Abstract
Background
Pulmonary arterial hypertension (PAH) is a progressive disease which causes exercise limitation, heart failure, and death. We aimed to determine the safety and efficacy of aspirin and simvastatin in PAH.
Methods and Results
We performed a randomized, double-blind, placebo-controlled 2 × 2 factorial clinical trial of aspirin and simvastatin in patients with PAH receiving background therapy at four centers. A total of 92 patients with PAH were to be randomized to aspirin 81 mg or matching placebo and simvastatin 40 mg or matching placebo. The primary outcome was six-minute walk distance (6MWD) at six months. Sixty-five subjects were randomized when the trial was terminated by the DSMB after an interim analysis showed futility in reaching the primary end point for simvastatin. After adjustment for baseline 6MWD, there was no significant difference in the 6MWD at six months between aspirin (n = 32) and placebo (n = 33) [placebo-corrected difference = −0.5 m (95%CI, −28.4 – 27.4 m), p = 0.97] or between simvastatin (n = 32) and placebo (n = 33) [placebo-corrected difference = −27.6 m (95%CI, −59.6 – 4.3 m), p = 0.09]. There tended to be more major bleeding episodes with aspirin compared to placebo (4 events vs. 1 event, respectively, p = 0.17).
Conclusions
Neither aspirin nor simvastatin had a significant effect on the 6MWD, although patients randomized to simvastatin tended to have a lower 6MWD at six months. These results do not support the routine treatment of patients with PAH with these medications.
Keywords: pulmonary hypertension, clinical trial, anti-platelet agents, endothelial dysfunction
Pulmonary arterial hypertension (PAH) includes idiopathic (IPAH) and heritable forms, as well as PAH associated with connective tissue disease, portal hypertension, anorexigen use, HIV infection, congenital systemic-to-pulmonary shunts, and other conditions. In PAH, the small muscular pulmonary arteries show endothelial proliferation and smooth muscle hypertrophy, in situ thrombosis, and plexiform lesions. Right ventricular failure ensues, leading to exercise limitation and death.
Reduced prostacyclin (PGI2) production, elevated endothelin-1 (ET-1) levels, and deficits in nitric oxide (NO) are seen in PAH and have guided therapeutic development. Platelet activation, endothelial nitric oxide synthase (eNOS) dysfunction, oxidative stress, and inflammation are also present, but these mechanisms have not been specifically targeted. Activated platelets produce thromboxane (Tx) A2 which causes platelet aggregation, vasoconstriction, and vascular smooth muscle hypertrophy. Patients with PAH have increased TxA2 (and TxB2) production and decreased PGI2 production resulting in an increased Tx:PGI2 ratio, even with treatment.1–5 Clinical trials of intravenous epoprostenol6 and other prostacyclin analogs which are efficacious in PAH7–9 were predicated on the inference that low endogenous PGI2 (and increased Tx:PGI2 ratio) is detrimental in PAH. Investigators have visualized circulating platelet aggregates in the blood of patients with PAH,10 and increased platelet aggregation is associated with more severe PAH.11 Soluble P-selectin is elevated in patients with PAH, and platelet-released CD40 ligand increases across the pulmonary vascular bed suggesting trans-pulmonary platelet activation.12, 13 Thrombocytopenia due to platelet trapping in the lungs occurs during PAH “crises”14, 15 and is associated with worse hemodynamics.16 Aspirin lowers the Tx:PGI2 ratio in PAH (even in patients chronically treated with PGI2 analogues or other medications) and inhibits platelet activity,2 offering a potential therapeutic approach. A recent study showed that aspirin decreased pulmonary artery pressure, reduced right ventricular hypertrophy, and improved survival in the monocrotaline animal model of pulmonary hypertension.17
Reductions in NO production and eNOS activity and increases in oxidative stress contribute to endothelial dysfunction in patients with PAH.18, 19 Statins inhibit proliferation, induce apoptosis, and cause vasorelaxation in vascular smooth muscle cells, enhance expression and activity of eNOS in endothelium, and reduce oxidative stress and inflammation.20–24 Several studies have demonstrated the efficacy of statins in animal models of pulmonary hypertension.25–32 Uncontrolled human studies of simvastatin have also suggested benefit in patients with PAH.33, 34 One recently-published randomized clinical trial (RCT) showed that simvastatin decreased right ventricular mass and plasma N-terminus pro-brain natriuretic peptide (NT-proBNP), but had no effect on six-minute walk distance (6MWD) in patients with PAH.35
Aspirin and simvastatin have potential for the treatment of PAH by targeting platelet activation and endothelial dysfunction. We aimed to show the feasibility of studying these therapies and to get estimates of efficacy and safety in a Phase II RCT. We hypothesized that the 6MWD at six months would be greater in subjects with PAH assigned to aspirin or simvastatin than to the respective placebo after adjustment for baseline 6MWD.
Methods
Study Design
ASA-STAT was a four-center, randomized, double-blind, placebo-controlled 2 × 2 factorial study to determine the efficacy and safety of aspirin and simvastatin in patients with PAH. Details of the methods have been published elsewhere.36 (See Supplemental Material.) The initial protocol called for the recruitment of 128 subjects with PAH (anticipating 100 completers) over approximately three years. The first patient was randomized in January 2007 and a total of 65 were randomized by September 2009, when the study was terminated (See below).
The trial protocol was approved by the institutional review board at each participating center and the Data Safety and Monitoring Board (DSMB). The trial was registered at clinicaltrials.gov before initiating recruitment (NCT00384865).
Study Participants
We included patients > 18 years of age with PAH without an indication for aspirin or statin therapy and without risk factors for adverse events from these medications (Table E1, Supplemental Material). Participants were recruited from four pulmonary vascular disease clinics (Columbia University College of Physicians and Surgeons, New York City, NY; Johns Hopkins University, Baltimore, MD; Tufts Medical Center, Boston, MA; University of Pennsylvania School of Medicine, Philadelphia, PA). All participants provided written informed consent.
Study Procedures
Patients were identified by medical staff. Subjects were randomly assigned in a 1:1:1:1 ratio by a Web-based computerized system to enteric coated aspirin 81 mg (Tiny Tablets, Bayer HealthCare LLC, Morristown, NJ) once daily/simvastatin 40 mg (Zocor, Merck & Co., Whitehouse Station, NJ) once daily, aspirin 81 mg once daily/simvastatin placebo once daily, aspirin placebo once daily/simvastatin 40 mg once daily, or aspirin placebo once daily/simvastatin placebo once daily. The randomization scheme was random permuted block, stratified by type of PAH (idiopathic/heritable vs. other) and center. All subjects and study personnel (other than the Data Coordinating Center (DCC) Chair and research pharmacy) were masked to treatment assignment and were not unmasked until the study was completed. Subjects were evaluated at baseline, six weeks, three months, and six months.36
The primary outcome was the 6MWD at six months after randomization after adjustment for the baseline 6MWD. Secondary outcomes included 6MWD, measures of platelet activation (serum TxB2, plasma β-thromboglobulin (β-TG), and soluble P-selectin levels) and endothelial function (brachial artery flow-mediated dilation (FMD) and plasma von Willebrand factor (vWF) levels), NT-proBNP levels, C-reactive protein (CRP) levels, oxidized low-density lipoprotein levels, WHO functional class, Borg dyspnea score, and Short Form-36 (SF-36) scores at six weeks, three months and six months after randomization. We assessed time to clinical worsening (defined by the addition of new PAH therapies or dose increases in previously stable PAH therapy, hospitalization for right-sided heart failure, lung transplantation, atrial septostomy, and cardiovascular and all-cause death). End point and bleeding definitions and details of end point assessments are provided in the Supplemental Material and have been published.36
Study Monitoring
Details of study monitoring are provided in the Supplemental Material.36 The enrollment period lasted from January 2007 to September 2009. In July 2009, the DSMB requested a conditional power calculation by the DCC using the subjects enrolled to that point. This showed that the trial had a 4.9% chance of detecting a statistically significantly higher 6MWD for simvastatin vs. placebo. The DSMB determined that continuing the study with only the aspirin vs. placebo comparison would lead to a study with low scientific credibility and recommended study termination. The DSMB recommended that subjects currently active in the trial at that time (N = 16) should discontinue receiving all study drugs and should not be followed or undergo any additional study procedures. The DSMB reported that there were no significant safety concerns related to the recommendation for termination. The NHLBI accepted these recommendations, and active subjects stopped study medications and did not return to the Field Centers or have telephone follow-up after September 25, 2009.
