INTRODUCTION
Since the 2005 publication of International Anesthesiology Clinics issue addressing Regional Anesthesia for Ambulatory Surgery1, the predominant “paradigm shift” in regional anesthesia (RA) care has been entailed the use of ultrasound-guided regional anesthesia (UGRA). The present 2011 volume and issue of this journal has addressed these advances in detail, along with sections addressing newly available “tricks of the trade,” one of the most relevant being the effective treatment of local anesthetic systemic toxicity.
In many realms of professional and commercial life, fascination with refinement of current paradigms can usurp needed energy from the development of the next “paradigm shift” that is centered on the customer (in the world of commerce) or the patient (in health care). In business terminology, one runs the risk of focusing too much attention on “internal customers” (i.e., our colleagues and partners in the world of health care professionals) at the expense of “external customers” (i.e., our patients). Fascination with advancing technology, and contributing to the published research advancing such technology, is certainly essential to achieving quality outcomes. However, the limitations of infusing newly available research talent in our subspecialty (when such talent is rare 2) may run the risk of overemphasizing refinement and underemphasizing advancement.
Patient-centered care, dating back to our 2005 issue in this symposium journal1, focused on minimizing pain, nausea, and vomiting, the 3 symptoms that patients want to avoid most.3 Admittedly, this classic work by Macario et al. (1999) did not include RA-specific outcomes to avoid, such as needling time, vascular puncture, seizure/dysrhythmia, and long-term perineural/neuropathic sequelae. We know that UGRA effectively reduces the former 3 sequalae, and that there is no evidence of UGRA superiority in reducing the latter.4 The author of this chapter opines that local anesthetics are under-estimated in the extent to which they are responsible for long-term neuropathic sequelae in clinical practice, as alluded to in the recent review by Hogan (2008).5 Therefore, the remainder of this chapter will re-focus due attention on two patient-centered RA care concepts, (i) re-evaluate the role of local anesthetics in perineural analgesia, in an effort to maximize the duration of perineural analgesia and minimize patients’ short-term rebound pain6 after a block wears off, and (ii) to minimize patients’ long-term pain related to local anesthetic-induced peripheral neuropathy (in isolation, or as part of potential “double-crush” phenomena5,7). Using clinical applications of patient-centered RA care for surgery at and below the knee, this chapter will forecast the outcomes of a proposed paradigm shift in efforts to prolong perineural analgesia, to restrict the use of local anesthetics to specifically achieve perineural anesthesia to produce surgical conditions, and to use “novel” perineural analgesics to attenuate rebound pain and provide sustained perineural analgesia. In this discussion, neurolocation techniques and specific dosing strategies (e.g., bolus volume and infusion rate for local anesthetics) will not be considered.
REBOUND PAIN
Clinical manifestations
Clinically, rebound pain is defined as the “quantifiable difference in pain scores when the block is working, versus the increase in acute pain that is encountered during the first few hours after the effects of perineural single-injection or continuous infusion local anesthetics resolve.”6 There are 2 patient-centered ways to consider rebound pain, which is subjectively reported by the patient. First is rebound pain relative to the near-anesthetic state of the blocked extremity, and second is rebound pain relative to the patient’s preoperative baseline pain in the extremity having surgery. In practical experience, the former is psychologically traumatic to the patient after having developed a false sense of security that there may be no pain at all after surgery for the long-term future. The latter (rebound pain relative to baseline pain) is important to consider; baseline pain evaluations may prove valuable to psychologically prepare the patient for rebound pain to approach and hopefully not exceed baseline pain scores. Anecdotally, predictive counseling for rebound pain after surgery for an arthritic condition (e.g., total knee replacement) is commonly more successful since patients’ pain rebound will return to a level that was “higher than zero” baseline. Rebound pain counseling can be more difficult for patients having corrective surgery for instability in the absence of baseline pain, as is commonly the case with anterior cruciate ligament reconstruction (ACLR). In such cases, the initial injury was painful, but in the intervening weeks before injury and surgery, it is common for inflammation and swelling to sufficiently dissipate such that the patient’s knee is merely unstable but without significant pain.
Potential basic science correlation of rebound pain
Recently, Kolarczyk and Williams (20118) described transient heat hyperalgesia in rat behavioral testing after sciatic nerve block with 0.5% ropivacaine. No painful surgery was performed in the sciatic nerve distribution of the rats. During thermal and mechanical nociceptive testing at 1, 3, 5, and 7 hr after sciatic block placed during general anesthesia, rat hindpaws expectedly showed hindpaw anesthesia to heat and mechanical stimuli at 1 hr after block heat hyperalgesia. However, at 3 hr, the rat hindpaws showed an unexpected heat hyperalgesia with resolved mechanical anesthesia (Figure 1). This, to the authors’ knowledge, was the first basic science demonstration of a possible mechanism for rebound pain, in this example being carried by heat-specific pain fibers.
