Abstract
Objective
To analyze coronary artery vitamin D receptor (VDR) expression, plasma concentration of vitamin D3 [25OHD3], and their relationship with coronary artery atherosclerosis.
Methods
Premenopausal cynomolgus monkeys were fed atherogenic diets containing the equivalent of 1,000 IU/day of 25OHD3. Protein was derived from casein-lactalbumin (C/L, n=10), soy protein isolate (soy, n=10), or a combination (n=19). After 32 months consuming the diets, each monkey underwent surgical menopause. After 32 post-menopausal months, coronary atherosclerosis was measured in the left circumflex artery (LCX) and left anterior descending artery (LAD). VDR expression was determined for the LAD and 25OHD3 concentrations were assessed.
Results
Both the cross-sectional area of atherosclerotic plaques (mm2) and plaque thickness (mm) in the LCX as well as the LAD were analyzed in these monkeys. Those with higher plasma 25OHD3 concentrations and higher VDR were compared to those with higher plasma 25OHD3 concentrations and lower VDR. Significantly smaller plaque sizes were noted with higher plasma 25OHD3 concentrations and higher VDR. For the LCX, there was also a significantly lower plaque size (both plaque thickness and cross sectional area) in those with higher VDR and lower 25OHD3 concentrations versus those with lower quantities of VDR and higher plasma concentrations of 25OHD3, p=0.040 and p=0.009, respectively.
Conclusions
Cynomolgus monkeys with higher quantities of VDR have significantly less atherosclerosis than those with lower quantities of VDR and higher plasma 25OHD3 concentrations. If these findings translate to human beings, it might explain why some individuals with higher plasma concentrations of 25OHD3 have more coronary artery atherosclerosis.
Keywords: Vitamin D, Vitamin D Receptors, Coronary Artery Atherosclerosis, Cardio-protection, Menopause, Coronary heart disease
Introduction
The biologically active form of vitamin D (1,25(OH)2D3) is a fat soluble vitamin.1 It is the only vitamin that can be acquired via endogenous production from 7-dehydrocholesterol after exposure to UVB light.2-4 The measurement 1,25(OH)2D3 can be misleading with respect to whole body stores of vitamin D (25OHD) due to compensatory mechanisms [i.e. secondary hyperparathyroidism (SHPT)] which may mask low vitamin D concentrations.3-6 Consequently, the isomer 25OHD (both 25OHD2 and 25OHD3) is traditionally measured in plasma to determine the adequacy of vitamin D stores rather than the active form.
While the role of vitamin D and skeletal health is well understood,7 more recent evidence has suggested potential non-skeletal benefits to vitamin D. These benefits may include the prevention of various cancers,8 psoriasis,9 multiple sclerosis,10 and coronary heart disease (CHD).11,12
There are many well-established pathophysiological reasons to indicate that vitamin D may have a beneficial role in cardiovascular health. These include vitamin D’s ability to inhibit vascular smooth muscle cell proliferation, vascular calcification, and atherogenesis via anti-inflammatory pathways [including effects on C-reactive protein (CRP), cytokines, tumor necrosis factor (TNF), interleukin-6 (IL-6), and matrix metalloproteinase 9 (MMP-9)].13-17 Vitamin D has been shown to improve insulin sensitivity18,19 and to have a beneficial association with diabetes mellitus.20-24 Vitamin D has been associated with enhanced vascular reactivity25 and can control hypertension through its effect on intravascular volume via the renin-angiotensin-aldosterone system.26-27 Vitamin D deficiency also has been associated with heart failure28-29 and a worsening thrombogenic profile.30 Despite these mechanisms explaining a logical link between vitamin D and CHD, there are no good prospective studies to support this concept. In accordance, the recent IOM report emphasized the need to explore further evidence and/or knowledge about the potential association between vitamin D and CHD.7
While most physicians, as well as researchers, are focusing on 25OHD3 supplementation, an adequate/optimal dose and the target plasma concentration are not well defined. There are still many simple unanswered questions. For example, while a strong genetic link for the plasma concentration and physiologic variability of vitamin D has been suggested,31-33 it has not been well defined what parameters are under genetic influence. In addition, it is not known whether there are genetic variations in vitamin D metabolism, intestinal absorption, production of transport proteins, vitamin D receptor (VDR) concentrations or activity levels. Clinically, it is evident that responses to standard doses of 25OHD3 supplementation are variable. The results of such studies have suggested that 18 to 53% of individuals will not respond to vitamin D supplementation despite oral doses of between 2,000 and 8,000 IU per day of 25OHD3.34,35 Similarly, we have shown previously that plasma concentrations of 25OHD3 varied considerably (26 to 95 ng/mL) in monkeys consuming diets with identical amounts of 25OHD3 and where living conditions along with sun exposure were the same.36
The clinical implication(s) and meaning of such a wide range of plasma concentrations are not known. Recent data from investigations using mice suggest that vitamin D binding protein (DBP) can influence the plasma concentration of 1,25(OH)2D3 without affecting biologic activity.37 Mice deficient in DBP had significantly reduced plasma concentrations of 1,25(OH)2D3, yet their serum calcium (a marker of vitamin D activity) was normal and not significantly different from the control mice37. Studies also suggest that DBP is involved in the delivery of vitamin D to the nuclear site of vitamin D activation, the VDR37,38.
