Abstract
Rationale
Tobacco and alcohol are frequently used together, and this may be partly explained by a distinct profile of subjective effects associated with co-administration. Ecological Momentary Assessment studies have examined effects of naturally occurring co-use, but, to date, have not assessed differing effects as alcohol levels rise and fall.
Objectives
To describe subjective states and appraisals of cigarette and alcohol effects reported during the entirety of real-world drinking episodes.
Methods
Currently-smoking frequent drinkers (N = 255) carried electronic diaries for 21 days. Analyses focused on reports made during 2,046 drinking episodes. Signaled prompts intensively oversampled moments in the hours following consumption of the first drink in an episode. Multilevel regression analyses were used to predict ratings of buzz, dizziness, excitement, and sluggishness as a function of person-level and contextual covariates, estimated blood alcohol concentration (eBAC) level, ascending vs. descending eBAC, smoking, and their interactions. Appraisals of cigarette and alcohol effects were also examined within this framework.
Results
Buzz, excitement, and pleasure from alcohol and cigarettes were prominent features of real-world drinking episodes. Smoking was associated with enhanced buzz and excitement when eBAC was high and descending. Smoking slightly accentuated the relation between eBAC and ratings of drinking pleasure among women, but this relation was somewhat weakened by smoking among men.
Conclusions
Smoking during drinking episodes may be partly be explained by a persistence of stimulant alcohol effects beyond the BAC peak. Acute effects of nicotine and tobacco use on the descending limb deserve further scrutiny in experimental alcohol challenge research.
Keywords: smoking, tobacco, alcohol, craving, reinforcement, subjective states, Ecological Momentary Assessment
Cigarette smoking and alcohol use are associated with one another, both across persons and within persons across time (Shiffman and Balabanis, 1995). One prominent hypothesis for explaining the within-person, intertemporal covariation of alcohol and tobacco use is that co-administration might produce a distinct profile of subjective effects, such as enhanced reward or diminished aversive consequences compared to use of either substance alone (e.g., Perkins 1997; Littleton et al. 2007). Interview and survey studies indicate that users expect simultaneous use of alcohol and tobacco to enhance “buzz” and rewarding effects of alcohol and tobacco (e.g., Harrison et al. 2008; McKee et al. 2004; Stromberg et al. 2007). In laboratory experiments, alcohol administration primes positive smoking outcome expectancies (Kirchner and Sayette, 2007). Additionally, both alcohol administration and the belief that one has consumed alcohol have been shown to lead to more positive ratings of cigarettes (Glautier et al. 1996; King et al. 2009; McKee et al. 2010; Rose et al. 2004). There is also some laboratory evidence that nicotine/tobacco administration may increase euphoria and intoxication and lessen sedative effects following alcohol administration, but these findings have been somewhat inconsistent across studies (cf. Acheson et al. 2006; Barrett et al. 2005; Kouri et al. 2004; McKee, et al. 2008; Perkins et al. 2000; Perkins et al, 1995).
Recently, researchers have begun to use Ecological Momentary Assessment (EMA; Shiffman 2009) to extend the study of the subjective consequences of alcohol-tobacco co-use to users’ natural environments. These studies have been reasonably consistent in suggesting that real-world co-use episodes are associated with enhanced reports of rewarding drug effects (cf. Piasecki et al., 2008; Piasecki, et al., 2011; Shiffman and Kirchner, 2009). Though existing EMA investigations have provided a window onto naturally occurring effects of alcohol-tobacco co-use, they have not described these effects comprehensively. Subjective effects of alcohol can vary considerably as a function of the blood alcohol concentration (BAC) and whether the BAC is rising or falling. On the rising limb, hedonically positive stimulant effects tend to predominate, but sedative effects are more prominent at higher BAC levels and on the falling limb (Martin et al. 1993; Sher et al 2005). To date, EMA studies have assessed presence vs. absence of any alcohol consumption in the past 15-60 minutes (Piasecki et al. 2008; Shiffman and Kirchner, 2009) or have limited analyses to diary records completed after the first drink in an episode (Piasecki et al. 2011). Thus, the amount of alcohol consumed prior to these reports was either constrained or left uncharacterized. Because these investigations have focused on moments following recent alcohol use, they have likely been slanted toward capturing rising limb alcohol effects and lower blood alcohol levels.
