Abstract
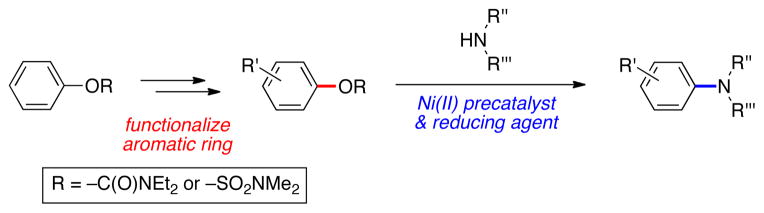
A facile nickel-catalyzed method to achieve the amination of synthetically useful aryl sulfamates and carbamates is reported. Contrary to most Ni-catalyzed amination reactions, this user-friendly approach relies on an air-stable Ni(II) precatalyst, which, when employed with a mild reducing agent, efficiently delivers aminated products in good to excellent yields. The scope of the method is broad with respect to both coupling partners and includes heterocyclic substrates.
Nickel-catalyzed cross-couplings of phenol-based electrophiles have received considerable attention in recent years.1 Attractive aspects of such processes include the low cost of Ni and the many benefits that pertain to utilizing phenol derivatives. Of the substrates widely explored, aryl carbamates and sulfamates are particularly attractive because of their pronounced stability and capacity to direct the installation of functional groups onto an aromatic ring through directed ortho-metallation1,2,3 or electrophilic aromatic substitution processes.2d Although carbon–carbon bond forming reactions using aryl sulfamates and carbamates have been most widely studied,4,5 several reports of carbon–nitrogen bond formation are available.6,7,8,9 Aminations of aryl sulfamates and carbamates are facile and proceed in synthetically useful yields; however, the air-sensitivity of the nickel precatalyst employed in all cases (i.e., Ni(cod)210) limits the widespread use of these C–N bond forming processes.
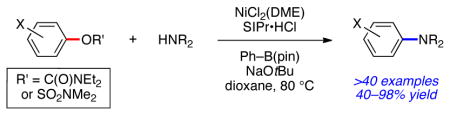
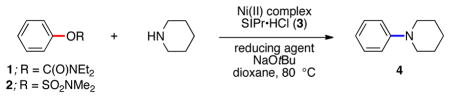
We report the development of sulfamate and carbamate aminations using an inexpensive air-stable nickel(II) precatalyst (Figure 1). When used in combination with phenylboronic acid pinacol ester (Ph–B(pin)) as a mild reducing agent, this procedure provides an efficient and user friendly means to achieve amination reactions across a range of aryl substrates and amine coupling partners.
Figure 1.
Amination of aryl carbamates and sulfamates using Ni(II) precatalyst.
A key challenge in developing the desired amination reaction using a Ni(II) precatalyst is the difficulty in reducing Ni(II) to Ni(0). Although Pd(II) precatalysts readily undergo in situ reduction with amines or phosphines in Pd-catalyzed Buchwald–Hartwig couplings, the corresponding reduction of Ni(II) is less facile. Ni-catalyzed amination methodologies that use Ni(II) precatalysts are only available for aryl halides, and typically use Zn, silanes, or hydrides as reducing agents.11
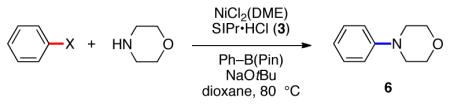
We selected phenylcarbamate 1 and phenylsulfamate 2 as substrates for the desired amination, and then surveyed Ni(II) complexes in the presence of the NHC ligand SIPr•HCl (3)12 and various reducing agents. Key results are summarized in Table 1, where piperidine was employed as the amine coupling partner. Reaction conditions utilizing Zn dust proved ineffective (entries 1 and 2), while the use of triethylsilane gave either poor or modest results (entries 3 and 4). Inspired by Suzuki–Miyaura coupling methodologies of sulfamates and carbamates, where boronic acids serve to reduce Ni(II) to Ni(0) in situ,4 we tested the use of Ph–B(OH)2 in the amination reaction. Gratifyingly, good to excellent yields could be obtained (entries 5 and 6), and NiCl2(DME) complex13 was identified as the optimal Ni precatalyst (entry 6). Although these results were promising, we found that the corresponding coupling of sulfamate 2 gave inconsistent results (entry 7). Nonetheless, it was observed that boronic esters could be used in place of Ph–B(OH)2 or boroxines to give more consistent results (entries 8 and 9). By using Ph–B(pin) as reducing agent with NiCl2(DME) as precatalyst, a 94% yield of the desired aminated product 4 was obtained. These conditions were also found to be useful for the coupling of carbamate 1 (entry 10).
