Abstract
Introduction
Burkitt lymphoma (BL) is endemic in parts of Tanzania, but there is scant country or region level data about burden and trends of BL in Tanzania over the past three decades. Here, we update baseline epidemiology of BL in northern Tanzania using recent data.
Procedure
Data for childhood BL diagnosed at six hospitals in Mara and Mwanza regions in northern Tanzania during 2000 – 2009 were compiled. Age, sex, and regional patterns were analyzed. Crude incidence rates of BL were calculated by sex, anatomic site, geographical region, and calendar year.
Results
Among 944 cases, 549 (58%) were male (male/female case ratio 1.4:1). Among those with known anatomic site (92%), facial only tumors represented a large proportion of tumors in boys than girls (50% versus 36%, P<0.002). Tumors occurred at a younger mean age in boys than girls (6.8 versus 7.6 years, P<0.01). Crude BL incidence was 4.2 per 100,000, but varied by region (3.0 in Mwanza versus 6.8 in Mara, P=0.01), by district (from 1.4 to 22), by gender (5.0 in boys versus 4.0 in girls) and by age group (2.0 in 0–4, 7.8 in 5–9, and 3.1 in 10–15 years). BL incidence peaked in 2001 and decreased gradually thereafter.
Conclusions
Our results indicate that male sex, young age, and geographical characteristics are risk factors for BL in Tanzania. BL incidence declined with calendar year, but the significance of this finding is uncertain. Well-designed epidemiological studies of BL in Tanzania may shed light on environmental characteristics underlying these patterns.
Keywords: Burkitt lymphoma, Malaria, Epstein Bar Virus, Epidemiology, Tanzania
Introduction
Burkitt lymphoma (BL) is endemic in sub-Saharan Africa, including parts of northern Tanzania [1, 2], where it accounts for 50 to 70% of all new childhood cancers diagnosed each year [3]. Endemicity of BL is linked to chronic and intense transmission of Plasmodium falciparum malaria [4] and infection with Epstein-Barr virus (EBV) at a very young age [5], when the immune system is still underdeveloped [5–7]. In support, ecological studies, at a global and local level, have demonstrated strong and positive correlation between BL endemicity and P. falciparum malaria indicating that malaria is a key determinant of population risk of BL [4, 8]. Because 95%–100% of endemic BL cases are EBV positive [9–11], EBV is generally considered necessary for endemic BL to develop.
Biologically, P. falciparum malaria is thought to influence BL risk by suppression of EBV-specific T-cell immune responses, which may lead to EBV reactivation and expansion of the EBV-infected B cell pool [12]. P. falciparum malaria may also influence risk directly by interacting with cystein-rich inter-domain region 1α (ICDR1α) receptor on B cells [13]. This interaction at a cellular level is postulated to selectively stimulate the proliferation of EBV-infected B cells.
Nonetheless, some questions remain about the nature of malaria (clinical or asymptomatic) or EBV (early age at infection or chronic viremia) that is immediately relevant to triggering BL onset. For example, hundreds of millions of children in Africa are infected by and harbor P falciparum malaria and EBV, but only relatively few develop BL [14]. The biological characteristics of malaria, EBV, or host genetics that influence the risk of BL in settings of high P. falciparum malaria endemicity are incompletely understood [8]. In addition we have little understanding of the relationship between biological markers of malaria, such as crude parasite prevalence, parasite load, mixed genotype infections and/or humoral immunity, and risk of BL. Also unexplained is why the risk of BL peaks between 5–9 years[16] in people who reside in malaria endemic areas, given that peak intensity of exposure is between 0–2 years and people continue to be exposed to sub-clinical and clinical malaria for life [14]. We would expect the biological effects of malaria to begin earlier and persist for life in holoendemic malaria areas.
As part of a plan to conduct an epidemiological study to examine the link between BL and malaria in East Africa [15], including Tanzania, we implemented a systematic search of BL cases diagnosed over a ten year period in Mara Region in northern Tanzania, where BL is historically known to be endemic [16], and in Mwanza Region, which is adjacent to Mara and has high malaria endemicity, but BL epidemiology has not been studied before. Knowledge of baseline patterns of BL in the region would guide our plans to implement the BL study and interpret findings, and the BL patterns and trends would provide clues about underlying risk factors that may be evaluated in future hypothesis-driven studies.