The aim of the current paper is to provide a more detailed analysis of how smoking behavior is related to alcohol responses in the natural environment. In the study described by Piasecki, et al. (2011), the diary protocol was designed to intensively time-sample experiences in the aftermath of the first drink. Here, we analyze these data, modeling current subjective states as a function of estimated BAC, etimated BAC limb, and momentary smoking. We also examine participants’ explicit appraisals of the motivational impact of cigarette and alcohol effects within this basic framework.
We expected that diary-measured intoxication effects would be related to estimated blood alcohol concentrations. We also expected that hedonically positive subjective experiences would tend to decrease and sedative effects would tend to increase when estimated blood alcohol was descending, as is commonly found in laboratory challenge research (e.g., King et al. 2002; Martin et al. 1993). Based on our prior co-use analyses, we expected smoking would be associated with enhanced stimulant alcohol effects when blood alcohol was ascending. Predictions concerning the influence of smoking behavior on alcohol-induced subjective states during the descending limb were less certain. One hypothesis might be that momentary covariation of alcohol and tobacco use are explained, in part, by a tendency for smoking to enhance the hedonic payoff of drinking by intensifying or extending stimulant effects. Another hypothesis stems from the fact that low response to alcohol, particularly on the descending limb, is thought to be an important risk factor for alcohol use disorders (cf. Morean and Corbin 2010; Newlin and Renton, 2010; Schuckit et al. 2010). If smoking dampens alcohol effects (e.g., Madden et al. 1995; McKee et al. 2008), particularly sedative effects on the descending limb, this could help account for the associations between smoking and drinking behaviors.
In our prior analyses of this data set limited to first drinks (Piasecki et al 2011), we found that couse was associated with enhanced ratings of the pleasurable effects of the most recent cigarette and drink, with effects of alcohol on tobacco pleasure significantly stronger compared to the converse. We expected that analyses of data from the entire drinking episode would reprise these effects, such that cigarette pleasure would tend to rise with estimated blood alcohol concentration and drink pleasure would tend to be higher after smoking.
Methods
Participants
A total of 259 current smokers, defined using a liberal threshold (self-report of at least one cigarette per week in the past month), were recruited in and around Columbia, Missouri using mass emails, posted fliers, and print advertisements. Additional eligibility criteria included (a) self-report of drinking alcohol at least 4 times in the past month, (b) age 18 years or older, (c) ability to read and write English, (d) not currently trying to quit smoking or currently taking smoking cessation pharmacotherapy, (e) not planning to quit smoking during the next 30 days, (f) not regularly using non-cigarette tobacco products, (g) not interested in seeking treatment for drinking problems or an alcohol use disorder, (h) no report of unsuccessful attempts to cut down or quit drinking, (i) no history of arrests for alcohol-related offenses (excluding status offenses), and (j) if female, not currently pregnant or planning to become pregnant. The current analyses excluded three smokers who did not log any drinking episodes during diary monitoring and one smoker with missing body weight data. The remaining sample of 255 was balanced with respect to gender (47% women) and consisted predominately of Whites (85%) and young adults (M = 25.0 years, SD = 8.6). Most of the sample (71%) reported smoking on a daily basis. These participants averaged 11.7 cigarettes per day (SD = 12.3). The remaining, nondaily smokers reported consuming an average of 2.9 cigarettes per smoking day (SD = 2.3) and smoking on an average of 3.1 days per week (SD = 1.8). Participants could earn up to $150 by completing all study visits and returning the electronic diary (ED). Data collection occurred between January, 2007 and November, 2008. The research protocol was approved by the Institutional Review Boards at the University of Missouri and Washington University School of Medicine and all participants provided written informed consent. Additional findings from this data set have been reported elsewhere (Piasecki, et al., 2011; Robertson, et al., 2012).
Procedure
Participants attended an initial, group (2 to 6 participants) baseline session during which they were weighed using a physician's scale and completed a battery of questionnaires. Approximately 1-2 days later, participants were scheduled individually or in small groups (up to 10 participants) to complete a 45-minute ED training session. Participants carried the ED for 21 days, starting immediately after the training session. During the course of the study, participants returned to the lab for 4 drop-in sessions focused on data review and troubleshooting.