Statistical Analysis
The primary end point of the trial was the 6MWD at six months after randomization after adjustment for the baseline 6MWD. Preliminary data suggested that a difference of > 50 m in 6MWD was associated with an improvement in symptoms and survival. A total of 100 subjects (25 in each of the four randomized groups, 50 in each active drug and placebo group) was necessary to detect a difference of 57 m with 80% power assuming no significant interaction between study drugs and without adjustment for the baseline 6MWD. With a significant interaction between study medications, we had 80% power to detect a difference of 80 m. Anticipating 20% attrition, we initially planned for the enrollment of 128 subjects. In May 2009, the DSMB requested revised sample size calculations (See Supplemental Material). The revised sample size of 92 was approved by the DSMB and NHLBI on June 4, 2009.
The primary analysis proceeded according to the intent-to-treat principle. Hypothesis testing for the primary and secondary end points was conducted using two-sided α = 0.05. There was no observed clinically relevant statistical interaction between the study medications (See Protocol),36 so all analyses proceeded “at the margins” by comparing patients assigned to aspirin to those who were assigned to aspirin placebo and comparing those assigned to simvastatin compared to those who were assigned to simvastatin placebo.
Continuous variables are presented as mean ± standard deviation or median (interquartile range (IQR)) and categorical variables as n (%). Skewed variables were natural log transformed before analysis. The analysis of the primary end point compared the absolute 6MWD at six month follow-up between active therapy and placebo groups while adjusting for the baseline 6MWD using linear regression models. Multiple imputation was performed for subjects without a six month 6MWD in the primary analysis (See Supplemental Material for details). Secondary analyses incorporated all of the available end point assessments (six weeks, three months, and six months) without imputation in linear mixed-effects models with adjustment for baseline values. There were no interim analyses or stopping rules planned a priori for the trial. Other analyses are described in the Supplemental Material.36
Results
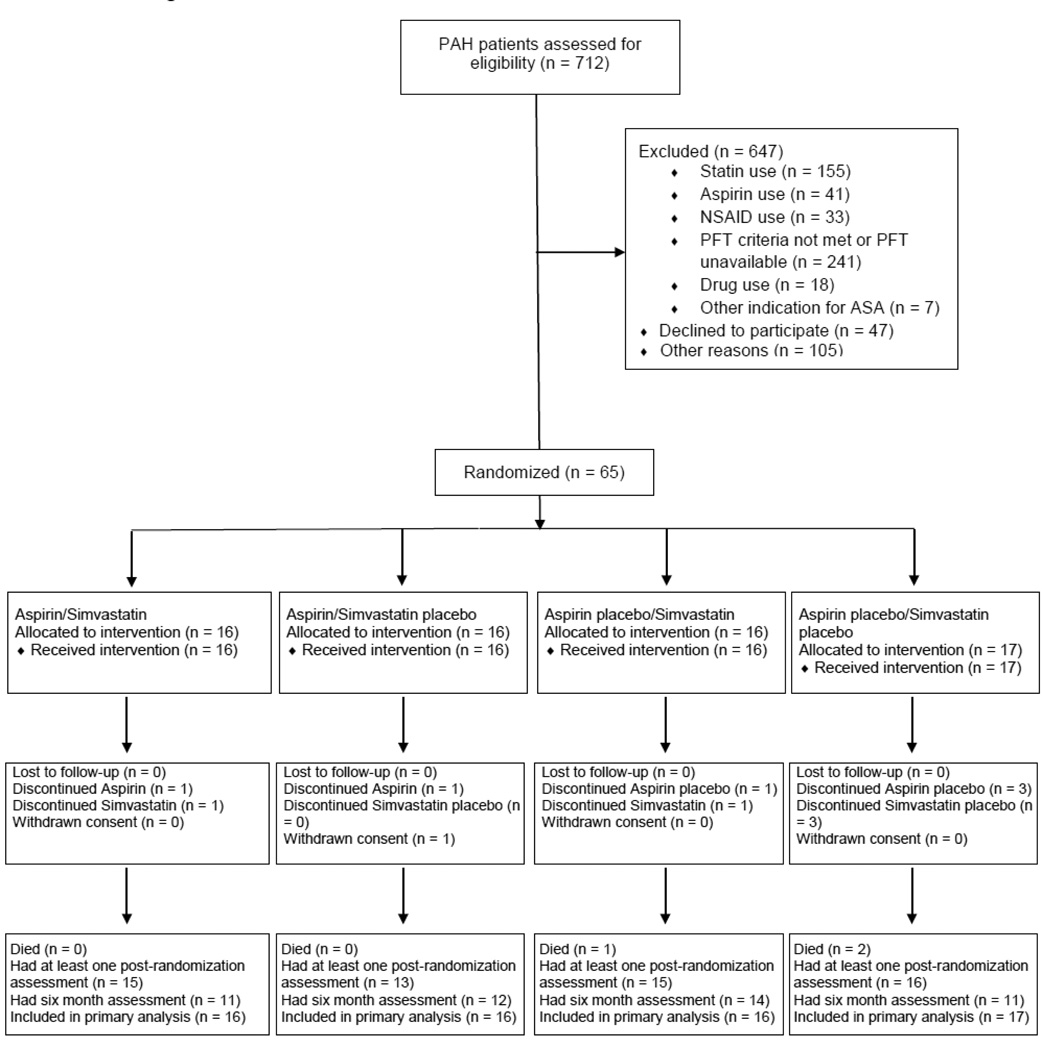
We screened 712 PAH patients during the enrollment period from January 2007 to September 25, 2009. Of the adjusted target sample of 92, 65 were randomized and 49 had completed the six month assessment at study termination (3 died, 1 withdrew consent, and 12 were active in the trial and had not yet had their six month study visits) (Figure 1). One subject completed the study but was unable to perform the walk at the six month assessment. At study termination, 59 of the 65 randomized subjects had undergone at least one outcome assessment.
Figure 1.
Flow diagram
The mean age of the participants was 50.5 ± 13.9 yrs and 56 (86.1%) were female. Thirty-nine (60.0 %) were non-Hispanic white, 9 (13.9%) were Hispanic, 13 (20.0%) were black, and 3 (4.6%) were Asian. Thirty-three (51.6%) had idiopathic PAH, 3 (4.7%) had heritable PAH, 12 (18.8%) had PAH associated with systemic sclerosis, 9 (15.3%) had PAH associated with other connective tissue diseases, and 6 (9.2%) had congenital systemic-to-pulmonary shunts. Those enrolled were somewhat younger, but similar in terms of gender and race, to those screened and not enrolled (Table E2, Supplemental Material).
Aspirin vs placebo
Thirty-two subjects were randomized to aspirin and 33 were randomized to placebo (Table 1). The two groups were similar in terms of demographics. Subjects randomized to aspirin had a somewhat higher prevalence of idiopathic/heritable PAH than other forms, more frequent use of sildenafil, somewhat lower WHO functional class, and somewhat higher baseline 6MWD. Combination therapy was common, and most patients were treated with warfarin. Six subjects discontinued the aspirin study drug (2 aspirin, 4 placebo) (Figure 1). One participant was started on non-study aspirin after she was diagnosed with coronary artery disease. One patient stopped aspirin study drug for epigastric pain, one had a decrease in hemoglobin without clinical bleeding, one had a new gastic ulcer with bleeding, one had light-headedness, and one without providing a reason. Compliance with the study drug was > 95%.
Table 1.
