Abstract
Tonic pain has been difficult to demonstrate in animals. Because relief of pain is rewarding, analgesic agents that are not rewarding in the absence of pain should become rewarding only when there is ongoing pain. We used conditioned place preference to concomitantly determine the presence of tonic pain in rats and the efficacy of agents that relieve it. This provides a new approach for investigating tonic pain in animals and for evaluating the analgesic effects of drugs.
Patients with neuropathic conditions commonly experience ongoing and/or paroxysmal spontaneous pain1–3. Although spontaneous pain is a common clinical complaint, and an important target for new drug development, most preclinical animal studies use hypersensitivity to evoked stimuli to evaluate new treatments4–6. Furthermore, drug modulation of evoked responses in humans does not always correlate with analgesic efficacy7. Consequently, reliance on reflex responses is a critical shortcoming of current animal models of neuropathic pain4–6.
Pain has both sensory and affective dimensions. The affective dimension (that is, unpleasantness)6,8 reflects pain’s aversive motivational power, which can serve as a teaching signal (punishment) that shapes subsequent behavior8. Conversely, relief of pain is rewarding (that is, elicits negative reinforcement9).
In animals, the validity of measures of spontaneous neuropathic pain is uncertain and controversial4–6. In fact, whether animals with experimental peripheral nerve injury experience spontaneous pain has not yet been definitively demonstrated. This makes it difficult to know whether mechanisms that mediate possible spontaneous pain are distinct from those mediating stimulus-evoked responses. We reasoned that manipulations that relieve spontaneous neuropathic pain will produce negative reinforcement and, consequently, contextual cues associated with pain relief should elicit approach following conditioning.
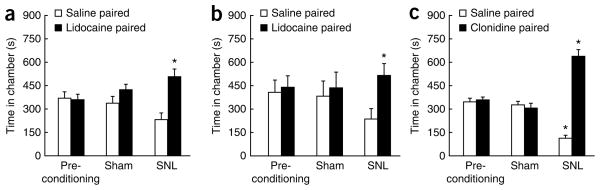
In rats with spinal nerve ligation (SNL), we tested whether drugs that have been proven to alleviate neuropathic pain in humans could reverse evoked tactile allodynia and concomitantly produce conditioned place preference (CPP) (see Supplementary Methods). On day 7 after surgery and before CPP conditioning, allodynia was confirmed and reversed by spinal clonidine or ω-conotoxin MVIIA (Fig. 1a,b). Following a 3-d pre-conditioning phase, rats underwent conditioning (6 d) using spinal drug or saline administration paired with alternate chambers on alternate days. Rats were restricted to the appropriate chamber within 2 min of drug administration. On test day, rats were placed drug-free in the CPP boxes with access to all of the chambers. Only SNL rats increased the time that they spent in the drug-paired chambers (Fig. 1c,d). All groups spent equivalent time in the neutral chamber (data not shown). Neither drug produced preference in sham-operated rats, indicating that spinal administration of these drugs at these doses are not rewarding in the absence of nerve injury.
Figure 1.
Inhibition of spontaneous neuropathic pain and evoked hypersensitivity can be dissociated by spinally administered drugs. (a,b) Spinal clonidine (10 μg, a) or ω-conotoxin (250 ng, b) reversed SNL-induced allodynia. * P < 0.05 (two-factor repeated-measure ANOVA, Student-Neuman-Keuls post hoc test versus pre-drug values). BL, baseline. (c,d) Spinal clonidine (c) or ω-conotoxin (d) increased the time the rats spent in their paired chamber, with a corresponding decrease in the saline-paired chamber. * P < 0.05 (two-factor repeated-measures ANOVA, Bonferroni post hoc test versus pre-conditioning values). (e) Spinal adenosine (10 μg) reversed SNL-induced allodynia. * P < 0.05 as above. (f) SNL rats did not increase the time that they spent in the adenosine-paired chamber. Sham-operated rats showed no chamber preference and no pre-conditioning chamber preferences existed (c,d,f). For all of the CPP experiments, pre-conditioning data was analyzed using two-factor ANOVA (chambers versus treatment). Statistical analysis for chamber preference before conditioning revealed no difference in the time spent in chambers between sham-operated and SNL-treated rats (P > 0.05), therefore baseline chamber data was pooled for graphical representation. For all analyses, significance was set at P < 0.05. All graphs represent mean ± s.e.m. (n = 6). All procedures involving rats were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Arizona and were in accordance with the US National Institutes of Health guidelines.
