Abstract
The microbiota contributes to the induction of both effector and regulatory responses in the gastrointestinal tract. However, the mechanisms controlling these distinct properties remain poorly understood. We previously showed that commensal DNA promotes intestinal immunity. Here, we find that the capacity of bacterial DNA to stimulate immune responses is species specific and correlated with the frequency of motifs known to exert immunosuppressive function. In particular, we show that the DNA of Lactobacillus species, including various probiotics, are enriched in suppressive motifs able to inhibit lamina propria DC activation. In addition, immunosuppressive oligonucleotides sustain Treg cell conversion during inflammation and limit pathogen-induced immunopathology and colitis. Altogether, our findings identify DNA suppressive motifs as a molecular ligand expressed by commensals and support the idea that a balance between stimulatory and regulatory DNA motifs contributes to the induction of controlled immune responses in the GI tract and gut immune homeostasis. Further, our findings suggest that the endogenous regulatory capacity of DNA motifs enriched in some commensal bacteria could be exploited for therapeutic purposes.
Introduction
The human intestine harbors 1000 trillion microbes composed of an estimated 4000 strains 1. The commensal flora plays a fundamental role in various aspects of host physiology including tissue development, metabolism and immunity. This symbiotic relationship is controlled, at least in part, by interaction of host cells with commensal derived microbe-associated molecular patterns. Paradoxically, commensal and pathogenic microbes interact with the host immune system through similar conserved ligands such as the ones signaling through the Toll like family of receptors (TLR) 2. TLR signaling in the intestinal epithelial compartment is critically involved in the maintenance of intestinal homeostasis and tissue repair 3. Further, these signals also positively regulate the sampling of luminal contents by DCs from the underlying lamina propria compartment and can mediate tolerance to food antigens 4, 5.
TLR9 recognizes unmethylated cytosine phosphate guanosine (CpG) dinucleotides, which are abundant in prokaryotic DNA found in intestinal flora. In mice, we found that constitutive gut flora DNA sensing in the gastrointestinal (GI) tract can act as an immunological adjuvant and critically controls the balance between regulatory and effector T cells 6. Although commensal communities can control immune system development and function, individual bacteria can have highly distinct properties. For instance, some bacteria, referred to as probiotics, can promote regulatory responses in the GI tract while, others referred to as pathobionts, can in some circumstances contribute to pathology 7, 8. Some of these distinct properties have been linked to the expression of defined ligands or expression of factors associated with enhanced adhesion with epithelial cells. However, the molecular basis of the functional disparities between commensal species remains poorly understood.
DNA from pathogenic bacteria are not all equal in their capacity to stimulate TLR9 and do so with various levels of efficiency that correlates with their frequency of CpG dinucleotides 9, 10. Thus, it could be proposed that increased CpG motifs in the DNA of defined commensal bacteria may contribute to their enhanced inflammatory properties. An alternative possibility is that the DNA of a subset of commensal bacteria may be more enriched in what have been referred to as suppressive motifs. These motifs are poly guanosine- or [GC]-rich sequences, are enriched in the telomere region of mammalian DNA, and have been shown to selectively block immune activation induced by CpG ODN 11–14. Although their presence has not been reported in bacteria, we hypothesized that increased frequency of these motifs at the DNA level may potentially contribute to the functional specificity of commensal bacteria on host immune responses.
Our present results reveal that the capacity of bacterial DNA to stimulate immune responses is bacteria specific. Strikingly, suppressive motifs, enriched in the DNA of the probiotic of the Lactobacillus family, potently prevent DC activation and maintain Treg cell conversion in the face of inflammation. Additionally, treatment of mice with suppressive oligonucleotides (sup-ODN) mitigates tissue damage and inflammation associated with oral Toxoplasma gondii infection and DSS-induced colitis. Altogether, our work provides evidence that DNA motifs in the microbiota may contribute to the hierarchy of commensal derived signals and maintenance of gut immune homeostasis. Further, our findings suggest that the endogenous regulatory capacity of DNA motifs enriched in specific commensal bacteria could be harnessed for therapeutic purposes.
Results
DNA from distinct commensal bacteria differentially stimulate intestinal immune responses
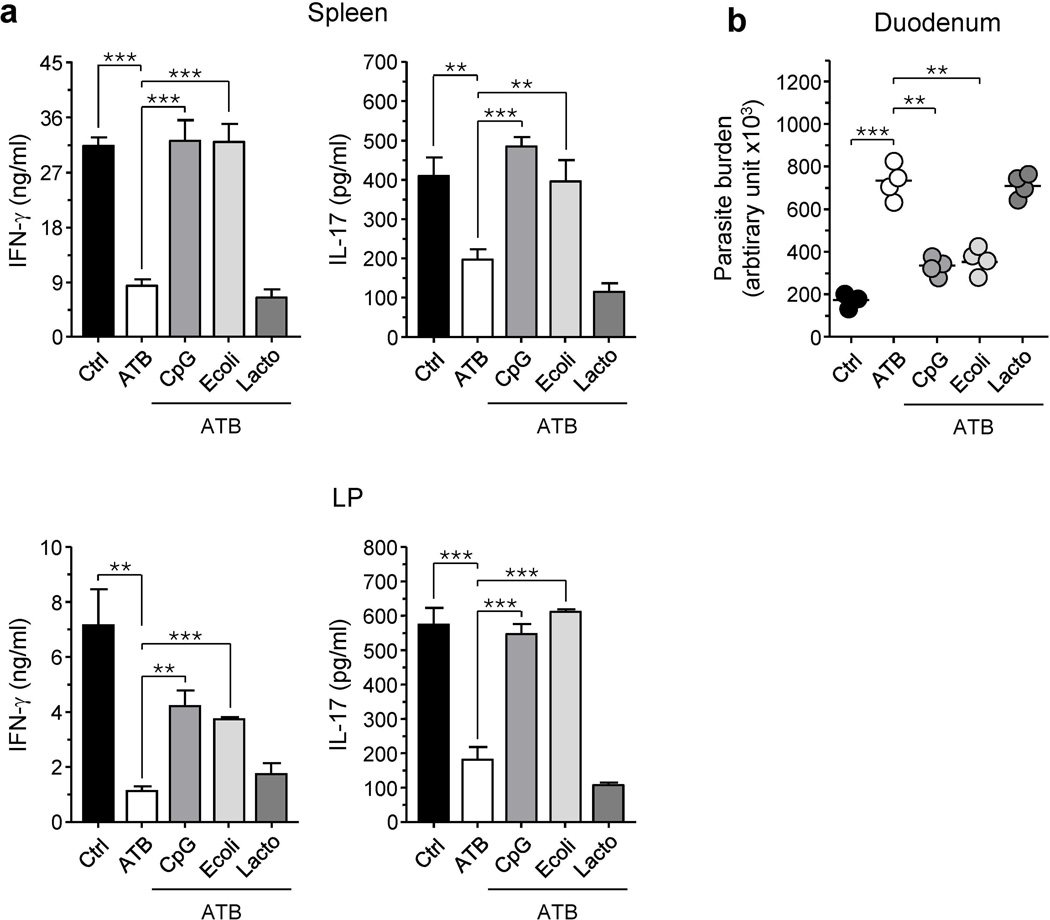
To assess if functional differences in response to commensals is apparent at their DNA level, we evaluated the proinflammatory capacity of the DNA from two prototypic commensal bacteria with known divergent effects on the host immune system. In particular, we used DNA isolated from the probiotic Lactobacillus paracasei and a commensal with pathogenic potential Escherichia coli 7. To ensure the predominance of these signals, we placed 3 week-old mice on a cocktail of antibiotics (ATB) 3. After six weeks of the ATB course, mice were treated orally once a week with CpG ODN or highly purified genomic DNA from E. coli or L. paracasei. Two weeks later, mice were orally infected with the gastro-intestinal parasite Encephalitozoon cuniculi that induces strong Th17 and Th1 responses, the latter required for host protection 6, 15. As previously shown, reduction of the gut flora significantly impaired both local and systemic IFN-γ and IL-17 responses and compromised parasite control (Figure 1a, b) 6. In a comparable manner to CpG ODN treatment, E. coli DNA significantly restored effector responses and parasite control (Figure 1a, b). In contrast, DNA from L. paracasei failed to rescue either IFN-γ or IL-17 responses and consequently had no effect on the parasite burden compared to ATB treatment alone (Figure 1a, b). Thus, at an equivalent dose, DNA from distinct bacterial species have a differential capacity to stimulate immune responses.
Figure 1. Lactobacillus paracasei and Escherichia coli DNA differ in their capacity to restore intestinal effector immune responses in antibiotic-treated mice.
A cocktail of antibiotics (ATB) in the drinking water was given to 3-week-old mice. Six weeks after the start of the ATB regimen, mice received oral weekly treatments consisting of PBS alone (ATB) or containing 100 µg of CpG ODN 1826 (CpG), 500 µg of DNA from Lactobacillus paracasei (Lacto) or 500 µg of DNA from Escherichia coli (E. coli). Control mice (Ctrl) received regular water and no treatment. All mice were orally infected with Encephalitozoon cuniculi 8 weeks after the beginning of the ATB course. (a) shows ELISA of IFN-γ and IL-17 in supernatants of bulk leukocyte preparations from spleen and small intestine lamina propria (LP) of 11 days post-infected mice, restimulated with E. cuniculi-infected BMDC for 72 hrs. Histograms represent the mean cytokine concentration of triplicate wells ± SD (** p < 0.01; *** p < 0.001). (b) Parasite burden was evaluated in duodenum 11 days after infection by quantitative real-time PCR. Each dot represents one mouse and each bar represents the mean of three of four mice analysed (** p < 0.01; *** p < 0.001). All data shown are representative of two independent experiments with similar results.
