Altered CpG DNA methylation contributes to phenotypic changes in smoker's alveolar macrophages.
Keywords: epigenetics, inflammation, emphysema
Abstract
Cigarette smoking is implicated in numerous diseases, including emphysema and lung cancer. The clinical expression of lung disease in smokers is not well explained by currently defined variations in gene expression or simple differences in smoking exposure. Alveolar macrophages play a critical role in the inflammation and remodeling of the lung parenchyma in smoking-related lung disease. Significant gene expression changes in alveolar macrophages from smokers have been identified. However, the mechanism for these changes remains unknown. One potential mechanism for smoking-altered gene expression is via changes in cytosine methylation in DNA regions proximal to gene-coding sequences. In this study, alveolar macrophage DNA from heavy smokers and never smokers was isolated and methylation status at 25,000 loci determined. We found differential methylation in genes from immune-system and inflammatory pathways. Analysis of matching gene expression data demonstrated a parallel enrichment for changes in immune-system and inflammatory pathways. A significant number of genes with smoking-altered mRNA expression had inverse changes in methylation status. One gene highlighted by this data was the FLT1, and further studies found particular up-regulation of a splice variant encoding a soluble inhibitory form of the receptor. In conclusion, chronic cigarette smoke exposure altered DNA methylation in specific gene promoter regions in human alveolar macrophages.
Introduction
Despite large-scale educational efforts that target smoking behavior, the health effects of smoking remain a significant problem. Currently, an estimated 21% of U.S. adults smoke [1]. Cigarette smoking is associated with increased overall morbidity and mortality and is the direct cause of major pulmonary diseases, including COPD and lung cancer [1–4]. By the year 2020, COPD (chronic bronchitis and emphysema) caused by cigarette smoke exposure will become the third-leading cause of death [5]. Alveolar macrophages are critical players in COPD pathogenesis [6–12]. Gene expression changes in alveolar macrophages from smokers are linked to inflammatory pathways and dysregulation of the tissue remodeling and repair processes [13, 14]. Mutations in a number of genes have been associated with COPD, including MMP12, SOD3, HO-1, and TGF-β [8, 10, 15–19]. However, despite years of epidemiologic, toxicologic, and genetic research, the mechanism(s) by which cigarette smoking causes disease remain unknown.
To understand smoking-related disease, it is important to understand the changes that occur with chronic cigarette smoke exposure. One of the early events with smoking is an accumulation of alveolar macrophages in the airways. Alveolar macrophages play an important role in the development of smoking-related disease, including COPD [6, 8, 10–14]. Both heavy smokers and patients with emphysema demonstrate an accumulation of alveolar macrophages around terminal airways [9, 12]. These macrophages have an altered gene expression profile, including activation of genes linked to COPD, such as MMP12 [8]. A number of studies have demonstrated changes in DNA methylation with smoking [20–22], but none of these has looked at primary lung cells. This study examines the role of DNA methylation in alveolar macrophage gene expression in active smokers.
Epigenetic changes, in particular, altered DNA methylation, are attractive candidates to understand the complex pathogenesis of smoking-related disease. Consistent with this hypothesis, smoking alters DNA methylation in nonlung cells [20–27]. Investigators have reported smoking-induced hypermethylation in genes, such as p16 and high-temperature requirement factor A3, in lung cancer biopsies [28–30] and in sputum samples from current and former smokers [20]. In addition, over the past several years, smoking-associated DNA methylation changes have been noted in DNA from a number of nonpulmonary, noncancerous cell sources, including lymphocyte, lymphoblast, and buccal smear DNA [22, 24, 31, 32]. Therefore, it seems reasonable to hypothesize that changes in pulmonary tissue DNA methylation may be occurring in response to smoking and that some of those changes may be responsible, at least in part, for the pathological changes noted in patients with lung disease.
Whereas it has been shown that cigarette smoke alters gene expression in alveolar macrophages [13, 14, 33], it is not known whether changes in DNA methylation play a role in these changes. This study shows profound differences in DNA methylation at CpG motifs in alveolar macrophages. Furthermore, methylation and gene expression data sets highlighted the gene FLT1 as altered with smoking. Further studies found a differential increase in a FLT1 splice variant that encodes a sVEGF inhibitor. This information may offer new insights into pathophysiology and novel targets for studying smoking-related lung disease.
MATERIALS AND METHODS
Ethics statement
All procedures and protocols described in this communication were approved by The University of Iowa Institutional Review Board (Iowa City, IA, USA). Written, informed consent was obtained, and all clinical investigation has been conducted according to the principles expressed in the Declaration of Helsinki.
Subject recruitment
Subjects were recruited from the community via advertisements and word-of-mouth. To be included, case subjects had to be actively smoking with at least a 10-pack-year history of smoking. To be included as a control, the subject had to deny ever smoking cigarettes. Subjects were excluded if they had any significant comorbid conditions, such as pregnancy, or if a baseline spirometry revealed that the FEV1 was <60% of predicted. Medical records and previous chest CT examinations were reviewed for evidence of comorbid conditions. Three case subjects had evidence of emphysema on a chest CT, which was confirmed by a board-certified radiologist from The University of Iowa. However, they were not diagnosed previously with obstructive lung disease.
BAL
After informed consent was obtained, subjects underwent standard flexible bronchoscopy. Local anesthesia was performed with lidocaine instillation into the upper airway, followed by BAL. The lavage was performed by instilling 20 ml normal saline into a tertiary bronchus up to five times in three different lung segments. The first collection out of five was discarded for possible contamination from upper airway secretions or lidocaine. The remaining lavage was transported to the laboratory, where fluid was filtered through sterile gauze and centrifuged at 200 g for 5 min to pellet cellular material. The resulting pellet was suspended in PBS and centrifuged at 16,000 g for 1 min. A sample of the cells was labeled with Wright stain and examined microscopically to ensure that the majority of the cells was macrophages [11, 34, 35]. The average macrophage concentration for this study was 97% macrophages with a sd of 5%. Aliquots were frozen at −80°C for later DNA and RNA isolation.
DNA and RNA isolation
DNA and RNA were isolated from alveolar macrophages using the Qiagen DNAeasy kit (Qiagen, Valencia, CA, USA) and MirVana (Applied Biosystems, Austin, TX, USA) reagents, according to the manufacturers ' instructions. After isolation, to assess the quantity and quality of our samples, Nanodrop and Experion (Experion Automated Electrophoresis System, Bio-Rad, Hercules, CA, USA) chips were used for DNA analysis and RNA analysis, respectively. The RQI of the RNA samples was above 8.1 in all samples except for one that was marginal, measuring 5.4. After preparation, RNA and DNA samples were stored in a −80°C freezer until use.
DNA methylation analysis
Determination of genome-wide methylation values was conducted under contract by the University of Minnesota BioMedical Genomics Center (Minneapolis, MN, USA) using the Illumina Infinium 27K Human Methylation array, which contains 27,038 probes that interrogate CpG residues in 14,475 RefSeq annotated genes (NCBI, Bethesda, MD, USA). The resulting microarray data were inspected for complete bisulfite conversion of the DNA. Average β-values (i.e., average methylation) for each CpG residue were determined using the GenomeStudio V2009.2, methylation module version 1.5.5., version 3.2 (Illumina, San Diego, CA, USA). Comparison of β-values (i.e., methylation) between cases and controls was conducted using Student's t-test, whereas comparisons of the relationship between overall values between individual arrays were conducted using Pearson's correlation coefficient [36–38].
