Abstract
Acidocalcisomes of Trypanosoma cruzi are acidic calcium-containing organelles rich in phosphorus in the form of pyrophosphate (PPi) and polyphosphate (poly P). Acidification of the organelles is driven by vacuolar proton pumps, one of which, the vacuolar proton pyrophosphatase (V-H+-PPase), is absent in mammalian cells. A Ca2+-ATPase is involved in calcium uptake and an aquaporin is important for water transport. Enzymes involved in the synthesis and degradation of PPi and poly P are present within the organelle. Acidocalcisomes function as storage sites for cations and phosphorus, participate in PPi and poly P metabolism, and volume regulation, and are essential for virulence. A signaling pathway involving cyclic AMP (cAMP) generation is important for fusion of acidocalcisomes to the contractile vacuole complex (CVC), transference of aquaporin, and volume regulation. This pathway is an excellent target for chemotherapy as shown by the effects of phosphodiesterase C (PDEC) inhibitors on parasite survival.
1. INTRODUCTION
Acidocalcisomes were first identified in bacteria and named metachromatic granules (Babes, 1895) because they had the property of changing the color of basic blue dyes. They were also named volutin granules (Meyer, 1904) because of their presence in Spirillum volutans. Volutin granules were later found in a number of microorganisms, including trypanosomes (Swellengrebel, 1908). Polyphosphate (poly P), a linear polymer of a few to several hundred orthophosphate residues discovered by Lieberman (Lieberman, 1888), was reported in the 1940′ as a component of these granules (Wiame, 1947), which then began to be known as poly P granules. More recent work in trypanosomatids and Apicomplexan parasites (Vercesi et al., 1994, Docampo et al., 1995, Moreno and Zhong, 1996) revealed that the poly P granules have proton and calcium pumps, which are responsible for their acidity and calcium content, and were given the name of acidocalcisomes. As these organelles have been found from bacteria (Seufferheld et al., 2004, Seufferheld et al., 2003) to human cells (Ruiz et al., 2004) it has been suggested that they have been conserved over evolutionary time or have appeared more than one time by convergent evolution (Docampo et al., 2010).
2. ACIDOCALCISOMES IN T. CRUZI
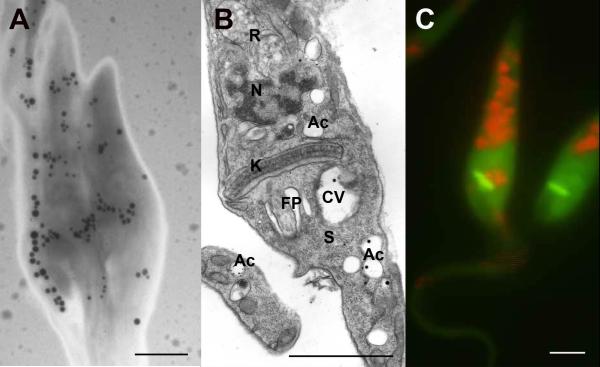
T. cruzi acidocalcisomes are electron-dense (Fig. 1A) and have a vacuolar appearance by conventional electron microscopy (Fig. 1B). At the light microscopy level they can be stained with DAPI, which labels poly P, and with dyes that accumulate in acidic compartments such as acridine orange (Fig. 1C) (Docampo et al., 1995), or cycloprodigiosin (Scott and Docampo, 2000). They are spherical with an average diameter of 0.2 μm, and randomly distributed in the cells (Fig. 1A).
Fig. 1.
Acidocalcisomes in T. cruzi. (A) electron micrograph of intact epimastigotes showing the electron-dense acidocalcisomes (black granules) distributed at random; (B) electron micrograph of epimastigote sections showing the acidocalcisomes (Ac) as empty vacuoles containing some electron dense material (dots and electron dense material on the membrane of the organelle); the contractile vacuole bladder (CV) and spongiome (S) are also apparent; (C) Staining of acidocalcisomes with Acridine Orange. The acidocalcisomes are shown in orange. Notations are flagellar pocket (FP), acidocalcisomes (Ac), kinetoplast (K), contractile vacuole bladder (CV), tubules forming the spongione (S), reservosomes (R), nucleus (N). Bars, A, B, C = 2.0 μm.
T. cruzi acidocalcisomes are rich in orthophosphate (Pi), pyrophosphate (PPi), and poly P complexed with cations (sodium, potassium, magnesium, calcium, zinc and iron) and basic amino acids (Ruiz et al., 2001b, Rohloff et al., 2003). T. cruzi is especially rich in short chain poly P such as poly P3, poly P4, and poly P5 (Moreno et al., 2000). On the basis of its total concentration (Ruiz et al., 2001b) and the relative volume of acidocalcisomes in the different stages of T. cruzi (about 1-2% of the total cell volume) (Miranda et al., 2000), it was calculated that the concentration in the organelles would be in the molar range (3-5 M) (Docampo et al., 2005). This is congruent with the detection of solid-state condensed phosphates by magic-angle spinning NMR techniques (Moreno et al., 2002), and with the very high electron density of acidocalcisomes (Scott et al., 1997, Miranda et al., 2000). Carbohydrates and lipids could be involved in maintaining these physical characteristics (Salto et al., 2008).
Some enzymatic activities have been detected in acidocalcisomes of T cruzi, such as a polyphosphate kinase, and an exopolyphosphatase (Ruiz et al., 2001b, Fang et al., 2007b). Synthesis of poly P in the yeast vacuole (Hothorn et al., 2009) and in acidocalcisomes of trypanosomatids (Fang et al., 2007a) is mediated by the “vacuolar transporter chaperone’ (Vtc) complex, which comprises four proteins in yeasts (Vtc 1- 4) anchored in the vacuole membrane and probably two (Vtc1 and Vtc4) anchored in the acidocalcisome membrane of trypanosomatids. Vtc4 has the catalytic activity and functions polymerizing and translocating the poly P chain through the vacuole membrane (Hothorn et al., 2009).
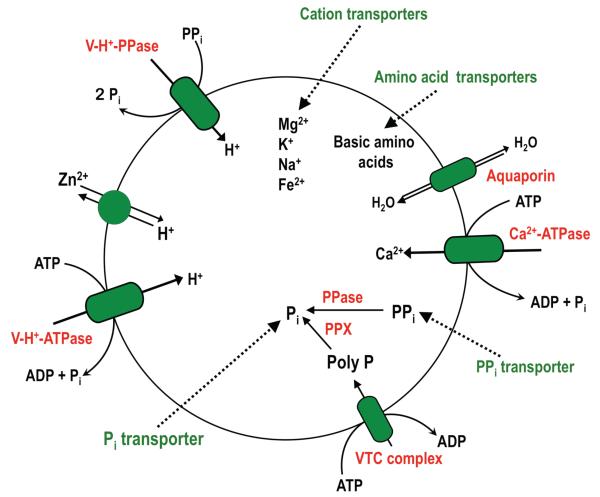
A scheme of all the enzymes and transporters identified in acidocalcisomes of T. cruzi is depicted in Fig. 2. Acidocalcisome membranes possess several pumps and at least one channel. A vanadate-sensitive Ca2+-ATPase was first detected in acidocalcisomes of T. cruzi permeabilized with digitonin (Docampo et al., 1995), and later found in the isolated organelles (Scott and Docampo, 2000). A gene encoding for the acidocalcisome Ca2+-ATPase was identified and used to complement yeast mutants that were deficient in the vacuolar Ca2+-ATPase gene PMC1, giving evidence of its functionality (Lu et al., 1998). This Ca2+-ATPase is closely related to the family of plasma membrane calcium ATPases (PMCA), although it does not have a typical calmodulin-binding domain. Two proton pumps have been detected in acidocalcisomes of T. cruzi. One is the multisubunit vacuolar-type H+-ATPase (Docampo et al., 1995) and the other is the vacuolar proton pyrophophatases (V-H+-PPase) (Scott et al., 1998, Hill et al., 2000), which uses PPi instead of ATP as energy source to transport protons. The V-H+-ATPase was shown, by immunofluorescence and immunoelectron microscopy, to co-localize to acidocalcisomes with the vacuolar-type Ca2+-ATPase (Lu et al., 1998).
