Abstract
Background
Deficits in smooth pursuit eye movements (SPEM) are an established phenotype for schizophrenia (SZ) and are being investigated as a potential liability marker for bipolar disorder. While the molecular determinants of this deficit are still unclear, research has verified deficits in predictive pursuit mechanisms in SZ. Because predictive pursuit may depend on the working memory system, we have hypothesized a relationship between the two in healthy controls (HC) and SZ, and here examine whether it extends to psychotic bipolar disorder (BDP).
Methods
Volunteers with SZ (n = 38), BDP (n = 31), and HC (n = 32), performed a novel eye movement task to assess predictive pursuit as well as a standard visuospatial measure of working memory.
Results
Individuals with SZ and BDP both showed reduced predictive pursuit gain compared with HC (p <.05). Moreover, each patient group showed worse performance in visuospatial working memory compared to controls (p <.05). A strong correlation (r = .53, p =.007) was found between predictive pursuit gain and working memory in HC, a relationship that showed a trend correlation within the BDP group but not among SZ.
Conclusions
Individuals with SZ and BDP showed similar deficits in predictive pursuit, suggesting that this alteration could be a characteristic trait of the psychosis domain. The correlation between predictive pursuit and working memory in HC supports the assumption that working memory is related to predictive pursuit eye movements; however, the degradation of working memory in people with psychosis disrupts its association with eye tracking behavior.
Keywords: psychosis, schizophrenia, bipolar disorder, smooth pursuit eye movement, predictive pursuit, working memory
Introduction
Smooth pursuit eye movements (SPEM) are used as biomarker measurements to reflect underlying brain alterations in neuropsychiatric conditions and to test specific biological hypotheses about disease mechanisms. An abundance of evidence implicates SPEM deficits in individuals with schizophrenia (SZ) (reviewed in 1;2) and their biological relatives (reviewed in 3). SPEM deficits have also been reported in affective disorders in some studies (4;5), but not all (6;7), providing mixed evidence for a shared phenotype across these disorders, potentially within the dimension of psychosis. Such data have lead to an increasing interest in refining the measurement of SPEM as a phenotype. The mechanisms of SPEM deficits have been variously explained by (1) a failure of saccade inhibition in the frontal eye fields and temporal-parietal cortex (8), (2) deficits in the motion processing system, mediated in the middle temporal and medial superior temporal areas (9), and (3) a dysfunction of predictive eye movements, implicating the prefrontal and parietal cortex (10;11). This is consistent with hypotheses implicating dysfunction of the frontal cortex in schizophrenia pathophysiology.
While a general deficit in cognition is associated with SZ (12;13), a selective dysfunction in working memory has been well established and is often proposed as a potential candidate phenotype. Park and Holzman (14) reported that individuals with SZ were impaired on a delayed response task adapted from Goldman-Rakic’s classic working memory paradigm (15). Park and Holzman also reported an association between working memory on an oculomotor delayed response task and SPEM (16), providing evidence for a relationship between these cognitive and oculomotor tasks of frontal functions. Several other studies have reported a relationship between cognition and eye movements in SZ. Snitz et. al (16) reported a deficit in SZ in the working memory component of a spatial delayed response task as well as a correlation between SPEM and spatial working memory. However, the evidence of a relationship between working memory and smooth pursuit is mixed. A recent study reported no correlation between verbal and spatial working memory as measured by the N-back task and predictive smooth pursuit in a sample of 1,100 healthy young men (17). Litman et al. (18) found a correlation between performance on the Wisconsin Card Sorting Test, a task of mental flexibility and set-shifting, and gain during smooth pursuit, although this has not been consistently replicated (19). Interestingly, considerable heterogeneity in cognitive function exists within the bipolar disorder (BD) population, with some BD individuals manifesting profound deficits in cognition, and others showing little or none. Bipolar individuals with a history of psychosis may have more severe impairments on measures of executive functioning and spatial working memory than non-psychotic bipolars (20). We hypothesize that BD with psychosis individuals will show working memory deficits similar to SZ individuals. Based on multiple-component working memory theory which suggests working memory is comprised of separate auditory and visual-spatial components controlled by a central executive controller (21), the current study chose a visual-spatial task1 to investigate this specific aspect of working memory.
The predictive mechanism of smooth pursuit has traditionally been measured by recording eye movement in response to a briefly masked (invisible) target (24;25). In the current study, we use a new paradigm which examines the predictive pursuit response without removing the target from visibility (26). This allows us to examine a subject’s ability to generate predictive pursuit using memory of the target’s previous velocity without disrupting the test process by removing the target from sight. The movement of the eye during this task is based on extra-retinal information, (i.e., the internal representation of target velocity). This extra-retinal processing of the target velocity information is considered part of the working memory system, suggesting that working memory dysfunction in psychosis is associated with the abnormal predictive response (27). The current study aims to characterize not only the nature of predictive pursuit in SZ and BD but also the relationship between predictive pursuit and working memory across the psychosis dimension and healthy comparison (HC) subjects, based on the hypothesis that working memory contributes to the predictive pursuit mechanism and that both are disordered in psychosis. These may be promising phenotypic markers of psychosis.
