Abstract
Treatment with ex vivo–generated regulatory T cells (T-reg) has been regarded as a potentially attractive therapeutic approach for autoimmune diseases. However, the dynamics and function of T-reg in autoimmunity are not well understood. Thus, we developed Foxp3gfp knock-in (Foxp3gfp.KI) mice and myelin oligodendrocyte glycoprotein (MOG)35–55/IAb (MHC class II) tetramers to track autoantigen-specific effector T cells (T-eff) and T-reg in vivo during experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis. MOG tetramer–reactive, Foxp3+ T-reg expanded in the peripheral lymphoid compartment and readily accumulated in the central nervous system (CNS), but did not prevent the onset of disease. Foxp3+ T cells isolated from the CNS were effective in suppressing naive MOG-specific T cells, but failed to control CNS-derived encephalitogenic T-eff that secreted interleukin (IL)-6 and tumor necrosis factor (TNF). Our data suggest that in order for CD4+Foxp3+ T-reg to effectively control autoimmune reactions in the target organ, it may also be necessary to control tissue inflammation.
Thymus-derived (natural) T-reg are crucial for preventing generalized multiorgan autoimmunity throughout the lifespan of an individual1,2. The importance of antigen-specific T-reg in conferring genetic resistance to organ-specific autoimmunity3 and in limiting autoimmune tissue damage has also been documented in disease models for multiple sclerosis4, type I diabetes5,6 and rheumatoid arthritis7. In humans, a decreased frequency of occurrence of T-reg8 or defects in their suppressor function9 have been demonstrated in multiple sclerosis and lupus10,11.
In vitro, natural T-reg are anergic and suppress T-eff responses in a cell contact–dependent manner12. In vivo, T-reg can proliferate and their mechanism of suppression remains largely unknown13,14. It was suggested that the encounter with autoantigens was required for their maintenance in vivo15. However, it is unclear whether expansion of antigen-specific T-reg can actually be triggered during an inflammatory autoimmune response. This is a fundamental question as T-reg that bear transgenic autoreactive T-cell receptors (TcRs) seem to suppress autoimmune diabetes better than a polyclonal T-reg population does16. Furthermore, it has been shown that naive T cells develop reciprocally into T-reg or pathogenic T-helper type 17 (TH17) cells depending on the absence or presence of IL-6 in the local cytokine milieu17–19. Thus, the origin and population dynamics of T-reg in autoimmune tissue inflammation are unclear. It has been proposed that in EAE, T-reg may not even reach the target organ but may prevent the trafficking of autopathogenic T-eff into the CNS (ref. 4). More recent studies have suggested that T-reg themselves might be targeted to the CNS (ref. 20).
The transcription factor Foxp3 has been identified as crucial for the commitment of thymocytes to the T-reg lineage21–23. We and others have generated Foxp3gfp.KI mice in order to reliably monitor T-reg in vivo based on the linked expression of green fluorescent protein (GFP) in Foxp3+ T cells19,24. Using Foxp3gfp.KI mice in combination with a MOG35–55/IAb-specific tetramer, we tracked antigen-specific T-reg and T-eff in vivo during the course of EAE and tested whether T-reg present in the target tissue were functional in suppressing autopathogenic T cells.
RESULTS
T-reg infiltrate the CNS during EAE
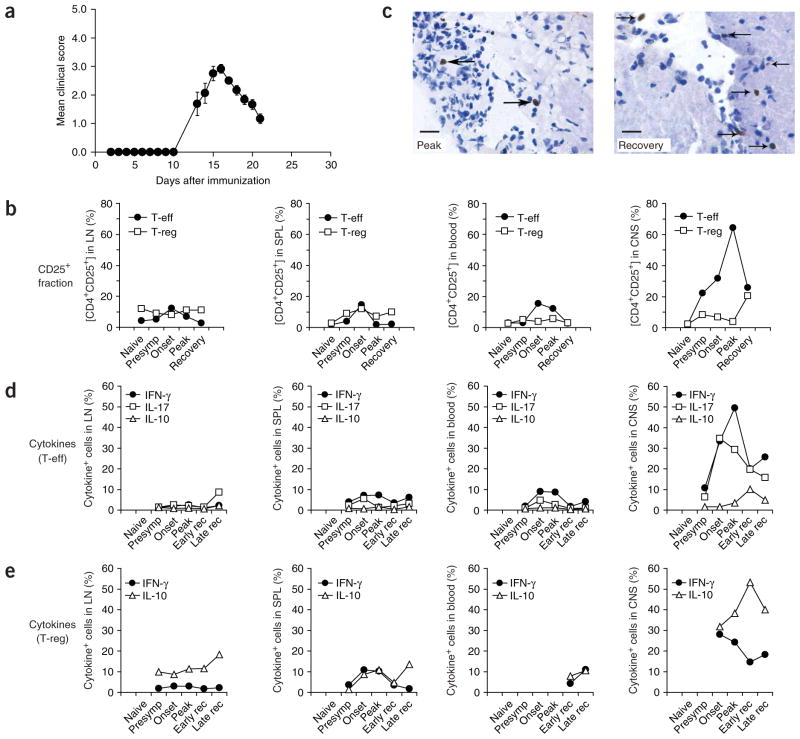
Timing of expansion and trafficking of T-reg during an autoimmune response have been unclear in a system that is not artificially biased by transfer of T-reg or transgenic expression of monoclonal TcRs. Using Foxp3gfp.KI mice, we addressed this question by tracking Foxp3+ T-reg based on their expression of GFP in different lymphoid compartments and the target organ during MOG35–55-induced EAE (Fig. 1a and Supplementary Fig. 1 online). Whereas the fraction of Foxp3/GFP+ T-reg within the CD4+ compartment was essentially constant in secondary lymphoid tissues and blood, the frequency of activated (CD25+Foxp3/GFP−) T-eff in the draining lymph nodes and spleen showed a moderate increase in the presymptomatic and initial disease phases and regressed to baseline at the peak of disease (Fig. 1b). However in the CNS, T-eff increased markedly about 3 d before the peak of disease and rapidly declined at the initiation of recovery (between day 16 and day 21, Fig. 1b). In contrast, the Foxp3/GFP+ T-reg population, which was detectable in the CNS as early as day 10, did not expand during the peak of disease but neither did it contract during the transition to recovery. Thus, the share of T-reg within the CD4+ T-cell population of the CNS increased fivefold (from 4 to 20, Fig. 1b) as the mice went into remission. Foxp3+ cells were detected in meningeal and brain parenchymal inflammatory foci. In 20 inflammatory foci at disease peak, there were 1.5 ± 0.2 Foxp3+ cells per focus (Fig. 1c), whereas in recovery there were 5.3 ± 1.1 Foxp3+ cells per focus (Fig. 1c, P < 0.001, t-test).
