Abstract
Background
Group differences in brain structure between methamphetamine-dependent and healthy research participants have been reported, but findings in the literature present discrepancies. Although most methamphetamine-abusing individuals also smoke cigarettes, the effects of smoking on brain structure have not been distinguished from those of methamphetamine. Changes with abstinence from methamphetamine have also been relatively unexplored. This study, therefore, attempted to account for effects of smoking and brief abstinence from methamphetamine on gray-matter measures in methamphetamine-dependent research participants.
Methods
Gray matter was measured using voxel-based morphometry in three groups: 18 Control Nonsmokers, 25 Control Smokers, and 39 Methamphetamine-dependent Smokers (methamphetamine-abstinent 4–7 days). Subgroups of methamphetamine-dependent and control participants (n = 12/group) were scanned twice to determine change in gray matter over the first month of methamphetamine abstinence.
Results
Compared with Control Nonsmokers, Control Smokers and Methamphetamine-dependent Smokers had smaller gray-matter volume in the orbitofrontal cortex and caudate nucleus. Methamphetamine-dependent smokers also had smaller gray-matter volumes in frontal, parietal and temporal cortices than Control Nonsmokers or Smokers, and smaller gray-matter volume in insula than Control Nonsmokers. Longitudinal assessment revealed gray matter increases in cortical regions (inferior frontal, angular, and superior temporal gyri, precuneus, insula, occipital pole) in methamphetamine-dependent but not control participants; the cerebellum showed a decrease.
Conclusions
Gray-matter volume deficits in the orbitofronal cortex and caudate of methamphetamine-dependent individuals may be in part attributable to cigarette smoking or pre-morbid conditions. Increase in gray matter with methamphetamine abstinence suggests that some gray-matter deficits are partially attributable to methamphetamine abuse.
Keywords: methamphetamine, cigarette smoking, longitudinal, voxel-based morphometry, prefrontal cortex, caudate nucleus
1. Introduction
Although studies using structural magnetic resonance imaging (sMRI) have generally shown less cortical gray matter and larger basal ganglia volumes in methamphetmaine(MA)-dependent than controls participants, the literature presents some discrepancies (Berman et al., 2008a). MA-dependent research participants, in a narrow epoch of early abstinence (4–7 days), exhibited smaller gray-matter volumes in the cingulate gyrus and hippocampus than in a control group (Thompson et al., 2004). When duration of abstinence from MA was highly variable, however, participants with past MA dependence had smaller gray-matter volume in dorsolateral prefrontal, orbitofrontal, and superior temporal cortices (Nakama et al., 2011), and lower gray-matter density in the middle frontal gyrus (Kim et al., 2006) and insula (Schwartz et al., 2010) than control subjects. MA-dependent participants who were abstinent for long periods (average > 90 days), showed larger gray-matter volumes in parietal cortex, caudate nucleus, lenticular nucleus, nucleus accumbens (Jernigan et al., 2005), putamen and globus pallidus (Chang et al., 2005) than control groups. In studies that reported the proportion of cigarette smokers, MA-dependent samples included more smokers (62%-89%) than controls (0%-39%). Therefore, inconsistencies in the literature may reflect effects of cigarette smoking or differences in durations of MA abstinence.
Although ~87–92% of MA-dependent research participants smoke cigarettes, effects of smoking in these individuals are untested (Weinberger and Sofuoglu, 2009). Smokers have smaller gray-matter volumes and/or lower densities than nonsmokers in prefrontal, cingulate, insular, parietal, temporal and occipital cortex, thalamus and cerebellum (Almeida et al., 2008; Brody et al., 2004; Gallinat et al., 2006; Kuhn et al., 2010; Zhang et al., 2011). One study found that on average, smokers had greater gray-matter density in insular cortex than nonsmokers (Zhang et al., 2011). Little has been done to dissociate the effects of smoking from other drug abuse on gray matter. In one study, participants who drank heavily and smoked had smaller brain volumes than nonsmokers who drank lightly or heavily; and brain volumes did not differ between groups who did not smoke but drank lightly or heavily (Durazzo et al., 2007). These findings suggest that if effects of smoking are not considered, gray-matter differences linked to smoking may be incorrectly attributed to other drug abuse.
Findings from cross-sectional research suggest that gray matter changes with abstinence from MA. MA-dependent participants who had achieved short-term MA abstinence (< 6 months) had lower gray-matter density in the right middle frontal gyrus than those who were abstinent longer (> 6 months; Kim et al., 2006). Furthermore, length of MA abstinence was positively correlated with gray-matter density in the amygdala, putamen, and left fusiform gyrus, but negatively correlated with density in the right middle frontal gyrus (Schwartz et al., 2010). These findings may reflect gray matter changes due to MA abstinence or pre-existing group differences related to the ability to maintain MA abstinence. Although not a perfect solution, longitudinal assessment of the trajectory of changes in gray matter during abstinence from MA can help clarify this issue and may help in determining whether differences from control are attributable to the effects of MA as opposed to other factors.
This study aimed to separate effects of cigarette smoking from those of MA abuse on gray-matter volume. As most previous studies found smaller cortical gray-matter volumes in smokers than nonsmokers (see above), we hypothesized that MA-dependent and control participants who smoke cigarettes would exhibit lower gray-matter volume in prefrontal, cingulate and insular cortices compared with control nonsmokers. MA-dependent participants exhibited larger volumes in the striatum and globus pallidus than control participants (Chang et al., 2005; Jernigan et al., 2005), but no group differences have been found between nonsmokers and smokers in these brain regions (Almeida et al., 2008; Brody et al., 2004a; Das et al., 2011; Gallinat et al., 2006; Zhang et al., 2011). We therefore hypothesized that in striatum and globus pallidus, the MA-dependent sample would differ from two control groups that did not abuse MA, but that the two control groups would not differ from one another. We mapped changes in gray-matter during the first month of MA abstinence, anticipating that gray matter would increase within regions where the early abstinent, MA-dependent participants had smaller gray-matter volumes than control smokers.