E. coli and L. paracasei DNA differentially activate lamina propria DC and influence Treg cell induction
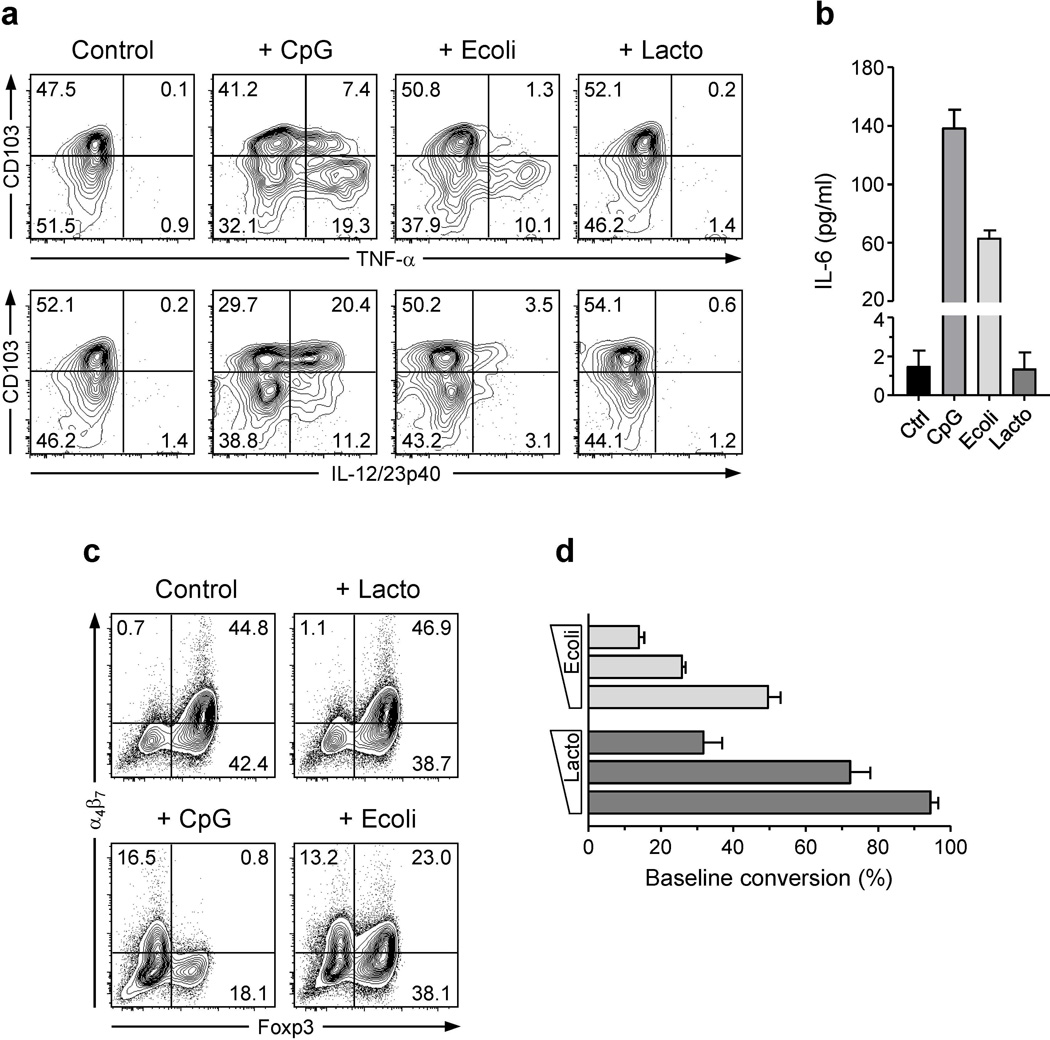
The intestinal mucosa contains a large number of antigen presenting cells that have an important role in shaping adaptive immunity in response to intestinal environmental cues 16. We therefore assessed if our in vivo observations could correlate with differential effects of L. paracasei and E. coli DNA on gut resident DCs by performing in vitro experiments using lamina propria DC (LpDC) purified from naïve mice. The small intestine lamina propria (LP) contains both resident and migratory DCs, the latter tending to express high levels of CD103 17, 18. Upon stimulation with CpG ODN, both LpDC subsets produced TNF-α and IL12/23p40 (Figure 2a). High levels of IL-6 were also measured in the supernatant of CpG ODN-stimulated cultures of purified CD11chi MHCII+ LpDCs (Figure 2b). Similarly, stimulation with E. coli DNA induced TNF-α, IL-12/23p40 and IL-6 secretion by LpDCs albeit to a lower degree than highly purified CpG ODN (Figure 2a, b). In contrast, a similar dose of L. paracasei DNA was not able to trigger proinflammatory cytokine production by LpDCs (Figure 2a, b).
Figure 2. DNA from Lactobacillus paracasei and Escherichia coli exert distinct modulatory effects on cytokine production by lamina propria DC and Treg conversion in vitro.
(a, b) E. coli DNA is a potent inducer of proinflammatory cytokine secretion by lamina propria DCs (LpDC). (a) LP leukocytes prepared from wild-type naïve mice were stimulated for 5 hrs in presence or absence of CpG ODN 1555 (CpG; 1 µg/ml), L. paracasei DNA (Lacto; 0.1 µg/ml) or E. coli DNA (Ecoli; 0.1 µg/ml) in complete media containing brefeldin A. Dot plots represent TNF-α or IL-12/23p40 versus CD103 expression on viable CD11chi MHCII+ DCs analyzed by flow cytometry after intracellular cytokine staining. Numbers in quadrant refer to the percentage of each subset. (b) FACS-purified CD11chi MHCII+ LpDCs were stimulated for 18 hrs in the presence or absence of CpG ODN 1555 (CpG; 1 µg/ml), L. paracasei DNA (Lacto; 1 µg/ml) or E. coli DNA (Ecoli, 1 µg/ml) in complete media containing 40 ng/ml of GM-CSF. IL-6 was then measured in the culture supernatant by ELISA. Histograms represent the mean cytokine concentration of triplicate wells ± SD. (c, d) FACS-sorted CD4+ CD25− CD44lo Foxp3− T cells isolated from Foxp3eGFP Tlr9−/− naïve mice were cultured in Treg cell-polarizing conditions with CD11chi MHCII+ wild-type LpDCs in the presence of the indicated treatments. As shown in (c), dot plots illustrate α4β7 versus Foxp3 expression on viable TCR-β+ CD4+ T cells after 5 days of culture in presence or absence of CpG ODN 1555 (CpG; 1 µM), E. coli DNA (Ecoli; 0.1 µg/ml) or L. paracasei (Lacto; 0.1 µg/ml). As shown in (d), E. coli DNA or L. paracasei DNA was added in the culture at starting concentrations of 10 µg/ml. Two subsequent 10-fold dilutions of bacterial DNA were tested (white edge). Results were normalized to conditions plated in the absence of any bacterial DNA (100% conversion). Cross bars indicate the highs and lows of duplicate cultures. All data shown in this figure are representative of at least three independent experiments with similar results.
Another property of intestinal LpDCs is their ability to enhance TGF-β-mediated Treg cell conversion 19–22. We previously showed that gut flora DNA impaired Treg cell conversion via its capacity to activate DCs 6. We utilized this feature as a functional read out for the ex vivo effect of the DNA from E. coli and L. paracasei. Purified LpDCs and naïve CD4+Foxp3− T cells were co-cultured in Treg cell-polarizing conditions, in the presence or absence of CpG ODN or the two types of bacterial DNA. To specifically assess the effect of DNA on DCs, we utilized T cells from Tlr9−/− mice. As we previously demonstrated, stimulation with CpG ODN led to a dramatic decrease in the frequency of Foxp3+ cells, whereas α4β7 expression, which mediates migration of cells to the gut was maintained on Foxp3-negative cells (Figure 2c). Similarly, the proportion of Foxp3+ cells was strongly reduced in a TLR9-dependent manner in the presence of E. coli derived DNA (Figure 2c and Supplementary Figure S1). In contrast, a similar amount of L. paracasei DNA had no effect on the induction of Treg cells (Figure 2c). To assess if such an effect was due to a complete inability of the probiotic DNA to activate LpDC we performed a similar assay in the presence of increasing doses of DNA. Using this approach, we found that L. paracasei DNA was not inert but 100 times less potent at inhibiting Treg cell induction compared to the DNA of E. coli (Figure 2d). Thus, the L. paracasei DNA has the capacity to stimulate antigen presenting cells but do so in a less efficient manner than the DNA from E. coli.
