Abstract
Tendon-to-bone healing is typically poor, with a high rate of repair-site rupture. Bone loss after tendon-to-bone repair may contribute to poor outcomes. Therefore, we hypothesized that the local application of the osteogenic growth factor BMP-2 would promote bone formation, leading to improved repair site mechanical properties. Intrasynovial canine flexor tendons were injured in Zone 1 and repaired into bone tunnels in the distal phalanx. BMP-2 was delivered to the repair site using either a calcium phosphate matrix (CPM) or a collagen sponge (COL) carrier. Each animal also received carrier alone in an adjacent repair to serve as an internal control. Repairs were evaluated at 21 days using biomechanical, radiographic, and histologic assays. Although an increase in osteoid formation was noted histologically, no significant increases in bone mineral density occurred. When excluding functional failures (i.e., ruptured and gapped repairs), mechanical properties were not different when comparing BMP-2/CPM groups with carrier controls. A significantly higher percentage of BMP-2 treated specimens were functional failures (maximum force < 20 N) compared to carrier controls. While tendon-to-bone healing can be enhanced by addressing the bone loss that typically occurs after surgical repair, the delivery of BMP-2 using the concentrations and methods of the current study did not improve mechanical properties over carrier alone. The anticipated anabolic effect of BMP-2 was insufficient in the short time frame of this study to counter the post-repair loss of bone.
Keywords: BMP-2, enthesis, animal model, growth factor
INTRODUCTION
Outcomes after tendon-to-bone repair are typically poor, with an insufficient healing response and a high rate of repair-site failure. Flexor digitorum profundus tendon repairs in Zone 1 (near insertion) are often unsatisfactory.1, 2 Similarly, both rotator cuff and anterior cruciate ligament repairs to bone fail frequently.3–5 Poor outcomes following repair of tendon to bone are due to a number of factors, including bone loss at the insertion site6–10, a lack of regeneration of the natural fibrocartilaginous transition between tendon and bone11, 12, and a lack of collagen fiber continuity across the healing interface11, 13, 14.
Bone loss after tendon-to-bone repair has been noted in a number of clinical and animal studies. In the canine flexor tendon model, a decrease in bone mineral density (BMD) was seen at the distal phalanx as early as 10 days following injury and repair, coincident with a decrease in repair-site mechanical properties.10 Similar results were noted in a rat rotator cuff model.8, 9 Reduced BMD was also observed in the humeri of patients after rotator cuff injury and repair.6 Similarly, a reduction in BMD was seen after ACL reconstruction.7 Furthermore, prevention of bone loss using bisphosphonates in the canine flexor tendon-to-bone model led to improved repair-site biomechanical properties.15 In a rat study, improving BMD at the rotator cuff footprint in ovariectomized animals enhanced failure stress of the tendon enthesis.8 These results provide strong evidence that bone loss is a significant factor contributing to poor outcomes.
Experimental studies showed that tendon-to-bone healing is enhanced by either promoting bone formation or suppressing bone resorption. The osteogenic growth factor bone morphogenetic protein 2 (BMP-2) was used in an animal study to improve healing after ACL reconstruction.16 Improved healing was achieved by promoting bone ingrowth from the tunnel wall into the graft. In a separate study, bisphosphonates were used to suppress osteoclast activity after flexor digitorum profundus repair into a distal phalanx bone tunnel.15 Decreased resorption led to improved biomechanical properties 3 weeks after repair. Based on this previous work, our objective was to improve flexor tendon-to-bone healing by promoting bone formation. We hypothesized that BMP-2, applied at the time of surgical repair, would lead to increased bone mineral density and improved repair site mechanical properties.
MATERIALS AND METHODS
Animal model
Using established methods, 58 flexor digitorum profundus (FDP) tendons were injured and repaired into bone tunnels in the distal phalanx in 29 female, purpose bred, mongrel canines (Covance, Princeton, NJ).14, 15, 17 Procedures complied with the Animal Studies Committee of Washington University and with the policies of the NIH. The 2nd and 5th FDP tendons were injured and repaired in each animal. Each dog received BMP-2 (provided as a gift from Pfizer) plus carrier at one repair site and carrier alone in the other repair site. BMP-2 plus carrier or carrier alone was assigned to either the 2nd or 5th FDP tendon randomly. The tendon was transected sharply at its insertion and grasped using a 4-strand modified Becker stitch (SUPRAMID EXTRA suture, S. Jackson, Inc.). A 5mm deep × 3.6mm diameter hole was then made into the distal phalanx. Two needle holes were drilled through the distal phalanx, and the sutures were passed through these holes. An additional peripheral stitch (also using 4-0 suture) was added to augment the repair strength, as described previously.17, 18 Careful consideration was given to the dosage of BMP-2 and the rate of its delivery. Although a low dose may be ineffective in stimulating bone formation, a high dose may also be detrimental by inciting an osteolytic response.19–23 Therefore, the following doses and delivery methods were chosen based on previous reports in other animal models.24–27 In phase I of the study, 2 doses of BMP-2 (high dose: 0.688µg/µL and low dose: 0.344µg/µL) were administered in calcium phosphate matrix in a total volume of 50µL (CPM, provided as a gift from Pfizer). The BMP-2/CPM was injected into the base of the bone tunnel using a spinal needle prior to inserting the tendon into the tunnel. For carrier controls, CPM alone was injected into the base of the bone tunnel in an identical manner. Based on previous reports, release of BMP-2 from CPM occurs over the course of ≥ 5 wks.26 In phase II of the study, 1 dose of BMP-2 (low dose: 0.344µg/µL) was administered in a volume of 58µL using a collagen sponge with dimensions 5×12×2.5mm (COL, provided as a gift from Pfizer). The BMP-2 was added to the sponge manually using a pipette. Apparent diffusion of the BMP-2 solution and hydration of the sponge indicated that the growth factor was evenly distributed throughout the sponge. Hydration of the sponge with the BMP-2 solution resulted in a reduction in its thickness, producing a thin hydrogel film. Release of BMP-2 from these scaffolds occurs much faster than CPM, with >90% of the growth factor released within 2 wks.21 Therefore, only a low dose of BMP-2 was tested to avoid too high a local dose. The BMP-2/COL was wrapped around the distal end of the tendon and secured using 6-0 Prolene suture prior to insertion of the tendon into the tunnel. There was no exudation of fluid after insertion. To complete the repair, the suture was tied over the dorsal surface of the toenail, pulling the tendon stump into the tunnel. For carrier controls, COL was wrapped around the tendon in an identical manner. The 29 animals were distributed as follows: BMP-2/CPM high dose N=13 (11 for biomechanics/bone densitometry and 2 for histology), BMP-2/CPM low dose N=6 (6 for biomechanics/bone densitometry), BMP-2/COL low dose N=10 (8 for biomechanics/bone densitometry and 2 for histology). Post-operatively, forelimbs were subjected to passive motion rehabilitation.14, 15, 17 Dogs were euthanized at 21 days.