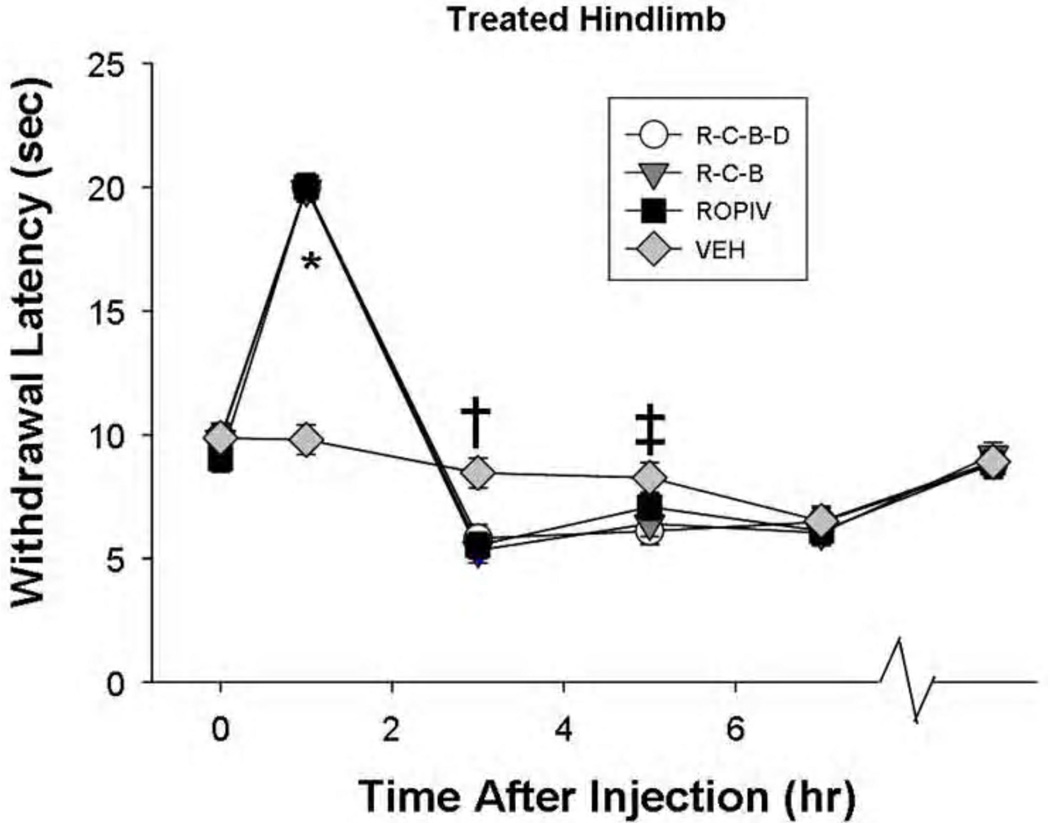
Figure 1.
Heat anesthesia-analgesia followed by shorter heat withdrawal latencies after sciatic nerve block with ropivacaine. This is an illustration of nociceptive responses of the treated (left) hindlimb to thermal stimuli as a function of treatment and time (P<0.001). The last time point (on the X-axis) represents behavioral testing before rats were euthanized (postoperative day 12–14). The 3 ropivacaine groups showed anesthesia at 1 hr after injection (P<0.001), compared to the vehicle group VEH, followed by a shorter response latency to thermal stimuli (†P≤0.003) at 3 hr after injection. At 5 hr, the only significant difference between treatments was the R-C-B-D group having a significantly shorter thermal response latency than did the VEH group (‡P=0.016). There was no evidence of long-term thermal hyperalgesia. ROPIV: ropivacaine; R-C-B: ropivacaine – clonidine – buprenorphine; R-C-B-D: R-C-B plus dexamethasone.
Unpublished preliminary data addressing rebound pain
The cited 8 findings authenticated consecutive preliminary experiments that demonstrated heat hyperalgesia after ropivacaine sciatic block. The first experiment’s results were presented as a meeting abstract, and showed tactile anesthesia after ropivacaine (0.56%) treatment (n=10 rats) for 5 hr, but heat hyperalgesia manifested after ropivacaine treatment by 3 hr.1 Saline-treated rats (n=10) showed neither anesthetic block nor heat hyperalgesia, and the treatments did not differ with respect to microscopic examination at 2–3 days or 3 weeks after the block.
A second preliminary experiment2 in rat compared sciatic nerve blocks with plain ropivacaine (0.625%), plain clonidine (0.03, 0.09, and 0.3 mcg), and saline vehicle (n=10 rats per treatment). Plain clonidine was used to detect perineural clonidine-induced sedation in behavioral testing in a dose-response fashion, but showed no analgesia or hyperalgesia effects (Figure 2). Transient heat hyperalgesia was manifest after ropivacaine treatment for 3–5 hr after block (Figure 2). In other words, a higher concentration of ropivacaine used in this experiment was associated with a longer duration of transient heat hyperalgesia than in the separate experiments reported above. No additional experiments were performed to compare dose-responses based on ropivacaine concentration.
