Abstract
We recently observed that naloxone, a non-specific opioid antagonist, attenuated operant responding to ethanol in infant rats. Through the use of an operant conditioning technique, we aimed to analyze the specific participation of mu, delta, and kappa opioid receptors on ethanol reinforcement during the second postnatal week. In Experiment 1, infant rats (PDs 14–17) were trained to obtain 5, 7.5, 10, or 15% ethanol, by operant nose-poking. Experiment 2 tested blood ethanol levels (BELs) attained by operant behavior. In Experiment 3, at PDs16–18, rats received CTOP (mu antagonist: 0.1 or 1.0 mg/kg), naltrindole (delta antagonist: 1.0 or 5.0 mg/kg) or saline before training. In Experiment 4, rats received nor-binaltorphimine (kappa antagonist: 10.0 or 30.0 mg/kg, a single injection after completion of PD15 operant training), spiradoline mesylate (kappa agonist: 1.0 or 5.0 mg/kg; at PDs16–18) or saline (PDs16–18), before the conditioning. Experiment 5 and 6 assessed possible side effects of opioid drugs in locomotor activity (LA) and conditioned taste aversion (CTA). Ethanol at 7.5 and 10% promoted the highest levels of operant responding. BELs were 12–15 mg/dl. In Experiment 3 naltrindole (dose response effect) and CTOP (the lowest dose) were effective in decreasing operant responding. Nor-binaltorphimine at 10.0 mg/kg and spiradoline at 5.0 mg/kg also blocked ethanol responding. The effects of opioid drugs on ethanol reinforcement cannot be explained by effects on LA or CTA. Even though particular aspects of each opioid receptor require further testing, a fully functional opioid system seems to be necessary for ethanol reinforcement, during early ontogeny.
Keywords: Ethanol reinforcement, infant rat, mu, delta and kappa opioid receptors, operant conditioning
1. Introduction
Epidemiological studies with humans and experimental studies with rodents agree that prenatal and/or early postnatal ethanol experience predicts later responsiveness to the drug [1–6]. This association emphasizes the importance of understanding the neurobiological mechanisms that regulate ethanol effects early in development, especially considering the growing number of studies showing a particular sensitivity to ethanol’s reinforcing effects during the infantile period of the rat (reviewed in: [3, 4, 7, 8]). Ethanol seeking and intake are modulated by the appetitive and aversive properties of the drug [9], and preweanling rats have proven valuable for assessing these phenomena. A pattern of high acceptance of ethanol early in ontogeny seems to be associated with the pharmacological effects of ethanol rather than its orosensory properties [4, 10]. Preweanling rats are also sensitive to locomotor activating effects of ethanol [11, 12]. The stimulating effect of ethanol is observed with relatively high ethanol doses (1.25 or 2.5 g/kg) during the rising phase of the blood ethanol curve [12]. When blood ethanol concentration reaches peak values, ethanol–induced locomotor activation is no longer observed [12].
This biphasic effect of ethanol is likely to be associated with the biphasic (appetitive and aversive) reinforcing effects induced by relatively high ethanol doses in preweanling rats [13], since both follow a similar time course. The use of operant techniques to evaluate the motivational effects of ethanol during early ontogeny has been recently documented in the literature [14–19]. This evidence strengthens the notion that the developing rat is highly sensitive to appetitive motivational effects of ethanol, as demonstrated through rapid and robust instrumental learning.
At this time, four major classes of opioid receptors have been identified: mu, delta, kappa and ORL1 [20, 21]. These subtypes are widely expressed in brain areas associated with the reinforcing effects of different drugs of abuse including ethanol [22]. Under normal conditions activation of mu and kappa receptors seem to have opposite effects, neurochemically and behaviorally. Intracerebral administration of mu and kappa receptor agonists respectively increase and decrease dopamine release in the ventral tegmental area [23]. Administration of N/OFQ, the natural ligand of ORL1 opioid receptors, also suppresses the activity of the mesocorticolimbic/dopaminergic system, whereas central antagonism of ORL1 receptors induces anxiolysis [24] and conditioned place preference [25].
Similar to adult rodents [22] ethanol reinforcement and acceptance in preweanling rats seems to be regulated, at least partly, by the opioid system. For example, non-specific opioid antagonists (such as naloxone or naltrexone) co-administered with ethanol during gestation disrupt future increases in appetitive responding towards ethanol [16, 26–28]. Furthermore, opioid antagonist administration prior to conditioning with ethanol disrupts appetitive reinforcement towards the drug [17]. In newborn and infant rats, the mu and kappa opioid system modulates ethanol-mediated appetitive reinforcement [29] with administration of selective mu and kappa antagonists inhibiting ethanol-induced reinforcement [29, 30]. Ethanol intake can also be reduced by non-specific or specific (mu or delta) opioid antagonists during the preweanling period [28, 31, 32].
In adults ethanol increases release of β-endorphins, especially in the ascending limb of the blood ethanol curve [33]. Ethanol intake is reduced by selective kappa opioid receptor (KOR) agonists, and delta and mu receptor antagonists [34–36]. Lower preference for ethanol is found in ORL1 knockout mice than in wild-type counterparts [25]. The opioid system is also involved in ethanol-induced appetitive conditioning. In addition to this evidence, it has been reported recently that the opioid receptors for mu, but not delta, are involved in the psychomotor stimulant effects of ethanol [31, 37, 38].
Recent research conducted in our laboratories indicates that very early in life, the opioid system is involved not only in ethanol consumption but also in operant behavior, (i.e., seeking of ethanol consumption [17]). In this work, self-administration of ethanol was established in terms of operant responding in preweanling rats with no previous exposure to the drug. Pairing of naloxone with ethanol, at a point separate in time from operant responding, reduced ethanol reinforcement, although separate contributions of each subtype of opioid receptors in this particular effect has not been tested at this early stage of development. The present experiments tested the involvement of specific opioid receptors (mu, delta and kappa) in ethanol-mediated operant behavior, in infant rats. Early in ontogeny mu, delta and kappa receptors follow different patterns of ontogenetic development, but all are functional by the second postnatal week of life [39]. The idea was to use a protocol of operant conditioning similar to that used by Miranda Morales et al. [17], in conjunction with selective blockade of ethanol reinforcement through the administration of CTOP (mu antagonist) or naltrindole (delta antagonist). For the kappa opioid system, we tested both an agonist (spiradoline mesylate) and an antagonist (norbinaltorphimine) to evaluate the participation of this sub-system in ethanol reinforcement. This idea is based on recent findings obtained by Pautassi and colleagues [56] in which either blockade or activation of kappa facilitated appetitive ethanol reinforcement depending on timing of antagonist administration.
The present study was conducted in a different laboratory, country, and strain of rat (Wistar vs. Sprague-Dawley) than used by Miranda Morales et al. [17]. The first step of this work was to confirm and extend the generalizability of the main findings obtained by Miranda-Morales et al. [17]. Strain-dependent differences have been observed occasionally between Wistar and Sprague-Dawley rats. Nevertheless, for both strains of rat, newborns prenatally exposed to an odor paired with ethanol intoxication exhibit heightened responsiveness postnatally to an artificial nipple in the presence of that odor [40]. In addition to this, ethanol-mediated operant reinforcement has been established in neonates of both rat strains [14, 15]. However, it remains to be established if the protocol of operant nose-poking procedure like that of Miranda-Morales et al. [17] can be replicated in pups derived from the Sprague-Dawley strain.
The plan of the present study was to: (i) reproduce infantile ethanol-mediated operant behavior (Experiments 1 and 2); (ii) analyze the specific participation of mu, delta and kappa opioid receptors in mediating ethanol reinforcement in infant rats (Experiments 3 and 4); and (iii) test whether non-specific effects of the opioid drugs (CTOP, Naltrindole, nor-BNI and spiradoline) used in Experiments 3–4 might mask effects on operant behavior for ethanol (Experiments 5 and 6).
2. General methods
2.1 Subjects
Sprague–Dawley infant rats (PDs 14–18) were used across all experiments. These animals were born and reared in the vivarium at the Center for Development and Behavioral Neuroscience (AAALAC-accredited facility, Binghamton University, Binghamton, NY, USA). Births were examined daily, and the day of birth was considered PD 0. The colony was maintained at 22–24 °C under a 12 hr/12 hr light/dark cycle. The experiments were approved by the Binghamton University Institutional Review Committee for the Use of Animal Subjects and were in compliance with the NIH Guide for the Care and Use of Laboratory Animals [41].
2.2 Cannulation procedures
Across experiments, intraoral infusion was conducted by means of polyethylene cannulae positioned in the pup’s cheek. Intraoral cheek cannulation is minimally stressful in preweanlings [42] and has been shown to be a useful tool for the assessment of responsiveness to tastants, early in life (e.g., [43, 44]). This intraoral cannulation procedure has been extensively described in previous studies (for detailed information please see [16, 17, 19, 45–48]).