Biomechanics
After euthanisia, FDP tendon-to-bone specimens were prepared. The 2nd and 5th digits from the left (surgically treated) forelimbs were disarticulated at the metacarpophalangeal joint, and the FDP tendons were transected proximally at the musculotendinous junction. After carefully removing the tendon sheath, isolated tendon-to-bone specimens were pulled in uniaxial tension until failure as described previously.13, 14, 16 After preconditioning (triangle wave form, 0–0.7mm, at a rate of 0.35mm/s), the specimens were tested in uniaxial tension using a material testing machine (5866; Instron Corp., Norwood, MA) at 0.2mm/s until failure. This slow rate ensured quasi-static testing conditions consistent with our previous studies.14, 15, 17 Force-elongation data were recorded with use of Bluehill software (Instron Corp., Norwood, MA), and optical strain was recorded using a digital video camera (DP70; Olympus, Center Valley, PA) with video recording software (StreamPix; NorPix Inc., Montreal, Canada). Video was calibrated and processed using a video correlation and tracking software (Video Analysis; Qualisys, Gothenburg, Sweden). From the force-elongation curves, we determined maximum force and stiffness (the slope of the linear portion). From the force-strain curves we determined strain at 20N force (a physiologically relevant load level28, 29) and rigidity (the slope of the linear portion).
Bone densitometry
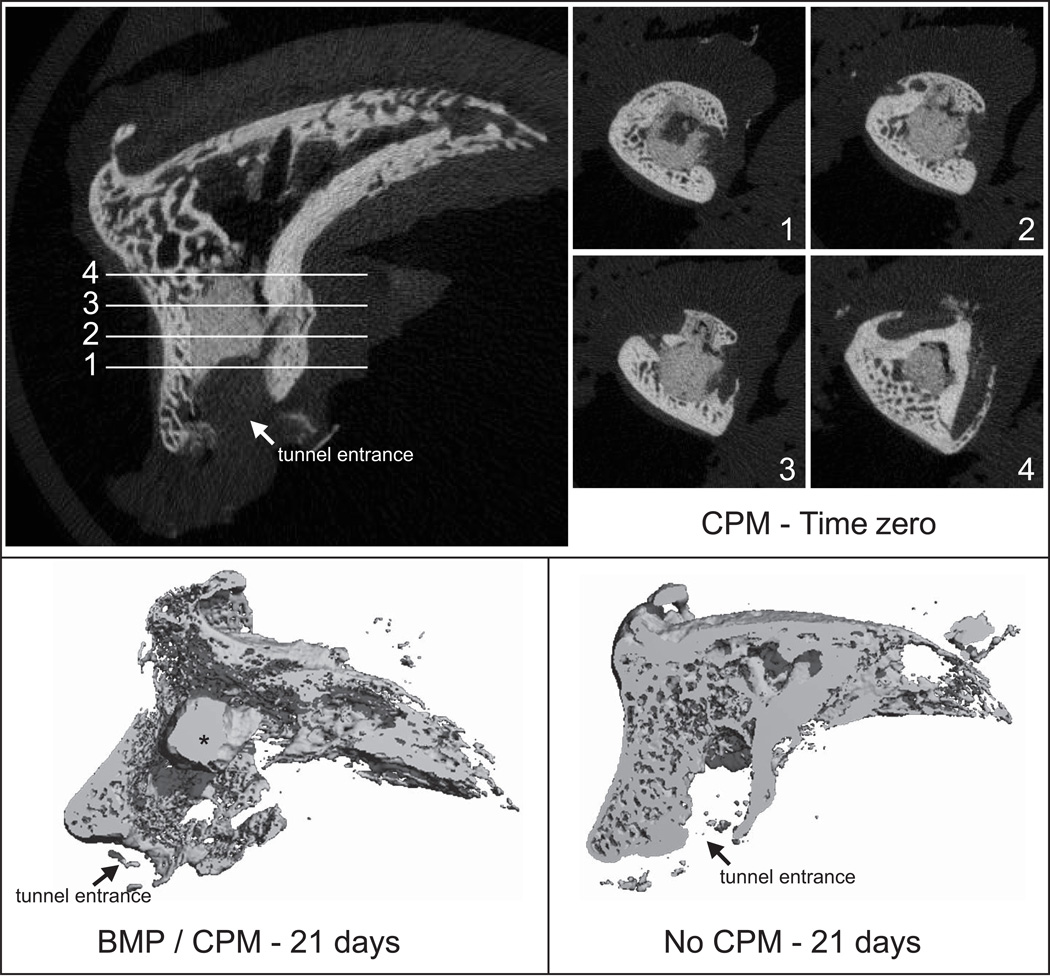
BMD of the distal phalanges was assessed after biomechanical testing using peripheral quantitative computed tomography (pQCT; XCT Research M, Stratec) as described previously.15 Biomechanical testing led to failure at the repair site followed by tendon pullout from the bone tunnel in all specimens. Therefore, BMD measures were not affected by tendon material within the tunnel. Three transverse slices were obtained 1, 2, and 5 mm distal to the FDP tendon-to-bone insertion site (0.5 mm thickness; 0.07 mm voxel size). The position of images was chosen to assess BMD around the repair and to avoid including residual CPM at the tendon stump. Using the manufacturer’s software (CALCBD routine) and a threshold of 280 mg/cm3 to segment bone from external soft tissues, we determined average volumetric BMD (mg/cm3) for the 2 slices. The BMD represents an “apparent” value, as the volume of interest is the entire cross-section of the distal phalanx including cortical and trabecular bone and the tunnel. A subset of samples (N=3–6 per group) was also assessed qualitatively using microCT (µCT 40; Scanco Medical, Bassersdorf, Switzerland) to visualize the tunnel and the CPM. 2 CPM only cadaver repairs were also assessed to visualize the CPM location at time zero. Scans were performed at a 30µm resolution and a bone threshold ≥ a linear attenuation coefficient of 1.70/cm or density of 293mg HA/ccm was used (Fig. 1).
