Abstract
Previously it has been reported that neural stem cells undergoing apoptotic stress have increased levels of Amyloid precursor protein (APP) and increased APP expression results in glial differentiation. APP activity was also shown to be required for staurosporine induced glial differentiation of neuroprogenitor cells. Monocyte chemoattractant protein-1 (MCP-1) is a chemokine that is expressed early during inflammation. The binding of MCP-1 to its chemokine receptor induces expression of novel transcription factor MCP-1 induced protein (MCPIP). MCPIP expression subsequently leads to cell death. Previous studies have shown that pro apoptotic factors have the ability to induce neural differentiation. Therefore, we investigated if MCPIP expression leads to differentiation of NT2 neuroprogenitor cells. Results showed that MCPIP expression increased glial fibrillary acid protein (GFAP) expression and also caused distinct morphological changes, both indicative of glial differentiation. Similar results were observed with MCP-1 treatment. Interestingly, APP expression decreased in response to MCPIP. Instead, we found APP activity regulates expression of both MCP-1 and MCPIP. Furthermore, inhibition of either p38 MAPK or JAK signaling pathways significantly reduced APP’s effect on MCP-1 and MCPIP. These data demonstrates the role APP has in glial differentiation of NT2 cells through MCP-1/MCPIP signaling. It is possible that increased APP expression after CNS injury could play a role in MCP-1 production, possibly promoting astrocyte activation at injured site.
Keywords: Amyloid precursor protein, Monocyte chemoattractant protein 1, glial differentiation, astrocyte, neuroprogenitor
INTRODUCTION
Chemokines are a family of conserved cytokines that have been shown to induce the migration of several leukocyte subpopulations into damaged tissues. In the adult brain, microglia and astrocytes are believed to be the main source of chemokine production[5,11,13,21]. The central nervous system (CNS) has been shown to produce chemokines in response to several inflammatory and disease conditions, including allergic encephalitis, Alzheimer’s disease, multiple sclerosis, ischemia-induced neurodegeneration, and trauma [7]. Monocyte chemoattractant protein-1 (MCP-1), a member of the C-C chemokine family, is a potent chemotactic factor for monocytes[26]. A recent study showed that the binding of MCP-1 to its receptor, CCR2, induces the expression of a novel transcription factor, MCP-induced protein or MCPIP. MCPIP expression up regulates several pro apoptotic genes, which leads to cell death[32]. Other than their neuro inflammatory properties, chemokine function in the CNS is still not fully understood.
Amyloid precursor protein (APP) is a ubiquitous glycoprotein receptor that is involved in the pathogenesis of Alzheimer’s disease. APP undergoes enzymatic processing to produce fragments with various functions[6]. One of the fragments, beta-amyloid peptide (AB), is a main constituent of senile plaques[6,8] seen in Alzheimer’s disease. While the physiological function of APP is not fully understood, its structure suggests that APP might function as a receptor via its G0 binding domain or as a ligand via its soluble N-terminal domain[30]. Previously, neural stem cells undergoing apoptotic stress showed an increase in expression of APP and the surrounding cells underwent glial differentiation[19]. APP expression is capable of inducing glial differentiation and that neural stem cells transplanted into APP transgenic mice differentiated predominantly into astrocytes [15]. APP was also shown to be required in pro apoptotic factor staurosporine induced glial differentiation of neuroprogenitor cells [16]. These results demonstrate that increased APP signaling under apoptotic conditions causes glial differentiation. In this study, we demonstrate that APP is capable of inducing expression of both MCP-1 and MCPIP. Increased levels of MCP-1 and MCPIP were able to activate glial differentiation in NT2 neuroprogenitor cells.
EXPERIMENTAL
Cell Culture
The NTera-2/Clone D1 cells (NT2) were plated at a density of 5 ×106 per 75cm2 in tissue culture treated flask (Corning). Cell culture media was Dulbecco’s modified Eagle’s medium with F-12 (DMEM/F12, Invitrogen), supplemented with 10% heat inactivated fetal bovine serum (Atlanta Biologics). An incubation chamber was used to maintain a humidified atmosphere of 5% CO2 and 37°C. NT2 cells were passed twice a week by 0.05% Trypsin/EDTA (Invitrogen) treatment.
Transfection
Transfection of plasmid DNA was performed with Lipofectamine 2000 (Invitrogen). Transfections were performed on subconfluent NT2 cells in 6 well plates (Corning) or 35 mm glass bottom dishes (MatTek). Transfection efficiency was determined by observation of GFP expression under fluorescence microscope or immunoblot analysis with GFP antibody. AICD plasmid transfection efficiency was determined by co-transfection with GFP plasmid. Plasmids: pEGFP-C1 (Clonetech), pCDNA4/His-Max TOPO (Invitrogen), pCEP-APP-GFP, MCPIP-GFP (provided by Dr. Pappachan E. Kolattukudy, University of Central Florida), AICD pCDNA3 APP-C59 (provided by Dr. Fuyuki Kametani, Tokyo Institute of Psychiatry).