RNA analysis
Measurement of genome-wide macrophage mRNA expression was conducted using the GeneChip Human Exon 1.0 ST (Affymetrix, Santa Clara, CA, USA) arrays under contract by The University of Iowa DNA Facility. The resulting data were analyzed using the Partek GS, version 6.5 (Partek, St. Louis, MO, USA), suite of programs. After inspection, data were assessed for quality and subjected to Robust Multichip Average normalization. The normalized data were analyzed using an ANOVA model with linear contrasts to find the P value and fold-changes between the two categories. The FDR step-up method [39] was applied to correct for multiple testing. The raw data have been deposited in a Minimum Information about a Microarray Experiment (MIAME) compliant database. The GEO (NCBI) accession number is GSE27002.
Pathway analysis
Gene pathway analysis was conducted using the GoMiner suite (Genomics and Bioinformatics Group, Bethesda, MD, USA) of algorithms, using the default settings (http://discover.nci.nih.gov/gominer/index.jsp) [40]. FDR was calculated by GoMiner using the default algorithm. Cluster analysis of the data was accomplished using the CIMminer web interface (Genomics and Bioinformatics Group) and the default Euclidean clustering algorithm.
FLT1 splice variant analysis
mRNA analysis of FLT1 was conducted in a nonredundant data set (eight nonsmokers and eight active smokers) to confirm and expand on the array data. Primers were chosen to specifically amplify the three transcripts (membrane variant 1 and soluble variants 2 and 3). RNA quality was assessed using the Experion system (Bio-Rad). RQI >8.5 was used in analysis. Total RNA (300 ng) was reverse transcribed to cDNA using the iScript cDNA synthesis kit (Bio-Rad), following the manufacturer's instructions. PCR reactions were performed as described previously [41]. Specificity of the amplification was confirmed using melting curve analysis. Data were collected and recorded and expressed as a function of CT. The relative quantity of the gene of interest was then normalized to relative quantity of HPRT (ΔΔCT). The sample mRNA abundance was calculated by the formula 2−(ΔΔCT). Based on the study by Thomas et al. [42], the following primers were used: membrane variant 1 FLT1 forward 5′-TGG CAG CGA GAA ACA TTC TTT TAT C-3′ and reverse 5′-CAG CAA TAC TCC GTA AGA CCA CAC-3′; soluble variant 2 sFLT1_i13 forward 5′-ACA ATC AGA GGT GAG CAC TGC AA-3′ and reverse 5′-TCC GAG CCT GAA AGT TAG CAA-3′; and soluble variant 3 sFLT1_e15a forward 5′-AC ACA GTG GCC ATC AGC AGT T-3′ and 5′-CCC GGC CAT TTG TTA TTG TTA-3.
FLT1 protein analysis
Release of sFLT1 from alveolar macrophage cultures was measured using an ELISA specific for sFLT1 from R&D Systems (Minneapolis, MN, USA; R&D DY321). Membrane-bound FLT1 levels were examined in alveolar macrophage whole cell lysates by Western blot techniques [43, 44]. Protein (25–30 μg) was mixed 1:1 with sample buffer (20% glycerol, 4% SDS, 10% 2-ME, 0.05% bromophenol blue, and 1.25 M Tris, pH 6.8), loaded onto a 12 SDS-PAGE gel, and run at 150 V for 90 min. Cell proteins were transferred to an Immunoblot PVDF membrane (Bio-Rad) with a Bio-Rad semidry transfer system, according to the manufacturer's instructions. The PVDF membrane was then incubated with primary antibody (FLT1, Abcam, Cambridge, MA, USA; #ab14601; 1:500) in 5% milk in TBS with 0.1% Tween 20 overnight. The blots were washed three times with TBS with 0.1% Tween 20, incubated for 1 h with HRP-conjugated secondary anti-IgG antibody (dilution 1:10,000), and washed as before. Immunoreactive bands were developed using the chemiluminescent substrate ECL Plus (Amersham Biosciences, Piscataway, NJ, USA). An autoradiograph was obtained with exposure times of 10 s–2 min. Equal loading of proteins was confirmed by reprobing blots for β-actin.
Other analyses
Comparison of proportion and rates for categorical variables was conducted using Exact testing [36].
RESULTS
Twenty-two subjects were recruited for this study—10 never smokers and 12 active smokers with a significant smoking history. Three of the smokers had CT-defined emphysema. For all of the subjects, FEV1 levels were above 60%. None of them was on medications (other than possible birth control for the women). The never smokers included four males and six females with an average age of 29.7 ± 8.5 years. The smokers included seven males and five females with an average age of 39.3 ± 3.81 years. Overall, the cases (six of 12) were more likely to be African-Americans than the controls (zero of 10; P<0.02). There was also a significant difference in age between cases and controls (P<0.04). However, if the three emphysema cases were excluded, there was no significant difference between the age of smokers and nonsmokers (P=0.14).
Genome-wide methylation status of the alveolar macrophage DNA samples was determined using the Illumina Infinium 27K Human Methylation array. This array contains 27,578 oligonucleotide probes that interrogate the methylation status of CpG residues in 14,475 RefSeq annotated genes. Overall, 20,006 (72.4%) of the probes interrogate sites within promoter-associated CpG islands, and 7572 (27.5%) probes assess methylation in the CpG island “shores”, which are the regions immediately flanking annotated CpG islands.
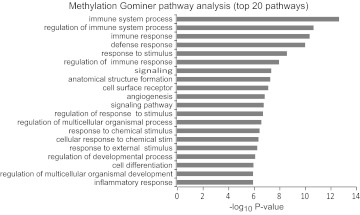
The average methylation (i.e., mean β-value) of the probes in the smoking cases (26.6%) and nonsmoking controls (26.5%) was fairly similar. The mean correlation of the genome-wide methylation values was more similar between those samples of similar status (average r2 between individual cases=0.985 and between individual controls, =0.983) than between cases and control (average r2=0.978; data not shown). Most importantly, the distribution of methylation in these probes differed significantly with respect to smoking status. Table 1 lists the gene regions with the strongest evidence of differential methylation. Overall, 4542, 1625, and 390 of the probes showed nominal evidence of differential methylation at the P < 0.05, P < 0.01, and P < 0.001 level, respectively. Consistent with prior literature suggesting a prominent role for CpG motifs in the regions flanking the islands, the percent of differentially methylated probes for the shores of the island increased with increasing stringency with 31.8% (P<0.05), 40.6% (at P<0.01), and 54.0% (at P<0.001). GoMiner pathway analysis of the 390 differentially methylated probes at the P < 0.001 level showed marked enrichment for probes found in pathways involved in inflammatory responses (Fig. 1).