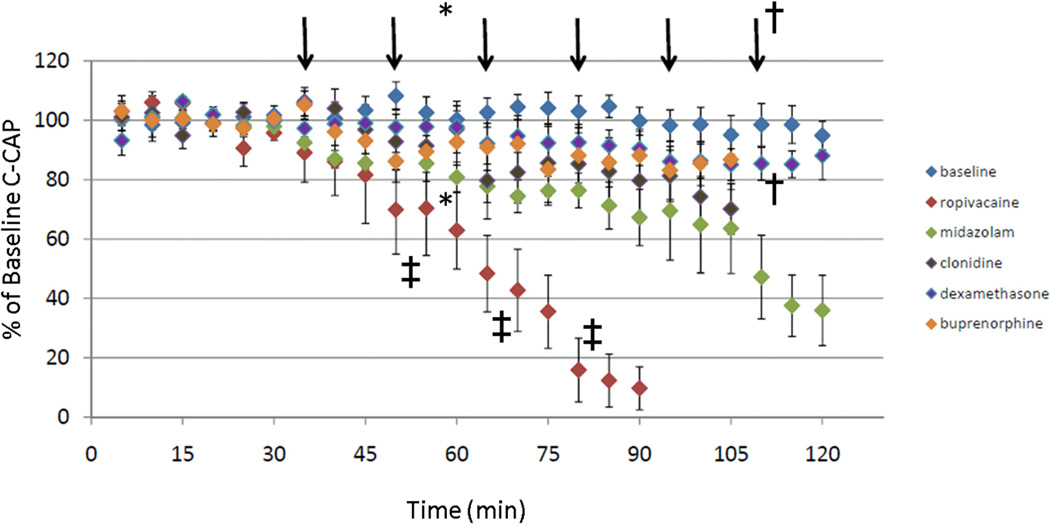
Figure 2.
Preliminary data regarding C-fiber compound action potentials (C-CAPs) after superfusion with increasing concentrations of ropivacaine or perineural analgesic single-drugs clonidine, buprenorphine, dexamethasone, and midazolam. Each vertical arrow indicates a semilog increase in molarity concentration from the previous drug-concentration.. These data are preliminary, are not intended to be interpreted as peer-reviewed, and are submitted as a 2011 poster presentation at the Annual Meeting for the Society for Ambulatory Anesthesia. When alternative analgesic adjuvants were applied to isolated nerve, we observed the following.: * Midazolam at 33 mcg/mL showed small but significant (P=0.03) reductions in C-wave conduction. †Clonidine 8 mcg/mL, a supra-clinical concentration, attenuates C-CAP by 15% (P<0.04). ‡Ropivacaine significantly attenuates C-CAPs at 0.3, 1, and 3 mM (low subclinical concentrations of 0.08, 0.027, and 0.8 mg/mL, all P<0.001).
The third unpublished preliminary experiment addressed changes in analgesic duration in rat when using plain ropivacaine 0.5% (n=14), or ropivacaine combined with (i) clonidine-buprenorphine (n=13) or (ii) clonidine-buprenorphine-dexamethasone (n=15).3 In this experiment, clonidine-buprenorphine had no effect on ropivacaine-induced heat hyperalgesia, whereas clonidine-buprenorphine-dexamethasone extended the duration of heat hyperalgesia to occur both at 3 hr (P<0.001) and 5 hr (P=0.016) after sciatic block (Figure 1). Interestingly, the additives did not prolong ropivacaine-induced mechanical anesthesia (Randall-Selitto assay9) from the one hour duration to a three hour duration. Future experiments will require smaller time intervals to assess duration of mechanical anesthesia-analgesia. Ropivacaine-induced transient heat hyperalgesia was a reproduced laboratory finding in 3 consecutive rat studies; there may be a suggestion of ropivacaine-dexamethasone mixtures, or higher concentrations of ropivacaine, extending the duration of the transient heat hyperalgesia – further research is needed.
Clinical correlation of nerve block duration and rebound pain: published and exploratory data
In our group’s first publication addressing rebound pain 6, patients underwent ACLR with spinal anesthesia, multimodal analgesia and antiemesis, and received a femoral perineural bolus of levo-bupivacaine 0.25% followed by saline placebo or levobupivacaine 0.25% at 5 mL/hr. The mean rebound pain score was 2.0; rebound pain scores on a 0–10 numeric rating scale score were reduced by 0.03 units per hour of nerve block duration, in the context of the described anesthesia-analgesia plan. In other words, 33 hr of additional nerve block duration were needed to reduce rebound pain by one point on a 0–10 scale.6 It is difficult to attribute clinical significance to such a finding, but at least the methodology is documented to determine rebound pain as a function of nerve block duration. Applying the same methodology to earlier clinical pathway data from our institution (1998–1999), outpatients undergoing shoulder surgery (n=213) reported a rebound pain score of 4.6 (4.2–5.1, 95%CI), while complex knee surgery outpatients (n=167) reported a rebound pain score of 3.6 (3.1–4.0).6 These data illustrate that nerve block recovery and rebound pain may not be identical from plexus to plexus. “Complex” (versus “less invasive”) knee surgery has been defined elsewhere.10
Rebound pain score and block duration clinical data from our institution (2008–2009) were gathered for quality control proceedings; with better knowledge of rebound pain and baseline pain informed by our 2007 manuscript.6 In these unpublished data, the author performed 50 blocks with ropivacaine, clonidine, and buprenorphine (duration = 23±1 hr [SEM]), while 117 other patients were blocked using ropivacaine only or ropivacaine and clonidine (duration = 17±0.5 hr). Each of the two perineural analgesic adjuvants were associated with a 3.0 (±0.9) hr increase in duration per adjuvant. Rebound pain scores were then evaluated using linear regression (Table 1) and the following associations were noted: (i) neither the number of adjuvants nor the use of UGRA had an associated effect on rebound pain score; (ii) every baseline pain score unit increased the rebound pain score by 0.22 numeric rating score units; and (iii) each additional hour of nerve block duration reduced rebound pain score by 0.1 numeric rating score units. Table 1 illustrates practical examples of the interactions of these independent associated effects on rebound pain score.
