Abstract
FOXO transcription factors have emerged as rheostats that coordinate the activities of Akt and target of rapamycin complexes (TORCs). This review summarizes the regulatory circuits mediated by the activation of FOXO, which in turn modulate Akt and TORCs activities. The biological significance of these regulatory circuits is discussed in this article.
Introduction
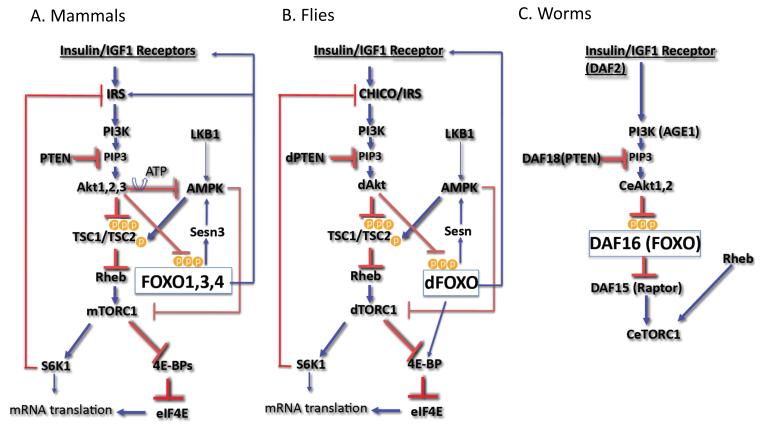
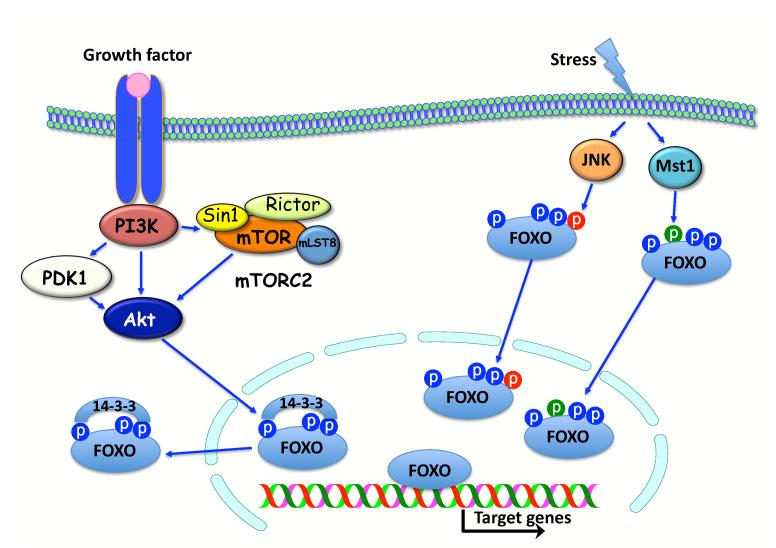
The phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway, which leads to the inhibition of the forkhead box O (FOXO) transcription factors is highly conserved across metazoans (Fig. 1). In both C. elegans and Drosophila, FOXO transcription factors are inhibited predominantly by the activation of insulin (Ins) or insulin-like growth factor receptors (Ins/IGF1R). Insulin and insulin-like growth receptors have similar functions across species. They exert their effects through the phosphorylation and activation of insulin receptor substrates (IRS), which provide docking sites for the interaction and activation of downstream effectors. In mammals, FOXO transcription factors are also inhibited by any extracellular signal that activates the serine/threonine kinase, Akt, which is also known as protein kinase B (PKB). Akt is activated by extracellular signals that activate PI3K. PI3K phosphorylates phosphoinositols to generate phosphatidylinositol 3′ phosphate (PIP3). The binding of PIP3 to the pleckstrin homology (PH) domain of Akt is the rate-limiting step in Akt activation. This binding elicits the translocation of Akt to the membrane, where it is fully activated by other kinases. Akt is phosphorylated at threonine residue 308 (Thr 308) by PDK1 and at a serine residue (Ser 473) by mTORC2, which is a rapamycin-resistant complex containing mammalian target of rapamycin (mTOR) (for review, see [1]) (Fig. 2). Antagonizing PI3K activity negatively regulates Akt activity. For instance, Akt activity is negatively regulated by phospholipid phosphatases that dephosphorylate PIP3. The major phospholipid phosphatase that regulates Akt activity is the tumor suppressor PTEN, which dephosphorylates the 3′ phosphate of PIP3 and thereby negates the activity of PI3K. The most evolutionarily conserved downstream effectors of Akt are the FOXO transcription factors, which include FOXO1, 3, 4, and 6 in mammals [2]. Another conserved target of Akt is the target of rapamycin complex 1 (TORC1) (reviewed in [1], which is indirectly activated by Akt (Fig. 1)).