Fig. 2.
Schematic representation of an acidocalcisome. A H+ gradient is established by a vacuolar ATPase (V-H+-ATPase) and a vacuolar pyrophosphatase (V-H+-PPase). Ca2+ transport is driven by a Ca2+-ATPase. Other transporters include a Zn2+/H+ antiporter, and a water channel or aquaporin. A vacuolar transporter chaperone (VTC) complex is involved in synthesis and translocation of poly P. Transporters for basic amino acids, Pi, PPi, and cations are potentially present (in green). The matrix is rich in PPi and polyphosphate (poly P) and enzymes involved in their metabolism (exopolyphosphatase (PPX), and pyrophosphatase (PPase).
The K+-stimulated V-H+-PPase of T. cruzi was the first example of this type of enzymes found in any organism different from bacteria or plants (Scott et al., 1998). The enzyme was also found in the Golgi apparatus and plasma membrane (Martinez et al., 2002) and in the contractile vacuole complex (Rohloff et al., 2004) of T. cruzi, although it is predominantly localized in the acidocalcisome and can be used as a marker for this organelle purification (Scott and Docampo, 2000). The gene for the T. cruzi V-H+-PPase could be functionally expressed in yeast (Hill et al., 2000). It was found recently that the N-terminal region of the T. cruzi V-H+-PPase, when fused to other protist enzymes enhance their functional expression in yeast (Drake et al., 2010). An aquaporin has also been identified in acidocalcisomes of T. cruzi (Montalvetti et al., 2004). The protein acts as a water channel and is unable to transport glycerol when expressed in Xenopus oocytes (Montalvetti et al., 2004). A zinc-transporter like protein was also identified by proteomic analysis and by co-localization of the tagged protein with the V-H+-PPase (Ferella et al., 2008).
3. VOLUME REGULATION
Any change in the intracellular and extracellular solute content generates the immediate flow of water into or out of the eukaryotic cell until equilibrium is achieved (Choe, 2009). This water flow through channels, driven by osmotic pressure gradients, causes swelling or shrinkage of the cells. Cells respond to these volume changes by activating volume-regulatory mechanisms. The processes by which swollen and shrunken cells return to a normal volume is known as regulatory volume decrease (RVD) and regulatory volume increase (RVI), respectively. Regulation of cell volume in most eukaryotic cells is by the gain or loss of osmotically active solutes that could be inorganic ions such as Na+, K+, and Cl− or small organic molecules known as organic osmolytes, such as polyols (e.g. sorbitol, myo-inositol), amino acids and their derivatives (e.g. taurine, alanine, and proline), and methylamines (e.g. betaine and glycerophosphorylcholine) (Choe, 2009). Some protists, however, rely on a contractile vacuole complex (CVC) to maintain their water balance both under normal environmental conditions as well as during dramatic osmotic changes in their environment (Allen, 2009). Both mechanisms, the release of ions and osmolytes and the function of the CVC are important for volume regulation in different stages T. cruzi (Rohloff and Docampo, 2008). It is possible that under normal environmental conditions the CVC is responsible for most water efflux needed to maintain the steady state volume of the cells.
RVD and RVI are important homeostatic mechanisms needed by all cells under steady state conditions and in addition they are essential for the adaptation of T. cruzi to the diverse environmental conditions that the parasite must encounter during its life cycle. For instance, there is a considerable increase in the osmolarity in the lower digestive tract of the insect vector. Osmolarity increases slightly from the feces to the urine, from 320 to 410 mosmol.Kg−1, but there is a very strong increase in the yellow rectal content, up to 1000 mosmol.Kg−1 (Kollien et al., 2001). When metacyclic trypomastigotes are introduced into a new host there is again a dramatic change, back to the normal osmolarity in the tissues and blood (330 mosm.Kg−1). Entry of the trypomastigote into a parasitophorous vacuole and its release into the cytosol could potentially involve changes in osmolarity although it is difficult to have a correct estimate of the osmolarity of different regions of a cell. Therefore, the parasite is subjected to both hyperosmotic and hyposmotic stresses during its life cycle and needs mechanisms to adapt to both stress conditions.
Physiological adaptations to hyposmotic stress in different stages of T. cruzi have been studied more extensively. The results have indicated that a RVD mechanism is present in all stages, amastigotes, epimastigotes and trypomastigotes (Rohloff et al., 2003). The process is rapid and essentially complete in all T. cruzi stages by 5 min. Amino acid efflux accounts for approximately 50% of the RVD at 150 mOsm in all stages of T. cruzi (Rohloff et al., 2003), while the rest depends on K+ efflux (7%) and the function of the CVC (43%) (Rohloff and Docampo, 2008). A number of uncharged or acidic amino acids are mobilized during hyposmotic stress in all three stages and there is a marked absence of mobilization of cationic amino acids. Glu, Gly, Pro, and Ala account for nearly 90% of the total amino acids mobilized (Rohloff et al., 2003). These results suggest that amino acid efflux in T. cruzi occurs through anion channels/transporters as proposed in other cells (Vieira et al., 1996; Lang, 2007). Acidocalcisomes contain high concentrations of amino acids but nearly 90% of them consist of Arg and Lys, minor components of the amino acids released extracellularly during regulatory volume decrease (Rohloff et al., 2003). A rise in intracellular Ca2+ occurs upon hyposmotic stress which is completely dependent on extracellular calcium and, although it plays a role in modulating the early phase of amino acid efflux, is not a key determinant of the final outcome of the regulatory volume decrease (Rohloff et al., 2003). Na+ and phosphate are not released extracellularly. Inositol efflux to the extracellular medium is negligible (Rohloff et al., 2003).
4. THE CONTRACTILE VACUOLE COMPLEX
The contractile vacuole complex (CVC) was first described in Paramecium more than 200 years ago (Spallanzani, 1799), and was later found in a wide range of amoeba, photosynthetic and nonphotosynthetic flagellates, and ciliates. Clark (Clark, 1959) was the first to describe the presence of a CVC in T. cruzi, and reported a pulsation period (time between contractions) in epimastigotes between 1 min and 1 min and 15 sec.