Table 1. The 30 Most Differentially Methylated Probes.
| Probe ID | Gene | CHR | CHR position | Average β-values |
P values |
||
|---|---|---|---|---|---|---|---|
| Smoker | Control | −log10 P | FDR | ||||
| 3830671 | WNT5B | 12 | 1596294 | 0.77 | 0.56 | −8.582 | 0.0001 |
| 5270092 | WFDC13 | 20 | 43764255 | 0.70 | 0.41 | −8.083 | 0.0001 |
| 2940609 | AOC3 | 17 | 38256798 | 0.76 | 0.46 | −7.765 | 0.0001 |
| 2060630 | FGR | 1 | 27822930 | 0.32 | 0.55 | −7.695 | 0.0001 |
| 6660487 | FLJ27365 | 22 | 44859555 | 0.78 | 0.42 | −7.554 | 0.0001 |
| 3780519 | WNT5B | 12 | 1596553 | 0.82 | 0.70 | −7.532 | 0.0001 |
| 2680088 | SERPINA1 | 14 | 93927028 | 0.43 | 0.29 | −7.237 | 0.0002 |
| 6960538 | IL1B | 2 | 113310256 | 0.15 | 0.30 | −7.164 | 0.0002 |
| 1690048 | ACSM1 | 16 | 20610738 | 0.23 | 0.40 | −6.759 | 0.0005 |
| 6380240 | PTK9L | 3 | 52249091 | 0.71 | 0.53 | −6.698 | 0.0006 |
| 5080743 | ARHGAP9 | 12 | 56169546 | 0.67 | 0.84 | −6.532 | 0.0007 |
| 1500142 | TRAK 1 | 3 | 42176902 | 0.36 | 0.54 | −6.490 | 0.0007 |
| 4220369 | CIDEC | 3 | 9897234 | 0.68 | 0.53 | −6.425 | 0.0008 |
| 2030228 | FLJ27365 | 22 | 44860687 | 0.47 | 0.22 | −6.418 | 0.0008 |
| 1170653 | C5AR1 | 19 | 52504359 | 0.57 | 0.35 | −6.249 | 0.0010 |
| 2600717 | NR1H3 | 11 | 47236881 | 0.62 | 0.40 | −6.179 | 0.0011 |
| 6280274 | ANK3 | 10 | 61818965 | 0.78 | 0.67 | −6.178 | 0.0011 |
| 6560360 | CSPG4 | 15 | 73792916 | 0.72 | 0.61 | −6.117 | 0.0012 |
| 5130121 | MR1 | 1 | 179269292 | 0.67 | 0.51 | −6.072 | 0.0012 |
| 70762 | MEFV | 16 | 3246525 | 0.49 | 0.36 | −6.029 | 0.0013 |
| 4730148 | ACVRL1 | 12 | 50587901 | 0.86 | 0.78 | −5.967 | 0.0014 |
| 1440053 | EMR1 | 19 | 6837962 | 0.80 | 0.68 | −5.908 | 0.0015 |
| 3850747 | PPM1M | 3 | 52255728 | 0.52 | 0.40 | −5.896 | 0.0015 |
| 1030717 | SP140 | 2 | 230798884 | 0.76 | 0.56 | −5.696 | 0.0023 |
| 3800451 | MAPK1 | 22 | 20552597 | 0.28 | 0.18 | −5.591 | 0.0027 |
| 940538 | GAS2L1 | 22 | 28033543 | 0.74 | 0.57 | −5.590 | 0.0027 |
| 3830626 | GPA33 | 1 | 165325989 | 0.72 | 0.46 | −5.570 | 0.0028 |
| 5090221 | GLYCTK | 3 | 52296306 | 0.04 | 0.06 | −5.438 | 0.0036 |
| 1450403 | C20orf55 | 20 | 764362 | 0.41 | 0.28 | −5.408 | 0.0037 |
| 780092 | ATP6V0D2 | 8 | 87180235 | 0.17 | 0.29 | −5.398 | 0.0037 |
Chromosome (CHR) position is per Genome Build 36.
Figure 1. Gominer pathway analysis of methylation data set.
Analysis was performed using the entire gene set list compared with gene regions with altered methylation at the P < 0.001 level. Shown are the top 20 pathways in terms of P value.
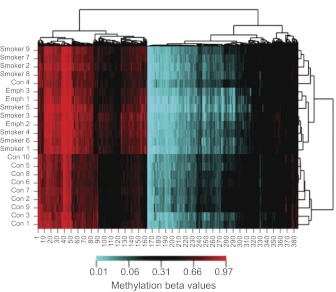
To determine whether these changes were uniform or overemphasized differential methylation in select individuals, we conducted cluster analysis of individual methylation values at all probes at non-X chromosome loci with evidence of differential methylation at the P < 0.001 level. As Fig. 2 demonstrates, the controls and cases segregated together with the exception of Control 4. In the smoking cases, there were three individuals with evidence of emphysema on a CT scan. Interestingly, two of the three individuals (Emph 1 and 3) clustered together, but the third segregated with some of the other smokers. In general, the CT-defined emphysema subjects did not appear to differ significantly from the other smoking subjects.
Figure 2. A clustered image map.
The map was generated by hierarchical clustering analysis of methylation values at the 400 most differentially methylated CpG residues to subject smoking status. Con, Control; Emph, emphysema. Portions in red have greater average methylation, whereas those in blue are the least methylated. Black is an intermediate intensity (see bar legend at bottom of cluster image map).
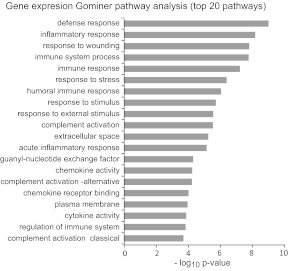
Analysis of mRNA expression also demonstrated widespread changes in alveolar macrophages isolated from the lungs of smokers. Overall, 1448, 567, and 201 genes were differentially expressed in alveolar macrophages from smokers as compared with those from matched controls at the P < 0.05, P < 0.01, and P < 0.001, respectively. Table 2 lists the top 30 of the most differentially expressed genes. Similar to the methylation analysis, pathway analysis of the differentially expressed genes revealed remarkable enrichment of immune system and inflammatory pathway genes (Fig. 3). These genes tended to map to the same pathways as those in the methylation data analysis, and seven of the top 20 most-enriched pathways were shared between the two sets of analyses.
Table 2. The 30 Most Differentially Expressed mRNAs (Smokers vs. Nonsmokers).