Table 1.
Description of Rebound Pain influenced by Block Duration and Baseline Pain, and Case Study Illustrations
| Raw data: | ||
| Author’s blocks (n=50) with ropivacaine-clonidine-buprenorphine: | 22.7 (20.8–24.5) hr duration | |
| Colleagues’ blocks (n=117) with ropivacaine with/without clonidine: | 17.4 (16.7–18.2) hr duration | |
|
Linear regression analysis demonstrating associations between block duration (hr) and/or preoperative baseline pain with rebound pain (on 0–10 numeric rating scale): | ||
| Rebound Pain Score (intercept): | 6.10 (95% CI: 4.00–8.20) units | |
| Baseline Pain Score (per unit) | 0.22 (95%CI: 0.04–0.40) units | (p=0.016) |
| Effect of Nerve block duration (per hr): | −0.1 (95% CI: 0.01–0.20) units | (p=0.031) |
| Case Study Illustration #1 – Patients with Zero Baseline Pain preop and 15 hr duration: | ||
| Patient with 15 hr block duration and preop baseline pain score of 0 had a rebound pain score of 4.6 | ||
| • 6.1 base units minus 1.5 units = 4.6 units on a 0–10 scale | ||
| Case Study Illustration #2 – Patients with Zero Baseline Pain preop and 25 hr duration: | ||
| Patient with 25-hour duration and preop pain score of 0 had a rebound pain score of 3.6 | ||
| • 6.1 base units minus 2.5 units = 3.6 units on a 0–10 scale | ||
| Case Study Illustration #3 – Patients with Baseline Pain preop of 5, with 15 hr duration: | ||
| Patient with 15-hour duration and preop pain score of 5 has a rebound pain score of 5.7 | ||
| • 6.1 base units plus (5 × 0.22=) 1.1, minus 1.5 units = 5.7 units on a 0–10 scale | ||
| Case Study Illustration #4 – Patients with Baseline Pain preop of 5, with 25 hr duration: | ||
| Patient with 25-hour duration and preop pain score of 5 had a rebound pain score of 4.7 | ||
| • 6.1 base units plus (5 × 0.22=) 1.1, minus 2.5 units = 4.7 units on a 0–10 scale | ||
|
Summary, 10 extra hours of nerve block duration was associated with a decreased Rebound Pain Score by 1 unit, on a scale of 0–10. | ||
To summarize, rebound pain score is an entity that can now be quantified. Perineural analgesia in the short term is quite different from long-term outpatient analgesia for chronic arthritic conditions, and it is important to measure pain using patient-centered methodology for the unique process that is perineural analgesia. At this time, based only on preliminary anecdotal clinical data, the adjuvants clonidine and buprenorphine (combined with ropivacaine) do not appear to have any associated independent properties to reduce rebound pain, other than by increasing duration in isolation.
CLINICAL ANECDOTES REGARDING PERINEURAL ADJUVANTS USED WITHOUT LOCAL ANESTHETICS
In our group’s case report from 200911, we described 2 patients who received perineural sciatic clonidine-buprenorphine for motor-sparing analgesia related to invasive (posterior cruciate ligament) knee surgery. One of these cases reflected a practice pattern of the author over the previous several years, specifically when the surgeons wanted to observe dorsiflexion immediately after surgery and before perineural sciatic ropivacaine infusions were begun. The author would preoperatively place femoral and sciatic perineural catheters, and provide a bolus dose of local anesthetic for the femoral catheter. For the sciatic catheter, in an effort to provide motor-sparing immediate postoperative analgesia, the author would inject clonidine (50–100 mcg) and buprenorphine (150–300 mcg), mixed together and diluted in 10 mL of preservative-free normal saline into the catheter, so that after surgery the patient would be able to demonstrate dorsiflexion pain-free either after the spinal anesthetic had dissipated, or after emergence from general anesthesia. In cases where there were delays of starting sciatic perineural infusions (e.g., infusion device not ready), the analgesic duration of the clonidine-buprenorphine injection (anecdotally) was approximately 8 hr.
FORECAST OF BASIC SCIENCE OUTCOMES RELATED TO PERINEURAL ADJUVANTS
Preliminary data from my group’s laboratory are emerging, when studying the effects of the described perineural drugs on compound action potentials of isolated rat sciatic nerves. As a frame of reference, not surprisingly, ropivacaine ablates 90% of A-fiber (data not shown) and C-fiber (Figure 2) conduction at 0.8 mg/mL (0.08%). When alternative analgesic adjuvants were applied to isolated nerve, we observed the following. Midazolam at 33 mcg/mL (a non-toxic dose to neurons12) showed small but significant (P=0.03) reductions in C-wave conduction (Figure 2); at the highest achievable concentration in vitro, midazolam did not completely ablate A-fiber or C-fiber conduction (data not shown). As single drugs, clonidine, buprenorphine, and dexamethasone had no effect on A-fiber conduction, but each demonstrated a dose-response trend toward attenuated C-fiber conduction, with (for example) clonidine’s attenuation of these responses reaching statistical significance at a supra-clinical concentration of 8 mcg/mL (P<0.04) nerve conduction in our model (Figure 2). It is acknowledged that the data in Figure 2 are presented as forthcoming abstract submissions at the time of this writing, and cannot be considered peer-reviewed or definitive.