Methods and Materials
Participants
Thirty-eight individuals with a diagnosis of SZ and 31 individuals with BDP were recruited from the Dallas county area through advertising and by referral from community mental health centers and from the University of Texas Southwestern Medical Center outpatient clinics. Thirty-two HC subjects without a history of Axis I or II diagnoses, and no reported family history of any psychotic illness in first or second degree relatives were similarly recruited. Patient volunteers were clinically stable outpatients with no hospitalizations in the past thirty days at the time of entering the study. Diagnoses were determined at consensus conferences using all available clinical data, including data from the Structure Clinical Interview for DSM-IV (SCID, Modules A – E) (28) as well a modified version of the Family History Research Diagnostic Criteria (FH-RCD) interview (29). Participants with a history of mood disturbance were classified per the FH-RDC as either schizoaffective – mainly schizophrenic type (n = 9), and included in the SZ group, or schizoaffective – mainly affective type (n = 1), and included in the BDP group. Only BDP with a lifetime history of psychotic symptoms, current or past, were included in this study. Inclusion criteria limited ages to between 16 and 58 years due to the known effects of age on eyetracking outside of this range (30). Subjects with a history of major neurological or decompensated medical illness, substance abuse within the last month, or substance dependence within the last 3 months were excluded. The study was approved by the institutional review board of UT Southwestern Medical Center and participants provided written informed consent of the full disclosure.
Experimental Procedures
Horizontal eye movements were recorded using a video-camera based system (EyeLink II eyetracker, SR Research, Osgoode, Canada) sampling at 500 Hz in a room with controlled illuminance of 2 lux. A target (a cross in a .25 × .25 degree box) was presented on a 22-inch flat screen monitor (ViewSonic, P225f Professional System) set to 150 Hz, placed 60 cm in front of the subject. Subjects were required to abstain from nicotine and caffeine for a minimum of 30 minutes before eye movement testing.
The digital data were filtered off-line using a low pass filter at two cutoff frequencies, 75 and 20 Hz, using data acquisition and analysis software (AcqKnowledge Version 3.7.3, Goleta, CA and IGOR Pro Version 5.0, Wavemetrics, Inc.). Data were all inspected visually to eliminate artifacts (blinks) and saccades. Saccades were identified based on velocity (>35°/sec) and acceleration (>600°/sec2) criteria. All saccades and artifacts were identified as missing data points.
Predictive Pursuit Task
The experimental procedures for this task have been described in detail elsewhere (26) and are summarized here. The predictive pursuit task consisted of two 4-minute sessions. Each session included 12 trials at 9.9°/sec and 12 trials at 18.7°/sec. A trial started with calibration steps at − 12, 0 and + 12 degrees of visual angle until the error between the target and the eye was less than .1 degree, followed by 1 to 3 seconds of center fixation. The target traversed horizontally across the screen at 24 degrees of visual angle at a steady velocity of either 9.9 or 18.7°/sec (a ramp). After 1–3 ramps, a virtual window was triggered to open during which the software would covertly switch the driver of the target from the computer to the eye if two conditions were met: the eye speed being near zero (−0.8°/sec to +0.8°/sec) and the target within − 50ms to + 200ms of the change in direction (26). Since the target image remains foveated, there is no motion of the target image on the retina and the memory of the previous target velocity drives the eye, which in turn drives the target on the monitor without the subject’s awareness. The duration of this virtual predictive window was 1 second. All subjects were naïve to the task, and no subjects reported any awareness of notable event in the target behavior when the stabilization occurred and their eye drove the target for the one second predictive window. The literature indicates differences between patient and control groups are more evident at higher target speeds ranging between 15 and 20°/sec , and that differences are not as robust at lower target speeds (10). The current task included trials at both 9.9 and 18.7°/sec. It was our prediction that differences would be more evident at the higher speed.
Working Memory Performance
Subjects completed a well-standardized visuospatial working memory task, the Spatial Span subtest from the Wechsler Memory Scale, Third edition (WMS-III) (22). An estimate of general intellectual level was obtained from the Wechsler Test of Adult Reading (WTAR) (31). Age-corrected scaled scores for total performance on the Wechsler Spatial Span task were obtained (mean = 10, standard deviation = 3). The Wechsler Test of Adult Reading IQ estimate is reported as a standard score with a mean of 100 and standard deviation of 15).
Statistical Analysis
Statistical analyses were conducted using SPSS version 12.0 (SPSS, Inc., Chicago IL). Descriptive statistics were examined for demographic characteristics of the three groups. A one-way analysis of variance (ANOVA) with a subsequent post hoc Tukey HSD test was used to examine between-group differences in socio-demographic characteristics. Yates corrected chi-square test was used to evaluate between-group differences on nominal variables.