2. Methods and materials
2.1. General experimental design
Gray-matter volumes were compared in three groups: Control Nonsmokers, Control Smokers, and MA-dependent Smokers (4–7 days abstinent). Then MA-dependent participants were scanned a second time [mean time between scans: 23.5 ± 1.6 (SD) days] and compared to a control sample that was matched for smoking [mean time between scans: 31.6 ± 13.1 (SD) days] and rescanned as well. This study focused on early abstinence because this period is critical for engagement in therapy and, therefore, for treatment outcomes (Brecht et al., 2000).
2.2. Participants and procedures
Participants were recruited through online and print advertisements, received a detailed explanation of the study (as approved by the University of California Los Angeles (UCLA) Institutional Review Board), and gave written informed consent. Eighty-two participants (ages 18–55 years) were recruited: Control Nonsmoker (n = 18), Control Smoker (n = 25), and MA-dependent Smoker (n = 39). In the longitudinal assessment, two groups were studied: Control and MA-dependent (n = 12 per group, smoking status described below). Sixty percent of the Control and 60% MA-dependent participants also participated in a previous study of gray-matter volume and inhibitory control (Tabibnia et al., 2011), and recruitment of participants continued to complete the present study.
A physical examination and medical history were used to exclude the following conditions: central nervous system, cardiovascular, pulmonary, or systemic disease; use of psychotropic medications, prior head trauma, HIV seropositivity, and pregnancy. Also exclusionary were any current Axis I diagnoses except for MA- or nicotine abuse or dependence [Structured Clinical Interview for DSM-IV (First et al., 1995)]. Drug use and demographic variables were collected using the Addiction Severity Index (McLellan et al., 2006) and a drug use survey prepared for this study. MA-dependent participants participated on a residential basis (UCLA General Clinical Research Center) and underwent daily urine toxicology to verify recent drug use history. Control Nonsmokers and Smokers participated on an outpatient basis and reported no drug use except for light alcohol or marijuana use. Self-reports were verified with urine testing at intake and at each subsequent visit. Smokers (Control or MA-dependent) used cigarettes on at least 25 of the 30 days before entering the study and Control Nonsmokers smoked fewer than 5 cigarettes in their lifetime. Individuals who had ever smoked more than 5 cigarettes and MA-dependent individuals who did not smoke cigarettes were permitted in the longitudinal but not the cross-sectional study (because only two of the MA-dependent participants did not smoke cigarettes).
2.3. MRI acquisition
High-resolution, whole-brain T1-weighted magnetic resonance images (MPRAGE, TR = 1900 ms, TE = 4.38 ms, flip angle = 15°, FOV = 256×256×160, 160 slices, thickness: 1-mm) were collected on a 1.5-Tesla Siemens Sonata scanner (Erlangen, Germany) with a standard quadrature head coil.
2.4. Voxel-based morphometry (VBM) analysis of cross-sectional data
All images were aligned to a standardized stereotactic space with the sagittal plane serving as the yz-plane, the axial-oblique plane normal to this and containing the anterior and posterior commissures (AC-PC plane) as the xy-plane, and the coronal-oblique plane normal to the sagittal AC-PC planes serving as the xz-plane. The origin of the space was set at the left-right and inferior-superior midpoint of the anterior commissure.
VBM (Ashburner and Friston, 2000) was conducted using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) for SPM8 (SPM8; Wellcome Department of Imaging Neuroscience, London) running on MATLAB® 7.9 (Mathworks, Sherborn, MA, USA). The toolbox is an extension of the unified segmentation model (Ashburner and Friston, 2005). As described previously (Koutsouleris et al., 2010), manually AC-PC aligned images are initially de-noised using an optimized block-wise non-local means de-noising filter (Coupe et al., 2006). To segment the images into three classifications (gray matter, white matter, and cerebrospinal fluid), an adaptive maximum a posteriori technique (Rajapakse et al., 1997) was extended by the addition of partial volume estimation (Manjon et al., 2008). Data were subsequently de-noised using a hidden Markov Random Field approach (Cuadra et al., 2005). Each image was registered to a standard template in Montreal Neurological Institute (MNI) space using Diffomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) (Ashburner, 2007).
The resulting images were maps of the probabilities that the voxel elements represented gray matter. These images were then modulated by a procedure in which the intensity value of each voxel was multiplied by the local value of the Jacobian determinant of the deformation used to register each brain to the standard template. Linear components of the deformations, reflecting scaling due to head size, were not considered during modulation so that differences in volume due to head size would not affect intensity values. In the resulting images, intensity at each voxel (“gray-matter volume”) reflected the probability that the voxel contained gray matter and the relative volume after adjusting for different brain sizes. Finally images were smoothed using a 8-mm FWHM Gaussian kernel.
For the overall test of differences in gray-matter volume between groups, smoothed images were compared using univariate analysis of covariance (ANCOVA) with gray-matter volume at each voxel as the dependent variable, group as a between-subjects factor, and age, gender, and frequency of marijuana use as covariates. Using the same covariates, T-tests were used for post hoc pair-wise comparisons of differences between groups and regression was used to test the association between gray-matter volume and drug use variables within groups. Statistical models were applied in an explicit mask of gray matter, created using an objective function that maximized the correlation between original and thresholded images (Ridgway et al., 2009). To assess statistical significance, a height threshold of p<0.001 was applied voxel-wise. Cluster sizes were adjusted to correct for the varying degrees of smoothness in different parts of the brain (Hayasaka et al., 2004) and family-wise error (FWE) was applied to correct for multiple comparisons in testing cluster extent significance (p<0.05).