At the time of this writing, we are currently engaged in similar nerve conduction experiments with drug mixtures, including and excluding local anesthetics. Knowing that (i) estimated clinical concentrations of clonidine-buprenorphine-dexamethasone-midazolam is nontoxic to isolated neurons12, (ii) clonidine-buprenorphine appears to lead to motor-sparing perineural analgesia11, (iii) corticosteroids suppress C-wave conduction (in rat) in vivo 13, and (iv) midazolam slightly attenuates nerve conduction (Figure 2), the potential exists for 3–4 drug perineural analgesic combinations as continuous infusions. If, for example, our clinical case reports11 of clonidine-buprenorphine motor-sparing sciatic analgesia is authenticated by reduced C-fiber conduction in vitro (with no effect on A-fibers) in the absence of local anesthetics, then this finding would certainly authenticate further clinical potential. It is important that midazolam not be combined with local anesthetics for perineural use based on profound synergistic neuronal cytotoxicity in vitro.12
FORECAST OF CLINICAL APPLICATIONS OF PERINEURAL ADJUVANTS TO OUTPATIENT KNEE SURGERY
Author’s Practice Summary Before Perineural Adjuvants are Considered
Table 2 lists categories of knee surgeries that we have previously reported 10,14,15, with respect to planning for RA care for knee surgery. “Category 1” entails procedures in which nerve blocks are unlikely indicated in advance of the surgery, but can be reserved for rescue pain. “Category 2” involves procedures with a short duration or a long duration of moderate-severe pain, Short pain durations (“2a” in Table 2) are typically managed by a preoperative single-injection femoral nerve block (e.g., bupivacaine 0.25% with 2.5–5 mcg/mL epinephrine, 15–30 mL); longer pain durations (“2b” in Table 2) are anticipated with a “preventive” (if not “pre-emptive”) femoral perineural catheter (e.g., ropivacaine 0.2% bolus with 20 mL, followed by an infusion of 0.2% ropivacaine).
Table 2.
Categories of Outpatient Knee Surgery for which Unique Regional Anesthesia Care Plans are Likely Beneficial
| Category 1: Diagnostic Knee Arthroscopy |
| Category 2: Surgical Pain Primarily Restricted to the Femoral Nerve Distribution |
| 2a: Duration of Moderate or Severe Pain Typically Restricted to 24 hr or less |
| Practical Examples: |
| • ACL with conventional single-bundle allograft (cadaver) |
| • Arthrotomy for Deep Hardware Removal |
| • Microfracture |
| • Mosaicplasty / Chondroplasty |
| • Meniscal repair with fibrin clot |
| 2b. Duration of Moderate or Severe Pain Extending Past 24 hr |
| Practical Examples: |
| • ACL patellar tendon autograft (a.k.a. bone-patellar tendon-bone) |
| Category 3: Surgical Pain Occurring in both Femoral and Sciatic Nerve Distributions |
| 3a. Least Invasive – Pain in both nerve distributions typically restricted to 24 hr or less |
| Practical Examples |
| • Distal patella realignment |
| • Knee manipulation for arthrofibrosis |
|
3b. Moderately Invasive - Pain in the sciatic nerve distributions typically restricted to 24 hr or less, while pain in femoral nerve distribution likely exceeding 24 hr |
| Practical Examples |
| • ACL with single-bundle hamstring autograft (a.k.a, semi-tendinosus – gracilis, or ST-G) |
| • ACL with double-bundle allograft |
| • Chondrocyte Transplant |
| • Medial Patellofemoral Ligament Reconstruction |
| 3b. Most Invasive - Pain in the femoral and sciatic nerve distributions typically exceeding 24 hr |
| Practical Examples |
| • Total Knee Replacement |
| • High Tibial Osteotomy |
| • Multi-ligament reconstruction including posterior cruciate ligament, posterior oblique ligament, poeterolateral corner reconstruction |
| • Unicompartmental Knee Arthroplasty |
| • Meniscal reconstruction |
ACLR: anterior cruciate ligament reconstruction
“Category 3” has three subsets: single injections for femoral and sciatic (“3a” in Table 2), femoral perineural infusion with sciatic single-injection (“3b” in Table 2), and dual femoral-sciatic perineural infusions (“3c” in Table 2). The single-injections in “Category 2a” would resemble the injections used for “Category 3a” (femoral and sciatic) and “Category 3b” (sciatic only), these being 0.25% bupivacaine with epinephrine, 20–30 mL (femoral), and 15–20 mL of 0.2% bupivacaine with low-concentration epinephrine (e.g., 2.5 mcg/mL or lower, sciatic). These bupivacaine (with epinephrine) single injections would likely approach 18–24 hr of analgesic duration; this author does not use ropivacaine for single-injection analgesic blocks (i.e., blocks not designed to produce surgical conditions) designed to give extended durations.
Table 3.
