Abstract
AMP-activated protein kinase (AMPK) is a key regulator of cellular and body energy homeostasis. We previously demonstrated that AMPK activation in osteoblasts increases in vitro bone formation while deletion of the Ampkα1 (Prkaa1) subunit, the dominant catalytic subunit expressed in bone, leads to decreased bone mass in vivo. To investigate the cause of low bone mass in the Ampkα1−/− mice, we analysed bone formation and resorption in the tibia of these mice by dynamic histomorphometry and determined whether bone turnover can be stimulated in the absence of the Ampkα1 subunit. We subjected 12-week-old Ampkα1+/+ and Ampkα1−/− mice to ovariectomy (OVX), intermittent PTH (iPTH) administration (80 μg/kg per day, 5 days/week) or both OVX and iPTH hormonal challenges. Tibiae were harvested from these mice and bone micro-architecture was determined by micro-computed tomography. We show for the first time that Ampkα1−/− mice have a high bone turnover at the basal level in favour of bone resorption. While both Ampkα1+/+ and Ampkα1−/− mice lost bone mass after OVX, the bone loss in Ampkα1−/− mice was lower compared with controls. iPTH increased trabecular and cortical bone indexes in both ovariectomised Ampkα1+/+ and Ampkα1−/− mice. However, ovariectomised Ampkα1−/− mice showed a smaller increase in bone parameters in response to iPTH compared with Ampkα1+/+ mice. By contrast, non-ovariectomised Ampkα1−/− mice responded better to iPTH treatment than non-ovariectomised Ampkα1+/+ mice. Overall, these data demonstrate that Ampkα1−/− mice are less affected by changes in bone turnover induced by OVX but respond better to the anabolic challenge induced by iPTH. These results suggest that AMPKα1 activation may play a role in the hormonal regulation of bone remodelling.
Introduction
AMP-activated protein kinase (AMPK) is a sensor and regulator of energy homeostasis not only at the cellular level but also at the whole-body level where it mediates the central and peripheral effects of many hormones on the metabolisms of appetite, fat and glucose (Minokoshi et al. 2002, Yamauchi et al. 2002, Andersson et al. 2004, Banerjee et al. 2004, Minokoshi et al. 2004, Han et al. 2005, Kola et al. 2005, Yamauchi et al. 2008 and for reviews see Hardie et al. 2006, Kola et al. 2006 and Lage et al. 2008). It is a highly conserved, ubiquitously expressed serine/threonine heterotrimeric protein kinase consisting of a catalytic α subunit and regulatory β and γ subunits, all of which have several isoforms with differential tissue-specific expression patterns (Hardie et al. 2006, Kola et al. 2006, Steinberg & Kemp 2009, Viollet et al. 2010, Hardie et al. 2011). AMPK senses the AMP/ATP ratio within the cell and is activated in response to environmental or nutritional stress factors that deplete intracellular ATP levels (Hardie et al. 2006, Hardie 2008, Steinberg & Kemp 2009). AMP binding activates AMPK by two mechanisms, phosphorylation of the Thr-172 residue in the α subunit by upstream kinases and inhibition of dephosphorylation of Thr-172 by phosphatases (Hawley et al. 1996, 2003, 2005, Xiao et al. 2011). Once activated, it switches on catabolic pathways that generate ATP and switches off anabolic pathways that consume ATP.
In the last few years, several in vitro and in vivo studies have established that the AMPK signalling pathway could also play a role in bone physiology (Kanazawa et al. 2008, 2009, Kasai et al. 2009, Lee et al. 2010, Molinuevo et al. 2010, Quinn et al. 2010, Shah et al. 2010, Zhen et al. 2010, Jang et al. 2011a,b, Mai et al. 2011, Wu et al. 2011). We demonstrated that the AMPKα1 subunit is the dominant catalytic isoform expressed in bone and that AMPK activators stimulate in vitro bone nodule formation (Shah et al. 2010). Several other studies have confirmed that the two main AMPK activators, 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) and metformin, are osteogenic in vitro. They stimulate proliferation, differentiation and mineralisation of MC3T3-E1 osteoblastic cells (Kanazawa et al. 2008, 2009, Jang et al. 2011a,b, Mai et al. 2011), primary osteoblasts (Shah et al. 2010, Zhen et al. 2010) and bone marrow progenitor cells (Molinuevo et al. 2010, Wu et al. 2011). However, it was also reported that osteoblast differentiation is functionally associated with decreased AMPK activity (Kasai et al. 2009). The relationship between AMPK activation and bone resorption is also unclear. Activation of AMPK was shown to inhibit osteoclast formation and bone resorption in vitro, AMPK acting as a negative regulator of RANKL (Lee et al. 2010). By contrast, in vivo studies have shown that AICAR stimulates bone loss and bone turnover in male mice (Quinn et al. 2010). The evidence for a role of AMPK signalling in the regulation of bone mass is best supported by genetic studies. Our work has shown that Ampkα1 (Prkaa1) knockout (Ampkα1−/−) mice have a very low bone mass compared with the WT (Ampkα1+/+) mice, both cortical and trabecular bone compartments being smaller in the Ampkα1−/− mice (Shah et al. 2010). Similarly, Quinn et al. (2010) showed that germline deletions of either the AMPKβ1 or β2 subunit resulted in reduced trabecular bone density and mass.
The underlying mechanism for the low bone mass in Ampkα1−/− mice and the exact role of AMPK in bone remodelling in vivo have not yet been investigated. Bone remodelling occurs constantly at multiple locations within the skeleton and bone needs to balance energy in response to nutrient availability with growth and turnover. To address the role of AMPK in bone turnover, we subjected Ampkα1−/− and Ampkα1+/+ mice to two types of hormonal challenges that increase bone turnover, ovariectomy (OVX) that induces a negative bone balance and intermittent PTH (iPTH) treatment that is anabolic, and examined their effects on bone architecture in these mice. We show that Ampkα1−/− mice have high bone turnover at basal level and that bone turnover in Ampkα1−/− mice is altered in response to OVX and iPTH, suggesting that AMPK activation may modulate the hormonal regulation of bone remodelling.
Materials and Methods
Animals
Ampkα1 knockout mice were generously provided by Dr Benoit Viollet (INSERM, U1016, Paris, France) and were generated as described previously (Jorgensen et al. 2004). Ampkα1+/+ and Ampkα1−/− mice in C57BL/6×129/Sv mixed background were used. All procedures were performed in accordance with the principles and guidelines established by the European Convention for the Protection of Laboratory Animals. All mice were maintained under a controlled temperature (21 °C) and lighting with 12 h light:12 h darkness cycle and received a standard mouse chow diet and water ad libitum. The knockout mice do not show any obvious metabolic phenotypes and their body weight was unaffected (Jorgensen et al. 2004).
Hormonal challenges
Three independent experiments were carried out to determine the response of bone to hormonal challenges in Ampkα1 knockout mice. In study 1, 12-week-old Ampkα1+/+ and Ampkα1−/− mice (n=7/group) were either ovariectomised or sham operated. Tibiae were collected from these mice 6 weeks after OVX for micro-computed tomography (micro-CT) analysis. For measurement of dynamic bone formation parameters, mice in this study were i.p. injected with calcein (Sigma–Aldrich) and alizarin red complexone (Sigma–Aldrich), at days 6 and 3, respectively, before killing. For study 2, 12-week-old Ampkα1+/+ and Ampkα1−/− mice (n=8/group) were all ovariectomised and immediately treated for 4 weeks with s.c. injection of either 80 μg/kg per day, 5 days/week, PTH (human PTH (1–34; Bachem, Inc., Torrance, CA, USA) dissolved in 1 mM HCl containing 0·2% BSA) or saline. For study 3, 12-week-old Ampkα1+/+ and Ampkα1−/− mice (n=10–11/group) were treated with s.c. injection of either 80 μg/kg per day, 5 days/week, iPTH or saline. For all mice, body weight was measured at the beginning of week 13 (i.e. on day of sham operation, OVX or at the beginning of iPTH treatment) and at the end of the experiment. Left and right tibiae were harvested from these mice for micro-CT (studies 1, 2 and 3) and bone histomorphometric analyses (study 1) respectively. Femora were collected for western blot and RT-PCR analyses.