2.3 Operant training procedures (PDs 14 to 17) and extinction session (PD 18)
Custom-made operant-conditioning chambers (12×12×15 cm) were employed [17–19]. One lateral wall of the chamber had a hole in it (diameter: 1 cm); behind the hole was an evaluation board equipped with a circular touch-sensitive sensor (Model E11x Evaluation Board; Quantum Research Group, Pittsburgh, PA). The target behavior under training was nose-poke. Specifically, each time the snout of an experimental subject touched the sensitive sensor, a signal went on and activated an infusion pump. A 50-cm section of PE-50 polyethylene tubing was connected to the end of the oral cannula of the animal and to a 2-ml micrometer Gilmont Syringe (Gilmont Instruments, Barrington, IL) mounted in a rotary syringe pump (Kashinsky-Rozboril, Model 5 2000, Binghamton, NY). The pump was set to deliver a solution at the rate of 5 μl/sec, directly into the oral cavity of the animal (the schedule of reinforcement was set as a fixed ratio 1). On each operant training day pups were removed from their maternal cages, cannulated (Section 2.2) and placed in pairs in a holding cage (45×20×20 cm) lined with pine shavings and warmed to approximately 35 ± 0.5 °C. Three hours later, operant training took place. First, the anogenital region of the preweanling was gently stroked with a cotton swab to stimulate defecation and void the subject’s bladder. The animal’s weight was then registered (±0.01 g; balance model BP410; Sartorius Corporation, Edgewood, NY). Operant training began by individually placing an experimental paired (Pd) pup and its corresponding yoked (Y) control (a same-sex pair of animals) into their respective operant conditioning chamber. Yoked-control pups received the infusion each time their paired animal did. In other words, Y controls and Pd pups received equivalent reinforcement but Y animals had no control over the contingency between target operant behavior and intraoral infusion. The duration of each daily training session was 15 min. Immediately after each training session, the weight of each pup was recorded again for measurement of intake. Nose-poking frequency was registered and recorded during each operant session (Simple Logger II, Model L404, AEMC Instruments, USA; sensitivity: 1 response / 0.01 s). Consumption of ethanol, in terms of grams per kilogram (g/kg) of ethanol ingested during operant training sessions, was calculated according to the following equation: ([(post-conditioning weight – pre-conditioning weight) × (ethanol solution concentration × 100)] × 0.81) / (pre-conditioning weight × 1000). At PD18, an extinction session was given: procedures and conditions of evaluation were similar to those of the previous operant training days, but the behavior of the infants did not yield delivery of the reinforcer.
2.4 Drug preparation and administration procedures
Across the experiments, different concentrations of ethanol solutions were used as reinforcers: 5.0, 7.5, 10.0 or 15.0% (190 proof ethanol, Pharmaco, Brookfield, CT; vehicle: distilled water).
The opioid antagonists/agonist used were the following: D-Pen-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) a mu opioid receptor (MOR) antagonist; naltrindole, a delta opioid receptor (DOR) antagonist; nor-binaltorphimine (nor-BNI), kappa opioid receptor (KOR) antagonist and spiradoline mesylate (U62,066E), a KOR agonist. All these drugs were administered via intraperitoneal (i.p) injection. Injections took less than 10 s and were performed in a region situated between the diaphragm and the genitalia. CTOP (TOCRIS, Ellisville, MO) was administered at a dose of 0.1 or 1.0 mg/kg and was derived from a 0.01 and 0.1 mg/ml solution, respectively. Naltrindole (Sigma-Aldrich, St. Louis, MO) was administered at a dose of 1.0 or 5.0 mg/kg and was derived from a 0.1 and 0.5 mg/ml solution, respectively. Nor-BNI (TOCRIS, Ellisville, MO) was administered at a dose of 10.0 or 30.0 mg/kg and was derived from a 1.0 and 3.0 mg/ml solution, respectively. U62,066E (Sigma-Aldrich, St. Louis, MO) was administered at a dose of 0.1 or 0.5 mg/kg and was derived from a 0.01 and 0.05 mg/ml solution, respectively. The injection volume was kept at 0.01 ml/g for all drugs and doses, and saline (ClNa 0.9% v/v) was used as the vehicle.
2.5 Locomotor Activity Assessment
On PD 16, pups were separated from the mother and placed in pairs in a holding cage. The animals remained in the cage for 3 h, then locomotor activity was evaluated for 15 min. The testing environment consisted of an open field chamber with Plexiglas walls (42×42×30 cm; VersaMax Animal Activity Monitoring System, Accuscan Instruments, Columbus, OH, USA). In this apparatus, locomotion was detected by interruption of eight pairs of intersecting photocell beams evenly spaced along the walls of the testing environment. This equipment was situated in sound-attenuating chambers (53×58×43 cm) equipped with a light and fan for ventilation and background noise. Consecutive photocell beam interruptions were translated to distance traveled in cm by the VersaMax software. This dependent variable takes into account the path the animal takes, and is an accurate indicator of ambulatory activity. Immediately after the locomotor activity test pups were returned to their home cage.
2.6 Conditioned Taste Aversion Test
The pups were separated from their dams on PD16, cannulated and placed in pairs in a holding cage. The animals remained in the cage for 3 h. After that time, pups were voided and weighed. The mean weight of all subjects was calculated and used as a benchmark for the volume of the intraoral infusion of saccharin (0.1% w/v) during conditioning. Each subject’s cannula was connected to a length of PE50 tubing that in turn was connected to a 5 ml syringe operated by an automatic infusion pump (KD Scientific, Holliston, MA). The subjects were then placed into a Plexiglas container divided into eight sections measuring 9 × 15 cm each. The bottoms of these containers were lined with absorbent paper and slightly heated (26–27°C). Subjects received an intraoral infusion of saccharin (2.5% of their body weight) over a 10 min period. These infusion parameters allowed pups to either accept or reject the infused solution [49]. Immediately following the intraoral infusion, pups were disconnected from the tubing, weighed to estimate saccharin consumption scores and injected i.p. with the opioid antagonist or agonist. One hour post injection, pups were returned to their dams. On PD17 (test day), subjects had the same manipulation as on PD16 but no injection after intake test.
2.7 Experimental design and data analysis
Across experiments, no more than one subject from each sex was assigned to the same treatment condition in a given litter. Number of males and females in each group was balanced, whenever possible [50]. Data obtained during the operant task were analyzed with analysis of variance (ANOVA). Separate ANOVAs were executed to analyze training and extinction sessions. In the case of significant three-way interactions, follow-up ANOVAs were employed before the use of post hoc tests. This procedure served to minimize the probability of Type I errors arising from multiple group comparisons. The loci of significant main effects or two-way interactions were further analyzed with Fisher’s LSD post hoc comparisons. A rejection criterion of p < 0.05 was adopted for all statistical analysis in the present study.
Preliminary analysis of the data of each experiment included sex as a variable. This factor consistently failed to exert any significant main effect, or to interact with any other factor under analysis. For this reason, further statistical analysis was performed after collapsing across sex in all conditions. In addition, other preliminary analyses were conducted to compare nose-poke behavior between experimental (Pd) vs. Y control infants for each experiment of operant conditioning (Experiment 1, 3 and 4). In these ANOVAs nature of reinforcer (Exp. 1), or dose of the opioid antagonist or agonist tested (Exp. 3 and 4) served as a between variable and days of operant training (PDs 14–17) were considered as a within variable. For all the cases, the nature of the reinforcer or the opioid antagonists/agonist failed to significantly affect nose-poking behavior of Yoked Pups. Only a significant effect of day of training could be observed: yoked pups significantly increased their number of sensor contacts from PD 14 to 15 (Exp. 3), 15 to 16 (Exp. 1) and 16 to 17 (Exp. 3). Taking this result into account and considering the analysis performed in previous studies of operant conditioning during infancy which also found that yoked-control animals were unaffected by the independent variables [17], Y-control animals were collapsed across the experimental conditions for statistical analysis.
3. Experiment 1
Previous studies indicate that preweanlings Wistar rats effectively increased the probability of nose-poking for a low-concentration ethanol solution (3.75% v/v; [17]). In addition, Sprague-Dawley rats, during early infancy, seem to perceive moderate-to-high ethanol concentrations as appetitive [14, 51, 52]. The present experiment was conducted to determine at which of four ethanol concentrations infant rats from the Sprague-Dawley strain would learn to perform a target behavior (nose-poke) to gain access to intraoral delivery of ethanol.
3.1 Methods
Eighty infants from 10 litters were employed. Groups were defined by the following factors: operant learning condition (paired or yoked) and concentration of ethanol solution used as the reinforcer (5.0, 7.5, 10.0 or 15.0% v/v). Each group was composed of 10 infants. Operant training procedures were conducted during PDs 14–17.
3.2 Results
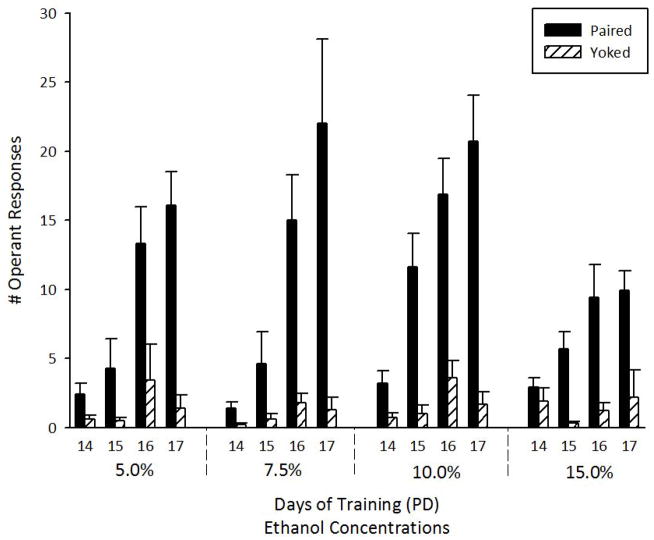
Results are depicted in Figure 1. A two-way analysis of variance (ANOVA) was employed to process nose-poke frequency during operant training sessions (PDs 14–17). The between-group factors were ethanol concentration (5.0, 7.5, 10.0, 15.0% or the yoked-control). Day of training (PDs 14–17) served as a within factor. Nose-poke frequency was significantly affected by each of the two factors under consideration (F(4, 75) = 31.95 and F(3, 225) = 53.57, both p’s < 0.001). The interaction of these two factors also achieved significance (F(12, 225) = 7.54, p < 0.001). Fisher post-hoc tests indicated that on PD16, Pd pups from all groups had a significantly higher frequency of nose-poking than the Yoked-collapsed group. Interestingly, on PD17, Pd pups reinforced with 7.5% or 10% ethanol had a significantly greater frequency of nose-poking than pups reinforced with 5 or 15% ethanol (the difference between Pd pups reinforced with 10% ethanol and Pd pups reinforced with 5% ethanol did not achieve significance, p = 0.09).