Small interference RNA
Small interference (siRNA) for human APP695 (Accession: A33292) was designed using Ambion software. Target sequence for APP695 (5′ATCTTTGGAACAGGAAGCAG3′). Preparation of siRNA was done using Silencer Expression (Ambion). SiRNA cassette was then subcloned into pCDNA4/His-Max TOPO plasmid (Invitrogen).
Reagents and Antibodies
Primary Antibodies: rabbit anti-GFAP (Promega); mouse anti-APP (22C11) (Chemicon); mouse anti-GFP (Invitrogen); rabbit anti-Beta Actin (Cell Signaling); mouse anti-Cleaved PARP (Cell Signaling); mouse anti-ERK; mouse anti-ERK1/2 (pT202/pY204) (BD Biosciences); rabbit ant-STAT3 (BD Biosciences); rabbit anti-STAT3 (pY705) (Cell Signaling); mouse anti-p38 MAPK; mouse anti-p38 MAPK (pT180/pY182) (BD Biosciences). Secondary antibodies: anti-mouse IgG and anti-rabbit IgG horseradish peroxidase-conjugated antibodies (Amersham); TRITC conjugated antibody (Jackson Immuno). Reagents and Kinase Inhibitors: Secreted type Amyloid Precursor protein alpha S9564-25UG (Sigma). Trypan blue (Sigma); JAK1 inhibitor (calbiochem); p38 MAPK inhibitor-SB203580 (Sigma); and ERK1/2 inhibitor-PD098059 (Sigma). Cells were pretreated with kinase inhibitors for 45 mins prior to plasmid transfection.
Immunoprecipitation and Immunoblot analysis
Protein samples were collected from cells lysed by ice cold lysis buffer consisting of 1% NP40, 150mM NaCl, 50mM Tris, pH 8.0 and protease inhibitor cocktail (Calbiochem). The protein concentration was measured using Bradord reagent (BioRad). For immunopreciptation experiment cell lysates were immunoprecipitated with an antibody against ERK, STAT3, p38 MAPK using protein G-Sepharose (Amersham Bioscience). Then precipitates or cell lysates were heated at 90 C for 10 min in a sample loading buffer and separated on NuPAGE 4–12% Bis-Tris Gel (Invitrogen) for 60 mins at 200 V and subsequently transferred to a PVDF membrane (30 V for 90 mins). Membranes were incubated in blocking solution (5% milk in PBS with 0.05% Tween 20 (PBS-T) (Invitrogen) for 2 hours at room temperature (RT). Membranes were probed with primary antibody in blocking solution overnight at 4°C. The membranes were washed three times for 5 mins with PBS-T and then incubated with horseradish peroxidase conjugated secondary antibodies in blocking solution for 2 hours at RT. After three washes with PBS-T protein bands were visualized with ECL plus chemilluminescence reagent (Amersham Bioscience). Immunoblot images were captured by KODAK Image Station 2000MM. Protein values were normalized to Beta Actin expression. Image analysis and data normalization was done using ImageJ 4.10 software (NIH).
Immunocyotohemistry
Cells were fixed in 4% paraformaldehyde 30 mins at RT, washed three times for 5 mins in PBS, then incubated in PBS-T for 1 hour at RT. Cells were then blocked with 3% Normal Donkey Serum in PBS-T (blocking solution). Next, samples were incubated with primary antibody diluted in blocking solution for 2 hours at RT. Cells were washed three times for 5 mins in PBS prior to adding secondary antibody, diluted in blocking solution. Secondary antibody incubation was done overnight at 4°C. Cells were then washed thoroughly and air-dried prior to adding Vectashield mounting media with DAPI (Vector).
Microscopy
Imaging was done using an inverted fluorescent microscope (Leica DMI 6000B) with differential interphase contrast (DIC). DAPI/Rhodamine/FITC filter cube (Chroma) was utilized for fluorescent microscopy. Image analysis was done using Openlab Software 4.0.1 (Improvision)
Quantitative RT-PCR
Total RNA was extracted from the cells with Trizol reagent (Invitrogen). cDNA synthesis and RT-PCR reactions were performed using iQ SYBER Green Supermix (Biorad). RT-PCR conditions were pre denaturation for 2 mins at 94°C; 35 cycles of 94°C for 15 s: 55–60°C for 30 s; and 72°C for 30 s; and a post extension at 72°C for 2 mins. RT-PCR reaction was performed using iCycler (Biorad). Fold change was determined using the pfaffl method [23].
RT-PCR Primers
GFAP (+) 5-AAGCAGTCTACCCACCTCAG-3,
(−)5-ATCCCTCCCAGCACCTCATC-3;
APP (+) 5-CTTGAGTAAACTTTGGGACATGGCGCTGC-3,
(−)5-GAACCCTACGAAGAAGCC-3;
Beta Actin (+) 5-GACAGGATGCAGAAGGAGAT-3,
(−) 5-TTGCTGATCCACATCTGCTG-3;
MCP-1 (+) 5-GCGAGCTATAGAAGAATCACC-3,
(−) 5-ATAAAACAGGGTGTCTGGGG-3;
MCPIP (+) 5-AACTGGAGAAGAAGAAGATCCTGG-3,
(−) 5-ATTGACGAAGGAGTACATGAGCAG-3
Statistical analysis
Experiments were done in triplicate and standard deviation was determined. A T-test analysis was performed to determine statistical significance of experimental group compared to control group.