| Probe ID | Gene Symbol | CHR | Log10 P-value | FDR | Ratio S/N | Fold-change S/N |
|---|---|---|---|---|---|---|
| 2869096 | SLCO4C1 | 5 | −7.53 | 0.000211 | 2.1856 | 2.1856 |
| 3134511 | SNAI2 | 8 | −7.49 | 0.000211 | 0.3946 | −2.5345 |
| 3105749 | ATP6V0D2 | 8 | −7.48 | 0.000211 | 6.1924 | 6.1924 |
| 3714177 | CYTSB | 19 | −7.41 | 0.000211 | 1.9061 | 1.9062 |
| 2540157 | ODC1 | 2 | −6.89 | 0.000462 | 1.5088 | 1.5088 |
| 2926802 | MYB | 6 | −6.84 | 0.000462 | 0.4023 | −2.4860 |
| 2515933 | ZAK | 2 | −6.82 | 0.000462 | 1.4122 | 1.4122 |
| 3507282 | FLT1 | 13 | −6.74 | 0.000462 | 3.1959 | 3.1959 |
| 2439478 | OR6K3 | 1 | −6.72 | 0.000462 | 2.0234 | 2.0234 |
| 3553998 | TDRD9 | 14 | −6.50 | 0.000686 | 2.2476 | 2.2476 |
| 2548699 | CYP1B1 | 2 | −6.35 | 0.000880 | 5.4828 | 5.4829 |
| 2783916 | TNIP3 | 4 | −6.11 | 0.001411 | 0.5671 | −1.7633 |
| 2955827 | PLA2G7 | 6 | −5.82 | 0.002505 | 5.6804 | 5.6804 |
| 3935486 | S100B | 1 | −5.58 | 0.004135 | 3.9850 | 3.9850 |
| 3722195 | AOC3 | 17 | −5.52 | 0.004382 | 0.4303 | −2.3239 |
| 2489172 | MTHFD2 | 2 | −5.41 | 0.005260 | 1.4517 | 1.4517 |
| 2374345 | CAMSAP1L1 | 1 | −5.34 | 0.005783 | 0.7546 | −1.3252 |
| 2403215 | FGR | 1 | −5.29 | 0.005783 | 1.3526 | 1.3526 |
| 3166477 | ACO1 | 22 | −5.28 | 0.005783 | 0.6748 | −1.4820 |
| 3432090 | ALDH2 | 12 | −5.27 | 0.005783 | 0.7596 | −1.3164 |
| 3463727 | LIN7A | 12 | −5.25 | 0.005858 | 0.4692 | −2.1312 |
| 3379326 | CHKA | 11 | −5.15 | 0.006572 | 0.6723 | −1.4872 |
| 2421995 | GBP4 | 1 | −5.15 | 0.006572 | 1.4873 | 1.4873 |
| 3256590 | PAPSS2 | 4 | −5.14 | 0.006572 | 1.6941 | 1.6941 |
| 2413685 | SSBP3 | 1 | −5.12 | 0.006572 | 2.3296 | 2.3296 |
| 2530713 | CCL20 | 2 | −5.08 | 0.006584 | 0.4108 | −2.4340 |
| 3339346 | FOLR3 | 11 | −5.06 | 0.006908 | 0.3979 | −2.5133 |
| 2422035 | GBP5 | 1 | −5.05 | 0.006908 | 0.4868 | −2.0540 |
| 2773947 | CXCL9 | 4 | −5.00 | 0.006908 | 0.3498 | −2.8589 |
| 3622386 | GATM | 15 | −4.99 | 0.007228 | 0.5872 | −1.7029 |
S, Smokers, including emphysema subjects; N, nonsmokers (controls).
Figure 3. Gominer pathway analysis of mRNA data set.
Analysis was performed using the entire gene set list and the gene regions with altered mRNA expression at the P < 0.01 level. Shown are the top 20 pathways in term of P value.
To determine the potential effects of differential gene methylation on gene expression, we analyzed the most differentially expressed genes (mRNA) and methylated probe sets (P<0.01; Table 3). Overall, 72 genes were found on both lists. In 30 of those 72 cases, increasing methylation was associated with decreasing gene expression. In 20 of those 72 cases, decreasing methylation was associated with increasing gene expression. As a composite, 50 of the differentially regulated methylation and mRNA data points were counter-regulated (P<0.001 by χ2 analysis), suggesting that changes in methylation could be driving the changes in mRNA. Thirty-one of the 50 counter-regulated genes have methylation changes in shores (defined as regions adjacent to CpG islands) [45, 46]; the remaining are found in CpG islands. In six of the 22 cases with gene expression and methylation changing in parallel, the level of methylation in cases and controls was below 10%, suggesting that methylation is not playing a role or that its relationship to gene expression may be complex.
Table 3. Common Genes in Methylation and mRNA Datasets at the P < 0.01 Level (Top 30 Based on mRNA P Values).
| Gene symbol | mRNA | Methylation | Island or shore |
|---|---|---|---|
| FLT1 | up | down | shore |
| CCL20 | down | up | shore |
| FGR | up | down | shore |
| MS4A6A | down | up | shore |
| CYP1B1 | up | down | island |
| ATP6V0D2 | up | down | shore |
| ZAK | up | down | island |
| FOLR3 | down | up | shore |
| SCGB1A1 | down | up | shore |
| AOC3 | down | up | shore |
| C1QA | down | up | shore |
| GPR109B | down | up | island |
| SLC19A3 | down | up | island |
| CFB | down | up | shore |
| ME3 | up | down | island |
| INHBA | down | up | shore |
| MMP2 | up | down | island |
| CD3D | down | up | shore |
| KCNAB1 | down | up | island |
| CD4 | down | up | shore |
| CD80 | down | up | shore |
| CCL5 | down | up | shore |
| TREM2 | up | down | shore |
| KCNE1 | up | down | shore |
| C2 | down | up | shore |
| PPP2R3A | down | up | island |
| IFI27 | down | up | shore |
| AGRP | down | up | shore |
| CYBRD1 | up | down | island |
| MX2 | down | up | island |
Up, Up in smokers compared with nonsmokers; down, down in smokers compared with nonsmokers.
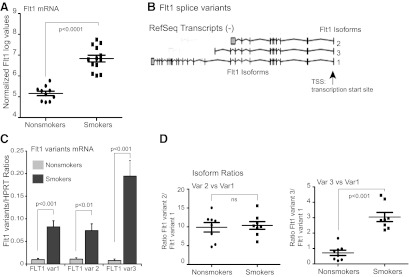
Examining the genes identified in the gene expression/methylation correlation (Table 3), the FLT1 gene was the top hit. The FLT1 gene encodes the full-length transmembrane VEGFR1, FLT1. There are also two major splice variants that encode soluble, secreted forms (sVEGFR1, sFLT1), which are transcribed from the same gene locus [42] (Fig. 4B). They share the extracellular N-terminal ligand-binding domain but lack the transmembrane and cytosolic portions of the full-length protein. sFLT1 serves as a decoy receptor for VEGF, blocking VEGF binding to its receptor on endothelial cells [47, 48]. Smoking has not been identified previously as increasing FLT1 in the lung. Figure 4A shows individual data points from the mRNA microarray analysis. It is clear that smoking increases FLT1 gene expression. To further characterize the smoking effect on FLT1, we analyzed mRNA using primers specific for the full-length membrane form of Flt1 (FLT1, variant 1) and two soluble forms (FLT1, variants 2 and 3) [49]. Figure 4B and C shows that smoker alveolar macrophages have increased levels of all three variants. Figure 4C shows that there is a differential increase in mRNA for FLT1 variant 3, sFlt1-e15a. The CpG motif used in the methylation array is less methylated in smokers and occurs 700 bp upstream of the transcription start site. Interestingly, this particular CpG motif is within a DNAse I hypersensitivity site and an H3K27 acetylation-enriched area. There is a CpG island in the FLT1 promoter region, and the particular probe with decreased methylation is found in this CpG island.
Figure 4. Smoking alters expression of Flt1 in alveolar macrophages.
(A) Flt1 expression data from the mRNA array. Shown are normalized log values for the study subjects. (B) Flt1 variants image from University of California Santa Cruz (Santa Cruz, CA, USA) Human (Homo sapiens) Genome Browser Gateway (http://genome.ucsc.edu/cgi-bin/hgGateway). (C) Analysis of Flt1 variant expression in a nonredundant set of eight never smokers and eight active smokers. Primers for quantitative RT-PCR were chosen from unique 3′ sequences in each of the variants (var1–3). (D) Data from quantitative RT-PCR in C, shown as a ratio between variant 2 and variant 1 (upper) or variant 3 and variant 1 (lower).