Predictive pursuit gain was calculated using all velocities measured at 50 ms epochs during the 1-second predictive window using the artifact-free (saccades and blinks removed) eye velocity divided by the target velocity. Maintenance pursuit gain was calculated in the same manner using velocities collected over 1 second during the preceding ramp with the regular pursuit target. One-way analyses of variance (ANOVA) with a subsequent post hoc Tukey HSD test were used to examine between-group differences in gain at both 18.7 and 9.9°/sec. Based on our hypothesis that group differences would be more evident at higher speeds, we examined the relationship between working memory and predictive pursuit gain during the 18.7°/sec condition, using a one-tailed Pearson product moment correlation, adjusted for age.
To further characterize smooth pursuit, eye velocity was averaged across trials by 50-ms epochs of the 1–second predictive windows for each trial at the two target speeds, 9.9°/sec and 18.7°/sec. For comparison, maintenance pursuit velocity from the preceding ramp was also analyzed by 50-ms epochs at each of the two target speeds. In analysis, subjects whose values were based on less than 5 trials with valid data were not included as this is the threshold below which results may be unreliable. In the 18.7°/sec condition, applying this threshold excluded 5 SZ, 4 BDP, and 8 HC; in the 9.9°/sec condition, 4 SZ, 6 BDP, and 7 HC were excluded. Eye velocity during predictive and maintenance pursuit at each target speed was evaluated for the three groups using individual repeated measures ANOVAs, with subsequent post hoc Tukey HSD tests for significant results. Velocity was a within-subjects factor; group was the between-subjects factor. For this analysis of 50 ms epochs, epoch was included as a within-subjects factor. Degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity. Age was used as a covariate in these analyses due to its known influence on eyetracking performance. For all analyses, probability values below .05 were considered significant.
Results
Demographic and clinical characteristics
Summary characteristics of the samples are provided in Table 1. Age was significantly different between the patient groups and thereafter used as a covariate in relevant subsequent analyses (F (2, 83) = 4.542, p < .001; post hoc Tukey HSD: SZ vs. BDP, p = .014; SZ vs. HC, p = .140; BDP vs. HC, p = .895).
Table 1. Demographics, Working Memory, and Pursuit Gain Measures.
Age was significantly different between the patient groups and thereafter used as a covariate in relevant subsequent analyses (F (2, 83) = 4.542, p < .001; post hoc Tukey HSD: SZ vs. BDP, p = .014; SZ vs. HC, p = .140; BDP vs. HC, p = .895). BPRS data was missing for 3 SZP and 2 BDP.
| SZ ( n = 38) | BDP (n = 31) | HC (n = 32) | X2 or F 2,83 value | P value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Sex, % Male | 60.5 | 35.5 | 46.8 | 4.34 | .11 |
| Ethnicity, C:AA:H:O | 24:12:1:1 | 22:3:4:2 | 27:3:2:0 | 12.42 | .053 |
| Age, mean ± SD | 41.9 ± 9.5 | 35.3 ± 9.7 | 37.8 ± 10.5 | 4.54 | < .001* |
| Education (years), mean ± SD | 13.58 ± 2.27 | 13.65 ±2.39 | 13.75 ± 1.5 | .008 | .992 |
| WTAR Estimated IQ | 99.58 ± 13.35 | 101.24 ± 10.71 | 102.56 ± 11.23 | .270 | .764 |
| Clinical Symptoms: Brief Psychotic Rating Scale Scores | |||||
| Positive subscale | 13.97 ± 5.9 | 9.4 ± 3.7 | N/A | 12.9 | .001* |
| Affective subscale | 7.89 ± 2.5 | 9.86 ± 2.9 | N/A | 8.33 | .005* |
| Negative subscale | 11.2 ± 5.07 | 5.59 ± 2.49 | N/A | 29.6 | < .001* |
| Total | 48.26 ± 12.5 | 43.38 ± 10.1 | N/A | 2.85 | .096 |
| Working Memory | |||||
| Spatial Span scaled score, mean ± SD | 9.7 ± 3.06 | 9.24 ± 2.89 | 12.13 ± 2.5 | 5.88 | .004* |
| Eyetracking at 18.7 degrees/second | |||||
| Predictive pursuit gain, mean ± SD | 0.36 ± .12 | 0.36 ± .13 | 0.45 ± .13 | 4.18 | .019* |
| Maintenance pursuit gain, mean ± SD | 0.70 ± 0.11 | 0.69 ± 0.12 | 0.73 ± 0.11 | 0.786 | .459 |
| Eyetracking at 9.9 degrees/second | |||||
| Predictive pursuit gain, mean ± SD | 0.47 ± .14 | 0.47 ± .17 | 0.56 ± .11 | 3.29 | .042* |
| Maintenance pursuit gain, mean ± SD | 0.75 ± .07 | 0.75 ± .10 | 0.74 ± .09 | 0.131 | .877 |
The three study groups did not differ in estimated intelligence or education. Specifically, no significant differences emerged in WTAR estimated full scale IQ (F (2, 66) = .270, p = .76), with each of the three groups scoring in the average range (mean ± SD: SZ = 99.58 ±13.35, BDP = 101.24 ±10.71, HC = 102.56 ±11.23). Further, mean education years were similar across groups (SZ= 13.58 ± 2.27, BDP = 13.65 ± 2.39, and HC = 13.59 ± 1.58; F (2, 83) = .008, p = .992).