L. paracasei genome is enriched in immunosuppressive motifs
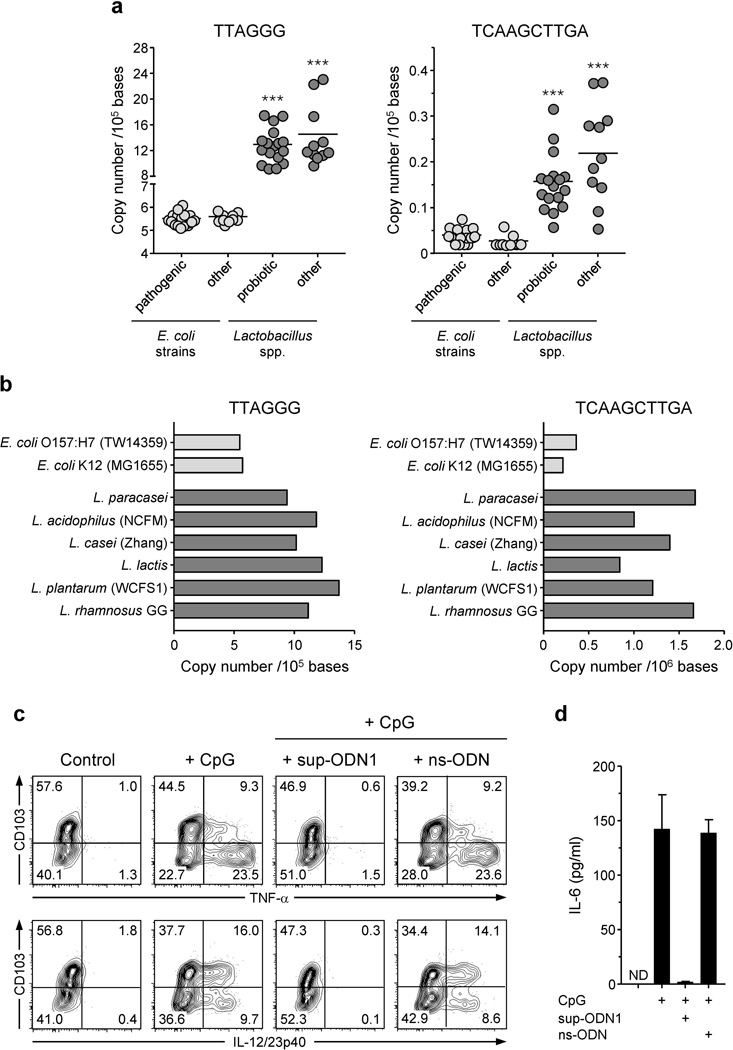
Previous studies have shown that, in vitro, DNA from distinct pathogenic bacteria stimulate TLR9 with varying levels of efficiency that correlate with the frequency of unmethylated cytosine-phosphate-guanosine dinucleotides (CpG) in their DNA 9, 10. However, assessment of the frequency of prototypic stimulatory motifs AACGAT and ATCGAT in the sequenced genome of 27 strains of E. coli and 28 Lactobacillus species showed similar relative expression between E. coli and bacteria of the Lactobacillus genus (Supplementary Tables 1–2 and Supplementary Figure S2a). An alternative but not exclusive explanation for our aforementioned results is that DNA from L. paracasei would have increased regulatory capacity. We next compared the frequency of suppressive motifs 23, 24. Notably, we found that the suppressive sequences, TTAGGG and TCAAGCTTGA, were enriched in the DNA of all Lactobacillus species compared to E. coli, including those with reported probiotic activities (Figure 3a, b and Supplementary Figure S2b, c). Of note, reduced levels of suppressive motifs do not correlate with the pathogenicity of E. coli strains (Figure 3a, b and Supplementary Figure S2b, c). These findings suggest that a shift in the ratio of regulatory versus stimulatory motifs might contribute to the differential effect of commensal DNA on immune responses.
Figure 3. Lactobacillus paracasei DNA is enriched in suppressive motifs able to inhibit intestinal lamina propria DC activation.
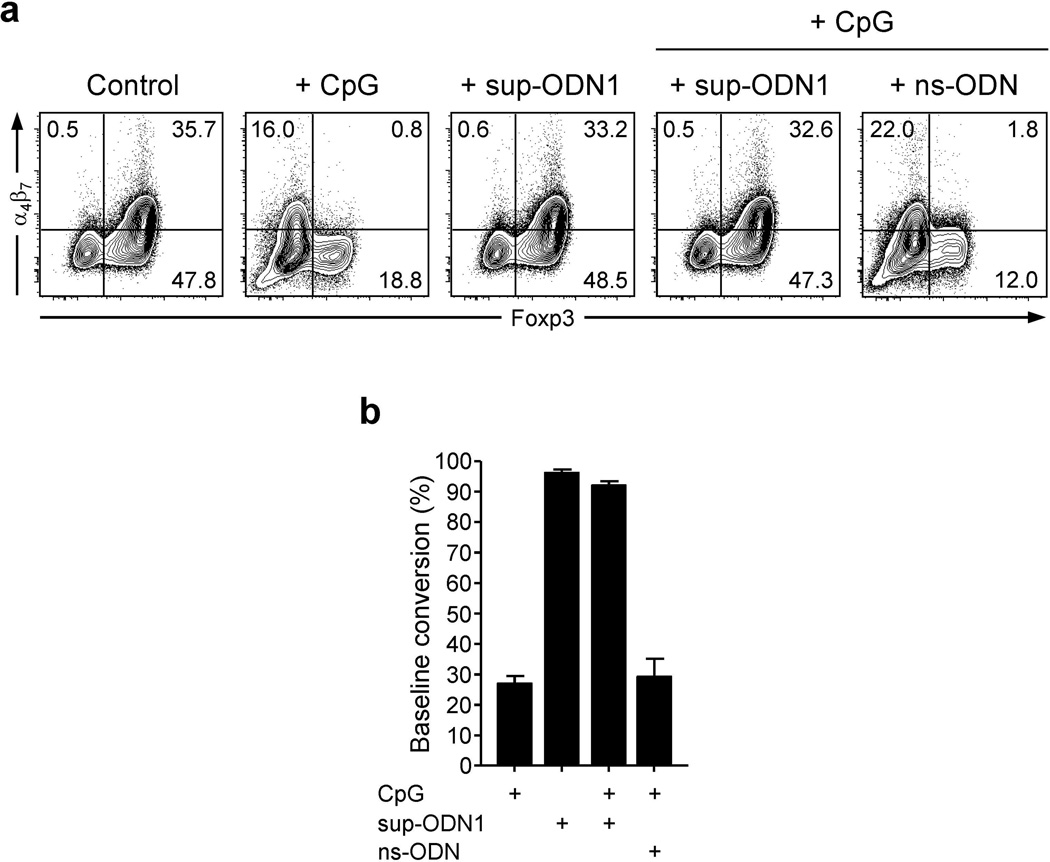
(a, b) The sequenced genomes of 27 strains of E. coli and 28 species of Lactobacillus bacteria were analyzed for the presence of two suppressive motifs: TTAGG and TCAAGCTTGA. Each dot or graph bar represents the number of suppressive motifs per 105 or 106 bases for each bacteria. (a) DNA from Lactobacillus spp. bacteria contains higher frequencies of immunosuppressive motifs than DNA from different E. coli strains. *** p < 0.001 (compared to pathogenic or other E. coli strains). (b) Number of TTAGG and TCAAGCTTGA motifs in two E. coli strains and 6 probiotic Lactobacillus bacteria, including L. paracasei. (c and d) Suppressive ODN1 (sup-ODN1) inhibits LpDC cytokine secretion induced by CpG stimulation. (c) LP leukocytes prepared from wild-type naïve mice were stimulated for 5 hrs in presence or absence of CpG ODN 1555 (CpG; 1 µM) alone or in combination with sup-ODN1 (1 µM) or control ODN (ns-ODN; 1 µM), in complete media containing brefeldin A. Dot plots represent TNF-α or IL-12/23p40 versus CD103 expression on viable CD11chi MHCII+ DCs analyzed by flow cytometry after intracellular cytokine staining. Numbers in quadrant refer to the percentage of each subset. (d) FACS-purified CD11chi MHCII+ LpDCs were stimulated for 18 hrs in presence or absence of CpG ODN 1555 (CpG; 1 µM) alone or in combination with sup-ODN1 (1 µM) or control ODN (ns-ODN; 1 µM) in complete media containing 40 ng/ml of GM-CSF. IL-6 was then measured in the culture supernatant by ELISA. Histograms represent the mean cytokine concentration of triplicate wells ± SD.
Suppressive ODN reduce inflammatory cytokine production by gut APCs
Previous work has established that suppressive ODN (sup-ODN) could inhibit the production of proinflammatory cytokines by CpG ODN-stimulated splenic cells 13, 14, 25. To address the possibility that suppressive motifs could interfere with the adjuvant property of CpG ODN at mucosal sites, we synthesized two ODNs containing repeats of the suppressive sequences TTAGG or TCAAGCTTGA: A151 (sup-ODN1) and H154 (sup-ODN2), respectively 23, 24. Addition of sup-ODN1 to the CpG ODN-treated cultures of DC purified from the small intestine lamina propria significantly blocked their production of TNF-α, IL-12/23p40 and IL-6 (Figure 3c, d). Of note, co-treatment with control scrambled ODN (ns-ODN) that do not contain stimulatory or suppressive motifs had no effect on the secretion of TNF-α, IL-12/23p40 and IL-6 by LpDCs following stimulation with CpG ODN (Figure 3c, d). Based on the profound inhibitory effect of sup-ODN on gut DC inflammatory cytokine production, we next assessed the physiological consequences of these motifs on gut immune responses and pathology.