Figure 1.
Total number of responses during operant training (PDs 14–17), as a function of learning condition (paired or yoked) and reinforcers (5.0, 7.5, 10.0 or 15.0% Ethanol). Vertical lines represent standard errors of the mean.
To analyze consumption scores during training sessions, a preliminary ANOVA indicated no difference in body weight gains between experimental and yoked-controls across the different ethanol concentrations. In other words, ingestion of any specific fluid did not differ between experimental groups (paired vs. yoked). The subsequent statistical analyses focused on experimental animals, exclusively. A two-way ANOVA included reinforcer (5.0, 7.5, 10.0 and 15.0%) as a between factor and days of training (PDs 14, 15, 16 and 17) as a within factor. This ANOVA showed a significant main effect of reinforcer (F(3, 36) = 4.09, p < 0.025) and of days of training (F(3, 108) = 16.46, p < 0.001). The interaction between these factors had a borderline significance (F(9, 108) = 1.81, p = 0.07). Fisher post-hoc tests indicated that consumption of ethanol solutions increased significantly after PD15. Consumption of 10% ethanol was significantly higher when compared with the other solutions (comparison against 7.5% was not significant, p = 0.08). These results are summarized in Table 1.
TABLE 1.
Estimated ethanol intake (g/Kg) after each daily session of operant conditioning as a function of ethanol concentration (Experiment 1); mu or delta opioid receptor blockade (Experiment 3); or kappa opioid receptors blockade or agonism (Experiment 4).
| EXPERIMENT 1
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Reinforcer | PD 14 | PD 15 | PD 16 | PD 17 | ||||
| Paired | Yoked | Paired | Yoked | Paired | Yoked | Paired | Yoked | |
| 5.0% Ethanol | 0.005±0.003 | 0.003±0.002 | 0.014±0.009 | 0.018±0.014 | 0.055±0.014 | 0.057±0.018 | 0.057±0.019 | 0.047±0.016 |
| 7.5% Ethanol | 0.002±0.002 | 0.003±0.003 | 0.030±0.025 | 0.027±0.022 | 0.080±0.026 | 0.093±0.027 | 0.185±0.080 | 0.106±0.032 |
| 10.0% Ethanol | 0.018±0.018 | 0.018±0.012 | 0.068±0.030 | 0.085±0.031 | 0.171±0.034 | 0.122±0.031 | 0.245±0.063 | 0.167±0.049 |
| 15.0% Ethanol | 0.012±0.012 | 0.024±0.007 | 0.029±0.013 | 0.044±0.024 | 0.052±0.027 | 0.076±0.047 | 0.080±0.033 | 0.086±0.026 |
|
| ||||||||
|
EXPERIMENT 3
| ||||||||
| Opioid Antagonist (mg/Kg) | PD 14 | PD 15 | PD 16 | PD 17 | ||||
| Paired | Yoked | Paired | Yoked | Paired | Yoked | Paired | Yoked | |
| CTOP 0.1 | 0.000±0.000 | 0.000±0.000 | 0.031±0.017 | 0.019±0.013 | 0.059±0.038 | 0.067±0.030 | 0.051±0.038 | 0.070±0.038 |
| CTOP 1.0 | 0.005±0.004 | 0.004±0.002 | 0.029±0.010 | 0.026±0.019 | 0.117±0.028 | 0.098±0.038 | 0.186±0.066 | 0.189±0.072 |
| Naltrindole 1.0 | 0.014±0.009 | 0.014±0.008 | 0.027±0.018 | 0.039±0.017 | 0.066±0.027 | 0.071±0.033 | 0.069±0.022 | 0.056±0.025 |
| Naltrindole 5.0 | 0.004±0.003 | 0.004±0.003 | 0.017±0.009 | 0.063±0.047 | 0.058±0.022 | 0.025±0.010 | 0.040±0.013 | 0.016±0.007 |
| Saline | 0.009±0.007 | 0.021±0.016 | 0.035±0.016 | 0.029±0.013 | 0.081±0.033 | 0.060±0.027 | 0.126±0.063 | 0.157±0.068 |
|
| ||||||||
|
EXPERIMENT 4
| ||||||||
| Opioid Antagonist /Agonist (mg/Kg) | PD 14 | PD 15 | PD 16 | PD 17 | ||||
| Paired | Yoked | Paired | Yoked | Paired | Yoked | Paired | Yoked | |
| Nor-BNI 10.0 | 0.000±0.000 | 0.000±0.000 | 0.017±0.010 | 0.013±0.009 | 0.020±0.014 | 0.022±0.015 | 0.026±0.023 | 0.030±0.028 |
| Nor-BNI 30.0 | 0.000±0.000 | 0.000±0.000 | 0.030±0.015 | 0.009±0.007 | 0.028±0.017 | 0.032±0.023 | 0.066±0.027 | 0.102±0.037 |
| U62,066E 1.0 | 0.000±0.000 | 0.000±0.000 | 0.007±0.005 | 0.003±0.002 | 0.004±0.003 | 0.005±0.003 | 0.031±0.017 | 0.009±0.008 |
| U62,066E 5.0 | 0.000±0.000 | 0.000±0.000 | 0.002±0.002 | 0.009±0.007 | 0.018±0.018 | 0.025±0.025 | 0.003±0.003 | 0.006±0.006 |
| Saline | 0.000±0.000 | 0.000±0.000 | 0.031±0.021 | 0.015±0.015 | 0.044±0.018 | 0.063±0.021 | 0.102±0.039 | 0.069±0.027 |
The results of Experiment 1 indicate that the present experimental preparation, previously used only in Wistar rats [17, 19], is also suitable for analysis of operant conditioning in Sprague-Dawley infants. The ethanol-concentrated solutions used as reinforcers exerted differential effects on infants, reflected in nose-poking behavior and consumption scores with each concentration. Importantly, operant behavior of control (Y) animals did not differ across the different solutions used as reinforcer. Of major importance for the goal of this study was the observation that consumption scores of these animals are similar to those observed before in Wistar-derived infants [17], even when employing higher concentrations of the drug. In addition, these levels of ethanol self-administration are similar to those indicated in the literature during early ontogeny [14, 17, 53–55]. These results are summarized in Table 1.
4. Experiment 2
According to Experiment 1, 7.5 and 10% ethanol solutions were more effective than lower or higher concentrations in terms of both appetitive and consummatory behaviors. The present study was conducted to estimate blood ethanol concentrations (BECs) resulting from the levels of consumption observed in Experiment 1. The goal of this experiment was to determine whether detectable BECs would be achieved in the process of operant training. Infants were intraorally infused with 7.5 or 10.0% v/v ethanol using timing and amount of infusion that mirrored those of the Pd animals in Experiment 1, using a protocol applied previously to measure BECs in similar circumstances [14].
4.1 Methods
57 infants from 6 litters were employed. Groups were defined by the ethanol solution used as reinforcer (7.5 and 10.0% v/v) and the day of training (14–15, 14–16 or 14–17). Each group was composed of 9–10 infants. Pups underwent the same treatment as animals in Experiment 1. During each daily operant session pups were provided with infusions of 7.5 or 10.0% ethanol, mimicking the rate of administration of Pd infants from the previous experiment. Pups were sacrificed after completion of operant training at PD15, 16 or 17 (in other words, pups underwent 2, 3 or 4 operant training sessions) and trunk blood was obtained through decapitation. Samples were collected using a 1.5-ml Eppendorf tube and immediately centrifuged at high speed (6,000 rpm [roughly 2,200 g]; micro-Haematocrit Centrifuge, Hawksley & Sons LTD, Sussex, England). Serum was removed from the samples and stored at 70°C for later analysis. An AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA) was used to process blood ethanol contents. BECs calculations are made in terms of conversion rate of ethanol oxidation to acetaldehyde in the presence of ethanol oxidase. The apparatus measures the rate of oxygen required by this process, which is proportional to ethanol concentration, and produces a printout 20 to 30 seconds after the plasma is injected. All BEC values were expressed as milligrams of ethanol per deciliter of body fluid (mg dl or mg%).
4.2 Results
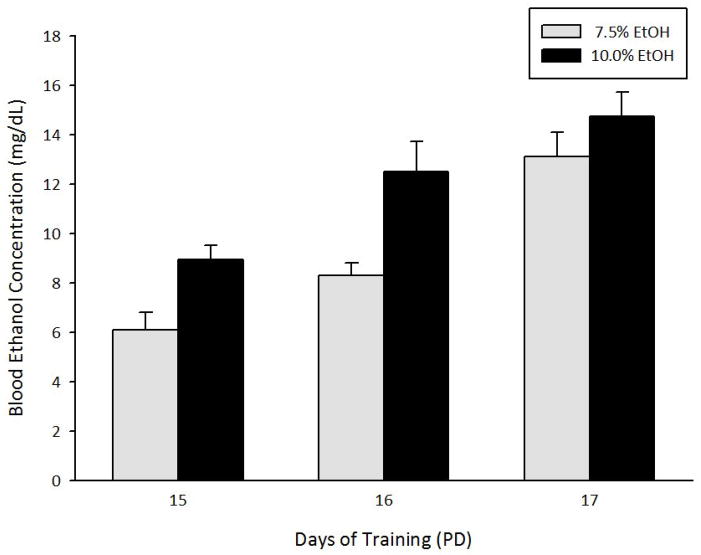
As indicated by the 2-way ANOVA (2 ethanol concentrations × 3 number of training sessions), BECs varied significantly as a function of solution and number of training sessions (F(1, 51) = 16.44, p < 0.001; F(2, 51) = 20.19, p < 0.001, respectively). BECs achieved by self-administration of 10% ethanol were significantly higher than those recorded with 7.5% ethanol. In addition, BECs significantly increased across the training sessions. These results are summarized in Figure 2.