Figure 1.
MicroCT demonstrated the location of CPM in the bone tunnel. CPM was evident in specimens that received that carrier, regardless of BMP-2 treatment or timepoint (* CPM: calcium phosphate matrix).
Histology
Samples were processed using standard plastic-embedding protocols and stained with H&E/Von Kossa and Masson’s trichrome. Slides were qualitatively assessed by 2 investigators, blinded to group, for regeneration of a fibrocartilaginous transition, osteoclasts, appositional new bone formation, and inflammatory cells.
Statistics
The average load across the repair site during the passive motion rehabilitation protocol we employed is ~10N.28, 29 Therefore, repairs with maximum force < 10N were considered functional failures, i.e., these repairs were likely to have ruptured or elongated excessively. For failure load, stiffness, rigidity, and strain at 20N, group means were compared using an ANOVA followed by a Fisher’s least squares differences post-hoc test. Samples with a maximum force < 10N were excluded from the ANOVA and from the descriptive statistics. Significance was set to p < 0.05. Another set of samples had failure forces between 10 and 20 N; these we considered to be “at risk” for failure, as loads associated with unopposed active motion rehabilitation in patients is ~20N.30 To compare the incidence rates of tendons that were either functional failures or at risk for failure, we binned samples into those above and below 20 N maximum force, and then performed Chi-square tests. Based on a power analysis using our previous results in this model,1, 14, 15, 17 N=8 was deemed sufficient for clinically significant biomechanical differences of ~30% between groups.
RESULTS
3 of the 29 animals developed post-operative complications (2 wound breakdowns followed by infection, 1 cast loosening) and were euthanized early and not included in the analysis. 2 were in the BMP-2/COL group and 1 was in the BMP-2/CPM group. All 3 had been allocated for biomechanics and bone densitometry, resulting in 10 canines (20 samples) for the BMP-2/CPM high dose group, 6 canines (12 samples) for the BMP-2/CPM low dose group, and 6 canines (12 samples) for the BMP-2/COL low dose group for those assays.
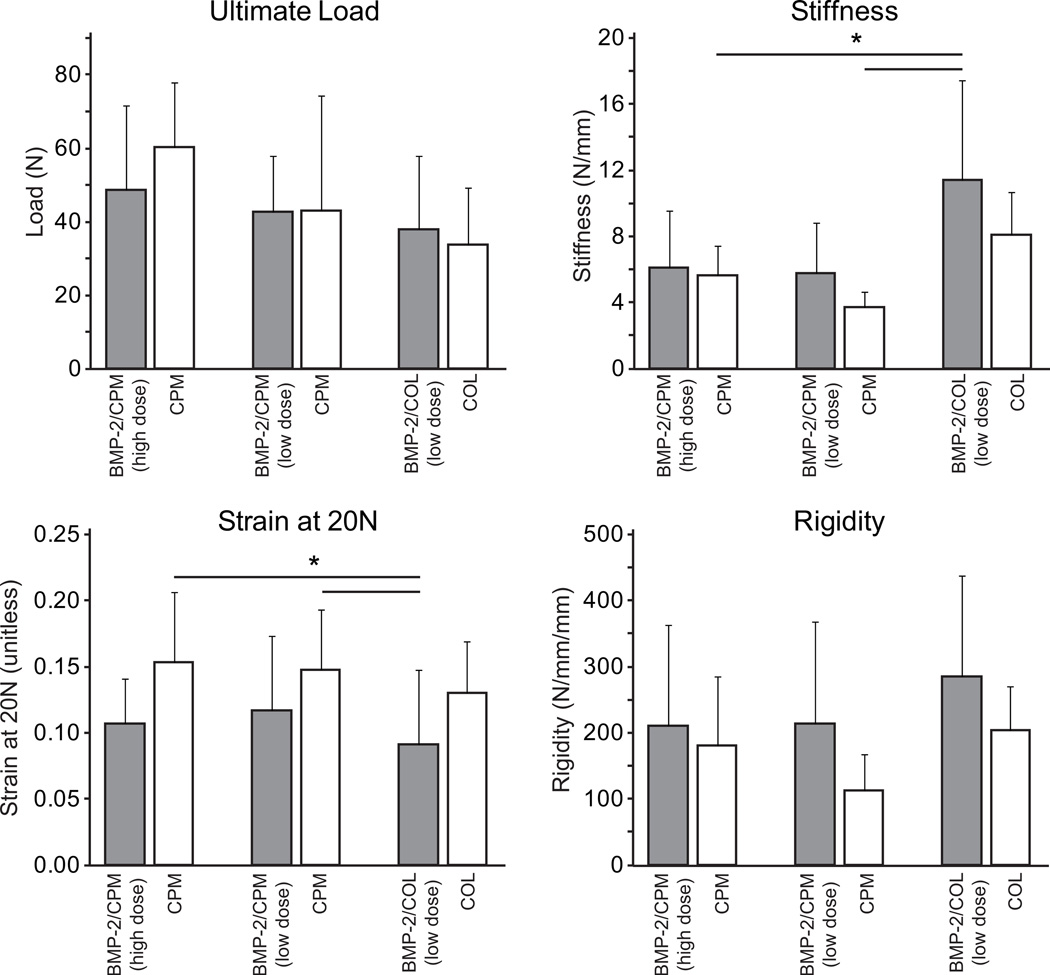
10 of the 44 biomechanics samples had Fmax < 10N (“functional failure”). The group breakdown s was as follows: BMP-2/CPM high dose = 3/10, CPM high dose = 1/10, BMP-2/CPM low dose = 2/6, CPM low dose = 1/6, BMP-2/COL = 2/6, and COL = 1/6. For the subsequent biomechanical and BMD analyses, the final sample sizes were: BMP-2/CPM high dose N=7, CPM high dose N=9, BMP-2/CPM low dose N=4, CPM low dose N=5, BMP-2/COL N=4, and COL N=5. Of the remaining samples, mechanical properties were not significantly different when comparing BMP-2 groups with their respective carrier controls (Table 1, Fig. 2). Stiffness was significantly higher and strain at 20N was significantly lower in the BMP-2/COL group compared to the CPM alone group (Fig. 2). No significant differences in mechanical properties were observed when comparing carrier controls. BMP-2 did not significantly affect BMD compared to carrier controls (Table 1). BMD was significantly lower than normal in all injury and repair groups, consistent with prior findings.1, 10, 15
Table 1.