Figure 1.
T-reg infiltrate the CNS during EAE. (a) EAE was induced in Foxp3gfp.KI mice by immunization with MOG35–55 in complete Freund adjuvant (CFA). The course of EAE in these mice is shown as mean clinical score (± s.e.m., n = 8). (b) T-eff and T-reg population dynamics in the draining lymph nodes (LN), spleen (SPL), blood and the CNS at different stages of EAE. Mononuclear cells were prepared and analyzed by flow cytometry. CD4+Foxp3/GFP− (T-eff) and CD4+Foxp3/GFP+ (T-reg) are shown according to their expression level of CD25. (c) Immunohistochemical analysis of Foxp3+ cells (arrows) in inflammatory foci in a mouse at the peak of disease (left) and during recovery (right). Scale bars, 15 μm. (d,e) Mononuclear cells were isolated from LN, SPL, blood and the CNS, activated with phorbol 12-myristate 13-acetate (PMA)/ionomycin, and stained for intracellular cytokines. Data represent the percentage of cytokine+ cells within the T-eff population (CD4+Foxp3/GFP−) (d) and the T-reg population (CD4+Foxp3/GFP+) (e) at different phases of the disease.
The CNS-infiltrating T-eff (CD4+Foxp3/GFP−) produced massive amounts of IL-17 and interferon (IFN)-γ, whereas IL-17 and IFN-γ production from peripheral T-eff was one order of magnitude lower (Fig. 1d and Supplementary Fig. 2 online). Notably, Foxp3/GFP+ T-reg also produced IFN-γ, but this was transient and rapidly declined even before the peak of disease (Fig. 1e). However, approximately one-third of all T-reg in the CNS were IL-10+ at the onset of disease (day 12) and more than half of the Foxp3+ T-reg in the CNS were IL-10+ during recovery (day 21, Fig. 1e). At no stage of the disease could we recover T-cell populations from blood or peripheral lymphoid compartments that exhibited such marked cytokine phenotypes. Thus, T-reg and T-eff dynamics and their functional phenotype in the CNS, but not in the lymphoid compartment or blood, closely correlated with the clinical disease course.
T-reg expand from natural T-reg in autoimmune inflammation
T-reg not only occur as thymus-derived natural T-reg but can be induced upon encounter of T cells with antigen in a specific cytokine milieu25. To characterize the origin of Foxp3/GFP+ T-reg upon immunization with MOG35–55 emulsified in complete Freund adjuvant (CFA), we used different adoptive transfer systems. In the first set of experiments, we transferred either unsorted splenocytes from Foxp3gfp.KI donor mice (CD45.2) comprising a fraction of 2% Foxp3+ cells or FACS-sorted Foxp3/GFP− splenocytes into congenic hosts (CD45.1). The recipients were immunized with MOG35–55/CFA. After 6 d, we analyzed draining lymph nodes for Foxp3/GFP expression in the CD45.2+ donor population. We detected a distinct CD4+Foxp3/GFP+ population when the mice received unsorted splenocytes. However, Foxp3/GFP expression was not induced in donor cells when Foxp3/GFP− splenocytes were transferred (Fig. 2a). This indicated that Foxp3− T cells were not likely to be converted into Foxp3+ T cells in the peripheral lymphoid compartment by sensitization with autoantigen in vivo.
Figure 2.
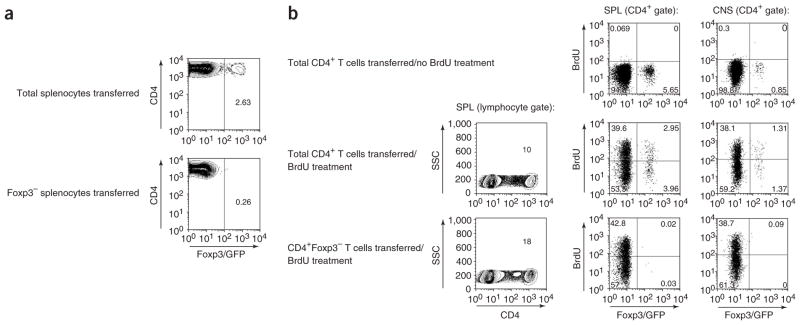
Foxp3/GFP− T cells are not converted into T-reg during EAE. (a) Total splenocytes or 5 × 106 FACS-purified Foxp3/GFP− splenocytes from CD45.2 Foxp3gfp.KI donor mice were transferred intravenously (i.v.) into CD45.1 congenic hosts. After 1 d, the recipient mice were immunized with MOG35–55/CFA. After 6 d, draining lymph node cells were isolated and analyzed for Foxp3/GFP expression in the CD4+ T-cell population positive for the donor-specific marker CD45.2. Numbers indicate percentages within the CD4+CD45.2+ gate. (b) We transferred 10 × 106 total CD4+ T cells or 5 × 106 FACS-sorted CD4+Foxp3/GFP− T cells from naive Foxp3gfp.KI donor mice intraperitoneally (i.p.) into Rag2−/− recipients. After 5 d, the recipients were immunized with MOG/CFA. Starting from day 9 after immunization, the mice were treated with 2 mg BrdU i.p. every other day up to a cumulative dose of 8 mg BrdU as indicated. Eight hours after the last BrdU injection, CD4+ T cells recovered from the spleen and the CNS were analyzed by flow cytometry for the expression of Foxp3/GFP and the incorporation of BrdU. Representative flow cytometric analyses, which indicate percentages of BrdU+ cells in the corresponding gates, are shown for one individual mouse of each group. Each group consisted of two or three mice. Two independent experiments were performed.