2.5. VBM analysis of longitudinal data
As described for analysis of the cross-sectional data, images were aligned to the AC-PC plane, and preprocessed using the VBM8 toolbox. Data were preprocessed using the default parameters for processing longitudinal data described in the VBM8 manual (http://dbm.neuro.uni-jena.de/vbm/download/). Briefly, for each subject, scans from each time-point were realigned and averaged to create a mean image. Then, the original scans from the two time periods were realigned to the mean image, bias-corrected, and segmented. Nonlinear deformation parameters, calculated by registering the mean image to MNI space using DARTEL, were applied to segmented gray-matter images from both time points to account for individual differences in head size. Images were smoothed using an 8-mm FWHM Gaussian kernel. A repeated measures analysis of variance model (flexible factorial model in SPM8), with the group as a between-subject factor and time as a within-subjects factor, was used to test for a Group-by-Time interaction and for the effect of time in each of the two groups. The statistical model was applied to voxels within an explicit mask of the gray matter (Ridgway et al., 2009). A height threshold for significance was set at p<0.001, uncorrected, with a cluster extent of at least 100 contiguous voxels.
3. Results
3.1. Research participants in the cross-sectional study
The three groups did not differ in age (ANOVA: F(2,79)=2.10, p=0.13), sex distribution (Chi-Square=0.87), or frequency of recent alcohol consumption (ANOVA: F(2,79)=1.2, p=0.31). MA-dependent Smokers completed fewer years of education than Control Nonsmokers and Smokers (ANOVA: F(2,79)=8.52, p<0.001; post hoc t-tests, p’s<0.003). The age at which a participant began using MA was positively correlated with education (those that initiated MA abuse later in life achieved higher levels of education; p < 0.05). This finding supported our previous report that the quality and quantity of educational attainment is interrupted by MA abuse (Dean et al., 2011). As education and patterns of MA abuse are intertwined, education may be a poor proxy for cognitive functioning. In MA-dependent participants, parental education (but not participant education) relates to cognitive functioning (Dean et al., 2011), and in the current study, the groups did not differ significantly on education attained by the participants’ mothers (ANCOVA: F(2,75)=1.74, p=0.18).
The groups differed on frequency of marijuana use (ANOVA: F(2,79)=4.55, p=0.01). MA-dependent Smokers used marijuana more often than Control Nonsmokers and Control Smokers (p’s<0.02). Frequency of marijuana use, therefore, was included in the statistical models. Control Smokers and MA-Dependent Smokers did not differ on frequency of cigarette use, number of cigarettes per day, pack years, score on the Fagerström Nicotine Dependence Test (Fagerstrom, 1978) or in age of first cigarette use (all p’s > 0.2; Table 1).
Table 1.
Characteristics of Research Participants
| Cross-Sectional | Longitudinal | ||||
|---|---|---|---|---|---|
|
| |||||
| Control Nonsmoker (n=18) | Control Smoker (n=25) | MA Smoker (n=39) | Control (n=12) | MA (n=12) | |
| Age (years)a | 30.1 ± 2.2 | 35.4 ± 1.8 | 34.8 ± 1.5 | 37.5 ± 2.3 | 33.8 ± 2.4 |
| Sex (# male) | 8 | 13 | 20 | 7/5 | 5/7 |
| Mother’s Education (years) | 13.2 ± 0.6 | 13.6 ± 0.5 | 12.3 ± 0.4 | 15.0 ± 1.0 | 13.0 ± 0.7 |
| Education (years)* | 14.6 ± 0.4 | 14.1 ± 0.3 | 12.8 ± 0.3 | 14.8 ±0.5 | 12.7 ± 0.4 |
| Alcohol Use | |||||
| Days used in the last 30 d | 2.4 ± 1.7 | 5.3 ± 1.4 | 5.4 ± 1.1 | 6.5 ± 1.9 | 8.1 ± 2.3 |
| Marijuana Use | |||||
| Days used in the last 30 d | 0.1 ± 0.4 | 0.2 ± 0.3 | 1.1 ± 0.2*** | 0.0 ± 0.0 | 2.5 ± 1.5 |
| Tobacco Use (# current user) | 8 | 10 | |||
| Days used in the last 30 d | 29.6 ± 0.3 | 29.8 ± 0.1 | 29.6 ± 0.4 | 28.8 ± 1.2 | |
| Cigarettes per day | 14.1 ± 1.2 | 13.4 ± 1.5 | 11.5 ± 1.4 | 9.7 ± 2.0 | |
| Pack years | 11.5 ± 1.9 | 13.2 ± 2.3 | 9.8 ±4.0 | 11.1 ± 3.8 | |
| Fagerstrom Score | 3.8 ± 0.4 | 3.6 ± 0.4 | 2.9 ± 0.7 | 2.8 ± 0.8 | |
| Age of first use (year) | 16.4 ± 1.1 | 15.0 ± 0.7 | 13.5 ± 1.4 | 14.5 ± 1.3 | |
| Methamphetamine Use | |||||