Clinical symptom ratings from the Brief Psychiatric Rating Scale (BPRS) are also provided in Table 1. BDP had higher mean Affective subscale scores than SZ (9.86 vs. 7.89, respectively), and SZ had higher mean Positive subscale scores (13.97 vs. 9.4, respectively), and Negative subscale scores than BDP (11.2 vs. 5.9, respectively). There were no significant differences in overall BPRS score for SZ (48.26 ± 12.5) and BDP (43.38 ± 10.1), p = .096.
Eyetracking performance
Predictive and maintenance pursuit gain can be found in Table 1. There were no differences in maintenance pursuit gain at either 18.7°/sec (p = .459) or 9.9°/sec (p = .877). Predictive pursuit gain was significantly different at 18.7 (p = .019; post hoc Tukey HSD: SZ vs. BDP, p = .980; SZ vs. HC, p = .037; BDP vs. HC, p = .031) and 9.9°/sec (p = .042; post hoc Tukey HSD: SZ vs. BDP, p = .993; SZ vs. HC, p = .065; BDP vs. HC, p = .074).
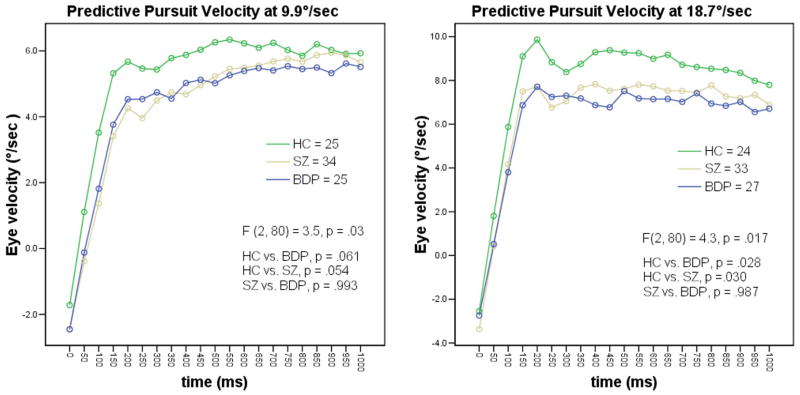
Analysis of the velocities during the predictive pursuit window at 18.7 °/sec showed significant differences in eye velocity (F (2, 80) = 4.3, p < 0.05). Post hoc comparisons showed that the predictive pursuit velocity was lower in both BDP and SZ compared to HC (p < 0.05); SZ and BDP did not differ on predictive velocity (p = 0.98). A similar pattern emerged during the slower 9.9°/sec condition (F (2, 80) = 3.54, p = 0.04). Again, post hoc comparisons revealed volunteers with psychosis did not differ from each other (SZ vs. BDP, p = 0.98), while HC showed faster velocity than SZ at a trend level (p = 0.06) (Figure 1). The three groups did not differ in maintenance pursuit velocity at either 18.7°/sec (F (2, 80) = 1.02, p = .36) or at the slower speed of 9.9 °/sec (F (2, 80) = .262, p = .77). These analyses examined all 50-ms epoch velocities over the course of the 1000 ms predictive window. We also examined the initial smooth pursuit response (velocity of the eye during the first 100 ms of the predictive window, measured during the first three 50-ms epochs – e.g. at 0, 50, and 100ms.) A one-way ANOVA showed a significant between group difference on this additional measurement of eye behavior (F (2, 67) = 4.438, p =.015).
Figure 1. Eye velocity during predictive pursuit at 18.7 and 9.9 degree/second.