RESULTS
Cell death caused by MCPIP expression
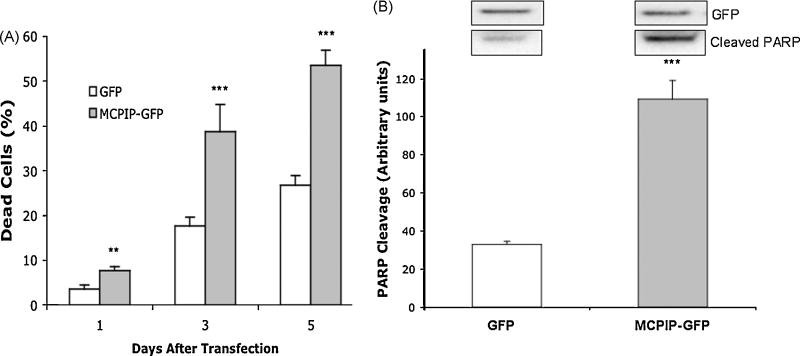
The transcription factor MCPIP has previously been shown to induce apoptosis in various cell lines[10,32]. To determine the effect MCPIP has on NT2 cells, we performed a cell death dye exclusion assay utilizing trypan blue. NT2 cells were transfected with either GFP (control) or MCPIP-GFP and analyzed 1, 3, and 5 days post transfection. Results showed that MCPIP expression caused a significant increase in cell death compared to control at all indicated time points (Figure 1). Day 5 showed the most significant increase in cell death, with MCPIP at 53.65% compared to 26.7% for GFP. As expected, plasmid transfection caused low levels of cell death as seen in GFP transfected cells. We also showed that MCPIP induced apoptosis in NT2 cell by immunoblot analysis with anti cleaved PARP antibody. These results demonstrate that MCPIP expression in NT2 neuroprogenitor cells induces apoptosis, as previously seen in other cell lines.
Figure 1. MCPIP increases cell death in NT2 cells.
(A) Percent cell death after MCPIP-GFP or GFP (Control) transfection. Cells were harvested at 1, 3, 5 days post transfection. Cell death was detected by trypan blue. (B) Immunoblot analysis of GFP expression and PARP cleavage in NT2 cells transfected with MCPIP-GFP or GFP. Analyzed 5 days post transfection. Protein values were normalized to Beta Actin expression. GFP expression demonstrates equal transfection efficiency. Note: MCPIP-GFP was approximately 90kDa compared to GFP alone (27 kDa). * Indicates statistical significance (**P<0.01, ***P<0.001).
Induction of glial differentiation by MCPIP expression in NT2 cells
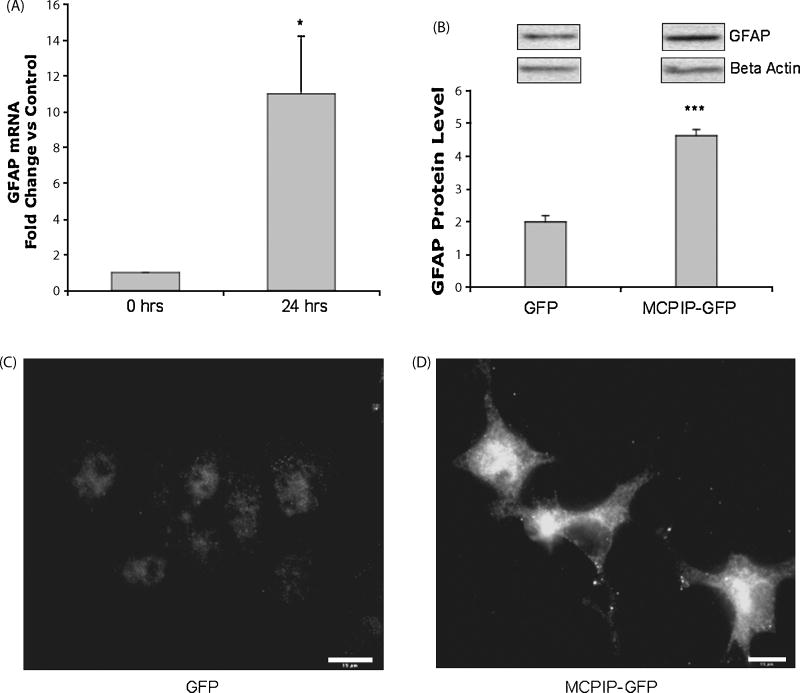
Previously, pro apoptotic factors have been shown to be involved neural differentiation [16]. Therefore, we investigated if the pro apoptotic nature of MCPIP could induce differentiation in NT2 neuroprogenitor cells. The results showed that MCPIP caused a significant increase in GFAP mRNA, indicative of glial differentiation (Figure 2A). Immunoblot analysis with GFAP specific antibody confirmed these results (Figure 2B). Immunocytochemistry showed increased levels of GFAP expression, as well as distinct astrocyte morphology for MCPIP transfected cells (Figure 2C and 2D). Taken together, these results demonstrate that MCPIP expression is capable of inducing glial differentiation in NT2 cells.