As Foxp3/GFP+ T-reg were readily detected in the CNS during EAE, we wondered whether the local milieu in the target organ might promote the de novo generation of Foxp3/GFP+ cells, thus accounting for the marked increase in T-reg fraction in the CNS during recovery. To address this, we transferred either total CD4+ T cells containing a fraction of 8% Foxp3+ T cells or FACS-sorted CD4+Foxp3/GFP− T cells from Foxp3gfp.KI donor mice into Rag-deficient recipients. This was followed by immunization with MOG/CFA after 5 d. Also, in order to compare the rate of proliferation of T-eff and T-reg, the recipients were treated with bromodeoxyuridine (BrdU). After 16 d, a distinct CD4+Foxp3/GFP+ T-cell population was isolated from the spleen and the CNS of hosts that had received total unsorted CD4+ T cells (Fig. 2b). However, Foxp3/GFP+ T cells were not detected either in the spleen or in the CNS of mice that had received FACS-sorted CD4+Foxp3/GFP− T cells (Fig. 2b). The immunized recipients were tested for BrdU incorporation into Foxp3/GFP+ T cells. About 50% of the CD4+Foxp3/GFP+ T cells recovered from hosts that had received total CD4+ T cells or FACS-sorted CD4+Foxp3/GFP+ T-reg had incorporated BrdU (Fig. 2b and Supplementary Fig. 3 online), confirming that T-reg proliferated in vivo13,26. In recipients of total CD4+ T cells, the fraction of proliferating (BrdU+) T-reg was similar in the spleen and the CNS, with a trend of higher proliferation in the CNS (Fig. 2b). There was no significant CD4+ T-cell infiltration into the CNS of hosts that had received CD4+Foxp3/GFP+ T-reg only (data not shown). Thus, whereas natural T-reg readily proliferated, conversion of Foxp3− into Foxp3+ T cells was unlikely to occur following immunization with MOG/CFA.
Immunization with MOG35–55 expands myelin-specific T-reg
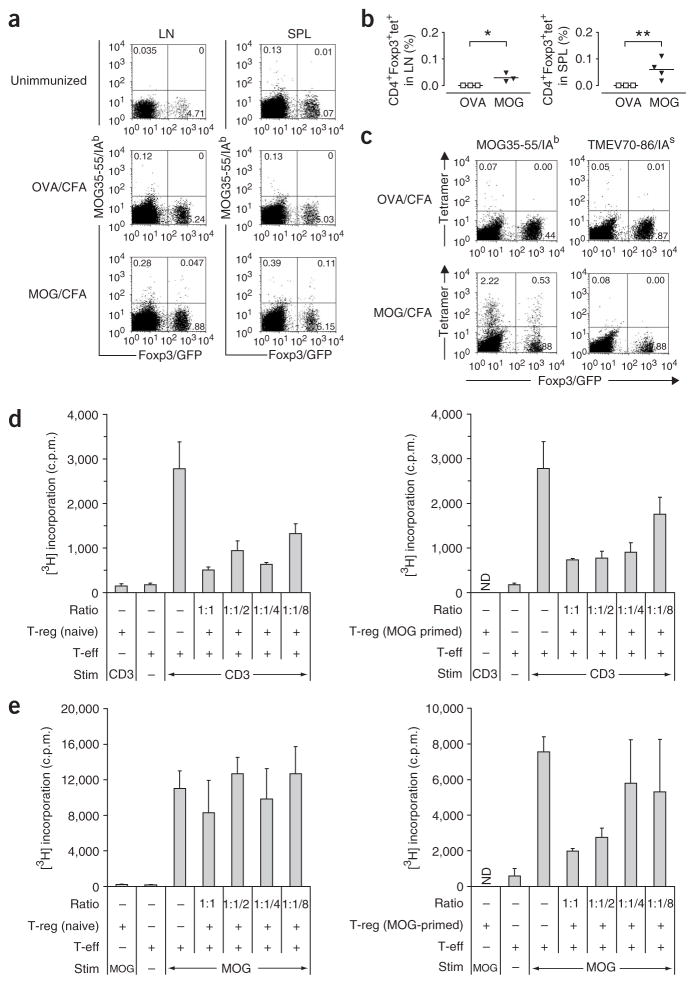
It is unknown whether autoantigen-specific T-reg expand in vivo following immunization with myelin antigens. Thus, we combined the T-reg analysis in Foxp3gfp.KI mice with the use of MOG35–55/IAb tetramers and tracked T-eff (CD4+Foxp3/GFP−) and T-reg (CD4+Foxp3/GFP+) based on their myelin antigen specificity. As expected, ex vivo staining with MOG tetramers of draining lymph node cells and splenocytes revealed a fraction of tetramer-positive T-eff in mice immunized with MOG35–55 but not in naive mice or mice immunized with a control peptide (OVA323–339, Fig. 3a). However, MOG tetramers consistently also stained a distinct population of T-reg in the secondary lymphoid tissue of MOG-sensitized mice (Fig. 3a,b). The frequency of MOG tetramer–positive T cells both in the T-eff and T-reg compartments was markedly amplified after short-term in vitro culture (Fig. 3c).
Figure 3.
