Abstract
The chemical and isotopic compositions of oceanic biogenic and authigenic minerals contain invaluable information on the evolution of seawater, hence on the history of interaction between tectonics, climate, ocean circulation, and the evolution of life. Important advances and greater understanding of (a) key minor and trace element cycles with various residence times, (b) isotopic sources and sinks and fractionation behaviors, and (c) potential diagenetic problems, as well as developments in high-precision instrumentation, recently have been achieved. These advances provided new compelling evidence that neither gradualism nor uniformitarianism can explain many of the new important discoveries obtained from the chemistry and isotopic compositions of oceanic minerals. Presently, the best-developed geochemical proxies in biogenic carbonates are 18O/16O and Sr/Ca ratios (possibly Mg/Ca) for temperature; 87Sr/86Sr for input sources, Cd/Ca and Ba/Ca ratios for phosphate and alkalinity concentrations, respectively, thus also for ocean circulation; 13C/12C for ocean productivity; B isotopes for seawater pH;, U, Th isotopes, and 14C for dating; and Sr and Mn concentrations for diagenesis. The oceanic authigenic minerals most widely used for chemical paleoceanography are barite, evaporite sulfates, and hydrogenous ferromanganese nodules. Marine barite is an effective alternative monitor of seawater 87Sr/86Sr, especially where carbonates are diagenetically altered or absent. It also provides a high-resolution record of seawater sulfate S isotopes, (evaporite sulfates only carry an episodic record), with new insights on factors affecting the S and C cycles and atmospheric oxygen. High-resolution studies of Sr, Nd, and Pb isotopes of well-dated ferromanganese nodules contain invaluable records on climate driven changes in oceanic circulation.
Before the first global oceanographic HMS Challenger expedition (1873–1876) and the publication by Murray and Renard (1) on deep-sea sediments that was based on the expedition, little was known about oceanic minerals, their origin, distribution, or relevance. The first recovery of long cores on the Albatross Swedish expedition (1947–1949) revolutionized our understanding of the Pleistocene-Holocene ice ages recorded in oceanic minerals. The enormous potential of these minerals, especially of their chemical and isotopic tracers, containing information on the chemical history of seawater, thus on the record of interaction between tectonics, climate, ocean circulation, and evolution of life was realized. Since, great advances have been made in our understanding of the chemical and isotopic signatures of marine minerals, and therefore, of their applicability for unraveling Earth system’s evolution and operation. The history of the rates and nature of interactions of physical, chemical, and biological processes in the ocean, which reflect the interplay between Earth’s internal and external environmental processes, is recorded only in oceanic minerals.
Although detrital minerals also are discussed, first-order invaluable information on Earth system processes is recorded and stored in oceanic minerals; hence, in this synthesis the emphasis is on biogenic and authigenic minerals. Ocean-atmosphere climate models depend on such data. In addition, the role of biology in oceanic authigenic mineral formation is briefly evaluated. The oceanic minerals francolite (carbonate-F-apatite), hydrothermal Fe-Cu-Zn sulfides, and possibly Mn-Fe oxyhydroxides also may have economic significance. Characterizing active processes of formation and understanding modes of occurrence of these minerals in the modern ocean are necessary for assessing their potential economic importance and for guiding exploration for similar deposits in older geologic terrains. The potential influence of the large amount of clathrate hydrate of methane in continental margins on global warming and on its status as a future energy resource is discussed by Kvenvolden (124) in this issue of the Proceedings.
Sediment Types, Sources, and Distribution in the Ocean
The sediments in the ocean, which consist of three major components of detrital, biogenic, and authigenic origins, contain direct and/or indirect evidence of chemical and material inputs to the ocean and recycling within it. Even though each of the detrital minerals in the marine sedimentary record contains significant environmental information (Table 1), the focus of this paper is on the oceanic minerals. Because oceanic minerals record the history of the ocean-atmosphere system and hydrothermal mineralization strongly impacts seawater composition, the formation and occurrence of hydrothermal sulfide minerals is summarized as well.
Table 1.
Oceanic minerals, sources, transport, occurrence, and significance
| Mineral type | Sources and transport | Occurrence and significance |
|---|---|---|
| Detrital | ||
| Terrigenous, mostly clay minerals, feldspars, and heavy minerals | Weathering and erosion on land, transported as suspended matter of water, ice, and wind | Highest concentrations in continental margins but spread over all ocean floor. Indicative of source material, type and intensity of weathering and erosion, glaciation, wind patterns and intensities. |
| Volcanic glass (ash) and minerals | Subaerial or submarine, volcanic ejecta transported by wind and water | Occur throughout the ocean. Some concentrated at both divergent and convergent plate boundaries. Indicate history of volcanism and links to climate, valuable for dating. |
| Cosmogenic, magnetic metallic spherules and silicates | Extraterrestrial interplanetary dust, meteoritic and cometary material; gravitational input | Occur over all of the ocean floor. Indicate history of input and implications for climate, and indicate bolide-related catastrophies. |
| Biogenic | ||
| Calcite Aragonite Opal-A Celestite Apatite Magnetite Organic matter | Utilizing dissolved chemical species from weathering and erosion on land, hydrothermal submarine plumes, and elemental recycling in the ocean | Calcite and opal-A as oozes that transform diagenetically to chalk, limestone, porcellanite, and chert. Widespread in ocean and concentrated below high productivity zones. Corals consist of aragonite, most grow in tropical seas at the sea-surface, and some in the deep geochemistry and biologic evolution of the calcite and opal-A biogenic oozes and on coral aragonite. The ultramorphology of diatom opal-A is valuable for filtration. Apatite occurs primarily as fish teeth is used for stratigraphy in red-clay deep sea regions devoid of biogenic calcite and opal-A. Its geochemistry is highly informative of chemical paleoceanography. Magnetotactic bacteria, that form single domain magnetite crystals, are useful for magnetic stratigraphy. Organic matter is concentrated beneath high productivity regions; highly enriched strata may evolve into prolific hydrocarbon producers. |
| Authigenic | ||
| Sulfates and phosphate Barite Evaporites | Formation in situ from dissolved matter from weathering and erosion on land and hydrothermal submarine plumes, and by diagenesis of detrital and biogenic particular matter. | Barite concentrates beneath high productivity regions and its geochemistry provides critical information on the history of seawater chemistry, paleoceanography, especially on paleo-productivity. Evaporites for special tectonic-climate events and desiccation in semi-isolated basins and seas, i.e., the Mediterranean Sea. |
| Francolite | ” | Francolite is an indicator of high productivity zones at continental margins with medium sedimentation rates. Forms phosphorites, the most important natural fertilizer, by enrichment through winnowing and redeposition with fluctuating sea levels, and by replacement of carbonate on some oceanic islands rich in guano. |
| Mn-Fe oxyphydroxides | ” | As nodules, crusts, and coatings. Scavenge metals from seawater that are extremely important for chemical paleoceanography. |
| Silicates | ” | |
| SiO2 phases Clay minerals Zeolites | SiO2 as opal-CT porcellanite or quartz chert mostly from diagenesis of biogenic opal-A. Some opal forms at hydrothermal vents from elevated silica concentrations and hydrothermal quartz cements sulfide stockworks. By reacting with Fe opal-A may transform to nontronite. The smectites nontronite and saponite are the most important authigenic clay minerals in slow-sedimentation deep-sea environments, and form by diagenetic alteration of volcanic matter and oceanic basement. The zeolites phillipsite and analcime mostly reflect on diagenesis of volcanic matter; clinoptilote and heulandite occur in Si-rich diagenetic environments. | |
| Carbonates | ” | |
| Calciate Dolomite Rhodochrosite Siderite | Diagenetic calcite forms from biogenic calcite recrystallization beneath equatorial high productivity regions. The other carbonates indicate diagenetic formation in modified seawater by primary precipitation or replacement of calcite and aragonite; mostly in both active and passive continental margins where pore waters become suboxic to anoxic and Fe-Mn are mobilized. Dolomite also forms in evaporite settings. | |
| Sulfides Pyrite Greigite | ” | Present mostly as pyrite, but also greigite that occurs in excess-Fe or S-deficient anoxic pore waters. Pyrite is widespread in continental margin sediments with medium to high sedimentation rates and detritus input that supplies the Fe. Greigite may effect the magnetic properties of sediments. |
| Hydrothermal Fe, Cu, Zn sulfides | Formation in situ from discharging hydrothermal fluids—highly enriched in dissolved metals from seawater-oceanic basement interaction. | In association with submarine hydrothermal systems at spreading centers, including back arcs, with and without sediment cover, some may be of economic significance; provide new insights on the origin and evolution of sulfide deposits that should aid mineral exploration. |
Detrital Minerals.
