shengelia T., Yao K., Nussenzweig M. (2008) The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat. Immunol. 9, 676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu K., Waskow C., Liu X., Yao K., Hoh J., Nussenzweig M. (2007) Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 8, 578–583 [DOI] [PubMed] [Google Scholar]
- 52. Geissmann F., Prost C., Monnet J. P., Dy M., Brousse N., Hermine O. (1998) Transforming growth factor β1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J. Exp. Med. 187, 961–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Randolph G. J., Inaba K., Robbiani D. F., Steinman R. M., Muller W. A. (1999) Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11, 753–761 [DOI] [PubMed] [Google Scholar]
- 54. Varol C., Vallon-Eberhard A., Elinav E., Aychek T., Shapira Y., Luche H., Fehling H. J., Hardt W. D., Shakhar G., Jung S. (2009) Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 31, 502–512 [DOI] [PubMed] [Google Scholar]
- 55. Merad M., Ginhoux F., Collin M. (2008) Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 8, 935–947 [DOI] [PubMed] [Google Scholar]
- 56. Merad M., Manz M. G., Karsunky H., Wagers A., Peters W., Charo I., Weissman I. L., Cyster J. G., Engleman E. G. (2002) Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3, 1135–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ginhoux F., Tacke F., Angeli V., Bogunovic M., Loubeau M., Dai X. M., Stanley E. R., Randolph G. J., Merad M. (2006) Langerhans cells arise from monocytes in vivo. Nat. Immunol. 7, 265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shortman K., Liu Y. J. (2002) Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2, 151–161 [DOI] [PubMed] [Google Scholar]
- 59. Ito T., Liu Y. J., Kadowaki N. (2005) Functional diversity and plasticity of human dendritic cell subsets. Int. J. Hematol. 81, 188–196 [DOI] [PubMed] [Google Scholar]
- 60. Wu L., Liu Y. J. (2007) Development of dendritic-cell lineages. Immunity 26, 741–750 [DOI] [PubMed] [Google Scholar]
- 61. Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D. N., Leenen P. J., Liu Y. J., MacPherson G., Randolph G. J., Scherberich J., Schmitz J., Shortman K., Sozzani S., Strobl H., Zembala M., Austyn J. M., Lutz M. B. (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116, e74–80 [DOI] [PubMed] [Google Scholar]
- 62. Dzionek A., Fuchs A., Schmidt P., Cremer S., Zysk M., Miltenyi S., Buck D. W., Schmitz J. (2000) BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 165, 6037–6046 [DOI] [PubMed] [Google Scholar]
- 63. MacDonald K. P., Munster D. J., Clark G. J., Dzionek A., Schmitz J., Hart D. N. (2002) Characterization of human blood dendritic cell subsets. Blood 100, 4512–4520 [DOI] [PubMed] [Google Scholar]
- 64. Lindstedt M., Lundberg K., Borrebaeck C. A. (2005) Gene family clustering identifies functionally associated subsets of human in vivo blood and tonsillar dendritic cells. J. Immunol. 175, 4839–4846 [DOI] [PubMed] [Google Scholar]
- 65. Segura E., Valladeau-Guilemond J., Donnadieu M. H., Sastre-Garau X., Soumelis V., Amigorena S. (2012) Characterization of resident and migratory dendritic cells in human lymph nodes. J. Exp. Med. 209, 653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Doulatov S., Notta F., Eppert K., Nguyen L. T., Ohashi P. S., Dick J. E. (2010) Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat. Immunol. 11, 585–593 [DOI] [PubMed] [Google Scholar]
- 67. Angel C. E., Chen C. J., Horlacher O. C., Winkler S., John T., Browning J., MacGregor D., Cebon J., Dunbar P. R. (2009) Distinctive localization of antigen-presenting cells in human lymph nodes. Blood 113, 1257–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McIlroy D., Troadec C., Grassi F., Samri A., Barrou B., Autran B., Debre P., Feuillard J., Hosmalin A. (2001) Investigation of human spleen dendritic cell phenotype and distribution reveals evidence of in vivo activation in a subset of organ donors. Blood 97, 3470–3477 [DOI] [PubMed] [Google Scholar]
- 69. Kadowaki N., Ho S., Antonenko S., Malefyt R. W., Kastelein R. A., Bazan F., Liu Y. J. (2001) Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194, 863–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. De Saint-Vis B., Fugier-Vivier I., Massacrier C., Gaillard C., Vanbervliet B., Ait-Yahia S., Banchereau J., Liu Y. J., Lebecque S., Caux C. (1998) The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J. Immunol. 160, 1666–1676 [PubMed] [Google Scholar]
- 71. Poulin L. F., Salio M., Griessinger E., Anjos-Afonso F., Craciun L., Chen J. L., Keller A. M., Joffre O., Zelenay S., Nye E., Le Moine A., Faure F., Donckier V., Sancho D., Cerundolo V., Bonnet D., Reis e Sousa C. (2010) Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8α+ dendritic cells. J. Exp. Med. 207, 1261–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bachem A., Guttler S., Hartung E., Ebstein F., Schaefer M., Tannert A., Salama A., Movassaghi K., Opitz C., Mages H. W., Henn V., Kloetzel P. M., Gurka S., Kroczek R. A. (2010) Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 207, 1273–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Crozat K., Guiton R., Contreras V., Feuillet V., Dutertre C. A., Ventre E., Vu Manh T. P., Baranek T., Storset A. K., Marvel J., Boudinot P., Hosmalin A., Schwartz-Cornil I., Dalod M. (2010) The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8α+ dendritic cells. J. Exp. Med. 207, 1283–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jongbloed S. L., Kassianos A. J., McDonald K. J., Clark G. J., Ju X., Angel C. E., Chen C. J., Dunbar P. R., Wadley R. B., Jeet V., Vulink A. J., Hart D. N., Radford K. J. (2010) Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 207, 1247–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nestle F. O., Zheng X. G., Thompson C. B., Turka L. A., Nickoloff B. J. (1993) Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J. Immunol. 151, 6535–6545 [PubMed] [Google Scholar]