The contractile vacuole of T. cruzi has a bipartite structure, consisting of a central vacuole or bladder and a surrounding loose network of tubules and vesicles known as the spongiome (Rohloff et al., 2004) (Fig. 1B and Fig. 3). The spongiome and central vacuole form a stable interconnected network that collapses during systole. Functional distinctions between the peripheral and central components of the contractile vacuole complex were evidenced by the localization of different proteins to each compartment. Recent proteomic analysis and fluorescence studies of GFP-tagged proteins have revealed the presence of the vacuolar H+-ATPase, Rab11, Rab32, AP180, VAMP1 and a putative phosphate transporter to the bladder while calmodulin, and two SNAREs are localized to the spongiome (Ulrich et al., 2010).
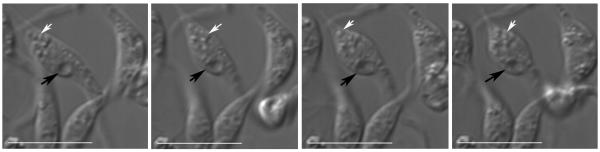
Fig. 3.
Live cell imaging of the contractile vacuole. T. cruzi epimastigotes were submitted to hyposmotic stress (150 mOsm) to detect swelling of the contractile vacuole bladder (arrows). Note that the cells become rounded after the stress and there is also swelling of smaller vacuoles in the posterior part of the cells. Pictures were taken at 5 sec intervals after hyposmotic stress for a total time of 6 min. Initial 20 seconds of the response are shown in the figure. Bars = 10 μm.
It has been pointed out that because no contractile vacuole from any organism characterized to date is significantly acidic (for example, one study (Stock et al., 2002) calculated the pH of the CV in Paramecium multimicronucleatum to be 6.4), the H+-ATPases most likely provides instead the primary electrochemical gradient for the movement of other ions (Allen and Naitoh, 2002). Interestingly, the CVC of T. cruzi also possesses a vacuolar H+-pyrophosphatase (Rohloff et al., 2004), which would provide a redundant mechanism for generating an electrochemical potential. The roles of the contractile vacuoles in protists, though, extend beyond regulation of cell volume to maintenance of Ca2+ homeostasis (Xie et al., 1996, Moniakis et al., 1999, Malchow et al., 2006) and transport of proteins to the plasma membrane (Sesaki et al., 1997), although these functions have not been investigated in T. cruzi. Recently, Hasne et al. (Hasne et al., 2010) demonstrated that the contractile vacuole of T. cruzi houses a polyamine transporter that can be transferred to the plasma membrane when the incubation media is deficient in polyamines. The CVC of T. cruzi also possesses an aquaporin involved in its periodic filling (Montalvetti et al., 2004, Rohloff et al., 2004).
5. ACIDOCALCISOMES AND THE CONTRACTILE VACUOLE COMPLEX
The acidocalcisome was initially postulated as an osmotically-active reservoir linked to the contractile vacuole function in both Chlamydomonas reinhardtii (Ruiz et al., 2001a) and Dictyostelium discoideum (Marchesini et al., 2002). Inorganic osmolytes, such as Pi released from hydrolyzed poly P, could be transferred from the acidocalcisome to the contractile vacuole, setting up a favorable osmotic gradient and facilitating net water flux into the CVC for subsequent water elimination (Marchesini et al., 2002). It was proposed that acidocalcisomes, which usually appear as indistinctive empty vacuoles by electron microscopy (Fig. 1B), could correspond to the “vesicles” or “vacuoles” that were identified in free-living protozoa as dynamically fusing with the spongiome portion of the contractile vacuole. More recent work established that hyposmotic stress conditions results in a significant increase in cyclic AMP (cAMP), swelling of acidocalcisomes, and displacement of green fluorescent protein (GFP)-labeled aquaporin immunofluorescent labeling from the acidocalcisomes to the CVC in a microtubule-and cAMP-dependent fashion (Rohloff et al., 2004). Fusion of acidocalcisomes to the CVC was initially suggested by their apparent continuity in intact cells and subcellular fractions and by electron microscopy observation of similar electron-dense material in both organelles, and was detected by video microscopy (Rohloff et al., 2004). In addition hyposmotic stress induces a rapid rise in intracellular ammonia, up to 1 mM in whole cell terms, which is rapidly sequestered into acidocalcisomes (Rohloff and Docampo, 2006).
The translocation mechanism of an aquaporin from vesicles to other membranes is similar to that described in mammalian cells; a cAMP-mediated event is involved in fusion of AQP2- (Nielsen et al., 1995) and AQP8- (Garcia et al., 2001) containing vesicles to the plasma membrane. On the other hand, an acetylcholine-induced rise in Ca2+ induces fusion of AQP5 vesicles, probably through a protein kinase C-mediated phosphorylation event (Ishikawa et al., 1998).
6. SIGNALING PATHWAYS INVOLVED IN VOLUME REGULATION
The study of the cAMP pathway in T. cruzi has been limited. The predicted structure of T. cruzi adenylyl cyclases consists of a large presumably extracellular N-terminal domain, followed by a single membrane-spanning helix and an intracellular catalytic domain (Taylor et al., 1999). This is different from the typical 12-transmembrane spanning structure of G-protein coupled adenylyl cyclases. The structure suggests that these adenylyl cyclases might function as catalytic receptors, ruling out the participation of heterotrimeric G-proteins or other regulatory factors. Furthermone, heterotrimeric G-proteins have not been identified in T. cruzi (Parsons and Ruben, 2000). Only one adenylyl cyclase gene of the near 30 genes annotated in the genome of T. cruzi (TriTryp.DB) has been studied in detail. The protein product localizes to the flagellum and is activated by calcium (D’Angelo et al., 2002). The increase in cAMP levels after cells swelling might suggest the activation of either a mechanosensitive adenylyl cyclase like the one that occurs in coronary vascular smooth cells (Mills et al., 1990) or of a mechanosensitive channel (Xiao and Xu, 2010) that could lead to the influx of ions, such as Ca2+, and activation of the adenylyl cyclase upon hyposmotic stress. How cAMP exerts its action is unknown. Homologs of a protein kinase A catalytic (Huang et al., 2002) and regulatory (Huang et al., 2006) subunits have been identified in T. cruzi but it is still uncertain whether this PKA is stimulated by cAMP. T cruzi AQP1 could be phosphorylated in vitro by a bovine PKA (Bao et al., 2008) but no experiments were done with the T. cruzi enzyme.