Aluminosilicate minerals, ultimately derived from the weathering and erosion of rocks on land, comprise the bulk of detrital sediments. Detrital sediments are transported by water, wind, or ice. Their distribution in the ocean thus depends on climate and weathering at the source, the geographic disposition of rivers, existence of glaciers, and prevailing winds. They are most abundant in continental margins but occur over the entire seafloor. Detrital minerals and their spatial and temporal distribution contain extremely important information on climate and tectonics (refs. 2–6 and references therein) such as the changing patterns and intensities of winds (refs. 7–9 and references therein), the nature and intensity of weathering and erosion, and detritus transport by rivers and ice. A prime example are pulses of massive detritus transport by icebergs from northeast Canada into the Labrador Sea and North Atlantic Ocean during glacial retreat. These episodes are known as Heinrichs events (i.e., ref. 10). Fresh water from the melting of these icebergs disrupted the oceanic conveyor circulation and Heinrichs events correspond in time with important climate changes; for example, one makes the end of the last glacial cycle.
Volcanic ejecta comprise another type of detrital sediment carried by water and wind into the ocean. Volcanic sediments provide important information on periods of intense island arc and submarine volcanic activities, which can be compared with records of submarine tectonics and with climate and biological productivity records (11, 12).
About 4–6 × 104 tons/year of cosmogonic particles survive the Earth’s atmosphere and enter the sedimentary record. They include recognizable magnetic spherules (13), which have distinct geochemical signatures, in particular, extreme 3He concentrations and 3He/4He ratios (14–16), and high Ir and Os concentrations and distinct Os isotope ratios (17–19). They also provide crucial information on meteorite impacts and impact catastrophes (i.e., refs. 18 and 20) and on the history of cosmogonic bombardment, which may be one of the controls on climatic fluctuations (21).
Biogenic Minerals.
Dissolved weathering products constitute most of the salt in the sea. The major- and minor-element chemistry of biogenically produced minerals, most importantly calcite, aragonite, and opal-A (amorphous silica) reflect the geologic processes and rates that control the chemistry of seawater, ocean circulation, and biologic evolution. The less reactive, long residence time elements such as Cl, Na, K, Mg, Ca, and Sr do not vary throughout the ocean. Others vary with depth and between oceans because of biological cycling and scavenging by particles (22). Plants and organisms preferentially extract some elements, primarily C, N, P, Ca, and Si, from seawater to form soft tissues and minerals. Some of these elements are consumed by other organisms or redissolve, thus they are internally recycled. The rest reach the seafloor mostly as calcite and opal-A. At the seafloor, the rather segregated distribution of calcite, formed by coccolithophores (phytoplankton) and foraminifera (zooplankton), and of opal-A formed by diatoms (phytoplankton) and radiolaria (zooplankton), are important indicators of the history of productivity and ocean circulation. The trace element chemistry and isotopic compositions of these skeletal components are the most powerful tools available to unravel the effects of the interplay between tectonic and surficial processes on the chemical history of seawater (i.e., refs. 23–32 and references therein).
In addition to calcite and opal-A, aragonite is an important indicator of chemical paleoceanography. Corals form aragonitic skeletons. Because of their seasonal growth bands and formation near the sea surface, the isotopic and trace element compositions of coral heads are important as recorders at high resolution (decadal or longer) of late Quaternary sea level fluctuations, sea surface paleo-temperatures, and rainfall data (i.e., refs. 33–36). Another important mineral is celestite, which forms biogenically by acantharians (zooplankton); however, its high solubility prevents its preservation as fossils. Despite the absence of celestite from the geologic record, its importance lies in the influence on recycling of Sr and Ba in the uppermost km of the ocean, with important implications for chemical paleoceanographic interpretations of Sr/Ca and Ba/Ca ratios in corals and planktonic foraminifera.
Fish-teeth apatite is highly useful for both stratigraphy and chemical paleoceanography, especially in red clay deep-sea sediments where biogenic calcite and opal-A are absent because of dissolution (37, 38).
Magnetite biomineralization first was identified in chiton teeth (39), as it hardens their surface. The geologically important magnetite consists of the morphologically distinct single domain crystals formed by magnetotactic bacteria (39, 40). This magnetite is responsible for much of the stable magnetic remanence in many marine sediments, which is valuable in paleomagnetic studies.
Organic matter-rich sediments signify periods of higher productivity and/or higher organic C preservation in low-oxygen waters, either in a more intense and expanded oxygen minimum zone or in low-oxygen bottom waters (i.e., ref. 41). The assumed link between organic C preservation and water oxygen content has been challenged (i.e., ref. 42) and presently is being tested. Nevertheless, the stable carbon isotopic composition of organic matter in marine sediments helps to identify periods of high productivity or of high terrestrial organic matter input from enhanced continental weathering. This information is essential for modeling the oceanic C cycle and atmospheric CO2 and O2 fluctuations (i.e., refs. 22, 43, and 44 and references therein). Prime examples of periods with widely distributed organic C-rich sediments are (a) the mid-Cretaceous abnormally organic C-rich “black” sediments in all major ocean basins, concentrated primarily in Aptian (115–110 Ma) and Cenomanian/Turonian (≈90 Ma) (i.e., refs. 45 and 46 and references therein), known as the Crateceous Anoxic Events (CAE), and (b) the late Neogene Mediterranean sapropels (i.e., ref. 47 and references therein). The mid-Cretaceous organic C burial during the CAE has significant implications for the ocean-atmosphere system C imbalance, as seen in the C isotopic composition of marine carbonates (48), hence on atmospheric O2 content. Such “black” shales are potential reservoir rocks. Worldwide, Cretaceous strata are known for being prolific hydrocarbon producers (i.e., refs. 49–51).
Authigenic Minerals.
Authigenic minerals form by in situ inorganic precipitation on the seafloor and within the sediment column. Barite is the only mineral so far reported to also form in the water column (52, 53). Some authigenic mineral reactions are bacterially mediated; the bacteria modify the immediate geochemical environment, inducing mineral formation. For Paleo-environmental studies, the most important marine authigenic minerals are barite, francolite (a carbonate fluor apatite), evaporites, especially anhydrite/gypsum and halite, and Mn-Fe-oxyhydroxide that occur as nodules or crusts. The concentrations and isotopic compositions of oceanic conservative components in these authigenic minerals that form at the seafloor are extremely informative for studies of the history of seawater chemistry. Nonconservative chemical components also reveal information about distinct water masses, thus about ocean circulation in the geologic past. In addition to diagenetic opal-CT and quartz that form from the dissolution of biogenic opal-A, other common authigenic alumino-silicate minerals are smectites and zeolites. Dolomite and pyrite are also widespread authigenic minerals.
Authigenic alumino-silicates from both close to the sediment-seawater interface and the sediment column mainly form by replacement of precursor minerals or mineraloids, but also can form by direct precipitation from pore fluids. On short time scales (decadal to millenial) the total amount of oceanic authigenic silicate remineralization seems to exert an extremely small impact on the global balance between CO2 degassing rate and atmospheric CO2 removal rate by continental weathering of silicates (43, 54, 55). Research on the contribution of silicate remineralization to the global CO2 budget in the modern ocean presently is being actively pursued (56).
