Abstract
The scanning electron microscope (SEM) studies on both male and female tick, Amblyomma gervaisi (Lucas 1847) inhabiting the snake Naja naja were made. Detail of the surface structures as seen under SEM exhibit sexual dimorphism and were compared with other tick species as reported. SEM observations show characteristic alloscutal ornamentation, inornate festoons, triangular licking organ, size of the hypostome, round Haller’s organ, genital plate oval in male and slit-like in female, and prominent marginated anal plate. The findings of the present study address the diversity in surface ultrastructure among snake ticks which would help in tick biosystematics.
Keywords: Amblyomma, Ectoparasite, Haematophagous, Tick, Naja, Surface ultrastructure
Introduction
Ticks are haematophagous ectoparasite of reptiles, mammals and even amphibians transmitting wide scale of infectious diseases. Tick transmits more infectious agents than any other blood feeding arthropods (Hoogstraal 1967). Ticks act as multiple and diverse disease agents (including virus, bacteria, and parasites). They are viaduct between animal reservoirs and humans. Continuous change in climate and weather pattern induces the distribution of ticks and associated disease transmission such as Lyme disease (cf. Barbour et al. 1996; Brownstein et al.2005; Paddock et al. 2008). In USA several species of Amblyomma act as vectors of pathogens, which are threat to human and livestock (Keirans and Durden 1998). It is very interesting that in the evolutionary history of ticks, the first pair of limbs forms the chelicerae (mandibles) for cutting into the skin of the host, the second pair of limbs forms the palps which position the capitulum for feeding, the third to sixth pairs of limbs are the actual legs used for walking whilst on and off the host. There is a complex sensory apparatus (Haller’s organ) located on the dorsal surface of the tarsus of leg I. This is very important, as it is the primary organ for determining host location, and detecting host odors, pheromones, etc. (Sonenshine 1991). Taxonomy of the ticks is discussed on the morphology and molecular basis (Dobson and Barker, 1999; Horak et al. 2002; Klompen et al. 2002; Barker and Murrell 2004) where the genus Aponomma is considered as junior synonym of Amblyomma. There are several studies on the light microscopy of reptilian ticks (Barnard and Durden 2000; Kenny et al.2004; Durden and Knapp 2005; Reeves et al.2006; Barros-Battesti et al. 2007). Limited studies on scanning electron microscopy (SEM) observation of reptile tick have been reported (Durden et al.2002; Barros-Battesti et al.2005a, b). However, SEM studies on Amblyomma inhabiting other than reptiles are available (Beati et al. 2008; Onofrio et al. 2008). So it is very essential to study of these amazing ticks to unravel detail body organization. Infestation of snakes by ticks is common in India (Pandit et al. 2011).
The present study is undertaken to study of the surface ultrastructure of a tick, Amblyomma gervaisi (Lucas 1847) infesting a poisonous snake, Naja naja to reveal detail of the surface characteristics. Indian Cobra (N. naja) or Spectacled Cobra is a species of the genus Naja found in the Indian subcontinent and a member of the “big four”, species inflicting the most snakebite in Indian subcontinent. The Cobra occurs in wild forest and in cultivated areas in Indian subcontinent.
Materials and methods
Material
Both male and female ixodid tick, Amblyomma gervaisi (Lucas 1847), previously known as Aponomma gervaisi.
The specimen was identified by The Zoological Survey of India, Calcutta.
Taxonomic position:
Phylum Arthropoda
Class Arachnida
Order Acarina
Sub order Ixodida
Family Ixodidae
GenusAmblyomma
Speciesgervaisi (Lucas).
Host: Naja naja (Family Elapidae).
Site of infestation: Dorsal side of the snake over the scale.
Procedure of collection: By hand picking.
Site of collection: Payradanga, Dist. Nadia, West Bengal, India.
Scanning electron microscopy preparation
The smallest adult fixed individuals with the features (1–2 mm3) were taken for studying the surface structures. The ticks of both sexes were killed by benzene vapor and immediately placed in 70 % ethanol. Any extra cellular debris, such as the mucus, blood, other body fluids and tissue fragments may obscure the surface to be examined, were removed carefully. The samples were fixed in buffered 2 % glutaraldehyde (pH 7.2) for 12 h. The fixed ticks were washed in phosphate buffer (pH 7.2) for thrice and then in double distilled water followed by acetone dehydration.
After acetone dehydration, the specimens were dried in a critical point drier using liquid CO2. Samples were then attached in aluminum stub by adhesive tapes. Ticks were then gold coated in a sputter coater (Polaron-SC7620) and were examined under SEM (FEI-Quanta200, operated at 20 kV), at Regional Sophisticated Instrumentation Center (RSIC), Bose Institute, Kolkata.
Observation
Altogether 20 N. naja were examined for ticks of which 12 ticks (7 ♀♀ and 5 ♂♂) were isolated from eight snakes. Both the male and female adult ticks, A. gervaisi (Lucas 1847) differ in their dorsal and ventral surface ultrastructure as well as most of the appendages, which are described separately.