Table 1.
Optimization of amination using Ni(II) precatalysta
| ||||
|---|---|---|---|---|
| entry | substrate | Ni source | reducing agent | yieldb |
| 1 | 1 | Ni(acac)2 | Zn dustc | 0% |
| 2 | 1 | NiCl2(DME) | Zn dustc | 0% |
| 3 | 1 | Ni(acac)2 | H–SiEt3c | 0% |
| 4 | 1 | NiCl2(DME) | H–SiEt3c | 51% |
| 5 | 1 | Ni(acac)2 | Ph–B(OH)2 | 57% |
| 6 | 1 | NiCl2(DME) | Ph–B(OH)2 | 98% |
| 7 | 2 | NiCl2(DME) | Ph–B(OH)2 | variable |
| 8 | 2 | NiCl2(DME) | (Ph–BO)3 | 58% |
| 9 | 2 | NiCl2(DME) | Ph–B(pin) | 94% |
| 10 | 1 | NiCl2(DME) | Ph–B(pin) | 92% |
Conditions: Ni(II) complex (5 mol %), 3 (10 mol %), sulfamate/carbamate substrate (1 equiv), piperidine (1.2 equiv), reducing agent (0.55 equiv), NaOtBu (1.85 equiv), hexamethylbenzene (0.1 equiv), 3 h.
Yield determined by 1H NMR analysis of the crude reaction mixtures using hexamethylbenzene as an internal standard.
Reducing agent (0.8 equiv) and NaOtBu (1.4 equiv).
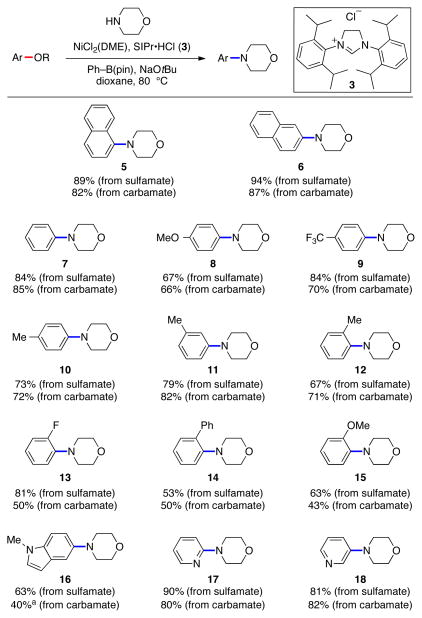
Having identified optimal reaction conditions,14 we examined the scope of aryl sulfamates and carbamates, using morpholine as the amine coupling partner (Figure 2). Fused arenes were tolerated, as demonstrated by the smooth formation of 5 and 6. The ability to form 7–12 in good yields shows the methodology’s tolerance to non-fused arenes with a variety of substituent patterns. It should also be noted that ortho-substituted substrates, which are readily accessible by ortho-functionalization of phenyl sulfamates or carbamates,2,3 underwent the desired coupling to give 12–15. Heterocycles such as indoles and pyridines were also tolerated, as revealed by the formation of products 16–18. In many cases, sulfamates and carbamates perform equally well in this amination methodology.
Figure 2.
Amination of aryl sulfamates and carbamates using morpholine. Reaction conditions: NiCl2(DME) (5–20 mol %), 3 (10–40 mol %), sulfamate/carbamate substrate (1 equiv), morpholine (1.2–2.4 equiv), Ph–B(pin) (0.15–1.4 equiv), NaOtBu (1.4–3.75 equiv), 3 h. Unless otherwise noted, yields reflect those of isolated product. aYield determined by 1H NMR analysis with hexamethylbenzene as internal standard.
