Abstract
Pulmonary paragonimiasis is an important zoonotic disease reported from many parts of the world. It is an endemic problem in human population in north-eastern states of India. There seems no report of pulmonary paragonimiasis in canine population from India. The present case describes first report of pulmonary paragonimiasis in a female dog suggesting possibility of this fluke becoming established in canine population in the country. The dog revealed mild coughing with serous nasal discharge. Faecal sample revealed eggs of Paragonimus spp. Treatment with fenbendazole resulted in marked improvement as revealed by clinical signs and chest radiography.
Keywords: Dog, Fenbendazole, Paragonimiasis, Paragonimus
Introduction
Pulmonary paragonimiasis in dogs is caused by lung fluke, Paragonimus kellicotti and is prevalent in many parts of North America, South Asia and South Africa (Pechman 2008). In many north-eastern hilly states of India including Manipur, Nagaland, and Arunachal Pradesh, pulmonary paragonimiasis is an endemic problem (Singh et al. 1982, 2009; Narain et al. 2003). In India, P. westernmani is reported to cause disease in many mammalian species including human beings (Singh et al. 1986). However, pulmonary paragonimiasis in dogs has not been reported so far from any part of India including Punjab. This is the first report of canine paragonimiasis caused by Paragonimus spp. and its successful treatment with fenbendazole in India, suggesting possibility of this fluke becoming established in canine population in the country.
Case description
A German shepherd, 8 year old female dog was presented to the Teaching Veterinary Hospital, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, Punjab, India with a month old history of intermittent coughing, vomiting, pica and tarry coloured faeces. No dose of deworming was given to the dog for the last more than 1 year, but the vaccination for common infectious diseases was up to date. Clinical examination revealed rectal temperature 101.6°F, heart rate 128 beats per minute and normal respiratory rate and pattern. However, serous nasal discharge was seen at the time of examination. Auscultation of lungs revealed increased lung sounds, on both inspiration and expiration. Evaluation of different haemato biochemical parameters (Table 1) revealed leukocytosis and mild increase in alkaline phosphatase activity. Faecal sample examination revealed eggs of Paragonimus spp. The eggs were large in size (72–90 μm long by 44–60 μm wide), yellowish brown in colour, with an operculum at one pole and a thickened shell at the opposite end (Fig. 1). Lateral radiograph of the chest showed thickened bronchi in the cranial lung lobe, caudal heart and accessory lung lobes, along with presence of multiple cavitations suggesting peribronchial infiltration (Fig. 2). A radio opaque round density was present near the diaphragmatic line. Size and position of the heart appeared normal.
Table 1.
Levels of different haemato biochemical parameters
| Parameters | Pre-treatment | Post-treatment | Reference rangea |
|---|---|---|---|
| Haemoglobin (g%) | 13.6 | 13.1 | 12–18 |
| Total leukocyte count (per mm3) | 24,420 | 17,400 | 6,000–17,000 |
| Differential leukocyte count (%) | |||
| Neutrophils | 60 | 64 | 60–70 |
| Lymphocytes | 38 | 34 | 12–30 |
| Monocytes | 02 | 0 | 3–10 |
| Eosinophils | 0 | 02 | 2–10 |
| Basophils | 0 | 0 | Rare |
| Platelet count (×105 cells/μL) | 2.52 | 2.93 | 2–9 |
| Alanine aminotransferase U/L) | 26 | 19 | 8.2–57.3 |
| Aspartate aminotransferase (U/L) | 24 | 12 | 8.9–48.5 |
| Alkaline phosphatase (U/L) | 242 | 213 | 10.6–100.7 |
| Total proteins (g/dL) | 7.7 | 7.8 | 5.5–7.5 |
| Albumin (g/dL) | 2.6 | 2.6 | 2.6–4.0 |
| Blood urea nitrogen (mg/dL) | 6 | 2 | 8.8–25.9 |
| Creatinine (mg/dL) | 1 | 0.8 | 0.5–1.6 |
aAdopted from Kahn CM, Line S (2005) The Merc veterinary manual, 9th edn. Merck and Co. INC, N.J, pp 2584–2589
Fig. 1.