The important marine clay minerals are smectites. The iron smectite, nontronite, is widespread in the Pacific ocean floor where hydrothermal activity is prevalent and sedimentation rates are slow <1 cm/kyr. It forms at the seafloor from hydrothermal Fe oxyhydroxides and opal-A (57). In addition, nontronite and saponite replace precursor volcanic components, especially volcanic glass. Diagenetic illitic clays are celadonite and glauconite. Celadonite is associated primarily with volcanic matter alteration. Glauconite forms in subtropical and tropical margins in shallow to intermediate water depths, near the sediment-water interface beneath high productivity upwelling regions. These areas have intermediate to slow sedimentation rates, and both ferric and ferrous Fe are simultaneously available in pore waters. The zeolite Phillipsite is common at the seafloor or at shallow burial (58, 59). It replaces volcanic material in slow sedimentation environments such as the South Pacific deep sea. Clinoptilolite is also common and forms within the sediment column where silica concentrations are elevated to stabilize it, mostly from opal-A dissolution (59, 60). Occasionally clinoptilolite forms pseudomorphs after radiolaria tests (60). Analcime is significantly less abundant and typically forms in volcanic ash-rich sediments (59).
Authigenic calcite is widespread in the ocean. It forms mostly from biogenic calcite recyrstalization and is geochemically distinct in its minor and trace element concentrations from its precursor. This recrystallization process ultimately transforms calcareous ooze into chalk and limestone. Dolomite forms where pore-fluid seawater is modified bacterially or by physical-chemical processes. In continental margins where pore fluids become suboxic to anoxic, dolomite forms both as a primary precipitate and by replacing precursor Ca and Ca-Mg carbonates (61, 62). It also occurs in carbonate platforms where dolomite replaces CaCO3 by the thermally driven convective flow of seawater that is being inorganically and bacterially modified along the flow path. The chemical and isotopic compositions, primarily of carbon and oxygen, reveal the origins of the various dolomites. Rhodochrosite and siderite are uncommon marine minerals. Manganese and Fe mostly substitute for Mg in the dolomite structure in suboxic to anoxic pore fluid environments where they are mobilized. In these environments, only in the presence of much detrital Mn and Fe, rhodochrosite and/or siderite may form during late diagenesis where the reactions have driven Mg/Ca ratios in the pore fluids to levels below dolomite stability.
Pyrite forms where sulfate is bacterially reduced. In these environments Fe+3 is reduced and mobilized, reacting with the sulfide to form pyrite. Because of the large isotopic fractionation by the bacterial reduction of sulfate to sulfide (≈40%) the amount of pyrite formation modulates the seawater-sulfate sulfur isotopic composition and the atmospheric oxygen content, as discussed below. Greigite, another Fe sulfide, may form kinetically instead of pyrite, especially in the presence of Fe excess or in S-deficient anoxic pore waters. The variability in pyrite versus greigite formation reflects the pH and Fe/S ratio of the pore fluid, which originally was modified by sulfate-reducing bacteria. Greigite has distinct magnetic properties.
The most important economic authigenic mineral is francolite, which forms phosphorite deposits when tectonic-oceanographic conditions are favorable (63, 64). Similar to C, about 80% of the global P burial occurs in continental margins despite the much greater area of the deep ocean. Understanding the origin of francolite and phosphorites, used principally as fertilizer in detergents and other industrial applications, is essential for effective exploration for new phosphorite deposits. Oceanic phosphorites form more than 80% of the world phosphate, whereas guano deposits are considerably less economically important. Based on recent research, francolite forms in continental margins at slow to intermediate sedimentation rates and more efficiently in suboxic to oxic bottom waters where Fe-redox cycling of P prevails (64–66). Its occurrence in this environment is characteristic of high sea-level stands during interglacial periods, where it is enriched by winnowing and redeposition during glacial periods (67). Repeated formation and enrichment of this mineral is necessary to form economic deposits. Secular variations in the occurrence of large phosphorite deposits in the geologic record reflect shifts in global regional patterns of P deposition that are controlled by tectonics and climate. The chemical and isotopic signatures in phosphorite francolite that forms at or close to the sediment-seawater interface are those of the bottom water oceanic environment. Because of slow formation and sedimentological reworking and mixing, the chemical paleoceanographic information stored in phosphorite francolite, although extremely valuable, is of moderate to low resolution.
Submarine Hydrothermal Sulfide Deposits.
Submarine hydrothermal massive sulfide deposits forming at divergent plate boundaries have been discovered only in the past ≈20 years. These deposits primarily consist of the sulfides chalcopyrite, sphalerite and/or wurtzite, pyrrhotite, pyrite and/or marcasite, and the associated minerals anhydrite, barite, and opal-A. At the seafloor in oxidizing low-temperature environments hydrothermal sulfides are unstable, they are oxidized to form Fe oxides and secondary sulfides such as covellite, digenite, and bornite, except if rapidly covered by new volcanic eruptions (i.e., refs. 68 and 69). As yet, observations mainly of surface seafloor sites have been conducted, and the magnitude and structures of these sulfide deposits have not yet been thoroughly characterized. The discovery of massive sulfide deposits has had a profound impact on our understanding of ore-forming processes, but their economic significance is still uncertain.
At fast- and slow-spreading centers, seawater penetrates several km into oceanic basement (maximum depth is above the top of the magma chamber), where it is heated to 350–400°C and interacts with oceanic basement and sediments. This fluid ascends by buoyancy to form hydrothermal vents and sulfide deposits. The discharging fluids support rich chemosynthetic, previously unknown biological communities. The suggestion that the deep-sea hydrothermal vents environment was possibly the most conducive environment for the origin of life on Earth is controversial.
About 30% of the oceanic heat budget is removed by hydrothermal circulation (70, 71). Chemical and isotopic exchange reactions between the heated seawater and oceanic basement have a profound influence on seawater chemistry (72, 73), and thus also effect the mineralogy, geochemistry, and physical properties of the altering oceanic basement (74, 75). Hot and acidic, pH 3–4, discharging hydrothermal fluids are strongly enriched, not only in metal complexes and 3He, but also in Li, K, Rb, Ca, Ba, and Si, and have no Mg and sulfate (72). Overall the fluxes of these elements out and into the midocean ridges are comparable to those from river fluxes. Precipitation of minerals occurs when the hot acid, sulfide, and metal-rich fluids mix with cold oxidizing alkaline seawater. The entire ocean volume circulates through oceanic basement at spreading centers in 5–7 million years. Especially the common newly formed hydrous silicate minerals, smectite, ordered mixed layer smectite/illite, epidote, chlorite, serpentine, and amphiboles exert important controls on the chemical and physical behaviors of the oceanic plate when it is subsequently subducted. In particular, as a result of mineral dehydration reactions, these minerals determine the amount and timing (temperature) of fluids released in the seismogeneic zone in relation to earthquake cycles and arc volcanism (76–78).
In 1994, on Ocean Drilling Program Leg 158 drilling into an actively forming large sulfide mound in the Trans-Atlantic Geotraverse (TAG) area of the Mid-Atlantic Ridge, at ≈26°N, a modern analog of fossil ophiolite-hosted deposits such as those exposed on Cyprus or Oman, revealed that this sulfide deposit has been forming for at least 20,000 years by successive hydrothermal episodes, including brecciation and cementation by anhydrite and quartz of previous precipitates. Brecciation of these deposits is induced by anhydrite dissolution between mineralization episodes. The widespread occurrence of anhydrite indicates seawater entraintment and its heating to >150°C. Upon cessation of the hydrothermal activity the anhydrite dissolves and does not survive in the geologic record. Pyrite is abundant in the upper flow region together with the anhydrite and with quartz. The hottest hydrothermal chimneys (black smokers) are composed of mostly chalcopyrite and anhydrite; the cooler ones (white smokers) are dominated by sphalerite. Estimates are that 2.7 million tons of massive sulfides exist above the seafloor and at least 1.2 million tons beneath it in the fluid upflow zones at TAG (79). This is comparable to an average-sized Cyprus-type ophiolite sulfide deposit (80). The basalt below is chloritized. In sedimented and back arc spreading centers, barite is a common cementing mineral together with anhydrite. Examples are the Guaymas Basin, Juan de Fuca Ridge, and Mariana Trough. Unlike anhydrite, barite is more likely to survive burial and resist diagenesis and dissolution.