Antigen-specific T-reg are expanded upon immunization with MOG35–55 and can be triggered by MOG35–55 to suppress T-eff responses. (a) Lymph node cells and splenocytes were isolated from unimmunized Foxp3gfp.KI mice or from Foxp3gfp.KI mice that had been in vivo–sensitized with OVA323–339/CFA or MOG35–55/CFA 8 d earlier. The cells were stained ex vivo with MOG35–55/IAb tetramers. Live (7AAD−) CD4+ T-cell populations are shown. The numbers in each quadrant represent percentages. (b) MOG tetramers detected a significant population of myelin-specific T-reg in the draining lymph nodes (*P < 0.05) and spleens (**P < 0.03) of MOG-immunized, but not OVA-immunized, mice. (c) After short-term culture of splenocytes in the presence of the immunizing antigen, MOG-specific T-eff and T-reg were visualized by MOG35–55/IAb tetramer staining. (d) CD4+Foxp3/GFP+ T-reg isolated by FACS sorting from the spleens of naive or MOG35–55-immunized mice were tested for their suppressive capacity in vitro. A fixed number of naive 2D2 responder T cells (CD4+CD62L+CD25−) was cultured with titrated numbers of T-reg in the presence of irradiated syngeneic splenocytes as antigen-presenting cells (APCs) and a monoclonal antibody to CD3. (e) In an analogous assay, naive and MOG-primed T-reg were compared for their capacity to suppress MOG35–55-specific recall responses. Mean [3H]thymidine incorporation indicated as c.p.m. (+ s.d.) in triplicate wells. The ratios indicated in d and e represent the ratios of T-eff:T-reg. ND, not done.
Next we compared the capacity of purified CD4+Foxp3/GFP+ T cells from naive and MOG-sensitized mice to suppress an auto-antigen-driven T-cell response. Both T-reg populations (naive and MOG-primed) were equally effective in suppressing the proliferation of responder T cells in a suppression assay driven by antibody to CD3 (Fig. 3d). However, in a MOG-driven suppression assay, naive T-reg did not inhibit proliferation (Fig. 3e). In contrast, MOG-primed Foxp3/GFP+ T cells were effective in suppressing the MOG35–55-specific proliferation of naive 2D2 responder T cells27 and MOG-primed splenic CD4+Foxp3/GFP−T-eff (Fig. 3e and data not shown). Thus, in vitro stimulation with the specific antigen (MOG) was insufficient to trigger the suppressive function of naive Foxp3/GFP+ T-reg, indicating that the frequency of MOG35–55-specific T-reg in the naive repertoire was too low to mediate antigen-specific suppression.
MOG-specific T-reg in the CNS do not contract
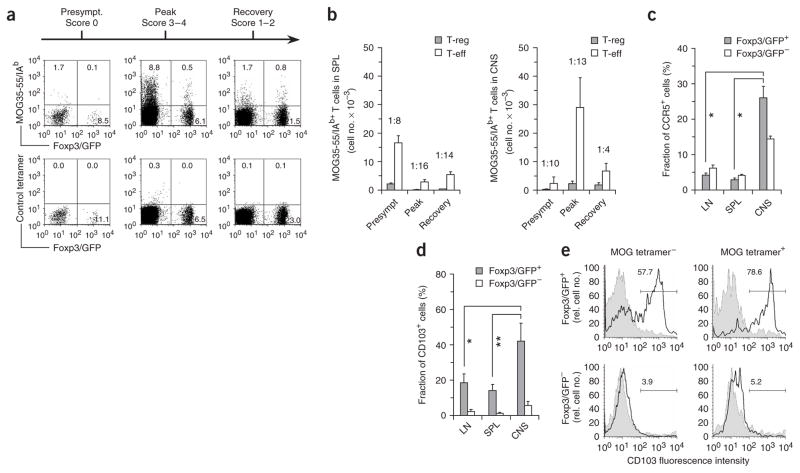
Consistent with our current understanding, the frequency of MOG-specific T-eff in the CNS (CD4+Foxp3/GFP−tetramer+) was highest (approximately 10%) around the peak of disease and dropped to less than 2% during recovery (Fig. 4a). Notably, at the peak of disease, 0.5% of all CD4+ T cells in the CNS were MOG-specific T-reg (Foxp3/GFP+tetramer+), and this fraction increased to approximately 1% during recovery (Fig. 4a). Regarding the absolute numbers of MOG-specific T cells, both MOG-specific T-reg and T-eff in the spleen were most abundant during the presymptomatic disease phase (priming) but were markedly decreased when the mice were overtly sick (Fig. 4b). However in the target organ, the number of MOG-specific T-eff paralleled the clinical disease score, in that the highest numbers of antigen-specific T-eff were found at the peak of disease with a marked decrease in the recovery phase (Fig. 4b). Notably, at the onset of recovery, the number of MOG-specific T-reg in the CNS did not decline. This resulted in a substantial change in the ratio of MOG-specific T-reg/T-eff, which increased from 1:13 at the peak of disease to 1:4 during remission (Fig. 4b).
Figure 4.
Myelin-specific T-reg accumulate in the CNS. Mononuclear cells were prepared from the CNS of MOG/CFA-immunized mice at different stages of EAE as indicated. (a) CD4+ T cells stained with MOG35–55/IAb tetramer or a control-tetramer. The numbers indicate percentages within the CD4+ T-cell gate. (b) Absolute numbers of MOG tetramer–positive T-reg versus T-eff recovered from the spleen (SPL) and the CNS before disease onset (Presympt), at the peak of disease and during recovery. The ratio of MOG-specific T-reg:T-eff is indicated for each disease phase. (c) Comparison of CCR5 expression on CD4+Foxp3/GFP+ T cells (T-reg) and CD4+Foxp3/GFP− T cells (T-eff) isolated from the lymph nodes (LN), spleen (SPL) and CNS of EAE mice at the peak of disease (day 14). Mean (+ s.d.) of isotype-controlled expression of CCR5. n = 3. *P < 0.004, t-test. (d) CD103 (integrin αEβ7) expression on T-reg (CD4+Foxp3/GFP+) versus T-eff (CD4+Foxp3/GFP−) at the peak of disease. Mean (+ s.d.) of isotype-controlled expression of CD103. n = 4–7. *P < 0.003, **P < 0.002, t-test. (e) CD103+ cells are enriched in MOG tetramer–positive T-reg. Three-color staining for CD4, MOG tetramer and CD103 in CNS mononuclear cells from EAE mice. The fraction (as a percentage) of CD103+ cells (black line) versus control IgG (shaded curve) is shown in the MOG tetramer–negative and MOG tetramer–positive T-reg (top) and T-eff (bottom) populations.