| Days used in the last 30 d | 22.4 ± 1.3 | 21.1 ± 2.9 | |||
| Grams per week | 2.6 ± 0.4 | 1.9 ± 0.5 | |||
| Years of heavy useb | 8.4 ± 1.3 | 6.8 ± 2.2 | |||
| Years of usec | 11.5 ± 1.5 | 9.3 ± 2.4 | |||
| Age of first use (year) | 21.7 ± 1.2 | 21.6 ± 2.2 | |||
Data shown are means ± SEM;
n=38 for cross-sectional;
n=33 for cross-sectional, n=11 for longitudinal
Cross-sectional: Significant differences between groups by ANOVA (F=8.5; p<0.001) MA-dependent Smoker significantly different from Control (nonsmoker and tobacco smoker) by Student’s t-test (p<0.001). Longitudinal: Significant differences between groups by Student’s t-test (p<0.005)
Significant differences between the groups by ANVOA (F=6.210; p=0.003). Significantly different from Control Nonsmoker and Control Smoker by Student’s t-test (p<0.001)
3.2. Differences in gray-matter volume: cross-sectional study
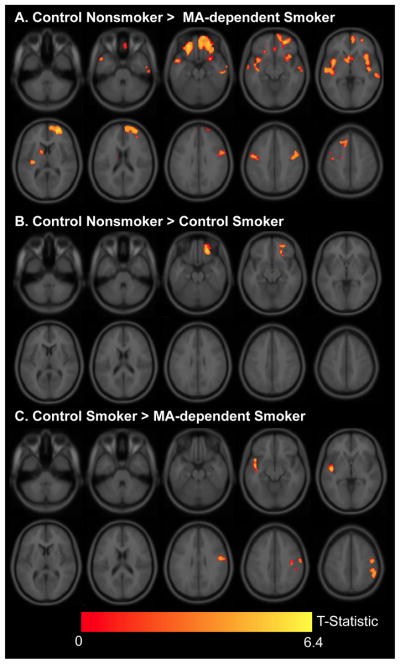
ANCOVA revealed differences in gray-matter volume among the three groups in bilateral orbitofrontal and precental gyri, right frontal pole, left superior temporal gyrus and superior frontal gyrus (Table 2). Subsequent comparisons (Figure 1) showed that Control Nonsmokers had larger gray-matter volumes in the right orbitofrontal cortex than Control and MA-Dependent Smokers (p’s < 0.05 FWE corrected). There were no other brain regions where Control Smokers differed from Control Nonsmokers, but MA-dependent Smokers had smaller gray-matter volume than Control Nonsmokers in bilateral precentral gyrus; right frontal pole, middle temporal gyrus, precuneus and cingulate gyrus; and left orbitofrontal gyrus, superior frontal gyrus, insula, and caudate (p < 0.05 FWE-corrected). Control Smokers had larger gray-matter volumes than MA-dependent Smokers in left superior temporal gyrus, left precentral gyrus, right inferior temporal gyrus and right supramarginal gyrus (p < 0.05 FWE-corrected). There was no region where Control Smokers had smaller gray-matter volume than MA-dependent Smokers. Measures of cigarette smoking and methamphetamine abuse (listed in Table 1) were not significantly related to gray-matter measures.
Table 2.
Cross-Sectional Gray-matter volume Differences
| P value* | Cluster Extent | F or T | Z | X | Y | Z | Region |
|---|---|---|---|---|---|---|---|
|
Differences Between Three Groups
| |||||||
| 0.000 | 1925 | 20.19 | 5.21 | 26 | 54 | 12 | R Frontal Pole |
| 0.000 | 1957 | 17.33 | 4.85 | 18 | 38 | −15 | R Orbitofrontal Cortex |
| 0.000 | 1428 | 15.55 | 4.59 | −46 | −10 | −6 | L Superior Temporal Gyrus |
| 0.021 | 475 | 15.39 | 4.57 | −24 | 41 | −23 | L Orbitofrontal Cortex |
| 0.015 | 515 | 13.13 | 4.21 | −14 | 0 | 58 | L Superior Frontal Gyrus |
| 0.050 | 364 | 12.83 | 4.16 | −42 | −10 | 42 | L Precentral Gyrus |
| 0.030 | 426 | 11.92 | 4.00 | 48 | −3 | 31 | R Precentral Gyrus |
|
| |||||||
|
Control Nonsmoker > Methamphetamine-Dependent Tobacco Smoker
| |||||||
| 0.000 | 7314 | 6.35 | 5.67 | 26 | 54 | 12 | R Frontal Pole/Orbitofrontal Gyrus |
| 0.004 | 962 | 5.54 | 5.06 | −24 | 41 | −23 | L Orbitofrontal Cortex |
| 0.000 | 2387 | 4.66 | 4.36 | −14 | 0 | 58 | L Superior Frontal Gyrus |
| 0.000 | 2790 | 4.64 | 4.34 | −40 | 3 | −3 | L Insula |
| 0.007 | 836 | 4.62 | 4.33 | −42 | −10 | 40 | L Precentral Gyrus |
| 0.010 | 755 | 4.48 | 4.21 | 50 | −3 | 34 | R Precentral Gyrus |
| 0.003 | 1014 | 4.31 | 4.06 | 69 | −24 | −6 | R Middle Temporal Gyrus |
| 0.021 | 615 | 4.27 | 4.03 | 10 | −48 | 66 | R Precuneus |
| 0.018 | 646 | 4.25 | 4.01 | −3 | 10 | 1 | L Caudate |
| 0.027 | 569 | 4.18 | 3.95 | 4 | −34 | 42 | R Cingulate |
|
| |||||||
|
Control Nonsmoker > Control Tobacco Smoker
| |||||||
| 0.000 | 1043 | 5.59 | 5.10 | 18 | 38 | −15 | R Orbitofrontal Cortex |
|
| |||||||
|
Control Tobacco Smoker > Methamphetamine-Dependent Tobacco Smoker
| |||||||
| 0.005 | 919 | 5.10 | 4.71 | −46 | −10 | −5 | L Superior Temporal Gyrus |
| 0.026 | 575 | 4.45 | 4.18 | 38 | −7 | −47 | R Inferior Temporal Gyrus |
| 0.013 | 708 | 4.43 | 4.43 | 50 | −34 | 45 | R Supramarginal Gyrus |
| 0.020 | 620 | 4.24 | 4.24 | −48 | −3 | 30 | L Precentral Gyrus |
Family Wise Error corrected for cluster extent
L, left hemisphere; R, right hemisphere
Figure 1. Group differences in gray-matter volume.