Cyclic AMP phosphodiesterases (PDEs) are responsible for the termination of cyclic AMP signals by hydrolyzing cyclic AMP to 5′-AMP. There are four groups of PDEs in T. cruzi: A (two genes), B (two genes), C (one gene), and D (two genes). All four PDEs belong to the Class I group of PDEs, similar to the large number of Class I PDEs found in mammals (Laxman and Beavo, 2007). TcrPDEC is a novel and rather unusual PDE that, unlike all other class I PDEs, has its catalytic domain localized in the middle of the polypeptide chain (Kunz et al., 2005, Alonso et al., 2006). In contrast, PDEs have unique N-terminal regulatory domains and the catalytic domain is usually located near their C-terminus. TcrPDEC is the only trypanosome PDE identified to date capable of hydrolyzing cGMP, although it prefers cAMP as a substrate (cAMP Km 30 μM, and cGMP Km 80 μM) (Kunz et al., 2005). Additionally, TcrPDEC is unusual in that its N-terminal region contains a FYVE-type domain, a functional domain that has not been found in any PDE so far. T. cruzi PDEC was recently found to localize to the CVC (Schoijet, 2010). The FYVE domain was shown to be important for the activity of the enzyme and for its localization in the CVC (Schoijet, 2010). This is the PDE involved in osmoregulation since the same compounds that inhibit the activity of the recombinant enzyme (Kunz et al., 2005) inhibit CVC swelling that occurs after hyposmotic stress (Rohloff et al., 2004).
Another enzyme potentially involved in volume regulation is the class III phosphatidylinositol 3-kinase (PI3K), related to the yeast vacuolar protein sorting 34, Vps34p, and their homologs from other eukaryotes. These kinases specifically phosphorylate phosphatidylinositol to produce phosphatidylinositol 3-phosphate (PI 3-P) and are associated with a Vps15p-like protein kinase. T. cruzi PI3K was functionally characterized and was able to complement yeast deficient in Vsp34p (Schoijet et al., 2008). Parasites overexpressing TcPI3K showed an enlarged CVC and protection against inhibition of the RVD by the PI3K inhibitors wortmannin and LY294,000 (Schoijet et al., 2008) suggesting a role in volume regulation.
7. DRUG TARGETING OF THE VOLUME REGULATORY PATHWAY
Phosphodiesterases are validated pharmacological targets for the treatment of several human diseases, such as erectile dysfunction (sildenafil, tadalafil, vardenafil), congestive heart failure (milrinone), platelet aggregation and intermittent claudication (cilostazol), and pulmonary hypertension (sildenafil). TcrPDEC has sequence similarity to human PDE4. Since many PDE4 inhibitors are currently under development for the treatment of inflammatory diseases, such as asthma, chronic obstructive pulmonary disease, and psoriasis, as well as treating depression and serving as cognitive enhancers (Houslay et al., 2005), an extensive literature and a number of compounds with potentially little activity against human PDE4s but potentially effective against TcrPDEC are possibly available that could be tested against T. cruzi.
A number of compounds originally synthesized as potential PDE4 inhibitors (Zheng et al., 2008) were tested on T. cruzi amastigote growth, and several useful hits were obtained (King-Keller et al., 2010). An homology modeling of T. cruzi PDEC based on the structure of PDE4B was constructed and other potential inhibitors were obtained through virtual screening (King-Keller et al., 2010). Testing of these compounds on amastigote growth and on the recombinant TcrPDEC activity resulted in several potent inhibitors that caused no toxicity to the host cells (King-Keller et al., 2010). The TcrPDEC was chemically validated as the target for these inhibitors by following the increase in cAMP in the parasites submitted to these inhibitors and the inhibition of their response to hyposmotic stress (King-Keller et al., 2010). The lethal effect of TcrPDEC inhibitors under isosmotic conditions suggests that the cAMP signaling pathway is necessary not only to overcome dramatic changes in osmolarity that occur after hyposmotic stress but also under isosmotic steady-state conditions. Continuous cAMP oscillations, which are known to occur in other cells that have contractile vacuole mechanisms of water extrusion, such as D. discoideum (Monk and Othmer, 1989), could be responsible for the periodic contraction and water expulsion by the CVC that occurs under isosmotic conditions, and explain the absolute essentiality of this mechanism of water regulation in these organisms (King-Keller et al., 2010).
8. MODEL FOR VOLUME REGULATION
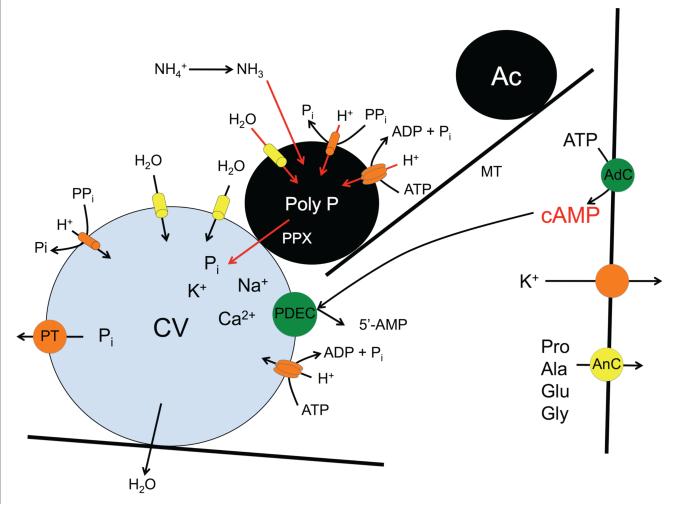
A model was proposed (Rohloff and Docampo, 2008) in which the stimulus of cell swelling causes a spike in intracellular cAMP through an as yet unidentified adenylyl cyclase, resulting in a microtubule-dependent fusion of acidocalcisomes with the contractile vacuole and translocation of aquaporin. A simultaneous rise in ammonia, and its sequestration in acidocalcisomes as NH +4, activates an acidocalcisomal exopolyphosphatase, which cleaves poly P, releasing inorganic phosphate residues and also the various poly P-chelated osmolytes, such as basic amino acids and calcium. The resulting osmotic gradient sequesters water through the aid of the aquaporin, which is subsequently ejected into the flagellar pocket. This process is terminated by the action of a PDE (Fig. 4).
Fig. 4.
Model proposed for regulatory volume decrease in T. cruzi. Cell swelling causes activation of an adenylyl cyclase (AdC) that results in a spike of intracellular cAMP, resulting in microtubule-dependent movement of acidocalcisomes (Ac) and fusion with the contractile vacuole (CV) with translocation of an aquaporin. A rise in ammonia and its sequestration in acidocalcisomes activates an exopolyphosphatase (PPX) that cleaves poly P, releasing inorganic phosphate residues and also various phosphate-chelated osmolytes, such as calcium and other cations. The resulting osmotic gradient sequesters water, through the aid of the aquaporin, which is subsequently ejected into the flagellar pocket. RVD is completed by the release of amino acids through an anion channel (AnC) and K+ through a potassium channel both localized in the plasma membrane. Termination of this cycle is by hydrolysis of cAMP through a phosphodiesterase C (PDEC) located in the contractile vacuole (CV). Phosphate is transporter back to the cytosol by a phosphate transporter (PT). Proton pumps (V-H+-ATPase and H+-PPase) maintain an electrochemical gradient in both the CV and Ac.
Since this model was proposed several supporting results have been reported. For instance, TcrPDEC was shown to localize to the CVC (Schoijet, 2010) and inhibitors of this enzyme affect the RVD of T. cruzi (King-Keller et al., 2010). This PDE has a FYVE domain, which is a phosphoinositide binding motif able to bind to PI3P, the product of a PI3K (Alonso et al., 2006, Kunz et al., 2005). Overexpression of TcPI3K was shown to affect the RVD (Schoijet et al., 2008). Several proteins important for vacuolar fusion such as SNAREs and VAMP1 were shown to localize to the CVC and a putative phosphate transporter was found in the bladder of the CVC (Ulrich, 2010). This transporter could be involved in recycling of phosphate produced by the hydrolysis of poly P during RVD (Fig. 4).