- 76. Angel C. E., Lala A., Chen C. J., Edgar S. G., Ostrovsky L. L., Dunbar P. R. (2007) CD14+ antigen-presenting cells in human dermis are less mature than their CD1a+ counterparts. Int. Immunol. 19, 1271–1279 [DOI] [PubMed] [Google Scholar]
- 77. Jaensson E., Uronen-Hansson H., Pabst O., Eksteen B., Tian J., Coombes J. L., Berg P. L., Davidsson T., Powrie F., Johansson-Lindbom B., Agace W. W. (2008) Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 205, 2139–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Collin M. P., Hart D. N., Jackson G. H., Cook G., Cavet J., Mackinnon S., Middleton P. G., Dickinson A. M. (2006) The fate of human Langerhans cells in hematopoietic stem cell transplantation. J. Exp. Med. 203, 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Haniffa M., Ginhoux F., Wang X. N., Bigley V., Abel M., Dimmick I., Bullock S., Grisotto M., Booth T., Taub P., Hilkens C., Merad M., Collin M. (2009) Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J. Exp. Med. 206, 371–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kanitakis J., Morelon E., Petruzzo P., Badet L., Dubernard J. M. (2011) Self-renewal capacity of human epidermal Langerhans cells: observations made on a composite tissue allograft. Exp. Dermatol. 20, 145–146 [DOI] [PubMed] [Google Scholar]
- 81. Watowich S. S., Liu Y. J. (2010) Mechanisms regulating dendritic cell specification and development. Immunol. Rev. 238, 76–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kingston D., Schmid M. A., Onai N., Obata-Onai A., Baumjohann D., Manz M. G. (2009) The concerted action of GM-CSF and Flt3-ligand on in vivo dendritic cell homeostasis. Blood 114, 835–843 [DOI] [PubMed] [Google Scholar]
- 83. McKenna H. J., Stocking K. L., Miller R. E., Brasel K., De Smedt T., Maraskovsky E., Maliszewski C. R., Lynch D. H., Smith J., Pulendran B., Roux E. R., Teepe M., Lyman S. D., Peschon J. J. (2000) Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 95, 3489–3497 [PubMed] [Google Scholar]
- 84. Naik S. H., Proietto A. I., Wilson N. S., Dakic A., Schnorrer P., Fuchsberger M., Lahoud M. H., O'Keeffe M., Shao Q. X., Chen W. F., Villadangos J. A., Shortman K., Wu L. (2005) Cutting edge: Generation of splenic CD8+ and CD8− dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J. Immunol. 174, 6592–6597 [DOI] [PubMed] [Google Scholar]
- 85. Maraskovsky E., Brasel K., Teepe M., Roux E. R., Lyman S. D., Shortman K., McKenna H. J. (1996) Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 184, 1953–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. O'Keeffe M., Hochrein H., Vremec D., Pooley J., Evans R., Woulfe S., Shortman K. (2002) Effects of administration of progenipoietin 1, Flt-3 ligand, granulocyte colony-stimulating factor, and pegylated granulocyte-macrophage colony-stimulating factor on dendritic cell subsets in mice. Blood 99, 2122–2130 [DOI] [PubMed] [Google Scholar]
- 87. Vollstedt S., O'Keeffe M., Odermatt B., Beat R., Glanzmann B., Riesen M., Shortman K., Suter M. (2004) Treatment of neonatal mice with Flt3 ligand leads to changes in dendritic cell subpopulations associated with enhanced IL-12 and IFN-alpha production. Eur. J. Immunol. 34, 1849–1860 [DOI] [PubMed] [Google Scholar]
- 88. Choi J. H., Cheong C., Dandamudi D. B., Park C. G., Rodriguez A., Mehandru S., Velinzon K., Jung I. H., Yoo J. Y., Oh G. T., Steinman R. M. (2011) Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity 35, 819–831 [DOI] [PubMed] [Google Scholar]
- 89. Esashi E., Wang Y. H., Perng O., Qin X. F., Liu Y. J., Watowich S. S. (2008) The signal transducer STAT5 inhibits plasmacytoid dendritic cell development by suppressing transcription factor IRF8. Immunity 28, 509–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R. M. (1992) Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176, 1693–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vremec D., Lieschke G. J., Dunn A. R., Robb L., Metcalf D., Shortman K. (1997) The influence of granulocyte/macrophage colony-stimulating factor on dendritic cell levels in mouse lymphoid organs. Eur. J. Immunol. 27, 40–44 [DOI] [PubMed] [Google Scholar]
- 92. King I. L., Kroenke M. A., Segal B. M. (2010) GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J. Exp. Med. 207, 953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fancke B., Suter M., Hochrein H., O'Keeffe M. (2008) M-CSF: a novel plasmacytoid and conventional dendritic cell poietin. Blood 111, 150–159 [DOI] [PubMed] [Google Scholar]
- 94. Borkowski T. A., Letterio J. J., Farr A. G., Udey M. C. (1996) A role for endogenous transforming growth factor β 1 in Langerhans cell biology: the skin of transforming growth factor β 1 null mice is devoid of epidermal Langerhans cells. J. Exp. Med. 184, 2417–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D. (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345, 442–444 [DOI] [PubMed] [Google Scholar]
- 96. Dai X. M., Ryan G. R., Hapel A. J., Dominguez M. G., Russell R. G., Kapp S., Sylvestre V., Stanley E. R. (2002) Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99, 111–120 [DOI] [PubMed] [Google Scholar]
- 97. Kabashima K., Banks T. A., Ansel K. M., Lu T. T., Ware C. F., Cyster J. G. (2005) Intrinsic lymphotoxin-β receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity 22, 439–450 [DOI] [PubMed] [Google Scholar]