Predictive pursuit task at 18.7°/sec: the three groups showed significant differences (F (2, 80) = 4.3, p < 0.05). Post hoc comparisons showed both BDP and SZ had slower eye velocity compared to HC (p < 0.05); SZ and BDP did not differ on predictive velocity (p > 0.98). A similar pattern emerged during the 9.9°/sec condition (F (2, 80) = 3.54, p < 0.04). Again, post hoc comparisons revealed volunteers with psychosis did not differ from each other (SZ vs. BDP, p > 0.98), while HC showed faster velocity than SZ at a trend level (p < 0.06)
Working memory performance
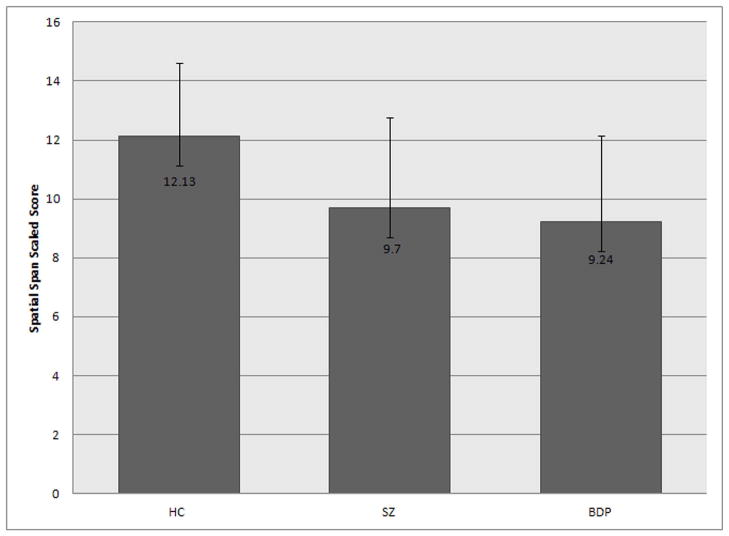
On the Spatial Span visuospatial working memory task, both SZ and BDP performed worse than the HC group (F (2, 75) = 5.88, p = .004; post hoc Tukey HSD: SZ vs. BDP, p = .934, SZ vs. HC, p = .010, BDP vs. HC, p = .005), although all groups scored within the normal range on average (Figure 2).
Figure 2. Working Memory Task Performance.
Both SZ and BDP performed worse than the HC group (F (2, 75) = 5.88, p = .004; post hoc Tukey HSD: SZ vs. BDP, p = .934, SZ vs. HC, p = .010, BDP vs. HC, p = .005), although all groups scored within the normal range of functioning.
Relationship between eye tracking and neuropsychological performance
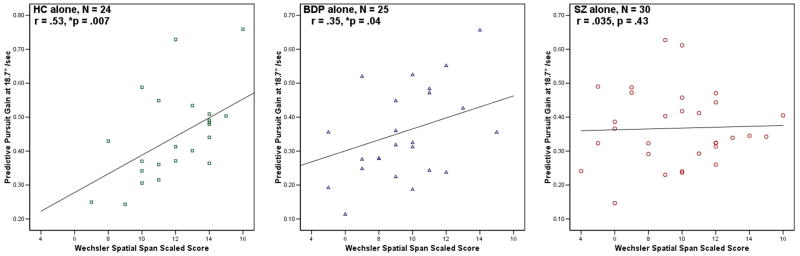
In order to examine the relationship between eye tracking deficits and working memory performance, correlations between the Spatial Span task and the predictive component of eye tracking performance were calculated. When examining the sample as a whole, there was a modest but significant correlation between predictive pursuit gain and performance on Spatial Span (r = 0.37, p = .001). When the sample was further examined by diagnostic group, a strong, significant correlation between working memory and predictive pursuit was found in the HC group (r = 0.53, p = .007); while, in the BDP group, a trend relationship was seen (r = .35, p =.04), and no relationship was found between predictive pursuit and working memory performance in the SZ sample (r = .035, p = .43) (Figure 3).
Figure 3. Scatterplots of correlations between Predictive Pursuit Gain and Working Memory, Separated by Diagnosis.
A strong, significant correlation between working memory and predictive pursuit was found in the HC group (r = 0.53, p = .007); in the BDP group, a trend relationship was seen (r = .35, p =.04). No relationship was found between predictive pursuit and working memory performance in the SZ sample (r = .035, p = .43)
Medication
Medication information is listed in Table 2. Exploratory correlational analyses were run between patients taking each of the five classes of medication and predictive pursuit gain at both 9.9 and 18.7°/sec and working memory performance. There were no significant correlations found between drug class and these outcome measures, with the exception of subjects on mood stabilizers and predictive pursuit gain at 9.9°/sec (r = − 0.29, p = 0.019); however due to the large number of correlations run this result is inconclusive.
Table 2. Medication Information for SZ and BDP.