VDR’s have been localized and identified in many tissues including pancreas,39 lung,40 myocardium,41 skeletal muscle,42 brain43, bone44, and ovary45. The potential importance of coronary VDR in the pathogenesis of coronary artery atherosclerosis has not been studied previously nor has the possible interaction between coronary artery VDR expression, plasma concentrations of vitamin D and coronary artery atherosclerosis. For these reasons, we quantified the expression of VDR in coronary arteries of cynomolgus monkeys, measured their plasma concentrations of 25OHD3 and correlated those results with the degree of coronary artery atherosclerosis.
Methods
Animals and Diets
After arriving at the Wake Forest University Primate Center from Indonesia, our cohort of 39 female cynomolgus monkeys (Macaca fascicularis) were housed indoors with no UV exposure for 3 months prior to the beginning of the study. Beginning at arrival, they were fed a diet which contained a women’s equivalent of 1,000 IU/day of 25OHD3 and 1,200 mg/day of calcium. Furthermore, and throughout the study, they were fed atherogenic diets with a women’s equivalent of 1,000 IU/day of 25OHD3 and 1,200 mg/day of calcium.
All animals were housed indoors during the entire length of the study. Dentition and evidence of physeal closure were used to determine adult status in the monkeys which on average was 11.5 years. After 32 months consuming diet A [casein-lactalbumin (C/L) or soy protein isolate (soy)], each monkey underwent oophorectomy to induce surgical menopause. Each diet group was then re-randomized to receive diet B [C/L or soy]. A total of 10 received C/L-soy, 10 received C/L-C/L, 9 received soy-C/L, and 10 received soy-soy, enhancing the likelihood of a broad range of coronary artery atherosclerosis. After 32 postmenopausal months and necropsy, coronary artery atherosclerosis, coronary artery VDR expression, and 25OHD3 concentrations were assessed. Both the C/L and soy diets contained 19% of calories from protein, 35% calories from fat, 46% calories from carbohydrate, and 0.20 mg cholesterol/cal. Both diets used wheat flour as a source for protein. The soy diet contained 1.85 mg aglycone isoflavones/g protein (SUPRO ® SOY Isolated Soy Protein, Solae St. Louis, MO).
All procedures involving animals in this study were conducted in compliance with state and federal laws, standards of the US Department of Health and Human Services, and guidelines established by the Wake Forest University Institutional Animal Care and Use Committee, where the animals were housed. Wake Forest University is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. An adiposity index for the monkeys was derived by measuring body weight by trunk length, and has a normal range of 35 to 55 kg/m2. Trunk length is measured from the sternal notch to the pubic symphysis.
Plasma Vitamin D3 Assessment
Plasma (500 μL) was collected from all cynomolgus monkeys (n=39). The samples were frozen (−70°C), packaged such that they were protected from sunlight, transported to The Reading Hospital and Medical Center, Reading, PA36,46, and had never been thawed prior to the vitamin D assessments. HPLC/tandem mass spectrometry, utilizing Shimadzu liquid chromatography - mass spectrometry/mass spectrometry (LC-MS/2) technology was used for determination of 25OHD3. Liquid chromatography - mass spectrometry allows the sample to be ionized, through the physical separation abilities of liquid chromatography, for mass analysis with the AB Sciex 3200 Q Trap mass spectrometer.