Figure 2.
Blood ethanol concentration (mg/dL) achieved after two (PDs 14–15), three (PDs 14–16) or four (PDs 14–17) daily operant training sessions as a function of ethanol solutions used as reinforcers (7.5 or 10.0%). Vertical lines represent standard errors of the mean.
In summary, these results indicate that detectable and dose-dependent BECs are obtained immediately after completing a procedure similar to that employed in Experiment 1, in which 7.5 or 10% of ethanol was infused. These solutions were also those observed to exert positive reinforcing effects in the previous experiment. Of major importance, BECs obtained during PDs 16 and 17 (12 to 15 mg dl) coincide with those reported to exert reliable positive reinforcing effects when newborn rat pups are tested in terms of Pavlovian conditioning with ethanol as the US [53, 54] or operant responding for ethanol [14], or when infants (2–3 weeks old) are tested in operant conditioning [17].
5. Experiment 3
The present experiment assessed the involvement of the opioid system in performance of an operant task with 10% ethanol as the source of reinforcement. A recent study from this group [17] indicated that blockade of the opioid system attenuates operant behavior towards ethanol reinforcement. A non-selective opioid antagonist (naloxone) was employed in this study; however, the specific subtype of opioid receptor involved in this effect is unclear. In the present experiment, infant rats were injected with specific opioid antagonists of mu or delta opioid receptors (MOR and DOR, respectively) before operant training and extinction. These manipulations were intended to assess participation of these two opioid receptor systems in ethanol-related operant behavior.
5.1 Methods
A total of 120 infant rats were tested. Methodological and technical procedures were similar to those described in General Methods and Experiment 1. Operant training procedures occurred during PDs 14–17, using 10.0% v/v ethanol as reinforcer. An extinction session was added on PD18. On PDs 16, 17 and 18, thirty minutes prior to the operant training session, pups were weighed and i.p. injected with CTOP (0.1 or 1.0 mg/Kg), naltrindole (1.0 or 5.0 mg/Kg) or vehicle (saline, 0.9%). Following injection, pups were returned to the holding chambers where they remained until the operant training session took place.
5.2 Results
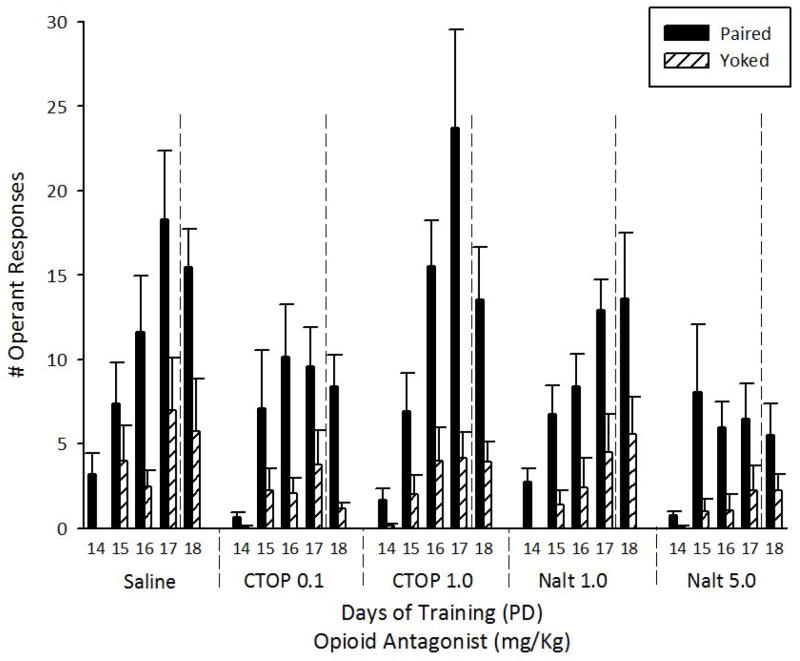
A two-way mixed ANOVA (6 opioid drug treatments × 4 training session days) indicated that nose-poking behavior significantly varied as a function of both drug treatment and training day (F(5, 114) = 15.21; F(3, 342) = 38.49, both p’s < 0.001, respectively). The drug treatment x training day interaction was also significant (F(15, 342) = 4.08, p < 0.001). The locus of the interaction was further analyzed with a Fisher post hoc test. On PD15 all pups given the contingency between nose-poking and ethanol delivery differed significantly from control Y pups; there was no significant difference among rats assigned to subsequent opioid antagonist groups during the two days before administration of the antagonist. On PD16, all the experimental groups, except NATL 5, displayed a significantly higher number of operant responses than Y-control animals. It is important to point out that the Y-control in this case is the total of all yoked conditions across the whole experiment. In addition, operant responsiveness among pups given NALT 5 was significantly lower than for those given CTOP 1 (p < 0.025); and tended to be lower than in the SAL control condition (p = 0.068). On PD17, the profile observed was quite similar, with highest responding in the SAL and CTOP 1 groups and no difference between the NALT 5 group and the Y-control. Only the SAL and CTOP 1 groups had greater responding than on the previous training day and both groups significantly differed from all other groups, but not from each other.
During extinction (PD18) operant behavior was similar to that observed during PD17: opioid antagonists differed in their effect (F(5, 114) = 7.95, p < 0.001); the NALT 5 group did not differ significantly from the Y-control (CTOP 0.1 vs. Y-control, p = 0.064); in addition, the highest numbers of responses during extinction were seen in the SAL, CTOP 1 and NALT 1 groups. These results are depicted in Figure 3.
Figure 3.
Total number of operant responses of experimental (Paired) and control (Yoked) animals towards a 10% ethanol solution, across training (PDs 14–17) and extinction sessions (PD 18) as a function of opioid antagonist injection during PDs16–18 (CTOP: 0.1 or 1.0 mg/Kg, Naltrindole: 1.0 or 5.0 mg/Kg or saline). Vertical lines represent standard errors of the mean.
Analysis of intake scores among Pd animals indicated only a significant main effect of training days (F(3, 165) = 11.77, p < 0.001). After PD 16, consumption scores of 10% ethanol significantly increased. Opioid antagonists did not significantly affect the consumption profile of the drug (p = 0.12). Results of consummatory tests are summarized in Table 1.
Overall, the results from Experiment 3 showed that both classes of opioid receptors, MOR and DOR, participated in mediating ethanol’s reinforcing effects through operant self-administration. The effects of DOR blockade seem to be dose dependent with greater effect of the higher dose, but surprisingly, only the lower dose of CTOP was effective in blocking operant behavior for ethanol reinforcement.
6. Experiment 4
Recent data established a role of the kappa opioid receptor (KOR) system in ethanol reinforcement during early ontogeny [56, 57]. In Experiment 4 the KOR system was manipulated through the use of agonists and antagonists. The question was whether this opioid sub-system is implicated in ethanol-mediated operant responding during infancy.
5.1 Methods
A total of 110 infant rats were tested. Methodological and technical procedures are described in General Methods and in Experiment 1. Operant training procedures occurred during PDs 14–17, using 10.0% v/v ethanol as reinforcer. On PD18 an extinction session was conducted. Thirty minutes prior to the operant training session on PDs 16, 17 and 18, pups were weighed and injected i.p. with the kappa agonist U62,066E (0.1 or 0.5 mg/Kg) or vehicle (saline 0.9%). Following injection, pups were returned to the holding chambers where they remained until operant training took place. Another group of animals was injected with the kappa antagonist nor-BNI (10.0 or 30.0 mg/Kg) 24 h before PD16 operant conditioning. These animals were injected only once with the antagonist. The rationale for the administration of nor-BNI was that detectable doses of nor-BNI have a slow but persistent onset of action. During the first hours after its administration nor-BNI does not selectively affect KOR differently than MOR and achieves its peak selective KOR antagonistic action approximately 24 h after administration [58]. This antagonist was injected only once because it has long lasting effects that persist for 72 h or more [59–61].
5.2 Results
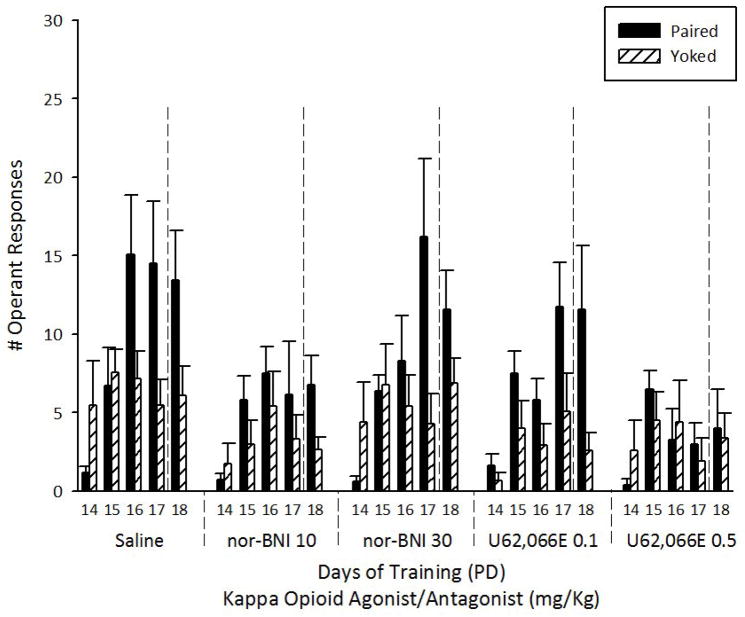
A two-way ANOVA was employed to compare nose-poke frequency during operant training (PDs 14–17). Between-group factors included dose of the of kappa opioid agonist (U62,066E 0.1 or 1.0 mg/Kg) or antagonist (nor-BNI 10 or 30 mg/Kg) plus the Y-control group. Day of training (PDs 14–17) served as a within factor. As Figure 4 shows, nose-poke frequency was significantly affected by the two factors under consideration [F(5, 104) = 3.62, p < 0.01 and F(3, 312) = 24.36, p < 0.001, respectively]. The interaction of these factors also achieved significance (F(15, 312) = 3.92, p < 0.001). Fisher LSD post-hoc tests indicated that, at PD 16, SAL experimental pups executed a significantly higher number of operant responses than all remaining groups. In addition, SAL was the only group that differed significantly from the Y-control pups. At PD17, SAL, nor-BNI 10 and U62,066E 0.1 significantly differed from the Y-control.