Participants were on a combination of medications; therefore the N in each class does not indicate the number taking that drug exclusively. Medication data was missing for 3 SZP and 1 BDP. 1 SZ and 1 BDP reported not taking any psychoactive medications.
| SZ (N = 38) | BDP (N = 31) | |
|---|---|---|
| No psychoactive medication – N (%) | 1 (2.6) | 1(3.2) |
| Antipsychotics (Typical/Atypical) –N (%) | 9 (23.7) / 25 (65.8) | 0 (0) / 17 (54.8) |
| Antidepressants – N (%) | 16 (42.1) | 15 (48.4) |
| Lithium – N (%) | 0 (0) | 12 (38.7) |
| Mood Stabilizers – N (%) | 5 (13.2) | 16 (51.6) |
| Anxiolytics & Hypnotics – N (%) | 10 (26.3) | 16 (51.6) |
Due to the potential negative effects of lithium on eye tracking performance (32), we conducted an exploratory analysis within the BDP group, comparing those taking lithium (n = 9)2 and those who were not (n = 16). No differences were found in predictive gain at 18.7°/sec (t (23) = .451, p = .65) or at 9.9°/sec (t (21) = −.116, p = .909), or in maintenance gain at 18.7°/sec (t (23) = .411, p = .685) or maintenance gain at 9.9°/sec (t (21) = .348, p = .731). An additional exploratory analysis comparing BDP taking antipsychotics (n = 15)3 and BDP who were not (n = 11) showed no differences in predictive gain at 18°/sec (t (24) = .552, p = .586 or 9.9°/sec (t (21) = .051, p = .959). No differences between BDP on and off antipsychotics were seen in maintenance gain at 18.7 (t (24) = −.011 p = .991), or at 9.9°/sec (t (21) = −.723, p = .478).
Discussion
The results show that predictive pursuit is impaired in SZ as measured with a new behavioral approach, a replication of the findings of Hong et al. (26). In addition, predictive pursuit is also impaired in BDP, an outcome consistent with the idea that poor predictive pursuit could characterize the psychosis phenotype. In the current study, neither SZ nor BDP showed impaired maintenance pursuit compared with HC. This is consistent with a recent report demonstrating intact maintenance pursuit gain in both SZ and psychotic BD on a constant speed oscillating task (29). Hong et al. found no difference in maintenance pursuit between SZ and HC at 9.9 but did see a difference at 18.7°/sec (26). The similar pattern of performance on the eye movement task between the two patient groups in the current study suggests that SZ and BDP may share critical biological dysfunctions which are associated with impaired predictive pursuit.
Results on the visuospatial working memory task again show similar performance deficits in both SZ and BDP, an observation which challenges previous reports of more severe cognitive disturbances in SZ compared to BD (33–36). Possibly, the lifetime psychosis characteristics of our sample could be the key contributor to this finding, whereas the majority of previous studies used both psychotic and non-psychotic BD individuals (37–39). These results are consistent with previous reports of the negative effect of lifetime psychosis on cognitive function in bipolar disorder (20;40). While both SZ and BDP volunteers scored significantly lower on the Spatial Span task compared with healthy volunteers, their scores still fell within the average range of functioning (SZ = 9.7, BDP = 9.24). It is also worth noting that the estimated IQ scores for these groups were in the average range, and did not differ across the groups. Therefore, this analysis of visuospatial working memory performance compared individuals who were similar in intellectual functioning.
Our a priori prediction that the predictive pursuit task would associate significantly with working memory performance was supported by the moderately strong correlation between predictive gain and visuospatial working memory performance in HC. Interestingly, this relationship failed to obtain in the sample of volunteers with SZ. It is possible other compensatory mechanisms in SZ disrupt the linear relationships observed between working memory and predictive response in other groups. As extra-retinal processing during smooth pursuit is mediated by a neuronal circuit that includes the prefrontal cortex, this is consistent with evidence of prefrontal dysfunction in SZ. This deficit could be due to the disease process itself, disrupting the normal association of working memory with eyetracking performance. The current study is limited by a modest sample size. Also, the majority of patients were on a combination of medications, and all but one volunteer with SZ and 17 out of 31 BDP were treated with antipsychotic medication at the time of testing; therefore, the effects of chronic medication use on cognition cannot be ruled out, actions which may have affected the overall results. Including patients with bipolar disorder without psychosis would allow for further exploration of psychosis related phenotypes.
In conclusion, both SZ and BDP showed impaired predictive pursuit and lower working memory performance compared with HC. The direct examination of the relationship between working memory performance and pursuit eye movements revealed a strong correlation in healthy volunteers and a degradation of that relationship in schizophrenia. Evidence of overlapping phenotypic patterns of deficits in individuals within the dimension of psychosis is advancing.
Acknowledgments
This work was supported by NIMH (MH07785) and Stanley Medical Research Institute (05-RC-001). The NIMH and Stanley Medical Research Institute had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
We would like to thank Ira Bernstein, Ph.D. for assistance with analyses; Darwynn Cole, B.A., Kate Burns, M.S., Regena Mitschke, B.A., Judy Shaw M.S., and Dorothy Denton, B.A. for assistance with data collection and manuscript preparation; Bradley Witte and Amie Elliott for assistance with data management, and all the volunteers that took part in this study.