Examples of Recent Advances in Studies of Oceanic Minerals and Implications for Chemical Paleoceanography and Global Change
Changes in ocean chemistry respond to climate-producing variations in the exogenic cycle and are recorded in oceanic minerals, but the record is far from simple and bulk chemical analyses are inadequate for paleoceanographic studies. Having well-dated materials is essential for placing geochemical records in a firm chronology. Important advances and greater understandings of (a) key minor and trace element cycles, (b) isotopic sources and sinks and fractionation behaviors especially of the light stable isotopes (with isotope masses <40), (c) the potential diagenetic problems of mineral diagenesis, and (d) important developments in high-resolution analytical instrumentation recently have been achieved. These advances have clearly reaffirmed that neither gradualism nor uniformitarinism can explain many of the recent important discoveries in paleoceanography.
Evidence for Rapid Climate Shifts.
Recent results obtained from the oxygen isotopes of foraminifera tests from deep-sea cores indicate that the evolutions of seawater chemistry, ocean circulation, biology, and climate are punctuated by periods of rapid (decadal to millennial) changes. Among the most well-known and geochemically clearly marked punctuating events are the rapid cooling and warming events that lasted a few thousand to a few hundred years during the longer Pleistocene-Holocene glacial-interglacial cycles, for example, the younger Dryas (i.e., refs. 81–84). Similar results, but at even higher resolution and frequencies, have been obtained from ice core studies (i.e., refs. 85–87). A second prime example is the abrupt and major extinction at the Cretaceous-Tertiary boundary when a bolide struck Earth (i.e., refs. 18–20). Oceanic minerals hold the key to understanding how the records left by such events and the resulting feedbacks might be used to gain knowledge about how Earth works. These event studies leave us better prepared to predict rapid global warming and may allow us to avoid surprises in climatic response to anthropogenic perturbations.
The Mediterranean Salinity Crisis.
The notable finding of a thick evaporative formation of halite and anhydrite/gypsum underlain and overlain by deep-sea sediments, in the deep Mediterranean Sea (and also the Red Sea), at >3 km water depths, indicates that the Mediterranean Sea almost completely dried up in the late Miocene Messinian stage 6–5 Ma when the Gibraltar isthmus tectonically separated it from the Atlantic Ocean. When the strait of Gibraltar barrier broke at the end of the Messinian, the Mediterranean Sea was abruptly filled with Atlantic Ocean water (within ≈100 years) (i.e., ref. 88).
Minor and Trace Elements in Biogenic Calcite and Aragonite and Their Significance.
To understand the unique geochemical records stored in oceanic minerals it is necessary to develop an in-depth understanding of biogeochemical processes in the modern oceans and establish new and redundant proxies for the most significant oceanographic processes. To be a reliable monitor, a mineral must inherit the chemical and isotopic composition of seawater at the time of its formation, must retain this signal intact, and remain inert to chemical exchange after burial. Another prerequisite for an effective paleoceanographic proxy is that the chemical behavior and oceanic distribution of the elements are rather well known.
The prime mineral used for paleoceanographic studies is biogenic calcite. Foraminifera shells in core tops are composed of low Mg-calcite (<1–4 mol% MgCO3) that contains minor and trace element concentrations, most importantly, Mg, Sr, Ba, Cd, (and possibly Na). These elements substitute for Ca. Li, B, REE, U are important trace elements in biogenic calcite that act as oceanographic environmental indicators. It has been shown that, similar to paleothermometry using the oxygen isotope ratios of these tests (e.g., ref. 23), the concentrations of these tracers in the foraminifera tests, although not representative of the thermodynamic distribution coefficients, generally reliably and consistently reflect the seawater composition and the biogeochemical conditions of formation. The biogeochemical controls on the incorporation of these minor and trace elements in the biogenic calcite and aragonite, however, are poorly known. Over the past several years, through difficult and persistent studies, it has been demonstrated that minor and trace elements in biogenic calcite can be used as proxies for a variety of important chemical oceanographic properties. The pioneering work by Boyle (89, 90) on cleaning procedures for studies of Cd in planktonic and benthonic foraminifera as a proxy for seawater phosphate, and for using Cd/Ca ratios in foraminifera shells for ocean circulation studies opened the field for new developments of trace element proxies of very low concentrations in the CaCO3 lattice. Improvements in culturing foraminifera, and especially in analytical techniques for accurate chemical and isotopic analyses of trace components and isotope ratios in small purified samples, such as the multicollector inductively coupled plasma mass spectrometry (MC-ICPMS) or the ion probe, provide great promise for documenting variations in seawater chemistry and sea surface temperature (SST) at very high resolution. The application of such techniques also should help to distinguish between physical-chemical versus biogeochemical controls on the trace element distributions in the calcites and aragonites.
Presently, the best developed and most used geochemical proxies in foraminifera calcite shells are:
Oxygen isotope ratios and Sr/Ca ratios. Both are primarily important as temperature indicators; the latter also indicates seawater Sr concentrations. Oxygen isotopes, however, are influenced by both temperature and ice volume (i.e., refs. 24, 91, and 92).
Sr isotope compositions hold information about fluctuations in the inputs of Sr into the ocean, through time, by climate, tectonics, weathering processes, and hydrothermal activity. It is very useful for stratigraphic correlation and dating (i.e., refs. 93–95 and references therein).
Cd/Ca and Ba/Ca ratios can be used as indicators of phosphate and alkalinity concentrations, respectively (i.e., refs. 31, 89, and 90). The C cycle is directly linked to nutrient concentrations and productivity, for example to phosphate concentration, therefore both Cd and Ba are important for paleoceanography (22).
Carbon stable isotope ratios are used to study ocean productivity (i.e., refs. 26 and 44).
The B isotope ratios of foraminifera (11B and 10B) reflect variations in seawater pH, an important parameter influencing atmospheric CO2 (i.e., refs. 32 and 96), because the boric acid to borate ratio is strongly pH dependent, and the two dissolved B species have distinct B isotopic signatures.
U/Th isotopes can be used for dating (i.e., refs. 97 and 98).
Sr, and possibly Mn, concentrations are excellent indicators of diagenesis (i.e., refs. 99 and 100). This is extremely important for monitoring the long-term integrity of mineral species in chemical oceanographic studies.
Because Mg readily substitutes for Ca in the calcite structures and is a major conservative component of seawater, the Mg/Ca ratio in foraminifera shells may be realized as a valuable palaeotemperature indicator. This possibility is being pursued at several laboratories (i.e., ref. 101).
Strontium, Ba, and Mg can substitute for Ca in aragonite. The large Sr and Ba are more abundant in aragonite, and the smaller Mg is more abundant in calcite. As a result, seasonal banded corals that grow at the sea surface, composed of aragonite, show great promise for preserving records of ocean chemistry and temperature over a few centuries with a seasonally to biweekly resolution. Questions like the “exact” timing of the last glaciation or the causes and feedbacks of the very rapid climate changes observed in deep sea and ice cores are important for our understanding of the dynamics of the large ice sheets, their effect on ocean circulation, climate, and SST anomalies in the tropics. The latter is of a key parameter in paleoclimate analysis, thus, for understanding the causes of past rapid climate fluctuations such as those responsible for the El Niño Southern Ocean phenomenon.
For climate studies, U-Th dated bands of coral species that live in the upper 5–20 m of the ocean are being used. For the SST record, in addition to oxygen isotope ratios, a measure of both SST and ice volume, the same coral bands also are analyzed for Sr/Ca and Mg/Ca ratios. These ratios are thought to provide more reliable SST data than oxygen isotopes. Corals from the equatorial Atlantic, Pacific and Indian oceans thus have been analyzed for these components (i.e., refs. 36, 83, and 102–106). The Sr/Ca and Mg/Ca ratios in the aragonite structure are controlled by the activity of Sr, Mg, and Ca in seawater and the Sr/Ca and Mg/Ca distribution coefficients between aragonite and seawater that also depend on temperature. At present it is assumed that the biological controls on these distribution coefficients are minimal and that the Sr/Ca and Mg/Ca ratios in seawater are conservative. However, especially for high-resolution temperature data based on Sr/Ca ratios, the effect of the precipitation of acantharians SrSO4 shells in the upper water column on these ratios in corals needs to be better constrained.