Figure 4.
Total number of operant responses of experimental (Paired) and control (Yoked) animals towards a 10% ethanol solution, across training (PDs 14–17) and extinction sessions (PD 18) as a function of kappa opioid agonist (U62,066E: 1.0 or 5.0 mg/Kg), antagonist (nor-BNI: 10.0 or 30.0 mg/Kg) or vehicle (saline) injection during PDs16–18. Vertical lines represent standard errors of the mean.
The ANOVA employed for analyzing operant seeking behavior, during extinction, indicated that both kappa opioid antagonist and agonist significantly affected nose-poking (F(5, 104) = 4.85, p < 0.001). SAL, nor-BNI 10 and U62,066E 0.1 groups had the highest numbers of responses during extinction and significantly differed from the Y-control group (all p’s < 0.01). U62,066E 0.5 and nor-BNI 10 did not differ from controls.
Statistical analyses of consumption scores focused on experimental animals. A two-way mixed ANOVA included kappa opioid agonist/antagonist as a between factor and day of training (PDs 15, 16 and 17) as a within factor. The PD 14 data were not included in this analysis because no significant change in body weight, as a function on ethanol consumption, was observed. The ANOVA showed significant main effects of both drug and day [F(4, 50) = 2.93, p < 0.05; and F(2, 100) = 4.09, p < 0.025, respectively]. The Fisher LSD post-hoc test indicated that pups injected with saline during PDs 16–18 consumed significantly more ethanol than animals injected with nor-BNI 10 (p < 0.05), U62,066E 0.1 (p < 0.025) or U62,066E 0.5 mg/Kg (p < 0.01). In other words, both the kappa agonist and antagonist (in the lower dose) decreased ethanol intake. Consumption of ethanol significantly increased after PD15 and, on PD17, consumption of the drug was significantly higher than before. Results are summarized in Table 1.
In summary, activation of kappa receptors by U62,066E during operant training/extinction produced a dose-dependent decrement in the rate of responding for ethanol. When KORs were blocked by nor-BNI there was a similar reduction in operant responding, although only with the low dose of nor-BNI. The higher dose of this KOR antagonist seemed to have no effect on operant responding, at least in terms of comparison with the saline control group. However, starting on PD17 and continuing into extinction on PD18 both the nor-BNI 30 and the U62,066E 0.1 paired subjects had greater responding for ethanol than their yoked controls.
6. Experiment 5
It is known that ethanol administration promotes changes in activity, density, or affinity of specific classes of opioid receptors in distinct regions of the brain. These ethanol-induced changes may be important in mediating some of the neurobehavioral effects of ethanol (including ethanol reinforcement) and may play a role in controlling ethanol consumption [22]. Experiment 3 indicated that both MOR and DOR are involved in ethanol-mediated operant responding during infancy. Both the lower dose of CTOP and the higher dose of naltrindole seemed to block the increment in operant behavior observed in the saline group. Nonetheless there exists the possibility that these effects could be due to other nonspecific opioid-antagonist-induced effects, such as depression in locomotor activity or alteration in the taste value of ethanol. To assess these alternative explanations, four control experiments were conducted. First, we tested sensitivity to changes in locomotor activity induced by CTOP and naltrindole. In the second control experiment, locomotor sensitivity to nor-BNI and U62,066E was tested. In the third and fourth control experiments motivational properties of the present doses of these opioid drugs were tested in terms of conditioned taste aversion (CTA). In the first of these, CTOP and naltrindole were used as the unconditional stimulus (US) and, in the second, nor-BNI and U62,066E were employed as the US. In both CTA experiments, a low concentration of saccharin solution was employed as the conditional stimulus (CS).
6.1 Methods
6.1.1 Effects of CTOP and naltrindole on Locomotor Activity
Forty-eight infant rats were tested. On PD 16, pups were separated from the mother and placed in pairs in a holding cage where they remained for 2.5 h. After that time, their body weights were individually recorded (±0.01 g) and the rats were given an i.p. administration of CTOP (0.1 or 1.0 mg/Kg), naltrindole (1.0 or 5.0 mg/Kg) or saline. After the injection, pups were placed again in pairs in the holding cage. Thirty minutes later, locomotor activity was evaluated for 15 min (See Section 2.6).
6.1.2 Effects of Nor-BNI and U62,066E on Locomotor Activity
A second locomotor activity test evaluated the effects of an agonist and antagonist of KOR on motor activity. For this experiment 48 infant rats were tested. The experiment was similar to the previous experiment except that 30 minutes before testing, animals were given an i.p. injection of U62,066E (0.1 or 0.5 mg/Kg) or saline. Other animals were given a nor-BNI injection (10.0 or 30.0 mg/Kg) 24 h before their test on PD16. The intention was to mimic the drug treatments tested in the operant conditioning experiment.
6.1.3 Conditioned Taste Aversion mediated by Unconditional effects of CTOP and/or naltrindole
Sixteen-day-old pups were tested for CTA (See Section 2.7). Immediately following the intraoral infusion of saccharin, pups were disconnected from the tubing, weighed to estimate saccharin consumption scores and i.p. injected with one of the following: CTOP (0.1 or 1.0 mg/Kg), naltrindole (1.0 or 5.0 mg/Kg) or saline. One hour post injection, pups were returned to their dams. On PD17 (test day), subjects were treated as on PD16 except that no injection was given after the intake test.
6.1.4 Conditioned Taste Aversion mediated by Unconditional effects of nor-BNI or U62,066E
A CTA test was conducted to evaluate motivational effects of the kappa agonist (U62,066E) and the kappa antagonist (nor-BNI). The experiment was similar to the one described before (Section 6.1.3) except that immediately after saccharin ingestion, animals were given an i.p. injection of U62,066E (0.1 or 0.5 mg/Kg), nor-BNI (10.0 or 30.0 mg/Kg) or saline. The administration time of nor-BNI was different from that used for operant conditioning. The rationale of changing the injection time in this paradigm was that the only way to test its effects as a US was to inject the drug after the CS, and it was important to ensure that the animal first perceived the CS and later the US.
6.2 Results
6.2.1 Locomotor Activity Assessment
A one-way ANOVA indicated that CTOP and naltrindole did not affect the locomotor activity of the infants. A similar result was obtained when pups were tested after injection of nor-BNI or U62,066E (see Table 2).
TABLE 2.
Effects of opioid drugs (CTOP, Naltrindole, nor-BNI or U62,066E) on locomotor activity (second column) or conditioned saccharin intake (third column).
| EXPERIMENT 6 - LOCOMOTOR ACTIVITY AND CTA
| ||
|---|---|---|
| Opioid Antagonist (mg/Kg) | Distance Travelled (cm) | Saccharin Intake (%BWG) |
| CTOP 0.1 | 1490±304 | 1.72±0.11 |
| CTOP 1.0 | 1278±206 | 1.65±0.18 |
| Naltrindole 1.0 | 1191±205 | 1.50±0.06 |
| Naltrindole 5.0 | 824±143 | 1.46±0.18 |
| Saline | 1151±329 | 1.47±0.13 |
| Nor-BNI 10.0 | 945±194 | 1.87±0.05 |
| Nor-BNI 30.0 | 982±167 | 1.59±0.19 |
| U62,066E 1.0 | 1395±321 | 1.59±0.15 |
| U62,066E 5.0 | 1435±265 | 1.48±0.21 |
| Saline | 1172±210 | 1.92±0.14 |
These results indicate that, at least for the procedures and doses of opioid agonist and antagonist used in this study, changes observed in operant behavior for ethanol as a result of these drugs cannot be explained as a drug-induced alteration in motor reactivity, but are due to effects of these opioid drugs on neural circuits that underlie ethanol reinforcement.
6.2.2 Conditioned Taste Aversion
Percentage of body weight gained during the testing day (PD 17) was analyzed through a one-way ANOVA of an index of saccharin intake. In this analysis, neither the opioid receptor antagonists nor the agonist (CTOP, naltrindole, nor-BNI and U62,066E) significantly affected consumption of saccharin on test day (see Table 2).
These results suggest that the association between infusions of saccharin and the present opioid drugs does not modify later consumption of saccharin. Moreover, extrapolation of these results to those obtained in operant conditioning indicate that the decrease in operant responding after injection of any of the opioid drugs cannot be explained in terms of an unconditional effect of the opioid antagonists or agonist on ingestion.
7. Discussion
Results of the present experiments are in concordance with previous evidence that ethanol reinforcement in infant rats is mediated, at least in part, by the opioid system [16, 17, 26, 62]. The first two experiments of this study indicated that Sprague-Dawley infants rapidly learn an operant response to obtain ethanol. An interesting point derived from Experiment 1 is that concentrations of ethanol higher than the 3.75% concentration previously employed [17, 19] were effective in eliciting operant behavior in this rat strain (Sprague-Dawley). Even though numbers of operant responses in the present study were considerably lower than those executed by Wistar-derived infants [17, 19], ethanol consumption as a function of operant responsiveness was very similar in both strains.