Ultimate strain and bone mineral density.
| Group | Ultimate Strain (unitless) |
Bone Mineral Density (mg/m3) |
|---|---|---|
| BMP-2 / CPM (high dose) | 0.37 ± 0.21 | 387 ± 19 |
| CPM | 0.43 ± 0.22 | 395 ± 45 |
| BMP-2 / CPM (low dose) | 0.23 ± 0.10 | 369 ± 45 |
| CPM | 0.28 ± 0.19 | 377 ± 31 |
| BMP-2 / COL (low dose) | 0.29 ± 0.29 | 406 ± 61 |
| COL | 0.34 ± 0.17 | 478 ± 87 |
| Normal (uninjured) | 654 ± 53 * | |
p < 0.05 compared to all other groups
Figure 2.
Stiffness was higher and strain at 20N was lower in the BMP-2/COL group compared to the CPM group. Mechanical properties were not significantly different when comparing BMP-2/CPM groups with carrier controls. No differences were observed when comparing carrier controls (i.e., CPM vs. COL). [* p < 0.05]
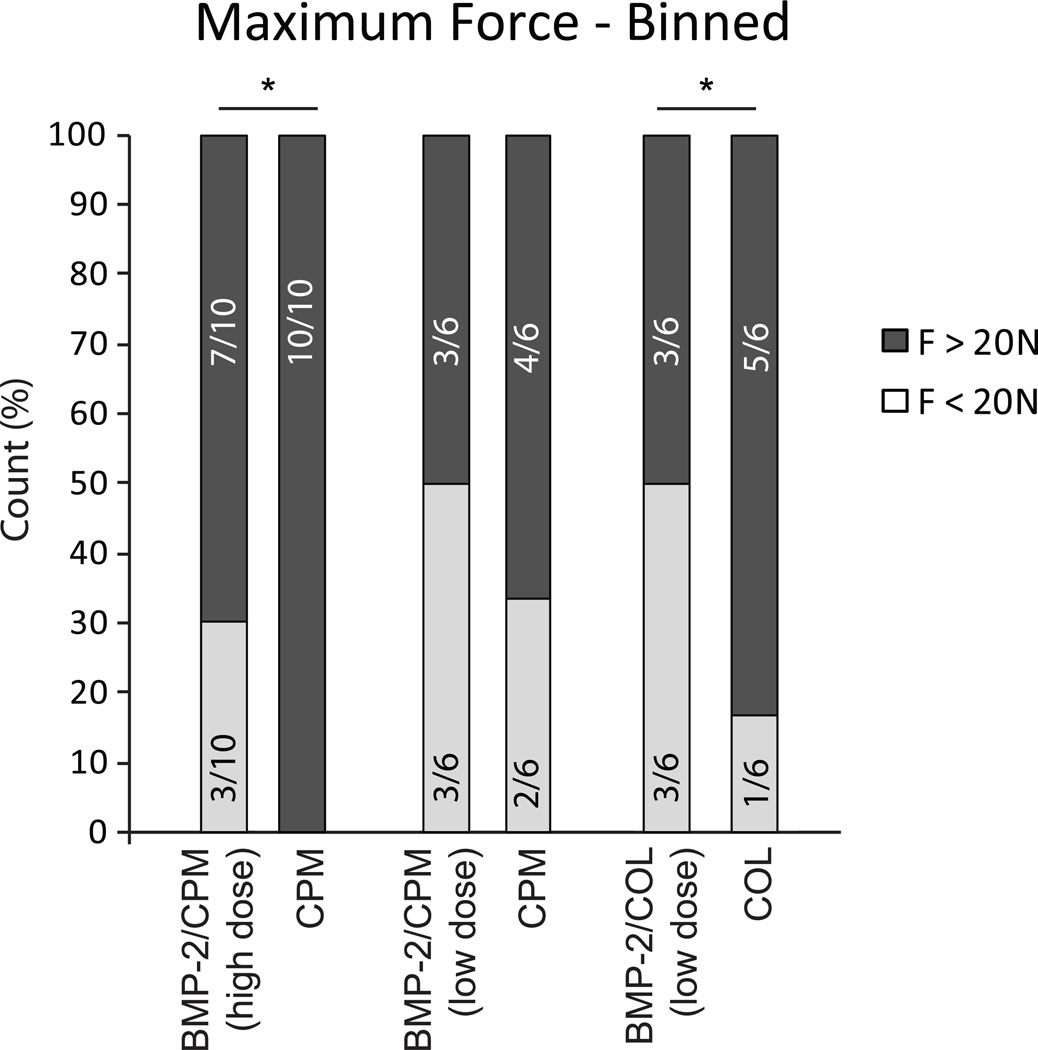
When comparing the incidence of Fmax below and above 20 N (“at risk” threshold), a significantly higher percentage of BMP-2/CPM (high dose) and BMP-2/COL treated specimens had Fmax < 20N compared to their carrier controls (30% vs. 0% and 50% vs. 17%, respectively, Fig. 3). All specimens failed at the repair site followed by tendon pullout from the tunnel.
Figure 3.
When comparing binned results for Fmax < 20N, and Fmax > 20N, a significantly higher percentage of BMP-2 treated specimens had Fmax < 20N compared to carrier controls.
MicroCT did not reveal qualitative differences between BMP-2 groups and controls. The tunnels contained little to no new bone. CPM carrier was clearly evident in all specimens that received that carrier, regardless of BMP-2 treatment (Fig. 1).