Baseline characteristics of subjects randomized to aspirin and placebo
| Placebo (n = 33) |
Aspirin (n = 32) |
||
|---|---|---|---|
| Age,yrs | 51.7 ± 13.0 | 49.2 ± 14.7 | |
| Gender, Female | 30 (90.9) | 26 (81.2) | |
| Body mass index, kg/m2 | 28.6 ± 7.8 | 27.4 ± 6.2 | |
| Race/ethnicity | |||
| White (Non-Hispanic) | 17 (51.5) | 22 (68.8) | |
| Hispanic or Latino | 6 (18.2) | 3 (9.4) | |
| Black | 8 (24.2) | 5 (15.6) | |
| Asian | 2 (6.1) | 1 (3.1) | |
| Other | 0 | 1 (3.1) | |
| PAH diagnosis | |||
| Idiopathic | 14 (42.4) | 19 (61.3) | |
| Heritable | 2 (6.1) | 1 (6.2) | |
| Congenital systemic-to-pulmonary shunt | 4 (12.1) | 2 (6.3) | |
| Systemic sclerosis | 8 (24.2) | 4 (12.9) | |
| Other connective tissue disease | 5 (15.1) | 4 (12.5) | |
| Drugs/toxins | 0 | 2 (6.5) | |
| Right ventricular systolic pressure by echocardiography |
(n=57) | 69.5 (53–91) | 70.0 (56–87) |
| Concomitant medications | |||
| Ambrisentan | 8 (24.2) | 10 (31.3) | |
| Bosentan | 12 (36.4) | 6 (18.8) | |
| Epoprostenol | 8 (24.2) | 7 (21.9) | |
| Iloprost (inhaled) | 4 (12.1) | 5 (15.6) | |
| Sildenafil | 16 (48.5) | 26 (81.3) | |
| Treprostinil (intravenous) | 3 (9.1) | 2 (6.3) | |
| Combination therapy | 17 (51.5) | 23 (71.9) | |
| Warfarin | 28 (84.8) | 23 (71.9) | |
| WHO functional class | |||
| Class I | 4 (12.1) | 1 (3.1) | |
| Class II | 17 (51.5) | 24 (75) | |
| Class III | 12 (36.4) | 7 (21.9) | |
| Six minute walk distance, m | 418.3 ± 131.7 | 447.3 ± 96.1 | |
| Post-test Borg dyspnea score | 3 (2–3) | 3 (2–3) | |
Data shown as mean ± standard deviation, median (interquartile range), or n (%).
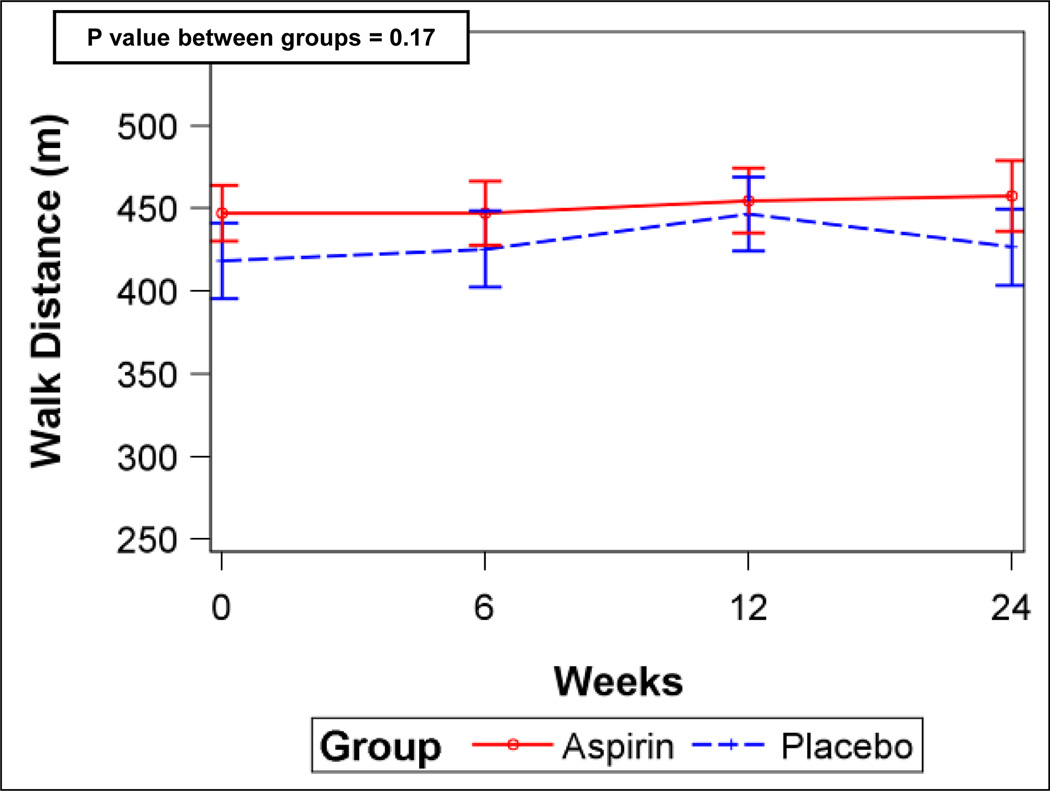
There was no difference in 6MWD between aspirin and placebo groups at six months after adjustment for baseline 6MWD [least squares means, 438.0 m (95%CI, 415.9–460.1 m) vs. 438.5 m (95%CI, 419.2–457.8 m), respectively, p = 0.97] (n = 65). The placebo-corrected difference in 6MWD at six months was −0.5 m (95%CI, −28.4– 27.4 m). Median post-walk Borg dyspnea scores at six months were similar between the groups (aspirin, 3 (IQR, 2–4) vs placebo, 3 (IQR, 2–4), p = 0.39). Analyses including the 6MWD from all post-randomization visits without imputation after adjustment for baseline showed similar results (Figure 2A).
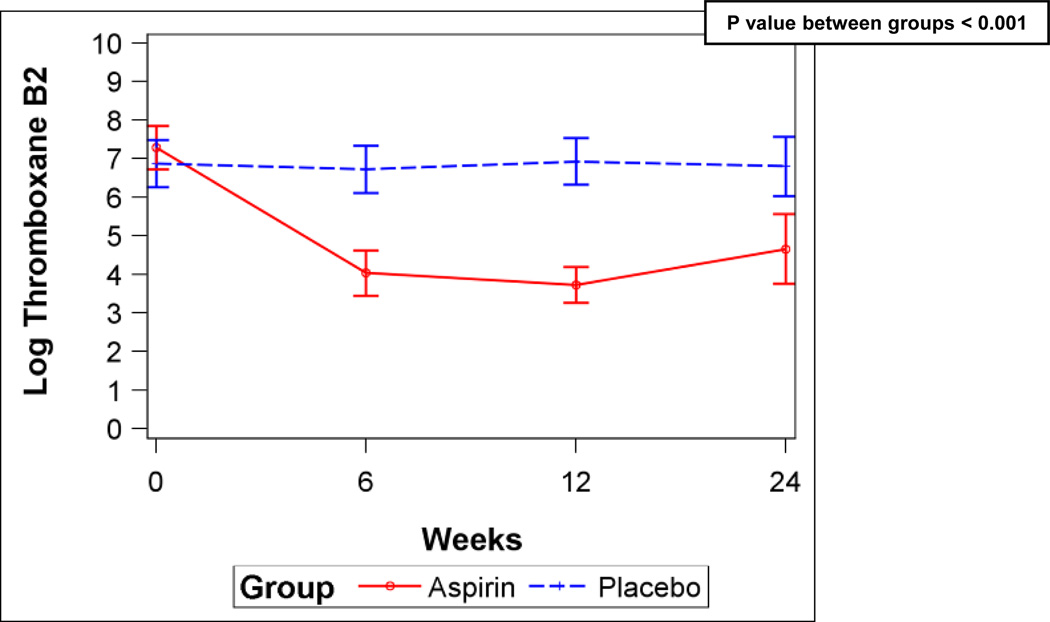
Figure 2.
A) Six-minute walk distance for aspirin and placebo (error bars are 95% confidence intervals). B) Serum ln (TxB2) levels for aspirin and placebo. P values from linear mixed-effects models.
Aspirin reduced serum TxB2 levels by 93% compared to placebo (Figure 2B), suggesting that the study drug had the expected effect on eicosanoid metabolism. However, there were no differences in soluble P-selectin, β-TG, or NT-proBNP levels between the groups (Figures E1A–C, Supplemental Material). There were no differences in any scales of the SF-36 (Table E3, Supplemental Material) or in WHO functional class (Data not shown).
All clinical worsening events are shown in Table E4 in the Supplemental Material. Subjects receiving aspirin had two clinical worsening events and subjects receiving placebo had six events (p = 0.19) There was no difference in time to clinical worsening between the groups (p = 0.35) (Figure E2, Supplemental Material).
We performed prespecified subset analyses in patients with baseline 6MWD < 450 m (n = 32); there were no differences in the results from the full sample (Data not shown). In patients with idiopathic or heritable PAH, we found that aspirin may have lowered soluble P-selectin levels (p = 0.07), but did not impact on the 6MWD (Figure E3, Supplemental Material). Other results were similar to those from the main analysis (Data not shown).