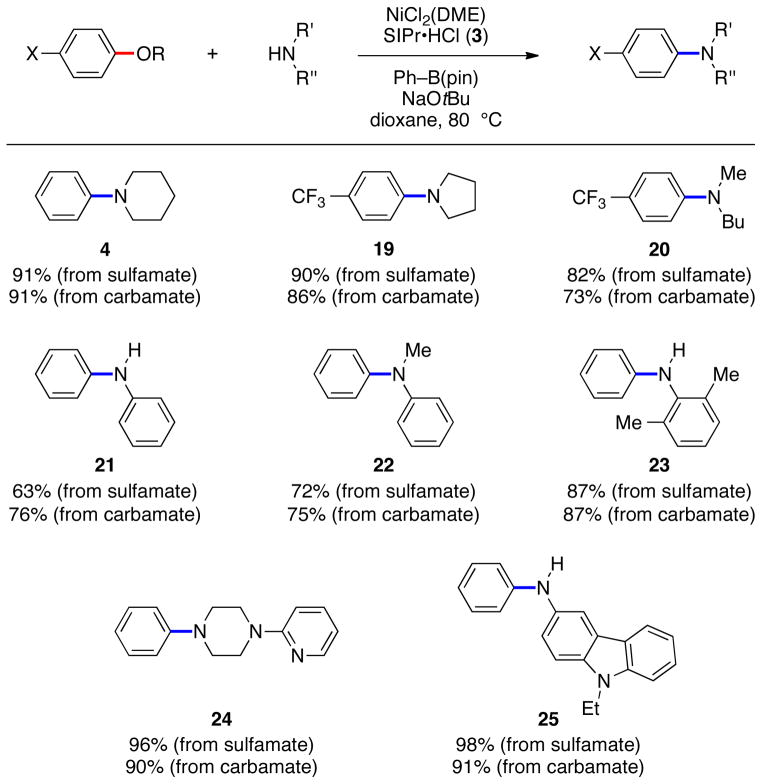
The scope with respect to the amine coupling partner is provided in Figure 3. In addition to morpholine, the cyclic amines piperidine and pyrrolidine underwent the desired coupling to furnish 4 and 19. Acyclic secondary amines and anilines were also tolerated, as shown by the formation of 20–23. Finally, amines with appended heterocycles were also tolerated, thus giving rise to 24–25.
Figure 3.
Amination of various amines. Reaction conditions: NiCl2(DME) (5–20 mol %), 3 (10–40 mol %), sulfamate/carbamate substrate (1 equiv), amine (1.2–2.4 equiv), Ph–B(pin) (0.15–1.05 equiv), NaOtBu (1.4–3.75 equiv), 3 h. Unless otherwise noted, yields reflect those of isolated product.
Given that most Ni-catalyzed amination reactions employ Ni(0) precatalysts, we tested the generality of our optimal reaction conditions on other electrophilic substrate classes using morpholine as the coupling partner (Table 2). Although modest results were obtained using phenyl-t-Bu-carbonate and phenyl pivalate (entries 1 and 2), the use of phenyl tosylate was more promising, giving a 63% yield of product (entry 3). Phenyl triflate was not a suitable coupling partner (entry 4), and low yields were observed using iodobenzene or bromobenzene as substrate (entries 5 and 6). On the other hand, chlorobenzene coupled smoothly under our reaction conditions to furnish the desired aminated product in 98% yield (entry 7).
Table 2.
Survey of Halide and Pseudohalide Substratesa
| ||
|---|---|---|
| entry | X | yieldb |
| 1 | OCO2tBu | 15% |
| 2 | OPiv | 44% |
| 3 | OTs | 63% |
| 4 | OTf | 4% |
| 5 | I | 25% |
| 6 | Br | 33% |
| 7 | Cl | 98% |
Conditions: NiCl2(DME) (5 mol %), 3 (10 mol %), substrate (1 equiv), morpholine (1.8 equiv), Ph–B(pin) (0.35 equiv), NaOtBu (2.25 equiv), hexamethylbenzene (0.1 equiv), 3 h.
Yield determined by 1H NMR analysis with hexamethylbenzene as internal standard.
In summary, we have developed a facile nickel-catalyzed method to achieve the amination of synthetically useful aryl sulfamates and carbamates. Our user-friendy approach employs NiCl2(DME) as a bench-stable Ni(II) precatalyst, in addition to the mild reducing agent Ph–B(pin), to furnish aminated products in good to excellent yields. Given the attractive features of aryl sulfamates and carbamates, coupled with the transformation’s broad scope, this Ni(II)-based methodology is expected to find use in various applications that require C–N bond construction.
Supplementary Material
Acknowledgments
The authors are grateful to Boehringer Ingelheim, DuPont, Eli Lilly, Amgen, AstraZeneca, Roche, the A. P. Sloan Foundation, the Foote family (fellowship to S.D.R), and the University of California, Los Angeles for financial support. We thank Materia Inc. for chemicals. These studies were supported by shared instrumentation grants from the NSF (CHE-1048804) and the National Center for Research Resources (S10RR025631).