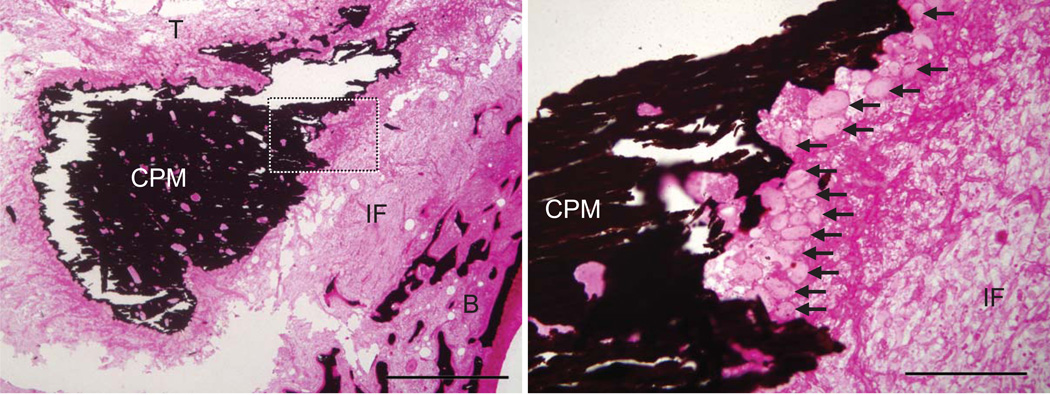
Qualitative evaluation of the histology demonstrated a zone of fibrovascular scar tissue in all samples between the tendon and the tunnel wall (Figs. 4–5). Regeneration of a fibrocartilaginous transition was not observed in any sample. CPM remained in the tunnels that received that carrier (Figs. 1 and 4). The CPM was surrounded by osteoclasts, demonstrating high resorptive activity due to the presence of the foreign mineral matrix. More osteoclasts were noted near the carrier in the BMP-2/CPM group compared to the CPM alone group. There were no apparent differences in osteoclasts when comparing BMP-2/COL to COL alone. Appositional new bone formation was seen in all BMP-2 treated specimens (regardless of carrier) on trabeculae adjacent to tendon compared to control (Fig. 5). While BMP-2 treatment appeared to lead to increased localized new bone formation, no mature bone formation or bone ingrowth onto the portion of the tendon situated inside the tunnel was observed. There were no apparent differences between groups when examining the number of inflammatory cells.
Figure 4.
CPM was clearly evident at the base of the tunnel at 21 days (mineral stained in black, dotted box in the left panel is magnified and shown in the right panel). The CPM was surrounded by osteoclasts in all cases (black arrows, right panel), demonstrating high resorptive activity due to the presence of the foreign mineral matrix. [CPM: calcium phosphate matrix, IF: fibrovascular interface, B: trabecular bone adjacent to bone tunnel wall; T: tendon stump; left: 1mm scale bar, right: 200um scale bar; Von Kossa / Hematoxylin & Eosin stain]
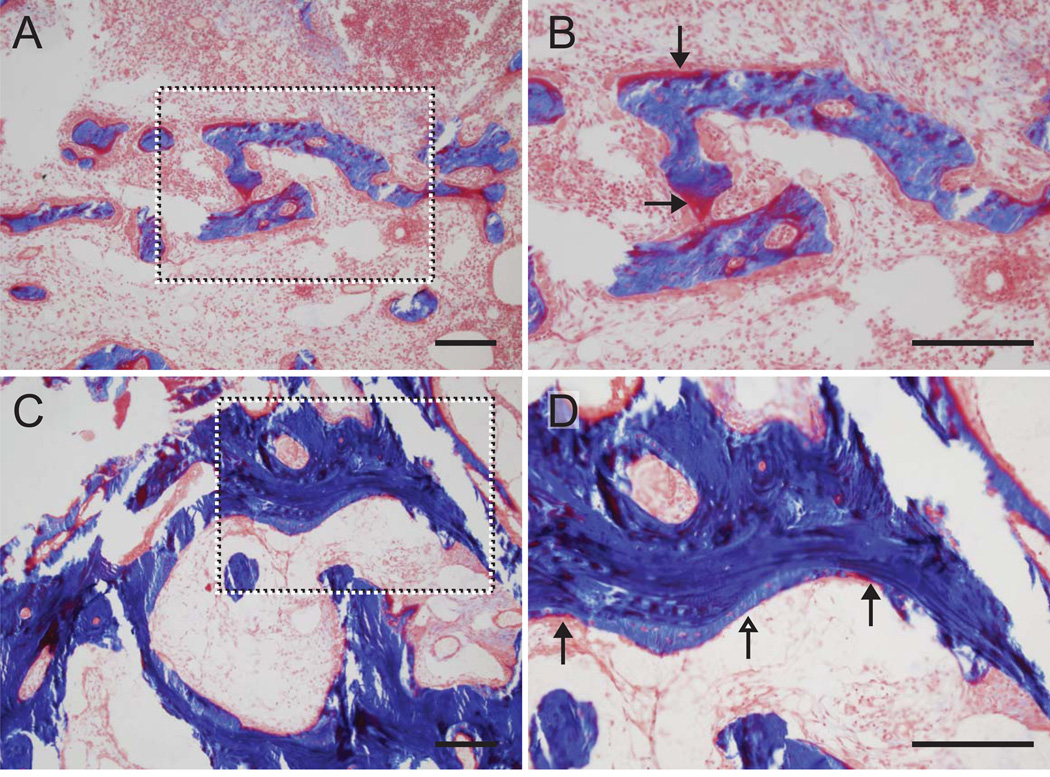
Figure 5.