T statistic maps showing brain regions where (1A) Control Nonsmokers have greater gray-matter volume than MA-dependent Smokers and (1B) Control Smokers. (1C) Control Smokers have greater gray-matter volume than MA-dependent Smokers (R: right hemisphere).
Previous findings show that MA-dependent participants had larger gray-matter volumes in caudate nucleus than controls (Chang et al., 2005; Jernigan et al., 2005). To determine whether voxel-wise assessment of gray-matter volume within the striatum led to the discrepancy with previously published results, we delineated the caudate nucleus using a semi-automatic method (Supplemental Methods)1. Results show that MA-Dependent and Control Smokers have smaller bilateral and left caudate volumes than Control Nonsmokers (p’s<0.05), but we did not detect statistically significant differences between Control and MA-dependent Smokers (Supplemental Results; Supplemental Table 1).
3.3. Characteristics of research participants in the longitudinal study
The two groups did not differ in age, sex distribution, recent alcohol and marijuana use use, or years of education attained by participants’ mothers (p’s>0.1), but did differ in average years of participant education (p<0.005). Among individuals who were currently smoking cigarettes, there were no differences in smoking behavior (p>0.5; Table 1).
3.4. Changes in gray matter during MA abstinence
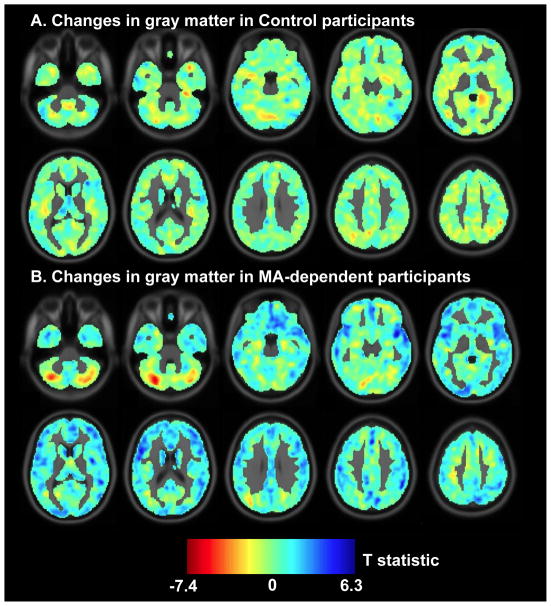
Group-by-time interactions were detected in bilateral superior temporal gyrus, right angular gyrus, right insula, left precuneus, left cerebellum, left inferior frontal gyrus, and left occipital pole. To explain these interactions, subsequent analyses were performed to determine the effect of time in each of the two groups. Between the first and fourth weeks of MA abstinence, gray-matter increased in the MA-dependent group in all of the cortical regions exhibiting Group-by-Time interactions; in the cerebellum, gray matter decreased. There were no brain regions where the Control group showed changes over time at the specified statistical threshold. In MA-dependent participants, increased gray matter was also detected in bilateral middle temporal gyrus and in the right hemisphere in precuneus, middle frontal gyrus, frontal operculum, inferior frontal gyrus, and ventromedial prefrontal cortex, but Group-by-Time interactions did not reach significance in these brain regions. Qualitative comparison of t statistic maps, denoting the effect of time in Control and MA-dependent participants, shows that the Control group exhibited relatively small changes in gray matter while the MA-dependent participants exhibited notable increases and decreases in gray matter (Figure 2).
Figure 2. Changes in gray matter during the first month of abstinence from methamphetamine.
T statistic maps showing brain regions where gray matter increased over time (shades of blue) and where it decreased over time (shades of red; R: right hemisphere) in Control (A) and MA-dependent participants (B).
4. Discussion
The present findings help begin to disentangle the various factors that may influence gray-matter volumes in MA-dependent individuals. The results are largely consistent with those of previous studies (Kim et al., 2006; Nakama et al., 2011; Schwartz et al., 2010; Thompson et al., 2004), but they also provide new evidence that after 4–7 days of abstinence MA-dependent Smokers have smaller caudate and parietal volumes than nonsmokers, and that smaller orbitofrontal and caudate volumes associated with cigarette smoking may help explain similar deficits in MA-dependent samples. Preliminary evidence, indicating that gray matter changes during the first month of MA abstinence, suggests that some brain regions may be affected by MA abuse itself.
Previous studies found that on average MA-abusing research participants (who were abstinent from MA for several days to a few years) had larger gray-matter volume in caudate and parietal cortex than control subjects (Jernigan et al., 2005). This report is the first to show smaller gray-matter volume in parietal cortex and caudate nucleus in MA-dependent participants as compared with controls, suggesting that deficits in gray-matter volumes during active abuse or early abstinence (4–7 days MA abstinent) precede volumetric expansion with longer sustained abstinence. Findings of increased gray matter in precuneus and angular gyrus, with abstinence from MA, support this hypothesis. These increases in gray-matter may relate to changes in brain function. A previous study showed that after 4 weeks of supervised abstinence, a group of MA-dependent participants showed increased glucose metabolism in parietal cortex while performing a vigilance task (Berman et al., 2008b). Larger samples or more sensitive methods for assessing changes in small subcortical structures may be needed to detect significant increases in caudate nucleus volume in MA-dependent participants (subthreshold increase in caudate gray matter seen in Figure 2).