9. POLYPHOSPHATE, STRESS RESPONSE AND VIRULENCE
Rapid hydrolysis or synthesis of acidocalcisome poly P occur during hypo- or hyperosmotic stress (Ruiz et al., 2001b). Overexpression of T. cruzi exopolyphosphatase (TcPPX) depletes poly P and affects the RVD (Fang et al., 2007b). The use of RNAi to reduce the expression of the acidocalcisomal soluble pyrophosphatase of T. brucei (TbVSP1) also resulted in trypanosomes that were deficient in poly P and in their response to hyposmotic stress (Lemercier et al., 2004). Ablation of a vacuolar transporter chaperone (VTC1) in T. brucei by RNAi resulted in abnormal morphology of acidocalcisomes, decrease in their poly P content, and deficient response to hyposmotic stress (Fang et al., 2007a).
In addition to its role in volume regulation acidocalcisome poly P could have a role in nutritional stress. Knockdown of a TOR-like 1 kinase in T. brucei using RNAi leads to growth arrest and accumulation of poly P and PPi inside acidocalcisomes, which increase in size and become heavily stained at the electron microscopy level (de Jesus et al., 2010). It has been suggested that these phenotypic changes would be similar to those occurring during nutritional stress in bacteria (de Jesus et al., 2010). This is known as the “stringent response”: amino acid starvation leads to coupled cessation of protein synthesis and stable RNA synthesis. Together with the cessation of nucleic acid synthesis and continued assimilation of Pi from the medium, this results in large accumulations of poly P (Brown and Kornberg, 2004). Knockout of the homologous gene in Leishmania major (LmTOR3), resulted in alteration of acidocalcisome morphology, deficient RVD, lower ability to respond to glucose starvation, and decreased virulence in vitro and in vivo (Madeira da Silva and Beverley, 2010). In contrast to the results in T. brucei (de Jesus et al., 2010), however, these phenotypic changes were accompanied by an apparent decrease in poly P content of acidocalcisomes although this was not detected by quantitative methods (Madeira da Silva and Beverley, 2010). No studies on this TOR-like kinase 1 (TOR3) have been done on T. cruzi. Interestingly, alterations in both acidocalcisomes and poly P content have been associated with reduced in vivo virulence in a number of parasites (Lemercier et al., 2004, Zhang et al., 2005, Luo et al., 2005, Madeira da Silva and Beverley, 2010, Besteiro et al., 2008).
10. CONCLUSIONS
In T. cruzi, acidocalcisomes are rich in short chain poly P complexed with cations and basic amino acids, and possess a number of transporters (Ca2+-ATPase, V-H+-PPase, V-H+-ATPase, zinc transporter) and a channel (aquaporin) involved in the uptake of several ions and water, as well as enzymes involved in poly P metabolism. Acidocalcisomes and their major component, poly P, are essential for the response of T. cruzi and other trypanosomatids to different stress conditions. Reduced levels of poly P are associated to decreased ability to respond to osmotic or nutritional stresses and decreased virulence in vitro and in vivo. A microtubule- and cAMP-dependent signaling pathway is stimulated by hyposmotic stress and results in the transfer of the aquaporin from acidocalcisomes to the CVC. A PI3K is also involved in the response to hyposmotic stress. Acidocalcisomes alkalinize due to ammonia accumulation and also increase their volume in response to hyposmotic stress. A T. cruzi PDEC was localized to the CVC and demonstrated to be essential for volume regulation and survival of the parasite providing a novel target for chemotherapy.
ACKNOWLEDGEMENTS
This work was supported in part by grants AI-077538 (to RD), and AI-079625 (to SNJM) from the U.S. National Institutes of Health. V.J. was supported by a postdoctoral fellowship from the American Heart Association, and S.K.K. was supported by U.S. NIH training grant AI-060546 to the Center for Tropical and Emerging Global Diseases.
Abbreviations used
- Ca2+-ATPase
calcium ATPase
- cAMP
cyclic AMP
- CVC
contractile vacuole complex
- PDE
phosphodiesterase
- PMCA
plasma membrane type Ca2+-ATPase
- poly P
polyphosphate
- PPi
pyrophosphate
- RVD
regulatory volume decrease
- RVI
regulatory volume increase
- V-H+-ATPase
vacuolar type proton ATPase
- V-H+-PPase
vacuolar type proton pyrophosphatase
- Vtc
vacuolar transporter chaperone
REFERENCES
- ALLEN RD, NAITOH Y. Osmoregulation and contractile vacuoles of protozoa. Int Rev Cytol. 2002;215:351–94. doi: 10.1016/s0074-7696(02)15015-7. [DOI] [PubMed] [Google Scholar]
- ALLEN RD, TOMINAGA T, NAITOH Y. The contractile vacuole complex and cell volume control in protozoa. In: EVANS DH, editor. Osmotic and Ionic Regulation. Cells and Animals. CRC Press; Boca Raton: 2009. [Google Scholar]
- ALONSO GD, SCHOIJET AC, TORRES HN, FLAWIA MM. TcPDE4, a novel membrane-associated cAMP-specific phosphodiesterase from Trypanosoma cruzi. Mol Biochem Parasitol. 2006;145:40–9. doi: 10.1016/j.molbiopara.2005.09.005. [DOI] [PubMed] [Google Scholar]
- BABES V. Beobachtungen über die metachomatischen köperschen, sporenbidung, verzwiegung, kolben-und kapsel-bildung pathogener bakterien. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. 1895:20. [Google Scholar]
- BAO Y, WEISS LM, BRAUNSTEIN VL, HUANG H. Role of protein kinase A in Trypanosoma cruzi. Infect Immun. 2008;76:4757–63. doi: 10.1128/IAI.00527-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BESTEIRO S, TONN D, TETLEY L, COOMBS GH, MOTTRAM JC. The AP3 adaptor is involved in the transport of membrane proteins to acidocalcisomes of Leishmania. J Cell Sci. 2008;121:561–70. doi: 10.1242/jcs.022574. [DOI] [PubMed] [Google Scholar]
- BROWN MR, KORNBERG A. Inorganic polyphosphate in the origin and survival of species. Proc Natl Acad Sci U S A. 2004;101:16085–7. doi: 10.1073/pnas.0406909101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOE K, STRANGE K. Volume regulation and osmosensing in animal cells. In: EVANS DH, editor. Osmotic and Ionic Regulation. Cells and Animals. CRC Press; Boca Raton: 2009. [Google Scholar]