The most important result obtained from recent studies of the δ18O values and Sr/Ca (and Mg/Ca) ratios in tropical corals is that SST fluctuation between glacial and interglacial periods in the tropics were considerably larger, by 2–5°C, than previously recorded by using the oxygen isotopic ratios of foraminifera. The resulting implications for climate modeling are profound.
Aragonite is unstable at surface seawater and transforms to calcite. Therefore the above-discussed coral studies are primarily applicable for Holocene corals. Even in Holocene corals aragonite may undergo diagenesis. As yet very little is known about the behavior of trace elements during diagenesis of coral aragonite. The absence of calcite is the only reliable test available that indicates that coral aragonite is pristine; this, however, is inadequate. To being able to extend in time the unique and high-resolution climatic records stored in coral aragonite, several laboratories have begun to pursue this problem.
Marine Barite as a Monitor of Seawater Sr and S Isotope Compositions and Ocean Productivity.
Marine barite is an ubiquitous minor phase in Pelagic sediments (52, 53), particularly underlying regions of high biological productivity where it reaches concentrations in the sediment of over 2 weight percent (carbonate free). The exact mechanism of barite formation is not known, although there are indications that it precipitates inorganically directly from seawater, in micro-environments containing decaying organic matter, acantharian shells, and siliceous frustules and tests (i.e., ref. 53). This authigenic barite forms micro-crystals or aggregates ranging from 0.5 to 5 mm that is very different in habit from hydrothermal barite (Fig. 1). The water column is slightly undersaturated with respect to barite (107), therefore, some barite must dissolve at greater depths in the water column. This is observed in dissolved Ba concentration-depth profiles in the ocean. In most sediments, especially those beneath high productivity zones, pore-waters rapidly become saturated with respect to barite; hence, most of this barite is preserved in oxic marine sediments (108, 109). Sr isotope analyses of marine barite and its crystal habit suggest no significant diagenetic alteration of this mineral occurs after burial in oxic pelagic sediments (109). The presence of morphologically and geochemically unaltered marine barite in calcareous sediments with clear indications of carbonate diagenesis (for example, Deep Sea Drilling Project Leg 85 Site 574, at >250 m burial depth), further supports this suggestion. Moreover, the 226Ra activities of marine barite indicate that the mineral behaves as a closed system in oxic environments (110). Thus, marine barite is a highly suitable mineral for a range of chemical paleoceanographic studies, for example, for seawater Sr and S isotopic compositions and paleo-productivity.
Figure 1.
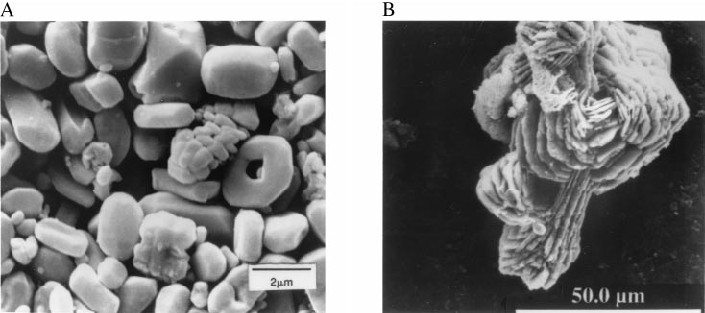
Scanning electron microscopy micrographs of (A) marine barite separated from late Miocene (9.6 Ma) equatorial Pacific calcareous sediment, Deep Sea Drilling Project, Site 575. (B) Hydrothermal barite from a submarine sulfide chimney at Juan de Fuca Ridge.
Minor amounts of particulate barite also form in submarine hydrothermal systems (111). Barite dissolves in sulfate-reducing sediments and reprecipitates at the oxic-anoxic boundaries of such sediments. These hydrothermal and diagenetic barites may have Sr isotope ratios that are different from contemporaneous sea water; they therefore are unsuitable for chemical paleoceanographic studies, but they are relatively easily recognized (Fig. 1).
As discussed earlier, the strontium isotope ratios in sea water are influenced by climate, tectonics, weathering, and hydrothermal activity at ocean ridges. The evolution of this ratio through time, determined primarily by measuring the strontium isotope composition of marine carbonates, holds information about variations in these processes. It is also useful for stratigraphic correlation and age dating. Carbonates, however, are absent from some marine sediments, such as siliceous oozes and red clays, and can be significantly diagenetically altered in others, especially in Eocene and older sediments. Like calcite marine barite is an effective monitor of seawater 87Sr/86Sr. The microcrystals of marine barite separated from Holocene Pacific, Atlantic, and Indian ocean sediments all record the modern seawater 87Sr/86Sr value, and the 87Sr/86Sr values of barite from sediment samples spanning the past 35 million years all fall within the range of published data for carbonates over this time period (109).
Global changes in climate and atmospheric chemistry also are intimately related to the sedimentary sulfur cycle, and seawater sulfate is one of the main reservoirs of the sulfur exogenic cycle. Hence, knowledge of the chemical and isotopic composition of sulfate is of great importance for understanding coupled geochemical cycles on Earth, especially of C, S, O, and P. Evidence for large-scale transfers of S between different sedimentary reservoirs is provided by the evaporite-based isotope record for oceanic sulfate (112, 113). Because the geological record of marine evaporites is episodic, with gaps of tens of million years and evaporites are susceptible to diagenesis, a continuous seawater sulfate sulfur isotope curve for the Cenozoic recently has been generated at ≈1 million year resolution (114). The new curve fills significant gaps in the evaporite-based data set. A comparison between the new sulfate sulfur isotope data and the carbon isotope record of marine carbonates does not reveal the clear systematic coupling between the S and C cycles assumed in the existing major global geochemical cycles models. The new data indicate that changes in pyrite sulfur and organic carbons burial rates did not balance each other over a few to tens of millions of years. This has important implications for modeling of the atmospheric oxygen concentration over Phanerozoic time.
Ocean productivity influences organic carbon supply to the sediment and its burial efficiency, thus affecting the partitioning of CO2 between the ocean and atmosphere and influencing climate. Large glacial to interglacial fluctuations in atmospheric CO2 concentrations have been observed in ice cores and related to variations in ocean productivity (85, 87). To discern the coupling between ocean circulation, productivity, and climate, it is important to be able to estimate past ocean productivity and to reconstruct its history from the record of marine sediments. Upwelling of CO2-rich waters in the equatorial Pacific provides the largest natural source of CO2 to the atmosphere.
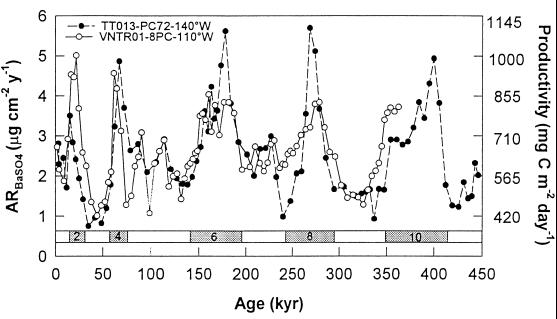
As indicated above, because of its relative stability in the oxic pelagic environment marine barite accumulation rate in sediments is suitable for reconstructing changes in ocean biological productivity. Fluctuations in barite accumulation rates down-core (shown in Fig. 2) indicate that during glacial periods of the past 45 × 104 years, the productivity in the central and eastern equatorial Pacific was about two times that during intervening interglacial periods (115). This result is consistent with other evidence that productivity was high in the eastern and central equatorial Pacific during the last glacial.
Figure 2.