Footnotes
Supporting Information Available: Experimental details and compound characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For recent reviews regarding the cross-coupling of phenolic derivatives, see: Rosen BM, Quasdorf KW, Wilson DA, Zhang N, Resmerita AM, Garg NK, Percec V. Chem Rev. 2011;111:1346–1416. doi: 10.1021/cr100259t.Li BJ, Yu DG, Sun CL, Shi ZJ. Chem Eur J. 2011;17:1728–1759. doi: 10.1002/chem.201002273.Yu DG, Li BJ, Shi ZJ. Acc Chem Res. 2010;43:1486–1495. doi: 10.1021/ar100082d.Knappke CEI, Jacobi von Wangelin A. Angew Chem Int Ed. 2010;49:3568–3570. doi: 10.1002/anie.201001028.Goossen LJ, Goossen K, Stanciu C. Angew Chem Int Ed. 2009;48:3569–3571. doi: 10.1002/anie.200900329.
- 2.(a) Snieckus V. Chem Rev. 1990;90:879–933. [Google Scholar]; (b) Hartung CG, Snieckus V. In: Modern Arene Chemistry. Astruc D, editor. Wiley–VCH; New York: 2002. pp. 330–367. [Google Scholar]; (c) Macklin T, Snieckus V. In: Handbook of C–H Transformations. Dyker G, editor. Wiley–VCH; New York: 2005. pp. 106–119. [Google Scholar]; (d) Smith MB, March J. March’s Advanced Organic Chemistry. 6. John Wiley & Sons, Inc; New Jersey: 2007. p. 670. [Google Scholar]; (e) Macklin TK, Snieckus V. Org Lett. 2005;7:2519–2522. doi: 10.1021/ol050393c. [DOI] [PubMed] [Google Scholar]
- 3.Aryl carbamates may also be ortho-functionalized through transition metal-catalyzed processes; see: Bedford RB, Webster RL, Mitchell CJ. Org Biomol Chem. 2009;7:4853–4857. doi: 10.1039/b916724m.Zhao X, Yeung CS, Dong VM. J Am Chem Soc. 2010;132:5837–5844. doi: 10.1021/ja100783c.Nishikata T, Abela AR, Huang S, Lipshutz BH. J Am Chem Soc. 2010;132:4978–4979. doi: 10.1021/ja910973a.Yamazaki K, Kawamorita K, Ohmiya H, Sawamura M. Org Lett. 2010;12:3978–3981. doi: 10.1021/ol101493m.Feng C, Loh TP. Chem Commun. 2011;47:10458–10460. doi: 10.1039/c1cc14108b.Gong TJ, Xiao B, Liu ZJ, Wan J, Xu J, Luo DF, Fu Y, Liu L. Org Lett. 2011;13:3235–3237. doi: 10.1021/ol201140q.
- 4.For the use of aryl carbamates in C–C bond forming processes, see: Quasdorf KW, Riener M, Petrova KV, Garg NK. J Am Chem Soc. 2009;131:17744–17749. doi: 10.1021/ja906477r.Finch AA, Blackburn T, Snieckus V. J Am Chem Soc. 2009;131:17750–17752. doi: 10.1021/ja907700e.Xi L, Li BJ, Wu ZH, Lu XY, Guan BT, Wang BQ, Zhao KQ, Shi ZJ. Org Lett. 2010;12:884–887. doi: 10.1021/ol9029534.Yoshikai N, Matsuda H, Nakamura E. J Am Chem Soc. 2009;131:9590–9599. doi: 10.1021/ja903091g.Quasdorf KW, Antoft-Finch A, Liu P, Silberstein AL, Komaromi A, Blackburn T, Ramgren SD, Houk KN, Snieckus V, Garg NK. J Am Chem Soc. 2011;133:6352–6363. doi: 10.1021/ja200398c.Baghbanzadeh M, Pilger C, Kappe CO. J Org Chem. 2011;76:1507–1510. doi: 10.1021/jo1024464.Dallaire C, Kolber I, Gingras M. Org Synth. 2002;78:42.Sengupta S, Leite M, Raslan DS, Quesnelle C, Snieckus V. J Org Chem. 1992;57:4066–4068.
- 5.For the use of aryl sulfamates in C–C bond forming processes, see: Civicos JF, Gholinejad M, Alonso DA, Najera C. Chem Lett. 2011;40:907–909.Shirbin SJ, Boughton BA, Zammit SC, Zanatta SD, Marcuccio SM, Hutton CA, Williams SJ. Tetrahedron Lett. 2010;51:2971–2974.Albaneze-Walker J, Raju R, Vance JA, Goodman AJ, Reeder MR, Liao J, Maust MT, Irish PA, Espino P, Andrews DR. Org Lett. 2009;11:1463–1466. doi: 10.1021/ol802381k.Ackermann L, Barfüsser S, Pospech J. Org Lett. 2010;12:724–726. doi: 10.1021/ol9028034.Leowanawat P, Zhang N, Resmerita AM, Rosen BM, Percec V. J Org Chem. 2011;76:9946–9955. doi: 10.1021/jo202037x.When PM, Du Bois J. Org Lett. 2005;7:4685–4688. doi: 10.1021/ol051896l.Chen GJ, Han FS. Eur J Org Chem. 2012:3575–3579.Zhang N, Hoffman DJ, Gutsche N, Gupta J, Percec V. J Org Chem. 2012;77:5956–5964. doi: 10.1021/jo300547v.; see also references 2e, 4a, 4e, and 4f.