As compared to LN- or SPL-T-reg, T-reg from the CNS expressed significantly elevated levels of CCR5 (Fig. 4c) and CD103 (αEβ7, Fig. 4d), which have been associated with the targeting of T-reg into sites of inflammation28–30. Therefore, the pool of T-reg in the CNS might not only be maintained by proliferation of MOG-specific T-reg in situ, but also by trafficking of T-reg from the periphery. CD103 was particularly enriched in MOG tetramer–positive relative to MOG tetramer–negative T-reg (Fig. 4e). Thus, T-reg recovered from the inflamed CNS comprised a high fraction of MOG-specific T-reg and displayed an ‘activated/migratory’ phenotype that was clearly distinct from the phenotype of T-reg present in lymphoid tissue.
CNS-T-eff are resistant to suppression at the disease peak
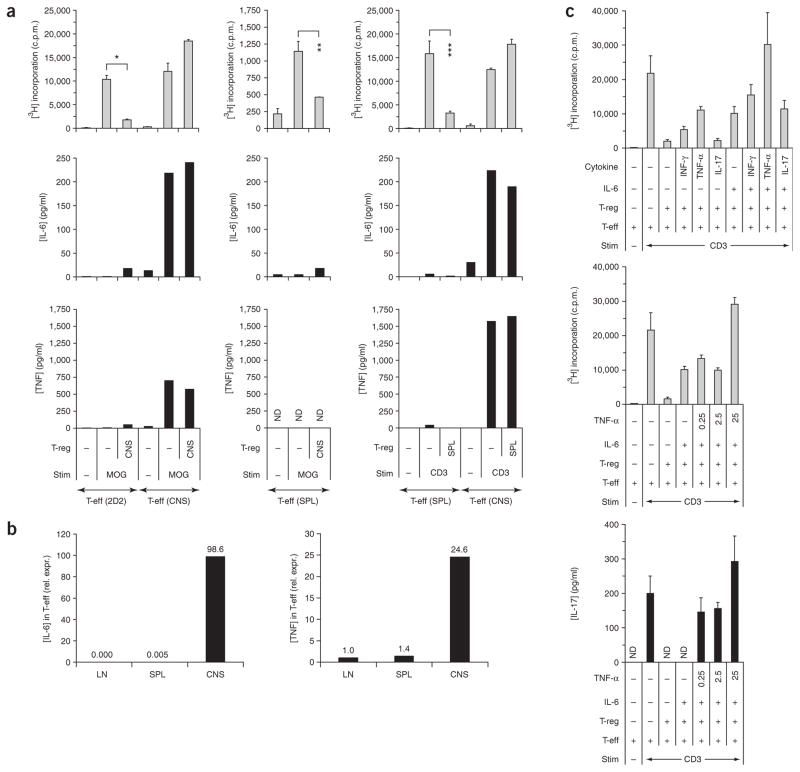
In accordance with the high fraction of MOG tetramer–positive cells, T-reg isolated from the CNS were very potent in suppressing naive (CD4+CD62LhighCD25−) MOG TcR-transgenic (2D2) T cells (Fig. 5a). However, the question remained as to why T-reg that accumulated in the target tissue failed to efficiently suppress T cell–driven autoimmune reactions in vivo. Therefore, we asked whether T-reg isolated from the CNS would be able to suppress T-eff that were derived from the inflamed target tissue. As our model system allowed us to differentiate between T-eff and T-reg on the basis of Foxp3 expression, we could circumvent the problem that cell surface markers that are commonly used to identify T-reg are also expressed on activated T-eff, which has marred this analysis in the past. In contrast to what we observed in naive 2D2 responder cells, CNS-derived T-reg were unable to suppress the proliferation of T-eff obtained from the CNS at the peak of disease (Fig. 5a). T-eff obtained from the spleen at the peak of disease did not proliferate strongly upon MOG stimulation but could clearly be suppressed by CNS-T-reg (Fig. 5a). To further test the properties of splenic T-eff and CNS-T-eff in this disease phase, we compared the proliferation of splenic T-eff and CNS-T-eff in the presence of spleen-derived T-reg upon stimulation with antibody to CD3. Again, splenic T-eff were suppressible whereas CNS-T-eff could not be suppressed by splenic T-reg (Fig. 5a).
Figure 5.
T-eff isolated from the acutely inflamed CNS are not suppressible by T-reg. Mononuclear cells from the spleen and the CNS of EAE mice were isolated at the peak of disease and FACS sorted for CD4+Foxp3/GFP− (T-eff) and CD4+Foxp3/GFP+ (T-reg). (a) The T-reg populations were tested in MOG-specific suppression assays and in suppression assays driven by antibody to CD3 in a 1:1 ratio with naive MOG TcR-transgenic T cells (2D2) or with CNS-T-eff or splenic T-eff from EAE mice as indicated. The proliferation of responder cells was measured by 3[H]thymidine incorporation. Mean of triplicate assays (+ s.d.). *P < 2 × 10−9, **P < 3 × 10−4, ***P < 0.008, t-test. Supernatants were taken after 48 h and the cytokines IL-6 and TNF were analyzed by cytometric bead array. (b) Cytokine expression in T-eff from lymph nodes (LN), spleen (SPL) and the CNS at the peak of disease (day 14). Mononuclear cells were isolated as described and FACS purified based on the T-eff phenotype (CD4+Foxp3/GFP−). cDNA was prepared and quantitative PCR was performed for IL-6 and TNF. One of two independent EAE experiments. (c) The combination of IL-6 and TNF-α induced a complete block in T-reg–mediated inhibition of T-eff from unimmunized mice. CD4+Foxp3/GFP+ T-reg were isolated from naive Foxp3gfp.KI mice and tested in a ratio of 1:1 for suppression of CD4+Foxp3/GFP− T-eff from unimmunized Foxp3gfp.KI mice upon polyclonal stimulation with antibody to CD3. Cytokines were added at a concentration of 25 ng/ml or titrated as indicated (in ng/ml). Proliferation was determined as 3[H]thymidine incorporation. Mean (+ s.d.) of triplicate cultures. Supernatants were collected after 48 h and assessed for IL-17 by ELISA (ND, not detectable).