Electronic Diary
EDs ran on palmtop computers (Palm m500, Palm Inc., Sunnyvale, CA) programmed with customized software designed by invivodata, inc. (Pittsburgh, PA). The current report is confined to data collected during drinking episodes. Participants were instructed to initiate a diary entry (Drinking Record) upon completion of the first drink in a drinking episode. Completion of this report triggered a sequence of time-based Drinking Follow-Ups (DFUs) intended to intensively sample subjective states during the post-drinking period. The ED delivered audible prompts at 30, 90 and 150 minutes after the triggering report. Each DFU asked whether the participant had consumed any additional drinks since last report. When new drinks were reported, an additional DFU alarm was scheduled for 60 minutes after the last currently scheduled alarm. This allowed the ED system to track effects of extended drinking episodes. Participants completed a diary entry when retiring to bed; these bedtime reports terminated the follow-up sequence, pre-empting any as-yet undelivered prompts. Smokers also initiated recordings when smoking cigarettes and responded to up to 5 randomly timed diary prompts per day. These assessments asked whether the participant had consumed alcohol since last report. Answering this question affirmatively also triggered the DFU sequence.
Measures
Person-level Covariates
Self-reported typical smoking rate (cigarettes per day) was measured using a smoking history questionnaire administered at baseline. For nondaily smokers, this was estimated by multiplying self-reports of the average cigarettes per smoking day by smoking days per week and dividing the result by seven. The Alcohol Use Disorders Identification Test (AUDIT; Babor, et al. 2001) was also administered at baseline. Item 1 of the AUDIT asks participants to rate how frequently they consume alcohol on a scale from 0 (never) to 4 (4+ times/week). Item 2 asks how many drinks the respondent typically consumes on a drinking day on a scale from 0 (1 or 2 drinks) to 4 (10+ drinks). These two items were multiplied to yield an index of typical alcohol use quantity/frequency for each participant. Dichotomous variables were constructed to represent participant gender (males coded 1, females coded 0) and daily smoking status (daily smokers coded 1, nondaily 0).
Contextual Covariates
Time/date stamps automatically recorded by the ED were used to categorize the time of day during which diary entry was made; for data analyses, we used a set of dummy-coded variables, with the 8pm – 12 pm interval serving as the reference category and each code representing additional four-hour spans (12am to 4am, 4am to 8 am, 8 am to 12pm, 12 pm to 4 pm, 4 pm to 8 pm). A dichotomous variable indexed whether a drinking episode was initiated between 6 pm Thursday and 6 pm Sunday (i.e., the weekend; coded 1) or not (coded 0). In the ED records triggering DFUs, participants were asked to indicate via checklists their locations and social contacts in the past 15 minutes. The social contacts item was coded into a dichotomous variable indicating the participant had been either alone (scored 0) or in presence of others (scored 1) at the outset of the episode. A set of dummy-coded variables was constructed to represent location at the outset of the drinking episode. Assessed locations were work/school (reference category) bar/restaurant, home, outside, vehicle, other location. During the period when data were collected, smoking was legally prohibited inside local bars and restaurants. Many of these establishments have outdoor patios where smoking is permitted.
Estimated Blood Alcohol Concentration (eBAC)
In order to analyze subjective states with reference to a plausible biological gradient, we computed estimated blood alcohol concentrations (eBAC) at each moment. Triggering records were assumed to have captured one drink. DFUs asked participants “Since last recording, how many drinks have you had?” Participants responded using a scale ranging from 0 (none) to 6 (6 or more drinks). The “6 or more” response was endorsed in fewer than 2% of the DFUs and was counted a 6 drinks in the analyses. Together, these data permitted calculation of a cumulative sum of reported drinks up to each observation within the episode. Time stamps indicated the latency of each report since completion of the first drink. Time spent consuming the first drink was not explicitly assessed. To make eBAC calculations more realistic, we assumed 20 minutes were spent consuming the first drink, then added 20 minutes to each of the follow-up latencies to estimate time since initiation of drinking. The equation of Matthews and Miller (1979; see also Hustad and Carey 2005) was then used to estimate BAC at each moment. Specifically:
where c = number of drinks consumed up to the moment, GC = gender constant (7.5 for males, 9.0 for females), w = weight in pounds, β60 = the alcohol metabolism rate per hour (we assumed the population average rate of .017 g/dl per hour, following Hustad & Carey, 2005) and t = time in hours since initiation of drinking. As found in prior investigations (e.g., Rutledge et al. 2008), this formula produced some negative eBAC estimates; these were recoded to zero.
Descending eBAC
We computed successive differences in eBAC across moments within each drinking episode. A dichotomous variable indicating descending eBAC was then computed, such that negative changes were coded 1 and positive or zero change was coded 0. First drink triggering events were always coded 0.