Figure 1. The interplay between FOXO, TOR, and Akt.
A. The signaling pathway from Insulin/IGF1 receptor through IRS, PI3K, and Akt in mammalian cells. Akt inhibits FOXO through direct phosphorylation, and indirectly activates mTORC1, which in turn elevates protein synthesis. mTORC1 and its downstream effector, S6K, elicit negative feedback loops to inhibit Akt. When activated FOXO induces the expression of Sestrin 3, which activates AMPK to inhibit mTORC1. FOXO also induces Insulin/IGF1 receptors, IRS2, and Rictor expression to activate Akt (see text for details). B. The signaling pathway from Insulin/IGF1 receptor through IRS, PI3K, and Akt in flies. The mechanisms of FOXO inhibition and mTORC1 activation by Akt are conserved in flies and mammals. In flies, the mechanism of TORC1 inhibition by FOXO through Sestrin and AMPK is conserved. In addition, FOXO elevates the expression of 4E-BP downstream of TORC1, and induces the expression of Insuin/IGF1 receptor to activate Akt (see text for details). C. The signaling pathway from Insulin/IGF1 receptor through PI3K, and Akt in worms. The inhibition of FOXO by Akt is conserved in worms. However, the mechanism of TORC1 activation by Akt is not conserved. Akt can activate TORC1 through inhibition of FOXO because FOXO inhibits the expression of Raptor, which is required for TORC1 activity (see text for details).
Figure 2. The regulation of FOXO activity by Akt activation, and by JNK and MST1 activation.
Akt is activated by PI3K downstream of tyrosine kinase growth factor receptors. PI3K also activates PDK1 and mTORC2, which composed of mTOR, Rictor, Sin1 and mLST8. PDK1 and mTORC2 phosphorylate Akt for full activation. Upon activation Akt phosphorylates FOXO and creates docking site for 14-3-3. The binding of 14-3-3 to FOXO excludes FOXO from the nucleus. Oxidative and genotoxic stresses activate JNK and MST1. JNK and MST1 phosphorylate FOXO at two different sites. The phosphorylation by JNK or MST1 promotes the nuclear localization of FOXO despite phosphorylation by Akt (see text for details).
FOXO transcription factors, across species, have highly conserved phosphorylation sites that are phosphorylated by Akt. The phosphorylation of FOXOs by Akt in the nucleus creates a 14-3-3 binding site. The binding of 14-3-3 to FOXO masks the nuclear localization signal (NLS) and prevents nuclear translocation, thereby inhibiting the activities of FOXO (reviewed in [3]). FOXO transcription factors, across species, possess other phosphorylation sites that can be phosphorylated by the stress inducible kinases, Jun N-terminus kinase (JNK) and STE20-like protein kinase 1 (MST1). The activation of FOXO, initiated by the phosphorylation by JNK and MST1, is dominant to the inhibitory phosphorylation by Akt (reviewed in [3], Fig. 2).
The PI3K/Akt/FOXO pathway was delineated in C. elegans in a genetic screen for lifespan extension [4-7]. Mutations that attenuate the activities of the insulin receptor ortholog, DAF-2, and the PI3K ortholog, AGE-1, inhibit Akt activity and can also extend the lifespan of worms. The increase in lifespan by the inhibition of PI3K and Akt activities is largely a consequence of DAF-16 activation, which is the C-elegans ortholog of FOXO. Mutations that inhibit the activity DAF-16 revert the lifespan extension phenotype [8, 9]. The role of the PI3K/Akt/FOXO signaling in longevity appears to be conserved across species (reviewed in [3, 10].