Suppressive ODN inhibit the adjuvant effect of CpG on intestinal immune responses
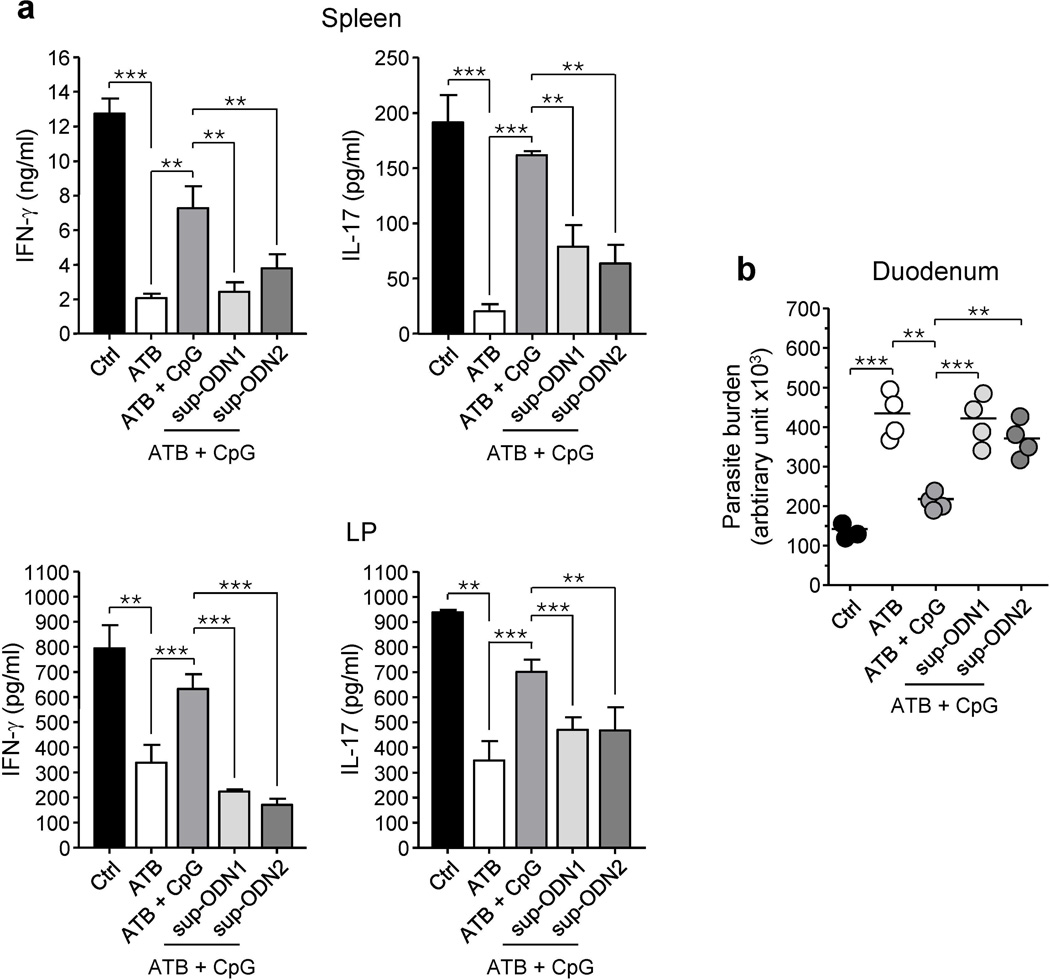
We first addressed the possibility that suppressive motifs could interfere with CpG adjuvant property at mucosal sites. A group of antibiotic treated mice received CpG ODN alone or in combination with sup-ODN1 or sup-ODN2 starting a week prior to E. cuniculi infection. Strikingly, sup-ODN1 or sup-ODN2 treatment interfered with the protective role of CpG ODN and limited parasite control (Figure 4a, b). Thus suppressive motifs naturally enriched in some commensal species can limit the adjuvant effect of CpG on intestinal immune responses.
Figure 4. Suppressive ODN limit the adjuvant effect of CpG on intestinal immune responses in antibiotic-treated mice.
A cocktail of antibiotics (ATB) in the drinking water was given to 3-week-old mice. Seven weeks after the beginning of the ATB regimen, all mice were infected orally with Encephalitozoon cuniculi. In addition some mice were treated with PBS alone (ATB) or containing CpG ODN 1826 (25 µg), sup-ODN1 (25 µg) and/or sup-ODN2 (25 µg), by gavage on day -6 and -3 before the infection and on day 1, 4 and 8 after the infection. Control mice (Ctrl) received regular water and no treatment. At day 11 post-infection, mice were euthanized and immune responses and parasite loads were analyzed. (a) Levels of IFN-γ and IL-17 were measured by ELISA in supernatants of bulk leukocyte preparations from infected spleen and small intestine lamina propria (LP) restimulated with E. cuniculi-infected BMDC for 72 hrs. Histograms represent the mean cytokine concentration of triplicate wells ± SD (** p < 0.01; *** p < 0.001). Data shown are representative of two independent experiments with similar results. (b) Parasite burden was evaluated in duodenum by quantitative real-time PCR. Each dot represents one mouse and each bar represents the mean of three of four mice analysed (** p < 0.01; *** p < 0.001). All data shown are representative of two independent experiments with similar results.
We and others previously showed that inflammation interferes with Treg cell conversion in vitro and in vivo 26, 27. To evaluate if sup-ODN could prevent such an outcome, we first tested its potential effects in vitro. To this end, CpG ODN was added with or without sup-ODN1 to Treg cell-polarizing co-cultures of LpDCs and naïve CD4+Foxp3− T cells. After 5 days, the percentages of CD4+ cells expressing Foxp3 were measured by flow cytometry. As expected, the addition of CpG ODN strongly suppressed Treg cell conversion (Figure 5a, b). Strikingly, sup-ODN1 interfered with the inhibitory effect of CpG ODN while control ns-ODN, devoid of stimulatory or suppressive motifs, had no effect (Figure 5a, b).
Figure 5. Suppressive ODN reverses the inhibition of Treg cell conversion by CpG in vitro.
FACS-sorted CD4+ CD25− CD44lo Foxp3− T cells isolated from Foxp3eGFP Tlr9−/− naïve mice were cultured in Treg cell-polarizing conditions with CD11chi MHCII+ wild-type LpDCs. The following treatments were added alone or in combination: CpG ODN 1555 (CpG; 1 µM), sup-ODN1 (1 µM), control ODN (ns-ODN; 1 µM). As shown in (a), dot plots illustrate α4β7 versus Foxp3 expression on viable TCR-β+ CD4+ T cells after 5 days of culture in presence of the indicated treatments. (b) shows the summary of (a) normalized to baseline Treg cell conversion. Cross bars indicate the highs and lows of duplicate cultures. All data shown in this figure are representative of at least three independent experiments with similar results.
Treatment with suppressive ODN sustains Treg cell conversion and ameliorates the intestinal immunopathology during T. gondii infection and DSS-induced colitis
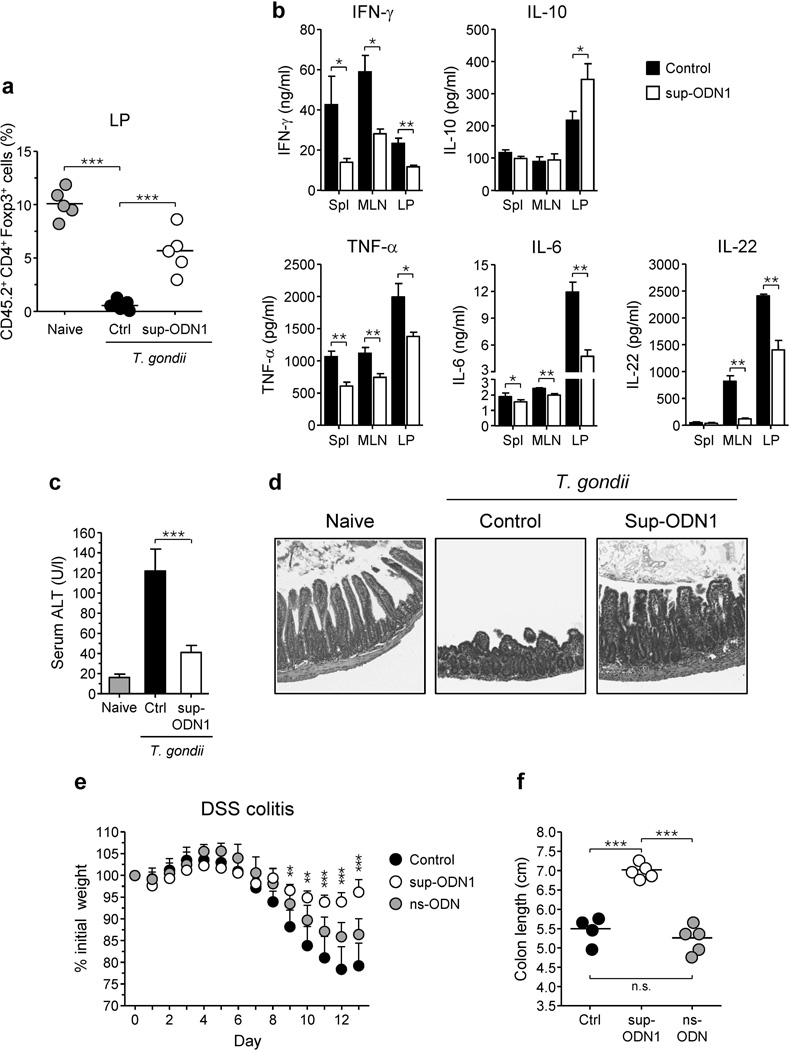
We next assessed the effect of sup-ODN on Treg cell induction during an inflammatory response in vivo. To this end, we utilized the well-characterized model of acute infection with the intracellular protozoan parasite Toxoplasma gondii. Following oral infection, T. gondii induces a strong inflammatory response in the GI tract, and control of the parasite and development of immunopathology are under the control of IFN-γ producing CD4+ T cells and IL-22, respectively 28, 29. Congenic CD4+Foxp3− T cells from OT-II Tg x Foxp3eGFP mice were adoptively transferred into recipients that were infected, or not, with 10 cysts of the type II T. gondii strain ME-49. Recipient mice were then fed ovalbumin (OVA) antigen dissolved in drinking water and treated every other day with sup-ODN1. As we have previously shown, the frequency of cells expressing Foxp3 de novo was dramatically reduced in the lamina propria after infection (Figure 6a) 27. In contrast, infected recipient mice treated with sup-ODN1 sustained Treg cell conversion to a greater extent compared to untreated infected animals (Figure 6a). Collectively, these data demonstrate that suppressive ODNs are sufficient to maintain a cardinal feature of gut physiology, Treg cell conversion, during acute infection.
Figure 6. Treatment with suppressive ODN restores Treg cell conversion and decreases intestinal pathology during oral infection with Toxoplasma gondii and DSS-induced colitis.