New bone formation was evident in the trabeculae of the tunnel wall adjacent to the tendon in COL (A and B) and BMP-2/COL samples (C and D). The dotted boxes in the left panels are magnified and shown in the right panels. Osteoid can be seen (red stain, black arrowheads) in carrier control (COL) samples (A and B). Osteoid can be seen at the leading edges (red stain, black arrowheads) of newly mineralized bone (light blue stain, white arrowheads) in BMP-2/COL samples (C and D). There was more newly mineralized bone in the BMP-2 samples compared to controls. [COL: collagen carrier, 200um scale bars; Masson’s trichrome]
DISCUSSION
BMP-2 at the concentrations and methods of administration that we used failed to improve tendon-to-bone healing in a canine flexor tendon repair model at 21 days. Although increases in localized bone formation were seen histologically in the BMP-2 treated groups, there were no significant increases in BMD. When considering all repairs, including those that were functional failures, BMP-2 treatment may have been detrimental to healing.
The critical time period for Zone 1 flexor tendon repair with proximal tendon retraction is 7 to 10 days following injury, when a decrease in tendon and bone mechanical properties occurs.1, 10, 12 In an attempt to obviate the structural changes that occur following injury and to accelerate repair during this early time period, we applied BMP-2 to the repair site. Failure to note improvements in BMD may have been due to the short time interval between tendon-to-bone repair and the performance of both imaging and biomechanical testing. This was especially relevant when CPM was used to deliver BMP-2. The CPM carrier was radiopaque from the time of implantation and was only partially absorbed over the 3 weeks. BMP-2 release depends on resorption of the mineralized matrix, so the local concentration of growth factor at the repair site may have been low. Although prolonged BMP-2 release may be beneficial in the long run, an insufficient amount of growth factor was possibly released during the short time course to promote adequate new bone formation at the flexor tendon repair site.
Previous studies demonstrated that decreases in BMD during healing are due to high levels of resorption, and not necessarily due to low bone formation.1, 10, 15 Suppression of osteoclast activity (e.g., using bisphosphonates) may be necessary before BMP-2 mediated increases in bone formation can be effective. However, a combined bisphosphonate/BMP-2 treatment may not be as effective using the CPM carrier, as release of the growth factor from that matrix depends on resorption of the CPM.
BMP-2 in the current study was potentially detrimental to healing, as evidenced by an increase in the number of repairs with Fmax < 20N in 2 of the 3 BMP-2-treated groups. This may be due to osteoblast-osteoclast cross-talk leading to increased localized osteoclast activity.16, 31–34 This result is consistent with recent studies demonstrating that BMP-2 delivered in a collagen sponge can lead to osteoclastogenesis and increased resorption.19–23 Transient bone resorption followed by bone formation was observed in nonhuman primate core defects treated with BMP/collagen sponge compared to collagen sponge alone and no treatment.21 In that study, the growth factor was delivered rapidly (i.e., within 2 wks) via collagen sponges, leading to high initial local BMP-2 concentrations. Bone resorption was seen as early as 1 wk after BMP-2 delivery and peaked by 2 wks. This was followed by bone formation at later timepoints. Similar results were reported at other anatomic sites in other animal models and clinically.19–23 The process of high resorption preceding formation is in contrast to results following slower delivery of BMP-2 administered in CPM. In this case, bone formation typically proceeds without a transient bone resorption phase.
Increased resorption due to BMP-2 may be amplified in inflammatory settings. Koide et al. demonstrated in vitro that BMP-2 stimulates osteoclast formation only in the presence of interleukin 1 alpha.31 The relatively high level of inflammation typically seen in the tunnels of healing tendon-to-bone interfaces14, 35 may have exacerbated BMP-2-dependent increases in bone resorption. Therefore, control of the inflammatory environment may be important for successful application of BMP-2 for flexor tendon healing in a distal phalanx bone tunnel. Although we did not find histologic evidence of increased numbers of inflammatory cells, any inflammatory infiltrate would likely have resolved by 21 days.
Rodeo et al. reported improved tendon-to-bone healing with BMP-2 treatment in a canine model.16 The long digital extensor tendon was transplanted into a bone tunnel in the proximal tibia. Radiography, histology, and biomechanics were performed from 3 days through 8 wks of healing. Increased bone formation was seen at all timepoints, with significant improvements in mechanical properties at 2 wks. Improvements in outcomes were attributed to increased bone ingrowth into the interface tissue between the tunnel and the tendon. Similar results were reported by Pan et al. in a rabbit ACL model using a crude extract of BMP.36 These results are in contrast to ours, which showed no increases in bone formation and limited improvements in mechanical properties due to BMP-2. Notably, resorption was observed along the bone tunnel in the high-dose BMP-2 group of the previous study.16 Consistent with other reports19–23, resorption was followed by new bone formation at later times. Although careful consideration was given to dosage and delivery method prior to performing our study, the 2 doses and 2 delivery methods did not lead to increases in bone formation or to improvements in biomechanical properties. Perhaps the dosage and/or release rate was not optimal. Apart from dosing considerations, the anatomy of the tunnel, and therefore the distribution of CPM relative to the tunnel, differed from the study of Rodeo et al.16 In our study, the tunnel was relatively short (~5 mm) and the BMP-2/CPM was applied at the base; the trabecular bone density in the canine distal phalanx at the base of the tunnel is quite low. This application method was necessitated by the size of the tendon relative to the distal phalanx tunnel; i.e., the largest achievable bone tunnel diameter did not allow sufficient space between the tendon surface and the tunnel surface for application of CPM. In contrast, the BMP-2/CPM was applied along the length of the tunnel (~15 mm) in the prior study16, 37, providing much greater interface area for bone formation and bone-tendon integration. Despite the overall lack of effect on BMD and mechanical properties, we did note new bone formation in the trabecular bone adjacent to the tendon, indicating the BMP-2 released from the CPM at the base stimulated bone formation at the tendon-to-bone interface. Further study is required to determine if other doses and release profiles can enhance bone formation and improve tendon-to-bone healing after Zone 1 flexor tendon repair.
To make appropriate comparisons between groups for the biomechanical outcomes, data were binned into relevant categories for analysis. As loads across the repair site during rehabilitation are between 10 N and 20 N,28, 29 repairs with maximum forces less than these values would be at high risk for rupture during rehabilitation. These load levels are clinically relevant thresholds that repairs must reach for success. A fully ruptured repair would have an effective failure load of 0 N. It would be inappropriate to include these in calculating group mean. Similarly, repairs that were “functional failures” should not be included, as they likely have different healing characteristics and were already excessively gapped prior to testing. This concept is similar to the apparent threshold in gap formation that is seen in flexor tendon mid-substance healing.38, 39 Repairs that form gaps > 3mm have dramatically lower mechanical properties compared to repairs that form gaps < 3mm. Our data were therefore analyzed and presented in 2 forms: (1) means and std devs for each group, excluding functional failures (Fig. 2) and (2) the entire data set for maximum force, presented in relative bins (Fig. 3).