Methods
Study population
BL data for children (0–15 years) during the ten years from 2000 to 2009 were compiled from six hospitals in Mara (Bunda and Shirati) and Mwanza (Sumve, Bugando Medical Center, Sengerema and Bukambi) regions. A research assistant compiled case information, including age at diagnosis, sex, calendar month and year of diagnosis, primary anatomic site of the tumor, method of diagnosis, histopathology, and address of residence (village and district) through hospital chart abstraction. The information was keyed into an epidemiological database for analysis. We restricted analysis to cases with a home address in the Mara and Mwanza Regions. These regions lie on the south-eastern shores of Lake Victoria, about 2 degrees south of the Equator and experience hyperendemic or holoendemic malaria. The regions are comprised of twelve districts (5 in Mara and 7 in Mwanza) and are home to about 4.3 million people, including two million (44.2%) children (0–15years) (Tanzania National Census, 2002). Cases were diagnosed clinically only. In a few cases, clinical diagnosis was supplemented by fine needle aspiration (FNA). The methods available for diagnosis of BL did not change during the period covered by the study. In addition, use of FNA was influenced mostly by availability a doctor to interpret FNA and not ability to perform FNA at the local facility. The study included all hospitals in the region with capacity to diagnose and treat BL in the regions. The casual impression of the local doctors was that the cases were representative of cases from the region. We assumed that, given the severity and relentless nature of BL, most cases will seek hospital treatment at some point during their disease, usually at a hospital within the region. However, given the rapidly growing nature of the disease, it is plausible that some children might die before reaching hospital and some might reach a hospital but could die before being diagnosed. Thus, the completeness of these case series for the regions is uncertain. No effort was made to search for cases from these two regions that may have been treated at hospital outside the region
Statistics
We used frequency tables to analyze distribution of BL by sex, tumor anatomic site (only face or head tumors, abdominal tumors with or without face or head involvement, and others sites, including unspecified), age group (0–3, 4–6, 7–9, 10–12, and 13–15), and calendar year period (2000–2004 and 2005–2009). We used Chi-squared tests to test the null hypothesis that categorical variables are independent from each other, and the Student’s t-test to test the null hypothesis that the means for variables with a continuous distribution are equal. Age of BL diagnosis and calendar year were considered to be important proxies of exposures related to developmental biology or the environment (intrinsic and extrinsic exposures), so we investigated the proportional distribution of BL cases according to age of diagnosis and of calendar year measured in single years. These patterns were assessed for all cases combined and by tumor anatomic site (facial only or abdominal with or without facial involvement).
Incidence rates provide the best measure of risk. We estimated crude incidence rates of BL by sex, age group, calendar year, and geography (district and region). We estimated the population at risk during the study period by extrapolating from the population of the region obtained in the 2002 national census. Starting from 2002, the annual (mid-year) age- and sex-specific population for each calendar year was extrapolated, backwards to 2000 and forwards to 2009. The extrapolation was done separately for each region using population growth rates of 2.6% for Mara and 3.2% for Mwanza regions (Tanzania National Census, 2002) to obtain estimates of the population count of the children residing in the study area during the study period, by age, sex, and region. The numbers obtained from these calculations were summed up to get an estimate of the population count (or person-years at risk) for the two regions for the period covered by the study. Because population counts by single year of age were not available for Mara and Mwanza, we estimated the population at risk in these age groups by applying the age-group specific proportions for the Tanzanian population in 2002 to the calculated population of children aged 0–15 years in Mwanza and Mara regions. These calculations were done based on the assumption that the average population growth rate for Tanzania was not significantly different for males and females or for children living in rural or urban areas. P values < 0.05 were considered statistically significant.
Results
There were 944 BL cases identified. Of these, 549 (58%) were in boys and 390 (42%) were in girls (Table I). The overall mean age at diagnosis was 7.1 years (standard deviation [SD] 2.9 years); mean age at diagnosis was lower in boys than girls (6.8 years [SD 2.7] versus 7.6years [SD 3], P<0.01). The proportion of cases at different ages increased from <1% in children aged less than 1 year to 8% in children aged 1–3 years and peaked at 39% in children aged 5–9 years and then decreased gradually to 4.6% by age 13–15 years. The male/female case ratio was 1.4:1, but it varied with age from 1.2:1 in children aged 0–3 years to 1.9:1 in children aged 4 to 6 years and then it reversed to less than unity (0.6:1) in children aged 13–15 years.
Table I.