Histomorphometry analysis of tibia
Right tibia from sham-operated Ampkα1+/+ and Ampkα1−/− mice from study 1 were fixed in 10% neutral-buffered formalin for 24–72 h, dehydrated and embedded in pure methyl methacrylate at low temperature to preserve enzymatic activity (Chappard et al. 1987). Unstained 8 μm-thick sections were used for fluorescence microscopy to assess mineral apposition rate (MAR, μm/day). Mineralising surfaces were expressed as double+half single labelled surfaces per bone surfaces (MS/BS, %) and the bone formation rate was calculated as MS/BS×MAR (BFR/BS, μm3/μm2 per day; Chavassieux et al. 1997). Alternatively, sections were stained for tartrate-resistant acid phosphatase (TRAP; Leucognost SP, Merck) and counterstained with Mayer's hemalum solution. Goldner's trichrome staining was performed to determine adipocyte number per tissue area. Histomorphometric parameters were measured on the trabecular bone of the metaphysis, on a region of interest consisting of 2 mm width below the growth plate. Measurements were performed using an Image Analysis Software (Bone, Explora Nova, La Rochelle, France). Histomorphometric parameters were reported in accordance with the ASBMR Committee nomenclature (Parfitt et al. 1987).
Micro-CT analysis of tibia
Left tibia was fixed in 10% neural-buffered formalin for 24–72 h and stored in 70% ethanol at 4 °C. They were scanned with high-resolution (5 μm pixel size) micro-CT (Skyscan 1172, Kontich, Belgium), as described previously (Shah et al. 2010). The whole tibia was reconstructed using NRecon v.1.4.4.0 (Skyscan) and bone histomorphometric analyses in 2- and 3-dimensions (2D and 3D) were performed by Skyscan Software (CT-Analyser v.1.5.1.3). For the analysis of trabecular bone, the cortical shell was excluded by operator-drawn regions of interest and 3D algorithms were used to determine the relevant parameters that included bone volume (BV) percentage (BV/tissue volume (TV), %), direct trabecular thickness (Tb.Th) and spacing, trabecular number (Tb.N), structure model index (SMI), trabecular bone pattern factor (TBPf) and the degree of anisotropy (DA). Analysis of cortical bone was performed using a 0·49 mm long segment (or 100 tomograms) at 37% of the tibias' length from the proximal end. For analysis of the cortical bone compartment, 2D computation was used and parameters were determined for each one of the 100 tomograms and then averaged. They included periosteal perimeter (Ps.Pm), endosteal perimeter (Ec.Pm) and cortical thickness (Ct.Th).
RNA extraction and RT-PCR analysis
Total RNA was isolated from femora and femoral muscles of Ampkα1+/+ and Ampkα1−/− mice and amplified using subunit-specific primers, as described previously (Shah et al. 2010).
Protein extraction and western blot analysis
For the isolation of total proteins, right and left femora from Ampkα1+/+ and Ampkα1−/− mice were carefully dissected, all their surrounding musculature removed leaving the periosteum intact. The cartilaginous ends of the bones were separated and the remaining femoral shafts were flushed with PBS to remove the marrow. The femoral shafts were then snap-frozen and pulverised under liquid nitrogen using a mortar and pestle and then lysed in cold denaturing lysis buffer (2% SDS, 2 M urea, 8% sucrose, 20 mM sodium glycerophosphate, 1 mM sodium fluoride, and 5 mM sodium orthovanadate). Proteins were denatured by boiling for 5 min and concentrations were determined by BCA protein assay. Two micrograms of proteins were size fractionated using SDS–PAGE and electrotransferred onto Protran nitrocellulose membranes (Schliecher and Schuell, Dassel, Germany). Membranes were blocked for 1 h in 0·2% (w/v) I-block (Topix, Bedford, MA, USA), before being incubated with primary antibodies. The blots were incubated overnight at 4 °C with antibodies against total AMPKα1/2 (tAMPK α1/2, rabbit), phospho-(Thr-172)-AMPKα1/2 (pAMPKα1/2, rabbit) (New England Biolabs, Hitchin, UK) and β-actin (goat) (Dako, Ely, UK), all added at a 1:1000 dilution. The following secondary antibodies were used: goat anti-rabbit (New England Biolabs) against tAMPK and pAMPKα1/2 and rabbit anti-goat (Dako) against β-actin antibody, both at 1:2500 dilution at room temperature for 1 h. Proteins were visualised using the enhanced chemiluminescence (ECL) detection system (GE Healthcare UK Ltd., Little Chalfont, UK). The intensity of the specific bands was quantified by densitometry using Image J Software.
Statistical analysis
The results are presented as mean±s.e.m. Comparisons between groups for all the data were performed using nonparametric Mann–Whitney U test. Differences were considered statistically significant at P<0·05. All statistical analyses were performed using GraphPad Prism Software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Female Ampkα1−/− mice have decreased bone mass and increased bone remodelling