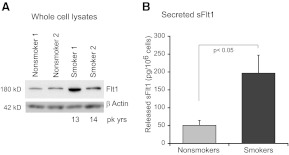
As a composite, these data suggest that smoking increases expression of FLT1, including differential increases in one of the soluble variants of FLT1. We next asked whether these changes in mRNA translated into protein changes. Full-length FLT1 levels were examined in alveolar macrophages. Whole cell protein from two nonsmokers and two active smokers (average pack years, 13.5) was run on a SDS-PAGE gel, transferred, and blotted using an antibody specific for FLT1. Figure 5A shows a smoking-linked increase in full-length FLT1 in lysates from alveolar macrophages. To examine the effect of smoking on release of the truncated sFLT1, we cultured eight sets of alveolar macrophages (four from nonsmokers and four from smokers) overnight. Twenty-four-hour culture supernatants were harvested and sFLT1 levels measured by ELISA, which detects both sFlt1 variants. Figure 5B shows that alveolar macrophages from smokers constitutively release more sFLT1 into the extracellular milieu, confirming what was predicted from the microarray data.
Figure 5. Smoking increases cellular Flt1 protein and sFlt1 protein.
(A) Alveolar macrophages from smokers and nonsmokers were lysed shortly after isolation and protein isolated. Western analysis was performed for Flt1. Equal loading of the samples was verified by reprobing the blot for β-actin. pk yrs, Pack years. (B) Release of sFlt1 from alveolar macrophages. Macrophages from active smokers and never smokers were cultured for 24 h and supernatants harvested. sFlt1 was measured by ELISA.
The data presented here show significant changes in DNA methylation and gene expression in smoker alveolar macrophages. A number of genes show reciprocal regulation, i.e., up-regulated methylation and down-regulated gene expression or vice versa. One gene of note from the gene/methylation comparison is FLT1. We show that smoking leads to increased FLT1 expression of the three major splice variants. The increased expression of all of the variants may be a result of epigenetic changes at the promoter. The reason for the differential up-regulation of variant 3 (the soluble form) remains to be elucidated. It may be caused by epigenetic changes, but it may also be a result of changes in mRNA stability.
DISCUSSION
In this study, using genome-wide methylation and expression approaches, we found strong evidence for alterations in gene pathways related to the immune system and inflammation in alveolar macrophages isolated from the lungs of smokers. It is evident that smoking alters alveolar macrophage gene expression in a significant manner. The consistent methylation pattern changes that we observed in DNA from smoker's alveolar macrophages support this epigenetic mechanism as an important regulatory force in gene expression. This study demonstrates for the first time that alveolar macrophages from smokers have significant alterations in DNA methylation patterns and mRNA expression, which are regulated in a gene-specific, divergent manner (i.e., one is up, and the other is down).
An interesting question is whether the changes in the methylation are directly caused by the chemical effects of cigarette smoke or by transcription-mediated epigenetic remodeling. Prior studies have demonstrated that cigarette smoke forms adducts with DNA [50, 51]. However, based on our prior research on monoamine oxidase A methylation and smoking [22], we believe that many of the changes observed in this manuscript may be a result of activity-dependent epigenetic remodeling. A more complete picture of the relationship between gene expression and methylation with smoking will help to address this question.
The smoking-induced alteration of mRNAs involved in inflammation and immune responses has been described in prior studies [13, 14, 52]. The array-based studies from the Erle and Crystal laboratories found significant alterations in inflammation-related genes [13, 14]. Consistent with our observations, the authors found a remarkably consistent pattern of gene expression in smokers. The paper by Shaykhiev et al. [13]analyzed inflammation-related gene expression and concluded that smoking converts alveolar macrophages from an M1 phenotype (inflammatory) to an M2 phenotype (alternatively activated). It is interesting to speculate that the consistency and stability of the smoking-induced changes might be a result of the fact that expression changes are mediated by altered methylation in the promoter-enhancer regions. The current gene expression results identify CYP1B1 (Table 2), PLA2G7 (Table 2), MMP12, syndecan 2, platelet-derived growth factor D, and IGF1 from the Erle paper and CXCL11, CXCL9 (Table 2), CCL5, MMP2, and c-mer proto-oncogene tyrosine kinase from the Crystal paper among the most significantly, differentially regulated RNAs (data available in the GEO database, Accession Number GSE27002). Thus, our gene expression data validate prior observations and support a hypothesis that inflammation-related genes and MMPs are important in the pathogenesis of smoking-related lung disease.
Our results complement previous genotype-based investigations of the etiology of COPD. For example, studies have demonstrated the association of genetic variations in SERPIN1A (α-1 antitrypsin) in emphysema [53–57]. We found that SERPIN1A is one of the most markedly methylation-altered genes in smokers as compared with nonsmokers in alveolar macrophages (Table 1). However, hepatocytes are the major producers of α-1 antitrypsin, and the alveolar macrophages, despite the changes in methylation, did not exhibit altered gene expression of SERPINA1. There may be a number of possible reasons for this apparent inconsistency. It is possible that α-1 antitrypsin expression by macrophages is not relevant to smoking-related disease processes in the lung. The data in alveolar macrophages do suggest that examining methylation changes at the SERPINA1 locus in hepatocytes may be of interest in determining disease susceptibility in smokers. Furthermore, there may be other functions for these serine proteinases relevant to macrophage biology in the lung.
Reassuringly, GoMiner pathway analysis of the methylation and gene expression data was mutually complementary. In both analyses, inflammatory response, complement activation, and response to foreign antigen pathways figure prominently. In fact, 13 of the top 30 pathways overlap between the two analyses, and the remainder of the pathways share a great deal of semantic overlap as well. Given the thousands of individual pathways listed in the GoMiner database, this overlap suggests that smoking-associated DNA methylation has a prominent role in the differential gene expression observed in the alveolar macrophages in the current study and in the report by Woodruff et al. [14] and Shaykhiev et al. [13].
The hierarchical clustering algorithm accurately classified individuals according to smoke exposure, with the exception of one individual, Control 4. Review of the subject's medical records did not reveal any clear reasons for this anomaly. This subject could be an outlier for any number of reasons, including passive smoke exposure, surreptitious smoking, occupational exposures, or inhalation of other agents. Nevertheless, despite the limited number of subjects in the current study, the overall results are promising and suggest that alveolar macrophage DNA methylation profiles may provide biomarkers for smoke exposure and/or tools for exploring the pathophysiology of smoking-related lung diseases. We are currently expanding this data set to include DNA methylation patterns derived from BAL and PBMCs of nonsmokers, current smokers, and ex-smokers as a first step in investigating biomarkers that could be used to characterize biologically relevant smoke exposure.
Consistent with recent observations by Feinberg and others [45, 46], stressing the importance of non-CpG island methylation in regulating tissue-specific gene expression, more shore than “island” areas were differentially methylated in the smokers. In our study, the degree of shore enrichment increased with increasing statistical stringency, and over one-half of the differentially methylated probes at the P < 0.001 level were found in shore regions. As methylation in the gene body may have different effects than methylation in the CpG island, and these effects may be tissue-specific [59, 60], it will be important to map these regions more densely in larger sample sets.