Atherosclerosis Evaluation
The cross-sectional area of atherosclerotic plaques (intimal area in mm2) and maximal plaque thickness (mm) were determined for the left circumflex artery (LCX) and the left anterior descending artery (LAD) from the proximal portions of those arteries obtained at necropsy. Sections of each artery, 0.5 cm in length, were placed into 70% ethanol, dehydrated, and embedded into paraffin blocks. Blocks were cut to 5 μm sections which were then deparaffinized and stained with Verhoeff and Van Gieson stain. Plaque size (IA) and plaque thickness (IT) were assessed by computer-assisted histomorphometry using Image Pro Plus software (Media Cybernetics, Inc., Silver Springs, MD). Measurements were conducted by an experienced technician using a well-established protocol.47
Identification of the VDR and Quantification using the H-Score Method
For the quantification of VDR, to eliminate the potential of intra or interobserver variation and interpretation bias, one consistent and blinded observer was used for all measurements. In order to assure validity and reproducibility, however, the following steps were taken before quantification and observations were carried out. The designated observer met with other trained observers to co-analyze specimens assuring consistent and reproducible results.
The quantity of coronary artery VDR’s was subsequently determined for the LAD by determining the intimal H-Score. Artery blocks were cut to 5 μm sections, which were deparaffinized and immunohistochemically stained for the VDR. Sections were treated with rat anti-Vitamin D Receptor Monoclonal Antibody (Thermo Scientific, Rockford, IL) and then treated with biotinylated goat anti-rat (Serotec, Raleigh, NC) with the enzyme conjugate steptavidin-alkaline phosphatase (BioGenex, San Ramon, CA) and enzyme substrate vector red (Vector Laboratories, Burlingame, CA). For counting, only a hematoxylin counterstain was used; the internal elastic lamina was visible as a refractile line. For demonstration of the internal elastic lamina for photomicroscopy (Figure 2), slides were further stained for elastin using a modified Weigert resourcin-fuchsin method48, hematoxylin, and metanyl yellow.
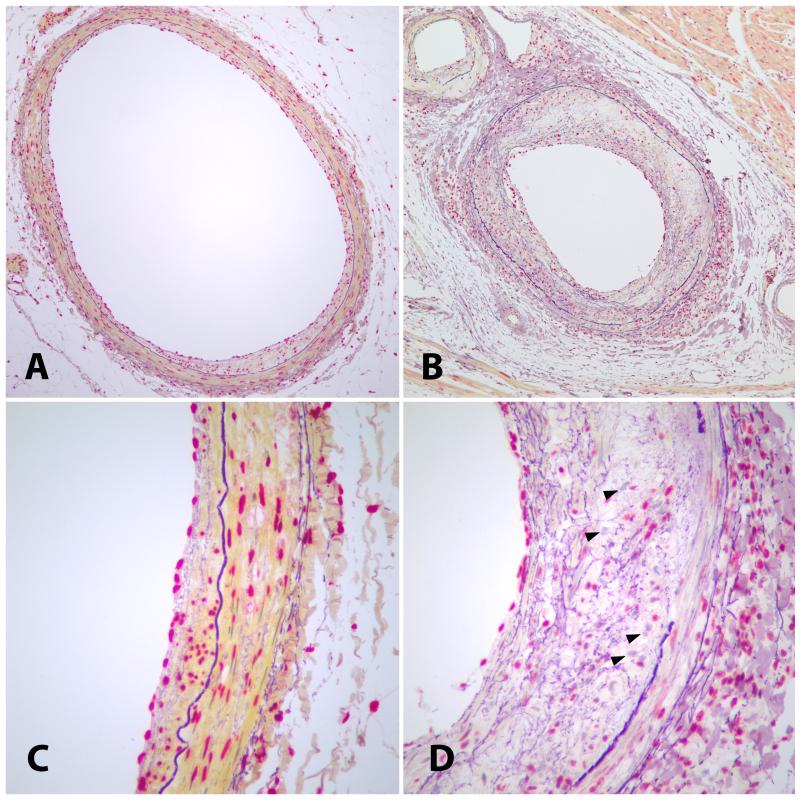
Figure 2.