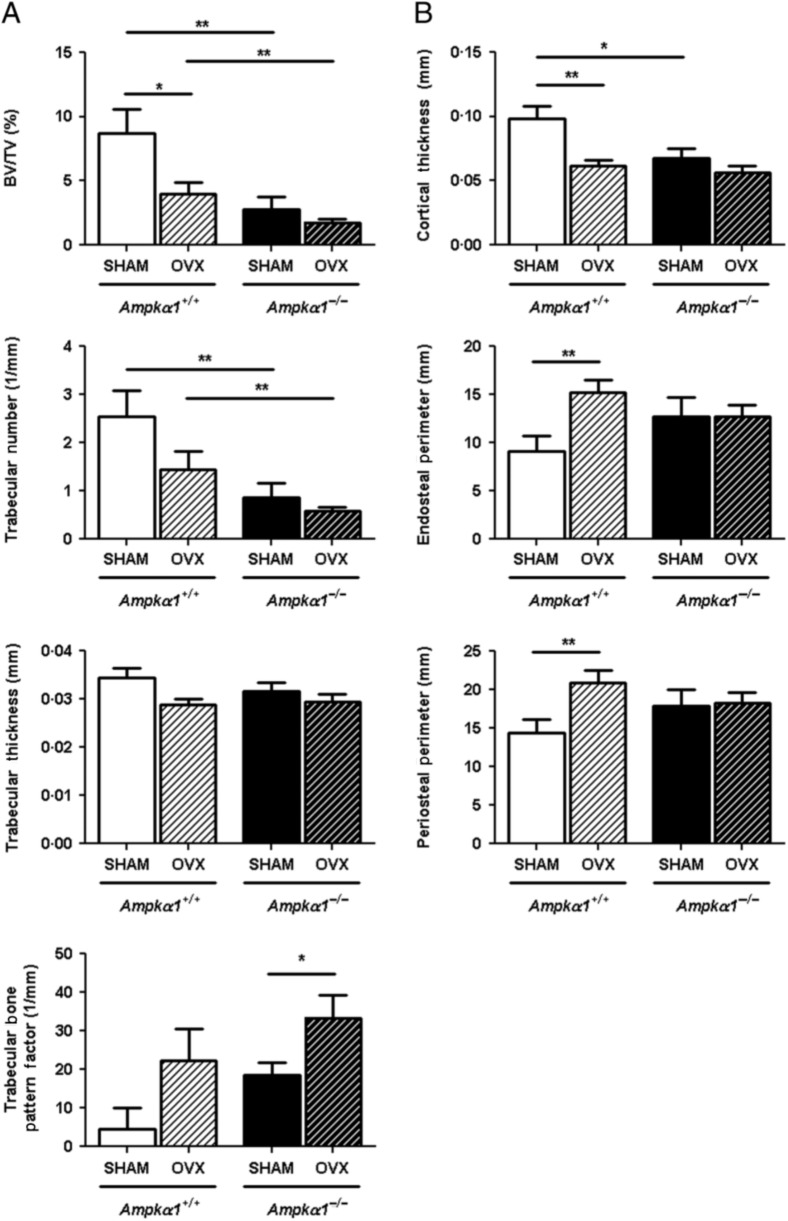
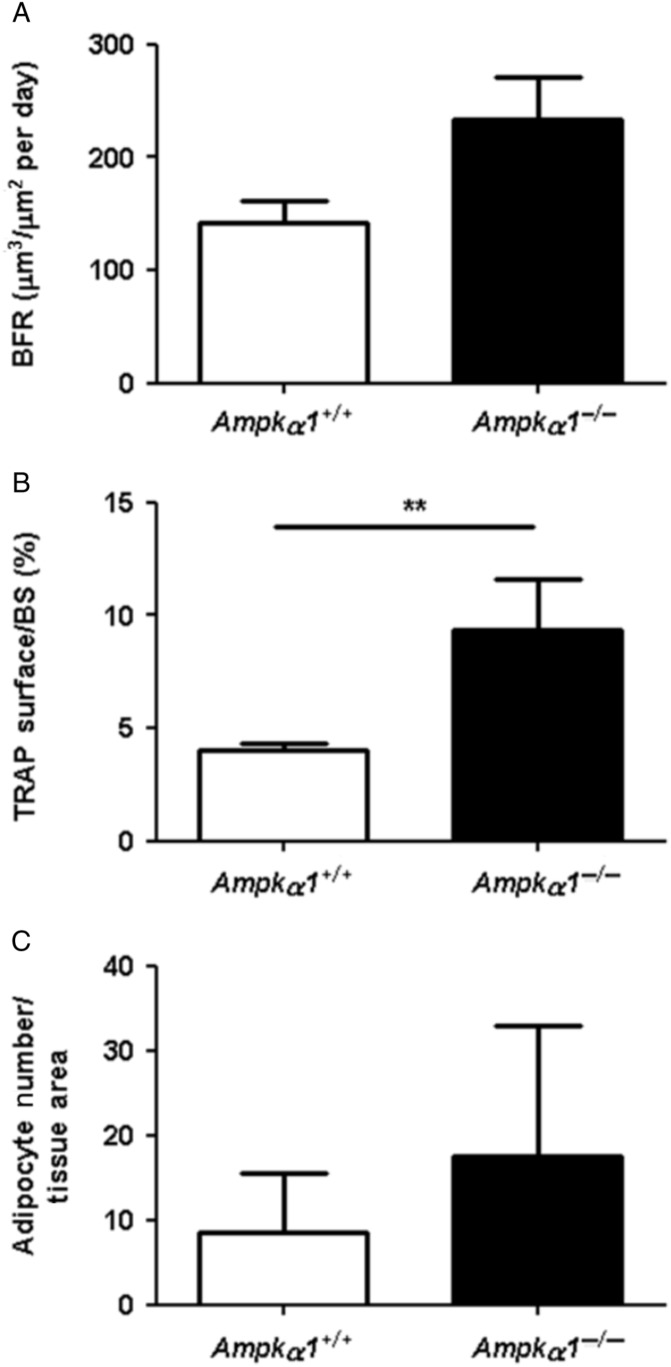
While our previous analysis of bone architecture in Ampkα1−/− mice was done in males (Shah et al. 2010), we confirmed with the present experiments that female Ampkα1−/− mice have a similar low bone mass phenotype compared with Ampkα1+/+ mice. The micro-CT measurements of trabecular and cortical parameters in tibia of non-OVX adult female mice (Fig. 1A and B) showed that the Ampkα1−/− mice had significantly lower BV/TV, Tb.N and Ct.Th compared with Ampkα1+/+ mice. Ampkα1−/− mice also showed a significant increase in SMI, a parameter reflecting trabecular shape, plate to rod elements (Ampkα1+/+, 1·19±0·15 vs Ampkα1−/−, 1·69±0·07; P<0·05), compared with Ampkα1+/+ mice. There were statistically non-significant increases in trabecular separation (Ampkα1+/+, 0·30±0·05 mm vs Ampkα1−/−, 0·47±0·05 mm; P=0·073) and TBPf (Fig. 1A) in Ampkα1−/− mice, suggesting poor trabecular interconnection. The DA reflecting trabecular structure (Ampkα1+/+, 1·64±0·10 vs Ampkα1−/−, 2·57±0·90; P=1·000) and Ps.Pm and Ec.Pm (Fig. 1B) were not significantly different between the Ampkα1+/+ and Ampkα1−/− mice. These changes in the trabecular and cortical parameters of female Ampkα1−/− mice are similar to those observed in male Ampkα1−/− mice (Shah et al. 2010). The trabecular architecture in Ampkα1−/− mice, characterised by increased SMI, trabecular separation and TBPf and lower BV/TV are similar to the changes seen during ageing and osteoporosis, suggesting an altered bone remodelling at basal level in these mice. To determine the cause of the low bone mass in Ampkα1−/− mice and whether bone remodelling is affected in these mice at basal level, we examined bone formation and resorption in the tibia of those mice, using bone histomorphometry. Analysis of BFR using double fluorescence labelling showed that the Ampkα1−/− mice had a higher BFR than Ampkα1+/+ mice (Fig. 2A), but this was not significant (P=0·095). The percentage of TRAP-positive surfaces (osteoclasts surfaces) was significantly higher in the Ampkα1−/− mice compared with Ampkα1+/+ mice (Fig. 2B). These results suggest that the Ampkα1−/− mice have a higher bone turnover compared with their Ampkα1+/+ littermates in favour of bone resorption. In addition, the Ampkα1−/− mice had twice the number of adipocytes than the Ampkα1+/+ mice within the bone marrow but, due to a large variation between animals, the difference was not significant (Fig. 2C).
Figure 1.
Effect of ovariectomy (OVX) on trabecular and cortical bone parameters in tibia of Ampkα1+/+ and Ampkα1−/− mice. (A) Trabecular bone parameters in Ampkα1+/+ and Ampkα1−/− mice that have undergone OVX or sham operation. (B) Cortical bone parameters in Ampkα1+/+ and Ampkα1−/− mice subjected to OVX or sham operation. Values are mean±s.e.m. of n=7 mice/group, *P<0·05, **P<0·01.
Figure 2.
Dynamic bone histomorphometry analyses in Ampkα1+/+ and Ampkα1−/− mice. (A) BFR, (B) percentage of TRAP surfaces and (C) number of adipocytes per tissue area in 16-week-old Ampkα1+/+ and Ampkα1−/− mice. Values are mean±s.e.m. of n=7 mice/group, **P<0·01.
Ovariectomy (OVX) induces bone loss in Ampkα1+/+ and Ampkα1−/− mice
To investigate the skeletal response of Ampkα1−/− mice to OVX, known to stimulate bone remodelling, OVX or sham operations were performed in Ampkα1+/+ and Ampkα1−/− mice. All the mice from these groups have gained weight throughout the treatment period. However, comparison of weight changes (from the day of sham or OVX operation to day of sacrifice, i.e. over the 6-week period) between the groups did not detect any statistically significant differences (sham-Ampkα1+/+, 1·37±0·61 g vs OVX–Ampkα1+/+, 1·66±0·42 g; sham-Ampkα1−/−, 1·76±0·25 g vs OVX–Ampkα1−/−, 1·54±0·30 g). In addition, there was no significant difference in weight change between sham-Ampkα1+/+ and sham-Ampkα1−/− mice. Tibial bone length was also not significantly different between Ampkα1+/+ or Ampkα1−/− mice (data not shown).
As expected, OVX induced bone loss in Ampkα1+/+ mice. These mice showed a significant decrease in BV/TV after OVX (Fig. 1A). Tb.N (P=0·097) and thickness (P=0·073) were also decreased in the Ampkα1+/+ mice after OVX, while TBPf was increased (P=0·165), although these parameters were not statistically significant (Fig. 1A). Analysis of cortical parameters demonstrated a significant increase in Ec.Pm and Ps.Pm in Ampkα1+/+ mice after OVX (Fig. 1B). By contrast, Ct.Th was significantly decreased after OVX in these mice (Fig. 1B).