Control Smokers and MA-dependent Smokers did not differ in right orbitofrontal gray-matter volume, but both groups exhibited smaller gray-matter volume in right orbitofrontal cortex than Control Nonsmokers. Our findings are consistent with a previous report indicating that compared with nonsmokers, smokers have focal gray-matter deficits in orbitofrontal cortex (Kuhn et al., 2010); however, other studies have found more widespread differences in cortex between smokers and nonsmokers (Almeida et al., 2008; Brody et al., 2004; Gallinat et al., 2006). Discrepancies may be attributable to the modest sample sizes or to the differential patterns of smoking behavior assessed across studies. Like previous studies using voxel-wise approaches, VBM did reveal group differences in caudate nucleus (Almeida et al., 2008; Gallinat et al., 2006), but three-dimensional delineation of the caudate nucleus using FSL FIRST shows that Control Smokers have smaller volume in caudate nucleus than Control Nonsmokers.
Consistent with previous findings, our results also showed that MA-dependent Smokers have smaller gray-matter volume in cingulate, superior temporal gyrus, insula and dorsolateral prefrontal cortex than Control Nonsmokers (Kim et al., 2006; Nakama et al., 2011; Schwartz et al., 2010; Thompson et al., 2004), but only the difference in superior temporal gyrus was significant when comparing MA-Dependent Smokers to Control Smokers. Between the first and fourth weeks of MA abstinence, increase gray-matter in superior temporal gyrus in the MA-dependent but not healthy control participants provides converging evidence for an MA-specific effect in this brain region. We did not replicate a previous finding from our laboratory of smaller hippocampal volumes in MA-dependent individuals than in healthy controls, perhaps owing to the different methodologies employed (Thompson et al., 2004).
Group differences in gray-matter volume may reflect premorbid biological risk factors for drug abuse or effects of the drugs themselves. While there is evidence to suggest that MA (Cadet and Krasnova, 2009; Steinkellner et al., 2011), nicotine (Ferrea and Winterer, 2009), and other compounds in cigarette smoke (Mactutus, 1989) are neurotoxic, there were no significant relationships between drug exposure and gray-matter volumes. This lack of correspondence between brain volume and drug abuse has been reported before in studies of MA (Jernigan et al., 2005; Nakama et al., 2008) and cocaine (Franklin et al., 2002; Matochik et al., 2005). It may be interpreted as evidence for gray-matter abnormalities that predate drug use, but may also reflect a complex and multi-factorial relationship between exposure and structural abnormality, with a threshold for structural change. It is also possible that MA abuse interacts with cigarette smoking to affect brain structure. For example, pre-exposure to nicotine protects against MA-induced loss of striatal dopamine terminals in mice that express the α4 nicotinic acetylcholine receptor subunit (nAChR) but not in α4-knockout mice (Ryan et al., 2001). This finding suggests that interactions between cigarette smoking and MA abuse may vary depending on regional expression of nAChRs. We could not test this hypothesis because a group of MA-dependent individuals who do not smoke cigarettes was not recruited, as these individuals are rare.
This study extends our understanding of the neurobiological changes taking place with MA abstinence. Previous work has shown increased dopamine transporter levels (Volkow et al., 2001) and increased cerebral glucose metabolism (Wang et al., 2004) with abstinence from chronic MA (Berman et al., 2008b), but this study provides the first evidence of changes in gray-matter during MA abstinence. More work will be necessary to determine how these changes in gross anatomy map onto microstructural changes at the cellular level. One possibility is that increased gray-matter reflects MA-induced inflammation or reactive gliosis (Chang et al., 2007), which has been associated with MA exposure in preclinical models (Asanuma et al., 2004; Thomas et al., 2004; Yamamoto and Bankson, 2005) and in human imaging studies (Ernst et al., 2000; Sekine et al., 2008). Future studies with larger samples will be needed to link structural, molecular, and functional changes in brain to potential improvements in mood, behavior and cognition associated with MA abstinence (Simon et al., 2010; Zorick et al., 2010).
While the present study extends our understanding of morphological differences associated with MA-dependence, it is not without limitations. One of these, modest sample size, may have prevented detection of the full range of potential cross-sectional and longitudinal differences in gray matter. Although small, the sample in the longitudinal assessment was comparable to (Berman et al., 2008b) or exceeded the samples studied in other within-subject assessments of MA-dependence using positron emission tomography to assess brain metabolism and dopamine transporter levels (Volkow et al., 2001; Wang et al., 2004). This likely reflects difficulties in recruiting MA-dependent individuals willing to participate in sustained abstinence.
Some differences in drug use and lifestyle, not accounted for in this study design, may also affect brain structure. As MA abuse interrupts education (Dean et al., 2011), it is difficult to disentangle the independent effects of each on brain structure. Since MA abuse and participant education are related, inclusion of participant education in the statistical model may account for some of the variance associated with MA dependence itself. Despite this, we obtained results that supported those obtained without including education in the model, when a more liberal statistical threshold (p<0.005 uncorrected) was used. Furthermore, potentially confounding effects of education on brain structure may be mitigated by the fact that groups did not differ on mother’s education, which is a better proxy for general cognitive functioning in MA-dependent participants than participant’s education (Dean et al., 2011).
Substantial abuse of marijuana (daily or almost daily) and alcohol has been associated with structural abnormalities in various brain regions, and there were some differneces between groups in the use of these substances (Buhler and Mann, 2011; Lorenzetti et al., 2010). While MA-dependent Smokers reported more marijuana use than controls, on average, they used marijuana on fewer than 2 days a month; and there were no significant group differences in alcohol consumption. In addition, individuals meeting criteria for either cannabis or alcohol abuse or dependence were excluded from study. Therefore, it is unlikely that marijuana or alcohol abuse factors substantially confounded the findings.