Categories of Outpatient Knee Surgery, and Foot-Ankle Surgery and Forecast for Potential Dosing Strategies of Alternative Perinerual Analgesics Clonidine, Buprenorphine, Dexamethasone, and Midazolam (all “off-label”; none of these are expressed or implied to be approved by the Food and Drug Administration)
| All listed drugs are assumed to be preservative-free (no benzyl alcohol diluent, no EDTA, etc.) |
| Knee surgery categories are assumed to be accompanied by a definitive intraoperative anesthetic (ipsilateral hyperbaric spinal anesthesia is recommended for minimized postoperative pain, when compared with general anesthetic techniques |
| Knee Category 1: Diagnostic Knee Arthroscopy |
Consideration for preoperative femoral nerve block if warranted by baseline preoperative pain scores of 3+ (out of 10) if baseline pain is arthritic (as opposed to structural, such as a loose body)
|
| Knee Category 2: Surgical Pain Primarily Restricted to the Femoral Nerve Distribution |
2a: Duration of Moderate or Severe Pain Typically Restricted to 24 hr or less
|
2b. Duration of Moderate or Severe Pain Extending Past 24 hr
|
| Knee Category 3: Surgical Pain Occurring in both Femoral and Sciatic Nerve Distributions |
3a. Least Invasive – Pain in both nerve distributions typically restricted to 24 hr or less
|
3b. Moderately Invasive - Pain in the sciatic nerve distributions typically restricted to 24 hr or less, while pain in femoral nerve distribution likely exceeding 24 hr
|
3c. Most Invasive - Pain in the femoral and sciatic nerve distributions typically exceeding 24 hr
|
| Foot-ankle surgery is assumed to be accomplished by definitive surgical nerve block (popliteal fossa block of the sciatic nerve, assuming calf tourniquet placed by surgeons). Emphasis is placed on the sciatic nerve; saphenous nerve block coverage is assumed for indicated cases, and is not discussed here. |
Foot and Ankle Soft Tissue Surgery
|
Bony-Ligamentous-Tendinous Foot-Ankle Surgery with Moderate-Severe Pain limited to 24–30 Hours
|
Bony Foot-Ankle Surgery with Moderate-Severe Pain extending beyond 24–30 Hours
|
“Category 3b” perineural femoral infusions resemble those of Category 2b above, while the sciatic nerve single-injection resembles the Category 3a single-injection (respectively: femoral ropivacaine 0.2% bolus with 20 mL, followed by an infusion of 0.2% ropivacaine, and sciatic of bupivacaine 0.2% with 15–20 mL with low-concentration epinephrine [e.g., 2.5 mcg/mL or lower]).
“Category 3c” perineural femoral and sciatic infusions differ slightly in that perineural sciatic infusions may (anecdotally) allow for lower starting ropivacaine infusion concentrations (such as 0.1%, versus 0.2% for femoral). This author typically uses ropivacaine 0.2% for the initial preoperative bolus for both nerves. Certainly if the ropivacaine 0.1% concentration provides neither motor block nor analgesia in the sciatic distribution, then the concentration of the infusion can be increased.
However, many “Category 3c” surgical procedures involve dissection at or near the common peroneal nerve. As a result, surgeons often wish to preserve the foot-ankle dorsiflexion response for examination after anesthetic emergence, precluding dosing of any local anesthetic to allow for such an exam. This is not necessarily patient-centered from a pain management standpoint; the author has achieved “the best of both worlds” by using a preoperative perineural sciatic bolus of clonidine-buprenorphine to allow for motor sparing analgesia immediately postoperatively (as described above), followed by ropivacaine sciatic perineural infusion.
Author’s Forecast of Combined Alternative Perineural Analgesics based on surgical knee category
Implied in this section is the potential for perineural single-injections and infusions that (for Categories 1–2) do not contain local anesthetics. To do so, a paradigm shift is needed with respect to defining “block success.” “Block success” research to date has involved blocks that are successful for surgical anesthesia, or in which the patients subjectively report limb heaviness consistent with motor block. For example, perineural analgesia only with alternative analgesics would need to “re-target” the desired postoperative pain score as the preoperative baseline pain score. So, for a chronic (low-grade) pain patient with arthritic baseline pain scores of 5 (out of 10) would be “recalibrated” for their analgesic targets based on the complexity of the planned surgery. These considerations would not be viable until the present evidence of relative safety (to neurons in vitro) of clonidine-buprenorphine-dexamethasone-midazolam in combination sans local anesthetic, when compared with plain ropivacaine.12 If perineural femoral-sciatic infusions of clonidine-buprenorphine-dexamethasone (with or without midazolam) lead to patient pain scores that are equal to or minimally higher than patient baselines (say, pain scores of 5–7 out of 10), and if the patient is satisfied and analgesic with the absence of motor block, should this be considered a patient-centered treatment success? To reiterate, these forecasts will not involve co-administered midazolam with local anesthetics due to in vitro synergistic neurotoxicity. 12
This forecast includes the use of preoperative perineural femoral clonidine-buprenorphine (with or without dexamethasone) for “Category 1” knee surgery, in an effort to facilitate routine bypass of the post-anesthesia care unit. Studies would be useful to determine if such use would be better directed toward patients undergoing GA (with either propofol, or with volatile agents 16 since GA has been well-documented to lead to higher post-arthroscopic pain scores than has spinal anesthesia.