Characteristics of Burkitts lymphoma cases diagnosed in northern Tanzania
| Characteristic | Number | Percent (%) | P-value |
|---|---|---|---|
| All subjects | 944 | (100) | |
| Sex | 0.001 | ||
| Male | 549 | (58) | |
| Female | 390 | (42) | |
| Age group (years) | 0.005 | ||
| 1–3 | 74 | (7.9) | |
| 4–6 | 368 | (39.4) | |
| 7–9 | 294 | (31.4) | |
| 10–12 | 156 | (16.7) | |
| 13–14 | 43 | (4.6) | |
| Tumor anatomic site | 0.120 | ||
| Facial only | 387 | (44.5) | |
| Abdominal | 432 | (49.7) | |
| Other | 50 | (5.7) | |
| Diagnosis year | 0.001 | ||
| 2000–2004 | 540 | (57.2) | |
| 2005–2009 | 404 | (42.8) | |
| Season | 0.06 | ||
| Dry | 499 | (53.1) | |
| Wet | 441 | (46.9) | |
| District | |||
| Bunda | 65 | (7.1) | 0.007 |
| Musoma rural | 40 | (4.3) | |
| Musoma urban | 60 | (6.5) | |
| Serengeti | 45 | (4.9) | |
| Tarime | 272 | (29.5) | |
| Sengerema | 153 | (16.6) | |
| Geita | 90 | (9.8) | |
| Mwanza urban | 68 | (7.4) | |
| Kwimba | 45 | (4.9) | |
| Magu | 28 | (3.0) | |
| Misungwi | 34 | (3.7) | |
| Ukerewe | 21 | (2.3) |
P-value for heterogeneity
Anatomic site information was complete for 869 (92%) cases. Slightly more than half the cases involved abdominal organs, with or without face, compared to only face or head (49.7% versus 41.5%, P<0.12). The male/female case ratio was 1.9:1 for BL cases involving only face or head tumors and 1.1:1 for BL cases involving abdominal sites. Age of BL diagnosis was lower in boys than girls regardless of tumor anatomic site (6.3 versus 6.9 years, P=0.046, both for only face or head tumors and 7.2 years versus 8.0 years, P<0.004, for abdominal tumors).
The number of cases diagnosed during 2000–2004 was higher than the number diagnosed during 2005 to 2009 (57.2% versus 42.8%) (Table I). This pattern in case numbers was observed in Mara Region, but it was not apparent in Mwanza Region. The number of cases diagnosed varied slightly by season (53% in dry season versus 47% in rainy season, P=0.06), but not by region (52% in Mara Region and 48% in Mwanza Region, P=0.19).
The proportional distribution of BL cases showed non-specific variation by calendar year (Figure 1A). No long-term temporal trends were observed for tumors involving only face or head or abdominal sites. Conversely, anatomic site involvement showed contrasting patterns with age. BL cases involving only face or head peaked at 4 years and then tapered, whereas BL cases involving abdominal sites increased progressively with age (Figure 1B).
Fig. 1.
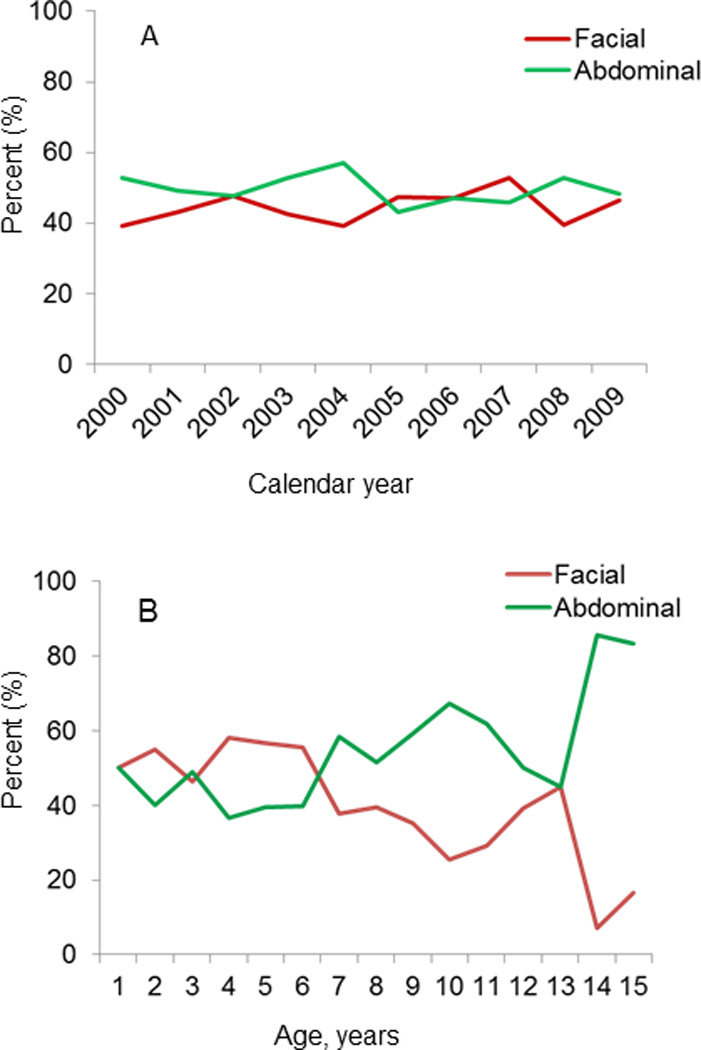
Line graph showing the proportional distribution of anatomic site of Burkitt lymphoma (facial only, or abdominal, with or without, facial involvement) by calendar years (A) or by age in single years (B).