Regulatory circuits that mediate interplays between FOXO, TOR and Akt
Like FOXO transcription factors, TOR complexes, which share TOR as their catalytic subunit, are highly conserved across evolution. TOR complex 1 (TORC1) is a conserved downstream effector of Akt, and TOR complex 2 (TORC2) is a conserved activator of Akt. The defining subunits of the two mammalian TORCs (mTORCs) are the regulatory associated protein of mTOR (Raptor) in mTORC1 and Rapamycin-insensitive companion of mTOR (Rictor) in mTORC2, which are also evolutionarily conserved. mTORC1 is composed of mTOR, Raptor, mLST8 as the core kinase complex, and the accessory factors PRAS40 and Deptor [11]. One mechanism by which Akt activates mTORC1 is through direct phosphorylation of tuberous sclerosis complex 2 (TSC2), which otherwise inhibits mTORC1 activity (Fig. 1; reviewed in [12]). Tuberous sclerosis complex 1 (TSC1) and TSC2 form a heterodimer that possesses GAP activity and inhibits the activity of Rheb, a small GTPase required for mTOR activation (Fig. 1; reviewed in [1]). TSC2 can be activated when intracellular levels of ATP are reduced and AMPK activity is elevated. AMPK directly phosphorylates TSC2, leading to the induction of mTORC1 inhibition [13]. Additionally, AMPK inhibits mTORC1 through direct phosphorylation of Raptor [14], which is a conserved mechanism that might be the major pathway by which AMPK affects TORC1 in flies (Fig. 1). However, AMPK phosphorylation sites are not fully conserved in the Drosophila TSC2. Akt also activates mTORC1 by maintaining intracellular ATP levels and reducing AMPK activity [15].
In TSC2- or TSC1-null cells, mTORC1 is constitutively activated, independent of growth factors and Akt, which is consistent with an inhibitory role for TSC2. In contrast, Akt activity is markedly reduced in these cells. This reduction has been attributed to a negative feedback mechanism involving an inhibitory effect of S6 Kinase (a downstream effector of mTORC1) on insulin receptor substrate-1 (IRS1) or IRS2, which mediates PI3K activation by insulin and IGF-1 [23]. Additional negative regulatory loops elicited by mTORC1 that inhibit Akt activity may also exist [1]
The other mTOR complex, mTORC2, is composed of Rictor, mLST8 and mSin1 as the core kinase complex (Fig. 2), and the accessory factors Deptor and Protor-1 [11]. mTORC2 is the carboxy-terminus hydrophobic motif (HM) kinase for Akt and other AGC kinases.
One major conserved function of mTORC1 is to increase mRNA translation (Fig. 1) via the phosphorylation and activation of S6 kinase and by the phosphorylation and inhibition of the eukaryotic translation initiation factor 4E (eIF4E) binding protein (4E-BP), which is a repressor of mRNA translation [12]. The hypophosphorylated active form of 4E-BP binds eIF4E and blocks the interaction of eIF4E with eIF4G, thereby inhibiting cap-dependent mRNA translation. In addition to protein synthesis, mTORC1 has another conserved anabolic activity that involves fatty acid biosynthesis through the activation of the sterol-regulatory-element-binding protein (SREBP1) [16]. SREBP1 is a transcription factor that facilitates fatty acid synthesis by regulating the expression of enzymes associated with fatty acids synthesis. Finally, mTORC1 has a conserved function in inhibiting autophagy by phosphorylating proteins that are required for its initiation [17].
Unlike in mammals and flies, the insulin/IGF1-Akt axis has not been shown to regulate C. elegans TORC1 (CeTORC1). The C. elegans genome lacks readily identifiable homologs of TSC1 and TSC2 [18], which mediate the effect of Akt on mTORC1, but C. elegans Akt (CeAkt) can indirectly increase CeTORC1 activity via the phosphorylation and inhibition of the C. elegans FOXO transcription factor, DAF-16. DAF-16 has a potent negative effect on DAF-15 (C. elegans Raptor) expression [19], and therefore, the inhibition of DAF-16 by CeAkt could maintain the availability of Raptor/DAF-15 to form CeTORC1. Thus, in nematodes, Akt indirectly activates TORC1 through the inhibition of DAF-16 and the elevation of Raptor expression (Fig. 1). In contrast, the activation of DAF-16 by the stress inducible kinase, JNK, would inhibit TORC1. It is not known if, like in mammals and flies, the activation of CeTORC1 elicits a negative feedback loop to inhibit IGF1/insulin signaling and Akt. If such a negative feedback loop exists in C. elegans, it would imply that the activation of DAF-16 by oxidative stress would lead to the inhibition of TORC1 and the activation of Akt.