Photomicrographs of monkey coronary arteries with vitamin D receptors (VDRs) identified with rat anti-monoclonal VDR antibody staining. The cells expressing VDR appear red while the cells not expressing VDR appear blue. The internal elastic lamina is dark purple. (A and C) Small atherosclerotic lesion with abundant VDR expression in all layers of the arterial wall. (B and D) Large atherosclerotic lesion with loss of VDR expression in all layers of the arterial wall, most markedly in the intima. VDR-negative cells are indicated by arrowheads. Modified Weigert counterstain. Objective magnification, 10X (A and B) or 40X (C and D).
All cells in all layers of the section were counted including the endothelium, intima, media, and adventitia. For this study, the adventitia was defined as half a 400X field width from the outermost portion of the media. Cells were counted using a light microscope at 400X magnification and a key counter. H-Score was calculated using a method described by Siboni A, et al49. All cells were assigned a grade of 0, 1, 2, or 3, where a grade 0 indicated a nucleus with no red stain, a grade 1 indicated a slightly red stained nucleus, a grade 2 indicated a moderately stained red nucleus, and a grade 3 indicated a dark and diffusely stained red nucleus. The intimal H-score was determined from calculating 3 times the percentage of grade 3 cells plus 2 times percentage of grade 2 cells plus 1 times the percentage of grade 1 cells plus 0 times the percentage of grade 0 cells in the intima. This summative score, with a possible minimum of 0 and a possible maximum of 300, will be referred to as the intimal H-Score.
Statistical Analyses
Descriptive statistics were comprised of means and standard deviations for continuous data (e.g., age, adiposity index). Data were evaluated for normality and had a Gaussian distribution. Analysis of Variance (ANOVA) was used to compare cross-sectional area of plaques as a function of high VDR, low VDR, and 25OHD3, while a post hoc Scheffé’s test was used to compare between-group differences. Correlations were analyzed with Pearson’s correlation coefficient (r).
SPSS v. 17.0 (SPSS, Inc., Chicago, IL 2009) was used for all analyses. An a priori alpha level of 0.05 was used such that all results yielding p<0.05 was deemed statistically significant.
Results
A total of 39 female cynomolgus monkeys all received moderately atherogenic diets with a women’s equivalent of 1,000 IU/day of 25OHD3 and 1,200 mg/day of calcium throughout the study. At necropsy, the mean age ± standard deviation [SD] and (range) was 21.6 ± 3.0 years (14.2 to 26.3); the mean weight was 3.6 kg ± 0.9 kg (1.9 to 5.4 kg), and the mean adiposity index was 49.8 ± 11.3 kg/m2 (28.8 to 70.5 kg/m2).
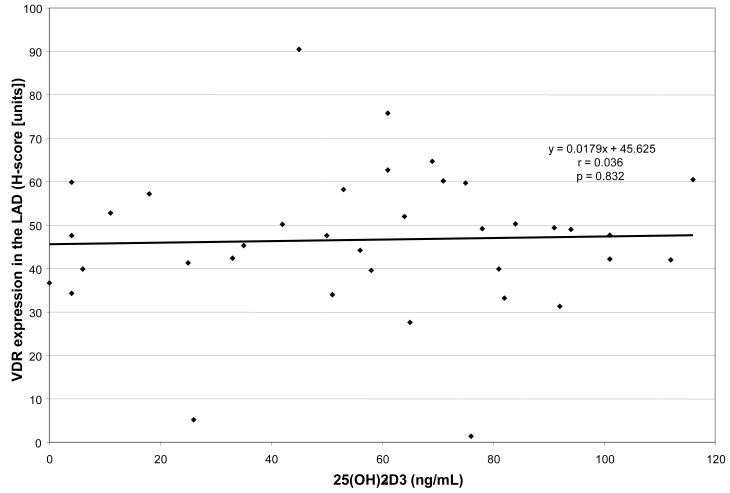
We analyzed the relationships between the plasma concentration of 25OHD3 and the expression of VDR in the LAD artery and there was no correlation, see figure 1. In addition to those measurements, coronary artery atherosclerosis was analyzed in all monkeys using both the cross-sectional area (mm2) and plaque thickness (mm) in the LCX as well as the LAD (see figure 2).
Figure 1.