Footnotes
The Spatial Span subtest of the Wechsler Memory Scale-III (22) is an adaptation of the Corsi Block-Tapping Test (23) which requires the subject to replicate a specified order of block tapping, and assesses the individual’s ability to hold a visual-spatial sequence of events in working memory.
In total, 12 BDP reported taking lithium, however 3 were already excluded from analysis at 18°/sec and 5 were excluded at 9.9°/sec due to having less than 5 usable trials of data.
In total 17 BDP reported taking antipsychotics, however 2 were already excluded from analysis at 18°/sec and 4 were excluded at 9.9°/sec due to having less than 5 usable trials.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Levy DL, Holzman PS, Matthysse S, Mendell NR. Eye tracking and schizophrenia: a selective review. Schizophr Bull. 1994;20(1):47–62. doi: 10.1093/schbul/20.1.47. [DOI] [PubMed] [Google Scholar]
- 2.O'Driscoll GA, Callahan BL. Smooth pursuit in schizophrenia: a meta-analytic review of research since 1993. Brain Cogn. 2008;68(3):359–370. doi: 10.1016/j.bandc.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Calkins ME, Iacono WG, Ones DS. Eye movement dysfunction in first-degree relatives of patients with schizophrenia: a meta-analytic evaluation of candidate endophenotypes. Brain Cogn. 2008;68(3):436–461. doi: 10.1016/j.bandc.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kathmann N, Hochrein A, Uwer R, Bondy B. Deficits in gain of smooth pursuit eye movements in schizophrenia and affective disorder patients and their unaffected relatives. Am J Psychiatry. 2003;160(4):696–702. doi: 10.1176/appi.ajp.160.4.696. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney JA, Luna B, Haas GL, Keshavan MS, Mann JJ, Thase ME. Pursuit tracking impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biol Psychiatry. 1999;46(5):671–680. doi: 10.1016/s0006-3223(99)00132-8. [DOI] [PubMed] [Google Scholar]
- 6.Abel LA, Friedman L, Jesberger J, Malki A, Meltzer HY. Quantitative assessment of smooth pursuit gain and catch-up saccades in schizophrenia and affective disorders. Biological Psychiatry. 1991;29(11):1063–1072. doi: 10.1016/0006-3223(91)90248-k. [DOI] [PubMed] [Google Scholar]
- 7.Tien AY, Ross DE, Pearlson G, Strauss ME. Eye movements and psychopathology in schizophrenia and bipolar disorder. Journal of Nervous & Mental Disease. 1996;184(6):331–338. doi: 10.1097/00005053-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Levin S. Frontal lobe dysfunctions in schizophrenia--II. Impairments of psychological and brain functions. J Psychiatr Res. 1984;18(1):57–72. doi: 10.1016/0022-3956(84)90047-5. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Nakayama K, Levy DL, Matthysse S, Holzman PS. Psychophysical isolation of a motion-processing deficit in schizophrenics and their relatives and its association with impaired smooth pursuit. Proc Natl Acad Sci U S A. 1999;96(8):4724–4729. doi: 10.1073/pnas.96.8.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thaker GK, Avila MT, Hong EL, Medoff DR, Ross DE, Adami HM. A model of smooth pursuit eye movement deficit associated with the schizophrenia phenotype. Psychophysiology. 2003;40(2):277–284. doi: 10.1111/1469-8986.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lencer R, Trillenberg P. Neurophysiology and neuroanatomy of smooth pursuit in humans. Brain Cogn. 2008;68(3):219–228. doi: 10.1016/j.bandc.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64(9):823–827. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keefe RS, Bilder RM, Harvey PD, Davis SM, Palmer BW, Gold JM, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31(9):2033–2046. doi: 10.1038/sj.npp.1301072. [DOI] [PubMed] [Google Scholar]
- 14.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49(12):975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 15.Goldman-Rakic PS. Prefrontal cortical dysfunction in working memory: the relevance of working memory. In: Caroll BJ, Barrett JE, editors. Psychopathology and the brain. New York: Raven; 1991. pp. 1–23. [Google Scholar]
- 16.Park S, Holzman PS. Association of working memory deficit and eye tracking dysfunction in schizophrenia. Schizophrenia Research. 1993;11:55–61. doi: 10.1016/0920-9964(93)90038-k. [DOI] [PubMed] [Google Scholar]
- 17.Kattoulas E, Smyrnis N, Stefanis NC, Avramopoulos D, Stefanis CN, Evdokimidis I. Predictive smooth eye pursuit in a population of young men: I. Effects of age, IQ, oculomotor and cognitive tasks. Exp Brain Res. 2011;215(3–4):207–218. doi: 10.1007/s00221-011-2887-5. [DOI] [PubMed] [Google Scholar]
- 18.Litman RE, Hommer DW, Clem T, Ornsteen ML, Ollo C, Pickar D. Correlation of Wisconsin Card Sorting Test performance with eye tracking in schizophrenia. Am J Psychiatry. 1991;148(11):1580–1582. doi: 10.1176/ajp.148.11.1580. [DOI] [PubMed] [Google Scholar]