Male (Figs. 1–6, 7–12, 13–14)
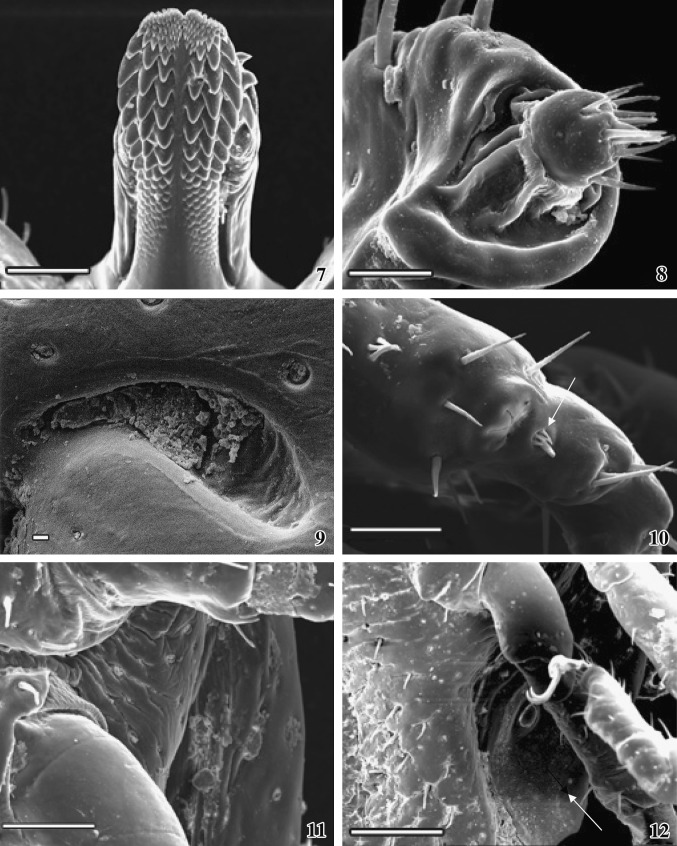
Figs. 1–6.
Scanning electron micrographs (SEM) of male A. gervaisi. 1Dorsal view of the whole body of a male tick. Scale = 1 mm. 2Ventral view of the whole body of the tick showing appendages, anus and genitalia. Scale = 1 mm. 3 Basis capituli (bold white arrow) and comma-shaped cervical grooves (arrow) and two lateral palps. Scale = 400 μm. 4 Licking organ at the tip of mandible (arrow) and central deep groove (white arrow) at the tip of the mandible. Scale = 10 μm. 5 Tip of the serrated hypostome, dorsal view. Scale = 50 μm. 6 Ventral surface of the basis capituli showing central hypostome and two lateral palps (arrows). Scale = 300 μm
Figs. 7–12.
SEM photographs of male A. gervaisi. 7Ventral view of the hypostome showing rows of denticle (3/3 arrangements). Scale = 100 μm. 8 Tip of the palp with densely packed sensory bristles. Scale = 30 μm. 9Magnified view of the elongated cervical groove. Scale = 10 μm. 10 The Haller’s organ (arrow). Scale = 50 μm. 11Magnified view showing attachment between coxa and trochanter. Scale = 100 μm. 12 Spiracle (arrow) and one pair of sharply curved pointed claw attached with pulvillus. Scale = 200 μm
Figs. 13–14.
SEM photographs of genital organ and anus of male snake tick. 13 The male genital organ showing hairless bulging aperture (arrow). Scale = 100 μm. 14 Anal plate with anal aperture, one pair of adanal plate and one pair of accessory adanal plate are seen. Scale = 400 μm
The male ixodid tick looks like a tear drop. It is dorsoventrally flattened and the posterior extremity is wider than that of the anterior portion. Body is divided into the anterior gnathosoma and posterior idiosoma. Gnathosoma extended from palpal apices to posterior margin of basis capituli and idiosoma extended immediate behind the basis capituli to posterior margin of festoons (Fig. 1). The dorsal surface of idiosoma is covered with a hard, smooth surface chitinous shield, which is known as scutum. Numerous pores are randomly arranged throughout the convex scutum surface (Fig. 1). One pair of cervical grooves is present on the scutum, just below the emergination line (Fig. 2). The floor of the cervical groove exhibits uneven ridges (Fig. 9).
Ventral surface of idiosoma consists of two pair and three unpaired plates, the surface of which is rough and bears numerous small hair-like projections (campaniforme sensilla). Hairs follow a definite arrangement pattern. These are arranged equidistantly from each other (Fig. 2). The emergination line, after the posterior margin of basis capituli, is very prominent, below of which many half circled striations are arranged densely. Small striations are fused together at the middle region (Fig. 6). Below the striated region, a smooth pregenital plate with two short spines follows the genital region (Fig. 2).
After the genital orifice the median plate extends to the anal region. The surface of the median plate is smooth and bears few hair-like projections (Fig. 2). Median plate follows the anal plate, which is half circled at its lower region. The junction between the median and anal plate is striated and bears spines. The surface of the anal plate is rough (Figs. 2, 14). The lower margin of the anal plate is the pre-anal groove followed by adanal plate. One pair of curved adanal plate, encircle anal plate, having a rough surface and possesses many short spines (Fig. 14). Equidistantly symmetrically placed spines are arranged in rows on adanal plate (Fig. 14). One pair of striated accessory adanal plate with rows of spines is present on the outer side of each adanal plate (Fig. 2).