Ovariectomy also induced alterations in both the trabecular and cortical bones in the Ampkα1−/− mice, but the effects were moderate, indicating that the bone response to OVX in these mice is attenuated (Fig. 1). Ampkα1−/− mice showed a significant increase in TBPf, but there were non-statistically significant decreases in BV/TV, Tb.N and thickness after OVX in these mice (Fig. 1A). Cortical parameters were not significantly affected in the Ampkα1−/− mice after OVX (Fig. 1B).
iPTH increases bone formation in ovariectomised Ampkα1+/+ and Ampkα1−/− mice
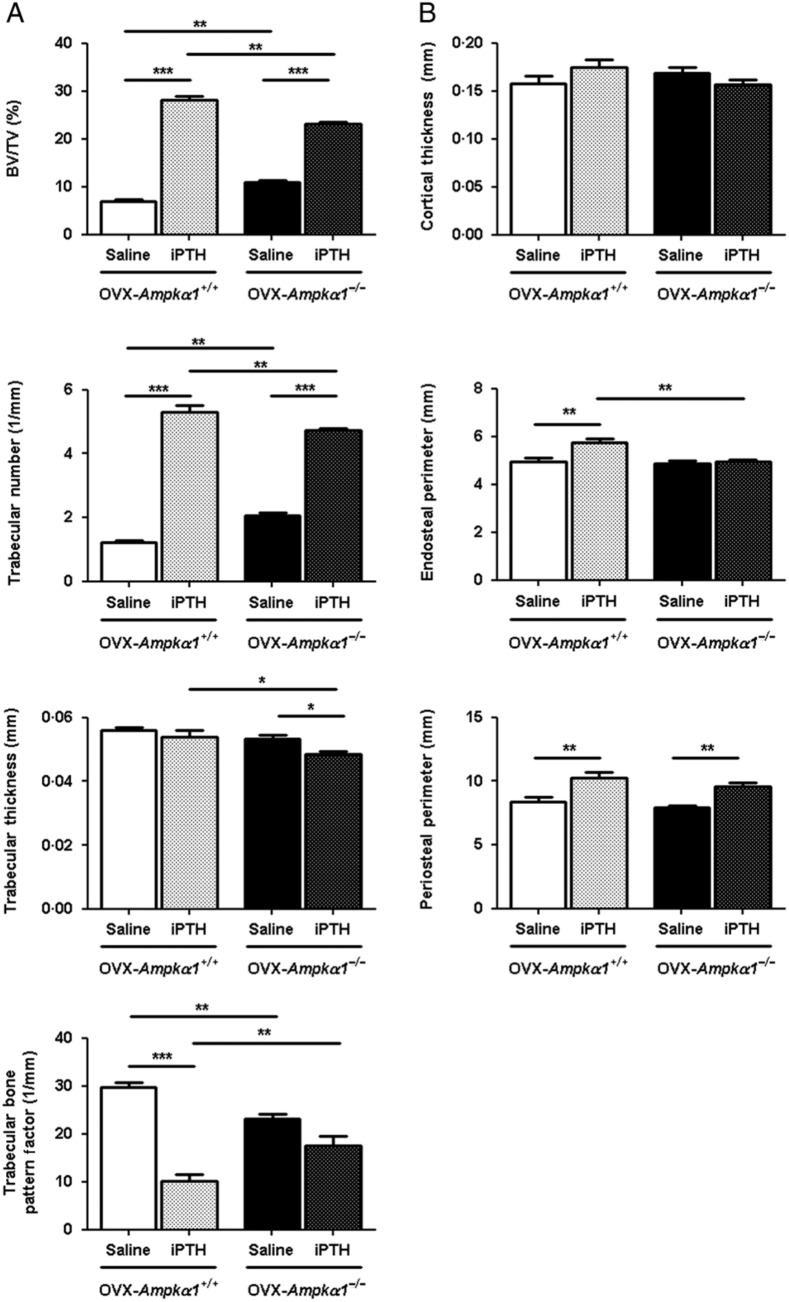
To determine whether PTH treatment overcomes the bone loss induced by OVX in Ampkα1+/+ and Ampkα1−/− mice, those mice were ovariectomised and then treated with iPTH or saline for 4 weeks. There was no difference in weight due to iPTH treatment in the ovariectomised Ampkα1+/+ and Ampkα1−/− mice (data not shown). Our results demonstrate that iPTH treatment alters trabecular and cortical bone indexes in both OVX–Ampkα1+/+ and OVX–Ampkα1−/− mice (Fig. 3).
Figure 3.
Effect of 4 weeks of iPTH treatment on trabecular and cortical bone parameters in tibia of ovariectomised Ampkα1+/+ and Ampkα1−/− mice. (A) Trabecular bone parameters in ovariectomised Ampkα1+/+ (OVX–Ampkα1+/+) and Ampkα1−/− (OVX–Ampkα1−/−) mice that have been treated with iPTH or saline. (B) Cortical bone parameters in OVX–Ampkα1+/+ and OVX–Ampkα1−/− mice subjected to iPTH or saline treatment. Values are mean±s.e.m. of n=8 mice/group, *P<0·05, **P<0·01, ***P<0·001.
In OVX–Ampkα1+/+ mice, iPTH induced a significant increase in BV/TV and Tb.N and a significant decrease in TBPf (Fig. 3A), trabecular separation (Saline OVX–Ampkα1+/+, 0·46±0·03 mm vs iPTH OVX–Ampkα1+/+, 0·20±0·01 mm; P<0·0005) and SMI (Saline OVX–Ampkα1+/+, 2·37±0·04 vs iPTH OVX–Ampkα1+/+, 1·38±0·09; P<0·0005). In the cortical compartment, Ps.Pm and Ec.Pm were significantly increased as a result of iPTH treatment in the OVX–Ampkα1+/+ mice, while Ct.Th was not affected (Fig. 3B).
Similarly, in OVX–Ampkα1−/− mice, iPTH significantly increased BV/TV and Tb.N, but significantly decreased Tb.Th (Fig. 3A), separation (Saline OVX–Ampkα1−/−, 0·29±0·01 mm vs iPTH OVX–Ampkα1−/−, 0·23±0·01 mm; P<0·05) and SMI (Saline OVX–Ampkα1−/−, 2·01±0·05 vs iPTH OVX–Ampkα1−/−, 1·53±0·13; P<0·05). Within the cortical compartment of these mice, Ps.Pm was also significantly increased by iPTH treatment but not the other parameters (Fig. 3B). Our results illustrate that the ovariectomised Ampkα1−/− mice have an attenuated response to iPTH treatment compared with Ampkα1+/+ mice.