Some strain-dependent differences have been observed between Wistar and Sprague-Dawley rats. Wistar pups have lower neonatal body weights than do the Sprague-Dawley rats; there seems also to be an apparent interaction between strain and effect of alcohol on body weight: Wistar but not Sprague-Dawley newborns had significantly lower body weights when they were prenatally exposed to alcohol than did those whose dams were treated with only water during gestation [40]. Some studies suggest that these genetically distinct rat strains can show differential sensitivity to opioids, more specifically to drug-seeking responses [63]. A recent work has shown that Wistar and Sprague-Dayley rats present differences in sensitivity to mu opioid receptor-mediated anti-nociception determined by cross-regulation between MOR and DOR [64]. In terms of operant behavior, ethanol-mediated reinforcement has been established in neonates of both rat strains [14, 15]. In Wistar rats, neonatal ethanol-mediated operant behavior only could be observed after prenatal exposure to the drug. In addition with this, relatively high ethanol concentrations seemed to exert different motivational effects in neonates of both strain. While Sprague-Dawley neonates displayed a reliable operant behavior towards 5.0 and 7.5% ethanol [14]; Wistar-derived pups seemed to show no differential response to a 6% ethanol solution, when compared to control subjects [15]. This result may be indicating that Sprague-Dawley strain could be more sensitive to higher ethanol concentrations [51].
Blood ethanol levels were not higher than 18 mg% as a function of ethanol self-administration (Experiment 2). Similar BELs have previously been found to exert pharmacologically significant reinforcing effects during early development. Indeed, preweanlings appear to be highly sensitive to the positive reinforcing effects of such low doses of ethanol [14, 17, 29, 53–55]. Studies conducted to analyze ethanol ingestion or sensitivity to the drug’s motivational effects during early ontogeny suggest that rapid accumulation of ethanol in blood or brain is conducive to high levels of ethanol ingestion or preference for stimuli that predict ethanol’s postabsorptive effects (e.g., see [13, 51, 53, 65, 66]). In other words, and in accordance with recent studies conducted in adult rats [67–69], it appears that acceptance of ethanol requires a balance between the sensory and postabsorptive effects of the drug.
A subsequent experiment (Experiment 3) tested participation of mu and delta opioid receptor (MOR and DOR) systems in operant behavior elicited by ethanol reinforcement. For this test, two different doses of specific MOR and DOR antagonists were injected just prior to operant training (PD 16 and 17) or extinction (PD 18) sessions. Data indicated that both receptor sub-systems mediate ethanol ingestion and nose-poking for ethanol. A clear reduction in operant responsiveness was evident for pups injected with the lower dose of CTOP or with NALT (in particular, the higher dose of NALT). These results are supported by evidence in adult rats: intra-cerebroventricular injections of both kinds of opioid antagonists (CTOP and naltrindole) were effective in suppressing ethanol responding in alcohol-preferring and heterogeneous strains of adult rats [34]. Arias et al. [31] recently found that locomotor activation induced by ethanol in two-week old rats is specifically modulated by MOR but not by DOR [31]. MOR as well as DOR are also implicated in ethanol-mediated conditioned place preference at about the same age [62]. MOR and DOR also regulate ethanol intake, but they seem to be implicated in regulating consummatory behavior in general during the preweanling period, since blockade of either of these receptors also suppressed acceptance of water and saccharin [31]. Hence, it seems that there is a partial overlap of neurochemical mechanisms involved in the appetitive, consummatory and stimulating effects of ethanol in preweanling rats. β-endorphin and the enkephalins are the endogenous ligands with the highest affinity for the MOR and DOR [70, 71]. In adults both endogenous peptides function as positive reinforcers and their release is increased after ethanol consumption [22]. The present experiments show that in infant rats, blocking receptors associated with opioid ligands for mu and delta attenuated operant responding, i.e., reduced ethanol reinforcement.
The next step was to evaluate the involvement of KOR by variation in dose of an antagonist and an agonist of these receptors (Experiment 4). When ethanol reinforcement was evaluated under the effects of a kappa agonist, a clear reduction of operant reinforcement was observed, in particular when employing the higher dose of U62,066E: pups injected with a lower dose of the KOR antagonist nor-BNI showed a reduction in responding for ethanol, but those given the higher dose of nor-BNI seemed unaffected.
The results with kappa opioid antagonists may seem puzzling at first glance. The kappa system is involved in many aspects of animal behavior, one being response to stress. Kappa activation is a stress-inducing event and blockade of the system has an anxiolytic effect (for review see [72]). One possible explanation for the set of results seen with nor-BNI is that increased operant responding for ethanol in the current preparation is partly due to the negative reinforcing (anxiolytic) properties of ethanol. Administration of the low dose of nor-BNI prior to testing would reduce the stress associated with the procedure and thus reduce responding for ethanol. On the other hand the higher dose of nor-BNI delayed expression of increased responding but eventually (one day later) produced clear appetitive reinforcement. In a recent study with preweanling rats, blockade of kappa function facilitated the expression of appetitive ethanol reinforcement in terms of tactile and taste conditioning, whereas kappa opioid activation potentiated ethanol’s motor-depressing effects [56]. Therefore, one explanation for the increased responding on PD17 in the group treated with the high dose of nor-BNI is increased appetitive reinforcement to ethanol’s pharmacological effects. The delay in increased responding would be expected given that Mitchell et al. [73] found an increase in ethanol self-administration by adult non-dependent rats four days after the administration of nor-BNI, with a dose (10 mg/Kg) similar to that used in the present study [73]. It should be noted, however, that in adult animals that are not ethanol-dependent, antagonists for KOR generally have had no effect on ethanol operant self-administration [74–77]. On the other hand, in ethanol-dependent animals, blocking KOR with nor-BNI reduces operant ethanol self-administration [76, 77].
The effects of kappa agonists in the present study can be explained more readily. It is known from older studies that KOR agonists can suppress ethanol intake in adults [36]. The fact that, in some cases, KOR agonists were found to be aversive [78–80] makes it difficult to determine if their effects on ethanol reinforcement can be separated from their direct aversive effects. Previous research suggests that endogenous dynorphin, the endogenous ligand for KOR, offers protection against drug abuse [81]. Enhancing the action of dynorphin with an agonist should therefore reduce the reinforcing value of ethanol. Collectively, activation of the kappa opioid system may have a general suppressant effect on ethanol reinforcement in nondependent animals (for a review, please see [82]). The controversial action of the kappa opioid sub-system remains to be completely understood for ethanol reinforcement in developing rats. One weakness of studies that employ long-acting KOR antagonists or agonists is that the organism is under the effects of these drugs for several hours, even days, after the injection. Despite this observation, indirect measures such as daily body weight have not been affected by these drugs.
Separate experiments were conducted in the present study to control the possibility that i.p. injections of antagonist/agonist opioid receptors exerted unconditional effects capable of masking ethanol reinforcement. In all cases, we failed to observe changes in locomotor responses elicited by any of these substances. Similarly, ingestion in general was not affected by any of the doses of MOR, DOR and KOR antagonists or the KOR agonist tested.
An interesting point that emerges from this set of results is that, as was the case of previous experiments [17], manipulation of the opioid system during extinction, when no ethanol was present, resulted in a profile of responding similar to that seen for operant behavior during test sessions, where ethanol was delivered.
The present study provides new evidence for the contribution of mu, delta, and kappa opioid receptors in ethanol-mediated operant behavior by developing rats. Overall we are able to conclude that, even though some particular aspects of each opioid sub-system require further testing, a fully functional opioid system is needed to promote ethanol reinforcement during the second postnatal week. Disruption by either a nonselective (i.e. naloxone) or specific opioid antagonist (mu, delta, kappa) or agonist (kappa) is sufficient for substantial reduction in consummatory and seeking behaviors associated with ethanol reinforcement.
As a whole, animal preclinical research brings information supporting the notion that early ontogeny is a vulnerable window in terms of increasing the likelihood of later risk of use and abuse of the drug. Exposure to alcohol in the womb ranks among the strongest predictors of alcohol consumption during adolescence and early adulthood [1, 2, 83, 84]. Animal models )[4, 85] also indicate heightened ethanol consumption following early pre- or postnatal ethanol exposure. In this sense, and according with Pautassi et al.[7], rat infancy is a developmental period of high sensitivity to ethanol’s positive and negative reinforcing (anti-anxiety) effects. Studies in this field provide substantial support for this assertion and provide theoretical and methodological basis for researchers interested in analyzing the neurobiology of conditioned reinforcement and ethanol intake, as well as, increase the body of knowledge in terms of human health and prevention on these issues.
Highlights.
Infant rats rapidly learn an operant task when receiving ethanol as reiforcer.
Opioid system is involved in ethanol reinforcement during infancy.
Mu, delta and kappa receptors modulate operant response to the drug.