In Drosophila, there are two major identified regulatory circuits by which FOXO regulates TORC1 and Akt activity (Fig. 1). It was shown that in Drosophila, 4E-BP is a transcriptional target of FOXO. Therefore, following the activation of FOXO in flies, 4E-BP is elevated and counteracts TORC1 activity on the initiation of cap-dependent mRNA translation. However, at the same time, FOXO upregulates the transcription of the insulin receptor (InsR) mRNA, which possesses an internal ribosome initiation site (IRES). Therefore, the high level of insulin receptor mRNA induced by FOXO is coupled to a high level of InsR protein through IRES-dependent mRNA translation, even though cap-dependent mRNA translation is inhibited. Consequently, the inhibition of cap-dependent mRNA translation by FOXO could be alleviated through the high levels of insulin receptor and the hyperactivation of Akt and TORC1 [20, 21].
The upregulation of InsR by FOXO appears to be conserved in mammals, as it was found that FOXO activates InsR transcription, at least in liver and muscle cells [20]. It was also shown that FOXO elevates IRS2 mRNA levels, thereby potentially elevating signaling downstream of InsR or IGF1R. Notably, IRS2 protein is degraded by a mechanism that is dependent on mTORC1 or its downstream effector, S6K1 [22]. More recently, it was shown that FOXO elevates HER2/HER3 tyrosine kinase receptor expression in several cancer cell lines [23] in addition to InsR and IGF1R. The elevation of tyrosine kinase receptors by FOXO establishes feedback mechanisms that amplify growth factor signaling and limit prolonged FOXO activation.
Another regulatory circuit through which FOXO affects TORC1 in Drosophila was recently uncovered [24]. It was found that FOXO indirectly activates AMPK, which in turn activates TSC2 and inhibits TORC1 activity. FOXO activates AMPK through the transcriptional upregulation of Sestrin. Sestrins are a family of highly conserved proteins that were originally discovered in mammals as antioxidants [25, 26]. However, it was found that they have an additional function that leads to the activation of AMPK, although the exact mechanism by which Sestrin activates AMPK is not fully understood [27]. This pathway, by which FOXO inhibits TORC1 in flies, manifests in conditions of oxidative stress, whereby FOXO is activated by stress inducible kinases, JNK or MST1.
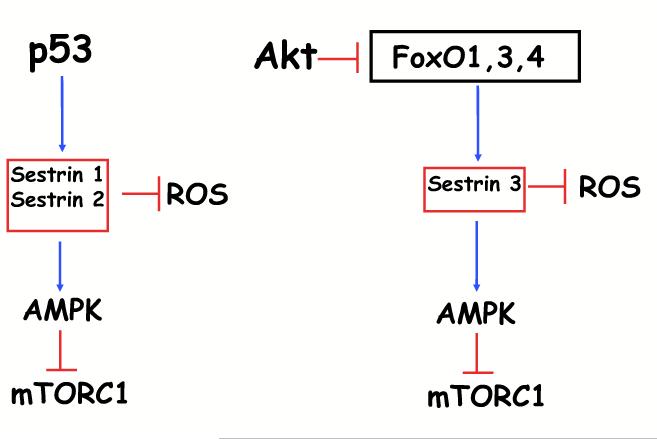
The FOXO-Sestrin-AMPK-TORC1 axis is conserved in mammalian cells (Fig. 1). Mammalian FOXO1 was shown to bind to the promoter region of Sestrin 3, and transcriptionally elevate Sestrin 3 expression [28]. Sestrin 3 is one of three members of the Sestrin family. Sestrin 1 and 2 were shown to be transcriptional targets of p53 [27], whereas Sestrin 3 is induced by FOXO [28, 29]. Sestrin 3 has a dual activity downstream of FOXO. In its role as a scavenger of ROS, Sestrin 3 mediates ROS detoxification by FOXO and inhibits cellular senescence [29], and as an activator of AMPK, it inhibits mTORC1 in a TSC2-depedent manner [28]. Thus, by analogy to p53, which induces the expression of Sestrin 1 and 2, reduces ROS levels and inhibits mTORC1, FOXO induces the expression of Sestrin 3 to reduce ROS and inhibit mTORC1 (Fig. 3). Notably, FOXO itself is subjected to regulation by the energy status of cells, as AMPK was shown to phosphorylate FOXO and facilitate its nuclear localization [30]. Therefore, the FOXO-Sestrin-AMPK axis could be further augmented by AMPK through a feed-forward mechanism.
Figure 3. Analogy between p53 and FOXO activities.
By analogy to p53, which induces Sestrin1 and Sestrin2 transcriptionally, FOXO induces Sestrin3 transcriptionally. p53 reduces ROS and inhibits mTORC1 through the induction of Sestrin1 and 2 expression, while FOXO reduces ROS and inhibits mTORC1 through the induction of Sestrin3 expression.