The methylation and gene expression data highlighted changes in the Flt1 gene. When we further examined the increase in Flt1 gene expression with smoking, we found up-regulation of the membrane and soluble forms of Flt1. In addition, we found differential up-regulation of Flt1 variant 3, sFlt1-e15a. sFlt1 is a known inhibitor of VEGF activity and a potent regulator of angiogenesis [61]. Increased sFlt1 in the lung with smoking has not been described. In a well-characterized system, placental expression of sFlt1 increases in pregnancy, and increased sFlt1 has been associated with preeclampsia [42, 62]. Paradoxically, smoking is associated with a decreased risk of preeclampsia and lower circulating levels of sFlt1 [63, 64]. This is the opposite of our findings of increased expression of Flt1 in smoker's macrophages. It is likely that sFlt1 regulation in the local lung environment differs from the regulation of circulating levels. There is precedence for a role for Flt1 in macrophage biology. Membrane-bound Flt1 has been shown to be a marker for the monocyte-macrophage cell lineages in humans [65]. Of note, a recent study by Kaplan et al. [66] found that the Flt1-positive hematopoietic bone marrow progenitor cell initiates the premetastatic niche. It is interesting to speculate that the increase in membrane (variant 1) and sFlt1 in smoker macrophages may play a role in lung cancer initiation.
The differential methylation found in Flt1 occurs in a CpG island at the promoter region. Our data suggest that differential methylation at the promoter region may explain the increase in Flt1 gene expression found in smoker alveolar macrophages. Secondary to the low-probe density, it is not possible to know whether differential methylation contributes to the altered splicing (increased expression of Flt1 variant 3, sFlt1-e15a). One interesting hypothesis is that changes in methylation result in recruitment (or lack of recruitment) of factors that influence splicing. The methyl-CpG-binding protein, meCP2, is thought to act as a splicing regulator [67]. The work by Young et al. [67] found that the meCP2 modified the splicing patterns of the CD44 minigene. It is interesting to speculate that changes in methylation at the Flt1 gene result in changes in meCP2 binding and subsequent changes in splicing.
In summary, our investigations support previous findings that smoking is associated with global alterations of immune and inflammatory gene pathways. We also suggest the novel hypothesis that differential DNA methylation may be a driver in this process. Further studies may lead to the use of an alveolar macrophage DNA methylation pattern to identify subjects who have a clinically relevant response to smoke or other respiratory toxicants and thus, identify patients at risk of developing, or in the early stages of, smoking-related lung disease.
ACKNOWLEDGMENTS
This study was supported by NIH R01 HL079901, NIH RO1 HL096625, and NIH R21HL109589 to M.M.M.; NIH MH080898 and P30DA027827 to R.A.P.; and NIH RO1 DK090053 to C.P.T. This publication was also made possible by grant number UL1RR024979 from the National Center for Research Resources (NCRR), a part of NIH, and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Clinical and Translational Science Awards or NIH.
Footnotes
- COPD
- chronic obstructive pulmonary disease
- CT
- threshold cycle
- CYP1B1
- cytochrome P450, family 1, subfamily B, polypeptide 1
- FDR
- false discovery rate
- FEV1
- forced expiratory volume in the first second
- FLT1
- fms-like tyrosine kinase 1/VEGFR 1
- GEO
- Gene Expression Omnibus
- HPRT
- hypoxanthine-guanine phosphoribosyltransferase
- meCP2
- methyl CpG-binding protein 2 (Rett syndrome)
- MMP2/12
- matrix metalloprotease 2/12
- NCBI
- National Center for Biotechnology Information
- PLA2G7
- phospholipase A2, group VII
- RefSeq
- Reference Sequence
- RQI
- RNA quality indicator
- s
- soluble
- SERPIN1A
- serpin peptidase inhibitor, clade A
AUTHORSHIP
R.A.P., R.A.S., and M.M.M. designed, implemented, and wrote the manuscript. L.S.P. isolated DNA and oversaw the technical side of the studies. E.N. performed the Flt1 experiments. T.B. analyzed the array data. A.K.G. and I.H. performed bronchoscopies and contributed to study design. C.P.T. assisted in design of the Flt1 experiments. T.J.G. directed subject recruitment and oversaw bronchoscopies. M.M.M. directed the studies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Samet J. M., Wipfli H. L. (2010) Globe still in grip of addiction. Nature 463, 1020–1021 [DOI] [PubMed] [Google Scholar]
- 2. Wipfli H. L., Samet J. M. (2011) Second-hand smoke's worldwide disease toll. Lancet 377, 101–102 [DOI] [PubMed] [Google Scholar]
- 3. Samet J. M. (2010) Estimating the burden of smoking: premature mortality, morbidity, and costs. Salud. Publica. Mex. 52 (Suppl. 2), S98–S107 [DOI] [PubMed] [Google Scholar]
- 4. Stampfli M. R., Anderson G. P. (2009) How cigarette smoke skews immune responses to promote infection, lung disease and cancer Nat. Rev. Immunol. 9, 377–384 [DOI] [PubMed] [Google Scholar]
- 5. Murray C. J., Lopez A. D., Black R., Mathers C. D., Shibuya K., Ezzati M., Salomon J. A., Michaud C. M., Walker N., Vos T. (2007) Global burden of disease 2005: call for collaborators. Lancet 370, 109–110 [DOI] [PubMed] [Google Scholar]
- 6. Churg A., Wang R. D., Tai H., Wang X., Xie C., Dai J., Shapiro S. D., Wright J. L. (2003) Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-α release. Am. J. Respir. Crit. Care Med. 167, 1083–1089 [DOI] [PubMed] [Google Scholar]
- 7. Green G. M. (1968) Cigarette smoke: protection of alveolar macrophages by glutathione and cysteine. Science 162, 810–811 [DOI] [PubMed] [Google Scholar]
- 8. Hautamaki R. D., Kobayashi D. K., Senior R. M., Shapiro S. D. (1997) Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277, 2002–2004 [DOI] [PubMed] [Google Scholar]
- 9. Hodge S., Hodge G., Ahern J., Jersmann H., Holmes M., Reynolds P. N. (2007) Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am. J. Respir. Cell. Mol. Biol. 37, 748–755 [DOI] [PubMed] [Google Scholar]
- 10. Houghton A. M., Quintero P. A., Perkins D. L., Kobayashi D. K., Kelley D. G., Marconcini L. A., Mecham R. P., Senior R. M., Shapiro S. D. (2006) Elastin fragments drive disease progression in a murine model of emphysema. J. Clin. Invest. 116, 753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monick M. M., Powers L. S., Walters K., Lovan N., Zhang M., Gerke A., Hansdottir S., Hunninghake G. W. (2010) Identification of an autophagy defect in smokers' alveolar macrophages. J. Immunol. 185, 5425–5435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tetley T. D. (2002) Macrophages and the pathogenesis of COPD. Chest 121, 156S–159S [DOI] [PubMed] [Google Scholar]
- 13. Shaykhiev R., Krause A., Salit J., Strulovici-Barel Y., Harvey B. G., O'Connor T. P., Crystal R. G. (2009) Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. J. Immunol. 183, 2867–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woodruff P. G., Koth L. L., Yang Y. H., Rodriguez M. W., Favoreto S., Dolganov G. M., Paquet A. C., Erle D. J. (2005) A distinctive alveolar macrophage activation state induced by cigarette smoking. Am. J. Respir. Crit. Care Med. 172, 1383–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shapiro S. D. (1999) The macrophage in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 160, S29–S32 [DOI] [PubMed] [Google Scholar]