There were a number of limitations to our study. First, only a single timepoint was studied. Although 21 days is a relevant time based on the typical presentation of clinical repair-site failures, an assessment at earlier and later times would be useful to elucidate the time course of healing and bone formation. Second, the study may have been underpowered for certain outcomes. Based on previous results, a sample size of 8 is typically necessary to find significant changes for an effect size of ~30%. In our study, after animals with post-operative complications and functional failures were excluded, the sample size in each group ranged from 4 to 10. Due to the financial and ethical constraints associated with a large animal model, we did not perform additional surgeries to reach the originally planned N=8. We felt it inappropriate to utilize additional animals when initial results showed no effect. Statistical comparisons using low sample sizes have an increased chance of a type II error. However, we were able to include the entire data set when considering failure load bins. This analysis revealed a potentially detrimental effect of BMP-2.
Tendon-to-bone healing at the flexor tendon repair site can be enhanced by addressing the bone loss that typically occurs after surgical repair.15 However, to address bone formation or prevention of bone loss, the use of BMP-2 at the concentrations evaluated and as administered in our study is contraindicated in canine Zone 1 digital flexor tendon repairs.22, 29–32 Biological augmentations may not have time to increase mineralized bone formation or prevent post-repair loss of bone leading to improved mechanical properties within the critical time frame for flexor tendon repair (i.e., 3 wks after surgery).
ACKNOWLEDGEMENT
This study was funded by the NIH (EB004347 and AR033097). Testing was conducted in a facility supported by Washington University Center for Musculoskeletal Research (NIH P30 AR057235). BMP-2 was provided as a gift from Pfizer. H.S. is an employee of Pfizer.
REFERENCES
- 1.Silva MJ, Boyer MI, Ditsios K, et al. The insertion site of the canine flexor digitorum profundus tendon heals slowly following injury and suture repair. Journal of Orthopaedic Research. 2002;20:447–453. doi: 10.1016/S0736-0266(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 2.Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. Journal of Hand Surgery - American Volume. 1977;2:66–69. doi: 10.1016/s0363-5023(77)80012-9. [DOI] [PubMed] [Google Scholar]
- 3.Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Harryman DT, 2nd, Mack LA, Wang KY, et al. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. Journal of Bone & Joint Surgery. 1991;73:982–989. [PubMed] [Google Scholar]
- 5.Fu FH, Bennett CH, Lattermann C, et al. Current trends in anterior cruciate ligament reconstruction. Part I: Biology and biomechanics of reconstruction. Am J Sports Med. 1999;27:821–830. doi: 10.1177/03635465990270062501. [DOI] [PubMed] [Google Scholar]
- 6.Kannus P, Leppala J, Lehto M, et al. A rotator cuff rupture produces permanent osteoporosis in the affected extremity, but not in those with whom shoulder function has returned to normal. J Bone Miner Res. 1995;10:1263–1271. doi: 10.1002/jbmr.5650100817. [DOI] [PubMed] [Google Scholar]
- 7.Leppala J, Kannus P, Natri A, et al. Effect of anterior cruciate ligament injury of the knee on bone mineral density of the spine and affected lower extremity: a prospective one-year follow-Up study. Calcif Tissue Int. 1999;64:357–363. doi: 10.1007/s002239900632. [DOI] [PubMed] [Google Scholar]
- 8.Cadet ER, Vorys GC, Rahman R, et al. Improving bone density at the rotator cuff footprint increases supraspinatus tendon failure stress in a rat model. J Orthop Res. 2010;28:308–314. doi: 10.1002/jor.20972. [DOI] [PubMed] [Google Scholar]
- 9.Galatz LM, Rothermich SY, Zaegel M, et al. Delayed repair of tendon to bone injuries leads to decreased biomechanical properties and bone loss. J Orthop Res. 2005;23:1441–1447. doi: 10.1016/j.orthres.2005.05.005.1100230629. [DOI] [PubMed] [Google Scholar]
- 10.Ditsios K, Boyer MI, Kusano N, et al. Bone loss following tendon laceration, repair and passive mobilization. J Orthop Res. 2003;21:990–996. doi: 10.1016/S0736-0266(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 11.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. Journal of Biomechanical Engineering. 2003;125:106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 12.Boyer MI, Harwood F, Ditsios K, et al. Two-portal repair of canine flexor tendon insertion site injuries: histologic and immunohistochemical characterization of healing during the early postoperative period. Journal of Hand Surgery - American Volume. 2003;28:469–474. doi: 10.1053/jhsu.2003.50091. [DOI] [PubMed] [Google Scholar]
- 13.Thomopoulos S, Hattersley G, Rosen V, et al. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. Journal of Orthopaedic Research. 2002;20:454–463. doi: 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 14.Silva MJ, Thomopoulos S, Kusano N, et al. Early healing of flexor tendon insertion site injuries: Tunnel repair is mechanically and histologically inferior to surface repair in a canine model. J Orthop Res. 2006;24:990–1000. doi: 10.1002/jor.20084. [DOI] [PubMed] [Google Scholar]
- 15.Thomopoulos S, Matsuzaki H, Zaegel M, et al. Alendronate prevents bone loss and improves tendon-to-bone repair strength in a canine model. J Orthop Res. 2007;25:473–479. doi: 10.1002/jor.20293. [DOI] [PubMed] [Google Scholar]
- 16.Rodeo SA, Suzuki K, Deng XH, et al. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. American Journal of Sports Medicine. 1999;27:476–488. doi: 10.1177/03635465990270041201. [DOI] [PubMed] [Google Scholar]