(A) Accumulation rate of barite (AR BaSO4) and productivity in the equatorial Pacific in the past 45 × 104 years; data from two piston cores VNTR01–08PC at 110°W (○) and TT013–72PC at 140°W (●). Modified from figure 3 in ref. 115.
Ocean Dynamics and Climate Records in Hydrogenous Ferromanganese Crusts and Nodules.
In the modern ocean, the thermohaline “conveyor” system circulation (in ≈1,500 years) controls the transfer of heat and solutes between the ocean basins and thus modulates climate. Dense cold surface seawater, formed in the North Atlantic, is carried throughout the ocean basins at depth and is replaced by northward transport of warmer water. Because circulation patterns most likely have been different in the past, the resulting global environmental, thus geochemical changes, are stored in biogenic and authigenic marine minerals.
Hydrogenous Mn-Fe oxyhydroxide nodules and crusts effectively scavenge and incorporate geochemically important trace elements from seawater, including Be and Th-U, making them suitable for age dating. Recent high-resolution studies especially of Sr, Nd, and Pb isotopes (i.e., refs. 116–119), of well-dated samples by 10Be/9Be isotope ratios (i.e., refs. 120–122) using a high-precision laser ablation, multicollector inductively coupled plasma mass spectrometry have successfully recorded important oceanic circulation changes. For example, the closure of the Central American isthmus, which changed an E-W ocean circulation to a N-S pattern, had clear geochemical consequences with evidence stored in Mn-crusts and nodules (123). Because of the slow growth of these nodules (1 to <10 mm/million year) high-precision sampling of them permits much higher resolution studies of paleoclimatic fluctuations than previously possible. Especially because of the very short residence time of Pb, the significant input variations in Pb isotopes in these minerals (i.e., refs. 117 and 122), on time scales similar to oceanic oxygen isotopes in sediments, provide important insights on the history of coupling between climate and input of Pb into the ocean, thus on continental erosion, atmospheric CO2, and climate-driven changes in ocean circulation through time.
The Role of Biology in Oceanic Authigenic Minerals Formation.
Questions about the role of biology in the formation of oceanic authigenic minerals have been raised. As yet, there is no evidence for a direct biological involvement in the formation of any of the known oceanic authigenic minerals. Bacterial processes induce the formation of the oceanic authigenic minerals francolite, pyrite, and/or greigite, and of the carbonates dolomite, siderite, and rhodochrosite, by modifying their immediate geochemical environment. Biology also controls the preservation of bacterially produced magnetite. For example, in organic-rich sediments with bacterial reduction of Fe3+ to Fe2+, single domain magnetite crystals are unstable and the remanent magnetization of these sediments is not preserved.
In continental margin sediments beneath high productivity regions with moderate sedimentation rates and burial of reactive organic matter, bacterial nitrate, Fe3+, and sulfate reduction occur, and reactive Fe3+ is bacterially reduced to Fe2+, setting in motion the Fe-redox cycling of P. This increases the availability of P for francolite and eventually promotes phosphorite formation, especially where bottom waters are oxic (64–66). Fe-redox cycling occurs in the uppermost sediment column close to the sediment-seawater interface. Below this zone, sulfide, produced by bacterial sulfate reduction, combines with the bacterially mobilized ferrous ion, which precipitates mostly as pyrite, but occasionally first as greigite, because of kinetic constraints. The first appearance of greigite occurs especially in areas of excess Fe and/or deficient S in sediments. Pore water pH, modulated by sulfate-reducing bacteria, also controls the formation of greigite versus pyrite. The sulfur isotope ratios of these sulfides indicate the important role of bacterial mediation in their formation.
In addition to P- and S-bearing minerals, biology also affects the formation of dolomite. Dolomite, although the thermodynamically stable carbonate mineral in seawater, does not form in the pelagic environment because the activation energy required for the formation of this ordered mineral at the prevailing low temperatures in the ocean is very high. It does, however, form in modified seawater with higher than normal alkalinity and/or Mg/Ca ratios that help to overcome the activation energy barrier. Dissolved sulfate kinetically inhibits the precipitation of carbonate minerals, especially of dolomite. Both its removal by bacterial sulfate reduction and the simultaneous alkalinity production promote dolomite formation (61, 62). As a result, dolomite forms inorganically in the marine environment in evaporative sub-basins where its formation is kinetically enhanced by high Mg/Ca ratios and elevated temperatures. It also forms by bacterial mediation in continental margins where most of the buried organic matter in the ocean occurs and pore fluid alkalinity is high. Consequently, on many margins authigenic calcite forms and pore fluid Mg/Ca ratios increase to between 6 to >20, compared with the seawater ratio of 5.4. Subsequently, at even higher alkalinity and lower sulfate concentrations dolomite forms instead of calcite. Dolomite also forms from precursor carbonates in carbonate platforms where thermally driven convective fluid flow drives reactions between modified pore fluids by the above mentioned bacterially mediated reactions. Bacterial sulfate reduction and alkalinity production is also widespread in evaporative sub-basins and is an important mechanism for dolomite formation in these environments.
An important consequence of the vertical sequence of bacterial redox reactions on other authigenic carbonate mineral formation is the continuous mobilization of available reactive Mn2+ and Fe2+ by iron-reducing bacteria. This can cause the formation of rhodochrosite, ankerite, and/or siderite, respectively, instead of dolomite, in pore fluids with lowered Mg/Ca (<1–2) from earlier carbonate and silicate diagenetic reactions. Because of the high Mg content in seawater siderite is less common as an authigenic oceanic mineral than in continental environments. In addition to carbonates, biological involvement also has been invoked in marine barite formation (i.e., refs. 52 and 53), to explain its formation in the ocean water column, which is undersaturated with respect to barite solubility (107); oxidation of organic S to sulfate in organic-rich micro environments was proposed (i.e., refs. 52, 53, and 108), but the biological involvement in marine barite formation is yet to be demonstrated unequivocally.