- 16. Sørheim I. C., DeMeo D. L., Washko G., Litonjua A., Sparrow D., Bowler R., Bakke P., Pillai S. G., Coxson H. O., Lomas D. A., Silverman E. K., Hersh C. P., International COPD Genetics Network Investigators (2010) Polymorphisms in the superoxide dismutase-3 gene are associated with emphysema in COPD. COPD 7, 262–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamada N., Yamaya M., Okinaga S., Nakayama K., Sekizawa K., Shibahara S., Sasaki H. (2000) Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am. J. Hum. Genet. 66, 187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Celedon J. C., Lange C., Raby B. A., Litonjua A. A., Palmer L. J., DeMeo D. L., Reilly J. J., Kwiatkowski D. J., Chapman H. A., Laird N., Sylvia J. S., Hernandez M., Speizer F. E., Weiss S. T., Silverman E. K. (2004) The transforming growth factor-β1 (TGFB1) gene is associated with chronic obstructive pulmonary disease (COPD). Hum. Mol. Genet. 13, 1649–1656 [DOI] [PubMed] [Google Scholar]
- 19. Hunninghake G. M., Cho M. H., Tesfaigzi Y., Soto-Quiros M. E., Avila L., Lasky-Su J., Stidley C., Melen E., Soderhall C., Hallberg J., Kull I., Kere J., Svartengren M., Pershagen G., Wickman M., Lange C., Demeo D. L., Hersh C. P., Klanderman B. J., Raby B. A., Sparrow D., Shapiro S. D., Silverman E. K., Litonjua A. A., Weiss S. T., Celedon J. C. (2009) MMP12, lung function, and COPD in high-risk populations. N. Engl. J. Med. 361, 2599–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belinsky S. A., Palmisano W. A., Gilliland F. D., Crooks L. A., Divine K. K., Winters S. A., Grimes M. J., Harms H. J., Tellez C. S., Smith T. M., Moots P. P., Lechner J. F., Stidley C. A., Crowell R. E. (2002) Aberrant promoter methylation in bronchial epithelium and sputum from current and former smokers. Cancer Res. 62, 2370–2377 [PubMed] [Google Scholar]
- 21. Breitling L. P., Yang R., Korn B., Burwinkel B., Brenner H. (2011) Tobacco-smoking-related differential DNA methylation: 27K discovery and replication. Am. J. Hum. Genet. 88, 450–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Philibert R. A., Beach S. R., Gunter T. D., Brody G. H., Madan A., Gerrard M. (2010) The effect of smoking on MAOA promoter methylation in DNA prepared from lymphoblasts and whole blood. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Breton C. V., Byun H-M., Wenten M., Pan F., Yang A., Gilliland F. D. (2009) Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am. J. Respir. Crit. Care Med. 180, 462–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang H. W., Ling G. S., Wei W. I., Yuen A. P. (2004) Smoking and drinking can induce p15 methylation in the upper aerodigestive tract of healthy individuals and patients with head and neck squamous cell carcinoma. Cancer 101, 125–132 [DOI] [PubMed] [Google Scholar]
- 25. Lin R. K., Hsieh Y. S., Lin P., Hsu H. S., Chen C. Y., Tang Y. A., Lee C. F., Wang Y. C. (2010) The tobacco-specific carcinogen NNK induces DNA methyltransferase 1 accumulation and tumor suppressor gene hypermethylation in mice and lung cancer patients. J. Clin. Invest. 120, 521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Philibert R. A., Gunter T. D., Beach S. R., Brody G. H., Madan A. (2008) MAOA methylation is associated with nicotine and alcohol dependence in women. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B, 565–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suter M., Abramovici A., Showalter L., Hu M., Shope C. D., Varner M., Aagaard-Tillery K. (2010) In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism 59, 1481–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beleford D., Liu Z., Rattan R., Quagliuolo L., Boccellino M., Baldi A., Maguire J., Staub J., Molina J., Shridhar V. (2010) Methylation induced gene silencing of HtrA3 in smoking-related lung cancer. Clin. Cancer Res. 16, 398–409 [DOI] [PubMed] [Google Scholar]
- 29. Merlo A., Herman J. G., Mao L., Lee D. J., Gabrielson E., Burger P. C., Baylin S. B., Sidransky D. (1995) 5′ CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat. Med. 1, 686–692 [DOI] [PubMed] [Google Scholar]
- 30. Belinsky S. A., Nikula K. J., Palmisano W. A., Michels R., Saccomanno G., Gabrielson E., Baylin S. B., Herman J. G. (1998) Aberrant methylation of p16INK4a is an early event in lung cancer and a potential biomarker for early diagnosis. Proc. Natl. Acad. Sci. USA 95, 11891–11896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Launay J-M., Del Pino M., Chironi G., Callebert J., Peoc'h K., Mégnien J-L., Mallet J., Simon A., Rendu F. (2009) Smoking induces long-lasting effects through a monoamine-oxidase epigenetic regulation. PLoS One 4, e7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suga Y., Miyajima K., Oikawa T., Maeda J., Usuda J., Kajiwara N., Ohira T., Uchida O., Tsuboi M., Hirano T., Kato H., Ikeda N. (2008) Quantitative p16 and ESR1 methylation in the peripheral blood of patients with non-small cell lung cancer. Oncol. Rep. 20, 1137–1142 [PubMed] [Google Scholar]
- 33. Carolan B. J., Harvey B. G., Hackett N. R., O'Connor T. P., Cassano P. A., Crystal R. G. (2009) Disparate oxidant gene expression of airway epithelium compared to alveolar macrophages in smokers. Respir. Res. 10, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Monick M. M., Powers L. S., Barrett C. W., Hinde S., Ashare A., Groskreutz D. J., Nyunoya T., Coleman M., Spitz D. R., Hunninghake G. W. (2008) Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity. J. Immunol. 180, 7485–7496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monick M. M., Powers L. S., Gross T. J., Flaherty D. M., Barrett C. W., Hunninghake G. W. (2006) Active ERK contributes to protein translation by preventing JNK-dependent inhibition of protein phosphatase 1. J. Immunol. 177, 1636–1645 [DOI] [PubMed] [Google Scholar]
- 36. Fleiss J. L. (1981) Statistical Methods for Rates and Proportions, John Wiley & Sons, New York [Google Scholar]
- 37. Beach S. R., Brody G. H., Todorov A. A., Gunter T. D., Philibert R. A. (2010) Methylation at SLC6A4 is linked to family history of child abuse: an examination of the Iowa Adoptee sample. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 710–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Philibert R. A., Beach S. R., Gunter T. D., Brody G. H., Madan A., Gerrard M. (2010) The effect of smoking on MAOA promoter methylation in DNA prepared from lymphoblasts and whole blood. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benjamini Y, Krieger A, Yekutieli D. (2001) Two-Staged Linear Step-Up FDR Controlling Procedure, Technical Report, Department of Statistics and Operation Research, Tel-Aviv University, Israel, and Department of Statistics, Wharton School, University of Pennsylvania, Philadelphia [Google Scholar]