The inhibition of mTORC1 by FOXO could lead to the activation of Akt via the inhibition of the negative feedback loop driven by mTORC1 and S6K1 (Fig. 1). However, FOXO also elevates transcriptional expression of Rictor through a DNA-binding independent mechanism. This elevation of Rictor by FOXO increases mTORC2 and Akt activity. The increase in Akt activity is due to the increase in assembly and activity of mTORC2, which occurs at the expense of the assembly and activity of mTORC1 [28]. Therefore, the elevation of Rictor by FOXO constitutes another mechanism by which FOXO could inhibit mTORC1 in a TSC-independent mechanism.
As indicated above, FOXO elevates both InsR and IRS2 mRNA but IRS2 protein is degraded by mTORC1. Therefore, the inhibition of mTORC1 by FOXO could further augment Akt activity through InsR and IRS2 (Fig. 1).
There are other potential mechanisms by which FOXO could regulate Akt and mTOR activities. For instance, FOXO suppresses the expression of the pseudokinase, tribbles 3 (Trb3)[31], and it was reported that Trb3 inhibits Akt activity (Du et al., 2003). Thus, by suppressing Trb3 expression, FOXO could activate Akt. It was also reported that FOXO elevates the expression of Bnip3 (Mammucari et al., 2007). Because Bnip3 was shown to inhibit mTORC1 activity downstream of TSC2 by interfering with Rheb activity (Li et al., 2007), FOXO could inhibit mTORC1 through the induction of Bnip3 expression. Finally, it was recently reported that FOXO3 induces the transcriptional expression of TSC1 and inhibit mTORC1 [32].
The biological significance of the FOXO, TOR, AKT interplay
The inhibitory effect of FOXO on TORC1 could explain, at least in part, some of FOXO activities that phenocopy TORC1 inhibition. For instance, the activation of FOXO extends lifespan, whereas the activation of TORC1 reduces it [33]. Thus, it is possible that one mechanism by which FOXO extends lifespan is through the inhibition of TORC1. Heterozygous DAF-15 (Raptor ortholog) worms and CeTOR RNAi-treated worms have an extended adult life span [19, 34], similar to DAF-2, the ortholog of insulin/IGF1 receptor and age-1 (CePI-3kinase), mutant worms and DAF-16 (FOXO ortholog) overexpressing strains. In addition, DAF-15 and CeTOR homozygous mutant larvae accumulate lipids, similar to DAF-2 mutant dauer larvae [19, 34]. Thus, at least some of the effects of the DAF-2/Akt/DAF-16 pathway (such as longevity) may be mediated through regulation of CeTOR/DAF-15.
In Drosophila, it was shown that 4E-BP extends lifespan and is required for the extension of lifespan by dietary restriction (DR) [33]. Although FOXO-null flies respond normally to DR, it cannot be completely excluded that the transcriptional induction of 4E-BP by FOXO, in flies, contributes to the ability of FOXO to extend lifespan. Supporting this possibility are the findings that show overexpression of 4E-BP in FoxO-null flies, which are sensitive to oxidative stress, restores oxidative stress resistance [35]. Furthermore, either the overexpression of 4E-BP or activation of FOXO delays proteostasis during muscle aging in flies, and FOXO exerts its effect on muscle aging through the induction of 4E-BP expression [36].
The FOXO-Sestrin-AMPK-TORC1 axis in flies was shown to alleviate several age-related pathologies in response to oxidative stress, such as muscle degeneration, cardiac arrhythmia, and lipid accumulation [24]. Because FOXO can also improve cardiac aging in flies by increasing 4E-BP levels [37], it could affect fly aging pathologies by both decreasing TORC1 activity and increasing 4E-BP levels.
FOXO transcription factors, across species, promote resistance to oxidative stress, premature aging, and cellular senescence. The activities of FOXO were attributed largely to their ability to induce the expression of antioxidants. By contrast, the anabolic activities of TORC1 induce oxidative stress, and chronic activation of mTORC1 induces premature senescence [33, 38-40]. Thus, the inhibition of TORC1 by FOXO, which occurs across species by different mechanisms, could add another way by which FOXO reduces oxidative stress and inhibits premature aging and cellular senescence.