The overall crude BL incidence was 4.2 per 100,000, and it was higher in boys than girls (5.0 versus 4.0, P=0.01). The incidence was higher in boys than girls in children aged 0–4 years (P=0.04), 5–9 years (P=0.04), but this pattern was reversed in children aged 13–15 years in whom the incidence was higher in girls than boys (P=0.05) (Figure 2). In regional comparisons, BL incidence was higher in Mara than Mwanza region (6.8 versus 3.0, P=0.001). Within regions, BL incidence was highest Tarime District in Mara Region (22 cases per 100,000, Figure 3A) and lowest in Magu District in Mwanza Region (<2 cases per 100,000) (Figure 3B). District-specific BL incidence rates were more variable in Mara Region and less variable in Mwanza Region (Figures 4A and B). The annual incidence of BL in both regions, overall as well as district-specific, declined with calendar period (Figures 4A and B).
Fig. 2.
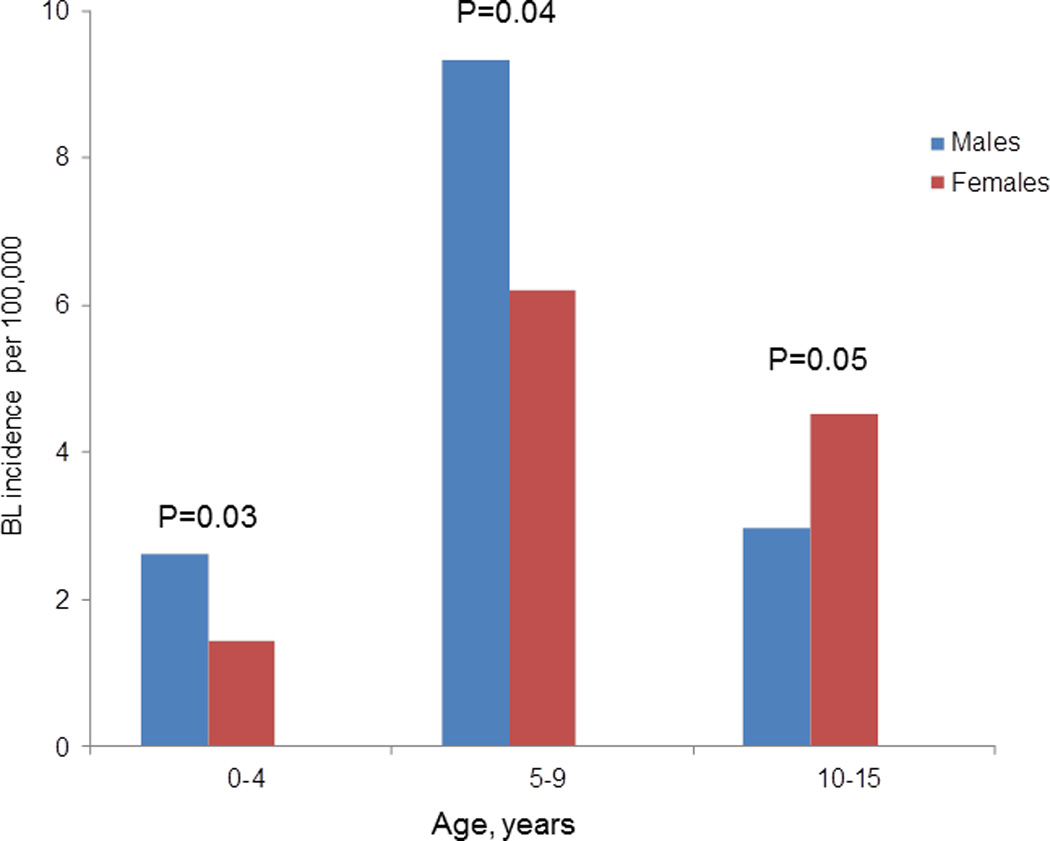
Age specific burkitt lymphoma incidence rate for male and female by age group. (P-Values are for evaluation of the hypothesis that the incidence rate of BL in males is equal to that in females, not adjusted for multiple comparisons).
Fig. 3.