Genital plate and median plate are less hairy than the adanal and accessory adanal plates (Fig. 2). The margin between the two adanal plates and between each adanal and accessory adanal plates is prominent (Fig. 14). Accessory adanal plate appears to originate from the second pair of coxae (Fig. 2). The arrangement of spines and striations on the paired plates maintains a symmetric pattern (Fig. 2).
Basis capituli (Figs. 2, 3, 6)
Dorso-ventrally rounded flask-shaped basis capituli bears the mouth parts, which consists of a mandible, a hypostome and a pair of sensory palp. It is connected by a soft articulating membrane that allows the capitulum to be fixed (ventrally) or extended (returned to the horizontal axis) from the emergination (Fig. 3). Dorsal surface is smooth, bear pores and few small sharply pointed spines. Two light, straight, diagonal striations are arranged equidistantly on the dorsal surface of basis capituli (Fig. 3). Ventral surface is smooth, have no pores and each lateral side consists of four to five spines (Fig. 6).
Mandible (Figs. 1, 4)
The anterior part of dorsal basis capituli extends to form the mandible. A central deep groove (furrow) extends to the tip of the mandible. The passage is narrower at the base and wide at the tip. A small triangular licking organ is projecting out from the tip of the groove (Fig. 4). Numerous scales are arranged sequentially in a definite manner which gives a beautiful architectural pattern on the dorsal surface. The scales are invisible at the base of the mandible.
Hypostome (Figs. 5, 6, 7)
It is the extending part of anterior portion of ventral basis capituli. Hypostome is a spatulate single structure lying ventral in close apposition to the mandibular sheath. Hypostome is longer than the mandible and the tip is serrated (Fig. 5), which is deep in middle portion than the lateral sides. The denticle is arranged in several rows, each of which overlaps on the succeeding row. Each tooth terminates distantly in crown or corona (Fig. 7). The hypostome shows a median toothless line running along its ventral surface. Each coronal surface is smooth and the dental arrangement is bilaterally symmetrical. The dental formula is 3/3. Several small teeth formed two clump regions at the tip of the hypostome. At the base of the hypostome the coronal size is so reduced that looks like scales (Fig. 7).
Palp (Figs. 3, 6, 8)
Two extending sensory palps originate from each side of the base of the hypostome and consist of four articles. Two articles (I and II) are fused together; hence only three segments are visible. Surface is wrinkled and consists of numerous sensory hairs, arranged in rows. The palp is longer than the dorsal mandible (Fig. 3). Each palpal base consists of two long spines. Sensory hairs are mainly located on the lateral sides. Tip of the palp is well organized and a rounded head like structure with densely packed sensory bristles are projecting outward from a comma-shaped depression (Fig. 8).
Appendages (Figs. 11, 12)
Four pairs of appendages are attached at the ventro-lateral side of the body. Each appendage consists of seven segments; coxa at the base, then trochanter, femur, patella, tibia, metatarsus, and tarsus. Coxa on the appendage II–IV is rigidly attached with the body than the first pair of coxa, which is comparatively loosely attached. Coxa of the first appendage possesses two (internal and external) broad, flat, and pointed spurs. Internal spur is slightly shorter than that of the external spur. Coxa on the II and III appendages possesses single short flat spur. Coxa on the IV appendage bears triangular pointed spur. Each appendicular segment bears few hairs on the distal end. Joints of the segments of each appendage are attached by a soft articulation membrane (Fig. 11). Tarsus of each appendage bears an apotele or pre-tarsus with a pair of highly curved, sharply pointed claws (Fig. 12). The base of the two claws of each pair originates from a special structure, called pulvillus, which is attached with the apotele or pre-tarsus.
Haller’s organ (Fig. 10)
Haller’s organ is located on the dorsal surface of first pair of tarsus. It is a complex bulging over growth, one end of which bears clumps of short halleral setae (four short and thin and one long and thick). Six long needle-like spines are present around the Haller’s organ. A deep depression with four to five grooves is found on smoothly surfaced Haller’s organ.
Spiracle (Figs. 2, 12)
One pair of respiratory openings, spiracle present ventro-laterally, behind each of fourth coxa. Each spiracle is an oval shaped concave structure, upper and lower portion of which is slightly tapering than the lateral portion. The upper surface bears a small circular bulging outgrowth. Margin around the spiracle is prominent and the surface is smooth and hair less.
Genital organ (Figs. 2, 13)
It is situated in the median line immediately below the basis capituli and between the second pair of coxa. The lateral sides of the genital region are striated and are sparsely distributed and bear short spines. The male genital orifice is oval in shape, hair less bulging aperture projecting outward from a cavity, upper end of which is fused with the pregenital plate.
Anus (Figs. 2, 14)
Below the genital organ, anus is located within the anal plate. Two valves are guarding the anus. Some bristles are projecting outward from the anal valves. Around the anal valves a prominent circular anal margin is present.