- CLARK TB. Comparative morphology of four genera of trypanosomatidae. J. Protozool. 1959;6:627–632. [Google Scholar]
- D’ANGELO MA, MONTAGNA AE, SANGUINETI S, TORRES HN, FLAWIA MM. A novel calcium-stimulated adenylyl cyclase from Trypanosoma cruzi, which interacts with the structural flagellar protein paraflagellar rod. J Biol Chem. 2002;277:35025–34. doi: 10.1074/jbc.M204696200. [DOI] [PubMed] [Google Scholar]
- DE JESUS TC, TONELLI RR, NARDELLI SC, DA SILVA AUGUSTO L, MOTTA MC, GIRARD-DIAS W, MIRANDA K, ULRICH P, JIMENEZ V, BARQUILLA A, NAVARRO M, DOCAMPO R, SCHENKMAN S. Target of rapamycin (TOR)-like 1 kinase is involved in the control of polyphosphate levels and acidocalcisome maintenance in Trypanosoma brucei. J Biol Chem. 2010;285:24131–40. doi: 10.1074/jbc.M110.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOCAMPO R, DE SOUZA W, MIRANDA K, ROHLOFF P, MORENO SN. Acidocalcisomes - conserved from bacteria to man. Nat Rev Microbiol. 2005;3:251–61. doi: 10.1038/nrmicro1097. [DOI] [PubMed] [Google Scholar]
- DOCAMPO R, SCOTT DA, VERCESI AE, MORENO SN. Intracellular Ca2+ storage in acidocalcisomes of Trypanosoma cruzi. Biochem J. 1995;310(Pt 3):1005–12. doi: 10.1042/bj3101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOCAMPO R, ULRICH P, MORENO SN. Evolution of acidocalcisomes and their role in polyphosphate storage and osmoregulation in eukaryotic microbes. Philos Trans R Soc Lond B Biol Sci. 2010;365:775–84. doi: 10.1098/rstb.2009.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAKE R, SERRANO A, PEREZ-CASTINEIRA JR. N-terminal chimaeras with signal sequences enhance the functional expression and alter the subcellular localization of heterologous membrane-bound inorganic pyrophosphatases in yeast. Biochem J. 2010;426:147–57. doi: 10.1042/BJ20091491. [DOI] [PubMed] [Google Scholar]
- FANG J, ROHLOFF P, MIRANDA K, DOCAMPO R. Ablation of a small transmembrane protein of Trypanosoma brucei (TbVTC1) involved in the synthesis of polyphosphate alters acidocalcisome biogenesis and function, and leads to a cytokinesis defect. Biochem J. 2007a;407:161–70. doi: 10.1042/BJ20070612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANG J, RUIZ FA, DOCAMPO M, LUO S, RODRIGUES JC, MOTTA LS, ROHLOFF P, DOCAMPO R. Overexpression of a Zn2+-sensitive soluble exopolyphosphatase from Trypanosoma cruzi depletes polyphosphate and affects osmoregulation. J Biol Chem. 2007b;282:32501–10. doi: 10.1074/jbc.M704841200. [DOI] [PubMed] [Google Scholar]
- FERELLA M, NILSSON D, DARBAN H, RODRIGUES C, BONTEMPI EJ, DOCAMPO R, ANDERSSON B. Proteomics in Trypanosoma cruzi-- localization of novel proteins to various organelles. Proteomics. 2008;8:2735–49. doi: 10.1002/pmic.200700940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA F, KIERBEL A, LAROCCA MC, GRADILONE SA, SPLINTER P, LARUSSO NF, MARINELLI RA. The water channel aquaporin-8 is mainly intracellular in rat hepatocytes, and its plasma membrane insertion is stimulated by cyclic AMP. J Biol Chem. 2001;276:12147–52. doi: 10.1074/jbc.M009403200. [DOI] [PubMed] [Google Scholar]
- HASNE MP, COPPENS I, SOYSA R, ULLMAN B. A high-affinity putrescine-cadaverine transporter from Trypanosoma cruzi. Mol Microbiol. 2010;76:78–91. doi: 10.1111/j.1365-2958.2010.07081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL JE, SCOTT DA, LUO S, DOCAMPO R. Cloning and functional expression of a gene encoding a vacuolar-type proton-translocating pyrophosphatase from Trypanosoma cruzi. Biochem J. 2000;351:281–8. doi: 10.1042/0264-6021:3510281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOTHORN M, NEUMANN H, LENHERR ED, WEHNER M, RYBIN V, HASSA PO, UTTENWEILER A, REINHARDT M, SCHMIDT A, SEILER J, LADURNER AG, HERRMANN C, SCHEFFZEK K, MAYER A. Catalytic core of a membrane-associated eukaryotic polyphosphate polymerase. Science. 2009;324:513–6. doi: 10.1126/science.1168120. [DOI] [PubMed] [Google Scholar]
- HOUSLAY MD, SCHAFER P, ZHANG KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10:1503–19. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- HUANG H, WEISS LM, NAGAJYOTHI F, TANOWITZ HB, WITTNER M, ORR GA, BAO Y. Molecular cloning and characterization of the protein kinase A regulatory subunit of Trypanosoma cruzi. Mol Biochem Parasitol. 2006;149:242–5. doi: 10.1016/j.molbiopara.2006.05.008. [DOI] [PubMed] [Google Scholar]
- HUANG H, WERNER C, WEISS LM, WITTNER M, ORR GA. Molecular cloning and expression of the catalytic subunit of protein kinase A from Trypanosoma cruzi. Int J Parasitol. 2002;32:1107–15. doi: 10.1016/s0020-7519(02)00085-1. [DOI] [PubMed] [Google Scholar]
- ISHIKAWA Y, EGUCHI T, SKOWRONSKI MT, ISHIDA H. Acetylcholine acts on M3 muscarinic receptors and induces the translocation of aquaporin5 water channel via cytosolic Ca2+ elevation in rat parotid glands. Biochem Biophys Res Commun. 1998;245:835–40. doi: 10.1006/bbrc.1998.8395. [DOI] [PubMed] [Google Scholar]
- KING-KELLER S, LI M, SMITH A, ZHENG S, KAUR G, YANG X, WANG B, DOCAMPO R. Chemical validation of phosphodiesterase C as a chemotherapeutic target in Trypanosoma cruzi, the etiological agent of Chagas’ disease. Antimicrob Agents Chemother. 2010;54:3738–45. doi: 10.1128/AAC.00313-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLLIEN AH, GROSPIETSCH T, KLEFFMANN T, ZERBST-BOROFFKA I, SCHAUB GA. Ionic composition of the rectal contents and excreta of the reduviid bug Triatoma infestans. J Insect Physiol. 2001;47:739–747. doi: 10.1016/s0022-1910(00)00170-0. [DOI] [PubMed] [Google Scholar]
- KUNZ S, OBERHOLZER M, SEEBECK T. A FYVE-containing unusual cyclic nucleotide phosphodiesterase from Trypanosoma cruzi. FEBS J. 2005;272:6412–22. doi: 10.1111/j.1742-4658.2005.05039.x. [DOI] [PubMed] [Google Scholar]