(a–d) C57BL/6 or congenic mice were orally infected or not with Toxoplasma gondii. In addition, some infected mice were orally treated with PBS containing sup-ODN1 (25 µg) every other day, starting 3 days before the infection. Control mice (Ctrl) received PBS only. (a) Treatment with sup-ODN1 sustains Treg cell conversion in mice orally infected with T. gondii. At 4 days post-infection, CD45.1+ congenic recipient mice received CD45.2+ CD4+ Foxp3− OT-II T cells and were then fed with OVA antigen in drinking water for 5 days. On day 9 post-infection, both naïve and infected mice were euthanized and TCR-β+ CD4+ CD45.2+-gated T cells from the small intestine lamina propria were assessed for intracellular Foxp3 expression. Each dot represents one mouse and each bar represents the mean of four mice analyzed (**, p < 0.01; ***, p < 0.001). (b–d) Inflammatory immune responses and pathology during T. gondii infection are decreased after treatment with sup-ODN1. At day 9 post-infection, mice were euthanized and immune responses, parasite loads and pathology were assessed. (b) ELISA of IFN-γ, IL-10, TNF-α, IL-6 and IL-22 in supernatants of spleen (Spl), mesenteric lymph nodes (MLN) or small intestine lamina propria (LP) CD90+ cells prepared from the different groups of mice and restimulated for 48 hrs with BMDC loaded with soluble T. gondii antigen. Histograms represent the mean cytokine concentration of triplicate wells ± SD (* p < 0.05; ** p < 0.01). (c) The level of hepatic alanine aminotransferase (ALT) was assessed in the serum of naïve or day 9 post-infection mice treated or not with sup-ODN1. Histograms show the mean enzyme concentration ± SD (4 mice per group, * p < 0.05). (d) Histological assessment of ileum from naïve and control or sup-ODN1-treated infected mice (hematoxylin and eosin stain, magnification ×100). All experiments shown in this figure are representative of three independent experiments with similar results. (e–f) Treatment with sup-ODN1 reduces weight loss and development of colitis upon DSS administration. Mice were given 2% DSS in drinking water for 8 days and changed to normal drinking water on day 9. Some mice received orally PBS containing sup-ODN1 (25 µg) or control ODN (ns-ODN, 25 µg) every other day, from day 0. Control mice (Ctrl) received PBS only. (e) shows weight loss measured as percentage of initial weight. Each dot represents the mean weight ± SD (4 or 5 mice per group). ** p < 0.01; *** p < 0.001 (sup-ODN1 treated mice compared to control). (f) Colon lengths from rectum to cecum were determined at day 13 following DSS treatment.
Suppressive ODNs have been shown to suppress inflammation in several experimental settings 23, 30–32. Based on the role of sup-ODN in limiting LpDC activation and sustaining Treg cell induction, we postulated that these suppressive motifs could represent a therapeutic approach to limit mucosal inflammation. C57BL/6 mice were infected orally with T. gondii and treated orally or not with sup-ODN1. At day 9 post-infection, pathology and antigen-specific cytokine responses from various sites were evaluated. As previously observed 33, 34, T. gondii oral infection induced the development of robust Th1 and pro-inflammatory cytokine responses (IFN-γ, TNF-α, IL-6 and IL-22), in the small intestine lamina propria (LP), mesenteric LN (MLN) and spleen (Figure 6b). As a byproduct of immune responses, mice developed a severe form of intestinal inflammation characterized by loss of epithelial architecture in the ileum, shortened villi, massive influx of inflammatory cells and scattered patches of necrosis, as well as severe liver damage, indicated by increased concentrations of serum alanine aminotransferase (Figure 6c, d). Strikingly, sup-ODN1 significantly reduced the Th1 response to T. gondii as well as the production of cytokines involved in T. gondii pathogenesis such as TNF-α, IL-6 and IL-22, in all the compartments analyzed (Figure 6b). In contrast, the immunoregulatory cytokine IL-10 required to limit tissue damage in this model 35 was selectively increased in the LP of sup-ODN1-treated animals compared to control mice (Figure 6b). Notably, the reduced inflammatory response was also associated with reduced intestinal and liver pathology (Figure 6c, d). These effects were specific to the presence of suppressive motifs as control ODN failed to down regulate inflammatory responses and pathology (Supplementary Figure S3 and data not shown). Further, the suppressive ODN used in this experiment specifically limited inflammatory responses mediated by TLR9 signaling since treatment with sup-ODN1 in Tlr9−/−mice had no effect (Supplementary Figure S4a, b). These findings show that treatment of mice with suppressive DNA motifs is sufficient to prevent the development of the catastrophic intestinal immunopathology observed during oral T. gondii infection.
To evaluate the suppressive role of sup-ODN in another model of mucosal inflammation, we utilized the well-established model of DSS-induced colitis. This model of colitis is associated with disruption of epithelial integrity which rapidly leads to acute gut inflammation and weight loss 36. Strikingly, treatment with sup-ODN1, but not with control ODN, significantly limited weight loss associated with DSS administration (Figure 6e). Further, colon size was significantly maintained following sup-ODN treatment compared to control treated animals (Figure 6f).
Altogether, these results demonstrate that sup-ODN can limit severe immunopathology in the GI tract.
Discussion
Here we showed that the DNA of defined commensal bacteria can be enriched in suppressive motifs able to exert strong immunoregulatory effects on the mucosal immune system. These findings reveal suppressive DNA motifs as an additional microbiota derived microbe-associated molecular pattern and support the idea that a balance between stimulatory and regulatory motifs at the commensal DNA level is likely to contribute to the maintenance of gut immune homeostasis and function. We propose a model in which the adjuvant properties of commensal DNA are tightly regulated by suppressive motifs allowing the induction of effector responses without triggering pathogenic responses.
The interaction between the host and the flora relies on a complex network of signals involving numerous bacterial species and microbial derived ligands. This multitude of signals converges to establish a tonic, regulated but responsive mucosal environment. Indeed, maintenance of responsiveness against pathogenic microbes as well as protection against commensal translocation relies on the establishment of a dynamic equilibrium sustained in part by the stimulatory capacity of the flora 37. Furthermore, recent work demonstrated that defined bacteria (e.g. SFB) or bacteria-derived products (e.g. bacterial polysaccharide A, DNA, ATP) could play a dominant role in promoting mucosal immune responses 6, 38–40. In some instances, defined bacteria species have been associated with enhanced pathogenesis during mucosal inflammation and are over-represented in the gut of patients suffering from inflammatory bowel disease 41. On the other hand, the microbiota can exert some strong immunoregulatory function via the induction and/or activation of Treg cells at mucosal sites. For instance, Bacteroides fragilis derived-polysaccharide A and indigenous bacteria from the genus Clostridium can expand or induce regulatory T cells 42–44. Recent data also demonstrate that a fraction of mucosal resident Treg are specific for commensal derived antigens 45. Further, some bacteria referred to as probiotics have been defined based on their beneficial effect to their host 8. Promising results have been obtained with probiotics in the treatment of human inflammatory diseases of the intestine and in the prevention and treatment of atopic eczema in neonates and infants 46, 47. The mechanisms behind these functional disparities between commensal species remain to date poorly understood, but has been associated at least in part with the induction of various regulatory T cell populations. Previous work identified an association between Crohn’s disease and a promotor polymorphism in the Tlr9 gene in humans 48. Such association supports the idea of a role for gut flora DNA sensing in the pathophysiology of inflammatory bowel diseases (IBD). We previously showed that the DNA of commensal bacteria can have a profound immunostimulatory role on mucosal immune responses 6. Strikingly, when used in isolation the DNA of L. paracasei is significantly less efficient at inducing immune responses and activating DCs than the DNA of E. coli, a commensal known for its stimulatory capacity. However, we found that these differences in the immunostimulatory capacity of L. paracasei and E. coli DNA were not associated with differences in CpG motifs
Krieg et al. were the first to document the existence of suppressive or neutralizing DNA motifs in the genome of certain strains of adenovirus 12. Other inhibitory sequences were later characterized in mammalian DNA. In humans and mice, telomeric regions of chromosomes contain large numbers of single-stranded guanosine-rich hexanucleotide repeats of the motif TTAGGG. Synthetic oligonucleotides composed of TTAGGG multimers have been developed and shown to have multiple effects on immune activation 13, 14, 23. In particular, these sequences down-regulate Th1 responses in the context of CpG ODN stimulation 11, 12, 24, 25. Importantly, we found that the genomes of bacteria from the Lactobacillus genus, including the genome of various probiotic such as L. paracasei, are enriched in several suppressive motifs. We find that enrichment in suppressive motifs is a general feature of bacteria from the Lactobacillus genus including bacteria with previously described probiotic activity. Although we cannot state that the relative enrichment of these suppressive motifs represents a unique feature to bacteria with known probiotic properties, we would like to mention that the regulatory functions of all members of the Lactobacillus genus have not been thoroughly studied at this time. These results support the idea that enrichment in suppressive DNA motifs is family specific. Our work reveals that these suppressive motifs have a potent immunoregulatory effect on the mucosal immune system. Indeed, these motifs potently inhibit CpG ODN mediated LpDC activation and sustain mucosal Treg cell conversion during inflammation both in vitro and in vivo. We also find that these motifs can limit immunopathology during acute intestinal infection. Complementing our results in an infectious model, we find that suppressive motifs potently inhibit the pathological consequences of DSS-induced colitis as well.
Despite their potent effect on immune responses, the exact mechanism of action of suppressive motifs remains poorly understood. Synthetic oligodeoxynucleotides composed of TTAGGG multimers can block the colocalization of CpG ODN with TLR9 in endosomal vehicles but not the binding or uptake of CpG ODN by immune cells 11. Further, their immunosuppressive ability depends on their ability to make complex structures and to prevent STAT1, STAT3 and STAT4 phosphorylation 25, 49. Consequently suppressive ODN impair the production of various inflammatory mediators such as IL-12 production 25. Interestingly, other nonimmunogenic nucleotides have been recently shown to strongly suppress CpG-induced innate responses by competing for high mobility group box proteins, known to be essential for triggering all nucleic acid receptor-mediated innate immune responses 50. Understanding how these motifs inhibit DC activation and immunopathology in the context of TLR9 stimulation in the GI tract remains to be addressed.