Adverse events in the aspirin and placebo groups are shown in Table 2. There were no differences in the numbers of minor (p = 0.87) bleeding episodes between the groups, but there may have been an increase in major bleeding episodes in subjects treated with aspirin (p = 0.17). There were no differences in safety laboratory results between the groups (Data not shown).
Table 2.
Frequency of adverse events of subjects randomized to aspirin and placebo
| Placebo (n = 33) |
Aspirin (n = 32) |
|
|---|---|---|
| Upper respiratory infection | 11 (33.3) | 10 (31.3) |
| Bruising | 8 (24.2) | 8 (25.0) |
| Myalgia | 5 (15.2) | 3 (9.4) |
| Headache | 3 (9.1) | 5 (15.6) |
| Other infections | 4 (12.1) | 4 (12.5) |
| Diarrhea | 4 (12.1) | 3 (9.4) |
| Dyspnea | 4 (12.1) | 2 (6.3) |
| Rash | 3 (9.1) | 3 (9.4) |
| Arthralgia | 3 (9.1) | 3 (9.4) |
| Chest pain | 3 (9.1) | 1 (3.1) |
| Increased transaminases | 3 (9.1) | 1 (3.1) |
| Insomnia | 2 (6.1) | 1 (3.1) |
| Number of bleeding episodes | ||
| Minor | 12 | 11 |
| Major | 1 | 4 |
Data shown as n (%).
Simvastatin vs placebo
Thirty-two subjects were randomized to simvastatin and 33 were randomized to placebo (Table 3). The two groups were similar in terms of demographics and type of PAH. Patients randomized to simvastatin had somewhat higher right ventricular systolic pressure by transthoracic echocardiography, but WHO functional class and 6MWD at baseline were similar between the groups. More than 60% of both groups were treated with sildenafil either as monotherapy or in combination therapy. Five subjects discontinued the simvastatin study drug (2 simvastatin, 3 placebo) (Figure 1). One participant was diagnosed with coronary artery disease and pravastatin was prescribed. Two patients discontinued for myalgias, one for headaches, and one without providing a reason. Compliance with the study drug was > 95%.
Table 3.
Baseline characteristics of subjects randomized to simvastatin and placebo
| Placebo (n = 33) |
Simvastatin (n = 32) |
||
|---|---|---|---|
| Age,yrs | 51.0 ± 13.6 | 50.0 ± 14.3 | |
| Gender, Female | 30 (90.9) | 26 (81.2) | |
| Body mass index, kg/m2 | 27.8 ± 6.5 | 28.1 ± 7.7 | |
| Race/ethnicity | |||
| White (Non-Hispanic) | 20 (60.6) | 19 (59.4) | |
| Hispanic or Latino | 2 (6.1) | 7 (21.9) | |
| Black | 8 (24.2) | 5 (15.6) | |
| Asian | 3 (9.1) | 0 | |
| Other | 0 | 1 (3.1) | |
| PAH diagnosis | |||
| Idiopathic | 17 (53.1) | 16 (50) | |
| Heritable | 1 (3.1) | 2 (6.3) | |
| Congenital systemic-to-pulmonary shunt | 1 (3) | 5 (15.6) | |
| Systemic sclerosis | 7 (21.2) | 5 (15.6) | |
| Other connective tissue disease | 5 (15.2) | 4 (12.5) | |
| Drugs/toxins | 2 (6.3) | 0 | |
| Right ventricular systolic pressure by echocardiography, mm Hg |
(n=57) | 63.5 (50–81) | 82.0 (59–100) |
| Concomitant medications | |||
| Ambrisentan | 10 (30.3) | 8 (25.0) | |
| Bosentan | 9 (27.3) | 9 (28.1) | |
| Epoprostenol | 6 (18.9) | 9 (28.1) | |
| Iloprost (inhaled) | 5 (15.2) | 4 (12.5) | |
| Sildenafil | 21 (63.6) | 21 (65.6) | |
| Treprostinil (intravenous) | 3 (9.1) | 2 (6.3) | |
| Combination therapy | 22 (66.7) | 18 (56.3) | |
| Warfarin | 23 (69.7) | 28 (87.5) | |
| WHO functional classification | |||
| Class I | 5 (15.2) | 0 | |
| Class II | 18 (54.6) | 23 (71.9) | |
| Class III | 10 (30.3) | 9 (28.1) | |
| Six minute walk distance, m | 442.0 ± 128.8 | 422.9 ± 101.4 | |
| Post-test Borg Dyspnea Score | 3 (2–3) | 3 (2–3) | |
Data shown as mean ± standard deviation, median (interquartile range), or n (%).
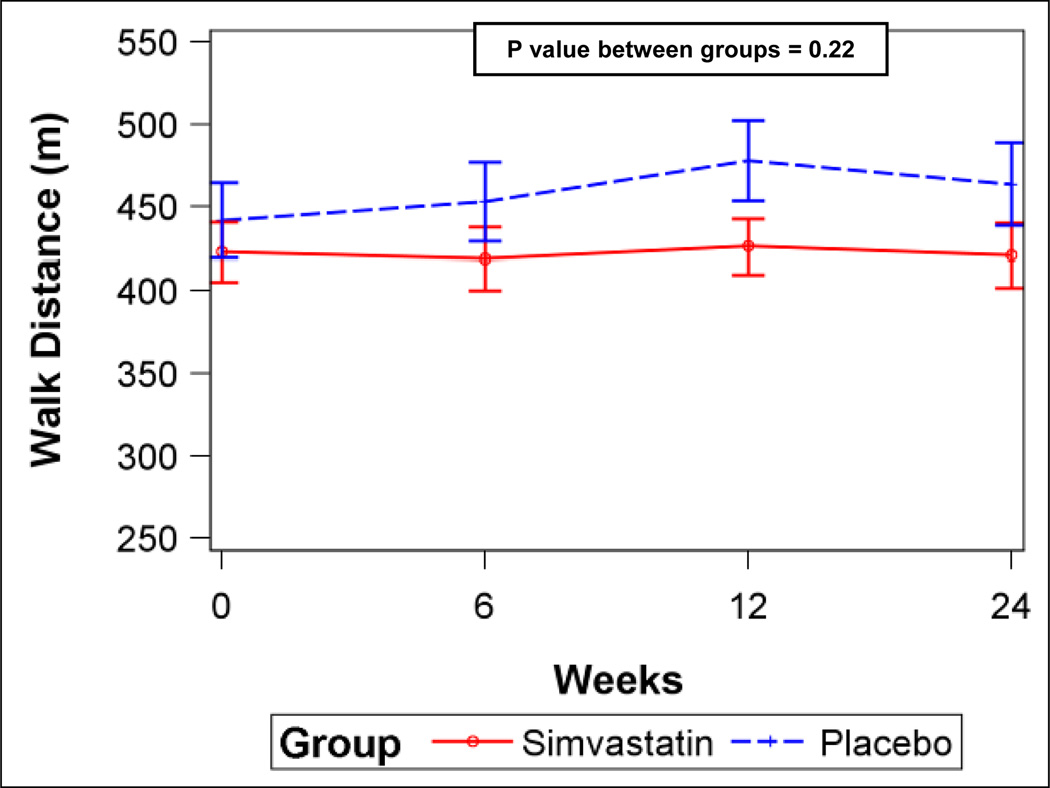
There was no difference in 6MWD between simvastatin and placebo groups at six months after adjustment for baseline 6MWD (least squares means, 425.0 m (95% CI, 400.2–449.9 m) vs 452.7 m (95% CI, 431.9 – 473.4 m), respectively, p = 0.09) (n = 65) with a placebo-corrected difference in 6MWD of −27.6 m (95%CI, −59.6–4.3 m), indicating that simvastatin may have reduced the 6MWD. Additionally, median post-walk Borg dyspnea scores at six months tended to be higher in the subjects assigned to simvastatin, suggesting greater breathlessness [simvastatin, 3 (IQR, 2–4) vs. placebo, 3 (IQR, 2–4) (p = 0.07)]. Analyses including the 6MWD from all post-randomization visits without imputation after adjustment for baseline 6MWD were also consistent with lower 6MWD in the group randomized to simvastatin (Figure 3A).
Figure 3.