- 19.Friedman L, Kenny JT, Jesberger JA, Choy MM, Meltzer HY. Relationship between smooth pursuit eye-tracking and cognitive performance in schizophrenia. Biol Psychiatry. 1995;37(4):265–272. doi: 10.1016/0006-3223(94)00170-8. [DOI] [PubMed] [Google Scholar]
- 20.Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, et al. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62(8):910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Baddeley AD. Working Memory. Oxford: Oxford University Press; 2010. [Google Scholar]
- 22.Wechsler D. Wechsler Memory Scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 23.Corsi PM. Unpublished Doctoral Dissertation. McGill University; 1972. Human memory and the medial temporal region of the brain. [Google Scholar]
- 24.Barnes GR, Asselman PT. Pursuit of intermittently illuminated moving targets in the human. J Physiol. 1992;445:617–637. doi: 10.1113/jphysiol.1992.sp018943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thaker GK, Ross DE, Cassady SL, Adami HM, LaPorte D, Medoff DR, et al. Smooth pursuit eye movements to extraretinal motion signals: deficits in relatives of patients with schizophrenia. Arch Gen Psychiatry. 1998;55(9):830–836. doi: 10.1001/archpsyc.55.9.830. [DOI] [PubMed] [Google Scholar]
- 26.Hong LE, Turano KA, O'Neill H, Hao L, Wonodi I, McMahon RP, et al. Refining the predictive pursuit endophenotype in schizophrenia. Biol Psychiatry. 2008;63(5):458–464. doi: 10.1016/j.biopsych.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull. 2008;34(4):760–773. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York, NY: New York State Psychiatric Institute, Biomedical Research Division; 1995. [Google Scholar]
- 29.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34(10):1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 30.Ross RG, Olincy A, Harris JG, Radant A, Adler LE, Compagnon N, et al. The effects of age on a smooth pursuit tracking task in adults with schizophrenia and normal subjects. Biol Psychiatry. 1999;46(3):383–391. doi: 10.1016/s0006-3223(98)00369-2. [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D. Wechsler Test of Adult Reading. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 32.Holzman PS, O'Brian C, Waternaux C. Effects of lithium treatment on eye movements. Biol Psychiatry. 1991;29(10):1001–1015. doi: 10.1016/0006-3223(91)90357-r. [DOI] [PubMed] [Google Scholar]
- 33.Altshuler LL, Ventura J, van Gorp WG, Green MF, Theberge DC, Mintz J. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biol Psychiatry. 2004;56(8):560–569. doi: 10.1016/j.biopsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Burdick KE, Goldberg JF, Harrow M, Faull RN, Malhotra AK. Neurocognition as a stable endophenotype in bipolar disorder and schizophrenia. The Journal of Nervous and Mental Disease. 2006;194(4):255–260. doi: 10.1097/01.nmd.0000207360.70337.7e. [DOI] [PubMed] [Google Scholar]
- 35.Dickerson F, Boronow JJ, Stallings C, Origoni AE, Cole SK, Yolken RH. Cognitive functioning in schizophrenia and bipolar disorder: comparison of performance on the Repeatable Battery for the Assessment of Neuropsychological Status. Psychiatry Res. 2004;129(1):45–53. doi: 10.1016/j.psychres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Seidman LJ, Kremen WS, Koren D, Faraone SV, Goldstein JM, Tsuang MT. A comparative profile analysis of neuropsychological functioning in patients with schizophrenia and bipolar psychoses. Schizophr Res. 2002;53(1–2):31–44. doi: 10.1016/s0920-9964(01)00162-1. [DOI] [PubMed] [Google Scholar]
- 37.Glahn DC, Bearden CE, Cakir S, Barrett JA, Najt P, Serap ME, et al. Differential working memory impairment in bipolar disorder and schizophrenia: effects of lifetime history of psychosis. Bipolar Disorder. 2006;8(2):117–123. doi: 10.1111/j.1399-5618.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- 38.Pearlson GD, Wong DF, Tune LE, Ross CA, Chase GA, Links JM, et al. In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Arch Gen Psychiatry. 1995;52(6):471–477. doi: 10.1001/archpsyc.1995.03950180057008. [DOI] [PubMed] [Google Scholar]
- 39.Potash JB. Carving chaos: genetics and the classification of mood and psychotic syndromes. Harvard Review of Psychiatry. 2006;14(2):47–63. doi: 10.1080/10673220600655780. [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Aran A, Torrent C, Tabares-Seisdedos R, Salamero M, Daban C, Balanza-Martinez V, et al. Neurocognitive impairment in bipolar patients with and without history of psychosis. J Clin Psychiatry. 2008;69(2):233–239. doi: 10.4088/jcp.v69n0209. [DOI] [PubMed] [Google Scholar]