FOXO transcription factors are thought to have tumor suppressive activity [41] whereas mTORC1 is frequently activated in cancer cells [1]. Both the activation of FOXO and the inhibition of mTORC1 elicit cell cycle arrest or attenuation of cell proliferation. Thus, the inhibition of mTORC1 could account for some of FOXO’s tumor suppressor activities.
Other consequences of FOXO activation and mTORC1 inhibition that phenocopy each other include cellular atrophy and autophagy. Both the activation of FOXO and the inhibition of mTORC1 elicit cellular atrophy [42-45]. Therefore, it is possible that the inhibition of mTORC1, by activated FOXO, contributes to cellular atrophy. The activation of mTORC1 is known to inhibit autophagy, and it was also shown that autophagy could be mediated by the activation of FOXO [46].
As described above, the inhibitory effect of FOXO on TORC1, at least in flies and mammals, is associated with simultaneous direct or indirect Akt activation, indicating that under normal physiological conditions, the effect of FOXO on TORC1 may occur in a temporal manner. In flies, at the organismal level, the induction of 4E-BP by FOXO in combination with the induction of Insulin/IGF-1 receptor transcription and mRNA translation was considered to be an adaptive response to nutrient availability (reviewed in [47]). When nutrients are limiting, insulin secretion is reduced and FOXO is activated. This inhibits cell proliferation and growth by elevating 4E-BP expression as well as Sestrin expression that inhibits TORC1. Consequently, cap-dependent mRNA translation and the other anabolic activities of TORC1 are repressed.
The anabolic activities of TORC1 consume nutrients and cellular energy, and thus, reducing these activities reduces demand for nutrients. The simultaneous induction of Insulin/IGF-1 receptor mRNA by FOXO, in conjunction with its IRES-dependent mRNA translation, increases the number of receptors at the cell surface, thereby increasing sensitivity to insulin. Thus, when nutrients become abundant, the cells are hypersensitized to the concomitant increase in insulin levels, which inhibit FOXO and promote cell growth and proliferation. A similar scenario could occur under other stress conditions, such as oxidative stress when FOXO is activated by the stress inducible kinases. If the stress conditions are prolonged, the inhibition of TORC1 by FOXO could induce autophagy as a rescue mechanism.
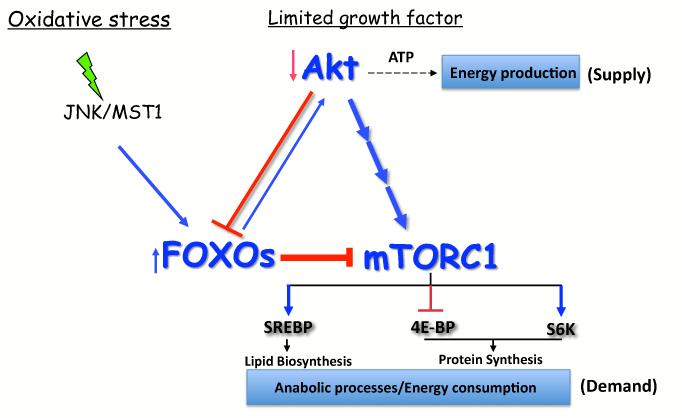
In mammalian cells, the activation of FOXO under stress conditions promotes the inhibition of mTORC1 by inducing the expression of Sesn3 and Rictor, which also leads to the activation of Akt. This mechanism was shown to maintain cellular energy homeostasis under stress conditions [28]. By shutting down the anabolic activities of mTORC1, such as protein synthesis and lipid biosynthesis, which consume energy, and by activating Akt, which increases energy production, FOXO maintains cellular energy homeostasis (Fig. 4). Through this mechanism, FOXO uncouples Akt and mTORC1 activities and prevents energy crisis. Under normal physiological conditions, this mechanism could constrain high mTORC1 activity. Under conditions of reduced growth factors, FOXO is activated due to reduced Akt activity. The regulatory circuit induced by FOXO, under conditions that inhibit mTORC1 and activate Akt, acts in a temporal manner because it does not permit prolonged FOXO activation and mTORC1 inhibition because of Akt activation. However, under oxidative stress conditions, the activation of FOXO by the stress inducible kinases is dominant to the inhibition of FOXO activity by Akt and, thus, could induce prolonged mTORC1 inhibition. By analogy to what was shown in flies, if stress conditions persist, the activation of mTORC1 by the FOXO-Sestrin3-AMPK axis could induce autophagy. Notably, as was recently reported, AMPK activation alone is sufficient to induce autophagy through the direct phosphorylation and activation of the ATG1 ortholog ULK1, which initiates the autophagic cascade [48]. Thus, the FOXO-Sestrin3-AMPK-mTORC1 axis could initiate autophagy both by inhibiting mTORC1, which otherwise inhibits ULK1, and by activating AMPK, which activates ULK1.