For the 39 female cynomolgus monkeys in this study, this figure shows that there was no correlation between the plasma concentration of 25OHD3 and the expression of VDR in the LAD.
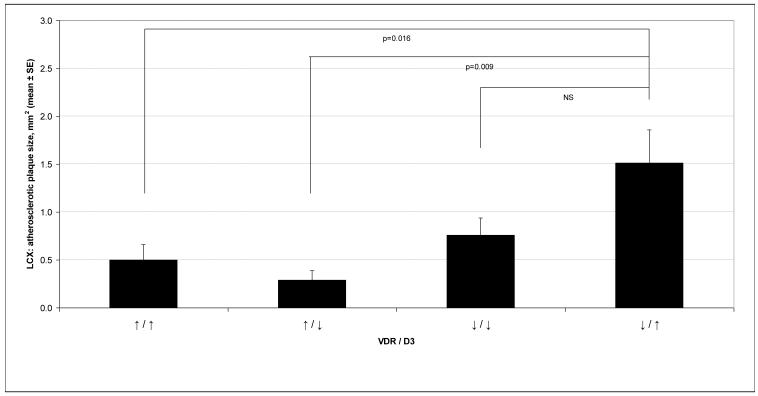
When monkeys with higher (above the median) plasma 25OHD3 concentrations and higher VDRs were compared to those with higher plasma 25OHD3 concentrations and lower VDR, we noted that monkeys with smaller plaque sizes in both the LAD and LCX (see figure 3), had higher plasma 25OHD3 concentrations and higher VDR (p = 0.016). For the LCX, there was also a significantly lower plaque size, for both plaque thickness and cross-sectional area (see figure 3) in those with higher VDR and lower plasma 25OHD3 concentrations versus those with lower quantities of VDR and higher plasma concentrations of 25OHD3, p=0.040 and p=0.009, respectively.
Figure 3.
This graph depicts the cross-sectional area of the Left Circumflex Artery according to the left anterior descending coronary artery VDR Quantity and Plasma Vitamin D3 Concentration (25OHD3) for the 39 female cynomolgus monkeys in this study. The higher (↑) categories indicate values ≥ the median while lower (↓) categories indicate values < the median. The symbols ↑↓ / ↑↓ (≥median<median, resp.) represent VDR expression and Plasma 25OHD3 concentration, respectively.
Discussion
Vitamin D is a fat-soluble vitamin that is bound to a carrier protein (DBP), which has been thought to safely transport hydrophobic vitamin D through the plasma environment to the VDR. VDR’s are localized to specific regions of target genes where 1,25(OH)2D3 [converted from 25OHD3] is subsequently able to modify their transcriptional abilities.37,50-52
While VDRs have been localized in many diffuse body organs,39-44 little is known about the expression of VDR and its association with disease in those organs. VDR null mice have been shown to have greater systemic inflammation53 and are more susceptible to autoimmune diseases as well as oncogenic and chemocarcinogenic induced tumors.54 It is not known, however, if this is related to a lack of 25OHD3 effect (i.e. 25OHD3 deficiency), a lack of VDR, or more importantly what role a low expression of VDR’s would have over time. Therefore, it can be viewed as a novel finding that a high VDR expression was associated with a significantly lower degree of coronary artery atherosclerosis compared with a low VDR expression and a high plasma concentration of 25OHD3 (but not a low concentration of 25OHD3; see figure 3).
The meaning of this finding, however, is less clear. The fact that there is no direct correlation between plasma 25OHD3 concentration and VDR expression (figure 1), along with our analysis of VDR and coronary atherosclerosis alone55, suggests the greater association is between VDR expression and coronary artery atherosclerosis. The results also indicate that plasma concentrations of vitamin D play a role. One potential explanation is an in vivo stimulation of enhanced 25OHD3 production, or absorption, in response to the greater coronary artery atherosclerosis, alone or in combination with low VDR quantity. There is also evidence that while low levels of vitamin D could be associated with cardiovascular compromise, there may also be an upper limit, above which detriment is again seen.11 This could be thought of as a therapeutic window, where low and high levels of plasma vitamin D were detrimental. This could also provide another potential reason for seeing a significantly worse coronary artery atherosclerosis result in those with low VDR expression (presumably detrimental), along with a high plasma vitamin D concentration (i.e., this may represent individuals above the therapeutic window).