Female (Figs. 15–18, 19–24, 25–30, 31–32)
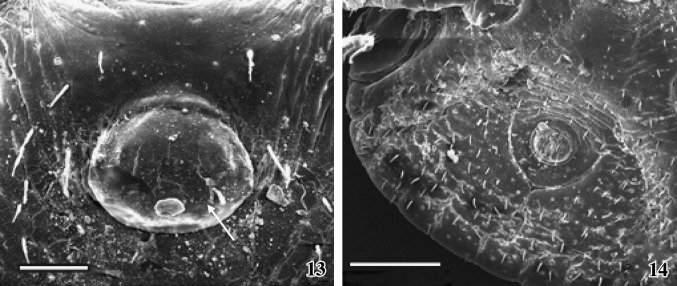
Figs. 15–18.
Scanning electron micrographs of snake inhabiting female A. gervaisi are presented. 15Dorsal view of the female tick. Scale = 1 mm. 16 The arrangement of festoons, dorsal view. Scale = 500 μm. 17Ventral view of the snake tick showing distinct anal region. Scale = 2 mm. 18 One pair of porous region (arrows) on the dorsal surface of basis capituli and one pair of elongated cervical grooves are present. Scale = 100 μm
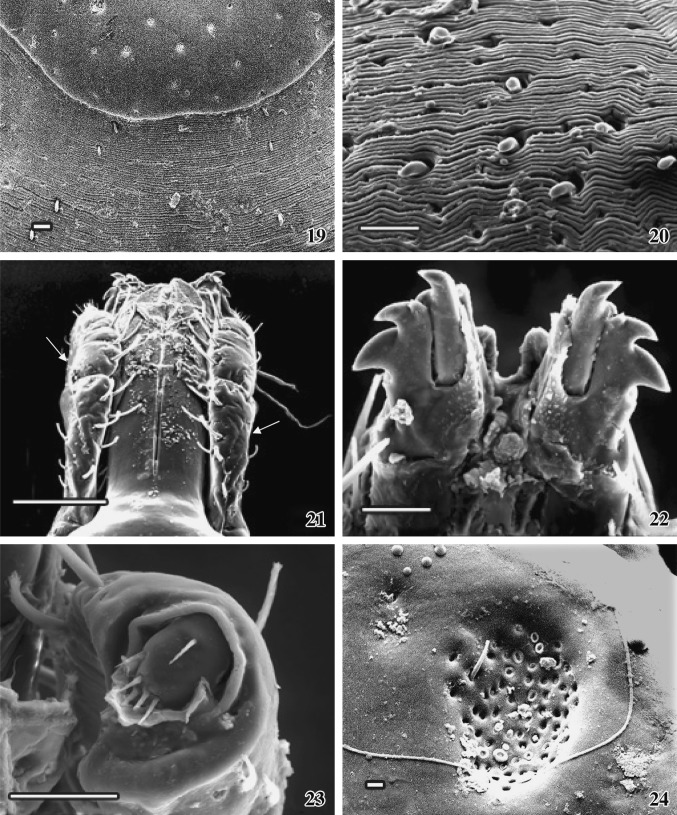
Figs. 19–24.
SEM photographs of female A. gervaisi.19 A close view of the dorsal aspect of scutum elaborating idiosoma zone. Scale = 30 μm. 20 Finely folded dorsal idiosoma with interspersed bristles are seen. Scale = 50 μm. 21 The association of mandible with one pair of palp (arrows), dorsal view. Scale = 200 μm. 22Ventral view of a pair of complex dental structure at the tip of mandible. Scale = 50 μm. 23 Tip of the palp with characteristic setae are seen. Scale = 50 μm. 24 A close view of porose region with a spine on dorsal basis capituli. Scale = 10 μm
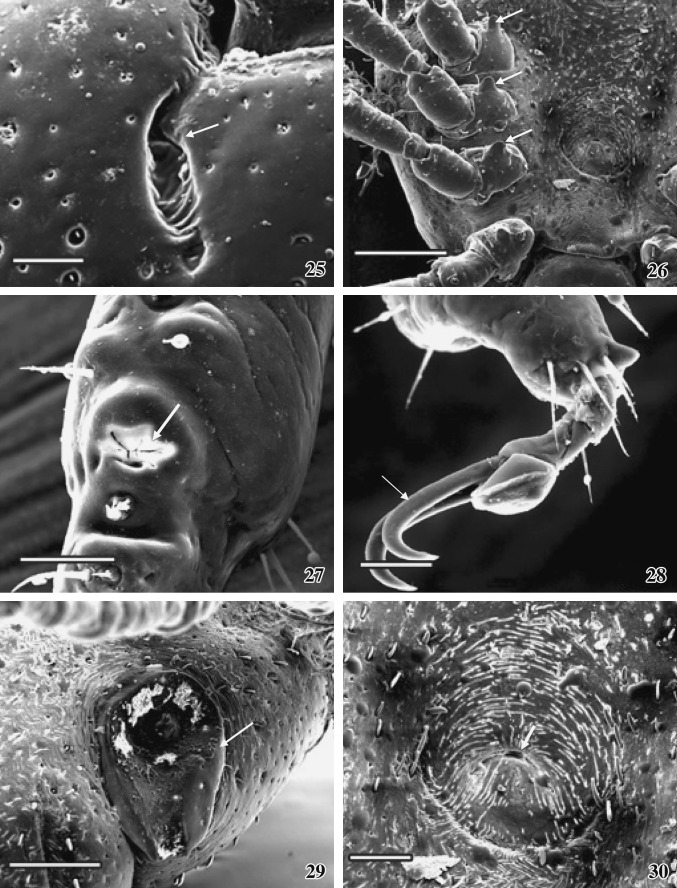
Figs. 25–30.