Figure 4. FOXO maintains cellular energy homeostasis by coordinating cellular supplies and demands.
Under conditions of growth factor limitation or other cellular stresses, FOXO transcription factors are activated, and inhibit the anabolic energy consuming functions of mTORC1, while activating Akt to facilitate energy producing processes. Prolonged stress conditions and activation of mTORC1 could induce autophagy (see text for details).
Concluding remarks
The picture that has emerged from the studies described in this review is that FOXO acts as a rheostat that, through fine-tuned mechanisms, coordinates intracellular supplies and demands. FOXO senses extracellular environment and responds accordingly. In the absence of extracellular stress, FOXO maintains a cellular homeostatic balance by preventing the hyperactivation of TORC1 relative to the extracellular signals mediated by Akt. Thus, in the absence of extracellular stress, it would be difficult to follow this FOXO activity in a cell population as this activity might not be synchronized. Consequently, if FOXO activity is followed in a population of cells in the absence of extracellular stress, it might be concluded that FOXO is relatively dormant. Therefore, to follow and quantify this oscillatory activity of FOXO, experiments should be designed to monitor the effect of FOXO on TORC1 and Akt at the single cell level using systems biology.
In response to environmental stress, FOXO may act in a temporal manner, but if the environmental stress is prolonged, FOXO activity might be shifted toward the inhibition of TORC1 to limit cellular demands under these conditions. In this respect, FOXO acts as a “gate keeper” that prevents cellular crisis. The activities of FOXO are consistent with its role in cellular lifespan and aging. It remains to be determined how much of FOXO’s effect on aging is determined by its effect on TORC1. Similarly, it should be determined how much of FOXO’s role in cellular atrophy, autophagy, and cell cycle is attributed to its effect on TORC1.
In light of the studies described in this review, the definition of FOXO as a tumor suppressor, in that its activation has a therapeutic advantage for cancer, becomes questionable. Although the suppressive effect of FOXO activation on mTORC1 could potentially have a positive impact on cancer therapy, the coupling Akt and upstream signaling activation, in particular, through tyrosine kinase receptors was shown recently [23], posing the question whether the inhibition of FOXO is preferred over its activation for cancer therapy.
Highlights.
The interactions between FOXO, Akt, and TOR in mammals, fly, and worms.
FOXO as rheostat that maintains cellular energy homeostasis by coordinating Akt and mTORC1 activities.
Implications of the FOXO, Akt, and mTORC1 interactions for aging and cancer.
Acknowledgements
Research in the author’s laboratory was supported by grants CA090764, AG016927, and AG025953 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhaskar PT, Hay N. The Two TORCs and Akt. Dev Cell. 2007;12(4):487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120(Pt 15):2479–87. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 3.Greer EL, Brunet A. FOXO transcription factors in ageing and cancer. Acta Physiol (Oxf) 2008;192(1):19–28. doi: 10.1111/j.1748-1716.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 4.Johnson TE. Increased life-span of age-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science. 1990;249(4971):908–12. doi: 10.1126/science.2392681. [DOI] [PubMed] [Google Scholar]
- 5.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366(6454):461–4. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 6.Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- 7.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an Insulin Receptor-Like Gene That Regulates Longevity and Diapause in Caenorhabditis elegans. Science. 1997;(277):942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 8.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278(5341):1319–22. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 9.Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389(6654):994–9. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 10.Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–12. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 11.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt 20):3589–94. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 13.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 14.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280(37):32081–9. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 16.Porstmann T, Santos CR, Lewis C, Griffiths B, Schulze A. A new player in the orchestra of cell growth: SREBP activity is regulated by mTORC1 and contributes to the regulation of cell and organ size. Biochem Soc Trans. 2009;37(Pt 1):278–83. doi: 10.1042/BST0370278. [DOI] [PubMed] [Google Scholar]
- 17.Neufeld TP. TOR-dependent control of autophagy: biting the hand that feeds. Curr Opin Cell Biol. 2010;22(2):157–68. doi: 10.1016/j.ceb.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long X, Spycher C, Han ZS, Rose AM, Muller F, Avruch J. TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Curr Biol. 2002;12(17):1448–61. doi: 10.1016/s0960-9822(02)01091-6. [DOI] [PubMed] [Google Scholar]