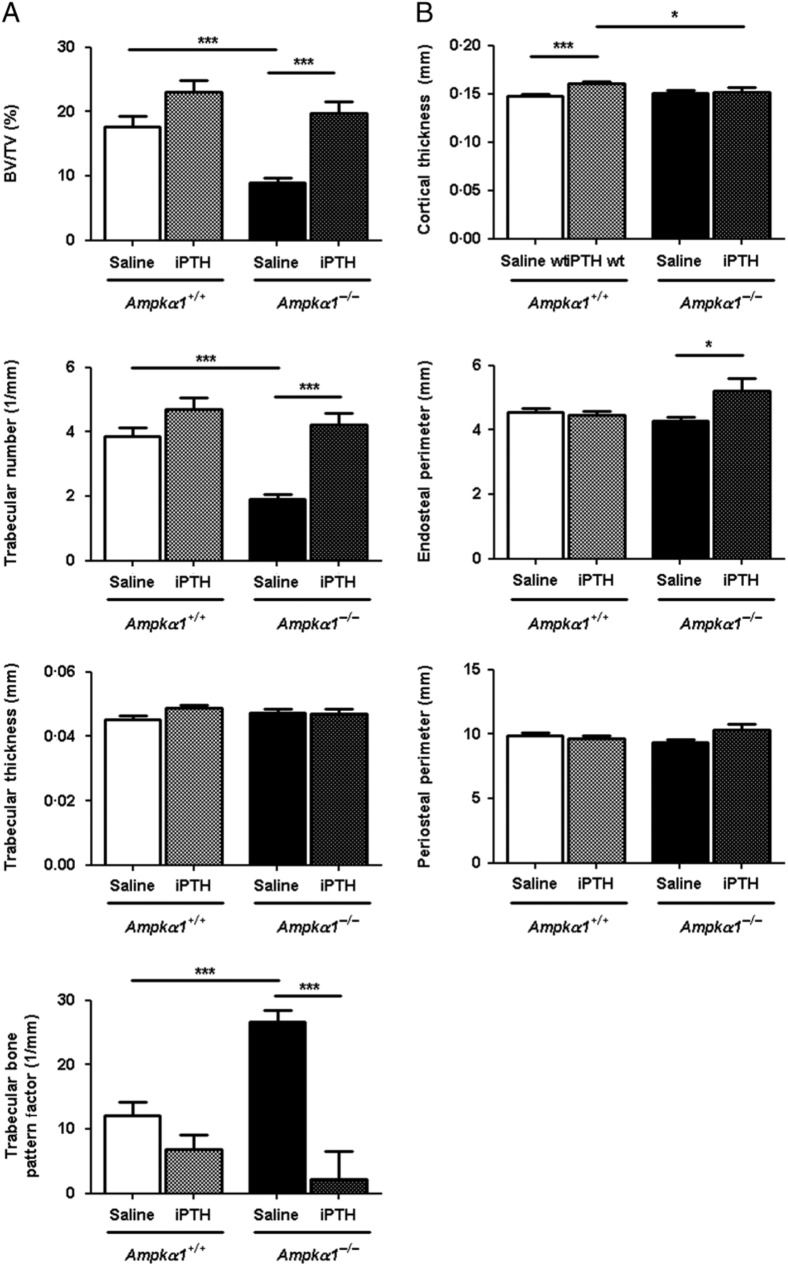
iPTH increases bone formation in Ampkα1+/+ and Ampkα1−/− mice
We then analysed the effect of iPTH in non-ovariectomised Ampkα1+/+ and Ampkα1−/− mice. Four weeks of iPTH treatment in the non-ovariectomised Ampkα1+/+ mice induced mild increases in bone formation in the trabecular and cortical compartments (Fig. 4). Within the trabecular compartment, the only statistically significant change induced by iPTH was a decrease in DA (saline-Ampkα1+/+, 1·81±0·04 vs iPTH-Ampkα1+/+, 1·69±0·04; P<0·05). There were non-significant increases in BV/TV (P=0·057), Tb.N (P=0·076) and thickness (P=0·066; Fig. 4A). iPTH also induced a significant increase in Ct.Th in the Ampkα1+/+ mice (Fig. 4B), while the other cortical bone parameters were not significantly affected. By contrast, in the Ampkα1−/− mice, iPTH induced a significant increase in BV/TV and Tb.N and a significant decrease in TBPf (Fig. 4A), trabecular separation (saline-Ampkα1−/−, 0·25±0·01 mm vs iPTH-Ampkα1−/−, 0·19±0·01 mm; P<0·005), SMI (saline-Ampkα1−/−, 2·07±0·08 vs iPTH-Ampkα1−/−, 1·12±0·18; P<0·0005) and DA (saline-Ampkα1−/−, 1·98±0·05 vs iPTH-Ampkα1−/−, 1·59±0·07; P<0·005). In the cortical compartment, it significantly increased Ec.Pm but had no effect on the other parameters (Fig. 4B). Surprisingly, in this experiment, Ct.Th was not decreased in the saline Ampkα1−/− mice compared with Ampkα1+/+ mice, in contrast to Fig. 1 and our previous results (Shah et al. 2010).
Figure 4.
Effect of 4 weeks of iPTH treatment on trabecular and cortical bone parameters in tibia of Ampkα1+/+ and Ampkα1−/− mice. (A) Trabecular bone parameters in Ampkα1+/+ and Ampkα1−/− mice that have been treated with either iPTH or saline for 4 weeks. (B) Cortical bone parameters in Ampkα1+/+ and Ampkα1−/− mice treated with iPTH or saline for 4 weeks. Values are mean±s.e.m. of n=10–11 mice/group, *P<0·05, ***P<0·001.
The comparison of changes in trabecular and cortical parameters due to iPTH treatment between Ampkα1+/+ and Ampkα1−/− mice shows that PTH induces a greater increase in bone in the non-ovariectomised Ampkα1−/− mice.
iPTH induces AMPKα phosphorylation in Ampkα1+/+ mice
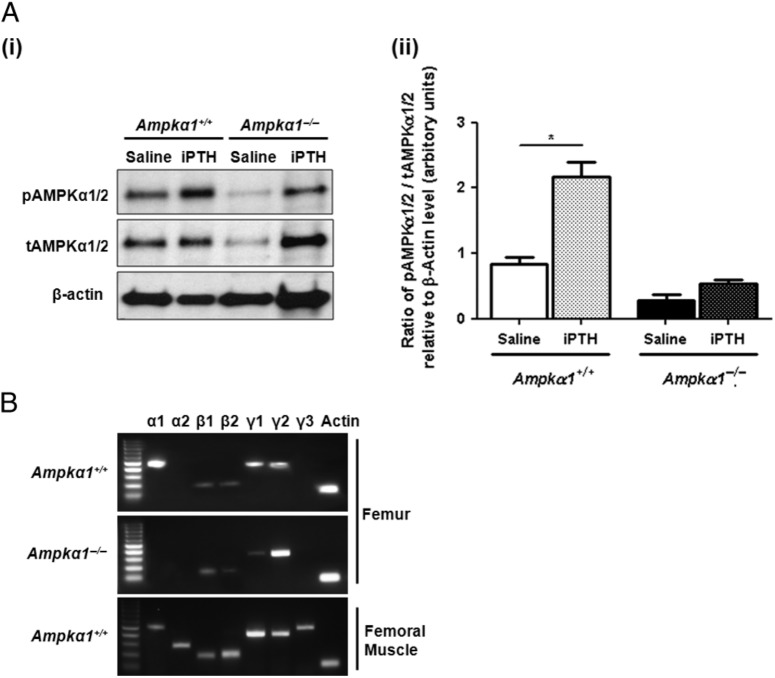
To determine whether iPTH treatment could affect bone AMPK activity, AMPKα1/2 subunit phosphorylation was determined by western blot analysis of proteins extracted from femora of mice from study 2 treated with saline or iPTH. The antibodies we used against phosphorylated AMPKα and total AMPKα do not differentiate between the α1 and α2 subunits. Our western blot analysis showed that iPTH induced a significant increase in pAMPKα1/2 levels in Ampkα1+/+ mice but not in Ampkα1−/− mice (Fig. 5Ai and ii). We previously showed that there is a very low level expression of α2 subunit in bone at basal conditions (Shah et al. 2010). To confirm that the absence of α1 transcript in the Ampkα1−/− mice does not induce a compensatory increase in α2 expression in bone, we examined the expression of α subunits in bones from Ampkα1−/− mice and showed that there was no overexpression of α2 in the bones of these mice (Fig. 5B).
Figure 5.
Effect of iPTH treatment on AMPKα1/2 phosphorylation in bone of ovariectomised Ampkα1+/+ and Ampkα1−/− mice. (A, i) Western blot analysis of pAMPKα1/2 and tAMPKα1/2. Proteins were extracted from femora of 16-week-old OVX–Ampkα1+/+ and OVX–Ampkα1−/− mice from study 2 and probed with polyclonal antibodies directed against pAMPKα1/2, tAMPKα1/2 and β-actin. Representative immunoblots are shown, which were repeated three times with similar results. (ii) Graph showing the ratio of pAMPK α1/2 to tAMPKα1/2 relative to β-actin determined by densitometry analysis of western blot data using Image J Software. Proteins were extracted from femora of two mice per group and western blot analyses were carried out in triplicates. Values are mean±s.e.m., *P<0·05. (B) RT-PCR analysis of RNA extracted from femora of Ampkα1+/+ and Ampkα1−/− mice showing differential subunit expression pattern. Expression pattern of AMPK subunits in femoral muscle from Ampkα1+/+ mice was carried as a control.