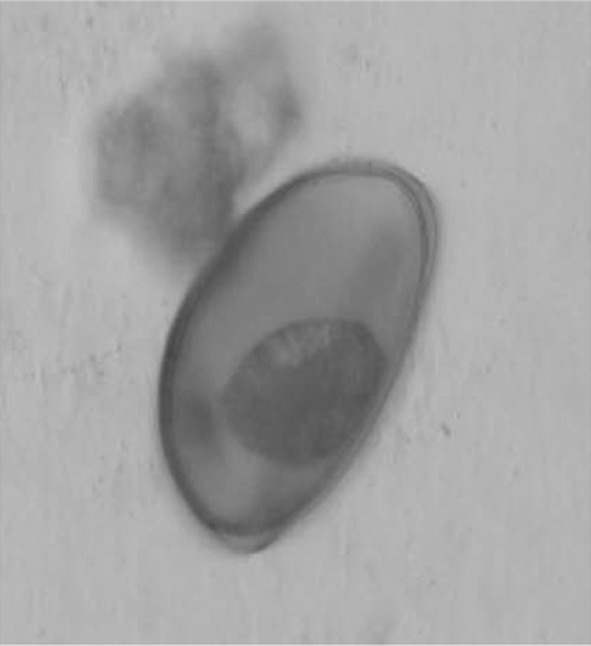
Paragonimus egg observed in faecal sample of the dog
Fig. 2.
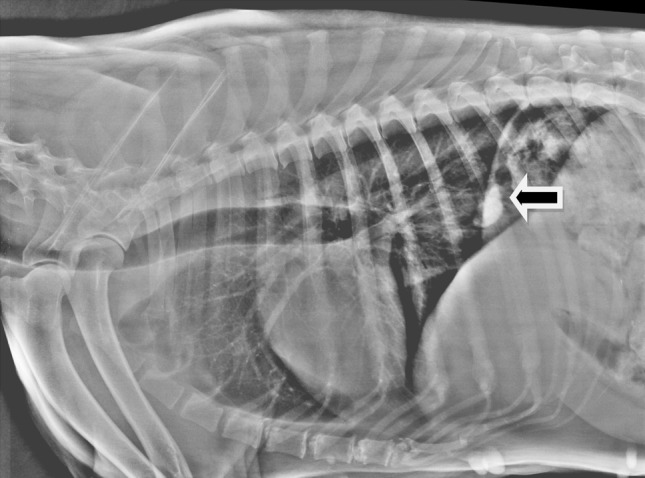
Pre-treatment chest radiograph showing multiple cavitations in lung along with one large calcified nodule (arrow)
Initially, the animal was treated symptomatically for gastric upsets with injectable antiemetics (metoclopramide) and H2 blockers (ranitidine) for 2 days. Thereafter, fenbendazole was given at 25 mg/kg body weight (bw) twice daily for 14 days. Oral liver supplement was also prescribed as supportive treatment. After completion of the therapy, marked improvement in condition was reported by the owner, which was substantiated by disappearance of all the clinical symptoms observed previously. Post therapy lung radiograph also revealed marked improvement in bronchial pattern (Fig. 3), but calcified mass was still present. Haemato biochemical parameters were improved, as total leukocyte count and alkaline phosphatase activity came towards the normal range (Table 1). No Paragonimus spp. eggs could be detected in repeated faecal sample examination conducted after the treatment.
Fig. 3.