SEM photographs of the female tick. 25 A comma-shaped cervical groove (arrow) with folded floor is seen. Scale = 100 μm. 26 Arrangement of spurs (arrows) on the base of the each coxa is very prominent. Scale = 400 μm. 27 The Haller’s organ on the first tarsus (arrow). Scale = 50 μm. 28 One pair of claw attached with pulvillus. Scale = 50 μm. 29 The prominent spiracle (arrow). Scale = 200 μm. 30 The female genital organ showing eccentric slit (arrow). Scale = 100 μm
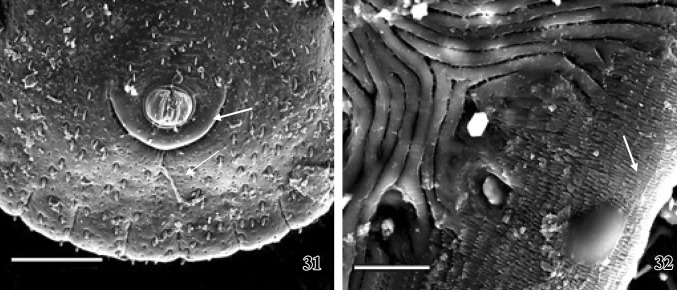
Figs. 31–32.
SEM photographs of female tick. 31 A clear picture of the bulging anus within anal plate (arrow) and the ventral view of festoon arrangement are seen. Scale = 400 μm. 32 A close view of the ventro-lateral surface of idiosoma showing series of scales (arrow). Scale = 20 μm
The adult female ixodid tick A. gervaisi is larger than that of the male tick, dorsoventrally flattened and the posterior extremity is wider than the anterior part (Fig. 15). Gnathosoma extended from palpal apices to posterior margin of the basis capituli and idiosoma extended immediate behind the basis capituli to posterior margin of festoons, which is deeply ridged and consists of eleven segments (Fig. 16). The dorsal gnathosoma contains one pair longitudinally elongated porose area (Figs. 18, 24). One-third of the total dorsal surface of idiosoma is covered with a hard, smoothly surfaced chitinous dorsal shield––the scutum. Numerous pores are randomly arranged throughout the convex surface of the scutum (Fig. 18). Below the scutum, the surface of the dorsal idiosoma is finely folded, appears as striations, which extends continuously (Figs.19, 20), on which several thick bristles-like projections are arranged in a row. The head of each projection is knob-like and bending downwards. One pair of elongated comma-shaped cervical groove is present on the scutum surface (Fig. 18). Several finger-like folding are arranged alternatively on both the sides on the floor of each cervical groove (Fig. 25).
Ventral surface of idiosoma does not possess any plates except the anal plate and the genital plate (Fig. 17). Ventral body surface is rough and bears numerous irregularly arranged small hair-like campaniforme sensilla. After the posterior margin of basis capituli many irregular striations are arranged densely throughout the overall ventral body surface (Fig. 26), which also bear several circular nodular over growth (Fig. 30).
Mandible (Figs. 21, 22)
The structure of the mandible is more all less similar to that of the male. Several irregular-shaped nodular structures are irregularly arranged throughout the mandibular surface (Fig. 21). Two movable triangular plates with numerous nodules are present at the tip of the mandible, which act as their licking purpose (Fig. 21).
Tip of the ventral mandible consists of a pair of complicated dental structures. Each of them consists of two fused tooth and a single, movable tooth, which is inserted freely at the lateral side of the fused tooth. Each tooth is curved laterally and sharply pointed. Several fused nodules form a complex structure in between the base region (Fig. 22).
Hypostome (Fig. 7)
It is the extending part of anterior portion of ventral basis capituli. It is a spatulate structure lying ventral in close apposition to the mandibular sheath. In female, the hypostome is shorter than the dorsal mandible. Several overlapping rows of denticle follow a typical architectural pattern. Each tooth terminates distantly in blunt crown or corona. The hypostome shows a median toothless line running along its ventral surface. The coronal surface is smooth and the arrangement is bilaterally symmetrical at both the sides of toothless transverse line. The dental formula is 3/3. Several small teeth formed two clumps at the tip of the hypostome. Corona at the base of the hypostome looks like scales.
Palp (Figs. 21, 23)
Two extending sensory palps originated from each side of the base of the hypostome consist of four articles. Middle articles I and II are fused together, and hence only three segments are visible. In comparison with male, palps are shorter in length than the dorsal mandible. Surface is wavy and consists of numerous sensory hairs, arranged in rows. Each palpal base consists of two long spines. Maximum sensory hairs are located at the lateral side. Tip of the palp is well organized and a rounded head like structure with few sensory bristles are projecting outward from a comma-shaped depression (Fig. 23).