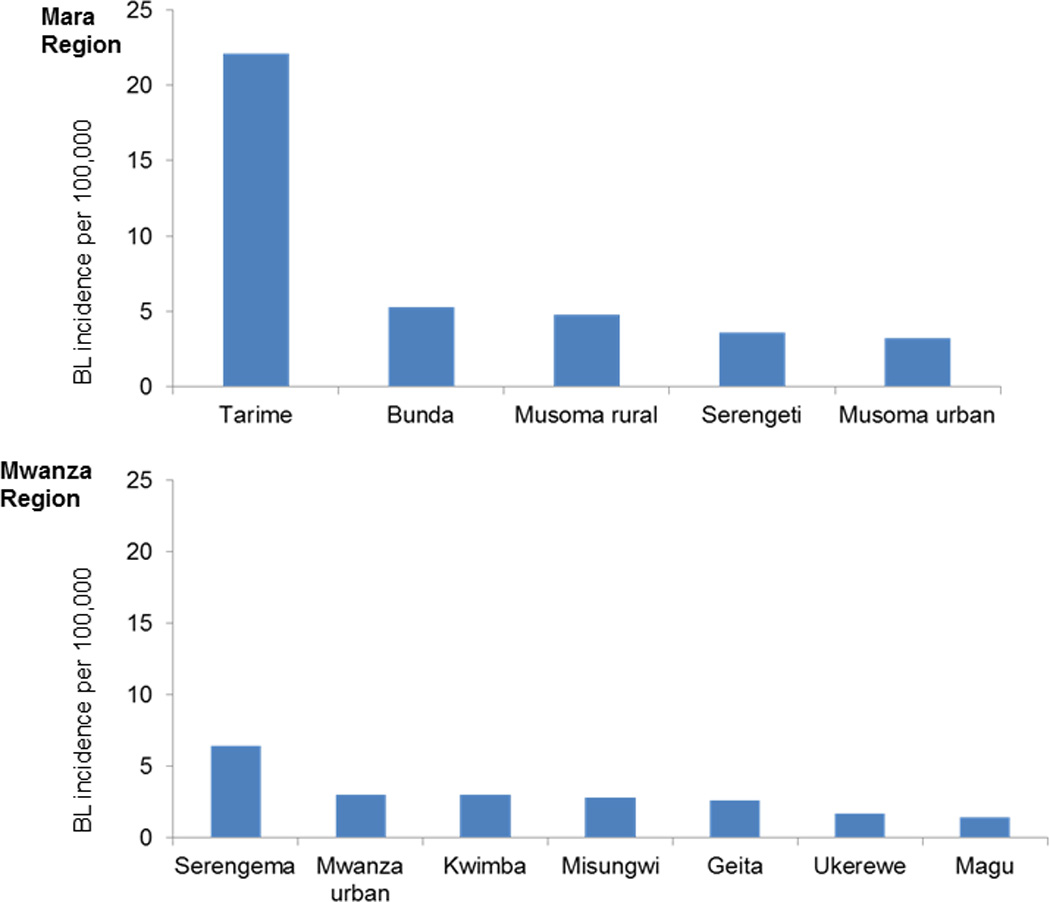
Crude district-specific Burkitt lymphoma incidence rates for Mara and Mwanza regions.
Fig. 4.
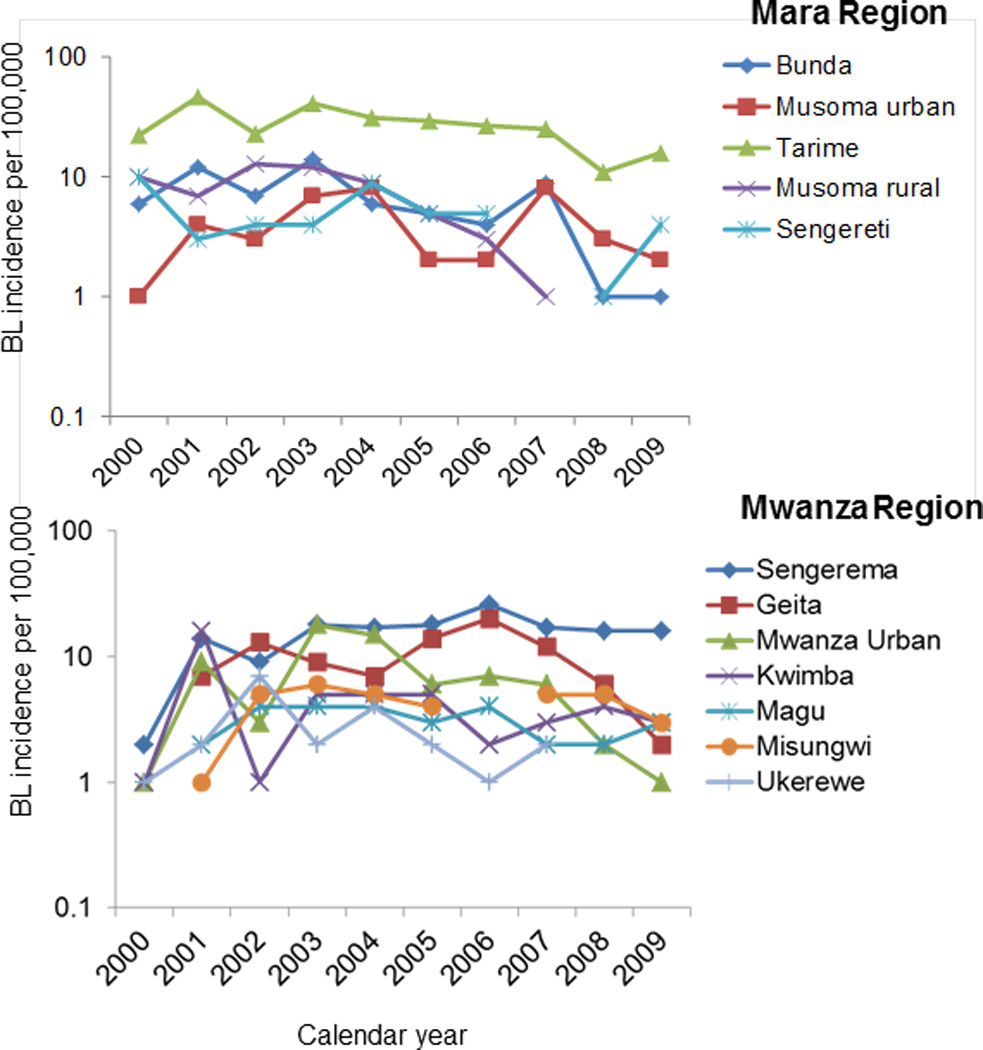
Crude district-specific Burkitt lymphoma incidence rates by calendar year for Mara Region and Mwanza Region.