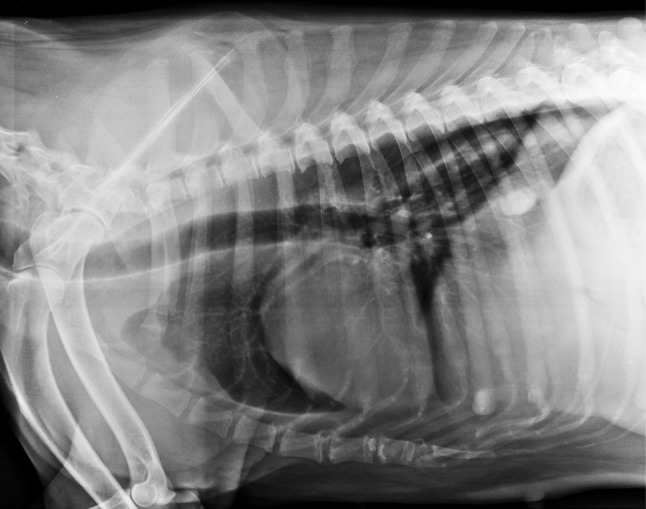
Post-treatment chest radiograph showing marked improvement in bronchial pattern
Discussion
Paragonimus spp. have complex life cycles that require two intermediate hosts, may be among snails, crustaceans and fish (Michael et al. 2009). The definitive host becomes infected by ingesting raw metacercariae contaminated shrimp or crayfish (Harrus et al. 1997). Paragonimus spp. can infect a variety of wild carnivores as well as dogs and cats (Michael et al. 2009) and is prevalent in North America, South Asia and South Africa (Harrus et al. 1997). Infection is more common in cats than in dogs (Nelson and Sellon 2005). The parasite has not been reported to cause disease in dogs so far from India. Humans are infected after eating fish containing metacercaria of the parasite (Mariano et al. 1986), where pulmonary lesions are caused by the penetration and growth of flukes (Dubey et al. 1979a). Clinical signs are often mild and hence frequently remain undiagnosed until severity of the disease increases. Clinical disease in humans is often confused with pulmonary tuberculosis (Singh et al. 1986). In human patients, chest radiographic findings include pneumothorax (Pachucki et al. 1984), pleural effusion (DeFrain and Hooker 2002; Madariaga et al. 2007) and consolidation (Castilla et al. 2003; Boe and Schwarz 2007). Leukocytosis and eosinophilia are common haematological changes in humans suffering from clinical disease (Singh et al. 1986; Shim et al. 1991). However, in present case, only leukocytosis with no increase in eosinophilia was observed in the dog.
Dogs also suffer from subclinical pulmonary infections where multiloculated cysts can be evident in chest radiograph (Pechman 2008). Radio opaque round density observed near the diaphragmatic line in the present case might be a focal granulomatous reaction caused by the lung fluke. Fenbendazole (Nelson and Sellon 2005) and praziquantel (Kirkpatrick and Shelly 1985) are reported to be effective against Paragonimus spp. Fenbendazole given at 25 mg/kg bw twice daily for 14 days showed good therapeutic response in the present case as evident from disappearance of clinical signs and improvement in chest radiographic findings. Fenbendazole in higher doses i.e., at 50–100 mg/kg bw for 3–8 days has been previously reported to be effective in canine paragonimiasis (Dubey et al. 1979b). Other drugs recommended for use in pulmonary paragonimiasis in man and animals include praziquantel, bithionol and albendazole (Pechman 2008; Michael et al. 2009).
This is the first report of Paragonimus spp. infection in a dog in India. Therefore, paragonimiasis may be added to the differential diagnosis whenever a dog is examined for chronic coughing and nasal discharges. Since overt clinical signs may be absent until the disease is in advanced stage, routine faecal and sputum examination should be performed, whenever nodular lung lesions are evident in lung radiograph. Paragonimus flukes do not have strong regional limitations and are increasingly being reported from many countries where snails of genus Melania and crabs of genus Potamon naturally occur (Harrus et al. 1997; Procop 2009). Both these two intermediate hosts are also found in aquatic habitat of Punjab (Khanna 1974), the state rich in water resources with many natural rivers and manmade canals, dams and ponds. Therefore, possibility of completing life cycle of this parasite in this region cannot be ruled out. In present case, it seems possible because the dog was born in Punjab and was never taken abroad or any other state of India. But since there is no report of Paragonimus spp. infection in dog or humans so far from Punjab, it may also be possible that this parasite was recently introduced from a geographically distinct area, where it is endemic. In view of the zoonotic potential of this parasite, there is an urgent need to create awareness among medical practitioners working in the Punjab state and other states of India regarding possible presence of lung flukes in human population. Fenbendazole is effective drug that can be used for treatment and control of lung worm infection in dogs.