Diary-Reported Smoking
ED reports were used to construct a dichotomous variable for the main analyses that coded whether each diary observation was preceded by smoking (scored 1) or not (scored 0). Smoking was counted as having occurred when: (a) a user-initiated cigarette log served as the triggering event, (b) a prompted assessment or user-initiated Drinking Report was the triggering event and the participant answered “yes” to an item asking whether the participant had smoked in the past 15 minutes, or (c) when participants provided any answer greater than zero to an item in the DFU assessment that asking “Since last recording, how many cigarettes did you smoke?”.
Current Subjective States
Participants used a 5-point scale (1 = “not at all” to 5 = “extremely”) to rate experiences with various subjective states during the past 15 minutes. The current analyses focused on a subset of items that were included in the diary protocol to tap intoxication effects (“buzzed,” “dizzy,” “excited,” and “sluggish”). “Buzzed” and “dizzy” were found to be synergistically affected by co-use in prior analyses from this sample (see Piasecki et al. 2011 for a complete list of assessed states). Piasecki et al. (2011) reported analyses demonstrating that buzz ratings were positively related to contemporaneous reports of pleasure from the last cigarette and the last drink, suggesting self-reported buzz reflects rewarding drug effects. “Excited” was included in the diary protocol because it is one of the items assessed by the Stimulant subscale of the Biphasic Alcohol Effects Scale (BAES; Martin et al. 1993). Similarly, “sluggish” was assessed because it is one of the items on the BAES Sedative subscale.
Appraisals of Alcohol and Cigarette Effects
When participants indicated they had consumed cigarettes or alcoholic drinks since last report, they were asked to appraise the effects of the drug using three items, worded identically for cigarettes and drinks and rated on a 1-5 scale. These probed: (a) positive reinforcement (“Was the last [cigarette/drink] pleasurable?”), (b) negative reinforcement (“Did the last [cigarette/drink] relieve unpleasant feelings or symptoms?”), and (c) punishment (“Did the last [cigarette/drink] make you feel worse?”).
Diary Compliance and Data Selection
The EDs captured data from 2,299 drinking episodes (1,412 triggered by user-initiated Drink Reports, 172 by user-initiated cigarette reports, and 715 triggered by randomly-prompted assessments). Participants completed 6,044 DFUs following triggering events but failed to respond to 1,123 delivered DFU prompts (84.3% compliance). A small number of implausibly high values for eBAC (up to .73) and smoking rate (up to 12 cigarettes/hr) were observed, likely attributable to mistakes in diary entries concerning consumption since last report. We sought to eliminate the most egregious errors (e.g., eBACs that, if veridical, would be have been expected to prove fatal) while minimizing deletion of real data points in dose-related regions of interest. More than 95% of eBAC values were .20 or lower, and more than 97% of smoking rate estimates were indicate 4 or fewer cigarettes per hour. The current analyses were limited records defined by the conjunction of these two upper bounds, a total of 7,765 records (93%) from 2,046 episodes.
Statistical Analysis
Linear mixed models were estimated using SAS software's PROC MIXED (SAS version 9.2, SAS Institute Inc., Cary, NC). Current subjective states were modeled as a function of person-level and contextual covariates, momentary eBAC, the descending indicator, smoking prior to the diary report, and the 2- and 3-way interactions among eBAC, descending, and smoking. The models used a three-level structure (moments nested in episodes nested in participants) with random intercepts and unstructured covariances. Estimated BAC, the descending indicator, and smoking were allowed to have random slopes across persons.
Items assessing appraisals of cigarettes and drinks were only administered when the participant indicated s/he had consumed the target substance. This required modifications to the model structure. Because cigarette appraisals were contingent on cigarette consumption, the smoking variable and associated interaction terms were omitted. Hence, cigarette appraisals were predicted as a function eBAC, the descending indicator, and their interactions (as well as covariates). Drink appraisal items were administered only when one or more new drinks were reported since the last diary entry. Due to this association with recent consumption, there were relatively few appraisals of new drinks against a backdrop of descending eBAC (1.9% of drink appraisals). Therefore, descending moments were omitted from the analyses of drink appraisals and these states were modeled as a function of eBAC level, smoking, and their interaction (in addition to covariates).