Inflammatory bowel disease constitutes a significant health burden in developed countries impacting the quality of life of some 1.4 million individuals in North America and 2.2 million individuals in Europe 51. There is now clear evidence that the microbiota is a major contributor to the initiation and amplification of mucosal inflammation 41. Here, we propose that the potential pathogenic outcome associated with microbial dysbiosis may be in part associated with increased ratio of immunostimulatory versus suppressive motifs at the DNA level, an event that could, in genetically predisposed individuals, tip the balance toward inflammation.
Our findings add an additional layer in our understanding of the complex host-microbiota interaction and propose a model in which the proinflammatory capacity of bacterial DNA can be harnessed by its endogenous content in suppressive motifs. Further, our data support the idea that suppressive ODN may represent an important therapeutic approach in the treatment of mucosal inflammatory responses.
Materials and Methods
Mice
C57BL/6 (WT) and B6.SJL mice were purchased from Taconic Farms. B6.129P2-Tlr9tmAki (Tlr9−/−) 52 mice were obtained from Dr. S. Akira (Osaka University, Japan) via Dr. R. Seder (Vaccine Research Center, NIH) and backcrossed 11 generations onto the C57BL/6 background (Taconic, Germantown, NY). Foxp3 eGFP reporter mice (Foxp3eGFP) were obtained from Dr. M. Oukka (Seattle Children’s Research Institute) 26. Tlr9−/− Foxp3eGFP mice were generated by crossing the F1 progeny of Tlr9−/− x Foxp3eGFP breeders. We generated OT-II Tg x Foxp3eGFP mice. All mice were bred and maintained under pathogen-free conditions at an American Association for the Accreditation of Laboratory Animal Care-accredited animal facility at the NIAID and housed in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals. All experiments were performed at the NIAID under an animal study proposal approved by the NIAID Animal Care and Use Committee. Mice between 6 and 12 weeks of age were used. Mice were sex- and age-matched for each experiment.
Parasite and infection protocol
A rabbit isolate of Encephalitozoon cuniculi obtained from Waterborne Inc. (New Orleans, LA) was used throughout the study. The parasites were maintained by continuous passage in rabbit kidney RK13 cells (ATCC #CCL-37). Spores were collected from the culture medium, resuspended in sterile PBS pH 7.2, and immediately used for inoculation of mice or cell cultures. Mice were infected by intragastric gavage with 5×106 fresh spores.
ME-49 clone C1 of Toxoplasma gondii (kindly provided by Dr. M.E. Grigg, NIAID/NIH) was obtained by electroporation of the parental ME-49 type II strain (ATCC #50840) with the red fluorescent protein (RFP) and was used for production of tissue cysts in C57BL/6 mice. Tissue cysts used in experiments were obtained from female mice that were perorally inoculated with 10 cysts two months earlier. Animals were sacrificed and their brains removed and homogenized in 1 ml of PBS pH 7.2. Cysts were counted under an inverted fluorescent microscope on the basis of 2 aliquots of 20 µl. For experiments, female mice were infected by intragastric gavage with 10 cysts of ME-49 C1.
Quantitation of parasite tissue loads
For experiments with E. cuniculi, duodenum was removed from infected mice and digested with proteinase K (Qiagen, Los Angeles, CA) after homogenization. DNA was subsequently extracted with the DNeasy Tissue kit from Qiagen. Quantitative real-time PCR was performed in triplicates with 50 ng of total tissue DNA, the iQ SYBR Green Supermix (BioRad, Richmond, CA), and the following primers specific for a 268-bp DNA sequence of the SSU rRNA gene from Encephalitozoon cuniculi: forward 5’-GTGAGACCCTTTGACGGTGT-3’ and reverse 5’-CTCAGACCTTCCGATCTTCG-3’. Real-time PCR was conducted on a Bio-Rad iCycler under the following conditions: 3 min at 95°C; 40 cycles of 45 s at 95°C; 60 s at 60°C; and 45 s at 72°C. Genomic DNA was extracted from known amounts of E. cuniculi spores with the QIAamp DNA stool mini-kit (Qiagen) and used as PCR standards. A standard curve was generated by linear regression on plotted cycle threshold (CT) values of the standards against the logarithms of parasite numbers using iCycler iQ Optical System software (version 3.1; Bio-Rad). Hs27 human fibroblast (ATCC #CRL-1634) cultures were used for parasite burden quantitation following infection with T. gondii. Tissue single-cell suspensions (104 to 106 cells) were added onto confluent fibroblast monolayers and titrated by looking at plaque-formation under an inverted fluorescent microscope 6 to 8 days later. Results of these titrations are reported as Plaque Forming Units (PFUs).
Genomic DNA extraction from bacteria
Escherichia coli strain K12 subtrain MG1655 was grown in tryptic soy broth medium (Fluka, St Louis, MO) under aerobic conditions at 37°C with constant shaking. Lactobacillus paracasei strain ATCC 25302 was grown in Lactobacilli MRS broth (Hardy Diagnostic, Santa Maria, CA) under anaerobic conditions at 37°C. Overnight cultures of E. coli or L. paracasei were centrifuged at 3300 g and 4°C for 20 min. Pellets were resuspended in lysis buffer (10 mM Tris/HCl, 50 mM EDTA, pH 8.0) containing lysozyme (0.5 mg/ml; Sigma-Aldrich, St Louis, MO). After incubation at 37°C for 2 hrs, 2 mg/ml of proteinase K and 1% SDS were added and the sample incubated at 60°C for 3 hrs. DNA was then purified by a series of 7 consecutive phenol-chloroform-isoamyl alcohol affinity extractions. The quality and the amount of purified DNA were measured and quantified using a NanoDrop 3300 spectrophotometer (Thermo Scientific, Wilmington, DE).
Oligonucleotides (ODN)
Oligonucleotides (ODN) with a nuclease-resistant phosphorothioate backbone were custom synthesized at the Center for Biologics Evaluation and Research core facility (Food and Drug Administration, Bethesda, MD). The following ODNs were used in experiments: stimulatory CpG ODN 1555, GCTAGACGTTAGCGT; suppressive ODN A151 (sup-ODN1) TTAGGGTTAGGGTTAGGGTTAGGG 23; suppressive ODN H154 (sup-ODN2) CCTCAAGCTTGAGGGG 24; and control ODN, GTCACGGCTGATGAGCG. ODNs contained <0.1 units of endotoxin/mg, as assessed by a Limulus amebocyte cell lysate assay. CpG ODN 1826 was purchased from Coley Pharmaceutical (Wellesley, MA). For injection in mice, all ODNs were diluted in PBS pH 7.2 and mice were orally injected with isotonic bicarbonate buffer 10 min prior to gavage with ODNs.
Bioinformatics
Genome data were downloaded from NCBI Genomes database for 28 different species of Lactobacillus bacteria and 27 strains of Escherichia coli (see Supplementary Tables 1 and 2). The presence of the following sequence motifs in the genome of different bacteria was analyzed: AACGTT and ATCGAT for the CpG motifs, TTAGGG and TCAAGCTTGA for the suppressive motifs 23, 24. “Perl” scripts were used to match the motif patterns to the bacteria sequences. Alternatively, the motifs were enumerated using the “fuzznuc” program of the EMBOSS package (http://emboss.sourceforge.net). Custom PHP scripts were written to extract and tabulate the results obtained using the above programs.
Antibiotic treatment
Male 3-week-old C57BL/6 mice were provided ampicillin (1 g/l), neomycin trisulfate (1 g/l), metronidazole (1 g/l) and vancomycin (500 mg/l) in drinking water as previously described 3. All antibiotics were purchased from Sigma-Aldrich (St Louis, MO). Six weeks after the start of the antibiotic course, mice also received orally various treatments once a week: CpG ODN 1826 (100 µg) in sterile PBS, DNA from E. coli (500 µg) or DNA from L. paracasei (500 µg) in sterile water. Two weeks later, mice were infected with E. cuniculi by gavage. In some experiments, mice were treated orally every three days with CpG ODN 1826 (25 µg) alone or in combination with suppressive ODN1 (25 µg) starting 6 weeks after the beginning of the antibiotic regimen. Mice were then gavaged with E. cuniculi 6 days later.
In vitro restimulation and intracellular cytokine detection
RPMI 1640 supplemented with 10% FBS, 2 mM L-glutamine, 20 mM HEPES, 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml gentamicin, 50 mM of β-mercaptoethanol, 1 mM sodium pyruvate and nonessential amino acids (complete medium) was used for in vitro restimulation. Single-cell suspension of leukocytes from spleen, mesenteric lymph nodes (MLN) and small intestinal lamina propria (LP) of naïve or infected mice were prepared as previously reported 22. For experiment with E. cuniculi, 5 × 105 leukocytes were co-cultured with 1 × 105 bone marrow-derived dendritic cells (BMDC) in a 96-well U-bottom plate. BMDC were previously incubated overnight with or without E. cuniculi spores (parasite:BMDC ratio, 10:1) in the presence of 20 ng/ml GM-CSF (Peprotech, Rocky Hill, NJ) and washed before culture with leukocytes. For experiment with T. gondii, leukocytes were stained with the CD90.2-positive selection kit and enriched for T cells with an autoMACS Pro (Miltenyi Biotec, Auburn, CA). After enrichment, 2.5 × 105 T cells were then co-cultured with 5 × 104 irradiated (3,000 rad) BMDC and with or without soluble T. gondii antigen (STAg, 5 µg/ml) in a 96-well U-bottom plate. After 2 or 3 days at 37°C in 5% CO2, culture supernatants were collected for cytokine assays. IFN-γ, IL-6, IL-10, IL-17, IL-22 and/or TNF-α were quantitated in culture supernatants using the DuoSet ELISA system (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol. For intracellular cytokine detection, LP single-cell suspensions were cultured in triplicate at 2 ×106 cells/ml in a 96-well U-bottom plate and stimulated with various amounts of CpG ODN 1826, CpG ODN 1555, DNA from E. coli or L. paracasei, suppressive ODN 1 and/or control ODN for 5 hrs at 37°C in 5% CO2. Brefeldin A (GolgiPlug, BD Biosciences, San Jose, CA) was added for the final 4 hrs of culture. After restimulation, cells were incubated with purified anti-mouse CD16/32 (clone 93) and with fluorochrome-conjugated antibodies against surface markers CD11b (M1/70), CD11c (N418), MHCII (M5/114.15.2), F4/80 (BM8) and CD103 (2E7), in HBSS for 20 min at 4°C and then washed. LIVE/DEAD Fixable Blue Dead Cell Stain Kit (Invitrogen, Carlsbad, CA) was used to exclude dead cells. Cells were then fixed for 15 min at room temperature using 2% paraformaldehyde solution (Electron Microscopy Sciences, Hatfield, PA), washed twice and then stained with fluorochrome-conjugated antibodies against IL-12/23p40 (C15.6), TNF-α (MP6-XT22), or isotype control rat IgG1κ (R3-34) for 30 min at 4°C in permeabilization buffer supplied with the BD Cytofix/Cytoperm kit (BD Biosciences) containing anti-mouse CD16/32, 0.2 mg/ml purified rat IgG and 1 mg/ml of normal mouse serum (Jackson Immunoresearch, West Grove, PA). All antibodies were purchased from eBioscience (San Diego, CA) or BD Biosciences. Cell acquisition was performed on an LSRII flow cytometer using FACSDiVa software (BD Biosciences). For each sample, at least 300,000 events were collected. Data were analyzed using FlowJo software (TreeStar).