Discussion
Our study provides a recent snapshot of BL epidemiology in two regions of northern Tanzania, including Mara where BL has historically been known to be endemic [16]. Several features of BL in northern Tanzania have remained stable relative to historical patterns, despite an alarming HIV/AIDS epidemic that spread in the region 1980s and 1990s [17]. These include male predominance, peak age at 5–9 years, slight preponderance of abdominal versus facial tumors, and lack of seasonal variation of BL.
Some of our observations are new. These include the first estimate of BL incidence for Mwanza region. We were surprised that BL incidence in Mwanza was lower than in Mara, although both regions lie on the shores of Lake Victoria, just south of Mara region, are traversed by numerous rivers and streams, and experience holoendemic malaria. This might be because Mwanza, being more urban, has lower malaria prevalence. Second, the BL incidence for Mara calculated in our study appears higher than that observed historically in that region. This might be due to improved case-ascertainment in the Mara Region. Interestingly, for both regions, we observed that BL incidence appeared to peak in 2001 and has been falling since. The fall in BL incidence was broad based in both regions and in all districts in each region, suggesting that these patterns may be valid. The casual impression of clinicians in Tanzania is that access to care and quality of care has been improving, thus, the decreasing BL incidence is unexpected. Improvements in access to and quality of care in Uganda associated with temporal increase in BL incidence have been reported [18, 19].
The temporal patterns in BL incidence reported here are intriguing. They could be a clue to recent changes in the underlying epidemiology of malaria [4, 8], a key determinant of BL incidence. A decline in BL incidence was reported by Geser et al., in the Mara Region during the 1980s [20] following an intervention to suppress malaria in children using chloroquine doses. Although the Government of Tanzania has implemented aggressive community malaria intervention programs, including indoor residual spraying, mosquito bed net provision, and supply of malaria medicines at the village level, these programs were introduced in northern Tanzania relatively recently during 2005 to 2010. The prevalence of malaria parasitaemia among febrile children in northeastern parts of Tanzania, which are adjacent to our study regions, fell from 78% in 2003 to 24% in 2006 in low-lying areas and from 25% to 7% in mountainous highland areas [21]. However, we lack similar data on for Mara and Mwanza regions to correlate with observed patterns in BL incidence.
Although unlikely, because BL in Africa is usually HIV negative [22], trends in HIV should be considered theoretically a possible explanation of declining incidence of BL. A significant association between BL and HIV has been reported in two case-control studies in East or central Africa [23, 24] and, while conflicting results, of non-statistically significant association with HIV in two other case-control studies cast doubt on this suggestion [25, 26], the confidence intervals in those studies were compatible with an elevated risk of BL with HIV. Thus, we are intrigued by the coincident decrease in HIV incidence in rural Tanzania, from a peak of 1.5% in the early 1990s to 0.5- 0.7% during 2008 [33]. We lack data on HIV prevalence in BL cases, which would be more informative, and numerous reports from the region indicating that BL cases are usually HIV negative [22] suggests that this explanation is unlikely to be valid. Perhaps, collaboration between HIV and malaria programs would make it possible to answer questions about the relationship between these conditions in populations where all the disease are relatively common [27].