Some prior investigations have reported significant gender differences in behavioral or subjective responses to co-administration of alcohol and tobacco (Acheson, et al., 2006; King, et al., 2009; Perkins, et al. 1995; Perkins, et al., 2000). To explore possible gender differences, a series of supplementary analyses were performed in which the models described above were expanded to include additional interactions terms testing moderation of the eBAC, descending, and smoking-related terms by gender.
Results
Descriptive Analyses
In the analyzed records, participants reported having smoked in 4,275 diary reports (55%). Overall, 1,888 of the analyzed moments (24.3%) were categorized as descending eBAC. This relatively low frequency of descending moments was not unexpected, despite the fact that the descending limb is typically longer in duration compared to the ascending limb. This is because the drinking event was sampled more frequently in the beginning phase and because subjects were likely to go to sleep during the descending limb, pre-empting DFU delivery and “censoring” the observation of the descending limb. Across all analyzed records, the mean eBAC was .042 (SD=.044, Mdn=.024). In ascending moments, the mean eBAC was .048 (SD=.045, Mdn=.030) and in observations classified as descending, the mean eBAC was .021 (SD=.033, Mdn=.009). On average, the last observation in the analyzed drinking episodes was made 2.2 hours after the first drink triggering record (SD = 1.6, Mdn = 2.5, range 0 [i.e., no DFUs completed] to10.5); 98% of the episodes spanned 5.5 hours or less. The average number of drinks per episode was 3.6 (SD = 2.9, Mdn = 3, range 1 to 18) and the average number of cigarettes per episode was 2.7 (SD = 3.0, Mdn = 2, range 0 to 26).
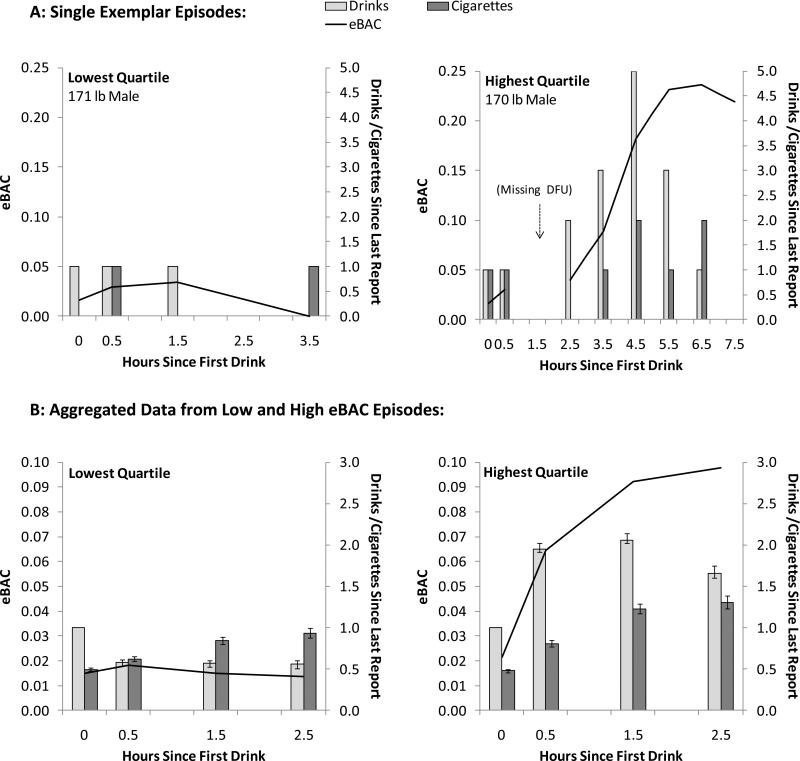
Given the novelty of the protocol, we sought to make the nature of the intra-episode assessments more tangible and to depict the variability across episodes. We computed the mean eBAC level within each episode and then divided episodes into quartiles based on mean eBAC. The top panels of Figure 1 show data from two individual exemplar drinking episodes, one each from the lowest and highest quartiles. These episodes were recorded by male participants with comparable body weights. Note that these two episodes differed in terms of the duration of follow-up. One DFU was missed during the high quartile episode. The drinking question asked about drinks since last completed report. In theory, the gap should be bridged by this wording, permitting an accurate resumption of BAC estimation despite the missed DFU. The bottom panels of Figure 1 show the means for eBAC, drinks, and cigarettes since last report across the triggering event and the first three follow-ups (i.e., those consistently scheduled by the diary regardless of drinking pattern) in the lowest and highest mean eBAC quartiles.