Acknowledgments
This work was supported by the U.S. National Institute on Alcohol Abuse and Alcoholism (AA11960, AA015992, and AA013098, N.E.S.), the U.S. National Institute of Mental Health (MH035219, N.E.S), Secretaría de Ciencia y Tecnología (SECyT, P.A.), Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 0923- CONICET, P.A.), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT-FONCyT, P.A.) and a fellowship from CONICET (to R.S.M.M.). R.S.M.M. is a student of the Ph.D. program of Doctorado en Ciencias Biológicas (F.C.E.F. y N. – U.N.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. Bibliography
- 1.Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the ethiology of adolescent alcohol problems. J Stud Alcohol. 1998;59:533–43. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- 2.Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Archives of general psychiatry. 2003;60:377–85h. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- 3.Chotro MG, Arias C, Laviola G. Increased ethanol intake after prenatal ethanol exposure: studies with animals. Neuroscience and biobehavioral reviews. 2007;31:181–91. doi: 10.1016/j.neubiorev.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Spear NE, Molina JC. Fetal or infantil exposure to alcohol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;25:909–29. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- 5.Alati R, Al Mamun A, Williams GM, O’Callaghan M, Najman JM, Bor W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: A birth cohort study. Archives of general psychiatry. 2006;63:1009–16. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- 6.Alati R, Clavarino A, Najman JM, O’Callaghan M, Bor W, Mamun AA, et al. The developmental origin of adolescent alcohol use: findings from the Mater University Study of Pregnancy and its outcomes. Drug and alcohol dependence. 2008;98:136–43. doi: 10.1016/j.drugalcdep.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Pautassi RM, Nizhnikov ME, Spear NE. Assessing appetitive, aversive, and negative ethanol-mediated reinforcement through an immature rat model. Neuroscience and biobehavioral reviews. 2009;33:953–74. doi: 10.1016/j.neubiorev.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abate P, Pueta M, Spear NE, Molina JC. Fetal learning about ethanol and later ethanol responsiveness: evidence against “safe” amounts of prenatal exposure. Exp Biol Med. 2008;233:139–54. doi: 10.3181/0703-MR-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham CL, Niehus JS, Noble D. Species difference in sensitivity to ethanol’s hedonic effects. Alcohol. 1993;10:97–102. doi: 10.1016/0741-8329(93)90087-5. [DOI] [PubMed] [Google Scholar]
- 10.Kozlov AP, Varlinskaya EI, Spear NE. Ethanol, sacharine, and quinine: early ontogeny of taste responsiveness and intake. Alcohol Clin Exp Res. 2008;32:294–305. doi: 10.1111/j.1530-0277.2007.00581.x. [DOI] [PubMed] [Google Scholar]
- 11.Arias C, Mlewski EC, Miller S, Molina JC, Spear NE. Novelty modulates the stimulating motor effects of ethanol in preweanling rats. Pharmacol Biochem Behav. 2009;92:448–56. doi: 10.1016/j.pbb.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arias C, Mlewski EC, Molina JC, Spear NE. Ethanol induces locomotor activating effects in preweanling Sprague-Dawley rats. Alcohol. 2009;43:13–23. doi: 10.1016/j.alcohol.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina JC, Pautassi RM, Truxell E, Spear N. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41:41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bordner KA, Molina JC, Spear NE. Analysis of ethanol reinforcement in 1-day-old rats: assessment through a brief and novel operant procedure. Alcohol Clin Exp Res. 2008;32:1–13. doi: 10.1111/j.1530-0277.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- 15.March SM, Abate P, Spear NE, Molina JC. Fetal exposure to moderate ethanol doses: heightened operant responsiveness elicited by ethanol-related reinforcers. Alcohol Clin Exp Res. 2009;33:1981–93. doi: 10.1111/j.1530-0277.2009.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miranda-Morales RS, Molina JC, Spear NE, Abate P. Participation of the endogenous opioid system in the acquisition of a prenatal ethanol-related memory: effects on neonatal and preweanling responsiveness to ethanol. Physiol Behav. 2010;101:153–60. doi: 10.1016/j.physbeh.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miranda-Morales RS, Molina JC, Spear NE, Abate P. Naloxone attenuation of ethanol-reinforced operant responding in infant rats in a re-exposure paradigm. Psychopharmacology (Berl) 2012;219:235–46. doi: 10.1007/s00213-011-2402-5. [DOI] [PubMed] [Google Scholar]
- 18.Pautassi RM, Truxell E, Molina JC, Spear NE. Motivational effects of intraorally-infused ethanol in rat pups in an operant self-administration task. Physiol Behav. 2008;93:118–29. doi: 10.1016/j.physbeh.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponce LF, Pautassi RM, Spear NE, Molina JC. Ethanol-mediated operant learning in the infant rat leads to increased ethanol intake during adolescence. Pharmacol Biochem Behav. 2008;90:640–50. doi: 10.1016/j.pbb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.New DC, Wong YH. The ORL1 receptor: molecular pharmacology and signalling mechanisms. Neuro-Signals. 2002;11:197–212. doi: 10.1159/000065432. [DOI] [PubMed] [Google Scholar]
- 21.Froehlich JC. Opioid peptides. Alcohol health and research world. 1997;21:132–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2009;9:999–1015. doi: 10.2174/156802609789630956. [DOI] [PubMed] [Google Scholar]
- 23.Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:9981–6. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duzzioni M, Duarte FS, Leme LR, Gavioli EC, De Lima TC. Anxiolytic-like effect of central administration of NOP receptor antagonist UFP-101 in rats submitted to the elevated T-maze. Behav Brain Res. 2011;222:206–11. doi: 10.1016/j.bbr.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 25.Sakoori K, Murphy NP. Endogenous nociceptin (orphanin FQ) suppresses basal hedonic state and acute reward responses to methamphetamine and ethanol, but facilitates chronic responses. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:877–91. doi: 10.1038/sj.npp.1301459. [DOI] [PubMed] [Google Scholar]
- 26.Arias C, Chotro MG. Increased palatability of ethanol after prenatal ethanol exposure is mediated by the opioid system. Pharmacol Biochem Behav. 2005;29:337–46. doi: 10.1016/j.pbb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Chotro MG, Arias C. Prenatal exposure to ethanol increases ethanol consumption: a condiitoned response? Alcohol. 2003;30:19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- 28.Chotro MG, Arias C. Ontogenetic difference in ethanol reinforcing properties: the role of the opioid system. Behavioural Pharmacology. 2007;18:661–7. doi: 10.1097/FBP.0b013e3282f00754. [DOI] [PubMed] [Google Scholar]
- 29.Nizhnikov ME, Varlinskaya EI, Petrov ES, Spear NE. Reinforcing properties of ethanol in neonatal rats: involvement of the opioid system. Behav Neurosci. 2006;120:267–80. doi: 10.1037/0735-7044.120.2.267. [DOI] [PubMed] [Google Scholar]
- 30.Nizhnikov ME, Pautassi RM, Truxell EM, Spear NE. Opioid antagonists block the acquisition of ethanol-mediated conditioned tactile preference in infant rats. Alcohol. 2009;43:347–58. doi: 10.1016/j.alcohol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arias C, Molina JC, Spear NE. Differential role of micro, delta and kappa opioid receptors in ethanol-mediated locomotor activation and ethanol intake in preweanling rats. Physiol Behav. 2010;99:348–54. doi: 10.1016/j.physbeh.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallmark RA, Hunt PS. Social learning about ethanol in preweanling rats: role of endogenous opioids. Dev Psychobiol. 2004;44:132–9. doi: 10.1002/dev.10163. [DOI] [PubMed] [Google Scholar]
- 33.de Waele JP, Gianoulakis C. Effects of single and repeated exposures to ethanol on hypothalamic beta-endorphin and CRH release by the C57BL/6 and DBA/2 strains of mice. Neuroendocrinology. 1993;57:700–9. doi: 10.1159/000126428. [DOI] [PubMed] [Google Scholar]
- 34.Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan–Sarin S, Portoghese PS, Li TK, Froelich JC. The delta 2-opioid receptor antagonist naltriben selectively attenuates alcohol intake in rats bred for alcohol preference. Pharmacol Biochem Behav. 1995;52:153–9. doi: 10.1016/0091-3057(95)00080-g. [DOI] [PubMed] [Google Scholar]
- 36.Lindholm S, Werme M, Brene S, Franck J. The selective kappa-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav Brain Res. 2001;120:137–46. doi: 10.1016/s0166-4328(00)00368-5. [DOI] [PubMed] [Google Scholar]
- 37.Pastor R, Aragon CM. The role of opioid receptor subtypes in the development of behavioral sensitization to ethanol. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:1489–99. doi: 10.1038/sj.npp.1300928. [DOI] [PubMed] [Google Scholar]
- 38.Pastor R, Sanchis-Segura C, Aragon CM. Effect of selective antagonism of mu(1)-, mu(1/2)-, mu(3)-, and delta-opioid receptors on the locomotor-stimulating actions of ethanol. Drug and alcohol dependence. 2005;78:289–95. doi: 10.1016/j.drugalcdep.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Volterra A, Brunello N, Restani P, Galli CL, Racagni G. Ontogenetic studies on mu, delta and kappa opioid receptors in rat brain. Pharmacological research communications. 1986;18:979–90. doi: 10.1016/0031-6989(86)90100-1. [DOI] [PubMed] [Google Scholar]
- 40.Abate P, Varlinkaya EI, Cheslock SJ, Spear NE, Molina JC. Neonatal activation of alcohol-related prenatal memories: impact on the first suckling response. Alcohol Clin Exp Res. 2002;26:1512–22. doi: 10.1097/01.ALC.0000034668.93601.8F. [DOI] [PubMed] [Google Scholar]