Several of the epidemiological features of BL that we observed, while not new, cannot be explained based on our current understanding of BL and the biology of its causal factors. For example, endemic BL incidence peaks during 5–9 years [19, 28], but this is true in sporadic [29] and AIDS-related BL as well [30]. Given that sporadic BL and AIDS-related BL are not related to malaria, the peak age of BL may be related to developmental age rather than environmental exposures such as malaria. Secondly, BL erupts at a younger mean age in boys than girls and when the tumor erupts on the face or head, the male/female case ratio is always extreme. This pattern is observed in endemic, sporadic, and AIDS-related BL. Thus, features common to all BL, regardless of epidemiological setting, suggest a role of intrinsic individual determinants of risk [31, 32], perhaps genetic or epigenetic factors that modulate developmental biology. A difference in age- and sex-adjusted incidence suggests a role of environmental factors, such as malaria and EBV.
Our results should be interpreted with caution. First, we used data compiled from routine hospital records, which may be incomplete and/or inaccurate. Second, our assumptions made to calculate BL incidence rates might be incorrect. For example, the assumption that BL cases present to hospital at some point during illness and that when they do, they will be diagnosed has not been verified. BL occurring on the face is relatively easy to suspect and a clinical diagnosis has high predictive value, but BL occurring in the abdomen often requires ultrasound to suspect and diagnose. We do not know to what extent non BL cases might have been included in our data, although we suspect the proportion is likely smaller than cases that were missed. The strength of our study includes gathering data from a well-defined geographic region, and relatively large case numbers. Our study also highlights the value of using routine clinical data to get data of public health significance. In particular, the quality of our analysis and impact of results would have been much improved if our data were derived from a population-based cancer registry, which is currently not available in Tanzania.
To conclude, our study of recent BL epidemiology in Northern Tanzania confirms high, but variable incidence rates in Mwanza and in Mara region. BL incidence decreased over the study period, starting in 2001, but the reasons for the decrease are unknown. Our study confirms, as we suspected, that there is large number of cases of BL in northern Tanzania, which are needed to conduct informative epidemiological studies of BL. Improvements on existing diagnosis and data capture infrastructure may be required to obtain reliable data.
Footnotes
Conflict of interest: Nothing to declare.
References
- 1.Carneiro PM, Kalokola FM, Kaaya EE. Paediatric malignancies in Tanzania. East Afr Med J. 1998;75:533–535. [PubMed] [Google Scholar]
- 2.Mgaya EM, Kitinya JN. Histopathology of malignant tumours of childhood in Tanzania. East Afr Med J. 2000;77:435–439. [PubMed] [Google Scholar]
- 3.Parkin DM, Sitas F, Chirenje M, et al. Part I: Cancer in Indigenous Africans--burden, distribution, and trends. Lancet Oncol. 2008;9:683–692. doi: 10.1016/S1470-2045(08)70175-X. [DOI] [PubMed] [Google Scholar]
- 4.Morrow RH., Jr Epidemiological evidence for the role of falciparum malaria in the pathogenesis of Burkitt's lymphoma. IARC Sci Publ. 1985:177–186. [PubMed] [Google Scholar]
- 5.de-The G, Geser A, Day NE, et al. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt's lymphoma from Ugandan prospective study. Nature. 1978;274:756–761. doi: 10.1038/274756a0. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter LM, Newton R, Casabonne D, et al. Antibodies against malaria and Epstein-Barr virus in childhood Burkitt lymphoma: a case-control study in Uganda. Int J Cancer. 2008;122:1319–1323. doi: 10.1002/ijc.23254. [DOI] [PubMed] [Google Scholar]
- 7.de-The G. Epstein-Barr virus and Burkitt's lymphoma worldwide: the causal relationship revisited. IARC Sci Publ. 1985:165–176. [PubMed] [Google Scholar]
- 8.Rainey JJ, Mwanda WO, Wairiumu P, et al. Spatial distribution of Burkitt's lymphoma in Kenya and association with malaria risk. Trop Med Int Health. 2007;12:936–943. doi: 10.1111/j.1365-3156.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- 9.Khanna R, Burrows SR, Moss DJ. Immune regulation in Epstein-Barr virus-associated diseases. Microbiol Rev. 1995;59:387–405. doi: 10.1128/mr.59.3.387-405.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geser A, de The G, Lenoir G, et al. Final case reporting from the Ugandan prospective study of the relationship between EBV and Burkitt's lymphoma. Int J Cancer. 1982;29:397–400. doi: 10.1002/ijc.2910290406. [DOI] [PubMed] [Google Scholar]
- 11.Shiramizu B, Barriga F, Neequaye J, et al. Patterns of chromosomal breakpoint locations in Burkitt's lymphoma: relevance to geography and Epstein-Barr virus association. Blood. 1991;77:1516–1526. [PubMed] [Google Scholar]