- 6.For examples of sulfamate and carbamate amination, see: Mesganaw T, Silberstein AL, Ramgren SD, Fine Nathel NF, Hong X, Liu P, Garg NK. Chem Sci. 2011;2:1766–1771. doi: 10.1039/c1sc00230a.Ramgren SD, Silberstein AL, Yang Y, Garg NK. Angew Chem Int Ed. 2011;50:2171–2173. doi: 10.1002/anie.201007325.Ackermann L, Sandmann R, Song W. Org Lett. 2011;13:1784–1786. doi: 10.1021/ol200267b.
- 7.For examples of the amination or aryl sulfonates, see: Hamann BC, Hartwig JF. J Am Chem Soc. 1998;120:7369–7370.Roy AH, Hartwig JF. J Am Chem Soc. 2003;125:8704–8705. doi: 10.1021/ja035835z.Ogata T, Hartwig JF. J Am Chem Soc. 2008;130:13848–13849. doi: 10.1021/ja805810p.Gao C-Y, Yang L-M. J Org Chem. 2008;73:1624–1627. doi: 10.1021/jo7022558.Fors BP, Watson DA, Biscoe MR, Buchwald SL. J Am Chem Soc. 2008;130:13552–13554. doi: 10.1021/ja8055358.So CM, Zhou Z, Lau CP, Kwong FY. Angew Chem Int Ed. 2008;47:6402–6406. doi: 10.1002/anie.200802157.
- 8.For the amination of aryl methyl ethers, see: Tobisu M, Shimasaki T, Chatani N. Chem Lett. 2009;38:710–711.
- 9.For the amination of aryl pivalates, see: Shimasaki T, Tobisu M, Chatani N. Angew Chem Int Ed. 2010;49:2929–2932. doi: 10.1002/anie.200907287.
- 10.Ni(cod)2 is commercially available from Strem Chemicals Inc. or Sigma-Aldrich (CAS # 1295-35-8). For more information on this catalyst, see: Wender PA, Smith TE, Zuo G, Duong HA, Louie J. Encyclopedia of Reagents for Organic Synthesis. 2006 doi: 10.1002/047084289X.rb118.pub2.
- 11.(a) Fan XH, Li G, Yang LM. J Organomet Chem. 2011;696:2482–2484. [Google Scholar]; (b) Tobisu M, Shimasaki T, Chatani N. Chem Lett. 2009;38:710–711. [Google Scholar]; (c) Gao CY, Cao X, Yang LM. Org Biomol Chem. 2009;7:3922–3925. doi: 10.1039/b911286c. [DOI] [PubMed] [Google Scholar]; (d) Manolikakes G, Gavryushin A, Knochel P. J Org Chem. 2008;73:1429–1434. doi: 10.1021/jo702219f. [DOI] [PubMed] [Google Scholar]; (e) Amrani RO, Thomas A, Brenner E, Schneider R, Fort Y. Org Lett. 2003;5:2311–2314. doi: 10.1021/ol034659w. [DOI] [PubMed] [Google Scholar]; (f) Desmarets C, Schneider R, Fort Y. J Org Chem. 2002;67:3029–3036. doi: 10.1021/jo016352l. [DOI] [PubMed] [Google Scholar]; (g) Gradel B, Brenner E, Schneider R, Fort Y. Tetrahedron Lett. 2001;42:5689–5692. [Google Scholar]
- 12.NHC ligand 3 has been employed in the Ni-catalyzed amination of sulfamates and carbamates; see refs 6a and 6b.
- 13.NiCl2(DME) is commercially available from Strem Chemicals Inc. (CAS #29046-78-4) at an approximate cost of $13 USD / gram.
- 14.Although the optimal reaction conditions shown in Table 1 (entries 9 and 10) are generally useful across a range of substrates, further optimization of reaction conditions for individual substrates led to improved yields. The optimized conditions and results are shown in Figures 2 and 3.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.