Despite these limitations, this study has several strengths. It focused on a relatively narrow period of MA abstinence, facilitating the detection of previously unreported deficits in caudate nucleus and parietal cortical volume that appear to be uniquely associated with early abstinence from MA. Notwithstanding any potential confounds, it remains clear that orbitofrontal and caudate nucleus gray-matter deficits seen in MA-dependent research participants are also seen in participants who smoke cigarettes but do not engage in notable illicit drug abuse. In addition, a longitudinal assessment showed that gray-matter changes during early abstinence from MA. Mapping the trajectory of these changes can provide an initial step towards developing a better understanding of the biological bases and effects of MA-dependence.
Supplementary Material
Table 3.
Longitudinal Changes in Gray Matter
| P value* | Cluster Extent | T | Z | X | Y | Z | Region |
|---|---|---|---|---|---|---|---|
|
Group x Time Interaction
| |||||||
| 0.000 | 195 | 6.33 | 4.73 | 51 | −48 | 46 | R Angular Gyrus |
| 0.000 | 186 | 6.07 | 4.60 | −3 | −60 | 34 | L Precuneus |
| 0.000 | 323 | 5.75 | 4.45 | 46 | 4 | −12 | R Superior Temporal Gyrus |
| 0.000 | 133 | 5.29 | 4.21 | 42 | −6 | 7 | R Insula |
| 0.000 | 296 | 4.94 | 4.01 | −50 | 5 | −5 | L Superior Temporal Gyrus |
| 0.000 | 145 | 4.87 | 3.97 | −38 | −69 | −56 | L Cerebellum |
| 0.000 | 121 | 4.61 | 3.81 | −50 | 17 | 21 | L Inferior Frontal Gyrus |
| 0.000 | 292 | 4.44 | 3.71 | −21 | −96 | 3 | L Occipital Pole |
| 0.000 | 125 | 4.30 | 3.63 | −36 | −70 | −39 | L Cerebellum |
|
| |||||||
|
MA-Dependence - Time 2 > Time 1
| |||||||
| 0.000 | 955 | 6.28 | 4.70 | 46 | 4 | −12 | R Superior Temporal Gyrus |
| 0.000 | 256 | 6.27 | 4.70 | 56 | −48 | 37 | R Angular Gyrus |
| 0.000 | 360 | 6.18 | 4.66 | −48 | 3 | −6 | L Superior Temporal Gyrus |
| 0.000 | 171 | 6.10 | 4.62 | 12 | −75 | 42 | R Precuneus |
| 0.000 | 594 | 6.09 | 4.61 | −50 | 14 | 21 | L Inferior Frontal Gyrus |
| 0.000 | 134 | 5.63 | 4.38 | 57 | −43 | 9 | R Middle Temporal Gyrus |
| 0.000 | 100 | 5.27 | 4.19 | −3 | 60 | 34 | L Precuneus |
| 0.000 | 119 | 5.19 | 4.15 | 42 | −4 | 9 | R Insula |
| 0.000 | 105 | 5.18 | 4.14 | −60 | −25 | −9 | L Middle Temporal Gyrus |
| 0.000 | 209 | 5.05 | 4.07 | −46 | 18 | −8 | R Frontal Operculum |
| 0.000 | 272 | 4.90 | 3.98 | 46 | 36 | 13 | R Inferior Frontal Gyrus |
| 0.000 | 143 | 4.64 | 3.83 | 4 | 51 | −14 | R Ventromedial Prefrontal Cortex |
| 0.000 | 271 | 4.46 | 3.72 | −21 | −96 | 3 | L Occipital Pole |
|
| |||||||
|
MA-Dependence - Time 1 > Time 2
| |||||||
| 0.000 | 1479 | 7.41 | 5.19 | −27 | −76 | −36 | L Cerebellum |
|
| |||||||
| 0.000 | 252 | 4.47 | 3.73 | 34 | −78 | −36 | R Cerebellum |
uncorrected voxel-level p-value
R, right hemisphere; L, left hemisphere
Acknowledgments
This research was supported by the following: NIH grants P20 DA022539, R01 DA015179, R01 DA020726 (EDL); institutional training grants T90 DA022768 and T32 DA024635; and M01 RR00865 (UCLA GCRC). Additional funding provided by the Eugene V. Cota-Robles Fellowship and endowments from the Thomas P. and Katherine K. Pike Chair in Addiction Studies and the Marjorie M. Greene Trust. The authors thank Catherine Sugar, PhD, for statistical advice and helpful comments on the manuscript; Sarah Wilson, MA, for performing psychometric evaluation and for coordination of the study; Todd Zorick, MD, PhD, for clinical oversight; Eugene Oh, Kristina Mouzakis and Greg Shipman for database support; and Christine Baker, Clayton Clement, Natalie DeShetler, Bahar Ebrat, Lisa Giragosian, Tom Hanson, Lindsey King, Natasha Moallem, and Andrew T. Morgan for participation in participant recruitment, screening and retention.
Role of Funding Source: Nothing Declared. None of the sponsors had any involvement with the design, collection, analysis, or interpretation of data, writing the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplemental methods can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors: Dr. London and Dr. O’Neill designed the study and wrote the protocol. Dr. Lee and Ms. Morales conducted data analysis. Dr. Hellemann and Ms. Morales conducted statistical analyses. Ms. Morales and Dr. London wrote the first draft of the manuscript and all authors contributed to subsequent drafts and approved the final manuscript.