ABBREVIATION
- SST
sea surface temperature
References
- 1.Murray J, Renard A F. Challenger Reports. London: Longmans; 1891. ; reprinted (1965) (Johnson, London). [Google Scholar]
- 2.Lisitzin A P. Sedimentation in the World: Ocean. Tulsa, OK: Soc. Econ. Paleont. Mineral.; 1972. , Spec. Pub., 17. [Google Scholar]
- 3.Berger W H. In: Chemical Oceanography. Riley J P, Chester R, editors. London: Academic; 1973. pp. 266–387. [Google Scholar]
- 4.Hay W W. Geol Rundsch. 1996;85:409–437. [Google Scholar]
- 5.Raymo M E, Ruddiman W F. Nature (London) 1992;359:117–122. [Google Scholar]
- 6.Edmond J M. Science. 1992;258:1594–1597. doi: 10.1126/science.258.5088.1594. [DOI] [PubMed] [Google Scholar]
- 7.Olivarez A M, Owen R M, Rea D K. Geochim Cosmochim Acta. 1991;55:2147–2158. [Google Scholar]
- 8.Prospero J M. In: Particle Flux in the Ocean. Ittekkott V, Honjo S, Depetris P J, editors. New York: Wiley; 1996. pp. 133–151. [Google Scholar]
- 9.Rea D K, Hilde S, Leah H J. Paleoceanography. 1998;13:215–224. [Google Scholar]
- 10.MacAyeal D R. Paleoceanography. 1993;8:767–773. [Google Scholar]
- 11.Larson R L. Geology. 1991;19:547–550. [Google Scholar]
- 12.Officer C B, Drake C L. Science. 1985;227:1161–1167. doi: 10.1126/science.227.4691.1161. [DOI] [PubMed] [Google Scholar]
- 13.Murrel M T, Davis P A, Nishizumi K, Millard H T. Geochim Cosmochim Acta. 1980;44:2067–2074. [Google Scholar]
- 14.Ozima M, Takayanagi M, Zashu S, Amari S. Nature (London) 1984;311:448–450. [Google Scholar]
- 15.Anderson D L. Science. 1993;261:170–176. doi: 10.1126/science.261.5118.170. [DOI] [PubMed] [Google Scholar]
- 16.Farley K A. Nature (London) 1995;376:153–156. [Google Scholar]
- 17.Barker J L, Anders E. Geochim Cosmochim Acta. 1968;32:627–645. [Google Scholar]
- 18.Alvarez L W, Alvarez W, Asaro F, Michel H V. Science. 1980;208:1095–1108. doi: 10.1126/science.208.4448.1095. [DOI] [PubMed] [Google Scholar]
- 19.Luck J M, Turekian K K. Science. 1983;222:613–615. doi: 10.1126/science.222.4624.613. [DOI] [PubMed] [Google Scholar]
- 20.Esser B K, Turekian K K. Geochim Cosmochim Acta. 1988;52:1383–1388. [Google Scholar]
- 21.Muller R A, MacDonald G J. Nature (London) 1995;377:107–108. [Google Scholar]
- 22.Broecker W S, Peng T-H. Tracers in the Sea. Palisades, NY: Eldigio; 1982. [Google Scholar]
- 23.Epstein S, Buchsbaum R, Lowenstam H A, Urey H C. Geol Soc Amer Bull. 1953;64:1315–1325. [Google Scholar]
- 24.Emiliani C. Earth Planet Sci Lett. 1978;37:349–352. [Google Scholar]
- 25.Savin S M, Douglas R G, Stehli F G. Geol Soc Amer Bull. 1975;86:1499–1510. [Google Scholar]
- 26.Shackleton N J, Opdyke N D. Nature (London) 1977;270:216–219. [Google Scholar]
- 27.Broecker W S. Geochim Cosmochim Acta. 1982;46:1689–1705. [Google Scholar]
- 28.Graham D W, Bender M L, Williams D F, Keigwin L D. Geochim Cosmochim Acta. 1982;456:1281–1292. [Google Scholar]
- 29.Burke W H, Denison R E, Hetherington E A, Koepnick R B, Nelson H F, Otto J B, et al. Geology. 1982;10:516–519. [Google Scholar]
- 30.Boyle E A. Paleoceanography. 1988;3:471–489. [Google Scholar]
- 31.Lea D W, Boyle E A. Nature (London) 1989;338:751–753. [Google Scholar]
- 32.Spivack A J, You C-F, Smith H J. Nature (London) 1993;363:149–151. [Google Scholar]
- 33.Emiliani C, Hudson J H, Shinn E A, George R Y. Science. 1978;202:627–629. doi: 10.1126/science.202.4368.627. [DOI] [PubMed] [Google Scholar]
- 34.Fairbanks R G. Nature (London) 1989;342:637–642. [Google Scholar]
- 35.Wellington G M, Dunbar R B. Coral Reefs. 1995;14:5–25. [Google Scholar]
- 36.De Villiers S, Nelson B K, Chivas A R. Science. 1995;269:1247–1249. doi: 10.1126/science.269.5228.1247. [DOI] [PubMed] [Google Scholar]
- 37.Doyle P S, Riedel W R. Micropaleontology. 1979;25:337–364. [Google Scholar]
- 38.Ingram B L, Coccioni R, Montanari A, Richter F M. Science. 1994;264:546–550. doi: 10.1126/science.264.5158.546. [DOI] [PubMed] [Google Scholar]
- 39.Lowenstam H A, Weiner S. On Biomineralization. Oxford: Oxford Univ. Press; 1989. [Google Scholar]
- 40.Chang S-B R, Kirschvink J L. Annu Rev Earth Planet Sci. 1989;17:169–195. [Google Scholar]
- 41.Emerson S, Hedges J I. Paleoceanography. 1988;3:621–634. [Google Scholar]
- 42.Calvert S E, Pedersen T F. In: Productivity, Accumulation and Preservation of Organic Matter in Recent and Ancient Sediments. Whelan J K, Farrington J W, editors. New York: Columbia Univ. Press; 1992. pp. 231–263. [Google Scholar]
- 43.Sundquist E T. Q Sci Rev. 1991;10:283–296. [Google Scholar]
- 44.Shackleton N J. In: Marine Petroleum Source Rocks. Brooks J, Fleet A J, editors. London: Geol. Soc.; 1987. pp. 423–434. [Google Scholar]
- 45.Schlanger S O, Cita M B, editors. Nature and Origin of Cretaceous Carbon-Rich Facies. London: Academic; 1982. [Google Scholar]
- 46.Jenkyns H C. J Geol Soc (London) 1980;137:171–188. [Google Scholar]
- 47.Calvert S E, Pedersen T F. Mar Geol. 1993;113:67–88. [Google Scholar]
- 48.Scholle P A, Arthur M A. Am Assoc Petrol Geol Bull. 1980;64:67–87. [Google Scholar]
- 49.Arthur M A, Schlanger J O. Am Assoc Petrol Geol Bull. 1979;63:870–885. [Google Scholar]
- 50.Demaison G J, Moore G T. Am Assoc Petrol Geol Bull. 1980;64:1179–1209. [Google Scholar]
- 51.Tissot B, Demaison G, Masson P, Delteil J R, Combaz A. Am Assoc Petrol Geol Bull. 1980;64:2051–2063. [Google Scholar]
- 52.Dehairs F, Chesselet R, Jewab J. Earth Planet Sci Lett. 1980;49:528–550. [Google Scholar]
- 53.Bishop J K B. Nature (London) 1988;332:341–343. [Google Scholar]
- 54.Berner R A, Lasaga A C, Garrels R M. Am J Sci. 1983;283:641–683. [Google Scholar]
- 55.Berner R A, Caldeira K. Geology. 1997;25:955–956. [Google Scholar]
- 56.Michalopoulos P, Aller R C. Science. 1995;270:614–617. [Google Scholar]
- 57.Alt J F. Mar Geol. 1988;81:227–239. [Google Scholar]
- 58.Bernat M, Bieri R H, Koide M, Griffin J J, Goldberg E D. Geochim Cosmochim Acta. 1970;34:1053–1071. [Google Scholar]
- 59.Kastner M, Stonecipher S A. In: Natural Zeolites, Occurrence, Properties, Use. Sand L B, Mumpton F A, editors. Oxford: Pergamon; 1978. pp. 199–220. [Google Scholar]
- 60.Kastner M. In: The Ocean Lithosphere: The Sea. Emiliani C, editor. Vol. 7. New York: Wiley; 1981. pp. 915–980. [Google Scholar]
- 61.Baker P A, Kastner M. Science. 1981;213:214–216. doi: 10.1126/science.213.4504.214. [DOI] [PubMed] [Google Scholar]
- 62.Burns S J, Baker P A. J Sed Petrol. 1987;57:128–137. [Google Scholar]
- 63.Bentor, Y. K. (1980) Soc. Econ. Paleontol. Mineral Spec. Publ., 3–18.