In humans with neuropathic pain, spinal adenosine blocks secondary hyperalgesia (measured with evoked stimuli) while having no effect on overall pain7. Similarly, we found that spinal adenosine reversed SNL-induced tactile allodynia (Fig. 1e), but failed to produce CPP in either SNL or sham-operated rats (Fig. 1f).
In rats, nerve injury–induced evoked hypersensitivity is dependent on descending facilitation from the rostral ventromedial medulla (RVM)10. Whether such descending influences modulate tonic spontaneous pain is unknown. RVM lidocaine induced CPP in SNL rats, but not in sham-operated rats (Fig. 2a). In fact, a single trial with RVM lidocaine induced CPP in SNL rats. Following preconditioning, rats received a morning RVM injection of saline paired with one chamber. The rats received RVM lidocaine paired with the other chamber 4 h later. RVM lidocaine selectively induced CPP in SNL rats (Fig. 2b). To the best of our knowledge, this is the first direct evidence that descending facilitation from the RVM can amplify tonic neuropathic pain. Single-trial conditioning with spinal clonidine also induced CPP in SNL rats (Fig. 2c).
Figure 2.
Repeated or single trial conditioning provides negative reinforcement and unmasks spontaneous neuropathic pain. (a) RVM lidocaine (4%, wt/vol, 0.5 μl) induced chamber preference in SNL rats. * P < 0.05 (two-factor repeated ANOVA, Bonferroni post hoc test versus pre-conditioning value). (b) Single-trial conditioning with RVM lidocaine produced chamber preference in SNL rats. (c) Single trial conditioning with spinal clonidine (10 μg) increased the time spent in the paired chamber of rats with SNL, but not sham-operated rats, with a corresponding decrease in the saline-paired chamber. Graphs represent mean ± s.e.m. (n = 6).
To examine the generality of this effect, we studied tonic pain with the spared nerve injury (SNI) model. Single-trial RVM lidocaine induced CPP (Fig. 3a) and reversed tactile allodynia (Fig. 3b) in SNI rats, but not sham-operated, rats.
Figure 3.
Spontaneous neuropathic pain requires descending modulation. (a) Single trial conditioning with RVM lidocaine selectively induced chamber preference in SNI rats. * P < 0.05 as above. (b) RVM lidocaine reversed SNI-induced allodynia. * P < 0.05 (two-factor repeated ANOVA, Student-Neuman-Keuls versus pre-drug value). Graphs represent mean ± s.e.m. (n = 6).
Pain is defined as a subjective experience6,8 and, consequently, animal studies of pain must be indirect. Currently, most pain assessment methods in animals use stimulus-evoked reflex responses4–6. However, clinical pains, especially chronic, difficult to treat pains, are both ongoing and spontaneous; that is, there is no temporally discrete objective stimulus that can be used to correlate intensity of input with behavioral response. The utility of stimulus-evoked pain as a predictor of analgesic efficacy is also called into question by the fact that some drugs that are not effective analgesics in humans can alleviate hypersensitivity.
The reduction of an aversive state (pain relief) provides a teaching signal termed negative reinforcement8,9. Accordingly, animals should approach contextual cues (for example, location) previously associated with pain relief. Here, we achieved CPP selectively in nerve-injured rats by blockade of descending pain modulatory circuits or by direct modulation of spinal cord neurons. These manipulations did not produce CPP in normal rats. The relief of an aversive state by manipulations that are not otherwise rewarding provides, to the best of our knowledge, the first evidence that widely used animal models of neuropathic pain have a tonic component. This approach may have greater predictive power than current methods used to assess the therapeutic potential of new analgesic agents.