- 41.National Research Council. Guide for the care and use of laboratory animals. 8. Washington, DC: National Academy Press; 2010. [Google Scholar]
- 42.Spear NE, Specht SM, Kirstein CL, Kuhn CM. Anterior and posterior, but not cheek, intraoral cannulation procedures elevate serum corticosterone levels in neonatal rat pups. Dev Psychobiol. 1989;22:401–12. doi: 10.1002/dev.420220407. [DOI] [PubMed] [Google Scholar]
- 43.Arias C, Chotro MG. Increased preference for ethanol in the infant rat after prenatal ethanol exposure, expressed on intake and taste reactivity tests. Alcohol Clin Exp Res. 2005;29:337–46. doi: 10.1097/01.alc.0000156115.35817.21. [DOI] [PubMed] [Google Scholar]
- 44.Pautassi RM, Godoy JC, Spear NE, Molina JC. Early responsiveness to stimuli paired with different stages within the state of alcohol intoxication. Alcohol Clin Exp Res. 2002;26:644–54. [PubMed] [Google Scholar]
- 45.Domínguez HD, Chotro MG, Molina JC. Alcohol in the amniotic fluid prior to cesarean delivery: effects of subsequent exposure to the drug’s odor upon alcohol responsiveness. Behav Neural Biol. 1993;60:129–38. doi: 10.1016/0163-1047(93)90229-b. [DOI] [PubMed] [Google Scholar]
- 46.Domínguez HD, López MF, Molina JC. Neonatal responsiveness to alcohol odor and infant alcohol intake as a function of alcohol experience during late gestation. Alcohol. 1998;16:109–17. doi: 10.1016/s0741-8329(97)00169-9. [DOI] [PubMed] [Google Scholar]
- 47.Pepino MY, Kraebel KS, López MF, Spear NE, Molina JC. Behavioral detection of low concentrations of ethanol in the preweanling rat. Alcohol. 1998;15:337–53. doi: 10.1016/s0741-8329(97)00154-7. [DOI] [PubMed] [Google Scholar]
- 48.Pepino MY, López MF, Spear NE, Molina JC. Infant rats respond differently to alcohol after nursing from an alcohol-intoxicated dam. Alcohol. 1999;18:189–201. doi: 10.1016/s0741-8329(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 49.Arias C, Chotro MG. Ethanol-induced preferences or aversions as a function of age in preweanlings rats. Behav Neurosci. 2006;120:710–8. doi: 10.1037/0735-7044.120.3.710. [DOI] [PubMed] [Google Scholar]
- 50.Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- 51.Sanders S, Spear NE. Ethanol acceptance is high during early infancy and becomes still higher after previous ethanol ingestion. Alcohol Clin Exp Res. 2007;31:1148–58. doi: 10.1111/j.1530-0277.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- 52.Truxell E, Spear NE. Immediate acceptance of ethanol in infant rats: ontogenetic differences with moderate but not high ethanol concentration. Alcohol Clin Exp Res. 2004;28:1200–11. doi: 10.1097/01.alc.0000134220.34842.18. [DOI] [PubMed] [Google Scholar]
- 53.Nizhnikov ME, Molina JC, Varlinskaya EI, Spear NE. Prenatal ethanol exposure increases ethanol reinforcement in neonatal rats. Alcohol Clin Exp Res. 2006;30:34–45. doi: 10.1111/j.1530-0277.2006.00009.x. [DOI] [PubMed] [Google Scholar]
- 54.Petrov ES, Varlinskaya EI, Spear NE. Reinforcement from pharmacological effects of ethanol in newborn rats. Alcoho Clin Exp Res. 2003;27:1583–91. doi: 10.1097/01.ALC.0000089960.62640.58. [DOI] [PubMed] [Google Scholar]
- 55.Cheslock SJ, Varlinskaya EI, Petrov ES, Silveri MM, Spear LP, Spear NE. Ethanol as a reinforcer in the newborn’s first suckling experience. Alcohol Clin Exp Res. 2001;25:391–402. [PubMed] [Google Scholar]
- 56.Pautassi RM, Nizhnikov ME, Acevedo MB, Spear NE. Early role of the kappa opioid receptor in ethanol-induced reinforcement. Physiol Behav. 2012;105:1231–41. doi: 10.1016/j.physbeh.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nizhnikov ME, Varlinskaya EI, Spear NE. The Central Reinforcing Properties of Ethanol Are Mediated by Endogenous Opioid Systems: Effects of Mu and Kappa Opioid Antagonists. Revista Argentina de ciencias del comportamiento. 2009;1:1–12. [PMC free article] [PubMed] [Google Scholar]
- 58.Metcalf MD, Coop A. Kappa opioid antagonists: past successes and future prospects. The AAPS journal. 2005;7:E704–22. doi: 10.1208/aapsj070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jewett DC, Woods JH. Nor-binaltorphimine: an ultra-long acting kappa-opioid antagonist in pigeons. Behav Pharmacol. 1995;6:815–20. [PubMed] [Google Scholar]
- 60.Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 1994;115:311–9. doi: 10.1007/BF02245071. [DOI] [PubMed] [Google Scholar]
- 61.Picker MJ, Mathewson C, Allen RM. Opioids and rate of positively reinforced behavior: III. Antagonism by the long-lasting kappa antagonist norbinaltorphimine. Behav Pharmacol. 1996;7:495–504. [PubMed] [Google Scholar]
- 62.Nizhnikov ME, Pautassi RM, Truxell E, Spear NE. Opioid antagonists block the acquisition of ethanol-mediated conditioned tactile preference in infant rats. Alcohol. 2009;43:347–58. doi: 10.1016/j.alcohol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shoaib M, Spanagel R, Stohr T, Shippenberg TS. Strain differences in the rewarding and dopamine-releasing effects of morphine in rats. Psychopharmacology (Berl) 1995;117:240–7. doi: 10.1007/BF02245193. [DOI] [PubMed] [Google Scholar]
- 64.Ballesta JJ, Cremades J, Rodriguez-Munoz M, Garzon J, Faura CC. Sensitivity to mu-opioid receptor-mediated anti-nociception is determined by cross-regulation between mu- and delta-opioid receptors at supraspinal level. Br J Pharmacol. 2012;166:309–26. doi: 10.1111/j.1476-5381.2011.01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Molina JC, Ponce LF, Truxel E, Spear NE. Infantile sensitivity to ethanol’s motivational effects: Ethanol reinforcement during the third postnatal week. Alcoho Clin Exp Res. 2006;30:1506–19. doi: 10.1111/j.1530-0277.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 66.Pautassi RM, Molina JC, Spear N. Infant rats exhibit aversive learning mediated by ethanol’s orosensory effects but are positively reinforced by ethanol’s post-ingestive effects. Pharmacol Biochem Behav. 2008;88:393–402. doi: 10.1016/j.pbb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fidler TL, Clews TW, Cunningham CL. Reestablishing an intragastric ethanol self-infusion model in rats. Alcohol Clin Exp Res. 2006;30:414–28. doi: 10.1111/j.1530-0277.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- 68.Kiefer SW, Hill KG, Coonfield DL, Ferraro FM., 3rd Ethanol familiarity and naltrexone treatment affect ethanol responses in rats. Alcohol. 2005;37:167–72. doi: 10.1016/j.alcohol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 69.Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. Int Rev Neurobiol. 2003;54:107–53. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- 70.Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267:495–9. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- 71.Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, et al. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Molecular pharmacology. 1994;45:330–4. [PubMed] [Google Scholar]
- 72.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology (Berl) 2005;182:384–92. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- 74.Williams KL, Woods JH. Oral ethanol-reinforced responding in rhesus monkeys: effects of opioid antagonists selective for the mu-, kappa-, or delta-receptor. Alcohol Clin Exp Res. 1998;22:1634–9. doi: 10.1111/j.1530-0277.1998.tb03960.x. [DOI] [PubMed] [Google Scholar]
- 75.Doyon WM, Howard EC, Shippenberg TS, Gonzales RA. Kappa-opioid receptor modulation of accumbal dopamine concentration during operant ethanol self-administration. Neuropharmacology. 2006;51:487–96. doi: 10.1016/j.neuropharm.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:643–52. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Walker BM, Zorrilla EP, Koob GF. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addiction biology. 2011;16:116–9. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mello NK, Negus SS. Effects of kappa opioid agonists on cocaine- and food-maintained responding by rhesus monkeys. The Journal of pharmacology and experimental therapeutics. 1998;286:812–24. [PubMed] [Google Scholar]
- 79.Kanarek RB, Homoleski BA, Wiatr C. Intake of a palatable sucrose solution modifies the actions of spiradoline, a kappa opioid receptor agonist, on analgesia and feeding behavior in male and female rats. Pharmacol Biochem Behav. 2000;65:97–104. doi: 10.1016/s0091-3057(99)00181-1. [DOI] [PubMed] [Google Scholar]
- 80.Cosgrove KP, Carroll ME. Effects of bremazocine on self-administration of smoked cocaine base and orally delivered ethanol, phencyclidine, saccharin, and food in rhesus monkeys: a behavioral economic analysis. The Journal of pharmacology and experimental therapeutics. 2002;301:993–1002. doi: 10.1124/jpet.301.3.993. [DOI] [PubMed] [Google Scholar]
- 81.Chen AC, LaForge KS, Ho A, McHugh PF, Kellogg S, Bell K, et al. Potentially functional polymorphism in the promoter region of prodynorphin gene may be associated with protection against cocaine dependence or abuse. American journal of medical genetics. 2002;114:429–35. doi: 10.1002/ajmg.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–35. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Streissguth A, Barr H, Kogan J, Bookstein F. Primary and secondary disabilities in Fetal Alcohol Syndrome. In: Streissguth A, Kanter J, editors. The Challenge of Fetal Alcohol Syndrome Overcoming Secondary Disabilities. Seattle, WA: University of Washington Press; 1997. pp. 25–39. [Google Scholar]
- 84.Yates WR, Cadoret RJ, Troughton EP, Stewart M, Giunta TS. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol, and drug dependence. Alcohol Clin Exp Res. 1998;22:914–20. [PubMed] [Google Scholar]
- 85.Lopez MF, Molina JC. Chronic alcohol administration in the rat pup: effects upon later consumption of alcohol and other palatable solutions. Addiction biology. 1999;4:169–79. doi: 10.1080/13556219971678. [DOI] [PubMed] [Google Scholar]