- LANG F. Mechanisms and significance of cell volume regulation. J Am Coll Nutr. 2007;26:613S–623S. doi: 10.1080/07315724.2007.10719667. [DOI] [PubMed] [Google Scholar]
- LAXMAN S, BEAVO JA. Cyclic nucleotide signaling mechanisms in trypanosomes: possible targets for therapeutic agents. Mol Interv. 2007;7:203–15. doi: 10.1124/mi.7.4.7. [DOI] [PubMed] [Google Scholar]
- LEMERCIER G, ESPIAU B, RUIZ FA, VIEIRA M, LUO S, BALTZ T, DOCAMPO R, BAKALARA N. A pyrophosphatase regulating polyphosphate metabolism in acidocalcisomes is essential for Trypanosoma brucei virulence in mice. J Biol Chem. 2004;279:3420–5. doi: 10.1074/jbc.M309974200. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN L. Uber das nuclein der hefe und kunstliche darstellung eines nucleus eiweiss und metaphosphatsaure. Ber. Chem-Ges. 1888:21. [Google Scholar]
- LU HG, ZHONG L, DE SOUZA W, BENCHIMOL M, MORENO S, DOCAMPO R. Ca2+ content and expression of an acidocalcisomal calcium pump are elevated in intracellular forms of Trypanosoma cruzi. Mol Cell Biol. 1998;18:2309–23. doi: 10.1128/mcb.18.4.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUO S, RUIZ FA, MORENO SN. The acidocalcisome Ca2+-ATPase (TgA1) of Toxoplasma gondii is required for polyphosphate storage, intracellular calcium homeostasis and virulence. Mol Microbiol. 2005;55:1034–45. doi: 10.1111/j.1365-2958.2004.04464.x. [DOI] [PubMed] [Google Scholar]
- MADEIRA DA SILVA L, BEVERLEY SM. Expansion of the target of rapamycin (TOR) kinase family and function in Leishmania shows that TOR3 is required for acidocalcisome biogenesis and animal infectivity. Proc Natl Acad Sci U S A. 2010;107:11965–70. doi: 10.1073/pnas.1004599107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALCHOW D, LUSCHE DF, SCHLATTERER C, DE LOZANNE A, MULLER-TAUBENBERGER A. The contractile vacuole in Ca2+-regulation in Dictyostelium: its essential function for cAMP-induced Ca2+-influx. BMC Dev Biol. 2006;6:31. doi: 10.1186/1471-213X-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCHESINI N, RUIZ FA, VIEIRA M, DOCAMPO R. Acidocalcisomes are functionally linked to the contractile vacuole of Dictyostelium discoideum. J Biol Chem. 2002;277:8146–53. doi: 10.1074/jbc.M111130200. [DOI] [PubMed] [Google Scholar]
- MARTINEZ R, WANG Y, BENAIM G, BENCHIMOL M, DE SOUZA W, SCOTT DA, DOCAMPO R. A proton pumping pyrophosphatase in the Golgi apparatus and plasma membrane vesicles of Trypanosoma cruzi. Mol Biochem Parasitol. 2002;120:205–13. doi: 10.1016/s0166-6851(01)00456-x. [DOI] [PubMed] [Google Scholar]
- MEYER A. Orientirende untersuchungen über verbreitung, morphologie und chemie des volutins. Bot. Zeitung. 1904;7:113–52. [Google Scholar]
- MILLS I, LETSOU G, RABBAN J, SUMPIO B, GEWIRTZ H. Mechanosensitive adenylate cyclase activity in coronary vascular smooth muscle cells. Biochem Biophys Res Commun. 1990;171:143–7. doi: 10.1016/0006-291x(90)91368-3. [DOI] [PubMed] [Google Scholar]
- MIRANDA K, BENCHIMOL M, DOCAMPO R, DE SOUZA W. The fine structure of acidocalcisomes in Trypanosoma cruzi. Parasitol Res. 2000;86:373–84. doi: 10.1007/s004360050682. [DOI] [PubMed] [Google Scholar]
- MONIAKIS J, COUKELL MB, JANIEC A. Involvement of the Ca2+-ATPase PAT1 and the contractile vacuole in calcium regulation in Dictyostelium discoideum. J Cell Sci. 1999;112(Pt 3):405–14. doi: 10.1242/jcs.112.3.405. [DOI] [PubMed] [Google Scholar]
- MONK PB, OTHMER HG. Cyclic AMP oscillations in suspensions of Dictyostelium discoideum. Philos Trans R Soc Lond B Biol Sci. 1989;323:185–224. doi: 10.1098/rstb.1989.0005. [DOI] [PubMed] [Google Scholar]
- MONTALVETTI A, ROHLOFF P, DOCAMPO R. A functional aquaporin co-localizes with the vacuolar proton pyrophosphatase to acidocalcisomes and the contractile vacuole complex of Trypanosoma cruzi. J Biol Chem. 2004;279:38673–82. doi: 10.1074/jbc.M406304200. [DOI] [PubMed] [Google Scholar]
- MORENO B, RODRIGUES CO, BAILEY BN, URBINA JA, MORENO SN, DOCAMPO R, OLDFIELD E. Magic-angle spinning 31P NMR spectroscopy of condensed phosphates in parasitic protozoa: visualizing the invisible. FEBS Lett. 2002;523:207–12. doi: 10.1016/s0014-5793(02)02977-0. [DOI] [PubMed] [Google Scholar]
- MORENO B, URBINA JA, OLDFIELD E, BAILEY BN, RODRIGUES CO, DOCAMPO R. 31P NMR spectroscopy of Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major. Evidence for high levels of condensed inorganic phosphates. J Biol Chem. 2000;275:28356–62. doi: 10.1074/jbc.M003893200. [DOI] [PubMed] [Google Scholar]
- MORENO SN, ZHONG L. Acidocalcisomes in Toxoplasma gondii tachyzoites. Biochem J. 1996;313(Pt 2):655–9. doi: 10.1042/bj3130655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIELSEN S, CHOU CL, MARPLES D, CHRISTENSEN EI, KISHORE BK, KNEPPER MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci U S A. 1995;92:1013–7. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARSONS M, RUBEN L. Pathways involved in environmental sensing in trypanosomatids. Parasitol Today. 2000;16:56–62. doi: 10.1016/s0169-4758(99)01590-2. [DOI] [PubMed] [Google Scholar]
- ROHLOFF P, DOCAMPO R. Ammonium production during hypo-osmotic stress leads to alkalinization of acidocalcisomes and cytosolic acidification in Trypanosoma cruzi. Mol Biochem Parasitol. 2006;150:249–55. doi: 10.1016/j.molbiopara.2006.08.010. [DOI] [PubMed] [Google Scholar]
- ROHLOFF P, DOCAMPO R. A contractile vacuole complex is involved in osmoregulation in Trypanosoma cruzi. Exp Parasitol. 2008;118:17–24. doi: 10.1016/j.exppara.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROHLOFF P, MONTALVETTI A, DOCAMPO R. Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi. J Biol Chem. 2004;279:52270–81. doi: 10.1074/jbc.M410372200. [DOI] [PubMed] [Google Scholar]
- ROHLOFF P, RODRIGUES CO, DOCAMPO R. Regulatory volume decrease in Trypanosoma cruzi involves amino acid efflux and changes in intracellular calcium. Mol Biochem Parasitol. 2003;126:219–30. doi: 10.1016/s0166-6851(02)00277-3. [DOI] [PubMed] [Google Scholar]