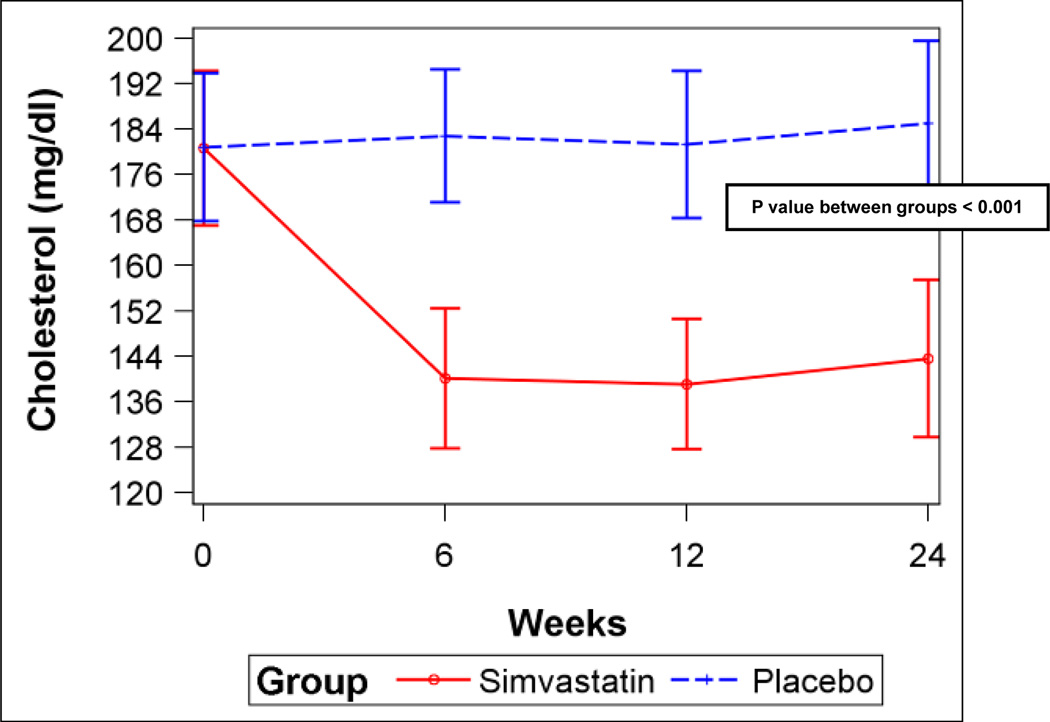
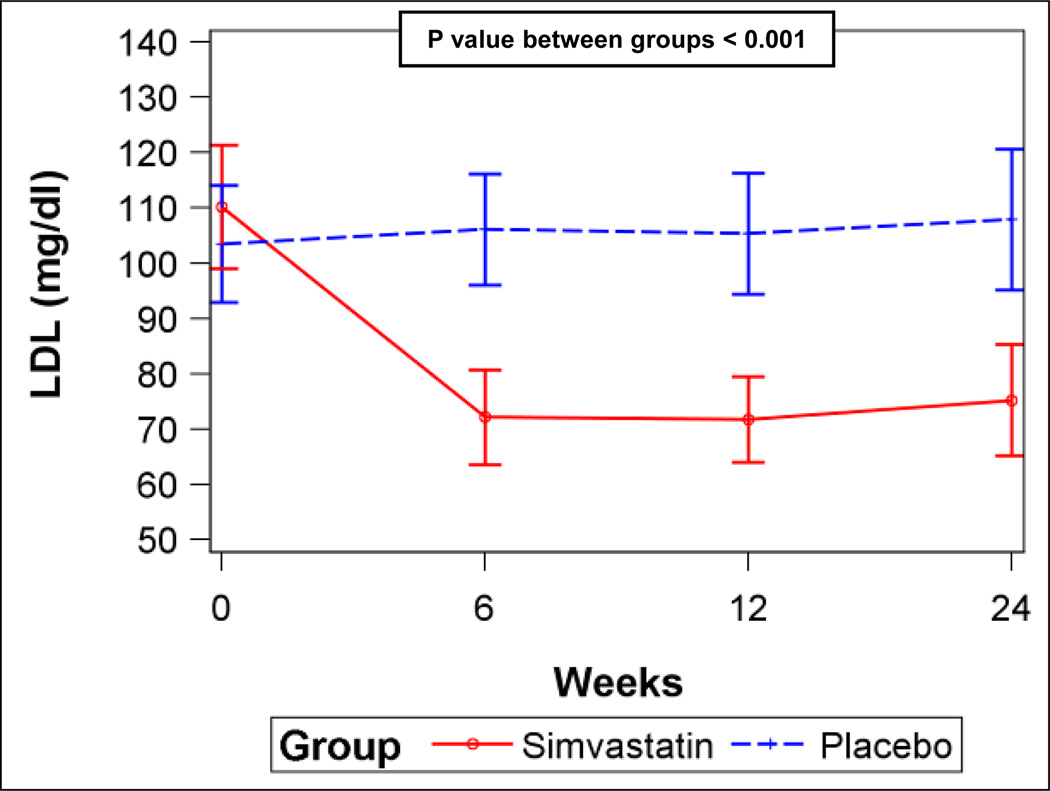
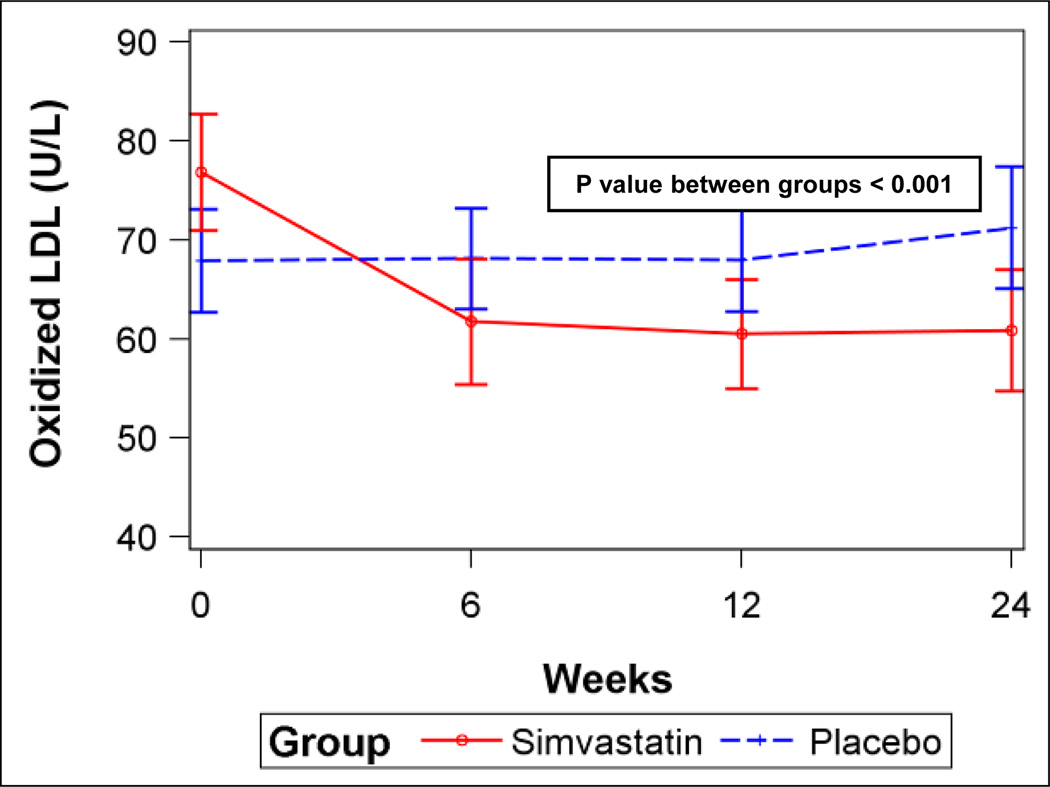
A) Six-minute walk distance for simvastatin and placebo (error bars are 95% confidence intervals). B) Serum total cholesterol levels for simvastatin and placebo. C) Serum low-density lipoprotein (LDL) levels for simvastatin and placebo. D) Plasma oxidized low-density lipoprotein (LDL) levels for simvastatin and placebo. P values from linear mixed-effects models.