- 12.Rogerson SJ, Wijesinghe RS, Meshnick SR. Host immunity as a determinant of treatment outcome in Plasmodium falciparum malaria. Lancet Infect Dis. 2010;10:51–59. doi: 10.1016/S1473-3099(09)70322-6. [DOI] [PubMed] [Google Scholar]
- 13.Chene A, Donati D, Orem J, et al. Endemic Burkitt's lymphoma as a polymicrobial disease: new insights on the interaction between Plasmodium falciparum and Epstein-Barr virus. Semin Cancer Biol. 2009;19:411–420. doi: 10.1016/j.semcancer.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter D, Rooth I, Farnert A, et al. Genetics of susceptibility to malaria related phenotypes. Infect Genet Evol. 2009;9:97–103. doi: 10.1016/j.meegid.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Baik S, Mbaziira M, Williams M, et al. A case-control study of Burkitt lymphoma in East Africa: are local health facilities an appropriate source of representative controls? Infect Agent Cancer. 2012;7:5. doi: 10.1186/1750-9378-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geser A, Brubaker G. A preliminary report of epidemiological studies of Burkitt's lymphoma, Epstein-Barr virus infection and malaria in North Mara, Tanzania. IARC Sci Publ. 1985:205–215. [PubMed] [Google Scholar]
- 17.Orroth KK, Korenromp EL, White RG, et al. Higher risk behaviour and rates of sexually transmitted diseases in Mwanza compared to Uganda may help explain HIV prevention trial outcomes. AIDS. 2003;17:2653–2660. doi: 10.1097/00002030-200312050-00013. [DOI] [PubMed] [Google Scholar]
- 18.Wabinga HR, Parkin DM, Wabwire-Mangen F, Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda, 1960–1997. Br J Cancer. 2000;82:1585–1592. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogwang MD, Bhatia K, Biggar RJ, Mbulaiteye SM. Incidence and geographic distribution of endemic Burkitt lymphoma in northern Uganda revisited. Int J Cancer. 2008;123:2658–2663. doi: 10.1002/ijc.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geser A, Brubaker G, Draper CC. Effect of a malaria suppression program on the incidence of African Burkitt's lymphoma. Am J Epidemiol. 1989;129:740–752. doi: 10.1093/oxfordjournals.aje.a115189. [DOI] [PubMed] [Google Scholar]
- 21.O'Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10:545–555. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- 22.Mbulaiteye SM, Parkin DM, Rabkin CS. Epidemiology of AIDS-related malignancies an international perspective. Hematol Oncol Clin North Am. 2003;17:673–696. doi: 10.1016/s0889-8588(03)00048-0. v. [DOI] [PubMed] [Google Scholar]
- 23.Mutalima N, Molyneux E, Jaffe H, et al. Associations between Burkitt lymphoma among children in Malawi and infection with HIV, EBV and malaria: results from a case-control study. PLoS One. 2008;3:e2505. doi: 10.1371/journal.pone.0002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newton R, Ziegler J, Beral V, et al. A case-control study of human immunodeficiency virus infection and cancer in adults and children residing in Kampala, Uganda. Int J Cancer. 2001;92:622–627. doi: 10.1002/1097-0215(20010601)92:5<622::aid-ijc1256>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 25.Mutalima N, Molyneux EM, Johnston WT, et al. Impact of infection with human immunodeficiency virus-1 (HIV) on the risk of cancer among children in Malawi - preliminary findings. Infect Agent Cancer. 2010;5:5. doi: 10.1186/1750-9378-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parkin DM, Garcia-Giannoli H, Raphael M, et al. Non-Hodgkin lymphoma in Uganda: a case-control study. AIDS. 2000;14:2929–2936. doi: 10.1097/00002030-200012220-00015. [DOI] [PubMed] [Google Scholar]
- 27.Mbulaiteye SM, Talisuna AO, Ogwang MD, et al. African Burkitt's lymphoma: could collaboration with HIV-1 and malaria programmes reduce the high mortality rate? Lancet. 2010;375:1661–1663. doi: 10.1016/S0140-6736(10)60134-1. [DOI] [PubMed] [Google Scholar]
- 28.Emmanuel B, Kawira E, Ogwang MD, et al. African Burkitt lymphoma: age-specific risk and correlations with malaria biomarkers. Am J Trop Med Hyg. 2011;84:397–401. doi: 10.4269/ajtmh.2011.10-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mbulaiteye SM, Biggar RJ, Bhatia K, et al. Sporadic childhood Burkitt lymphoma incidence in the United States during 1992–2005. Pediatr Blood Cancer. 2009;53:366–370. doi: 10.1002/pbc.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guech-Ongey M, Simard EP, Anderson WF, et al. AIDS-related Burkitt lymphoma in the United States: what do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood. 2010;116:5600–5604. doi: 10.1182/blood-2010-03-275917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14:32–38. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 32.Rose G. Sick individuals and sick populations. 1985. Bull World Health Organ. 2001;79:990–996. [PMC free article] [PubMed] [Google Scholar]
- 33.Global report: UNAIDS report on the global AIDS epidemic. 2010. [Google Scholar]