To understand why T-reg failed to inhibit T-eff from the inflamed target tissue, we determined the cytokines that were produced in the suppression cultures. In contrast to naive MOG-specific 2D2 T cells and T-eff isolated from the spleen of EAE mice, CNS-T-eff secreted large amounts of IL-6 and TNF (Fig. 5a,b). This seemed to be a unique property of T-eff obtained from the CNS, but not from lymph nodes or spleen (Fig. 5b). Therefore, we tested whether these two pro-inflammatory cytokines were responsible for abrogating T-reg–mediated suppression of naive T-eff. Whereas addition of exogenous IL-6 alone diminished suppression by about 50%, the combination of IL-6 and TNF was sufficient to completely reverse T-reg–mediated suppression of naive T-eff (Fig. 5c). With IL-6, TNF mediated a dose-dependent additive effect in abrogating suppression of proliferation and IL-17 production by T-eff (Fig. 5c). These data suggested that although T-reg recovered from the site of inflammation were effective in suppressing MOG-specific T-cell responses, T-eff derived from the CNS at the peak of inflammation were resistant to suppression partly due to their ability to produce IL-6 and TNF. We also confirmed the difference in susceptibility to T-reg–mediated suppression between splenic and CNS-T-eff in another EAE model, proteolipoprotein (PLP)139–151-induced EAE in Foxp3gfp.KI mice on the SJL genetic background (Supplementary Fig. 4 online). When T-eff (CD4+Foxp3/GFP−) were isolated from the spleen and the CNS of SJL Foxp3gfp.KI mice at the peak of the first disease episode (day 14) and tested in suppression cultures with spleen- or CNS-derived T-reg (CD4+Foxp3/GFP+), we observed that PLP-primed splenic T-eff could be inhibited by either splenic T-reg or CNS-T-reg whereas CNS-derived T-eff were resistant (Supplementary Fig. 4).
Collectively, these data demonstrated that irrespective of the driving autoantigen, T-eff obtained from the CNS at the peak of disease were not inhibited by T-reg isolated from either the CNS or the peripheral immune compartment. Furthermore, the inflammatory cytokine milieu, and not exclusively the number of T-reg, may determine whether or not autoimmune inflammation can be controlled by T-reg.
DISCUSSION
In this study, we used Foxp3gfp.KI mice in combination with MOG35–55/IAb tetramer staining to monitor antigen-specific T-reg and T-eff during EAE and to perform functional analyses at different disease stages. We show that (i) besides pathogenic T-eff, immunization with the autoantigen also triggers the expansion of MOG-specific T-reg from the natural T-reg repertoire; (ii) Foxp3+ T-reg accumulate in the CNS, but not in the peripheral lymphoid compartments; and (iii) the T-reg population in the CNS is efficient in suppressing MOG-specific responses of naive T cells, but fails to inhibit antigen-specific T-eff isolated from the target organ at the acute disease phase.
There has been a debate as to whether autoantigen-specific T-reg in vivo are generated from the natural T-reg repertoire or induced de novo from naive T cells. Antigen exposure without concurrent inflammation may promote conversion of Foxp3− T cells into Foxp3+ T-reg31. However, upon immunization with MOG/CFA, we did not observe conversion of Foxp3/GFP− T cells into Foxp3/GFP+ T cells, either in the lymphoid compartment or in the CNS. It has been proposed that encephalitogenic T cells interacting with neurons could be converted into Foxp3-expressing T-reg32. However in this study32, the Foxp3+ T cells recovered from the CNS were probably expanded from contaminating Foxp3+ T cells in the starting population, which was not rigorously selected for the lack of Foxp3. Thus, autoantigen presented in a pro-inflammatory milieu prevents conversion of Foxp3− T cells into Foxp3+ T-reg. Support for this hypothesis comes from recent data17,19,33 showing that whereas TGF-β can convert Foxp3− T cells into Foxp3+ T-reg, addition of IL-6, an acute phase protein induced during inflammation, suppresses the TGF-β–induced generation of Foxp3+ T-reg and results in the induction of pathogenic TH17. Therefore, IL-6 may be an important constituent of the inflammatory milieu that regulates differentiation of TH17 versus Foxp3+ T-reg in the peripheral immune compartment, but also in the CNS (ref. 34). Neutralizing IL-6 reduced clinical signs of EAE (ref. 35). However, the role of IL-6 in autoimmune inflammation is complex36, and it is important to define the cellular targets when discussing the effects of this cytokine in the context of tissue inflammation.