Appendages (Figs. 15, 17, 26, 28)
The structure of the appendages is more or less similar to those of the male. Spur present on coxa 4 is oval edged and slightly longer than the spur on coxa 2 and coxa 3. Base of each spur contains hair-like projection (Fig. 26). Each limb segment consists of distantly placed few hairs. Tip of the tarsus are surrounded by long needle-like spines (Fig. 28). Each trochanter surface contains two to three nodular outgrowths. Tarsus of each limb bears an apotele or pre-tarsus with a pair of highly curved, sharply painted claws. Claws are comparatively less curved than the male. Base of each pair of claw attached with a special structure called pulvillus, which is present at the tip of apotele (Fig. 28).
Haller’s organ (Fig. 27)
It is similar to that of the male but differs on the distribution of the halleral setae. Another bulging overgrowth along with setae is present ahead of the Haller’s organ (prehalleral setae).
Spiracle (Fig. 29)
Each spiracle has a small bulging portion and is centrally placed in the concave surface. Several minute nodular structures are scattered around the bulging portion on the spiracle. The ventro-lateral surface around the spiracle contains numerous fine folds with several needle-like spines, arranged in distinct way.
Genital organ (Figs. 26, 30)
It is situated in the medium line immediately post to the basis capituli and between the second pair of coxa (Fig. 26). Eccentric slit is centrally placed on the slightly concave circular genital plate. Spines are present around the margin. Several smaller striations are scattered randomly on the genital plate.
Anus (Fig. 31)
Bulging anus is present within the half circled anal plate and possesses two anal valves projecting outward from anal cavity. Around the anal valves a prominent circular anal margin is present. The ventro-lateral end of idiosoma possesses series of backwardly projected closely packed scales (Fig. 32).
Discussion
The general body organization of hard tick gives it a protection against the environmental odds. Hard outer surface gives it a natural protection. The sexual dimorphism of the species is prominent; the male is quite smaller than the female. The present observation is discussed against the available SEM studies on other ticks made by Robinson (1926) in Amblyomma goeldii, Kang and Jang (1985) in Ixodes persulcatus; Homsher et al. (1988) in eight species of the subgenus Sternalixodes of the genus Ixodes; Durden et al. (2002) in A. geochelone; Barros-Battesti et al. (2005a) in A. fuscum and Barros-Battesti et al. (2005b) in A. longirostre.
Numerous punctuations are uniformly distributed all over the scutal surface of both A. goeldii and A. gervaisi but concentrated in the lateral field in A. fuscum. The posterior end of idiosoma is broader in female A. gervaisi than the female A. fuscum. Scutal ornamentation is present in the both sexes of A. geochelone and the characteristics pattern of shallow grooves is present on the alloscutum of the female (Durden et al. 2002). Whereas these ornamentation and the shallow grooves are absent in the A. gervaisi. In female A. geochelone scutum is ornate with somewhat variable brown and yellowish markings darker than posterior markings, with a postero-medial dark extension. Punctations are numerous and deep in antero-lateral areas, otherwise almost absent. Alloscutum is distinctive with relatively consistent pattern of depressions. Alloscutal setae fairly numerous and somewhat clumped especially in and around depressions. While in the present study numerous punctations are randomly scattered throughout the convex scutum surface. The finely folded dorsal alloscutal surface consists of numerous thick bristles-like projections arranged in several rows.
Festoons are present in A. geochelone and the species under discussion while it is absent in I. persulcatus. The male A. geochelone possesses ornamented festoons while it is inornate in A. gervaisi. Number of campaniforme sensilla in the ventral surface is variable in A. gervaisi and A. longirostre inhabiting a rodent, Coendu prehensilis in Brazil (Barros-Battesti et al. 2005b).
The basis capituli of is rectangular in shape in A. fuscum (Barros-Battesti et al. 2005a), in Bothriocroton oudemansi (Beati et al. 2008) and in A. varium (Onofrio et al. 2008), spatulate in the larva of A. longirostre (Barros-Battesti et al. 2005b), while flask-shaped in A. geochelone, in A. gervaisi and trapezoidal in A. romitii (Barros-Battesti et al. 2007). In male A. geochelone, basis capituli is ornate dorsally with variable brownish and golden yellowish patterns, while it is smooth dorsally with pores and few small sharply pointed spines and two light, straight, diagonal striations are present in male A. gervaisi.
The mandibular tip of A.fuscum consists of two triangular structures, which is absent in A. gervaisi. A triangular licking organ is present at the tip of the A. gervaisi not mentioned in earlier studies.
The dental formula on the hypostome is 4/4 in A. goeldii, while it is 3/3 in, A. gervaisi, A. fuscum, and A. varium while is variable in A. romitii. The hypostome shows a median toothless line in case of the present species but not found in A. fuscum. Two extending complicated dental structures are present at the tip of the mandible of male A. fuscum. Hypostome is longer than the mandible in male A. gervaisi while is shorter than the mandible in male A. fuscum.