For “Category 2” knee surgery, the routine addition of low-dose dexamethasone to clonidine-buprenorphine analgesic blocks is forecasted, both for single injection blocks with local anesthetics for longer-duration perineurial analgesia, and without local anesthetics for continuous perineural analgesia. Combined single-injection bupivacaine (0.125–0.25%) with clonidine-buprenorphine-dexamethasone would seem likely to achieve 30 hours of analgesic duration, based on anecdotal experience. Caution is advised regarding a dose-response interaction regarding dexamethasone and ropivacaine in vitro (66 mcg/mL dexamethasone did not worsen ropivacaine neurotoxicity, but 133 mcg/mL dexamethasone did). 12 For continuous perineural femoral analgesia, it seems possible that low-concentration midazolam (e.g., 17 mcg/mL), which is not neurotoxic in vitro when co-administered with clonidine-buprenorphine-dexamethasone 12, would provide additional meaningful analgesia while avoiding significant motor block.
For “Category 3” knee surgery, specifically “Category 3c” surgery, as procedures yield more intense postoperative pain in the femoral and sciatic distributions, combining perineural adjuvants to low-concentration ropivacaine infusions (e.g., 0.05%) seems to be a rational forecast. Whether low-concentration midazolam can replace ropivacaine in these contexts should be interesting to evaluate.
Ultimately, although there would be potential for accelerated rehabilitation in the setting of motor-sparing analgesia, extreme caution should be applied during physical therapy efforts to not threaten the integrity of the surgical repairs, reconstructions, and arthroplasties described. Nothing discussed regarding improved analgesia should imply less vigilance during supervised rehabilitation and physical therapy.
Table 3 gives a forecast of the dosing strategy that may be encountered when considering the described paradigm shift.
FORECAST OF CLINICAL APPLICATIONS OF PERINEURAL ADJUVANTS TO OUTPATIENT FOOT AND ANKLE SURGERY
Spinal anesthesia is not considered first choice for foot-ankle surgery, in combination with an analgesic block. More commonly, RA practitioners would use a popliteal sciatic block (with a supplemental saphenous nerve block, when indicated) for surgical anesthesia for foot-ankle surgery. Postoperative pain typically increases with more surgical bony involvement. If one were to create categories for foot-ankle surgery and postoperative pain, four categories may include: (i) superficial procedures (neuroma excision), (ii) soft tissue procedures (such as endoscopic plantar fasciectomy), (iii) bony procedures (such as bunionectomy, which creates significant saphenous-distribution pain as well as expected sciatic-distribution pain), and (iv) extensive bony work (e.g., triple arthrodesis, multi-digit arthroplasty). If superficial procedures require more than surgical infiltration of local anesthetic, one must carefully consider the risk-benefit ratio of lost weight-bearing status secondary to long-duration nerve block (not to mention potential neurotoxic effects of local anesthetic nerve blocks). However, a short-duration motor block (e.g., 1.0–1.5% mepivacaine) combined with perineural adjuvants may “smooth out” the course of rebound pain related to adjuvant analgesia outlasting the effects of a resolved short-duration block. After soft tissue procedures, local anesthetic selection (mepivacaine versus ropivacaine) is likely influenced by surgical duration and the extent of bony suturing of soft tissue structures (tendons/ligaments). Assuming soft tissue and bony involvement, and the focus of postoperative pain primarily in the sciatic distribution, single-injection ropivacaine is logically combined with the aforementioned adjuvants (Table 3) for a popliteal sciatic block. Bunionectomy and both saphenous and sciatic-distribution pain would logically lead to a selection of ropivacaine and analgesic perineural adjuvants for dual single-injection blocks with the goal of achieving greater than 24 hr of analgesic duration in both nerve distributions. Finally, procedures leading to postoperative pain extending beyond 24 hr render sciatic perineural catheter placement as a logical choice, in which the “surgical dose” may be comprised of mepivacaine or ropivacaine, followed by a postoperative n infusion of the described combined perineural analgesics (with or without low-concentration local anesthetics in the same infusion). Table 3 presents a forecast of where the described alternative perineural analgesics in combination may fit into the clinical care of patients undergoing these varying degrees of foot-ankle surgery.
SUMMARY AND CONCLUSION
While much current clinical research is directed toward practitioner-centered refinement of RA techniques and technology, it is important to consider pharmacologic advances in perineural analgesia as the next major patient-centered advancement of our specialty. With all due respect to excellent bench science work with novel drugs and toxins that may not gain approval of the Food and Drug Administration (FDA) for many years, it is useful to know that four FDA-approved drugs are commercially available for potentially ground-breaking off-label use, pending ongoing research. The extent to which estimated clinical concentrations of clonidine, buprenorphine, dexamethasone, and midazolam appear to not influence A-fiber conduction holds significant progress for lower extremity perineural analgesia when weight-bearing may be desired, if not at least reducing the risk of falls after these surgeries using typical local anesthetic nerve blocks. Research is also needed to determine the extent to which these 4 drugs may reduce the needed local anesthetic concentration to achieve a surgical nerve block (upon bolus injection). Ongoing research in this direction seems to represent the next major advancement in the subspecialty, being distinguished from refinement research involving strictly techniques and technology.