Similar to T-eff37,38, the engagement of their myelin-specific TcR might be essential for T-reg to become reactivated in situ. Consistent with this concept, the BrdU+ T-reg fraction within the total T-reg population was slightly higher in the CNS than in the periphery, and MOG tetramer–positive T-reg were enriched in the CNS-derived T-reg pool. CNS-derived T-reg also showed enhanced production of IL-10. It has been proposed that natural T-reg only regulate T-eff bearing TcRs for the same antigen39. Also, adoptive transfer of myelin basic protein (MBP) TcR–transgenic CD4+CD25+ T-reg, but not of non-specific T-reg, into MBP TcR–transgenic Rag-deficient recipients prevents the development of spontaneous EAE when the cells are transferred before disease onset40. This suggests that before the onset of massive inflammation, T-reg–mediated regulation might be determined by the ratio of antigen-specific T-reg:T-eff in the peripheral immune compartment. However, our data demonstrate that as soon as autoimmunity has been triggered, T-reg are present in the target tissue in a relatively favorable T-reg:T-eff ratio at the onset of disease, but they still cannot prevent the expansion and function of pathogenic T-eff. This is not due to insufficient suppressive capacity of CNS-T-reg but because of the intrinsic resistance of CNS-derived T-eff to suppression, which is associated with their production of IL-6 and TNF. It has been shown that IL-6 renders naive T cells resistant to suppression and enables the initiation of an immune response in the presence of T-reg41. Here, IL-6 was produced by cells of the innate immune system that encountered bacterial constituents associated with infectious agents. In the context of organ-specific autoimmunity, pathogenic T cells themselves may be the source of IL-6 (ref. 42 and findings in the present study). Both IL-6 and TNF are induced in inflamed tissue by IL-17 (ref. 43). Thus, the early abundance of IL-17 in the CNS may indirectly dampen T-reg function. The present data also begin to shed light on why CD4+CD25+ T-reg are enriched in the inflamed joints of rheumatoid arthritis patients without inhibiting autoimmunity44–46. We believe that the therapeutic approach of neutralizing IL-6 and TNF in EAE (ref. 35) and in rheumatoid arthritis patients47 operates partly by enhancing suppressor functions of T-reg and by making T-eff susceptible to T-reg–mediated inhibition. Notably, during clinical recovery, the T-eff population in the target organ becomes partially susceptible to T-reg–mediated inhibition, which is paralleled by a marked reduction in IL-6 and TNF produced by CNS-T-eff at this disease stage (data not shown). This further strengthens the argument that after the decline of IL-17 levels, the feedback loop that drives IL-6 and TNF might collapse, resulting in the display of T-reg activity.
In summary, this report establishes that autoantigen-specific natural T-reg are targeted to the CNS during autoimmune encephalomyelitis, resulting in the accumulation of a T-reg population in situ. However, in order for T-reg to exert their suppressive function in established autoimmune inflammation, the local inflammatory cytokine milieu in the target tissue has to be controlled as well.
METHODS
Animals and induction of EAE
Foxp3gfp.KI mice were generated as described19. EAE was induced by injecting the mice subcutaneously (into the flanks) with 100 μl of an emulsion containing 100 μg of MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK) and 250 μg of M. tuberculosis extract H37 Ra (Difco) in incomplete Freund adjuvant oil. In addition, the mice received 200 ng of pertussis toxin (List Biological Laboratories via Cedarlane Ltd.) intraperitoneally (i.p.) on days 0 and 2. In some experiments, 107 T cells (or the indicated number and species of cells) were adoptively transferred i.p. before immunization. Clinical signs of EAE were assessed according to the following score: 0, no signs of disease; 1, loss of tone in the tail; 2, hind limb paresis; 3, hind limb paralysis; 4, tetraplegia; 5, moribund. Mice were kept in a conventional, pathogen-free facility at the Harvard Institutes of Medicine. All experiments were carried out in accordance with guidelines prescribed by the Institutional Animal Care and Use Committee (IACUC) at Harvard Medical School.
Preparation of CNS mononuclear cells
Mice were perfused through the left cardiac ventricle with cold PBS. The forebrain and cerebellum were dissected and spinal cords flushed out with PBS by hydrostatic pressure. CNS tissue was cut into pieces and digested with collagenase D (2.5 mg/ml, Roche Diagnostics) and DNaseI (1 mg/ml, Sigma) at 37 °C for 45 min. Mononuclear cells were isolated by passing the tissue through a cell strainer (70 μm), followed by a percoll gradient (70%/37%) centrifugation. Mononuclear cells were removed from the interphase, washed and resuspended in culture medium for further analysis.
T-cell proliferation and suppression assays
Cells were cultured in DMEM/10% FCS supplemented with 5 × 10−5 M β-mercaptoethanol, 1 mM sodium pyruvate, nonessential amino acids, L-glutamine, and 100 U penicillin/100 μg streptomycin per ml. Responder T cells were sorted (FACSAria™ cell sorter, BD Biosciences) from primary recall cultures of MOG35–55-stimulated splenocytes (CD4+Foxp3/GFP−) or from naive splenocytes of 2D2 mice (CD4+CD62L+ CD25−)27. T-reg were sorted as CD4+Foxp3/GFP+ T cells from naive Foxp3gfp.KI mice, from splenocytes of MOG-immunized mice or from the CNS of mice with overt EAE. For some experiments, myelin-specific T-reg were sorted from MOG-specific recall cultures. In this case, cells were rested in IL-2 containing medium for 5 d before setting up the suppression assay. For the suppression assays, 20,000 responder T cells were cocultured with the indicated number of T-reg and 100,000 irradiated (3,300 rad) syngeneic splenic antigen-presenting cells (APCs) per well in the presence of MOG35–55 (10 μg/ml) or antibody to mouse CD3 (0.5 μg/ml) for 72 h. During the last 16 h, cells were pulsed with 1 μCi of [3H]thymidine (PerkinElmer) followed by harvesting on glass fiber filters and analysis of incorporated [3H]thymidine in a β-counter (1450 Microbeta, Trilux, PerkinElmer). Where indicated in the figures, the following recombinant cytokines were added to the cultures: IFN-γ (25 ng/ml, R&D systems), TNF-α (25 ng/ml), IL-17 (25 ng/ml, eBioscience) and IL-6 (25 ng/ml, R&D systems).
Intracellular cytokine staining
For intracellular cytokine staining, cells were isolated as described and stimulated in culture medium containing phorbol 12-myristate 13-acetate (PMA, 50 ng/ml, Sigma), ionomycin (1 μg/ml, Sigma) and monensin (GolgiStop 1 μl/ml, BD Biosciences) at 37 °C, in a humidified 10% CO2 atmosphere for 4 h. After staining of surface markers (CD4 and CD25), cells were fixed and permeabilized using Cytofix/Cytoperm and Perm/Wash buffer from BD Biosciences according to the manufacturer’s instructions. All antibodies to cytokines (IFN-γ, IL-17, IL-10) including the corresponding isotype controls were obtained from BD Biosciences. Cells were incubated (1:100) at 25 °C for 20 min and washed twice in Perm/Wash before analysis.