Figure 2. Increased glial fibrillary acidic protein expression and morphological changes caused by MCPIP expression.
(A) RT-PCR analysis of GFAP in NT2 cells transfected with MCPIP-GFP or GFP (control) 24 hours post transfection. Figure represents fold change vs. control. (B) Immunoblot analysis of GFAP in cells transfected with MCPIP-GFP or GFP, analyzed 24 hours post transfection. Protein values were normalized to Beta Actin expression. * Indicates statistical significance (*P<0.05, ***P<0.001). Immunocytochemistry showing GFAP expression in NT2 cells transfected with GFP (C) or MCPIP-GFP (D). Analyzed 24 hours post transfection. Scale bar in panel represents 15 μm.
Induction of glial differentiation by MCP-1 treatment in NT2 cells
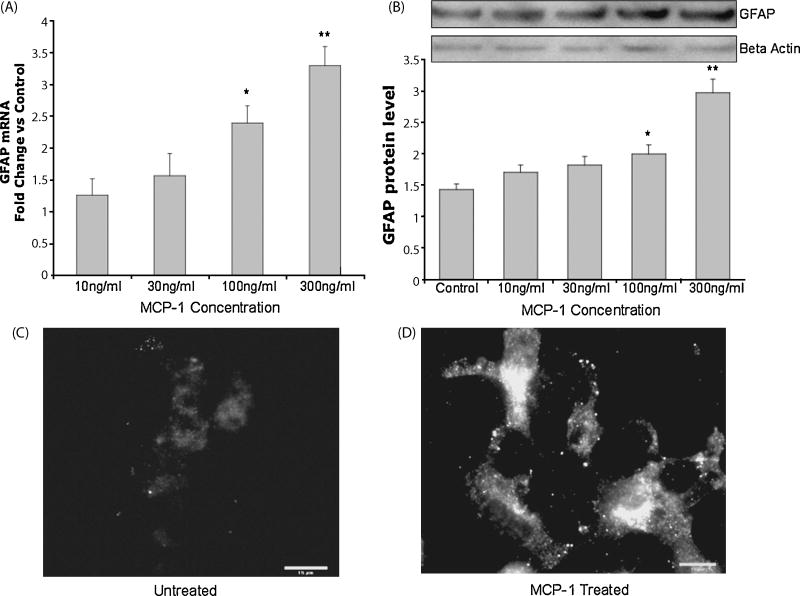
It is already shown that MCP-1 is responsible for activation of transcription factor MCPIP. Therefore, we tested MCP-1 for its ability to induce glial differentiation. The RT-PCR results showed that MCP-1 caused a significant increase in GFAP expression at 24 hours, especially for 100ng/ml and 300ng/ml concentrations (Figure 3A). Immunoblot analysis with anti GFAP antibody confirmed these results (Figure 3B). Increased GFAP expression and morphological changes indicative of astrocytic differentiation were also observed for MCP-1 treated cells (Figure 3C and 3D). These results demonstrate that MCP-1 activates glial differentiation in a similar manner as MCPIP.
Figure 3. Increased glial fibrillary acidic protein expression and morphological changes caused by MCP-1.
(A) RT-PCR analysis of GFAP in NT2 cells treated with MCP-1 at indicated concentrations. Analyzed 24 hours post treatment. Figure represents fold change vs. control (untreated). (B) Immunoblot analysis of GFAP in cells treated with MCP-1 or untreated after 24 hours. Protein values were normalized to Beta Actin expression. * Indicates statistical significance (*P<0.05, **P<0.01). Immunocytochemistry showing GFAP expression in NT2 cells untreated (C) or treated with MCP-1 (100ng/ml) (D). Analyzed 24 hours after treatment. Scale bar in panel represents 15 μm.
MCPIP expression decreases amyloid precursor protein levels
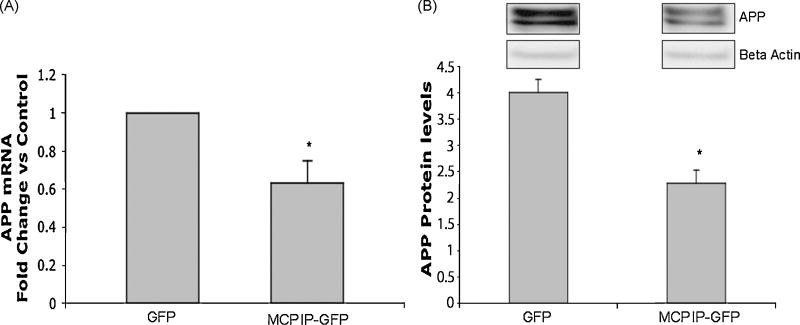
Previously, staurosporine induced glial differentiation of NT2 cells was shown to require APP expression[16]. Therefore, we expected that MCPIP expression might increase APP levels, and that APP activity could play a significant part in the differentiation process. Interestingly, results showed that MCPIP expression significantly decreased APP mRNA and protein levels in NT2 cells (Figure 4A and B). This data suggests that MCPIP induced glial differentiation does not require increased APP expression, as previously seen with staurosporine.