The structure of palp and the four pairs of limb of both A.fuscum and A. gervaisi are more all less similar, though the palp head is more hairy in the present species than that of A. fuscum. The length of the palp is almost equal with the mandible of male A. fuscum while the palp length is longer than the mandible of male A. gervaisi.
The male genital organ, anus, Haller’s organ, and spiracle of both A. fuscum and A. gervaisi are more all less similar in structure. The spiracular plate is sub-oval in shape, broadest posteriorly, with numerous small goblet cells and elongate macula in case of female A. geochelone. While in the present study it is oval shaped concave structure, upper and lower portion of which is slightly tapering with centrally placed small bulging portion and several minute nodular structures.
The Haller’s organ of A. gervaisi is somehow round than that of the genus Ixodes. Haller’s organ in eight species of the subgenus Sternalixodes of the genus Ixodes were studied under SEM (Homsher et al. 1988). Though the morphology of the organ is remarkably uniform throughout the subgenus Sternalixodes but some differences are observed. According to the description, the general shape of Haller’s organ is oval to elliptical in Ixodes anatis, I. dendrolagi, I. hirsti, and female I. holocyclus; roughly triangular in male I. holocyclus and I. trichosuri; oval in male but roughly elliptical in female I. confuses; roughler rectangular in female I. cordifer; elongate oval to elliptical in I. cornuatus, but more or less round in A. varium (Onofrio et al. 2008). In comparison of these studies, the Haller’s organ is round in both male and female A. gervaisi.
The well developed genital groove and specific, distinct anal groove, which is extending anterior around the anus of the species under discussion shows similarity with the I. persulcatus. The genital plate of the female I. persulcatus is elongated oval and the posterior region is deeply margined but this structure is nearly round in shape with deep margined anterior portion in female A. gervaisi. This is located between coxae II in A. varium (Onofrio et al. 2008) and at the level of coxae I–II in A. romitii (Barros-Battesti et al. 2007). The male genital aperture is broadly U-shaped in A. geochelone, but is oval in shape in the present species. The genital aperture of female A. geochelone is also broadly U-shaped, whereas it is eccentric slit-like structure situated central to the concave circular genital plate in female A. gervaisi. The anal plate is elongate to oval in shape with anterior margination in female I. persulcatus while the anal plate is circular in shape with prominent margination at posterior end in female A. gervaisi.
This is to be mentioned at the end that synonymy of Amblyomma and Aponomma is not yet conclusive as the status of “primitive Aponomma” still remains unclear (Barker and Murrell 2004). It is further argued that the genus Amblyomma was paraphyletic without the inclusion of the “typical” Aponomma species as inferred from 16S RNA (Dobson and Barker 1999). These authors claim that there is divergence at basal level between endemic Australian and other species in both metastriata and the prostriata. Further studies on molecular biology of reptilian ticks will through new light on this aspect.
The above discussion shows that species of ticks inhabiting snakes/reptiles exhibit important variations in their surface ultrastructure. Diversity in surface ultrastructure is important with regard to the adaptation of the ticks. Such studies on other snake ticks are necessary because many of them are carrier of Rickettsia and Lyme diseases (Stenos et al. 2003; Paddock et al. 2008).
References
- Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum––possible agent of a Lyme disease-like illness. J Infect Dis. 1996;173:403–409. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- Barker SC, Murrell A. Syatemaic and evolution of ticks with a list of valid genus and species names. Parasitology. 2004;129:S15–S36. doi: 10.1017/S0031182004005207. [DOI] [PubMed] [Google Scholar]
- Barnard SM, Durden LA. A veterinary guide to the parasites of reptiles, vol 2, arthropods (excluding mites) Malabar: Krieger Publishing Company; 2000. [Google Scholar]
- Barros-Battesti DM, Onofrio VC, Labruna MB, Martins R, Guglielmone AA. Redescription of Amblyomma fuscum Neumann, 1907 (Acari: Ixodidae), a rare South America tick confirmed in Brazil. Syst Parasitol. 2005;61:85–92. doi: 10.1007/s11230-004-6353-7. [DOI] [PubMed] [Google Scholar]
- Barros-Battesti DM, Arzua M, Rebello VM, S Barbieri F, Famadas KM. Description of the larva of Amblyomma longirostre (Koch,1844)(Acari: Ixodidae) by light and scanning electron microscopy. Rev Bras Parasitol Vet. 2005;14:51–57. [PubMed] [Google Scholar]