Conflict of Interest: Research support for projects other than the one reported here was supplied to Dr. Edythe London under UCLA contract (number 20063287) with Philip Morris USA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida OP, Garrido GJ, Lautenschlager NT, Hulse GK, Jamrozik K, Flicker L. Smoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:92–98. doi: 10.1097/JGP.0b013e318157cad2. [DOI] [PubMed] [Google Scholar]
- Asanuma M, Miyazaki I, Higashi Y, Tsuji T, Ogawa N. Specific gene expression and possible involvement of inflammation in methamphetamine-induced neurotoxicity. Ann N Y Acad Sci. 2004;1025:69–75. doi: 10.1196/annals.1316.009. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008a;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, Voytek B, Mandelkern MA, Hassid BD, Isaacson A, Monterosso J, Miotto K, Ling W, London ED. Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Mol Psychiatry. 2008b;13:897–908. doi: 10.1038/sj.mp.4002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht ML, von Mayrhauser C, Anglin MD. Predictors of relapse after treatment for methamphetamine use. J Psychoactive Drugs. 2000;32:211–220. doi: 10.1080/02791072.2000.10400231. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004a;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Buhler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res. 2011;35:1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN. Molecular bases of methamphetamine-induced neurodegeneration. Int Rev Neurobiol. 2009;88:101–119. doi: 10.1016/S0074-7742(09)88005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupe P, Yger P, Barillot C. Fast non local means denoising for 3D MR images. Med Image Comput Comput Assist Interv. 2006;9:33–40. doi: 10.1007/11866763_5. [DOI] [PubMed] [Google Scholar]
- Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Trans Med Imaging. 2005;24:1548–1565. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- Das D, Cherbuin N, Anstey KJ, Sachdev PS, Easteal S. Lifetime cigarette smoking is associated with striatal volume measures. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2010.00301.x. in press. [DOI] [PubMed] [Google Scholar]
- Dean AC, Hellemann G, Sugar CA, London ED. Educational attainment is not a good proxy for cognitive function in methamphetamine dependence. Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2011.11.019. Epub ahead of print 12/2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Cardenas VA, Studholme C, Weiner MW, Meyerhoff DJ. Non-treatment-seeking heavy drinkers: effects of chronic cigarette smoking on brain structure. Drug Alcohol Depend. 2007;87:76–82. doi: 10.1016/j.drugalcdep.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: a 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Ferrea S, Winterer G. Neuroprotective and neurotoxic effects of nicotine. Pharmacopsychiatry. 2009;42:255–265. doi: 10.1055/s-0029-1224138. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IP) American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24:1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim J, Kwon DH, Chang KH, Renshaw PF. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2006;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Patschurek-Kliche K, Scheuerecker J, Decker P, Bottlender R, Schmitt G, Rujescu D, Giegling I, Gaser C, Reiser M, Moller HJ, Meisenzahl EM. Neuroanatomical correlates of executive dysfunction in the at-risk mental state for psychosis. Schizophr Res. 2010;123:160–174. doi: 10.1016/j.schres.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Schubert F, Gallinat J. Reduced thickness of medial orbitofrontal cortex in smokers. Biol Psychiatry. 2010;68:1061–1065. doi: 10.1016/j.biopsych.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Lubman DI, Whittle S, Solowij N, Yucel M. Structural MRI findings in long-term cannabis users: what do we know? Subst Use Misuse. 2010;45:1787–1808. doi: 10.3109/10826084.2010.482443. [DOI] [PubMed] [Google Scholar]
- Mactutus CF. Developmental neurotoxicity of nicotine, carbon monoxide, and other tobacco smoke constituents. Ann N Y Acad Sci. 1989;562:105–122. doi: 10.1111/j.1749-6632.1989.tb21010.x. [DOI] [PubMed] [Google Scholar]
- Manjon JV, Tohka J, Garcia-Marti G, Carbonell-Caballero J, Lull JJ, Marti-Bonmati L, Robles M. Robust MRI brain tissue parameter estimation by multistage outlier rejection. Magn Reson Med. 2008;59:866–873. doi: 10.1002/mrm.21521. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Cacciola JC, Alterman AI, Rikoon SH, Carise D. The Addiction Severity Index at 25: origins, contributions and transitions. Am J Addict. 2006;15:113–124. doi: 10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- Nakama H, Chang L, Cloak C, Jiang C, Alicata D, Haning W. Association between psychiatric symptoms and craving in methamphetamine users. Am J Addict. 2008;17:441–446. doi: 10.1080/10550490802268462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakama H, Chang L, Fein G, Shimotsu R, Jiang CS, Ernst T. Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction. 2011 doi: 10.1111/j.1360-0443.2011.03433.x. Epub ahead of print, 5/2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans Med Imaging. 1997;16:176–186. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- Ridgway GR, Omar R, Ourselin S, Hill DL, Warren JD, Fox NC. Issues with threshold masking in voxel-based morphometry of atrophied brains. Neuroimage. 2009;44:99–111. doi: 10.1016/j.neuroimage.2008.08.045. [DOI] [PubMed] [Google Scholar]
- Ryan RE, Ross SA, Drago J, Loiacono RE. Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in alpha4 nicotinic receptor subunit knockout mice. Br J Pharmacol. 2001;132:1650–1656. doi: 10.1038/sj.bjp.0703989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, Hoffman WF. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage. 2010;50:1392–1401. doi: 10.1016/j.neuroimage.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Dean AC, Cordova X, Monterosso JR, London ED. Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. J Stud Alcohol Drugs. 2010;71:335–344. doi: 10.15288/jsad.2010.71.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkellner T, Freissmuth M, Sitte HH, Montgomery T. The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol Chem. 2011;392:103–115. doi: 10.1515/BC.2011.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, Lee B, London ED. Different forms of self-control share a neurocognitive substrate. J Neurosci. 2011;31:4805–4810. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Sofuoglu M. The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse. 2009;35:12–17. doi: 10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: cause and consequence of oxidative stress. Crit Rev Neurobiol. 2005;17:87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. 2011;54:42–48. doi: 10.1016/j.neuroimage.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorick T, Nestor L, Miotto K, Sugar C, Hellemann G, Scanlon G, Rawson R, London ED. Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction. 105:1809–1818. doi: 10.1111/j.1360-0443.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.