Lamina propria DC and T cell purification
Lamina propria dendritic cells (LpDCs) were purified from the small intestine of naïve wild-type or Tlr9−/− mice. After LP digests were passed through 70- and 40-µm cell strainers, cells were resuspended in 1.077 g/cm3 iso-osmotic animal cell separation medium (Accurate Chemical & Scientific Corp., Westbury, NY), and overlayed with RPMI 1640 medium. After centrifugation at 1,650 g for 15 min, cells were collected from the low-density fraction, washed and then incubated with a mixture of antibodies containing α-CD16/32 (2.4G2), α-CD11c (HL-3), α-MHCII (AF6-120.1), as well as the non-DC components α-TCR-β (H57-597) and 7-AAD viability staining solution (all from eBioscience). DCs were defined as TCR-β− CD11chi MHCII+ cells and sorted by flow cytometry on a FACSAria (BD Biosciences). The post-sort purity was higher than 95%. Purified CD11chi MHCII+ cells were used for in vitro conversion assay or stimulated for 18 hrs with CpG ODN 1826, DNA from E. coli or L. paracasei, suppressive ODN1 and/or non-sense ODN in presence of GM-CSF (50 ng/ml, Peprotech) in 5% CO2. For T cell purification, single-cell suspensions of spleen and peripheral lymph nodes extracted from Tlr9−/− Foxp3eGFP mice were enriched for CD4+ T cells by negative selection using an autoMACS Pro (Miltenyi Biotec). The enriched fraction was further labeled with fluorochrome-conjugated antibodies against CD4 (RM4-5), CD25 (7D4) and CD44 (IM7) (all from eBioscience), and sorted by flow cytometry on a FACSAria. Purified CD4+ CD25− CD44lo Foxp3− T cells (<0.5% Foxp3+) were used for in vitro conversion assays.
In vitro conversion assay
Our conversion protocol was performed as previously reported 26 with T cells and LpDCs obtained as described above. In brief, FACS-purified LpDCs and CD4+ T cells were co-cultured at a 1:10 ratio (1×105 CD4+ T cells) in complete medium and Treg cell polarizing conditions: soluble α-CD3 (1 µg/ml, BD Biosciences) and human recombinant TGF-β (0.3 ng/ml, R&D Systems). Co-cultures were supplemented with 5 ng/ml of recombinant human IL-2 (Peprotech) every 2 days. In some experiments, CpG ODN 1555, suppressive ODN1, control ODN, E. coli DNA and L. paracasei DNA were added individually or in combination at various concentrations at the start of the co-cultures in Treg cell polarizing conditions. On day 5, cells were stained with the LIVE/DEAD Fixable Blue Dead Cell Stain Kit and fluorochrome-conjugated antibodies against the cell surface markers CD4 (RM4-5) and α4β7 (DATK32) (all from eBioscience) in HBSS for 20 min at 4°C and then washed twice. Foxp3 staining was subsequently performed using the Foxp3 staining set (eBioscience) according to the manufacturer’s protocol.
Oral antigen administration
B6.SJL (CD45.1) mice were orally infected or not with 10 ME-49 C1 cysts of T. gondii. CD4+ Foxp3− T lymphocytes from the secondary lymph nodes and spleen of naïve OT-II Tg x Foxp3eGFP (CD45.2) mice were purified by cell sorting and adoptively transferred into naïve or infected (4 days post infection) B6.SJL recipient mice. Each mouse received 1.5 × 106 cells and was then fed with ovalbumin dissolved in the drinking water (1.5% solution; grade V from Sigma-Aldrich) for 5 consecutive days. On day 9 after infection, MLN and LP single-cell suspension were prepared from B6.SJL hosts as described above and intracellular Foxp3 expression was assessed among CD45.1+ transferred cells. In some experiments, B6.SJL recipient mice were injected by gavage with 25 µg of suppressive ODN1 on day -3 and -1 before infection with T. gondii and on days 1, 3, 5 and 7 post-infection.
Pathology assessment
Mice treated or not with suppressive ODN1 were euthanized 9 days post-oral infection with T. gondii. The ileum from each mouse was removed and immediately fixed in PBS containing 10% formalin. Paraffin-embedded sections were cut at 0.5 mm, stained with hematoxylin and eosin and examined histologically. Liver alanine aminotransferase (ALT) levels were measured in serum samples, using commercially available kits (Boerhinger Mannheim).
Induction of DSS colitis
Mice were given 2% (w/v) DSS (M.W. = 36,000–50,000 kDa, MP Biologicals) in their drinking water for 8 days, and subsequently switched to regular drinking water. The amount of DSS water drank was recorded for each cage and no differences in intake between cages were observed. Mice were weighted daily. Some mice were also treated orally every other day with suppressive ODN1 (25 µg) or control ODN (25 µg) starting at day 0 and for the duration of the experiments. Mice were euthanized on day 13 and colons were removed, flushed and the length was measured from rectum to cecum.
Statistical analysis
Groups were compared with Prism software (GraphPad) using the two-tailed unpaired Student’s t test. Data are presented as mean ± SD. P<0.05 was considered significant.
Supplementary Material
Figure S1. Effect of E. coli and L. paracasei DNA on Treg cell conversion is TLR9-dependent. FACS-sorted CD4+ CD25− CD44lo Foxp3− T cells isolated from Foxp3eGFP Tlr9−/− naïve mice were culture in Treg cell-polarizing conditions with CD11chi MHCII+ wild-type or Tlr9−/− LpDCs in the presence of the following treatments: E. coli DNA (Ecoli; 0.1 µg/ml or 10 µg/ml) or L. paracasei (Lacto; 0.1 µg/ml or 10 µg/ml). After 5 days of culture, Foxp3 expression on viable TCR-β+ CD4+ T cells was analysed by flow cytometry. Histograms represent the percentage of baseline conversion with 100% equalling conversion in Treg cell-polarizing conditions in the absence of any DNA. Cross bars indicate the highs and lows of duplicate cultures. Data shown are representative of two independent experiments with similar results.
Figure S2. L. paracasei and E. coli genomes have similar frequencies of CpG immunostimulatory motifs. The sequenced genomes of two strains of E. coli and six species of Lactobacillus bacteria were analyzed for the presence of immunostimulatory CpG and immunosuppressive motifs. (a) Graph bars represent the number of two CpG motifs (AACGTT and ATCGAT) per 105 bases for each bacteria. (b, c) show the ratio between the suppressive motifs TTAGG (b) or TCAAGCTTGA (c) and the two CpG motifs in the sequenced genome of each bacteria. A higher ratio reflects a higher enrichment of suppressive motif in the genome.
Figure S3. Treatment with control ODN has no effect on inflammatory immune responses and pathology during oral infection with Toxoplasma gondii. Mice were orally infected with T. gondii. In addition some mice were orally treated with PBS containing sup-ODN1 (25 µg) or control ODN (ns-ODN, 25 µg) every other day, starting 3 days before the infection. Control mice received PBS only. At day 9 post-infection, mice were euthanized and immune responses were assessed. ELISA of IFN-γ, IL-10, TNF-α, IL-6 and IL-22 in supernatants of spleen (Spl), mesenteric lymph nodes (MLN) or small intestine lamina propria (LP) CD90+ cells prepared from the different groups of mice and restimulated for 48 hrs with BMDC loaded with soluble T. gondii antigen. Histograms represent the mean cytokine concentration of triplicate wells ± SD.
Figure S4. Suppressive ODN has no effect on inflammatory immune responses and pathology during oral infection with Toxoplasma gondii in Tlr9−/− mice. Tlr9−/− mice were orally infected with T. gondii. In addition some mice were orally treated with PBS containing sup-ODN1 (25 µg) every other day, starting 3 days before the infection. Control mice (Ctrl) received PBS only. At day 9 post-infection, mice were euthanized and immune responses and liver pathology were assessed. (a) ELISA of IFN-γ, IL-10, TNF-α, IL-6 and IL-22 in supernatants of spleen (Spl), mesenteric lymph nodes (MLN) or small intestine lamina propria (LP) CD90+ cells prepared from the different groups of Tlr9−/− mice and restimulated for 48 hrs with BMDC loaded with soluble T. gondii antigen. Histograms represent the mean cytokine concentration of triplicate wells ± SD. (b) The level of hepatic alanine aminotransferase (ALT) was assessed in the serum of naïve or day 9 post-infection Tlr9−/− mice treated or not with sup-ODN1. Histograms show the mean enzyme concentration ± SD.