Figure 4. MCPIP decreases APP mRNA and protein levels.
(A) RT-PCR analysis of APP expression in cells transfected with MCPIP-GFP or GFP (control). Analyzed 24 hours post transfection. (B) Immunoblot analysis of APP expression in NT2 cells transfected with MCPIP-GFP or GFP control. Analyzed 24 hours post transfection. Protein values were normalized to Beta Actin expression. Figure represents fold change vs. control. * Indicates statistical significance (*P<0.05).
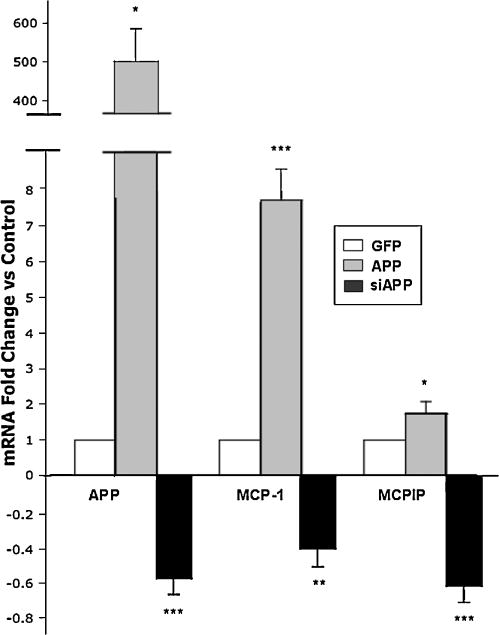
Amyloid precursor protein induces expression of MCP-1 and MCPIP
Contrary to our initial hypothesis, MCPIP expression decreased APP activity. It is possible that APP may act upstream of MCPIP and APP might be able to regulate MCPIP activity. Therefore, we investigated if APP expression is capable of increasing MCPIP and MCP-1. The results showed that 24 hours post APP transfection both MCP-1 and MCPIP expression significantly increased (Figure 5). Gene silencing of APP by siRNA significantly suppressed normal levels of both MCP-1 and MCPIP mRNA expression in NT2 cells (Figure 5). These results suggest that MCP-1 and MCPIP expression is effectively regulated by APP signaling.
Figure 5. APP expression regulates MCP-1 and MCPIP mRNA.
RT-PCR analysis of APP, MCP-1, and MCPIP in NT2 cells transfected with APP-GFP, siAPP, or GFP (control). Analyzed 24 hours post transfection. Figure represents fold change vs. control. * Indicates statistical significance (*P<0.05, **P<0.01, ***P<0.001).
Secreted type APP alpha (sAPPα) and APP intracellular domain (AICD) effect on MCP-1 and MCPIP
We next tested whether the processed forms of APP, secreted type APP alpha (sAPPα) and APP intracellular domain (AICD), have an effect on MCP-1 and MCPIP expression. We treated NT2 cells with sAPPα at 25, 50, and 100nM concentrations and measured MCP-1 and MCPIP expression by RT-PCR. Results showed that sAPPα significantly increased MCP-1, but not to the same extent as transfected full length APP (Figure 6A). Interestingly, MCPIP expression did not increase in response to either sAPPα or AICD. Increasing the concentration of sAPPα did not elevate MCP-1 levels any further, demonstrating its effect was not dose dependent (Figure 6A). Similar results were observed after NT2 cells were transfected with AICD plasmid (Figure 6B). In conclusion, these results show that both processed forms of APP, sAPPα and AICD, are capable of increasing expression of MCP-1, but not MCPIP.
Figure 6. Secreted APP alpha and APP intracellular domain (AICD) effect on MCP-1 and MCPIP.
(A) RT-PCR analysis of MCP-1 and MCPIP in NT2 cells treated with secreted type APP alpha (25, 50, 100nM) or transfected with AICD (B). RT-PCR analysis of MCP-1 and MCPIP in NT2 cells transfected with AICD or GFP (control). Figure represents fold change vs. control (GFP). Analyzed 24 hours post treatment/transfection. Indicates statistical significance (*P<0.05, **P<0.01)
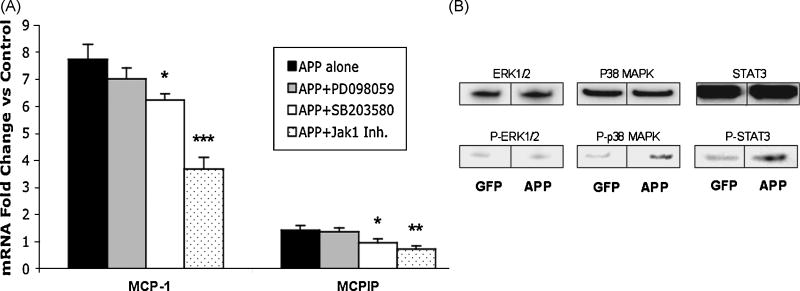
Amyloid precursor protein’s effect on MCP-1 and MCPIP expression relies on p38 MAPK and JAK/STAT signaling
We have shown that APP can increase MCP-1 and MCPIP expression. Next, we examined which signal transduction pathways are involved in this process. In order to determine what mechanism APP uses in its induction of MCP-1 and MCPIP we investigated the p38 mitogen-activated protein kinase (MAPK), extracellular signal-related protein kinase (ERK1/2), and Janus activated kinase (JAK) signal transduction pathways. NT2 cells were pretreated with p38 (SB203580), ERK1/2 (PD098059), and JAK1 inhibitors for 45 minutes at 25uM, 50uM, and 250nM, respectfully. Samples were then transfected with APP-GFP or GFP and analyzed 24 hours post transfection. Results showed that the p38 MAPK and JAK specific inhibitors caused a significant decrease in both MCP-1 and MCPIP (Figure 7A). The ERK1/2 inhibitor, PD098059, had no effect on either MCP-1 or MCPIP activity. Similar results were observed using increased concentrations of ERK1/2 inhibitor (data not shown). In addition, we also investigated the phosphorylated state of the specific proteins involved in these pathways. Results showed that APP expression increased phosphorylation of both p38 and STAT3 (Figure 7B). There was no increase in phosphorylation of ERK1/2 proteins, confirming the results obtained using PD098059. These results indicate that APP relies on activation of both p38 MAPK and JAK/STAT3 signaling pathways to induce MCP-1 and MCPIP expression.