Simvastatin significantly decreased total cholesterol, low-density lipoprotein (LDL), and oxidized LDL levels (Figures 3B–D) compared to placebo, but did not affect triglycerides or high-density lipoprotein (HDL) levels (Figures E4A–B, Supplemental Material), consistent with the expected effects. However, there were no differences in levels of vWF, CRP, or NT-proBNP or FMD between the groups (Figures E5A–D, Supplemental Material). There were no differences in any scales of the SF-36 (Table E5, Supplemental Material) or in WHO functional class (Data not shown).
All clinical worsening events are shown in Table E6 in the Supplemental Material. Subjects receiving simvastatin and subjects receiving placebo each had four events (p = 0.33). There was no difference in time to clinical worsening between the groups (p = 0.80) (Figure E6, Supplemental Material).
We performed prespecified subset analyses in patients with baseline 6MWD < 450 m (n = 32); there were no differences in the results from the full sample (Data not shown). In patients with idiopathic or heritable PAH, we found that simvastatin did not impact on the 6MWD (Figure E7, Supplemental Material). Other results were similar to those from the main analysis (Data not shown).
Adverse events in the simvastatin and simvastatin placebo groups are shown in Table 4. There was no difference in adverse events between the groups. There were no differences in safety laboratory results between the groups (Data not shown).
Table 4.
Frequency of adverse events of subjects randomized to simvastatin and placebo
| Placebo (n = 33) |
Simvastatin (n = 32) |
|
|---|---|---|
| Bleeding | 13 (39.4) | 14 (43.8) |
| Upper respiratory infection | 9 (27.3) | 12 (37.5) |
| Bruising | 6 (18.2) | 10 (31.3) |
| Myalgia | 5 (15.2) | 3 (9.4) |
| Headache | 3 (9.1) | 5 (15.6) |
| Other infections | 4 (12.1) | 4 (12.5) |
| Diarrhea | 4 (12.1) | 3 (9.4) |
| Dyspnea | 3 (9.1) | 3 (9.4) |
| Rash | 2 (6.1) | 4 (12.5) |
| Arthralgia | 2 (6.1) | 4 (12.5) |
| Chest pain | 3 (9.1) | 1 (3.1) |
| Increased transaminases | 2 (6.1) | 2 (6.3) |
| Insomnia | 2 (6.1) | 1 (3.1) |
Data shown as n (%).
Discussion
This is the first RCT of traditional cardiovascular disease therapies targeting platelet and endothelial function and the first NIH-funded RCT in patients with PAH. Neither aspirin nor simvastatin had a significant effect on the primary end point of 6MWD at six months. If anything, 6MWD may have decreased and post-walk Borg dyspnea scores increased with simvastatin. While aspirin lowered serum TxB2 levels, other traditional markers of platelet activation such as soluble P-selectin levels (except possibly in idiopathic/heritable PAH) and β-TG were not altered. There was a possible increased risk of major bleeding associated with aspirin use. While simvastatin lowered total cholesterol and LDL levels, there was no effect on other vascular or plasma markers of endothelial dysfunction or injury (FMD and vWF) or CRP. Simvastatin significantly lowered oxidized LDL levels, which reflect oxidative stress. Neither aspirin nor simvastatin affected NT-proBNP levels. The effects of aspirin and simvastatin on serum TxB2 and lipid levels respectively provided evidence that subjects were compliant with the study medication and that the medications had their traditional effects in patients with PAH. Early termination of the trial for futility in detecting a positive effect of simvastatin on the 6MWD limited the power of some analyses. However, none of the point estimates for the end points suggested benefit, and the precision of the estimates of 6MWD was good, making it less likely that low power accounted for the results.
Aspirin reduced production of Tx both in the current study and in our previous study in idiopathic PAH, in which platelet aggregation was also significantly inhibited by low-dose aspirin.2 Since Tx:PGI2 ratio is elevated in PAH and platelet aggregation is thought to contribute to disease pathogenesis, it was hypothesized that platelet inhibition with aspirin would translate to clinical benefit in terms of exercise capacity. While aspirin suppressed production of TxB2 by > 90%, there was no effect on 6MWD, NT-proBNP, or quality-of-life. This suggests that platelet Tx production does not contribute significantly to disease manifestations in PAH. One previous trial of a Tx synthase inhibitor was performed in PAH,37 but it was terminated prematurely due to an excess of side effects (leg pain) and did not show a clinical benefit.
While aspirin did lower serum TxB2, the effect was somewhat less than the 97–99% TxB2 suppression usually seen with aspirin.38 This suggests either aspirin resistance or another source of Tx unaffected by low dose aspirin (e.g., vascular endothelium). The lack of change of β-TG levels and borderline reductions in soluble P-selectin levels are also consistent with aspirin resistance. Ongoing vascular injury could also account for the smaller impact of aspirin on soluble P-selectin levels compared to that seen in normals.
There were no differences in health-related quality-of-life with aspirin. There tended to be fewer clinical worsening events in the aspirin group, but there was also a somewhat higher incidence of major bleeding events. We were unable to assess the interaction of aspirin with warfarin due to the small number of events.
Simvastatin has a significant effect on pulmonary vascular disease and RV changes in a variety of animal models. We have shown that statins impact on pulmonary hypertension and vascular remodeling in the chronically hypoxic rat model.25, 31, 39 Other studies have reproduced these findings in the monocrotaline animal model, but not universally.26, 40, 41 There have been two previous small RCTs of statins in patients with PAH. One studied the effect of rosuvastatin in patients with PAH which was idiopathic or associated with congenital heart disease.42 There were no statin-associated differences in 6MWD or other biomarkers. Wilkins et al. performed a placebo-controlled, double-blind RCT of simvastatin in 43 patients with PAH which was idiopathic, heritable, or associated with atrial septal defect or connective tissue disease.35 Simvastatin significantly reduced right ventricular mass and plasma NT-proBNP at six months, changes which were not maintained at one year. Notably, there was no effect of simvastatin on 6MWD, other RV parameters, quality-of-life, or other biomarkers.
In the current study, simvastatin did not improve 6MWD, biomarkers of endothelial dysfunction and injury, or quality-of-life. If anything, there were trends toward decreased 6MWD and more dyspnea in patients randomized to simvastatin. These results suggest that simvastatin is not effective as “add-on” therapy in PAH. Systemic side effects, such as myalgia and arthralgia, could have impacted on the measured response to the study medication. However, an increasing Borg dyspnea score after the six-minute walk suggested respiratory limitation.
Studying PAH patients receiving background therapy makes it more difficult to conduct trials of new therapies focused on clinically meaningful end points within a reasonable period of time. Since patients in clinical trials much receive the “standard-of-care”, this is the only ethically justifiable approach to intermediate or long-term placebo-controlled trials in the era of available effective therapy.
Our study has several limitations. The primary end point which should be used in early-stage clinical trials in PAH is uncertain. We chose the 6MWD because it is the one upon which all PAH therapies have been approved; an effective treatment should show some signal in terms of this measure. Available therapies for PAH which increase the 6MWD often improve long-term clinical outcomes, although 6MWD has not been validated as a surrogate end point.43 However, it remains possible that the active treatment had some favorable effect that went undetected. The development and validation of surrogate end points which change over a short period of time and reliably predict clinically meaningful outcomes is an unmet need in the field.
We did not include invasive hemodynamic end points. Measuring pulmonary hemodynamics as a secondary outcome would have been of interest, but for pragmatic reasons, could not be done. The requirement for multiple right heart catheterizations on otherwise stable, treated patients would have rendered the recruitment much more difficult and the study much more expensive, making the trial infeasible. While there is no specific biomarker “read-out” for pulmonary vascular function, the absence of any effect of the study drugs on NT-proBNP suggests that there was no impact on hemodynamics. Whether either agent had other long-term benefits beyond six months was not assessed.
This study was terminated due to a high likelihood of not rejecting the null hypothesis for the simvastatin arm even if fully recruited. Subjects were not followed or reassessed after study termination, leading to missing data for those active subjects in the trial at termination (n = 16). These missing data may have introduced bias, even though such data should be “missing completely at random”. The results and conclusions were similar whether we multiply imputed missing data or performed analyses with only the available data, making significant bias less likely. While even effective drugs may have reduced impact in the setting of background therapy in PAH, simvastatin was associated with a decrease in 6MWD and there was essentially no change in 6MWD with aspirin, making inadequate power an unlikely explanation for our results. Results from the subgroup with IPAH and heritable PAH did not differ from those of the main study; other disease subgroups were not large enough for meaningful analyses.