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank C. Eigsti and E. Stregevsky from the NIAID sorting facility and K. Beacht, V. Wang and T. Peterson for technical assistance. We thank D. Chou, M. Molloy, S. Naik, S. Spencer and E. Wohlfert for their critical reading of the manuscript.
Abbreviations used
- E. cuniculi
Encephalitozoon cuniculi
- GI
gastrointestinal tract
- IL
interleukin
- IFN-γ
interferon gamma
- LP
lamina propria
- LpDC
lamina propria dendritic cell
- MLN
mesenteric lymph node
- ODN
oligodeoxynucleotide
- STAg
soluble Toxoplasma antigen
- sup-ODN
suppressive ODN
- Teff cell
effector T cell
- Treg cell
regulatory T cell
- T. gondii
Toxoplasma gondii
- TNF
tumor necrosis factor
References
- 1.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Sansonetti PJ, Di Santo JP. Debugging how bacteria manipulate the immune response. Immunity. 2007;26:149–161. doi: 10.1016/j.immuni.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–6987. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 6.Hall JA, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan F, Polk DB. Probiotics and immune health. Curr Opin Gastroenterol. 2011;27:496–501. doi: 10.1097/MOG.0b013e32834baa4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalpke A, Frank J, Peter M, Heeg K. Activation of toll-like receptor 9 by DNA from different bacterial species. Infect Immun. 2006;74:940–946. doi: 10.1128/IAI.74.2.940-946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neujahr DC, Reich CF, Pisetsky DS. Immunostimulatory properties of genomic DNA from different bacterial species. Immunobiology. 1999;200:106–119. doi: 10.1016/S0171-2985(99)80036-9. [DOI] [PubMed] [Google Scholar]
- 11.Gursel I, et al. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol. 2003;171:1393–1400. doi: 10.4049/jimmunol.171.3.1393. [DOI] [PubMed] [Google Scholar]
- 12.Krieg AM, et al. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs. Proc Natl Acad Sci U S A. 1998;95:12631–12636. doi: 10.1073/pnas.95.21.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada H, et al. Effect of suppressive DNA on CpG-induced immune activation. J Immunol. 2002;169:5590–5594. doi: 10.4049/jimmunol.169.10.5590. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H, Cheng SH, Yew NS. Requirements for effective inhibition of immunostimulatory CpG motifs by neutralizing motifs. Antisense Nucleic Acid Drug Dev. 2000;10:381–389. doi: 10.1089/oli.1.2000.10.381. [DOI] [PubMed] [Google Scholar]
- 15.Moretto M, Weiss LM, Khan IA. Induction of a rapid and strong antigen-specific intraepithelial lymphocyte response during oral Encephalitozoon cuniculi infection. J Immunol. 2004;172:4402–4409. doi: 10.4049/jimmunol.172.7.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogunovic M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varol C, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 19.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 22.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klinman DM, et al. Therapeutic potential of oligonucleotides expressing immunosuppressive TTAGGG motifs. Ann N Y Acad Sci. 2005;1058:87–95. doi: 10.1196/annals.1359.015. [DOI] [PubMed] [Google Scholar]
- 24.Zeuner RA, et al. Reduction of CpG-induced arthritis by suppressive oligodeoxynucleotides. Arthritis Rheum. 2002;46:2219–2224. doi: 10.1002/art.10423. [DOI] [PubMed] [Google Scholar]
- 25.Shirota H, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides inhibit Th1 differentiation by blocking IFN-gamma- and IL-12-mediated signaling. J Immunol. 2004;173:5002–5007. doi: 10.4049/jimmunol.173.8.5002. [DOI] [PubMed] [Google Scholar]
- 26.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 27.Oldenhove G, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gazzinelli RT, et al. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 29.Munoz M, et al. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009;206:3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng X, et al. Suppressive oligodeoxynucleotides inhibit atherosclerosis in ApoE(−/𢈒) mice through modulation of Th1/Th2 balance. J Mol Cell Cardiol. 2008;45:168–175. doi: 10.1016/j.yjmcc.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Fujimoto C, et al. A suppressive oligodeoxynucleotide inhibits ocular inflammation. Clin Exp Immunol. 2009;156:528–534. doi: 10.1111/j.1365-2249.2009.03918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeuchi H, Kinjo T, Klinman DM. Effect of suppressive oligodeoxynucleotides on the development of inflammation-induced papillomas. Cancer Prev Res (Phila) 2011;4:752–757. doi: 10.1158/1940-6207.CAPR-10-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liesenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? J Infect Dis. 2002;185(Suppl 1):S96–S101. doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- 34.Mennechet FJ, et al. Lamina propria CD4+ T lymphocytes synergize with murine intestinal epithelial cells to enhance proinflammatory response against an intracellular pathogen. J Immunol. 2002;168:2988–2996. doi: 10.4049/jimmunol.168.6.2988. [DOI] [PubMed] [Google Scholar]
- 35.Gazzinelli RT, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 36.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 37.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 38.Atarashi K, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 39.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Saleh M, Elson CO. Experimental inflammatory bowel disease: insights into the host-microbiota dialog. Immunity. 2011;34:293–302. doi: 10.1016/j.immuni.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ochoa-Reparaz J, et al. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J Immunol. 2010;185:4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- 43.O'Mahony C, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000112. e1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atarashi K, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lathrop SK, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, Murrell DF, Tang ML. Probiotics for the treatment of eczema: a systematic review. Clin Exp Allergy. 2009;39:1117–1127. doi: 10.1111/j.1365-2222.2009.03305.x. [DOI] [PubMed] [Google Scholar]
- 47.Moayyedi P, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325–332. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 48.Torok HP, et al. Crohn's disease is associated with a toll-like receptor-9 polymorphism. Gastroenterology. 2004;127:365–366. doi: 10.1053/j.gastro.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 49.Shirota H, Gursel I, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides protect mice from lethal endotoxic shock. J Immunol. 2005;174:4579–4583. doi: 10.4049/jimmunol.174.8.4579. [DOI] [PubMed] [Google Scholar]
- 50.Yanai H, et al. Suppression of immune responses by nonimmunogenic oligodeoxynucleotides with high affinity for high-mobility group box proteins (HMGBs) Proc Natl Acad Sci U S A. 2011;108:11542–11547. doi: 10.1073/pnas.1108535108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 52.Hemmi H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of E. coli and L. paracasei DNA on Treg cell conversion is TLR9-dependent. FACS-sorted CD4+ CD25− CD44lo Foxp3− T cells isolated from Foxp3eGFP Tlr9−/− naïve mice were culture in Treg cell-polarizing conditions with CD11chi MHCII+ wild-type or Tlr9−/− LpDCs in the presence of the following treatments: E. coli DNA (Ecoli; 0.1 µg/ml or 10 µg/ml) or L. paracasei (Lacto; 0.1 µg/ml or 10 µg/ml). After 5 days of culture, Foxp3 expression on viable TCR-β+ CD4+ T cells was analysed by flow cytometry. Histograms represent the percentage of baseline conversion with 100% equalling conversion in Treg cell-polarizing conditions in the absence of any DNA. Cross bars indicate the highs and lows of duplicate cultures. Data shown are representative of two independent experiments with similar results.
Figure S2. L. paracasei and E. coli genomes have similar frequencies of CpG immunostimulatory motifs. The sequenced genomes of two strains of E. coli and six species of Lactobacillus bacteria were analyzed for the presence of immunostimulatory CpG and immunosuppressive motifs. (a) Graph bars represent the number of two CpG motifs (AACGTT and ATCGAT) per 105 bases for each bacteria. (b, c) show the ratio between the suppressive motifs TTAGG (b) or TCAAGCTTGA (c) and the two CpG motifs in the sequenced genome of each bacteria. A higher ratio reflects a higher enrichment of suppressive motif in the genome.
Figure S3. Treatment with control ODN has no effect on inflammatory immune responses and pathology during oral infection with Toxoplasma gondii. Mice were orally infected with T. gondii. In addition some mice were orally treated with PBS containing sup-ODN1 (25 µg) or control ODN (ns-ODN, 25 µg) every other day, starting 3 days before the infection. Control mice received PBS only. At day 9 post-infection, mice were euthanized and immune responses were assessed. ELISA of IFN-γ, IL-10, TNF-α, IL-6 and IL-22 in supernatants of spleen (Spl), mesenteric lymph nodes (MLN) or small intestine lamina propria (LP) CD90+ cells prepared from the different groups of mice and restimulated for 48 hrs with BMDC loaded with soluble T. gondii antigen. Histograms represent the mean cytokine concentration of triplicate wells ± SD.
Figure S4. Suppressive ODN has no effect on inflammatory immune responses and pathology during oral infection with Toxoplasma gondii in Tlr9−/− mice. Tlr9−/− mice were orally infected with T. gondii. In addition some mice were orally treated with PBS containing sup-ODN1 (25 µg) every other day, starting 3 days before the infection. Control mice (Ctrl) received PBS only. At day 9 post-infection, mice were euthanized and immune responses and liver pathology were assessed. (a) ELISA of IFN-γ, IL-10, TNF-α, IL-6 and IL-22 in supernatants of spleen (Spl), mesenteric lymph nodes (MLN) or small intestine lamina propria (LP) CD90+ cells prepared from the different groups of Tlr9−/− mice and restimulated for 48 hrs with BMDC loaded with soluble T. gondii antigen. Histograms represent the mean cytokine concentration of triplicate wells ± SD. (b) The level of hepatic alanine aminotransferase (ALT) was assessed in the serum of naïve or day 9 post-infection Tlr9−/− mice treated or not with sup-ODN1. Histograms show the mean enzyme concentration ± SD.