Figure 7. APP’s effect on MCP-1 and MCPIP expression relies on p38 MAPK and JAK/STAT signaling.
(A) RT-PCR analysis of MCP-1 and MCPIP in NT2 cells transfected with APP. NT2 cells were pretreated for 45 mins with 50uM of ERK1/2 inhibitor (PD098059), 25uM of p38 MAPK inhibitor (SB203580), 250nM of JAK1 inhibitor, or untreated. NT2 cells were then transfected with APP-GFP or GFP (control). Figure represents fold change over control (GFP). Analyzed 24 hours post transfection. (B) Immunoblot analysis of Phosphorylated ERK (pT202/pY204), p38 MAPK (pT180/pY182), and STAT3 (pY705). Prior to immunoblot analysis cell lysates were immunoprecipitated with anti ERK1/2, p38 MAPK or anti STAT3, respectively. Basal levels of phosphorylation was observed for controls, as expected. * Indicates statistical significance (*P<0.05, **P<0.01, ***P<0.001).
DISCUSSION
Our study characterizes the involvement APP and chemokine MCP-1 have in glial differentiation of NT2 neuroprogenitor cells. NT2 cells have the ability to develop into both mature neurons and astrocytes, making them an attractive in vitro model system for analyzing neural differentiation[1,16]. It has been reported that apoptosis was closely associated with neural differentiation[22] and that pro apoptotic factors could induce glial differentiation in NT2 cells[16]. Therefore, we investigated the effect transcription factor MCPIP has on cell death and differentiation in NT2 cells. Results showed that MCPIP expression not only induced cell death, but also promoted increased expression of GFAP and astrocyte cell morphology, indicative of glial differentiation. Similar results were seen with MCP-1 treatment, particularly at higher concentrations. Lower concentrations of MCP-1 were unable to induce expression of GFAP or cause any morphological changes. This suggests that low concentrations of MCP-1 may not be sufficient enough to activate MCPIP to the level needed to induce glial differentiation.
We have previously demonstrated APP’s involvement in glial differentiation of neural stem cells and that APP expression was required in staurosporine induced astrocytic differentiation in NT2 cells. Staurosporine treated cells underwent apoptosis, resulting in increased expression of APP. This increase in APP was responsible for induction of glial differentiation [16]. Therefore, we investigated if APP has a similar role in MCPIP induced cell death and glial differentiation. Unexpectedly, we found that MCPIP expression significantly reduced APP levels. This suggests that increased APP expression is not responsible for inducing glial differentiation, as previously seen with staurosporine treatment. Instead, we found that APP activity could directly regulate expression of both MCP-1 and MCPIP. Secreted type APPα or AICD were also able to significantly increase MCP-1 expression, but not to the extent that transfected APP obtained. Interestingly, both processed forms of APP had no effect on MCPIP activity. Given that MCP-1 is required to induce MCPIP activity, the increase resulting from sAPPα and AICD might not be sufficient to reach the threshold needed to induce MCPIP. This suggests that sAPPα and AICD are not solely responsible for MCP-1 and MCPIP activation. It is possible that their combined effect is needed or amyloid beta (Aβ), the other processed form of APP, is required to fully activate MCPIP activity. Further investigation is necessary to better understand the effect APP processing has on MCP-1/MCPIP expression. Previously, APP expression has been shown to increase in response to cell death [14,17,20]. The increased APP expression has been attributed to its anti-apoptotic abilities, where it functioned as a neuroprotective factor[14,17]. Activation of chemokine MCP-1 can be part of APP’s neuroprotective function, where it helps facilitate neuroinflammation and astrocyte activation at site of injury.