In summary, we have shown that while simvastatin 40 mg each day lowers total cholesterol, LDL, and oxidized LDL, there was no effect on 6MWD or biomarkers of endothelial dysfunction or injury in PAH. Similarly, while aspirin 81 mg each day reduced TxB2 production and may have lowered soluble P-selectin levels (in IPAH), there was no effect on 6MWD and possibly increased the risk of major bleeding. Based on these findings, neither drug can be recommended for the treatment of PAH. These drugs should be used according to usual indications in PAH.
Supplementary Material
Commentary.
Pulmonary arterial hypertension (PAH) is a progressive disease which causes exercise limitation, heart failure, and death. Aspirin and simvastatin have powerful effects on atherosclerosis, but have not been studied in PAH. We performed a randomized, double-blind, placebo-controlled 2 × 2 factorial clinical trial of aspirin and simvastatin in patients with PAH receiving background therapy at four centers. Sixty-five subjects were randomized when the trial was terminated by the DSMB after an interim analysis showed futility in reaching the primary end point for simvastatin. After adjustment for baseline 6MWD, there was no significant difference in the 6MWD at six months between aspirin (n = 32) and placebo (n = 33) [placebo-corrected difference = −0.5 m (95%CI, −28.4 – 27.4 m), p = 0.97] or between simvastatin (n = 32) and placebo (n = 33) [placebo-corrected difference = −27.6 m (95%CI, −59.6 – 4.3 m), p = 0.09]. This trial did not show any clinical benefit with the use of aspirin or simvastatin in patients with PAH. Traditional indications for these drugs should guide their use in patients with PAH.
Acknowledgements
We thank the following individuals for their assistance: Jude Aidam, MB, ChB, MPH, Rebekah Boyle, MS, Elaine Cornell, Nadine Al-Naamani, MD, Jeffrey Okun, MD, Kwabena Osei, MD, Diane Pinder, BS, Debbie Rybak, BS, and Karen Visnaw, RN.
Funding Sources
Funded by NIH R01 HL082895 and HL082895-S1. This publication was made possible by Grants UL1 RR025005, UL1 RR025752, UL1 RR024156, and UL1RR024134 from the NCRR and the NIH Roadmap for Medical Research. Aspirin and matching placebo were provided free of charge by Bayer HealthCare LLC. Supported in part by a research grant (in the form of simvastatin and matching placebo) from the Investigator-Initiated Studies Program of Merck & Co., Inc. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Bayer HealthCare LLC, Merck & Co., Inc, or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
SMK has consulted for Bayer, Gilead, Novartis, and Merck, served on grant review boards for Gilead and Pfizer, received fees from Gilead and Actelion for non-promotional lectures, received support for a CME conference from Actelion, Gilead, United Therapeutics, Lung Rx, and Pfizer, and received funding for contracted research from Actelion, Gilead, and Pfizer. EB has no conflicts of interest. DJL has served on advisory boards for Gilead and has received institutional funding from Gilead to conduct clinical trials. DS has no conflicts of interest. EMH has received funding for research from United Therapeutics, Gilead, Actelion, Pfizer, Medtronic, and Novartis, for advisory boards from United Therapeutics, Gilead, Actelion, Pfizer, for consulting Merck, and speaking fees from Gilead and Pfizer. KER has served on an advisory board for Gilead. NSH has received research grant support from Actelion, Bayer, Genzyme, Gilead, Pfizer and United Therapeutics. RGB has no conflicts of interest. EBR has received honoraria from Actelion, Gilead, and United Therapeutics for her counsel at scientific advisory board meetings and for balanced lectures on pulmonary hypertension. She has also received research grant support from Actelion, Gilead, United Therapeutics, Novartis, Pfizer, Eli Lily and Bayer. WP has no conflicts of interest. RPT has no conflicts of interest. HIP has served as a consultant, investigator, and/or lecturer for Actelion, GeNO, Gilead, Lung RX, United Therapeutics and Pfizer. PMH has served on advisory boards for Pfizer, Gilead, and Merck; has received research money from Actelion/United Therapeutics (REVEAL registry for PAH); has received honoraria for grant reviews for Gilead and Pfizer; is the recipient of NIH/NHLBI grants R01 HL049441 and P50 award no. HL084946. REG has received honoraria and consulting fees from Actelion, Gilead, and Pfizer and clinical research support from Actelion, Bayer, Gilead, United Therapeutics.
References
- 1.Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, Loyd JE. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992;327:70–75. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- 2.Robbins IM, Kawut SM, Yung D, Reilly MP, Lloyd W, Cunningham G, Loscalzo J, Kimmel SE, Christman BW, Barst RJ. A study of aspirin and clopidogrel in idiopathic pulmonary arterial hypertension. Eur Respir J. 2006;27:578–584. doi: 10.1183/09031936.06.00095705. [DOI] [PubMed] [Google Scholar]
- 3.Adatia I, Barrow SE, Stratton PD, Miall-Allen VM, Ritter JM, Haworth SG. Thromboxane A2 and prostacyclin biosynthesis in children and adolescents with pulmonary vascular disease. Circulation. 1993;88:2117–2122. doi: 10.1161/01.cir.88.5.2117. [DOI] [PubMed] [Google Scholar]
- 4.Barst RJ, Stalcup SA, Steeg CN, Hall JC, Frosolono MF, Cato AE, Mellins RB. Relation of arachidonate metabolites to abnormal control of the pulmonary circulation in a child. Am Rev Respir Dis. 1985;131:171–177. doi: 10.1164/arrd.1985.131.1.171. [DOI] [PubMed] [Google Scholar]
- 5.Ichida F, Uese K, Hamamichi Y, Hashimoto I, Tsubata S, Fukahara K, Murakami A, Miyawaki T. Chronic effects of oral prostacyclin analogue on thromboxane A2 and prostacyclin metabolites in pulmonary hypertension. Acta Paediatr Jpn. 1998;40:14–19. doi: 10.1111/j.1442-200x.1998.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 6.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH, Koerner SK, Langleben D, Keller CA, Murali S, Uretsky BF, Clayton LM, Jöbsis MM, Blackburn JSD, Shortino D, Crow JW. A comparison of continuous intravenous epoprostenol with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF, Robbins IM, Olschewski H, Rubenfire M, Seeger W. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2010;55:1915–1922. doi: 10.1016/j.jacc.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, Speich R, Hoeper MM, Behr J, Winkler J, Sitbon O, Popov W, Ghofrani HA, Manes A, Kiely DG, Ewert R, Meyer A, Corris PA, Delcroix M, Gomez-Sanchez M, Siedentop H, Seeger W. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 9.Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, Keogh A, Oudiz R, Frost A, Blackburn SD, Crow JW, Rubin LJ. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 10.Lopes AA, Maeda NY, Almeida A, Jaeger R, Ebaid M, Chamone DF. Circulating platelet aggregates indicative of in vivo platelet activation in pulmonary hypertension. Angiology. 1993;44:701–706. doi: 10.1177/000331979304400905. [DOI] [PubMed] [Google Scholar]
- 11.Nakonechnicov S, Gabbasov Z, Chazova I, Popov E, Belenkov Y. Platelet aggregation in patients with primary pulmonary hypertension. Blood Coagul Fibrinolysis. 1996;7:225–227. doi: 10.1097/00001721-199603000-00029. [DOI] [PubMed] [Google Scholar]
- 12.Damas JK, Otterdal K, Yndestad A, Aass H, Solum NO, Froland SS, Simonsen S, Aukrust P, Andreassen AK. Soluble CD40 ligand in pulmonary arterial hypertension: possible pathogenic role of the interaction between platelets and endothelial cells. Circulation. 2004;110:999–1005. doi: 10.1161/01.CIR.0000139859.68513.FC. [DOI] [PubMed] [Google Scholar]