While these data are clearly intriguing and innovative, it will be important to further elucidate what this association between VDR expression, plasma concentration of 25OHD3, and coronary artery atherosclerosis means. Since our data look at one fixed point in time (necrospy), we are unable to determine whether a lower VDR expression and/or higher plasma concentrations precede coronary artery atherosclerosis or vice versa. This knowledge would be tremendously valuable. The former, for instance, would suggest that the plasma concentration and VDR expression could be predictive, and potentially modifiable, factors in the development of coronary artery atherosclerosis. On the contrary, the latter finding could simply mean that after the development of coronary artery atherosclerosis, the body responds with changes in the expression of VDR locally, and perhaps systemic plasma 25OHD3 concentrations.
The ideal study would begin with women on a sub-therapeutic 25OHD3 dose (to prevent rickets) while measuring baseline arterial (e.g. iliac artery) VDR expression. Female participants would then be randomized to higher versus lower supplemental doses of 25OHD3 with the primary endpoint being degree of atherosclerosis progression, both in the contralateral iliac artery and coronary arteries.
Limitations of the current study include the relatively small sample size, and hence some of the apparent differences may not be significant due to a type II error. Additionally, because our data are all from one time point (necrospy), we are unable to determine a timeline, and hence potential predictive relationship, between VDR expression, 25OHD3 plasma concentrations and coronary artery atherosclerosis.
The strengths of the study include the novel approach to detecting VDR expression and correlating this with plasma 25OHD3 concentrations in association with coronary artery atherosclerosis. An additional strength is the fact that we were able to tightly control the diet, 25OHD3 dose, compliance, and housing/sun exposure. Finally, the ability to correlate the 25OHD3 and VDR data with histologic coronary artery tissue specimens is a major strength.
Conclusion
Cynomolgus monkeys with higher quantities of VDR have significantly less atherosclerosis than those with lower quantities of VDR and higher plasma 25OHD3 concentrations. The reason there is an increased plasma 25OHD3 concentration with a decrease in VDR in association with coronary artery atherosclerosis, and which came first, is not known. If these findings translate to human beings, it might explain why some individuals with higher plasma concentrations of 25OHD3 have more coronary artery atherosclerosis. The extent to which VDR may be an essential component related to 25OHD3 concentrations, and the role this plays in competitive feedback, is not established and should be a focus of future research. While VDR expressions in the coronary arteries have no direct association with plasma concentrations of 25OHD3, there is a significant association between plasma concentration of 25OHD3, VDR expression, and coronary artery atherosclerosis. Further elucidation of this association and prospective trials would be intriguing and important.
Acknowledgments
The funding sources for this research and manuscript preparation were the research budgets of the Wake Forest University Primate Center and The Reading Hospital and Medical Center. In addition, the original study was supported by the following grants from the National Institutes of Health: HL079421 (JRK) and PPG HL 45666 (TBC, JRK).
Footnotes
Conflict of Interest: Thomas B. Clarkson, DVM is a member of an advisory committee to Pfizer pharmaceuticals and has been supported with a research grant from Pfizer. He is also the recipient of a research grant from Merck. The other authors have no conflicts of interest.