- Barros-Battesti DM, Arzua M, Onofrio VC, Labruna MB. Validation and redescription of Amblyomma romitii Tonelli–Rondelli, 1939 (Acari: Ixodidae) Syst Parasitol. 2007;68:79–86. doi: 10.1007/s11230-006-9079-x. [DOI] [PubMed] [Google Scholar]
- Beati L, Keirans JE, Durden LA, Opiang MD. Bothriocroton oudemansi (Neumann, 1910) n. comb. (Acari: IxodidaIxodidae), an ectoparasite of the wester long-beaked echidna in Papua New Guinea redescription of the male and first description of the female and nymph. Syst Parasitol. 2008;69:185–200. doi: 10.1007/s11230-007-9115-5. [DOI] [PubMed] [Google Scholar]
- Brownstein JS, Holford TR, Fish D. Effect of climate change on Lyme disease risk in North America. Eco Health. 2005;2:38–46. doi: 10.1007/s10393-004-0139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson SJ, Barker SC. Phylogeny of the hard ticks (Ixodidae) inferred from 18SrRNA indicates that the genus Aponomma is paraphyletic. Mol Phylogenet Evol. 1999;11:288–295. doi: 10.1006/mpev.1998.0565. [DOI] [PubMed] [Google Scholar]
- Durden LA, Knapp CR. Ticks parasitizing reptiles in the Bahamas. Med Vet Entomol. 2005;19:326–328. doi: 10.1111/j.1365-2915.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- Durden LA, Keirans JE, Smith LI. Amblyomma geochelone, a new species of tick (Acari: Ixodidae) from the Madagascan Ploughshare tortoise. J Med Entomol. 2002;39:398–403. doi: 10.1603/0022-2585-39.2.398. [DOI] [PubMed] [Google Scholar]
- Homsher JP, Keirans JE, Robbins RG, Irwin-Punkley L, Sonneshine DE. Scanning electron microscopy of ticks for systematic studies: structure of Haller’s organ in eight species of the subgenus Sternalixodes of the genus Ixodes (Acari: Ixodidae) J Med Entomol. 1988;25:348–353. doi: 10.1093/jmedent/25.5.348. [DOI] [PubMed] [Google Scholar]
- Hoogstraal H. Ticks in relation to human diseases caused by Rickettsia species. Ann Rev Entomol. 1967;12:377–420. doi: 10.1146/annurev.en.12.010167.002113. [DOI] [PubMed] [Google Scholar]
- Horak IG, Camicas J-L, Keirans JE. The Argasidae, Ixododae an Nuttalliellidae (Acari: Ixodida): a world list of valid tick names. Exp Appl Acarol. 2002;28:27–54. doi: 10.1023/A:1025381712339. [DOI] [PubMed] [Google Scholar]
- Kang Y-B, Jang D-H. A description with scanning electron microscopy on the tick Ixodes persulcatus (Schulze, 1930) male and female specimens. Korean J Parasitol. 1985;23:306–312. doi: 10.3347/kjp.1985.23.2.305. [DOI] [PubMed] [Google Scholar]
- Keirans JE, Durden LA. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J Med Entomol. 1998;35:489–495. doi: 10.1093/jmedent/35.4.489. [DOI] [PubMed] [Google Scholar]
- Kenny MJ, Shaw SE, Hillyard PD, Forbes AB. Ectoparasites and haemoparasite risks associated with imported exotic reptiles. Vet Rec. 2004;154:435–436. doi: 10.1136/vr.154.14.434. [DOI] [PubMed] [Google Scholar]
- Klompen H, Dobson SJ, Barker SC. A new subfamily, Bothriocrotoninae n. subfam., for the genus Bothriocroton Keirans, King & Sharrad, 1994 status amend. (Ixodida Ixodidae), and the synonymy of Aponomma Neuman, 1899 with Amblyomma Koch 1844. Syst Parasitol. 2002;53:101–107. doi: 10.1023/A:1020466007722. [DOI] [PubMed] [Google Scholar]
- Onofrio VC, Barros-Battesti DM, Marques S, Faccini JLH, Labruna MB, Beati L, Guglielmone AA. Redescription of Amblyomma varium Koch, 1844 (Acari: Ixodidae) based on light and scanning electron microscopy. Syst Parasitol. 2008;69:137–144. doi: 10.1007/s11230-007-9128-0. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Fernandez S, Echenique GA, John W, Sumner JW, Reeves WK, Zaki SR, Remondegui CE. Rocky mountain spotted fever in Argentina. Am J Trop Med Hyg. 2008;78:687–692. [PubMed] [Google Scholar]
- Pandit P, Bandivdekar R, Geevarghese G, Pande S, Mandke O. Tick infestation on wild snakes in northern part of Western Ghats of India. J Med Entomol. 2011;48:504–507. doi: 10.1603/ME10164. [DOI] [PubMed] [Google Scholar]
- Reeves WK, Durden LA, Dasch GA. A spotted fever group Rickettsia from an exotic tick species, Amblyomma exornatum (Acari: Ixodidae), in a reptile breeding facility in the United States. J Med Entomol. 2006;43:1099–1101. doi: 10.1603/0022-2585(2006)43[1099:ASFGRF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Robinson LE. Ticks: a monograph of the Ixodoidea, part IV. The genus Amblyomma. Cambridge: Cambridge University Press; 1926. [Google Scholar]
- Sonenshine DE. Biology of ticks (as per Ixodes cookie) New York: Oxford University Press; 1991. [Google Scholar]
- Stenos J, Graves S, Popov VL, Walker DH. Aponomma hydrosauri, the reptile associated tick reservoir of Ricketisia honei on Flinders Island, Australia. Am J Trop Med Hyg. 2003;69:2003. [PubMed] [Google Scholar]