Through use of signaling pathway inhibitors, we investigated the mechanism by which APP regulated MCP-1 and MCPIP expression. Results showed that ERK1/2 activity, previously shown to be required for APP expression, was not required for APP’s activation of MCP-1 and MCPIP [16,18]. Interestingly, both JAK and p38 MAPK inhibitors were able to suppress APP function. Increased phosphorylation of both p38 MAPK and STAT3 was also observed, further confirming the results. It has been reported that JAK/STAT signaling was involved in regulating differentiation of cerebral cortical precursor cells [2,24]. STAT3 was shown to mediate astrocyte differentiation in the developing fetal mouse brain[31]. In addition, the p38 MAPK pathway was shown to be required for MCP-1 production [29] and APP mediated anti-apoptotic signaling [3]. Overall, both signaling pathways appear to play a specialized role in this APP/MCP-1 induced glial differentiation of NT2 neuroprogenitor cells.
Astrocytes have been identified as the primary source of MCP-1 in the brain in response to multiple sclerosis, ischemic brain injury, and brain trauma [7]. Increased expression of MCP-1 is present in senile plaques, microglia, and microvessels in Alzheimer’s disease[9,12]. It was shown that mice over expressing both APP and MCP-1 had microglia accumulation in the brain [27]. It is possible that MCP-1 is involved in the pathogenesis of several neuropathies by the recruitment of activated monocytes and/or microglia into the CNS, where they produced neurotoxic and inflammatory molecules. Although it has been shown that MCP-1 mediates inflammation in the CNS, it is still unknown if MCP-1 has alternative cellular functions in the brain[4,25]. Activation of glial differentiation could be another function of MCP-1 during neuroinflammation. It is already established that astrocytes play an important role in injury repair and are the main source of MCP-1 production[7,28]. Therefore, it is possible that MCP-1 induced glial differentiation is a repair mechanism that increases production of astrocytes, facilitating rapid recovery. Unfortunately, astrocyte activation after injury can also have negative effects. Increased astrocyte production can result in excess inflammation, toxic edema, cytotoxin production, and formation of glial scar, preventing effective repair[7,28]. It is essential to examine the connection between neuroinflammation and astrocyte activation and the role they might have in neuronal repair and protection.
In conclusion, we propose that elevated APP levels after CNS injury can result in increased MCP-1 production. This increase in MCP-1 can lead to astrocytic activation, eventually resulting in gliosis, causing excess damage. Further investigation is necessary to determine if this mechanism is responsible for the excess gliosis present in several neurodegenerative disorders. This information can help provide insight on the factors that regulate neural differentiation in the adult brain, particularly for use in stem cell therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bani-Yaghoub M, Felker JM, Naus CC. Human NT2/D1 cells differentiate into functional astrocytes. Neuroreport. 1999;10:3843–6. doi: 10.1097/00001756-199912160-00022. [DOI] [PubMed] [Google Scholar]
- 2.Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Stahl N, Yancopoulos GD, Greenberg ME. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–83. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- 3.Burton TR, Dibrov A, Kashour T, Amara FM. Anti-apoptotic wild-type Alzheimer amyloid precursor protein signaling involves the p38 mitogen-activated protein kinase/MEF2 pathway. Brain Res Mol Brain Res. 2002;108:102–20. doi: 10.1016/s0169-328x(02)00519-3. [DOI] [PubMed] [Google Scholar]
- 4.Cardona AE, Gonzalez PA, Teale JM. CC chemokines mediate leukocyte trafficking into the central nervous system during murine neurocysticercosis: role of gamma delta T cells in amplification of the host immune response. Infect Immun. 2003;71:2634–42. doi: 10.1128/IAI.71.5.2634-2642.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coughlan CM, McManus CM, Sharron M, Gao Z, Murphy D, Jaffer S, Choe W, Chen W, Hesselgesser J, Gaylord H, Kalyuzhny A, Lee VM, Wolf B, Doms RW, Kolson DL. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience. 2000;97:591–600. doi: 10.1016/s0306-4522(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 6.De Strooper B, Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci. 2000;113(Pt 11):1857–70. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- 7.Glabinski AR, Ransohoff RM. Chemokines and chemokine receptors in CNS pathology. J Neurovirol. 1999;5:3–12. doi: 10.3109/13550289909029740. [DOI] [PubMed] [Google Scholar]
- 8.Golde TE. Alzheimer disease therapy: can the amyloid cascade be halted? J Clin Invest. 2003;111:11–8. doi: 10.1172/JCI17527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol Aging. 2001;22:837–42. doi: 10.1016/s0197-4580(01)00276-7. [DOI] [PubMed] [Google Scholar]