These data were presented in abstract form September 21 - 24, 2011 at the NAMS 22nd annual Meeting in Washington, DC. These data and results, however, have not been published in manuscript form and have not been submitted previously to another journal.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:10805–65. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Lopez FR. Vitamin D: secosteroid hormone and reproduction. Gynecological Endocrinology. 2006;22:1–12. doi: 10.1080/09513590601045629. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689–96. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–72. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollis BW. Assessment of vitamin D nutritional and hormonal status: what to measure and how to do it. Calcif Tissue Int. 1996 doi: 10.1007/BF02509538. [DOI] [PubMed] [Google Scholar]
- 7.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Institute of Medicine; [accessed December 15, 2011]. Dietary Reference Intakes for Calcium and Vitamin D Available at: http://books.nap.edu/openbook.php?record_id=13050&page=R1. [Google Scholar]
- 8.Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holick MF. Clinical efficacy of 1,25-dihydroxyvitamin D3 and its analogues in the treatment of psoriasis. Retinoids. 1998;14:12–7. [Google Scholar]
- 10.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 11.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men. Arch Intern Med. 2008;168:1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong MS, Delansorne R, Man RY, Vanhoutte PM. Vitamin D derivatives acutely reduces endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2008;295:H289–96. doi: 10.1152/ajpheart.00116.2008. [DOI] [PubMed] [Google Scholar]
- 14.Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 15.Canning MO, Grotenhuis K, de Wit H, et al. 1-alpha,25-dihydroxyvitamin D3 (1,25(OH)(2)D(3)) hampers the maturation of fully active immature dendritic cells from monocytes. Eur J Endocrinol. 2001;145:351–7. doi: 10.1530/eje.0.1450351. [DOI] [PubMed] [Google Scholar]
- 16.Timms PM, Mannan N, Hitman GA, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95:787–96. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 17.Manson JE, Bassuk SS. Vitamin D and Cardiovascular Disease. Menopause Management. 2009 Jan-Feb;:28–31. [Google Scholar]
- 18.Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119:84–90. doi: 10.1210/endo-119-1-84. [DOI] [PubMed] [Google Scholar]
- 19.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–5. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 20.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. British Journal of Nutrition. 2010;103:549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 21.Pittas AG, Dawson-Hughes B. Vitamin D and Diabetes. J Steroid Biochem Mol Biol. 2010;121:425–429. doi: 10.1016/j.jsbmb.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathieu C, Waer M, Laureys J, et al. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia. 1994;37:552–8. doi: 10.1007/BF00403372. [DOI] [PubMed] [Google Scholar]
- 23.Gregori S, Giarratana N, Smiroldo S, et al. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–74. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 24.Gysemans CA, Cardozo AK, Callewaert H, et al. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology. 2005;146:1956–64. doi: 10.1210/en.2004-1322. [DOI] [PubMed] [Google Scholar]
- 25.Hatton DC, Xue H, DeMerritt JA, McCarron DA. 1,25(OH)2 vitamin D3-induced alterations in vascular reactivity in the spontaneously hypertensive rat. Am J Med Sci. 1994;307(Suppl 1):S154–8. [PubMed] [Google Scholar]
- 26.Li YC, Qiao G, Uskokovic M, et al. Vitamin D: a negative endocrine regulator of the reninangiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89-90(1-5):387–92. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maiya S, Sullivan I, Allgrove J, Yates R, Malone M, et al. Hypocalcaemia and vitamin D deficiency: an important, but preventable, cause of life-threatening infant heart failure. Heart. 2008;94:581–584. doi: 10.1136/hrt.2007.119792. [DOI] [PubMed] [Google Scholar]
- 29.Weber KT, Simpson RU, Carbone LD. Vitamin D and calcium dyshomoeostasis-associated heart failure. Heart. 2008;94:540–1. doi: 10.1136/hrt.2007.126359. [DOI] [PubMed] [Google Scholar]
- 30.Aihara K, Azuma H, Akaike M, et al. Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. J Biol Chem. 2004;279:35798–802. doi: 10.1074/jbc.M404865200. [DOI] [PubMed] [Google Scholar]
- 31.Hunter D, De Lange M, Snieder H, et al. Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. J Bone Miner Res. 2001;16:371–8. doi: 10.1359/jbmr.2001.16.2.371. [DOI] [PubMed] [Google Scholar]