- 98. Gilliet M., Boonstra A., Paturel C., Antonenko S., Xu X. L., Trinchieri G., O'Garra A., Liu Y. J. (2002) The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195, 953–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cisse B., Caton M. L., Lehner M., Maeda T., Scheu S., Locksley R., Holmberg D., Zweier C., den Hollander N. S., Kant S. G., Holter W., Rauch A., Zhuang Y., Reizis B. (2008) Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 135, 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Carotta S., Dakic A., D'Amico A., Pang S. H., Greig K. T., Nutt S. L., Wu L. (2010) The transcription factor PU.1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner. Immunity 32, 628–641 [DOI] [PubMed] [Google Scholar]
- 101. Schiavoni G., Mattei F., Borghi P., Sestili P., Venditti M., Morse H. C., 3rd, Belardelli F., Gabriele L. (2004) ICSBP is critically involved in the normal development and trafficking of Langerhans cells and dermal dendritic cells. Blood 103, 2221–2228 [DOI] [PubMed] [Google Scholar]
- 102. Schiavoni G., Mattei F., Sestili P., Borghi P., Venditti M., Morse H. C., 3rd, Belardelli F., Gabriele L. (2002) ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8α+ dendritic cells. J. Exp. Med. 196, 1415–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tamura T., Tailor P., Yamaoka K., Kong H. J., Tsujimura H., O'Shea J. J., Singh H., Ozato K. (2005) IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J. Immunol. 174, 2573–2581 [DOI] [PubMed] [Google Scholar]
- 104. Tsujimura H., Tamura T., Ozato K. (2003) Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. J. Immunol. 170, 1131–1135 [DOI] [PubMed] [Google Scholar]
- 105. Laouar Y., Welte T., Fu X. Y., Flavell R. A. (2003) STAT3 is required for Flt3L-dependent dendritic cell differentiation. Immunity 19, 903–912 [DOI] [PubMed] [Google Scholar]
- 106. Li H. S., Gelbard A., Martinez G. J., Esashi E., Zhang H., Nguyen-Jackson H., Liu Y. J., Overwijk W. W., Watowich S. S. (2011) Cell-intrinsic role for IFN-α-STAT1 signals in regulating murine Peyer patch plasmacytoid dendritic cells and conditioning an inflammatory response. Blood 118, 3879–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hildner K., Edelson B. T., Purtha W. E., Diamond M., Matsushita H., Kohyama M., Calderon B., Schraml B. U., Unanue E. R., Diamond M. S., Schreiber R. D., Murphy T. L., Murphy K. M. (2008) Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rathinam C., Geffers R., Yucel R., Buer J., Welte K., Moroy T., Klein C. (2005) The transcriptional repressor Gfi1 controls STAT3-dependent dendritic cell development and function. Immunity 22, 717–728 [DOI] [PubMed] [Google Scholar]
- 109. Scott E. W., Simon M. C., Anastasi J., Singh H. (1994) Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science 265, 1573–1577 [DOI] [PubMed] [Google Scholar]
- 110. Georgopoulos K., Bigby M., Wang J. H., Molnar A., Wu P., Winandy S., Sharpe A. (1994) The Ikaros gene is required for the development of all lymphoid lineages. Cell 79, 143–156 [DOI] [PubMed] [Google Scholar]
- 111. Anderson K. L., Perkin H., Surh C. D., Venturini S., Maki R. A., Torbett B. E. (2000) Transcription factor PU.1 is necessary for development of thymic and myeloid progenitor-derived dendritic cells. J. Immunol. 164, 1855–1861 [DOI] [PubMed] [Google Scholar]
- 112. Guerriero A., Langmuir P. B., Spain L. M., Scott E. W. (2000) PU.1 is required for myeloid-derived but not lymphoid-derived dendritic cells. Blood 95, 879–885 [PubMed] [Google Scholar]
- 113. Hacker C., Kirsch R. D., Ju X. S., Hieronymus T., Gust T. C., Kuhl C., Jorgas T., Kurz S. M., Rose-John S., Yokota Y., Zenke M. (2003) Transcriptional profiling identifies Id2 function in dendritic cell development. Nat. Immunol. 4, 380–386 [DOI] [PubMed] [Google Scholar]
- 114. Ghosh H. S., Cisse B., Bunin A., Lewis K. L., Reizis B. (2010) Continuous expression of the transcription factor e2-2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity 33, 905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jackson J. T., Hu Y., Liu R., Masson F., D'Amico A., Carotta S., Xin A., Camilleri M. J., Mount A. M., Kallies A., Wu L., Smyth G. K., Nutt S. L., Belz G. T. (2011) Id2 expression delineates differential checkpoints in the genetic program of CD8α+ and CD103+ dendritic cell lineages. EMBO J. 30, 2690–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Edelson B. T., Kc W., Juang R., Kohyama M., Benoit L. A., Klekotka P. A., Moon C., Albring J. C., Ise W., Michael D. G., Bhattacharya D., Stappenbeck T. S., Holtzman M. J., Sung S. S., Murphy T. L., Hildner K., Murphy K. M. (2010) Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J. Exp. Med. 207, 823–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bogunovic M., Ginhoux F., Helft J., Shang L., Hashimoto D., Greter M., Liu K., Jakubzick C., Ingersoll M. A., Leboeuf M., Stanley E. R., Nussenzweig M., Lira S. A., Randolph G. J., Merad M. (2009) Origin of the lamina propria dendritic cell network. Immunity 31, 513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ginhoux F., Liu K., Helft J., Bogunovic M., Greter M., Hashimoto D., Price J., Yin N., Bromberg J., Lira S. A., Stanley E. R., Nussenzweig M., Merad M. (2009) The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 206, 3115–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Honda K., Mizutani T., Taniguchi T. (2004) Negative regulation of IFN-α/β signaling by IFN regulatory factor 2 for homeostatic development of dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 101, 2416–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Suzuki S., Honma K., Matsuyama T., Suzuki K., Toriyama K., Akitoyo I., Yamamoto K., Suematsu T., Nakamura M., Yui K., Kumatori A. (2004) Critical roles of interferon regulatory factor 4 in CD11bhighCD8α− dendritic cell development. Proc. Natl. Acad. Sci. U. S. A. 101, 8981–8986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wu L., D'Amico A., Winkel K. D., Suter M., Lo D., Shortman K. (1998) RelB is essential for the development of myeloid-related CD8α− dendritic cells but not of lymphoid-related CD8α+ dendritic cells. Immunity 9, 839–847 [DOI] [PubMed] [Google Scholar]