Acknowledgments
Laboratory efforts that are cited in this chapter were contributed by Chris Yang, BS, Becky Y.K. Tsui, MPH, Eser Yilmaz, MS, Karen Hough, AS, Allison Garda, Lavinia Kolarczyk, MD, and Robert Garman, DVM. Clinical efforts, patient quality tracking data, and/or shared ideas cited in this chapter were contributed by Michael Kentor, MD, Steven Orebaugh, MD, Pamela Harding, PA-C, and Tammy Bregon, BS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Williams BA et al., Motor and Sensory Behavioral Models for Rat Sciatic Nerve Block – Ropivacaine versus Saline Vehicle. Proceedings of the 2009 Annual Meeting of the American Society of Anesthesiologists, New Orleans, LA; A-576
Williams BA, Yang CK, Garman RH. A Reliable Model of Ipsilateral Transient Thermal Hyperalgesia after Ropivacaine Sciatic Nerve Block in Rat. Unpublished manuscript in revision, 2009–2011.
Kolarczyk LM, Garda AE, Williams BA. Transient Thermal Hyperalgesia after Resolution of Ropivacaine Sciatic Nerve Block in Rat. Proceedings of the 2010 Annual Meeting of the American Society of Anesthesiologists, San Diego,CA; A-1698
REFERENCES
- 1.Williams BA, Hurford WE, editors. International Anesthesiology Clinics: Regional Anesthesia for Ambulatory Surgery. 3. Vol. 43. Philadelphia, Lippincott: Williams & Wilkins; 2005. (guest editor), (editor in chief) [Google Scholar]
- 2.Schwinn DA, Balser JR. Anesthesiology physician scientists in academic medicine: a wake-up call. Anesthesiology. 2006;104:170–178. doi: 10.1097/00000542-200601000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89:652–658. doi: 10.1097/00000539-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Neal JM, Brull R, Chan VW, Grant SA, et al. The ASRA evidence-based medicine assessment of ultrasound-guided regional anesthesia and pain medicine: Executive summary. Reg Anesth Pain Med. 2010;35:S1–S9. doi: 10.1097/AAP.0b013e3181d22fe0. [DOI] [PubMed] [Google Scholar]
- 5.Hogan QH. Pathophysiology of peripheral nerve injury during regional anesthesia. Reg Anesth Pain Med. 2008;33:435–441. doi: 10.1016/j.rapm.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams BA, Bottegal MT, Kentor ML, et al. Rebound pain scores as a function of femoral nerve block duration after anterior cruciate ligament reconstruction: retrospective analysis of a prospective, randomized clinical trial. Reg Anesth Pain Med. 2007;32:186–192. doi: 10.1016/j.rapm.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Upton AR, McComas AJ. The double crush in nerve entrapment syndromes. Lancet. 1973;2:359–362. doi: 10.1016/s0140-6736(73)93196-6. [DOI] [PubMed] [Google Scholar]
- 8.Kolarczyk LM, Williams BA. Transient heat hyperalgesia during resolution of ropivacaine sciatic nerve block in rat. Reg Anesth Pain Med. 2011 doi: 10.1097/AAP.0b013e3182176f5a. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anseloni VC, Ennis M, Lidow MS. Optimization of the mechanical nociceptive threshold testing with the Randall-Selitto assay. J Neurosci Methods. 2003;131:93–97. doi: 10.1016/s0165-0270(03)00241-3. [DOI] [PubMed] [Google Scholar]
- 10.Williams BA, Kentor ML, Vogt MT, et al. Femoral-sciatic nerve blocks for complex outpatient knee surgery are associated with less postoperative pain before same-day discharge: a review of 1200 consecutive cases from the period 1996 – 1999. Anesthesiology. 2003;98:1206–1213. doi: 10.1097/00000542-200305000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Whiting DJ, Williams BA, Orebaugh SL, Toshok RR. Case report: Postoperative analgesia and preserved motor function with clonidine and buprenorphine via a sciatic perineural catheter. J Clin Anesth. 2009;21:297–299. doi: 10.1016/j.jclinane.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Williams BA, Hough KA, Tsui BYK, et al. Neurotoxicity of adjuvants used in perineural anesthesia and analgesia in comparison with ropivacaine. Reg Anesth Pain Med. 2011 doi: 10.1097/AAP.0b013e3182176f70. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson A, Hao J, Sjolund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand. 1990;34:335–338. doi: 10.1111/j.1399-6576.1990.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 14.Williams BA, Spratt D, Kentor ML. Continuous nerve blocks for outpatient knee surgery. Techniques in Regional Anesthesia and Pain Management. 2004;8:76–84. [Google Scholar]
- 15.Williams BA, Matusic B, Kentor ML. Regional anesthesia procedures for ambulatory knee surgery: effects on in-hospital outcomes. Int Anesthesiol Clin. 2005;43:153–160. doi: 10.1097/01.aia.0000166332.15886.f5. [DOI] [PubMed] [Google Scholar]
- 16.Korhonen AM, Valanne JV, Jokela RM, et al. A comparison of selective spinal anesthesia with hyperbaric bupivacaine and general anesthesia with desflurane for outpatient knee arthroscopy. Anesth Analg. 2004;99:1668–1673. doi: 10.1213/01.ANE.0000139351.40608.05. [DOI] [PubMed] [Google Scholar]