The value of our approach is supported by the results of our experiments using spinal adenosine; consistent with human observations7, blockade of evoked responses did not predict production of CPP, suggesting that evoked hypersensitivity can be blocked by manipulations that are not effective for ongoing pain7,11. This result also indicates that reduction of pain possibly evoked by ambulation in the testing chamber does not produce substantial negative reinforcement.
Blockade of RVM activity produced CPP in two nerve-injury models, indicating that descending pain facilitatory pathways modulate both evoked hypersensitivity and injury-induced spontaneous tonic pain. The fact that RVM lidocaine–induced CPP in rats with SNI is of particular interest, as SNL and SNI elicit different behavioral phenotypes10,12. Although SNI animals show tactile, but not thermal, hypersensitivity12, SNL rats show both10, suggesting that there are possible differences in the mechanisms underlying evoked hypersensitivity. In contrast, mechanisms driving tonic pain may be similar in the two models.
In contrast with our results, SNL has been shown to reduce CPP produced by morphine and mu opioid receptor selective agonists13. Although this seems paradoxical, this previous study13 demonstrated that the functioning of reward circuitry is impaired in rodents with nerve injury. Morphine reward depends on activating dopamine neurons in the ventral tegmental area (VTA). Conversely, noxious input inhibits dopamine neurons14 and the ability of morphine to activate VTA dopamine neurons is impaired in rodents with SNL. This raises the possibility that relief of pain would activate VTA dopamine neurons. A substantial number of VTA dopamine neurons project to both the hypothalamus and amygdala, which in turn project directly to the peri-aqueductal gray, providing a robust connection between reward- and pain-modulating circuitry15.
Although spontaneous pain is one of the most important complaints of a majority of neuropathic pain patients1,4, the mechanisms mediating this aspect of pain are largely unknown. Knowledge of the mechanisms that are relevant to this condition could substantially increase translation of potentially relevant molecular targets into new therapies. Demonstration that a molecular target modulates spontaneous neuropathic pain will increase the probability that it is relevant for human analgesic development, regardless of whether this target shows activity against evoked tactile stimuli. The observation that a molecular target effective for evoked pain fails to modulate spontaneous neuropathic pain would also inform the decision to advance a candidate drug to human studies and would diminish the number of unsuccessful human clinical trials.
Supplementary Material
Footnotes
Supplementary information is available on the Nature Neuroscience website.
AUTHOR CONTRIBUTIONS
T.K. was partly responsible for design, data analysis, interpretation, writing and editing of the manuscript and figures. L.V.-P. was partly responsible for data collection and analysis. T.G. contributed to the SNI experiments. T.W.V., G.D. and J.L. provided conceptual input to the design and interpretation of the experiments. H.L.F. and F.P. jointly conceived and designed the studies and contributed to the interpretation, writing and editing of the manuscript.
Reprints and permissions information is available online at http://www.nature.com/reprintsandpermissions/.
References
- 1.Backonja MM, Stacey B. J Pain. 2004;5:491–497. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Boulton AJM. Clin Diabetes. 2005;23:9–15. [Google Scholar]
- 3.Rowbotham MC. Neurology. 2005;65:S66–S73. doi: 10.1212/wnl.65.12_suppl_4.s66. [DOI] [PubMed] [Google Scholar]
- 4.Campbell JN, Meyer RA. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rice AS, et al. Pain. 2008;139:243–247. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Vierck CJ, Hansson PT, Yezierski RP. Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Eisenach JC, Rauck RL, Curry R. Pain. 2003;105:65–70. doi: 10.1016/s0304-3959(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 8.Johansen JP, Fields HL, Manning BH. Proc Natl Acad Sci USA. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skinner BF. The Behavior of Organisms. Appleton Century Crofts; New York: 1938. [Google Scholar]
- 10.Burgess SE, et al. J Neurosci. 2002;22:5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC. Anesthesiology. 2007;106:312–322. doi: 10.1097/00000542-200702000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Decosterd I, Woolf CJ. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 13.Niikura K, et al. Neurosci Lett. 2008;435:257–262. doi: 10.1016/j.neulet.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 14.Ungless MA, Magill PJ, Bolam JP. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- 15.Fields H. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.