- 13.Sakamaki F, Kyotani S, Nagaya N, Sato N, Oya H, Satoh T, Nakanishi N. Increased plasma P-selectin and decreased thrombomodulin in pulmonary arterial hypertension were improved by continuous prostacyclin therapy. Circulation. 2000;102:2720–2725. doi: 10.1161/01.cir.102.22.2720. [DOI] [PubMed] [Google Scholar]
- 14.Stuard ID, Heusinkveld RS, Moss AJ. Microangiopathic hemolytic anemia and thrombocytopenia in primary pulmonary hypertension. N Engl J Med. 1972;287:869–870. doi: 10.1056/NEJM197210262871710. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki H, Nakasato M, Sato S, Yokoyama S, Katsuura M, Yamaki S, Hayasaka K. Microangiopathic hemolytic anemia and thrombocytopenia in a child with atrial septal defect and pulmonary hypertension. Tohoku J Exp Med. 1997;181:379–384. doi: 10.1620/tjem.181.379. [DOI] [PubMed] [Google Scholar]
- 16.Chin KM, Channick RN, de Lemos JA, Kim NH, Torres F, Rubin LJ. Hemodynamics and epoprostenol use are associated with thrombocytopenia in pulmonary arterial hypertension. Chest. 2009;135:130–136. doi: 10.1378/chest.08-1323. [DOI] [PubMed] [Google Scholar]
- 17.Shen L, Shen J, Pu J, He B. Aspirin attenuates pulmonary arterial hypertension in rats by reducing plasma 5-hydroxytryptamine levels. Cell Biochem Biophys. doi: 10.1007/s12013-011-9156-x. In press. [DOI] [PubMed] [Google Scholar]
- 18.Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. 2004;169:764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 19.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 20.Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. PNAS. 1998;95:8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez-Perera O, Perez-Sala D, Navarro-Antolin J, Sanchez-Pascuala R, Hernandez G, Diaz C, Lamas S. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest. 1998;101:2711–2719. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson JR, Dudek SM, Birukov KG, Ye SQ, Grigoryev DN, Girgis RE, Garcia JG. Cytoskeletal activation and altered gene expression in endothelial barrier regulation by simvastatin. Am J Respir Cell Mol Biol. 2004;30:662–670. doi: 10.1165/rcmb.2003-0267OC. [DOI] [PubMed] [Google Scholar]
- 23.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 24.Laufs U, Marra D, Node K, Liao JK. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing rho GTPase-induced down-regulation of p27(Kip1) J Biol Chem. 1999;274:21926–21931. doi: 10.1074/jbc.274.31.21926. [DOI] [PubMed] [Google Scholar]
- 25.Girgis RE, Mozammel S, Champion HC, Li D, Peng X, Shimoda L, Tuder RM, Johns RA, Hassoun PM. Regression of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1105–L1110. doi: 10.1152/ajplung.00411.2006. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura T, Vaszar LT, Faul JL, Zhao G, Berry GJ, Shi L, Qiu D, Benson G, Pearl RG, Kao PN. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation. 2003;108:1640–1645. doi: 10.1161/01.CIR.0000087592.47401.37. [DOI] [PubMed] [Google Scholar]
- 27.Kuang T, Wang J, Pang B, Huang X, Burg ED, Yuan JX, Wang C. Combination of sildenafil and simvastatin ameliorates monocrotaline-induced pulmonary hypertension in rats. Pulm Pharmacol Ther. 2010;23:456–464. doi: 10.1016/j.pupt.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee DS, Kim YK, Jung YW. Simvastatin, sildenafil and their combination in monocrotaline induced pulmonary arterial hypertension. Korean Circ J. 2010;40:659–664. doi: 10.4070/kcj.2010.40.12.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright JL, Zhou S, Preobrazhenska O, Marshall C, Sin DD, Laher I, Golbidi S, Churg AM. Statin Reverses Smoke-induced Pulmonary Hypertension and Prevents Emphysema but Not Airway Remodeling. Am J Respir Crit Care Med. 2011;183:50–58. doi: 10.1164/rccm.201003-0399OC. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura T, Faul JL, Berry GJ, Vaszar LT, Qiu D, Pearl RG, Kao PN. Simvastatin attenuates smooth muscle neointimal proliferation and pulmonary hypertension in rats. Am J Respir Crit Care Med. 2002;166:1403–1408. doi: 10.1164/rccm.200203-268OC. [DOI] [PubMed] [Google Scholar]
- 31.Girgis RE, Li D, Zhan X, Garcia JG, Tuder RM, Hassoun PM, Johns RA. Attenuation of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Heart Circ Physiol. 2003;285:H938–H945. doi: 10.1152/ajpheart.01097.2002. [DOI] [PubMed] [Google Scholar]
- 32.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Cool C, Wood K, Tuder RM, Burns N, Kasper M, Voelkel NF. Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;291:L668–L676. doi: 10.1152/ajplung.00491.2005. [DOI] [PubMed] [Google Scholar]
- 33.King WT, Day RW. Treatment of pediatric pulmonary hypertension with simvastatin: An observational study. Pediatr Pulmonol. doi: 10.1002/ppul.21361. (In Press) [DOI] [PubMed] [Google Scholar]
- 34.Kao PN. Simvastatin treatment of pulmonary hypertension: an observational case series. Chest. 2005;127:1446–1452. doi: 10.1378/chest.127.4.1446. [DOI] [PubMed] [Google Scholar]
- 35.Wilkins MR, Ali O, Bradlow W, Wharton J, Taegtmeyer A, Rhodes CJ, Ghofrani HA, Howard L, Nihoyannopoulos P, Mohiaddin RH, Gibbs JS. Simvastatin as a treatment for pulmonary hypertension trial. Am J Respir Crit Care Med. 2010;181:1106–1113. doi: 10.1164/rccm.2009111-699OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawut SM, Bagiella E, Shimbo D, Lederer DJ, Al-Naamani N, Roberts KE, Barr RG, Post W, Horn EM, Tracy R, Hassoun PM, Girgis RE. Rationale and design of a Phase II clinical trial of aspirin and simvastatin for the treatment of pulmonary arterial hypertension: ASA-STAT. Contemporary Clinical Trials. 2011;32:280–287. doi: 10.1016/j.cct.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langleben D, Christman BW, Barst RJ, Dias VC, Galie N, Higenbottam TW, Kneussl M, Korducki L, Naeije R, Riedel A, Simonneau G, Hirsch AM, Rich S, Robbins IM, Oudiz R, McGoon MD, Badesch DB, Levy RD, Mehta S, Seeger W, Soler M. Effects of the thromboxane synthetase inhibitor and receptor antagonist terbogrel in patients with primary pulmonary hypertension. Am Heart J. 2002;143:E4. doi: 10.1067/mhj.2002.121806. [DOI] [PubMed] [Google Scholar]
- 38.Clarke RJ, Mayo G, Price P, FitzGerald GA. Suppression of thromboxane A2 but not of systemic prostacyclin by controlled-release aspirin. N Engl J Med. 1991;325:1137–1141. doi: 10.1056/NEJM199110173251605. [DOI] [PubMed] [Google Scholar]
- 39.Girgis RE, Ma SF, Ye S, Grigoryev DN, Li D, Hassoun PM, Tuder RM, Johns RA, Garcia JG. Differential gene expression in chronic hypoxic pulmonary hypertension: effect of simvastatin treatment. Chest. 2005;128:579S. doi: 10.1378/chest.128.6_suppl.579S. [DOI] [PubMed] [Google Scholar]
- 40.McMurtry MS, Bonnet S, Michelakis ED, Bonnet S, Haromy A, Archer SL. Statin therapy, alone or with rapamycin, does not reverse monocrotaline pulmonary arterial hypertension: the rapamcyin-atorvastatin-simvastatin study. Am J Physiol Lung Cell Mol Physiol. 2007;293:L933–L940. doi: 10.1152/ajplung.00310.2006. [DOI] [PubMed] [Google Scholar]
- 41.Sun X, Ku DD. Rosuvastatin provides pleiotropic protection against pulmonary hypertension, right ventricular hypertrophy, and coronary endothelial dysfunction in rats. Am J Physiol Heart Circ Physiol. 2008;294:H801–H809. doi: 10.1152/ajpheart.01112.2007. [DOI] [PubMed] [Google Scholar]
- 42.Barreto AC, Maeda NY, Soares RP, Cicero C, Lopes AA. Rosuvastatin and vascular dysfunction markers in pulmonary arterial hypertension. Braz J Med Biol Res. 2008;41:657–663. doi: 10.1590/s0100-879x2008000800003. [DOI] [PubMed] [Google Scholar]
- 43.Ventetuolo CE, Benza RL, Peacock AJ, Zamanian RT, Badesch DB, Kawut SM. Surrogate and combined end points in pulmonary arterial hypertension. Proc Am Thorac Soc. 2008;5:617–622. doi: 10.1513/pats.200803-029SK. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.