- 10.Hausmann EH, Berman NE, Wang YY, Meara JB, Wood GW, Klein RM. Selective chemokine mRNA expression following brain injury. Brain Res. 1998;788:49–59. doi: 10.1016/s0006-8993(97)01160-8. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi M, Luo Y, Laning J, Strieter RM, Dorf ME. Production and function of monocyte chemoattractant protein-1 and other beta-chemokines in murine glial cells. J Neuroimmunol. 1995;60:143–50. doi: 10.1016/0165-5728(95)00064-9. [DOI] [PubMed] [Google Scholar]
- 12.Ishizuka K, Kimura T, Igata-yi R, Katsuragi S, Takamatsu J, Miyakawa T. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer’s disease. Psychiatry Clin Neurosci. 1997;51:135–8. doi: 10.1111/j.1440-1819.1997.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 13.Karpus WJ, Lukacs NW, McRae BL, Strieter RM, Kunkel SL, Miller SD. An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol. 1995;155:5003–10. [PubMed] [Google Scholar]
- 14.Kinoshita A, Whelan CM, Berezovska O, Hyman BT. The gamma secretase-generated carboxyl-terminal domain of the amyloid precursor protein induces apoptosis via Tip60 in H4 cells. J Biol Chem. 2002;277:28530–6. doi: 10.1074/jbc.M203372200. [DOI] [PubMed] [Google Scholar]
- 15.Kwak YD, Brannen CL, Qu T, Kim HM, Dong X, Soba P, Majumdar A, Kaplan A, Beyreuther K, Sugaya K. Amyloid precursor protein regulates differentiation of human neural stem cells. Stem Cells Dev. 2006;15:381–9. doi: 10.1089/scd.2006.15.381. [DOI] [PubMed] [Google Scholar]
- 16.Kwak YD, Choumkina E, Sugaya K. Amyloid precursor protein is involved in staurosporine induced glial differentiation of neural progenitor cells. Biochem Biophys Res Commun. 2006;344:431–7. doi: 10.1016/j.bbrc.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 17.Lesort M, Esclaire F, Yardin C, Hugon J. NMDA induces apoptosis and necrosis in neuronal cultures. Increased APP immunoreactivity is linked to apoptotic cells. Neurosci Lett. 1997;221:213–6. doi: 10.1016/s0304-3940(96)13310-3. [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Su Y, Li B, Ni B. Regulation of amyloid precursor protein expression and secretion via activation of ERK1/2 by hepatocyte growth factor in HEK293 cells transfected with APP751. Exp Cell Res. 2003;287:387–96. doi: 10.1016/s0014-4827(03)00152-6. [DOI] [PubMed] [Google Scholar]
- 19.Marutle A, Ohmitsu M, Nilbratt M, Greig NH, Nordberg A, Sugaya K. Modulation of human neural stem cell differentiation in Alzheimer (APP23) transgenic mice by phenserine. Proc Natl Acad Sci U S A. 2007;104:12506–11. doi: 10.1073/pnas.0705346104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura I, Takazaki R, Kuwako K, Enokido Y, Yoshikawa K. Upregulation and antiapoptotic role of endogenous Alzheimer amyloid precursor protein in dorsal root ganglion neurons. Exp Cell Res. 2003;286:241–51. doi: 10.1016/s0014-4827(03)00066-1. [DOI] [PubMed] [Google Scholar]
- 21.Oh JW, Schwiebert LM, Benveniste EN. Cytokine regulation of CC and CXC chemokine expression by human astrocytes. J Neurovirol. 1999;5:82–94. doi: 10.3109/13550289909029749. [DOI] [PubMed] [Google Scholar]
- 22.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 23.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajan P, McKay RD. Multiple routes to astrocytic differentiation in the CNS. J Neurosci. 1998;18:3620–9. doi: 10.1523/JNEUROSCI.18-10-03620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ransohoff RM, Tani M. Do chemokines mediate leukocyte recruitment in post-traumatic CNS inflammation? Trends Neurosci. 1998;21:154–9. doi: 10.1016/s0166-2236(97)01198-3. [DOI] [PubMed] [Google Scholar]
- 26.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 27.Sadowski M, Pankiewicz J, Scholtzova H, Ripellino JA, Li Y, Schmidt SD, Mathews PM, Fryer JD, Holtzman DM, Sigurdsson EM, Wisniewski T. A synthetic peptide blocking the apolipoprotein E/beta-amyloid binding mitigates beta-amyloid toxicity and fibril formation in vitro and reduces beta-amyloid plaques in transgenic mice. Am J Pathol. 2004;165:937–48. doi: 10.1016/s0002-9440(10)63355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–7. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- 29.Wong CK, Wang CB, Ip WK, Tian YP, Lam CW. Role of p38 MAPK and NF-kB for chemokine release in coculture of human eosinophils and bronchial epithelial cells. Clin Exp Immunol. 2005;139:90–100. doi: 10.1111/j.1365-2249.2005.02678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamatsuji T, Matsui T, Okamoto T, Komatsuzaki K, Takeda S, Fukumoto H, Iwatsubo T, Suzuki N, Asami-Odaka A, Ireland S, Kinane TB, Giambarella U, Nishimoto I. G protein-mediated neuronal DNA fragmentation induced by familial Alzheimer’s disease-associated mutants of APP. Science. 1996;272:1349–52. doi: 10.1126/science.272.5266.1349. [DOI] [PubMed] [Google Scholar]
- 31.Yanagisawa M, Nakashima K, Taga T. STAT3-mediated astrocyte differentiation from mouse fetal neuroepithelial cells by mouse oncostatin M. Neurosci Lett. 1999;269:169–72. doi: 10.1016/s0304-3940(99)00447-4. [DOI] [PubMed] [Google Scholar]
- 32.Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, Younce C, Binkley PF, Kolattukudy PE. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ Res. 2006;98:1177–85. doi: 10.1161/01.RES.0000220106.64661.71. [DOI] [PMC free article] [PubMed] [Google Scholar]