- 40. Zeeberg B. R., Feng W., Wang G., Wang M. D., Fojo A. T., Sunshine M., Narasimhan S., Kane D. W., Reinhold W. C., Lababidi S., Bussey K. J., Riss J., Barrett J. C., Weinstein J. N. (2003) GoMiner: a resource for biological interpretation of genomic and proteomic data. Genome Biol. 4, R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hansdottir S., Monick M. M. (2011) Vitamin D effects on lung immunity and respiratory diseases. Vitam. Horm. 86, 217–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thomas C. P., Andrews J. I., Raikwar N. S., Kelley E. A., Herse F., Dechend R., Golos T. G., Liu K. Z. (2009) A recently evolved novel trophoblast-enriched secreted form of fms-like tyrosine kinase-1 variant is up-regulated in hypoxia and preeclampsia. J. Clin. Endocrinol. Metab. 94, 2524–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hassan I. H., Zhang M. S., Powers L. S., Shao J. Q., Baltrusaitis J., Rutkowski D. T., Legge K., Monick M. M. (2012) Influenza A viral replication is blocked by inhibition of the inositol-requiring enzyme 1 (IRE1) stress pathway. J. Biol. Chem. 287, 4679–4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reisetter A. C., Stebounova L. V., Baltrusaitis J., Powers L., Gupta A., Grassian V. H., Monick M. M. (2011) Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles. J. Biol. Chem. 286, 21844–21852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doi A., Park I. H., Wen B., Murakami P., Aryee M. J., Irizarry R., Herb B., Ladd-Acosta C., Rho J., Loewer S., Miller J., Schlaeger T., Daley G. Q., Feinberg A. P. (2009) Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat. Genet. 41, 1350–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Irizarry R. A., Ladd-Acosta C., Wen B., Wu Z., Montano C., Onyango P., Cui H., Gabo K., Rongione M., Webster M., Ji H., Potash J. B., Sabunciyan S., Feinberg A. P. (2009) The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat. Genet. 41, 178–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cindrova-Davies T., Sanders D. A., Burton G. J., Charnock-Jones D. S. (2011) Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling. Cardiovasc. Res. 89, 671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kendall R. L., Wang G., Thomas K. A. (1996) Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem. Biophys. Res. Commun. 226, 324–328 [DOI] [PubMed] [Google Scholar]
- 49. Boeckel J. N., Guarani V., Koyanagi M., Roexe T., Lengeling A., Schermuly R. T., Gellert P., Braun T., Zeiher A., Dimmeler S. (2011) Jumonji domain-containing protein 6 (Jmjd6) is required for angiogenic sprouting and regulates splicing of VEGF-receptor 1. Proc. Natl. Acad. Sci. USA 108, 3276–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wiencke J. K. (2002) DNA adduct burden and tobacco carcinogenesis. Oncogene 21, 7376–7391 [DOI] [PubMed] [Google Scholar]
- 51. McCarty K. M., Santella R. M., Steck S. E., Cleveland R. J., Ahn J., Ambrosone C. B., North K., Sagiv S. K., Eng S. M., Teitelbaum S. L., Neugut A. I., Gammon M. D. (2009) PAH-DNA adducts, cigarette smoking, GST polymorphisms, and breast cancer risk. Environ. Health Perspect. 117, 552–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heguy A., O'Connor T. P., Luettich K., Worgall S., Cieciuch A., Harvey B. G., Hackett N. R., Crystal R. G. (2006) Gene expression profiling of human alveolar macrophages of phenotypically normal smokers and nonsmokers reveals a previously unrecognized subset of genes modulated by cigarette smoking. J. Mol. Med. (Berl.) 84, 318–328 [DOI] [PubMed] [Google Scholar]
- 53. Garver R. I., Jr., Mornex J. F., Nukiwa T., Brantly M., Courtney M., LeCocq J. P., Crystal R. G. (1986) α 1-Antitrypsin deficiency and emphysema caused by homozygous inheritance of non-expressing α 1-antitrypsin genes. New Engl. J. Med. 314, 762–766 [DOI] [PubMed] [Google Scholar]
- 54. Nukiwa T., Brantly M., Garver R., Paul L., Courtney M., LeCocq J. P., Crystal R. G. (1986) Evaluation of “at risk” α 1-antitrypsin genotype SZ with synthetic oligonucleotide gene probes. J. Clin. Invest. 77, 528–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Crystal R. G. (1989) The α 1-antitrypsin gene and its deficiency states. Trends Genet. 5, 411–417 [DOI] [PubMed] [Google Scholar]
- 56. Curiel D., Brantly M., Curiel E., Stier L., Crystal R. G. (1989) α 1-Antitrypsin deficiency caused by the α 1-antitrypsin Nullmattawa gene. An insertion mutation rendering the α 1-antitrypsin gene incapable of producing α 1-antitrypsin. J. Clin. Invest. 83, 1144–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chappell S., Daly L., Morgan K., Guetta Baranes T., Roca J., Rabinovich R., Millar A., Donnelly S. C., Keatings V., MacNee W., Stolk J., Hiemstra P., Miniati M., Monti S., O'Connor C. M., Kalsheker N. (2006) Cryptic haplotypes of SERPINA1 confer susceptibility to chronic obstructive pulmonary disease. Hum. Mutat. 27, 103–109 [DOI] [PubMed] [Google Scholar]
- 58. Woodruff P. G., Ellwanger A., Solon M., Cambier C. J., Pinkerton K. E., Koth L. L. (2009) Alveolar macrophage recruitment and activation by chronic second hand smoke exposure in mice. COPD 6, 86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. De Bustos C., Ramos E., Young J., Tran R., Menzel U., Langford C., Eichler E., Hsu L., Henikoff S., Dumanski J., Trask B. (2009) Tissue-specific variation in DNA methylation levels along human chromosome 1. Epigenetics Chromatin 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wu H., Coskun V., Tao J., Xie W., Ge W., Yoshikawa K., Li E., Zhang Y., Sun Y. E. (2010) Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science 329, 444–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kendall R. L., Thomas K. A. (1993) Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA 90, 10705–10709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thomas C. P., Andrews J. I., Liu K. Z. (2007) Intronic polyadenylation signal sequences and alternate splicing generate human soluble Flt1 variants and regulate the abundance of soluble Flt1 in the placenta. FASEB J. 21, 3885–3895 [DOI] [PubMed] [Google Scholar]
- 63. England L. J., Levine R. J., Qian C., Morris C. D., Sibai B. M., Catalano P. M., Curet L. B., Klebanoff M. A. (2002) Smoking before pregnancy and risk of gestational hypertension and preeclampsia. Am. J. Obstet. Gynecol. 186, 1035–1040 [DOI] [PubMed] [Google Scholar]
- 64. Jeyabalan A., Powers R. W., Durica A. R., Harger G. F., Roberts J. M., Ness R. B. (2008) Cigarette smoke exposure and angiogenic factors in pregnancy and preeclampsia. Am. J. Hypertens. 21, 943–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sawano A., Iwai S., Sakurai Y., Ito M., Shitara K., Nakahata T., Shibuya M. (2001) Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood 97, 785–791 [DOI] [PubMed] [Google Scholar]
- 66. Kaplan R. N., Riba R. D., Zacharoulis S., Bramley A. H., Vincent L., Costa C., MacDonald D. D., Jin D. K., Shido K., Kerns S. A., Zhu Z., Hicklin D., Wu Y., Port J. L., Altorki N., Port E. R., Ruggero D., Shmelkov S. V., Jensen K. K., Rafii S., Lyden D. (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Young J. I., Hong E. P., Castle J. C., Crespo-Barreto J., Bowman A. B., Rose M. F., Kang D., Richman R., Johnson J. M., Berget S., Zoghbi H. Y. (2005) Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc. Natl. Acad. Sci. USA 102, 17551–17558 [DOI] [PMC free article] [PubMed] [Google Scholar]