- 19.Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131(16):3897–906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- 20.Marr MT, 2nd, D’Alessio JA, Puig O, Tjian R. IRES-mediated functional coupling of transcription and translation amplifies insulin receptor feedback. Genes Dev. 2007;21(2):175–83. doi: 10.1101/gad.1506407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puig O, Marr MT, Ruhf ML, Tjian R. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 2003;17(16):2006–20. doi: 10.1101/gad.1098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo S, Dunn SL, White MF. The reciprocal stability of FOXO1 and IRS2 creates a regulatory circuit that controls insulin signaling. Mol Endocrinol. 2006;20(12):3389–99. doi: 10.1210/me.2006-0092. [DOI] [PubMed] [Google Scholar]
- 23.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT Inhibition Relieves Feedback Suppression of Receptor Tyrosine Kinase Expression and Activity. Cancer Cell. 2011;19(1):58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327(5970):1223–8. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peeters H, Debeer P, Bairoch A, Wilquet V, Huysmans C, Parthoens E, Fryns JP, Gewillig M, Nakamura Y, Niikawa N, Van de Ven W, Devriendt K. PA26 is a candidate gene for heterotaxia in humans: identification of a novel PA26-related gene family in human and mouse. Hum Genet. 2003;112(5-6):573–80. doi: 10.1007/s00439-003-0917-5. [DOI] [PubMed] [Google Scholar]
- 26.Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304(5670):596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 27.Budanov AV, Karin M. p53 Target Genes Sestrin1 and Sestrin2 Connect Genotoxic Stress and mTOR Signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, Park Y, Hay N. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18(4):592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14(6):458–70. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282(41):30107–19. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116(9):2464–72. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khatri S, Yepiskoposyan H, Gallo CA, Tandon P, Plas DR. FOXO3a regulates glycolysis via transcriptional control of tumor suppressor TSC1. J Biol Chem. 2010;285(21):15960–5. doi: 10.1074/jbc.M110.121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11(6):453–65. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426(6967):620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 35.Tettweiler G, Miron M, Jenkins M, Sonenberg N, Lasko PF. Starvation and oxidative stress resistance in Drosophila are mediated through the eIF4E-binding protein, d4E-BP. Genes Dev. 2005;19(16):1840–3. doi: 10.1101/gad.1311805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143(5):813–25. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wessells R, Fitzgerald E, Piazza N, Ocorr K, Morley S, Davies C, Lim HY, Elmen L, Hayes M, Oldham S, Bodmer R. d4eBP acts downstream of both dTOR and dFoxo to modulate cardiac functional aging in Drosophila. Aging Cell. 2009;8(5):542–52. doi: 10.1111/j.1474-9726.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schieke SM, Finkel T. Mitochondrial signaling, TOR, and life span. Biol Chem. 2006;387(10-11):1357–61. doi: 10.1515/BC.2006.170. [DOI] [PubMed] [Google Scholar]
- 39.Ruggero D, Montanaro L, Ma L, Xu W, Londei P, Cordon-Cardo C, Pandolfi PP. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004;10(5):484–486. doi: 10.1038/nm1042. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112(8):1223–33. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dansen TB, Burgering BM. Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol. 2008;18(9):421–9. doi: 10.1016/j.tcb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, Nishino I, Ezaki O. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem. 2004;279(39):41114–23. doi: 10.1074/jbc.M400674200. [DOI] [PubMed] [Google Scholar]
- 43.Ohanna M, Sobering AK, Lapointe T, Lorenzo L, Praud C, Petroulakis E, Sonenberg N, Kelly PA, Sotiropoulos A, Pende M. Atrophy of S6K1(-/-) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat Cell Biol. 2005;7(3):286–94. doi: 10.1038/ncb1231. [DOI] [PubMed] [Google Scholar]
- 44.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3(11):1009–13. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 45.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117(3):399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mammucari C, Schiaffino S, Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy. 2008;4(4):524–6. doi: 10.4161/auto.5905. [DOI] [PubMed] [Google Scholar]
- 47.Puig O, Mattila J. Understanding Forkhead Box Class O Function: Lessons from Drosophila melanogaster. Antioxid Redox Signal. 2011;14(4):635–47. doi: 10.1089/ars.2010.3407. [DOI] [PubMed] [Google Scholar]
- 48.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase Connects Energy Sensing to Mitophagy. Science. 2011;331(6016):456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]