- 122. Caton M. L., Smith-Raska M. R., Reizis B. (2007) Notch-RBP-J signaling controls the homeostasis of CD8− dendritic cells in the spleen. J. Exp. Med. 204, 1653–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Fainaru O., Woolf E., Lotem J., Yarmus M., Brenner O., Goldenberg D., Negreanu V., Bernstein Y., Levanon D., Jung S., Groner Y. (2004) Runx3 regulates mouse TGF-β-mediated dendritic cell function and its absence results in airway inflammation. EMBO J. 23, 969–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kel J. M., Girard-Madoux M. J., Reizis B., Clausen B. E. (2010) TGF-β is required to maintain the pool of immature Langerhans cells in the epidermis. J. Immunol. 185, 3248–3255 [DOI] [PubMed] [Google Scholar]
- 125. Lyman S. D., Jacobsen S. E. (1998) c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood 91, 1101–1134 [PubMed] [Google Scholar]
- 126. Blom B., Ho S., Antonenko S., Liu Y. J. (2000) Generation of interferon α-producing predendritic cell (Pre-DC)2 from human CD34+ hematopoietic stem cells. J. Exp. Med. 192, 1785–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Brasel K., De Smedt T., Smith J. L., Maliszewski C. R. (2000) Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood 96, 3029–3039 [PubMed] [Google Scholar]
- 128. Zhang Y., Wang Y., Ogata M., Hashimoto S., Onai N., Matsushima K. (2000) Development of dendritic cells in vitro from murine fetal liver-derived lineage phenotype-negative c-kit+ hematopoietic progenitor cells. Blood 95, 138–146 [PubMed] [Google Scholar]
- 129. Bjorck P. (2001) Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood 98, 3520–3526 [DOI] [PubMed] [Google Scholar]
- 130. Hochweller K., Miloud T., Striegler J., Naik S., Hammerling G. J., Garbi N. (2009) Homeostasis of dendritic cells in lymphoid organs is controlled by regulation of their precursors via a feedback loop. Blood 114, 4411–4421 [DOI] [PubMed] [Google Scholar]
- 131. Karsunky H., Merad M., Cozzio A., Weissman I. L., Manz M. G. (2003) Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J. Exp. Med. 198, 305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Manfra D. J., Chen S. C., Jensen K. K., Fine J. S., Wiekowski M. T., Lira S. A. (2003) Conditional expression of murine Flt3 ligand leads to expansion of multiple dendritic cell subsets in peripheral blood and tissues of transgenic mice. J. Immunol. 170, 2843–2852 [DOI] [PubMed] [Google Scholar]
- 133. Sallusto F., Lanzavecchia A. (1994) Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179, 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Caux C., Vanbervliet B., Massacrier C., Dezutter-Dambuyant C., de Saint-Vis B., Jacquet C., Yoneda K., Imamura S., Schmitt D., Banchereau J. (1996) CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF α. J. Exp. Med. 184, 695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Palma M., Adamson L., Hansson L., Kokhaei P., Rezvany R., Mellstedt H., Osterborg A., Choudhury A. (2008) Development of a dendritic cell-based vaccine for chronic lymphocytic leukemia. Cancer Immunol. Immunother. 57, 1705–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kadowaki N., Kitawaki T. (2011) Recent advance in antigen-specific immunotherapy for acute myeloid leukemia. Clin. Dev. Immunol. 2011, 104926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Burgdorf S. K., Fischer A., Myschetzky P. S., Munksgaard S. B., Zocca M. B., Claesson M. H., Rosenberg J. (2008) Clinical responses in patients with advanced colorectal cancer to a dendritic cell based vaccine. Oncol. Rep. 20, 1305–1311 [PubMed] [Google Scholar]
- 138. Zarei S., Schwenter F., Luy P., Aurrand-Lions M., Morel P., Kopf M., Dranoff G., Mach N. (2009) Role of GM-CSF signaling in cell-based tumor immunization. Blood 113, 6658–6668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Soiffer R., Hodi F. S., Haluska F., Jung K., Gillessen S., Singer S., Tanabe K., Duda R., Mentzer S., Jaklitsch M., Bueno R., Clift S., Hardy S., Neuberg D., Mulligan R., Webb I., Mihm M., Dranoff G. (2003) Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J. Clin. Oncol. 21, 3343–3350 [DOI] [PubMed] [Google Scholar]
- 140. Daro E., Pulendran B., Brasel K., Teepe M., Pettit D., Lynch D. H., Vremec D., Robb L., Shortman K., McKenna H. J., Maliszewski C. R., Maraskovsky E. (2000) Polyethylene glycol-modified GM-CSF expands CD11bhighCD11chigh but notCD11blowCD11chigh murine dendritic cells in vivo: a comparative analysis with Flt3 ligand. J. Immunol. 165, 49–58 [DOI] [PubMed] [Google Scholar]
- 141. Hahm B., Trifilo M. J., Zuniga E. I., Oldstone M. B. (2005) Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity 22, 247–257 [DOI] [PubMed] [Google Scholar]
- 142. Zuniga E. I., McGavern D. B., Pruneda-Paz J. L., Teng C., Oldstone M. B. (2004) Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat. Immunol. 5, 1227–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Swiecki M., Wang Y., Vermi W., Gilfillan S., Schreiber R. D., Colonna M. (2011) Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J. Exp. Med. 208, 2367–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Montoya M., Schiavoni G., Mattei F., Gresser I., Belardelli F., Borrow P., Tough D. F. (2002) Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 99, 3263–3271 [DOI] [PubMed] [Google Scholar]