Immunohistochemistry
Brain, spinal cord and spleen tissue samples from MOG-immunized mice at the peak and recovery phases of EAE were snap frozen in O.C.T. Tissue tek® cryomedium (Sakura Finetek) and immunostained with a biotinylated monoclonal antibody to mouse/rat Foxp3 (Ebioscience) using a standard avidin-biotin immunoperoxidase method. The sections were counterstained with hematoxylin. Spleen samples were used as a positive control. For semiquantitative analysis, numbers of Foxp3-immunopositive cells were counted in 20 brain inflammatory foci at 40× magnification in three immunostained sections from each mouse. Foci were selected on the basis of identification of at least one stained cell in an inflammatory focus. Data are expressed as mean positive cells ± s.e.m.
Statistical evaluation
Statistical evaluations of cell frequency measurements, proliferation data and immunohistochemical analyses (immunopositive cells within distinct inflammatory foci) were performed using the unpaired Student’s t-test for samples with unknown and potentially disparate variances.
Other methods
A detailed description of the generation of the Foxp3gfp.KI mice, the constructs for the MOG35–55/IAb monomers, the tetramer staining and the Taqman PCRs used in this report are provided in Supplementary Methods online.
Supplementary Material
Acknowledgments
We thank D. Kozoriz for performing the cell sorting. MOG35–55 peptide was provided by D. Teplow (David Geffen School of Medicine, University of California Los Angeles). This work was supported by the National Multiple Sclerosis Society (RG-2571-D-9 to V.K.K. and RG-3882-A-1 to M.O.), the US National Institutes of Health (1R01NS045937-01, 2R01NS35685-06, 2R37NS30843-11, 1R01A144880-03, 2P01A139671-07, 1P01NS38037-04 and 1R01NS046414) and the Juvenile Diabetes Research Foundation Center for Immunological Tolerance at Harvard Medical School. T.K. is supported by the Deutsche Forschungsgemeinschaft (KO 2964/1-1). V.K.K. is a recipient of the Javits Neuroscience Investigator Award from the US National Institutes of Health.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
AUTHOR CONTRIBUTIONS
T.K. conducted all experiments, prepared the figures and drafted the manuscript; J.R. generated the MOG tetramers; W.G. and M.O. generated the Foxp3gfp.KI mice; E.B., T.R.P. and B.T.B. helped in preparing the MOG35–55/IAb constructs; A.A. helped in generating the Foxp3gfp.KI and SJL Foxp3gfp.KI. mice; R.A.S. performed the immunohistological analyses; K.W.W. supervised the generation of the MOG35–55/IAb constructs; T.B.S. and M.O. supervised the project: V.K.K. initiated and supervised the project and edited the manuscript.
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 2.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 3.Reddy J, et al. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2004;101:15434–15439. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 5.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccirillo CA, et al. Control of type 1 autoimmune diabetes by naturally occurring CD4+CD25+ regulatory T lymphocytes in neonatal NOD mice. Ann NY Acad Sci. 2005;1051:72–87. doi: 10.1196/annals.1361.048. [DOI] [PubMed] [Google Scholar]
- 7.Morgan ME, et al. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–1460. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 8.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas J, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343–3352. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 10.Crispin JC, Martinez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2003;21:273–276. doi: 10.1016/s0896-8411(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 11.Alvarado-Sanchez B, et al. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2006;27:110–118. doi: 10.1016/j.jaut.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc Natl Acad Sci USA. 2003;100:8886–8891. doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 16.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Mangan PR, et al. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 19.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 20.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 21.Marson A, et al. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gavin MA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y, et al. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 24.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 26.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 27.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bagaeva LV, Williams LP, Segal BM. IL-12 dependent/IFNγ independent expression of CCR5 by myelin-reactive T cells correlates with encephalitogenicity. J Neuroimmunol. 2003;137:109–116. doi: 10.1016/s0165-5728(03)00079-1. [DOI] [PubMed] [Google Scholar]
- 29.Glabinski AR, Bielecki B, O’Bryant S, Selmaj K, Ransohoff RM. Experimental autoimmune encephalomyelitis: CC chemokine receptor expression by trafficking cells. J Autoimmun. 2002;19:175–181. doi: 10.1006/jaut.2002.0613. [DOI] [PubMed] [Google Scholar]
- 30.Huehn J, et al. Developmental stage, phenotype, and migration distinguish naive-and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- 33.Doganci A, et al. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005;115:313–325. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ’preferentially’ polarize CD4+ TH17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 35.Gijbels K, Brocke S, Abrams JS, Steinman L. Administration of neutralizing antibodies to interleukin-6 (IL-6) reduces experimental autoimmune encephalomyelitis and is associated with elevated levels of IL-6 bioactivity in central nervous system and circulation. Mol Med. 1995;1:795–805. [PMC free article] [PubMed] [Google Scholar]
- 36.Di Marco R, et al. Curative effects of recombinant human Interleukin-6 in DA rats with protracted relapsing experimental allergic encephalomyelitis. J Neuroimmunol. 2001;116:168–177. doi: 10.1016/s0165-5728(01)00301-0. [DOI] [PubMed] [Google Scholar]
- 37.Greter M, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 38.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 39.Tanchot C, Vasseur F, Pontoux C, Garcia C, Sarukhan A. Immune regulation by self-reactive T cells is antigen specific. J Immunol. 2004;172:4285–4291. doi: 10.4049/jimmunol.172.7.4285. [DOI] [PubMed] [Google Scholar]
- 40.Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc Natl Acad Sci USA. 2002;99:8213–8218. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 42.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 44.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4+CD25+ regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–2785. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 45.Cao D, et al. FOXP3 identifies regulatory CD25bright CD4+ T cells in rheumatic joints. Scand J Immunol. 2006;63:444–452. doi: 10.1111/j.1365-3083.2006.001755.x. [DOI] [PubMed] [Google Scholar]
- 46.Ruprecht CR, et al. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201:1793–1803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ehrenstein MR, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.