- 64.Schuffert J D, Kastner M, Jahnke R A. Mar Geol. 1998;146:21–31. [Google Scholar]
- 65.Ingall E, Jahnke R. Geochim Cosmochim Acta. 1994;58:2571–2575. doi: 10.1016/0016-7037(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 66.Van Cappellen P, Ingall E D. Science. 1996;271:493–496. doi: 10.1126/science.271.5248.493. [DOI] [PubMed] [Google Scholar]
- 67.Garrison R E, Kastner M, Kolodny Y. In: Cenozoic Basin Development of Coastal California. Ingersoll R V, Ernst W G, editors. VI. Englewood Cliffs, NJ: Prentice–Hall; 1987. pp. 348–381. [Google Scholar]
- 68.Haymon R M, Kastner M. Earth Planet Sci Lett. 1981;53:363–381. [Google Scholar]
- 69.Hannington M D, Jonnasson I R, Herzig P M, Petersen S. Geophys Monogr. 1995;91:115–157. [Google Scholar]
- 70.Sclater J G, Parsons B, Jaupart C. J Geophys Res. 1981;86:11522–11535. [Google Scholar]
- 71.Stein C A, Stein S. J Geophys Res. 1994;99:3081–3096. [Google Scholar]
- 72.Edmond J M, Measures C, McDuff R E, Chan L-H, Collier R, Grant B, Gordon L I, Corliss J B. Earth Planet Sci Lett. 1979;46:1–18. [Google Scholar]
- 73.East Pacific Rise Study Group. Science. 1981;213:31–40. doi: 10.1126/science.213.4503.31. [DOI] [PubMed] [Google Scholar]
- 74.Muehlenbacks K, Clayton R N. Can J Earth Sci. 1972;9:172–184. [Google Scholar]
- 75.Humphris S E, Thompson G. Geochim Cosmochim Acta. 1978;42:107–125. [Google Scholar]
- 76.Zindler A, Hart S R. Annu Rev Earth Planet Sci. 1986;14:115–118. [Google Scholar]
- 77.Tatsumi Y. J Geophys Res. 1989;94:4697–4707. [Google Scholar]
- 78.Alt J C, Teagle D A, Brewer T, Shanks W C, Halliday A. J Geophys Res. 1998;103:12365–12380. [Google Scholar]
- 79.Humphris S E, Herzig P M, Miller D J, Alt J C, Becker K, Brown D, Brügmann G, Chiba H, Fouquet Y, Gemmell J B, et al. Nature (London) 1995;377:713–716. [Google Scholar]
- 80.Richards J G, Cann J R, Jensenius J. Econ Geol. 1989;84:91–115. [Google Scholar]
- 81.Dansgaard W, White J W C, Johnsen S J. Nature (London) 1989;339:532–534. [Google Scholar]
- 82.Broecker W S. Q Res. 1992;38:135–138. [Google Scholar]
- 83.Fairbanks R G. Paleoceanography. 1990;5:937–948. [Google Scholar]
- 84.Bard B, Hamelin B, Arnold M, Montaggioni L, Gabioch G, Faure G, Rougerie F. Nature (London) 1996;382:241–244. [Google Scholar]
- 85.Neftel A, Oeschger H, Schwander J, Stauffer B, Zumbrunn R. Nature (London) 1982;295:220–223. [Google Scholar]
- 86.Brook E J, Sowers T, Orchardo J. Science. 1996;273:1087–1093. doi: 10.1126/science.273.5278.1087. [DOI] [PubMed] [Google Scholar]
- 87.Severinghaus J P, Sowers T, Brook E J, Alley R B, Bender M L. Nature (London) 1998;391:141–146. [Google Scholar]
- 88.Hsü K J, Montadert L, Bernoulli D, Cita M B, Erickson A, Garrison R E, Kidd R B, Mèlierés F, Müller C, Wright R. Nature (London) 1977;267:399–403. [Google Scholar]
- 89.Boyle E A. Paleoceanography. 1988;3:471–489. [Google Scholar]
- 90.Boyle E A. Annu Rev Earth Planet Sci. 1992;20:245–287. [Google Scholar]
- 91.Graham D W, Bender M L, Williams D F, Keigwin L D. Geochim Cosmochim Acta. 1982;46:1281–1292. [Google Scholar]
- 92.Delaney M L, B, Boyle E A. Geochim Cosmochim Acta. 1985;49:1327–1341. [Google Scholar]
- 93.Elderfield H. Paleogeog Paleoclimatol Paleoecol. 1986;57:71–90. [Google Scholar]
- 94.DePaolo D J, Ingram B L. Science. 1985;227:938–941. doi: 10.1126/science.227.4689.938. [DOI] [PubMed] [Google Scholar]
- 95.Hodell D A, Mueller P A, McKenzie J A, Mead G A. Earth Planet Sci Lett. 1989;92:165–178. [Google Scholar]
- 96.Sanyal A, Hemming N G, Hanson G N, Broecker W S. Nature (London) 1995;373:234–236. [Google Scholar]
- 97.Edwards R L, Chen J H, Wasserburg G J. Earth Planet Sci Lett. 1986;81:175–192. [Google Scholar]
- 98.Slowley N C, Henderson G M, Curry W B. Nature (London) 1996;383:242–244. [Google Scholar]
- 99.Baker P A, Gieskes J M, Elderfield H. J Sed Petrol. 1982;52:71–82. [Google Scholar]
- 100.Boyle E A. Geochim Cosmochim Acta. 1983;47:1815–1819. [Google Scholar]
- 101.Nurnberg D, Binja J, Hemleben C. Geochim Cosmochim Acta. 1996;60:803–814. [Google Scholar]
- 102.Hart S R, Cohen A L. Geochim Cosmochim Acta. 1996;60:3075–3084. [Google Scholar]
- 103.Shen G T, Cole J E, Lea D W, Linn L J, McConnaughey T A, Fairbanks R G. Paleoceanography. 1992;7:563–588. [Google Scholar]
- 104.Beck J W, Edwards R L, Ito E, Taylor F W, Recy J, Rougerie F, Joannot P, Henin C. Science. 1992;257:644–647. doi: 10.1126/science.257.5070.644. [DOI] [PubMed] [Google Scholar]
- 105.McCulloch M, Mortimer G, Esat T, Xianhua L, Pillans B, Chappel J. Earth Planet Sci Lett. 1996;138:169–178. [Google Scholar]
- 106.Mitsuguchi T, Matsumoto E, Abe O, Uchida T, Isdale P J. Science. 1996;274:961–963. doi: 10.1126/science.274.5289.961. [DOI] [PubMed] [Google Scholar]
- 107.Church T M, Wolgemuth K. Earth Planet Sci Lett. 1972;15:35–44. [Google Scholar]
- 108.Dymond J, Suess E, Lyle M. Paleoceanography. 1992;7:163–181. [Google Scholar]
- 109.Paytan A, Kastner M, Martin E E, Macdougall J D, Herbert T. Nature (London) 1993;366:445–449. [Google Scholar]
- 110.Paytan A, Moore W, Kastner M. Geochim Cosmochim Acta. 1996;60:4313–4319. [Google Scholar]
- 111.Feely R A. J Geophys Res. 1987;92:11343–11363. [Google Scholar]
- 112.Holser W T, Kaplan I R. Chem Geol. 1966;1:93–135. [Google Scholar]
- 113.Claypool G E, Holser W T, Kaplan I R, Sakai H, Zak I. Chem Geol. 1980;28:199–260. [Google Scholar]
- 114.Paytan A, Kastner M, Campbell D, Thiemens M H. Science. 1998;282:1459–1462. doi: 10.1126/science.282.5393.1459. [DOI] [PubMed] [Google Scholar]
- 115.Paytan A, Kastner M, Chavez F D. Science. 1996;274:1355–1357. doi: 10.1126/science.274.5291.1355. [DOI] [PubMed] [Google Scholar]
- 116.Albarede F, Goldstein S L. Geology. 1992;20:761–763. [Google Scholar]
- 117.Christensen J N, Halliday A N, Godfrey L V, Hein J R, Rea D K. Science. 1997;277:913–918. [Google Scholar]
- 118.Abouchami W, Goldstein S L, Galer S J G, Eisenhauer A, Mangini A. Geochim Cosmochim Acta. 1997;61:3957–3974. [Google Scholar]
- 119.O’Nions R K, Frank M, von Blanckenburg F, Ling H-F. Earth Planet Sci Lett. 1998;155:15–28. [Google Scholar]
- 120.von Blanckenburg F, O’Nions R K, Hein J R. Geochim Cosmochim Acta. 1996;60:4957–4963. [Google Scholar]
- 121.Ling H-F, Burton K W, O’Nions R K, Kamber B S, von Blanckenburg F, Gibbs A J, Hein J R. Earth Planet Sci Lett. 1997;146:1–12. [Google Scholar]
- 122.Frank M, O’Nions R K. Earth Planet Sci Lett. 1998;158:121–128. [Google Scholar]
- 123.Burton K W, Ling H-F, O’Nions R K. Nature (London) 1997;386:382–385. [Google Scholar]
- 124.Kvenvolden K A. Proc Natl Acad Sci USA. 1999;96:3420–3426. doi: 10.1073/pnas.96.7.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]