- 145. Longhi M. P., Trumpfheller C., Idoyaga J., Caskey M., Matos I., Kluger C., Salazar A. M., Colonna M., Steinman R. M. (2009) Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med. 206, 1589–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gonzalez-Navajas J. M., Lee J., David M., Raz E. (2012) Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 12, 125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ichikawa E., Hida S., Omatsu Y., Shimoyama S., Takahara K., Miyagawa S., Inaba K., Taki S. (2004) Defective development of splenic and epidermal CD4+ dendritic cells in mice deficient for IFN regulatory factor-2. Proc. Natl. Acad. Sci. U. S. A. 101, 3909–3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Franchini M., Hefti H., Vollstedt S., Glanzmann B., Riesen M., Ackermann M., Chaplin P., Shortman K., Suter M. (2004) Dendritic cells from mice neonatally vaccinated with modified vaccinia virus Ankara transfer resistance against herpes simplex virus type I to naive one-week-old mice. J. Immunol. 172, 6304–6312 [DOI] [PubMed] [Google Scholar]
- 149. Hamilton J. A. (2008) Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8, 533–544 [DOI] [PubMed] [Google Scholar]
- 150. MacDonald K. P., Rowe V., Bofinger H. M., Thomas R., Sasmono T., Hume D. A., Hill G. R. (2005) The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J. Immunol. 175, 1399–1405 [DOI] [PubMed] [Google Scholar]
- 151. Nefedova Y., Huang M., Kusmartsev S., Bhattacharya R., Cheng P., Salup R., Jove R., Gabrilovich D. (2004) Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in Cancer J. Immunol. 172, 464–474 [DOI] [PubMed] [Google Scholar]
- 152. Novak U., Marks D., Nicholson S. E., Hilton D., Paradiso L. (1999) Differential ability of SOCS proteins to regulate IL-6 and CSF-1 induced macrophage differentiation. Growth Factors 16, 305–314 [DOI] [PubMed] [Google Scholar]
- 153. Cao X., Tay A., Guy G. R., Tan Y. H. (1996) Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol. Cell. Biol. 16, 1595–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kaplan D. H., Li M. O., Jenison M. C., Shlomchik W. D., Flavell R. A., Shlomchik M. J. (2007) Autocrine/paracrine TGFβ1 is required for the development of epidermal Langerhans cells. J. Exp. Med. 204, 2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kobie J. J., Wu R. S., Kurt R. A., Lou S., Adelman M. K., Whitesell L. J., Ramanathapuram L. V., Arteaga C. L., Akporiaye E. T. (2003) Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res. 63, 1860–1864 [PubMed] [Google Scholar]
- 156. Yamaguchi Y., Tsumura H., Miwa M., Inaba K. (1997) Contrasting effects of TGF-β1 and TNF-α on the development of dendritic cells from progenitors in mouse bone marrow. Stem Cells 15, 144–153 [DOI] [PubMed] [Google Scholar]
- 157. Van de Laar L., van den Bosch A., Wierenga A. T., Janssen H. L., Coffer P. J., Woltman A. M. (2011) Tight control of STAT5 activity determines human CD34-derived interstitial dendritic cell and Langerhans cell development. J. Immunol. 186, 7016–7024 [DOI] [PubMed] [Google Scholar]
- 158. Cheers C., Haigh A. M., Kelso A., Metcalf D., Stanley E. R., Young A. M. (1988) Production of colony-stimulating factors (CSFs) during infection: separate determinations of macrophage-, granulocyte-, granulocyte-macrophage-, and multi-CSFs. Infect. Immun. 56, 247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Serbina N. V., Salazar-Mather T. P., Biron C. A., Kuziel W. A., Pamer E. G. (2003) TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19, 59–70 [DOI] [PubMed] [Google Scholar]
- 160. Maraskovsky E., Daro E., Roux E., Teepe M., Maliszewski C. R., Hoek J., Caron D., Lebsack M. E., McKenna H. J. (2000) In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood 96, 878–884 [PubMed] [Google Scholar]
- 161. Rosenzwajg M., Camus S., Guigon M., Gluckman J. C. (1998) The influence of interleukin (IL)-4, IL-13, and Flt3 ligand on human dendritic cell differentiation from cord blood CD34+ progenitor cells Exp. Hematol. 26, 63–72 [PubMed] [Google Scholar]
- 162. Miller J. S., McCullar V., Punzel M., Lemischka I. R., Moore K. A. (1999) Single adult human CD34+/Lin−/CD38− progenitors give rise to natural killer cells, B-lineage cells, dendritic cells, and myeloid cells Blood 93, 96–106 [PubMed] [Google Scholar]
- 163. Chen W., Antonenko S., Sederstrom J. M., Liang X., Chan A. S., Kanzler H., Blom B., Blazar B. R., Liu Y. J. (2004) Thrombopoietin cooperates with FLT3-ligand in the generation of plasmacytoid dendritic cell precursors from human hematopoietic progenitors. Blood 103, 2547–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Rickmann M., Krauter J., Stamer K., Heuser M., Salguero G., Mischak-Weissinger E., Ganser A., Stripecke R. (2011) Elevated frequencies of leukemic myeloid and plasmacytoid dendritic cells in acute myeloid leukemia with the FLT3 internal tandem duplication. Ann. Hematol. 90, 1047–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Choudhary C., Schwable J., Brandts C., Tickenbrock L., Sargin B., Kindler T., Fischer T., Berdel W. E., Muller-Tidow C., Serve H. (2005) AML-associated Flt3 kinase domain mutations show signal transduction differences compared with Flt3 ITD mutations. Blood 106, 265–273 [DOI] [PubMed] [Google Scholar]
- 166. Choudhary C., Brandts C., Schwable J., Tickenbrock L., Sargin B., Ueker A., Bohmer F. D., Berdel W. E., Muller-Tidow C., Serve H. (2007) Activation mechanisms of STAT5 by oncogenic Flt3-ITD. Blood 110, 370–374 [DOI] [PubMed] [Google Scholar]
- 167. Leischner H., Albers C., Grundler R., Razumovskaya E., Spiekermann K., Bohlander S., Ronnstrand L., Gotze K., Peschel C., Duyster J. (2012) SRC is a signaling mediator in FLT3-ITD but not in FLT3-TKD positive AML. Blood doi:10.1182/blood-2011-07-365726 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 168. Rocnik J. L., Okabe R., Yu J. C., Lee B. H., Giese N., Schenkein D. P., Gilliland D. G. (2006) Roles of tyrosine 589 and 591 in STAT5 activation and transformation mediated by FLT3-ITD. Blood 108, 1339–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Arima K., Watanabe N., Hanabuchi S., Chang M., Sun S. C., Liu Y. J. (2010) Distinct signal codes generate dendritic cell functional plasticity. Sci. Signal. 3, ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Caux C., Dezutter-Dambuyant C., Schmitt D., Banchereau J. (1992) GM-CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nature 360, 258–261 [DOI] [PubMed] [Google Scholar]