- RUIZ FA, LEA CR, OLDFIELD E, DOCAMPO R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–7. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- RUIZ FA, MARCHESINI N, SEUFFERHELD M, GOVINDJEE, DOCAMPO R. The polyphosphate bodies of Chlamydomonas reinhardtii possess a proton-pumping pyrophosphatase and are similar to acidocalcisomes. J Biol Chem. 2001a;276:46196–203. doi: 10.1074/jbc.M105268200. [DOI] [PubMed] [Google Scholar]
- RUIZ FA, RODRIGUES CO, DOCAMPO R. Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation, and environmental stress in Trypanosoma cruzi. J Biol Chem. 2001b;276:26114–21. doi: 10.1074/jbc.M102402200. [DOI] [PubMed] [Google Scholar]
- SALTO ML, KUHLENSCHMIDT T, KUHLENSCHMIDT M, DE LEDERKREMER RM, DOCAMPO R. Phospholipid and glycolipid composition of acidocalcisomes of Trypanosoma cruzi. Mol Biochem Parasitol. 2008;158:120–30. doi: 10.1016/j.molbiopara.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOIJET AC, MIRANDA K, GIRARD-DIAS W, DE SOUZA W, FLAWIA MM, TORRES HN, DOCAMPO R, ALONSO GD. A Trypanosoma cruzi phosphatidylinositol 3-kinase (TcVps34) is involved in osmoregulation and receptor-mediated endocytosis. J Biol Chem. 2008;283:31541–50. doi: 10.1074/jbc.M801367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOIJET AC, MIRANDA K, SOARES MEDEIROS C, DE SOUZA W, FLAWIÁ MN, TORRES HN, PIGNATARO OP, DOCAMPO R, ALONSO GD. Defining the role of a FYVE domain in the localization and activity of cAMP phosphodiesterase implicated in osmoregulation in Trypanosoma cruzi. Mol. Microbiol. 2010 doi: 10.1111/j.1365-2958.2010.07429.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT DA, DE SOUZA W, BENCHIMOL M, ZHONG L, LU HG, MORENO SN, DOCAMPO R. Presence of a plant-like proton-pumping pyrophosphatase in acidocalcisomes of Trypanosoma cruzi. J Biol Chem. 1998;273:22151–8. doi: 10.1074/jbc.273.34.22151. [DOI] [PubMed] [Google Scholar]
- SCOTT DA, DOCAMPO R. Characterization of isolated acidocalcisomes of Trypanosoma cruzi. J Biol Chem. 2000;275:24215–21. doi: 10.1074/jbc.M002454200. [DOI] [PubMed] [Google Scholar]
- SCOTT DA, DOCAMPO R, DVORAK JA, SHI S, LEAPMAN RD. In situ compositional analysis of acidocalcisomes in Trypanosoma cruzi. J Biol Chem. 1997;272:28020–9. doi: 10.1074/jbc.272.44.28020. [DOI] [PubMed] [Google Scholar]
- SESAKI H, WONG EF, SIU CH. The cell adhesion molecule DdCAD-1 in Dictyostelium is targeted to the cell surface by a nonclassical transport pathway involving contractile vacuoles. J Cell Biol. 1997;138:939–51. doi: 10.1083/jcb.138.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEUFFERHELD M, LEA CR, VIEIRA M, OLDFIELD E, DOCAMPO R. The H+-pyrophosphatase of Rhodospirillum rubrum is predominantly located in polyphosphate-rich acidocalcisomes. J Biol Chem. 2004;279:51193–202. doi: 10.1074/jbc.M406099200. [DOI] [PubMed] [Google Scholar]
- SEUFFERHELD M, VIEIRA MC, RUIZ FA, RODRIGUES CO, MORENO SN, DOCAMPO R. Identification of organelles in bacteria similar to acidocalcisomes of unicellular eukaryotes. J Biol Chem. 2003;278:29971–8. doi: 10.1074/jbc.M304548200. [DOI] [PubMed] [Google Scholar]
- SPALLANZANI L. Tracts on the nature of animals and vegetables. Edinburgh: 1799. [Google Scholar]
- STOCK C, GRONLIEN HK, ALLEN RD. The ionic composition of the contractile vacuole fluid of Paramecium mirrors ion transport across the plasma membrane. Eur J Cell Biol. 2002;81:505–15. doi: 10.1078/0171-9335-00272. [DOI] [PubMed] [Google Scholar]
- SWELLENGREBEL NH. La volutine chez les trypanosomes. C. R. Soc. Biol. Paris. 1908;64:38–43. [Google Scholar]
- TAYLOR MC, MUHIA DK, BAKER DA, MONDRAGON A, SCHAAP PB, KELLY JM. Trypanosoma cruzi adenylyl cyclase is encoded by a complex multigene family. Mol Biochem Parasitol. 1999;104:205–17. doi: 10.1016/s0166-6851(99)00154-1. [DOI] [PubMed] [Google Scholar]
- ULRICH PN, JIMENEZ V, PARK M, MARTINS VP, ATWOOD J, III, MOLES K, COLLINS D, ROHOLOFF P, TARLETON R, MORENO SNJ, ORLANDO R, DOCAMPO R. Identification of contractile vacuole proteins in Trypanosoma cruzi. 2010. submitted. [DOI] [PMC free article] [PubMed]
- VERCESI AE, MORENO SN, DOCAMPO R. Ca2+/H+ exchange in acidic vacuoles of Trypanosoma brucei. Biochem J. 1994;304:227–33. doi: 10.1042/bj3040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIEIRA LL, LAFUENTE E, GAMARRO F, CABANTCHIK Z. An amino acid channel activated by hypotonically induced swelling of Leishmania major promastigotes. Biochem J. 1996;319:691–7. doi: 10.1042/bj3190691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIAME JM. Etude d’une substance polyphosphorée, basophile et métachromatique chez les levures. Biochim. Biophys. Acta. 1947;1:234–255. [Google Scholar]
- XIAO R, XU XZ. Mechanosensitive channels: in touch with Piezo. Curr Biol. 2010;20:R936–8. doi: 10.1016/j.cub.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIE Y, COUKELL MB, GOMBOS Z. Antisense RNA inhibition of the putative vacuolar H+-ATPase proteolipid of Dictyostelium reduces intracellular Ca2+ transport and cell viability. J Cell Sci. 1996;109(Pt 2):489–97. doi: 10.1242/jcs.109.2.489. [DOI] [PubMed] [Google Scholar]
- ZHANG K, HSU FF, SCOTT DA, DOCAMPO R, TURK J, BEVERLEY SM. Leishmania salvage and remodelling of host sphingolipids in amastigote survival and acidocalcisome biogenesis. Mol Microbiol. 2005;55:1566–78. doi: 10.1111/j.1365-2958.2005.04493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHENG S, KAUR G, WANG H, LI M, MACNAUGHTAN M, YANG X, REID S, PRESTEGARD J, WANG B, KE H. Design, synthesis, and structure-activity relationship, molecular modeling, and NMR studies of a series of phenyl alkyl ketones as highly potent and selective phosphodiesterase-4 inhibitors. J Med Chem. 2008;51:7673–88. doi: 10.1021/jm701635j. [DOI] [PMC free article] [PubMed] [Google Scholar]