References
- Boe DM, Schwarz MI. A 31-year-old man with chronic cough and hemoptysis. Chest. 2007;132:721–726. doi: 10.1378/chest.07-0712. [DOI] [PubMed] [Google Scholar]
- Castilla EA, Jessen R, Sheck DN, Procop GW. Cavitary mass lesion and recurrent pneumothoraces due to Paragonimus kellicotti infection: North American paragonimiasis. Am J Surg Pathol. 2003;27:1157–1160. doi: 10.1097/00000478-200308000-00015. [DOI] [PubMed] [Google Scholar]
- DeFrain M, Hooker R. North American paragonimiasis: case report of a severe clinical infection. Chest. 2002;121:1368–1372. doi: 10.1378/chest.121.4.1368. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Miller TB, Sharma SP. Fenbendazole for treatment of Paragonimus kellicotti infection in dogs. J Am Vet Med Assoc. 1979;174:835–837. [PubMed] [Google Scholar]
- Dubey JP, Toussant MJ, Hoover EA, Miller TB, Sharma SP, Pechman RD. Experimental Paragonimus kellicotti infection in dogs. Vet Parasitol. 1979;5:325–337. doi: 10.1016/0304-4017(79)90024-4. [DOI] [Google Scholar]
- Harrus S, Nyska A, Colorni A, Markovics A. Sudden death due to Paragonimus kellicotti infection in a dog. Vet Parasitol. 1997;71:59–63. doi: 10.1016/S0304-4017(97)00007-1. [DOI] [PubMed] [Google Scholar]
- Khanna A (1974) Survey of molluscan fauna of Punjab. Department of Zoology, Punjab Agricultural University, Ludhiana, India (Ph. D thesis submitted)
- Kirkpatrick CE, Shelly EA. Paragonimiasis in a dog: treatment with praziquantel. J Am Vet Med Assoc. 1985;187:75–76. [PubMed] [Google Scholar]
- Madariaga MG, Ruma T, Theis JH. Autochthonous human paragonimiasis in North America. Wilderness Environ Med. 2007;18:203–205. doi: 10.1580/06-WEME-CR-063R2.1. [DOI] [PubMed] [Google Scholar]
- Mariano EG, Borja SR, Vruno MJ. A human infection with Paragonimus kellicotti. Am J Clin Pathol. 1986;86:685–687. doi: 10.1093/ajcp/86.5.685. [DOI] [PubMed] [Google Scholar]
- Michael AL, Marry CB, Carlos AS, Michael Y, Sam JL, Gary JW. Human paragonimiasis in North America following ingestion of raw crayfish. Clin Infect Dis. 2009;49:55–61. doi: 10.1086/599038. [DOI] [PubMed] [Google Scholar]
- Narain K, Devi RK, Mahanta J. Paragonimus and paragonimiasis: a new focus in Arunachal Pradesh, India. Curr Sci India. 2003;84:985–987. [Google Scholar]
- Nelson OL, Sellon RK. Pulmonary parenchymal disease. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. 5. Elsevier: St. Louis; 2005. pp. 1239–1266. [Google Scholar]
- Pachucki CT, Levandowski RA, Brown VA, Sonnenkalb BH, Vruno MJ. American paragonimiasis treated with praziquantel. N Engl J Med. 1984;3:582–583. doi: 10.1056/NEJM198408303110906. [DOI] [PubMed] [Google Scholar]
- Pechman RD. Pulmonary paragonimiasis in dog and cats: a review. J Small Anim Pract. 2008;21:87–95. doi: 10.1111/j.1748-5827.1980.tb01218.x. [DOI] [PubMed] [Google Scholar]
- Procop GW. North American paragonimiasis (caused by Paragonimus kellicotti) in the context of global paragonimiasis. Clin Microbiol Rev. 2009;22:415–446. doi: 10.1128/CMR.00005-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim YS, Cho SY, Han YC. Pulmonary paragonimiasis: a Korean perspective. Semin Respir Med. 1991;12:35–45. doi: 10.1055/s-2007-1006223. [DOI] [Google Scholar]
- Singh YI, Singh NB, Devi SS, Singh YM, Razaque M. Pulmonary paragonimiasis in Manipur. Indian J Chest Dis Allied Sci. 1982;24:304–306. [Google Scholar]
- Singh TS, Mutum SS, Razaque MA. Pulmonary paragonimiasis: clinical features, diagnosis and treatment of 39 cases in Manipur. Trans R Soc Trop Med Hyg. 1986;80:967–971. doi: 10.1016/0035-9203(86)90275-0. [DOI] [PubMed] [Google Scholar]
- Singh TS, Sugiyama H, Umehara A, Hiese S, Khalo K. Paragonimus heterotremus infection in Nagaland: a new focus of paragonimiasis in India. Indian J Med Microbiol. 2009;27:123–127. doi: 10.4103/0255-0857.49424. [DOI] [PubMed] [Google Scholar]