Discussion
We show in this study that the low bone mass observed in male Ampkα1−/− mice is also observed in female Ampkα1−/− mice and is due to an increase in bone formation and resorption with an imbalance in favour of resorption. In addition, our results reveal that bone turnover induced by OVX and iPTH hormonal challenges is moderately reduced in the Ampkα1 subunit knockout mice. However, the skeletal responses to OVX and iPTH in these mice were different from their WT littermate controls, suggesting that AMPK activation mediates the effects of these hormones on bone turnover.
The increased BFR and resorption surfaces in the Ampkα1−/− mice suggest increased bone remodelling. This accelerated bone turnover in favour of bone resorption could explain their low bone mass at basal level. Interestingly, deletion of AMPKβ subunits in mice also reduced bone mass and the authors did not observe any reduction in osteoblast or osteoclast numbers in these mice, suggesting that the low bone mass observed in Ampkβ1 and 2 knockout mice could be due to changes in bone cellular functions (Quinn et al. 2010). Our results, although not statistically significant, show an increase in BFR and MS, reflecting active bone formation, which could be due to an increase in the birth of new remodelling units and/or an increase in the lifespan of these remodelling units. At the cellular level, this could be the result of an enhanced differentiation of osteoprogenitors into mature osteoblasts or an increased lifespan of osteoblasts. This increase in bone formation was an unexpected finding as several in vitro studies indicate that AMPK activation stimulates bone formation (Kanazawa et al. 2008, 2009, Molinuevo et al. 2010, Shah et al. 2010, Jang et al. 2011a,b, Mai et al. 2011, Wu et al. 2011). We also show in this paper a trend towards an increase in marrow adipocyte numbers in bones of Ampkα1−/− mice, which could suggest a potential interaction between AMPK signalling in fat and bone, and this will need to be further investigated. Our results indicate that bone resorption is increased in Ampkα1−/− mice, suggesting that AMPKα1 activation inhibits bone resorption. Indeed, it has been shown that AMPK acts as a negative regulator of RANKL in bone marrow macrophages, and inhibition of AMPK increases RANKL-dependent formation of TRAP-positive multinucleated cells and bone resorption area (Lee et al. 2010). This could therefore explain the increase in percentage of TRAP surfaces in Ampkα1−/− mice.
To clarify whether changes in basal bone cellular activities in Ampkα1−/− mice affect their responses to changes in bone turnover, we first submitted these mice to OVX. Loss of bone mass, trabecular thinning and increased trabecular separation are general features of bone after OVX (Parfitt et al. 1987, Compston et al. 1989). This is due to increased bone resorption, which exceeds bone formation at the initial stages (Lambers et al. 2012). Consistent with this, we observed deteriorated trabecular bone architecture in the Ampkα1+/+ mice after OVX. Furthermore, OVX caused endosteal bone resorption and periosteal bone apposition in these mice, which is consistent with the known effect of OVX on cortical bone architecture (Turner et al. 1987a,b). Weight gain is typically observed after OVX in rats and in humans after menopause (Lobo 2008, Tezval et al. 2011a). Although all mice gained weight during the 6-week experimental period, OVX did not induce significant weight gain in both the Ampkα1+/+ and Ampkα1−/− mice. Body weight gain after OVX in mice is not always observed and may depend on the genetic background, as previously reported (Andersson et al. 2001, Bouxsein et al. 2005, Li et al. 2005, Iwaniec et al. 2006).
Our results illustrate that Ampkα1−/− mice, similar to Ampkα1+/+ mice, can lose bone after OVX, although this bone loss was moderate compared with Ampkα1+/+ mice. This suggests that AMPKα1 is likely to play a role in bone resorption and remodelling induced by OVX. As bone mass is already very low at basal levels in the Ampkα1−/− mice, there may be a protective mechanism to reduce the level of bone loss induced by OVX and to preserve the bone architecture without causing deleterious effects. One possibility for this mechanism could be the fact that there is less surface area for bone resorption. A study investigating OVX-induced bone loss in different inbred mouse strains revealed indeed that strains with low basal trabecular bone mass lose less bone compared with mice with high basal bone mass (Bouxsein et al. 2005). Alternatively, as bone remodelling is already very high in Ampkα1−/− mice, they may be less sensitive to an increase in bone remodelling induced by oestrogen withdrawal.
Intermittent administration of PTH is known to increase bone mass and improve bone architecture (Iida-Klein et al. 2002, Jiang et al. 2003, Brouwers et al. 2009, Recker et al. 2009). At the cellular level, iPTH has been shown to increase bone remodelling (Fox et al. 2006, Wade-Gueye et al. 2010) with bone formation exceeding resorption. To understand the bone responses of Ampkα1−/− mice to anabolic (iPTH) stimuli, we first subjected ovariectomised Ampkα1+/+ and Ampkα1−/− mice to iPTH treatment for 4 weeks (study 2). Our results confirm previous studies showing that iPTH (1–34) can increase bone formation in ovariectomised rodents (Alexander et al. 2001, Fox et al. 2006, Wade-Gueye et al. 2010, Tezval et al. 2011a). We show that similar to OVX–Ampkα1+/+ mice, OVX–Ampkα1−/− mice have an increase in trabecular BV and number as well as an augmented cortical bone mass in response to iPTH, confirming that osteoblast function is not severely affected in these Ampkα1−/− mice. OVX–Ampkα1−/− mice were, however, less affected than OVX–Ampkα1+/+ mice by the changes in bone turnover induced by iPTH, possibly because they lost less bone after OVX than their WT controls. We indeed found in this study that the trabecular bone mass in OVX–Ampkα1−/− mice was higher than that of OVX–Ampkα1+/+ mice (Fig. 3), which contrasts with our first study (Fig. 1) where OVX–Ampkα1−/− mice had lower trabecular bone mass than OVX–Ampkα1+/+ mice. This suggests that the Ampkα1−/− mice in study 2 did not lose as much bone mass after OVX as those in study 1. This discrepancy in the amount of bone loss after OVX between our two studies may be due to the time after OVX. In study 1, bone mass was determined 6 weeks after OVX while in study 2 it was investigated after 4 weeks, and there are studies supporting a time-dependent bone loss after OVX (Li et al. 2005, Iwaniec et al. 2006). It is also possible that the absence of AMPKα1 may have contributed to this delay in bone loss after OVX in the Ampkα1−/− mice.
To further investigate whether Ampkα1−/− mice responded to the sole anabolic effect of iPTH, non-ovariectomised Ampkα1+/+ and Ampkα1−/− mice were treated with iPTH for 4 weeks (study 3). In non-ovariectomised Ampkα1+/+ mice, iPTH induced a smaller increase in bone mass compared with mice that were ovariectomised, suggesting that OVX enhances the anabolic effect of iPTH on bone mass, as previously demonstrated (Andersson et al. 2001, Tezval et al. 2011b). By contrast, iPTH elicited a larger increase in bone mass in non-ovariectomised Ampkα1−/− mice compared with Ampkα1+/+ mice, possibly due to the low basal level of bone mass in these mice, enhancing the effect of iPTH. Interestingly, the comparison of the percentage of increased bone mass induced by iPTH in OVX–Ampkα1−/− mice (study 2) and non-OVX–Ampkα1−/− mice (study 3) showed that it is similar, in contrast to Ampkα1+/+ mice where there is about a tenfold decrease in the response to iPTH in non-OVX mice.
Our data suggest that the presence of the AMPKα1 subunit, and consequently AMPK activation in bone, is not essential for bone turnover but may contribute to the modulation of this process. We previously showed that the α2 subunit, in contrast to other tissues (Stapleton et al. 1996, Quinn et al. 2009, Shah et al. 2010), is not highly expressed in bone (Quinn et al. 2010, Shah et al. 2010). Furthermore, our results demonstrate that there is no compensatory up-regulation of α2 in bones of Ampkα1−/− mice. This is in contrast to the demonstration of an up-regulation of α2 in the soleus and extensor digitorum muscle in the Ampkα1−/− mice (Jorgensen et al. 2004). We cannot exclude, however, that this up-regulation of α2 in muscle and possibly in other tissues in these mice may have indirectly affected bone (Jorgensen et al. 2004).