- 171. Szabolcs P., Feller E. D., Moore M. A., Young J. W. (1995) Progenitor recruitment and in vitro expansion of immunostimulatory dendritic cells from human CD34+ bone marrow cells by c-kit-ligand, GM-CSF, and TNF α. Adv. Exp. Med. Biol. 378, 17–20 [DOI] [PubMed] [Google Scholar]
- 172. Steinman R. M. (1996) Dendritic cells and immune-based therapies. Exp. Hematol. 24, 859–862 [PubMed] [Google Scholar]
- 173. McKenna H. J. (2001) Role of hematopoietic growth factors/flt3 ligand in expansion and regulation of dendritic cells. Curr. Opin. Hematol. 8, 149–154 [DOI] [PubMed] [Google Scholar]
- 174. Dranoff G. (2002) GM-CSF-based cancer vaccines. Immunol. Rev. 188, 147–154 [DOI] [PubMed] [Google Scholar]
- 175. Waller E. K., Ernstoff M. S. (2003) Modulation of antitumor immune responses by hematopoietic cytokines. Cancer 97, 1797–1809 [DOI] [PubMed] [Google Scholar]
- 176. Cochran A. J., Huang R. R., Lee J., Itakura E., Leong S. P., Essner R. (2006) Tumour-induced immune modulation of sentinel lymph nodes. Nat. Rev. Immunol. 6, 659–670 [DOI] [PubMed] [Google Scholar]
- 177. Draube A., Klein-Gonzalez N., Mattheus S., Brillant C., Hellmich M., Engert A., von Bergwelt-Baildon M. (2011) Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta-analysis. PloS One 6, e18801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Garcia F., Climent N., Assoumou L., Gil C., Gonzalez N., Alcami J., Leon A., Romeu J., Dalmau J., Martinez-Picado J., Lifson J., Autran B., Costagliola D., Clotet B., Gatell J. M., Plana M., Gallart T. (2011) A therapeutic dendritic cell-based vaccine for HIV-1 infection. J. Infect. Dis. 203, 473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Verkade M. A., van de Wetering J., Klepper M., Vaessen L. M., Weimar W., Betjes M. G. (2004) Peripheral blood dendritic cells and GM-CSF as an adjuvant for hepatitis B vaccination in hemodialysis patients. Kidney Int. 66, 614–621 [DOI] [PubMed] [Google Scholar]
- 180. Wang K., Nishimoto K. P., Mehta R. S., Nelson E. L. (2006) An alternative flow cytometry strategy for peripheral blood dendritic cell enumeration in the setting of repetitive GM-CSF dosing. J. Transl. Med. 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Sathaliyawala T., O'Gorman W. E., Greter M., Bogunovic M., Konjufca V., Hou Z. E., Nolan G. P., Miller M. J., Merad M., Reizis B. (2010) Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity 33, 597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Zhang S., Broxmeyer H. E. (2000) Flt3 ligand induces tyrosine phosphorylation of gab1 and gab2 and their association with shp-2, grb2, and PI3 kinase. Biochem. Biophys. Res. Commun. 277, 195–199 [DOI] [PubMed] [Google Scholar]
- 183. Zhang S., Fukuda S., Lee Y., Hangoc G., Cooper S., Spolski R., Leonard W. J., Broxmeyer H. E. (2000) Essential role of signal transducer and activator of transcription (Stat)5a but not Stat5b for Flt3-dependent signaling. J. Exp. Med. 192, 719–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Takeda K., Noguchi K., Shi W., Tanaka T., Matsumoto M., Yoshida N., Kishimoto T., Akira S. (1997) Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. U. S. A. 94, 3801–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Melillo J. A., Song L., Bhagat G., Blazquez A. B., Plumlee C. R., Lee C., Berin C., Reizis B., Schindler C. (2010) Dendritic cell (DC)-specific targeting reveals Stat3 as a negative regulator of DC function. J. Immunol. 184, 2638–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Teglund S., McKay C., Schuetz E., van Deursen J. M., Stravopodis D., Wang D., Brown M., Bodner S., Grosveld G., Ihle J. N. (1998) Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93, 841–850 [DOI] [PubMed] [Google Scholar]
- 187. Cui Y., Riedlinger G., Miyoshi K., Tang W., Li C., Deng C. X., Robinson G. W., Hennighausen L. (2004) Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol. Cell. Biol. 24, 8037–8047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Yao Z., Cui Y., Watford W. T., Bream J. H., Yamaoka K., Hissong B. D., Li D., Durum S. K., Jiang Q., Bhandoola A., Hennighausen L., O'Shea J. J. (2006) Stat5a/b are essential for normal lymphoid development and differentiation. Proc. Natl. Acad. Sci. U. S. A. 103, 1000–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Meraz M. A., White J. M., Sheehan K. C., Bach E. A., Rodig S. J., Dighe A. S., Kaplan D. H., Riley J. K., Greenlund A. C., Campbell D., Carver-Moore K., DuBois R. N., Clark R., Aguet M., Schreiber R. D. (1996) Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84, 431–442 [DOI] [PubMed] [Google Scholar]
- 190. Durbin J. E., Hackenmiller R., Simon M. C., Levy D. E. (1996) Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84, 443–450 [DOI] [PubMed] [Google Scholar]
- 191. Biron C. A. (2001) Interferons α and β as immune regulators−a new look. Immunity 14, 661–664 [DOI] [PubMed] [Google Scholar]
- 192. Garcia-Sastre A., Biron C. A. (2006) Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312, 879–882 [DOI] [PubMed] [Google Scholar]
- 193. Stark G. R., Kerr I. M., Williams B. R., Silverman R. H., Schreiber R. D. (1998) How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 [DOI] [PubMed] [Google Scholar]
- 194. Darnell J. E., Jr., Kerr I. M., Stark G. R. (1994) Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264, 1415–1421 [DOI] [PubMed] [Google Scholar]
- 195. Van Boxel-Dezaire A. H., Rani M. R., Stark G. R. (2006) Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 25, 361–372 [DOI] [PubMed] [Google Scholar]
- 196. Dong C. (2008) TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 8, 337–348 [DOI] [PubMed] [Google Scholar]