- 32.Karohl C, Su S, Kumari M, et al. Heritability and seasonal variability of vitamin D concentrations in male twins. Am J Clin Nutr. 2010;92:1393–8. doi: 10.3945/ajcn.2010.30176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cherniack EP, Florez HJ, Hollis BW, Roos BA, Troen BR, Levis S. The response of elderly veterans to daily vitamin D3 supplementation of 2,000 IU: a pilot efficacy study. J Am Geriatr Soc. 2011;59:286–90. doi: 10.1111/j.1532-5415.2010.03242.x. [DOI] [PubMed] [Google Scholar]
- 35.Vashi PG, Trukova K, Lammersfeld CA, Braun DP, Gupta D. Impact of oral vitamin D supplementation on serum 25-hydroxyvitamin D levels in oncology. Nutritional Journal. 2010;9:60. doi: 10.1186/1475-2891-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnatz PF, Nudy M, O’Sullivan DM, Ethun K, Appt SE, Clarkson TB. Identification of a Mechanism for Increased Cardiovascular Risk among Individuals with Low Vitamin D Concentrations. Menopause. 2011;18(9):994–1000. doi: 10.1097/gme.0b013e318212539d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zella LA, Shevde NK, Hollis BW, Cooke NE, Pike JW. Vitamin D-binding protein influences total circulating levels of 1,25-dihydroxyvitamin D3 but does not directly modulate the bioactive levels of the hormone in vivo. Endocrinology. 2008;149:3656–67. doi: 10.1210/en.2008-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu-Wong JR. Potential for vitamin D receptor agonists in the treatment of cardiovascular disease. Br J Pharmacol. 2009;158(2):395–412. doi: 10.1111/j.1476-5381.2009.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am Physiological Soc. 1994;267:E356–E360. doi: 10.1152/ajpendo.1994.267.3.E356. [DOI] [PubMed] [Google Scholar]
- 40.Menezes RJ, Cheney RT, Husain A, et al. Vitamin D receptor expression in normal, premalignant, and malignant human lung tissue. Cancer Epidemiol Biomarkers Prev. 2008;17:1104–10. doi: 10.1158/1055-9965.EPI-07-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen S, Glenn DJ, Ni W, et al. Expression of the vitamin D receptor is increased in the hypertrophic heart. Hypertension. 2008;52:1106–12. doi: 10.1161/HYPERTENSIONAHA.108.119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bischoff-Ferrari HA, Borchers M, Gudat F, Durmuller U, Stahelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone and Mineral Res. 2004;19:265–9. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 43.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Langub MC, Reinhardt TA, Horst RL, Malluche HH, Koszewski NJ. Characterization of vitamin D receptor immunoreactivity in human bone cells. Bone. 2000;27:383–7. doi: 10.1016/s8756-3282(00)00335-5. [DOI] [PubMed] [Google Scholar]
- 45.Dokoh S, Donaldson CA, Marion SL, Pike JW, Haussler MR. The Ovary: A Target Organ for 1,25-Dihydroxyvitamin D3. Endocrinology. 1983;112(1):200–6. doi: 10.1210/endo-112-1-200. [DOI] [PubMed] [Google Scholar]
- 46.Lewis JG, Elder PA. Serum 25-OH vitamin D2 and D3 are stable under exaggerated conditions. Clin Chem Acta. 1960;5:609–17. doi: 10.1373/clinchem.2008.111526. [DOI] [PubMed] [Google Scholar]
- 47.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effect of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;86:41–7. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 48.Weigert C. Über eine Methode zur Färbung elastischer Fasern. Zentralblatt für Allgemeine Pathologie, und Pathologische Anatomie. 1898;9:289–292. [Google Scholar]
- 49.Siboni A, Mourits-Andersen T, Moesner J. Granulomatous bone marrow inflammation during treatment of chronic myeloid leukaemia with interferon alpha-2b. J Clin Pathol. 1995;48:878–880. doi: 10.1136/jcp.48.9.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Domingues CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–49. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 51.Sutton AL, MacDonald PN. Vitamin D: more than a “bone-a-fide” hormone. Mol Endocrinol. 2003;17:777–91. doi: 10.1210/me.2002-0363. [DOI] [PubMed] [Google Scholar]
- 52.Pike JW, Shevde NK. The vitamin D receptor. In: Feldman D, Pike JW, Glorieux F, editors. Vitmain D. 2nd ed Elsevier/Academic Press; New York: pp. 167–191. [Google Scholar]
- 53.Zwerina K, Baum W, Axmann R, Heiland GR, Distler JH, et al. Vitamin D receptor regulates TNF-mediated arthritis. Ann Rheum Dis. doi: 10.1136/ard.2010.142331. doi:10.1136/ard.2010.142331. [DOI] [PubMed] [Google Scholar]
- 54.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, et al. Vitamin D and Human Health: Lessons from Vitamin D Receptor Null Mice. Endocrine Reviews. 29(6):726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schnatz PF, Nudy M, O’Sullivan DM, Cline JM, Appt SE, Kaplan JR, Jiang X, Clarkson TB. The Quantification of Vitamin D Receptors in the Coronary Vasculature and Association with Atherosclerosis. Menopause. 2011;18(12):1345. [Google Scholar]