- 17.Thomopoulos S, Zampiakis E, Das R, et al. The effect of muscle loading on flexor tendon-to-bone healing in a canine model. J Orthop Res. 2008;26:1611–1617. doi: 10.1002/jor.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dovan TT, Gelberman RH, Kusano N, et al. Zone I flexor digitorum profundus repair: an ex vivo biomechanical analysis of tendon to bone repair in cadavera. J Hand Surg [Am] 2005;30:258–266. doi: 10.1016/j.jhsa.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Lewandrowski KU, Nanson C, Calderon R. Vertebral osteolysis after posterior interbody lumbar fusion with recombinant human bone morphogenetic protein 2: a report of five cases. Spine J. 2007;7:609–614. doi: 10.1016/j.spinee.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Pradhan BB, Bae HW, Dawson EG, et al. Graft resorption with the use of bone morphogenetic protein: lessons from anterior lumbar interbody fusion using femoral ring allografts and recombinant human bone morphogenetic protein-2. Spine (Phila Pa 1976) 2006;31:E277–E284. doi: 10.1097/01.brs.0000216442.12092.01. [DOI] [PubMed] [Google Scholar]
- 21.Seeherman HJ, Li XJ, Bouxsein ML, et al. rhBMP-2 induces transient bone resorption followed by bone formation in a nonhuman primate core-defect model. J Bone Joint Surg Am. 2010;92:411–426. doi: 10.2106/JBJS.H.01732. [DOI] [PubMed] [Google Scholar]
- 22.Toth JM, Boden SD, Burkus JK, et al. Short-term osteoclastic activity induced by locally high concentrations of recombinant human bone morphogenetic protein-2 in a cancellous bone environment. Spine (Phila Pa 1976) 2009;34:539–550. doi: 10.1097/BRS.0b013e3181952695. [DOI] [PubMed] [Google Scholar]
- 23.Shimer AL, Oner FC, Vaccaro AR. Spinal reconstruction and bone morphogenetic proteins: open questions. Injury. 2009;40(Suppl 3):S32–S38. doi: 10.1016/S0020-1383(09)70009-9. [DOI] [PubMed] [Google Scholar]
- 24.Chen CH, Liu HW, Tsai CL, et al. Photoencapsulation of bone morphogenetic protein-2 and periosteal progenitor cells improve tendon graft healing in a bone tunnel. The American journal of sports medicine. 2008;36:461–473. doi: 10.1177/0363546507311098. [DOI] [PubMed] [Google Scholar]
- 25.Li RH, Bouxsein ML, Blake CA, et al. rhBMP-2 injected in a calcium phosphate paste (alpha-BSM) accelerates healing in the rabbit ulnar osteotomy model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2003;21:997–1004. doi: 10.1016/S0736-0266(03)00082-2. [DOI] [PubMed] [Google Scholar]
- 26.Seeherman H, Li R, Bouxsein M, et al. rhBMP-2/calcium phosphate matrix accelerates osteotomy-site healing in a nonhuman primate model at multiple treatment times and concentrations. The Journal of bone and joint surgery. American volume. 2006;88:144–160. doi: 10.2106/JBJS.D.02453. [DOI] [PubMed] [Google Scholar]
- 27.Seeherman HJ, Bouxsein M, Kim H, et al. Recombinant human bone morphogenetic protein-2 delivered in an injectable calcium phosphate paste accelerates osteotomy-site healing in a nonhuman primate model. J Bone Joint Surg Am. 2004;86-A:1961–1972. doi: 10.2106/00004623-200409000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Lieber RL, Amiel D, Kaufman KR, et al. Relationship between joint motion and flexor tendon force in the canine forelimb. J Hand Surg [Am] 1996;21:957–962. doi: 10.1016/S0363-5023(96)80299-1. [DOI] [PubMed] [Google Scholar]
- 29.Lieber RL, Silva MJ, Amiel D, et al. Wrist and digital joint motion produce unique flexor tendon force and excursion in the canine forelimb. J Biomech. 1999;32:175–181. doi: 10.1016/s0021-9290(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 30.Schuind F, Garcia-Elias M, Cooney WP, 3rd, et al. Flexor tendon forces: in vivo measurements. J Hand Surg [Am] 1992;17:291–298. doi: 10.1016/0363-5023(92)90408-h. [DOI] [PubMed] [Google Scholar]
- 31.Koide M, Murase Y, Yamato K, et al. Bone morphogenetic protein-2 enhances osteoclast formation mediated by interleukin-1alpha through upregulation of osteoclast differentiation factor and cyclooxygenase-2. Biochemical and biophysical research communications. 1999;259:97–102. doi: 10.1006/bbrc.1999.0715. [DOI] [PubMed] [Google Scholar]
- 32.Abe E, Yamamoto M, Taguchi Y, et al. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2000;15:663–673. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- 33.Kanatani M, Sugimoto T, Kaji H, et al. Stimulatory effect of bone morphogenetic protein-2 on osteoclast-like cell formation and bone-resorbing activity. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1995;10:1681–1690. doi: 10.1002/jbmr.5650101110. [DOI] [PubMed] [Google Scholar]
- 34.Sotillo Rodriguez JE, Mansky KC, Jensen ED, et al. Enhanced osteoclastogenesis causes osteopenia in twisted gastrulation-deficient mice through increased BMP signaling. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24:1917–1926. doi: 10.1359/JBMR.090507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dagher E, Hays PL, Kawamura S, et al. Immobilization modulates macrophage accumulation in tendon-bone healing. Clin Orthop Relat Res. 2009;467:281–287. doi: 10.1007/s11999-008-0512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan W, Wei Y, Zhou L, et al. Comparative in vivo study of injectable biomaterials combined with BMP for enhancing tendon graft osteointegration for anterior cruciate ligament reconstruction. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29:1015–1021. doi: 10.1002/jor.21351. [DOI] [PubMed] [Google Scholar]
- 37.Rodeo SA, Arnoczky SP, Torzilli PA, et al. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. Journal of Bone & Joint Surgery - American Volume. 1993;75:1795–1803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Gelberman RH, Boyer MI, Brodt MD, et al. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J Bone Joint Surg Am. 1999;81:975–982. doi: 10.2106/00004623-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Kim HM, Nelson G, Thomopoulos S, et al. Technical and biological modifications for enhanced flexor tendon repair. J Hand Surg Am. 2010;35:1031–1037. doi: 10.1016/j.jhsa.2009.12.044. quiz 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]