- 197. Zhu J., Paul W. E. (2010) Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol. Rev. 238, 247–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Rothenberg E. V. (2011) T cell lineage commitment: identity and renunciation. J. Immunol. 186, 6649–6655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Lawrence T., Natoli G. (2011) Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11, 750–761 [DOI] [PubMed] [Google Scholar]
- 200. Dore L. C., Crispino J. D. (2011) Transcription factor networks in erythroid cell and megakaryocyte development. Blood 118, 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Orkin S. H. (2000) Diversification of haematopoietic stem cells to specific lineages. Nat. Rev. Genet. 1, 57–64 [DOI] [PubMed] [Google Scholar]
- 202. Rosenbauer F., Tenen D. G. (2007) Transcription factors in myeloid development: balancing differentiation with transformation. Nat. Rev. Immunol. 7, 105–117 [DOI] [PubMed] [Google Scholar]
- 203. Tenen D. G., Hromas R., Licht J. D., Zhang D. E. (1997) Transcription factors, normal myeloid development, and leukemia. Blood 90, 489–519 [PubMed] [Google Scholar]
- 204. Rosenbauer F., Koschmieder S., Steidl U., Tenen D. G. (2005) Effect of transcription-factor concentrations on leukemic stem cells. Blood 106, 1519–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Onai N., Obata-Onai A., Tussiwand R., Lanzavecchia A., Manz M. G. (2006) Activation of the Flt3 signal transduction cascade rescues and enhances type I interferon-producing and dendritic cell development. J. Exp. Med. 203, 227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. McKercher S. R., Torbett B. E., Anderson K. L., Henkel G. W., Vestal D. J., Baribault H., Klemsz M., Feeney A. J., Wu G. E., Paige C. J., Maki R. A. (1996) Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 15, 5647–5658 [PMC free article] [PubMed] [Google Scholar]
- 207. Rosenbauer F., Wagner K., Kutok J. L., Iwasaki H., Le Beau M. M., Okuno Y., Akashi K., Fiering S., Tenen D. G. (2004) Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU.1. Nat. Genet. 36, 624–630 [DOI] [PubMed] [Google Scholar]
- 208. Dakic A., Metcalf D., Di Rago L., Mifsud S., Wu L., Nutt S. L. (2005) PU.1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. J. Exp. Med. 201, 1487–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Bakri Y., Sarrazin S., Mayer U. P., Tillmanns S., Nerlov C., Boned A., Sieweke M. H. (2005) Balance of MafB and PU.1 specifies alternative macrophage or dendritic cell fate. Blood 105, 2707–2716 [DOI] [PubMed] [Google Scholar]
- 210. Laiosa C. V., Stadtfeld M., Xie H., de Andres-Aguayo L., Graf T. (2006) Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBPα and PU.1 transcription factors. Immunity 25, 731–744 [DOI] [PubMed] [Google Scholar]
- 211. Panopoulos A. P., Bartos D., Zhang L., Watowich S. S. (2002) Control of myeloid-specific integrin aMb2 (CD11b/CD18) expression by cytokines is regulated by Stat3-dependent activation of PU.1. J. Biol. Chem. 277, 19001–19007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Hegde S., Ni S., He S., Yoon D., Feng G. S., Watowich S. S., Paulson R. F., Hankey P. A. (2009) Stat3 promotes the development of erythroleukemia by inducing PU.1 expression and inhibiting erythroid differentiation. Oncogene 28, 3349–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Holtschke T., Lohler J., Kanno Y., Fehr T., Giese N., Rosenbauer F., Lou J., Knobeloch K. P., Gabriele L., Waring J. F., Bachmann M. F., Zinkernagel R. M., Morse H. C., 3rd, Ozato K., Horak I. (1996) Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell 87, 307–317 [DOI] [PubMed] [Google Scholar]
- 214. Becker A. M., Michael D. G., Satpathy A. T., Sciammas R., Singh H., Bhattacharya D. (2012) IRF-8 extinguishes neutrophil production and promotes dendritic cell lineage commitment in both myeloid and lymphoid mouse progenitors. Blood 119, 2003–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Tailor P., Tamura T., Morse H. C., 3rd, Ozato K. (2008) The BXH2 mutation in IRF8 differentially impairs dendritic cell subset development in the mouse. Blood 111, 1942–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Hambleton S., Salem S., Bustamante J., Bigley V., Boisson-Dupuis S., Azevedo J., Fortin A., Haniffa M., Ceron-Gutierrez L., Bacon C. M., Menon G., Trouillet C., McDonald D., Carey P., Ginhoux F., Alsina L., Zumwalt T. J., Kong X. F., Kumararatne D., Butler K., Hubeau M., Feinberg J., Al-Muhsen S., Cant A., Abel L., Chaussabel D., Doffinger R., Talesnik E., Grumach A., Duarte A., Abarca K., Moraes-Vasconcelos D., Burk D., Berghuis A., Geissmann F., Collin M., Casanova J. L., Gros P. (2011) IRF8 mutations and human dendritic-cell immunodeficiency. N. Engl. J. Med. 365, 127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Kanno Y., Kozak C. A., Schindler C., Driggers P. H., Ennist D. L., Gleason S. L., Darnell J. E., Jr., Ozato K. (1993) The genomic structure of the murine ICSBP gene reveals the presence of the γ interferon-responsive element, to which an ISGF3 α subunit (or similar) molecule binds. Mol. Cell. Biol. 13, 3951–3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Schmidt M., Nagel S., Proba J., Thiede C., Ritter M., Waring J. F., Rosenbauer F., Huhn D., Wittig B., Horak I., Neubauer A. (1998) Lack of interferon consensus sequence binding protein (ICSBP) transcripts in human myeloid leukemias. Blood 91, 22–29 [PubMed] [Google Scholar]
- 219. Lehtonen A., Lund R., Lahesmaa R., Julkunen I., Sareneva T., Matikainen S. (2003) IFN-α and IL-12 activate IFN regulatory factor 1 (IRF-1), IRF-4, and IRF-8 gene expression in human NK and T cells. Cytokine 24, 81–90 [DOI] [PubMed] [Google Scholar]