As our study was performed with Ampkα1−/− mice that are conventional whole-body knockout, we cannot exclude that the effects of the deletion of the α1 subunit observed in bone may be the result of indirect effects of AMPK deletion in other tissues. Ampkα1−/− mice have been reported to have significantly reduced inguinal and epididymal fat weights compared with Ampkα1+/+ mice and a tendency for lower body weights (Daval et al. 2005), although this was not observed in our study or in other studies (Jorgensen et al. 2004, Viollet et al. 2009). No other metabolic phenotype was reported in these Ampkα1−/− mice and these mice have no changes in oestrogen levels. It is, however, unknown whether PTH levels are altered in these mice.
While the hormonal regulation of AMPK activation is well characterised in several tissues (Xue & Kahn 2006, Dzamko & Steinberg 2009, Lim et al. 2010), it has not been extensively studied in bone. Our previous work has shown that AMPK activity in bone cells could be regulated by the same hormones that regulate food intake and energy expenditure through AMPK activation in the brain and peripheral tissues (Shah et al. 2010). While our preliminary data in the osteoblastic cell line UMR-106 have shown no effect of oestrogen on AMPK activation (data not shown), our results show for the first time that iPTH increased the level of pAMPKα1/2 in vivo in the ovariectomised Ampkα1+/+ mice, suggesting that PTH may activate AMPK signalling in bone, although this signalling pathway does not seem essential for the effect of PTH on bone formation. Further studies must, however, be carried out to elucidate downstream pathways and mechanism of action. PTH also induced a non-significant effect on phosphorylation of AMPKα1/2 in the Ampkα1−/− mice, likely due to the phosphorylation of the α2 subunit, expressed at very low levels in bone.
In conclusion, we demonstrate that Ampkα1−/− mice have an increased bone turnover compared with Ampkα1+/+ mice and can increase and decrease bone mass in response to anabolic and catabolic hormonal challenges, although these responses are modified. Taken together, our results indicate that AMPKα1 activity is not essential for bone turnover but may contribute to the regulation of bone remodelling.
Footnotes
(J Jeyabalan and M Shah are joint first authors)
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the Wellcome Trust grant (grant reference: 086630) and a joint exchange grant between the Royal Society and CNRS (Centre national de la recherche scientifique).
References
- Alexander JM, Bab I, Fish S, Muller R, Uchiyama T, Gronowicz G, Nahounou M, Zhao Q, White DW, Chorev M, et al. Human parathyroid hormone 1–34 reverses bone loss in ovariectomized mice. Journal of Bone and Mineral Research. 2001;16:1665–1673. doi: 10.1359/jbmr.2001.16.9.1665. [DOI] [PubMed] [Google Scholar]
- Andersson N, Lindberg MK, Ohlsson C, Andersson K, Ryberg B. Repeated in vivo determinations of bone mineral density during parathyroid hormone treatment in ovariectomized mice. Journal of Endocrinology. 2001;170:529–537. doi: 10.1677/joe.0.1700529. [DOI] [PubMed] [Google Scholar]
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. Journal of Biological Chemistry. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A, Scherer PE, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Myers KS, Shultz KL, Donahue LR, Rosen CJ, Beamer WG. Ovariectomy-induced bone loss varies among inbred strains of mice. Journal of Bone and Mineral Research. 2005;20:1085–1092. doi: 10.1359/JBMR.050307. [DOI] [PubMed] [Google Scholar]
- Brouwers JE, van Rietbergen B, Huiskes R, Ito K. Effects of PTH treatment on tibial bone of ovariectomized rats assessed by in vivo micro-CT. Osteoporosis International. 2009;20:1823–1835. doi: 10.1007/s00198-009-0882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappard D, Palle S, Alexandre C, Vico L, Riffat G. Bone embedding in pure methyl methacrylate at low temperature preserves enzyme activities. Acta Histochemica. 1987;81:183–190. doi: 10.1016/S0065-1281(87)80012-0. [DOI] [PubMed] [Google Scholar]
- Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. Journal of Clinical Investigation. 1997;100:1475–1480. doi: 10.1172/JCI119668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston JE, Mellish RW, Croucher P, Newcombe R, Garrahan NJ. Structural mechanisms of trabecular bone loss in man. Bone and Mineral. 1989;6:339–350. doi: 10.1016/0169-6009(89)90039-1. [DOI] [PubMed] [Google Scholar]
- Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, Hajduch E, Ferre P, Foufelle F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. Journal of Biological Chemistry. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- Dzamko NL, Steinberg GR. AMPK-dependent hormonal regulation of whole-body energy metabolism. Acta Physiologica. 2009;196:115–127. doi: 10.1111/j.1748-1716.2009.01969.x. [DOI] [PubMed] [Google Scholar]
- Fox J, Miller MA, Newman MK, Metcalfe AF, Turner CH, Recker RR, Smith SY. Daily treatment of aged ovariectomized rats with human parathyroid hormone (1–84) for 12 months reverses bone loss and enhances trabecular and cortical bone strength. Calcified Tissue International. 2006;79:262–272. doi: 10.1007/s00223-006-0108-1. [DOI] [PubMed] [Google Scholar]
- Han SM, Namkoong C, Jang PG, Park IS, Hong SW, Katakami H, Chun S, Kim SW, Park JY, Lee KU, et al. Hypothalamic AMP-activated protein kinase mediates counter-regulatory responses to hypoglycaemia in rats. Diabetologia. 2005;48:2170–2178. doi: 10.1007/s00125-005-1913-1. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. International Journal of Obesity. 2008;32(Suppl 4):S7–S12. doi: 10.1038/ijo.2008.116. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase – development of the energy sensor concept. Journal of Physiology. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Carling D, Gamblin SJ. AMP-activated protein kinase: also regulated by ADP? Trends in Biochemical Sciences. 2011;36:470–477. doi: 10.1016/j.tibs.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. Journal of Biological Chemistry. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. Journal of Biology. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metabolism. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Iida-Klein A, Zhou H, Lu SS, Levine LR, Ducayen-Knowles M, Dempster DW, Nieves J, Lindsay R. Anabolic action of parathyroid hormone is skeletal site specific at the tissue and cellular levels in mice. Journal of Bone and Mineral Research. 2002;17:808–816. doi: 10.1359/jbmr.2002.17.5.808. [DOI] [PubMed] [Google Scholar]
- Iwaniec UT, Yuan D, Power RA, Wronski TJ. Strain-dependent variations in the response of cancellous bone to ovariectomy in mice. Journal of Bone and Mineral Research. 2006;21:1068–1074. doi: 10.1359/jbmr.060402. [DOI] [PubMed] [Google Scholar]
- Jang WG, Kim EJ, Bae IH, Lee KN, Kim YD, Kim DK, Kim SH, Lee CH, Franceschi RT, Choi HS, et al. Metformin induces osteoblast differentiation via orphan nuclear receptor SHP-mediated transactivation of Runx2. Bone. 2011a;48:885–893. doi: 10.1016/j.bone.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Jang WG, Kim EJ, Lee KN, Son HJ, Koh JT. AMP-activated protein kinase (AMPK) positively regulates osteoblast differentiation via induction of Dlx5-dependent Runx2 expression in MC3T3E1 cells. Biochemical and Biophysical Research Communications. 2011b;404:1004–1009. doi: 10.1016/j.bbrc.2010.12.099. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. Journal of Bone and Mineral Research. 2003;18:1932–1941. doi: 10.1359/jbmr.2003.18.11.1932. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the α2 but not α1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. Journal of Biological Chemistry. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T. Metformin enhances the differentiation and mineralization of osteoblastic MC3T3-E1 cells via AMP kinase activation as well as eNOS and BMP-2 expression. Biochemical and Biophysical Research Communications. 2008;375:414–419. doi: 10.1016/j.bbrc.2008.08.034. [DOI] [PubMed] [Google Scholar]
- Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T. Activation of AMP kinase and inhibition of Rho kinase induce the mineralization of osteoblastic MC3T3-E1 cells through endothelial NOS and BMP-2 expression. American Journal of Physiology. Endocrinology and Metabolism. 2009;296:E139–E146. doi: 10.1152/ajpendo.90677.2008. [DOI] [PubMed] [Google Scholar]
- Kasai T, Bandow K, Suzuki H, Chiba N, Kakimoto K, Ohnishi T, Kawamoto S, Nagaoka E, Matsuguchi T. Osteoblast differentiation is functionally associated with decreased AMP kinase activity. Journal of Cellular Physiology. 2009;221:740–749. doi: 10.1002/jcp.21917. [DOI] [PubMed] [Google Scholar]
- Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, et al. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. Journal of Biological Chemistry. 2005;280:25196–25201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M. Expanding role of AMPK in endocrinology. Trends in Endocrinology and Metabolism. 2006;17:205–215. doi: 10.1016/j.tem.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Lage R, Dieguez C, Vidal-Puig A, Lopez M. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends in Molecular Medicine. 2008;14:539–549. doi: 10.1016/j.molmed.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Lambers FM, Kuhn G, Schulte FA, Koch K, Muller R. Longitudinal assessment of in vivo bone dynamics in a mouse tail model of postmenopausal osteoporosis. Calcified Tissue International. 2012;90:108–119. doi: 10.1007/s00223-011-9553-6. [DOI] [PubMed] [Google Scholar]
- Lee YS, Kim YS, Lee SY, Kim GH, Kim BJ, Lee SH, Lee KU, Kim GS, Kim SW, Koh JM. AMP kinase acts as a negative regulator of RANKL in the differentiation of osteoclasts. Bone. 2010;47:926–937. doi: 10.1016/j.bone.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Li CY, Schaffler MB, Wolde-Semait HT, Hernandez CJ, Jepsen KJ. Genetic background influences cortical bone response to ovariectomy. Journal of Bone and Mineral Research. 2005;20:2150–2158. doi: 10.1359/JBMR.050819. [DOI] [PubMed] [Google Scholar]
- Lim CT, Kola B, Korbonits M. AMPK as a mediator of hormonal signalling. Journal of Molecular Endocrinology. 2010;44:87–97. doi: 10.1677/JME-09-0063. [DOI] [PubMed] [Google Scholar]
- Lobo RA. Metabolic syndrome after menopause and the role of hormones. Maturitas. 2008;60:10–18. doi: 10.1016/j.maturitas.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Mai Q, Zhang Z, Xu S, Lu M, Zhou R, Zhao L, Jia C, Wen Z, Jin D, Bai X. Metformin stimulates osteoprotegerin and reduces RANKL expression in osteoblasts and ovariectomized rats. Journal of Cellular Biochemistry. 2011;112:2902–2909. doi: 10.1002/jcb.23206. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Molinuevo MS, Schurman L, McCarthy AD, Cortizo AM, Tolosa MJ, Gangoiti MV, Arnol V, Sedlinsky C. Effect of metformin on bone marrow progenitor cell differentiation: in vivo and in vitro studies. Journal of Bone and Mineral Research. 2010;25:211–221. doi: 10.1359/jbmr.090732. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. Journal of Bone and Mineral Research. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Quinn JMW, Tam S, Sims NA, Saleh H, McGregor NE, Poulton IJ, Walker EC, Scott J, Kemp BE, Gillespie MT, et al. Mice lacking AMP-activated kinase (AMPK) subunits β1 or β2 have low bone mass, while AICAR acts AMPK-independently to increase osteoclast formation. Bone. 2009;44:S136–S136. doi: 10.1016/j.bone.2009.01.299. [DOI] [Google Scholar]
- Quinn JM, Tam S, Sims NA, Saleh H, McGregor NE, Poulton IJ, Scott JW, Gillespie MT, Kemp BE, van Denderen BJ. Germline deletion of AMP-activated protein kinase β subunits reduces bone mass without altering osteoclast differentiation or function. FASEB Journal. 2010;24:275–285. doi: 10.1096/fj.09-137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recker RR, Bare SP, Smith SY, Varela A, Miller MA, Morris SA, Fox J. Cancellous and cortical bone architecture and turnover at the iliac crest of postmenopausal osteoporotic women treated with parathyroid hormone 1–84. Bone. 2009;44:113–119. doi: 10.1016/j.bone.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Shah M, Kola B, Bataveljic A, Arnett TR, Viollet B, Saxon L, Korbonits M, Chenu C. AMP-activated protein kinase (AMPK) activation regulates in vitro bone formation and bone mass. Bone. 2010;47:309–319. doi: 10.1016/j.bone.2010.04.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, et al. Mammalian AMP-activated protein kinase subfamily. Journal of Biological Chemistry. 1996;271:611–614. doi: 10.1074/jbc.271.45.28445. [DOI] [PubMed] [Google Scholar]
- Steinberg GR, Kemp BE. AMPK in health and disease. Physiological Reviews. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- Tezval M, Banhardt A, Sehmisch S, Kolios L, Schmelz U, Stuermer KM, Stuermer EK. The effects of parathyroid hormone applied at different regimes on the trochanteric region of the femur in ovariectomized rat model of osteoporosis. Journal of Osteoporosis. 2011a;2011 doi: 10.4061/2011/363617. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezval M, Serferaz G, Rack T, Kolios L, Sehmisch S, Schmelz U, Tezval H, Stuermer KM, Stuermer EK. Effect of parathyroid hormone on hypogonadism induced bone loss of proximal femur of orchiectomized rat. World Journal of Urology. 2011b;29:529–534. doi: 10.1007/s00345-011-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RT, Vandersteenhoven JJ, Bell NH. The effects of ovariectomy and 17β-estradiol on cortical bone histomorphometry in growing rats. Journal of Bone and Mineral Research. 1987a;2:115–122. doi: 10.1002/jbmr.5650020206. [DOI] [PubMed] [Google Scholar]
- Turner RT, Wakley GK, Hannon KS, Bell NH. Tamoxifen prevents the skeletal effects of ovarian hormone deficiency in rats. Journal of Bone and Mineral Research. 1987b;2:449–456. doi: 10.1002/jbmr.5650020513. [DOI] [PubMed] [Google Scholar]
- Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier L, Hebrard S, Devin-Leclerc J, Beauloye C, et al. AMPK: lessons from transgenic and knockout animals. Frontiers in Bioscience: a Journal and Virtual Library. 2009;14:19–44. doi: 10.2741/3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, Giri S, Andreelli F. AMPK inhibition in health and disease. Critical Reviews in Biochemistry and Molecular Biology. 2010;45:276–295. doi: 10.3109/10409238.2010.488215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade-Gueye NM, Boudiffa M, Laroche N, Vanden-Bossche A, Fournier C, Aubin JE, Vico L, Lafage-Proust MH, Malaval L. Mice lacking bone sialoprotein (BSP) lose bone after ovariectomy and display skeletal site-specific response to intermittent PTH treatment. Endocrinology. 2010;151:5103–5113. doi: 10.1210/en.2010-0091. [DOI] [PubMed] [Google Scholar]
- Wu W, Ye Z, Zhou Y, Tan WS. AICAR, a small chemical molecule, primes osteogenic differentiation of adult mesenchymal stem cells. International Journal of Artificial Organs. 2011;34:1128–1136. doi: 10.5301/ijao.5000007. [DOI] [PubMed] [Google Scholar]
- Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. Journal of Physiology. 2006;574:73–83. doi: 10.1113/jphysiol.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature Medicine. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Kambe F, Cao X, Lu X, Kozaki Y, Oiso Y, Seo H. Thyroid hormone activates adenosine 5′-monophosphate-activated protein kinase via intracellular calcium mobilization and activation of calcium/calmodulin-dependent protein kinase kinase-β. Molecular Endocrinology. 2008;22:893–903. doi: 10.1210/me.2007-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen D, Chen Y, Tang X. Metformin reverses the deleterious effects of high glucose on osteoblast function. Journal of Diabetes and its Complications. 2010;24:334–344. doi: 10.1016/j.jdiacomp.2009.05.002. [DOI] [PubMed] [Google Scholar]