- 220. Yang C. Y., Best J. A., Knell J., Yang E., Sheridan A. D., Jesionek A. K., Li H. S., Rivera R. R., Lind K. C., D'Cruz L. M., Watowich S. S., Murre C., Goldrath A. W. (2011) The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat. Immunol. 12, 1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Bigley V., Haniffa M., Doulatov S., Wang X. N., Dickinson R., McGovern N., Jardine L., Pagan S., Dimmick I., Chua I., Wallis J., Lordan J., Morgan C., Kumararatne D. S., Doffinger R., van der Burg M., van Dongen J., Cant A., Dick J. E., Hambleton S., Collin M. (2011) The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. J. Exp. Med. 208, 227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Hsu A. P., Sampaio E. P., Khan J., Calvo K. R., Lemieux J. E., Patel S. Y., Frucht D. M., Vinh D. C., Auth R. D., Freeman A. F., Olivier K. N., Uzel G., Zerbe C. S., Spalding C., Pittaluga S., Raffeld M., Kuhns D. B., Ding L., Paulson M. L., Marciano B. E., Gea-Banacloche J. C., Orange J. S., Cuellar-Rodriguez J., Hickstein D. D., Holland S. M. (2011) Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood 118, 2653–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Dickinson R. E., Griffin H., Bigley V., Reynard L. N., Hussain R., Haniffa M., Lakey J. H., Rahman T., Wang X. N., McGovern N., Pagan S., Cookson S., McDonald D., Chua I., Wallis J., Cant A., Wright M., Keavney B., Chinnery P. F., Loughlin J., Hambleton S., Santibanez-Koref M., Collin M. (2011) Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 118, 2656–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Tsai F. Y., Orkin S. H. (1997) Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 89, 3636–3643 [PubMed] [Google Scholar]
- 225. Rodrigues N. P., Janzen V., Forkert R., Dombkowski D. M., Boyd A. S., Orkin S. H., Enver T., Vyas P., Scadden D. T. (2005) Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood 106, 477–484 [DOI] [PubMed] [Google Scholar]
- 226. Vicente C., Conchillo A., Garcia-Sanchez M. A., Odero M. D. (2011) The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit. Rev. Oncol Hematol. 82, 1–17 [DOI] [PubMed] [Google Scholar]
- 227. Fujiwara T., O'Geen H., Keles S., Blahnik K., Linnemann A. K., Kang Y. A., Choi K., Farnham P. J., Bresnick E. H. (2009) Discovering hematopoietic mechanisms through genome-wide analysis of GATA factor chromatin occupancy. Mol. Cell 36, 667–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Wilson N. K., Foster S. D., Wang X., Knezevic K., Schutte J., Kaimakis P., Chilarska P. M., Kinston S., Ouwehand W. H., Dzierzak E., Pimanda J. E., de Bruijn M. F., Gottgens B. (2010) Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell 7, 532–544 [DOI] [PubMed] [Google Scholar]
- 229. Zhang P., Behre G., Pan J., Iwama A., Wara-Aswapati N., Radomska H. S., Auron P. E., Tenen D. G., Sun Z. (1999) Negative cross-talk between hematopoietic regulators: GATA proteins repress PU.1. Proc. Natl. Acad. Sci. U. S. A. 96, 8705–8710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Minegishi Y., Saito M., Tsuchiya S., Tsuge I., Takada H., Hara T., Kawamura N., Ariga T., Pasic S., Stojkovic O., Metin A., Karasuyama H. (2007) Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 448, 1058–1062 [DOI] [PubMed] [Google Scholar]
- 231. He J. L., Liu J. H., Liu F., Tan P., Lin T., Li X. L. (2012) Mutation screening of BMP4 and Id2 genes in Chinese patients with congenital ureteropelvic junction obstruction. Eur. J. Pediatr. 171, 451–456 [DOI] [PubMed] [Google Scholar]
- 232. Nagasawa M., Schmidlin H., Hazekamp M. G., Schotte R., Blom B. (2008) Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2-2 and the Ets factor Spi-B. Eur. J. Immunol. 38, 2389–2400 [DOI] [PubMed] [Google Scholar]
- 233. Saito M., Nagasawa M., Takada H., Hara T., Tsuchiya S., Agematsu K., Yamada M., Kawamura N., Ariga T., Tsuge I., Nonoyama S., Karasuyama H., Minegishi Y. (2011) Defective IL-10 signaling in hyper-IgE syndrome results in impaired generation of tolerogenic dendritic cells and induced regulatory T cells. J. Exp. Med. 208, 235–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Giacomelli M., Tamassia N., Moratto D., Bertolini P., Ricci G., Bertulli C., Plebani A., Cassatella M., Bazzoni F., Badolato R. (2011) SH2-domain mutations in STAT3 in hyper-IgE syndrome patients result in impairment of IL-10 function. Eur. J. Immunol. 41, 3075–3084 [DOI] [PubMed] [Google Scholar]
- 235. Murray P. J. (2006) Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr. Opin. Pharmacol. 6, 379–386 [DOI] [PubMed] [Google Scholar]
- 236. Spits H., Couwenberg F., Bakker A. Q., Weijer K., Uittenbogaart C. H. (2000) Id2 and Id3 inhibit development of CD34+ stem cells into predendritic cell (pre-DC)2 but not into pre-DC1. Evidence for a lymphoid origin of pre-DC2. J. Exp. Med. 192, 1775–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Schotte R., Nagasawa M., Weijer K., Spits H., Blom B. (2004) The ETS transcription factor Spi-B is required for human plasmacytoid dendritic cell development. J. Exp. Med. 200, 1503–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Allman D., Dalod M., Asselin-Paturel C., Delale T., Robbins S. H., Trinchieri G., Biron C. A., Kastner P., Chan S. (2006) Ikaros is required for plasmacytoid dendritic cell differentiation. Blood 108, 4025–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Wu L., Nichogiannopoulou A., Shortman K., Georgopoulos K. (1997) Cell-autonomous defects in dendritic cell populations of Ikaros mutant mice point to a developmental relationship with the lymphoid lineage. Immunity 7, 483–492 [DOI] [PubMed] [Google Scholar]
- 240. Aliberti J., Schulz O., Pennington D. J., Tsujimura H., Reis e Sousa C., Ozato K., Sher A. (2003) Essential role for ICSBP in the in vivo development of murine CD8α+ dendritic cells. Blood 101, 305–310 [DOI] [PubMed] [Google Scholar]