Figure 1.
(A) Data from two individual drinking episodes, one each from the lowest and highest quartiles based on mean intra-episode eBAC. Note the high quartile example had a longer duration and included a missing DFU. (B) Mean eBAC (solid line) after the first drink and the the first 3 follow up for the lowest and highest quartile of drinking episodes. Bars indicate means and standard errors of drinks and cigarettes since last report. The triggering event always involved one drink, so no error bars are presented. Note that, as described in text, 20 minutes (.33 hours) was added to each of the values on x-axis when computing eBAC.
Current Subjective States
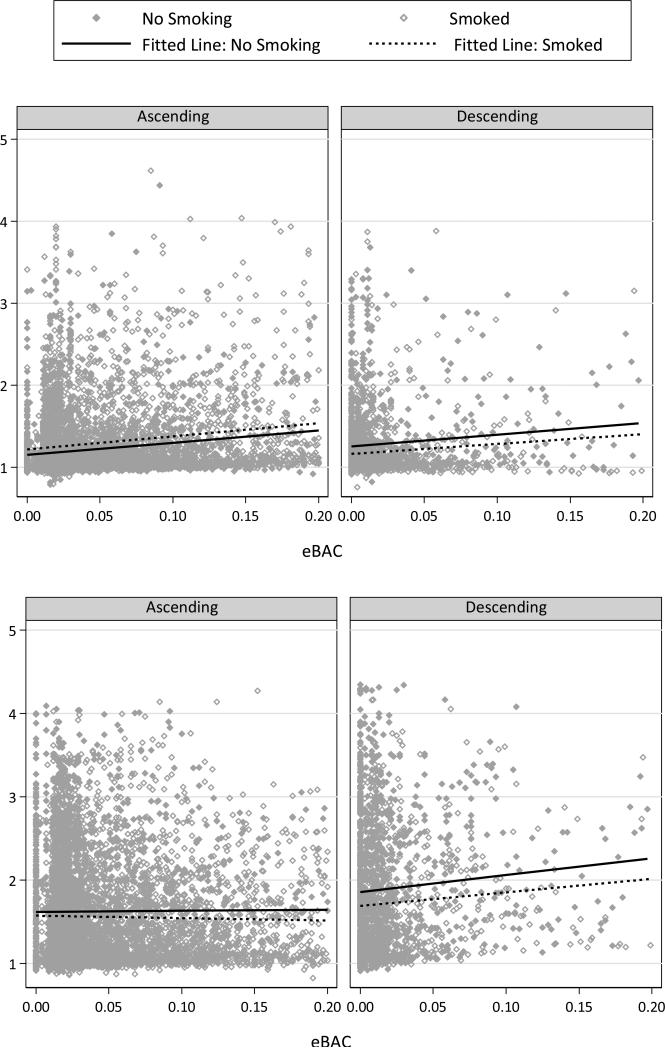
Table 1 summarizes fixed effects results from models predicting current buzz and excitement. F-statistics are also tabled as an indicator of the magnitude of the various effects. Buzzed ratings were higher among males, elevated in the bar/restaurant context, lower when alone, and varied significantly by time of day. Current buzz was strongly related to eBAC and higher after smoking. All two- and three-way interactions were significant. The top portion of Figure 2 presents model-predicted values as a function of eBAC, limb, and momentary smoking with fit lines to illustrate the trends. The three-way interaction indicated that, on the descending limb, the eBAC-buzz relation tended to be stronger when participants had smoked compared to when they had not.
Table 1.
Fixed effects from mixed models predicting ratings of buzzed and excited.
| Predictor |
Buzzed
|
Excited
|
||||||
|---|---|---|---|---|---|---|---|---|
| b | F | df | p | b | F | df | p | |
| Intercepta | 1.437 | -- | -- | -- | 2.910 | -- | -- | -- |
| Daily Smoker | -0.058 | 0.37 | 1, 249 | 0.541 | -0.047 | 0.13 | 1, 249 | 0.694 |
| Cigarettes per Dayb | 0.000 | 0.00 | 1, 249 | 0.957 | -0.012 | 6.82 | 1, 249 | 0.013 |
| Alcohol Use Q/Fb | 0.006 | 0.29 | 1, 249 | 0.594 | 0.036 | 7.95 | 1, 249 | 0.006 |
| Male | 0.197 | 5.34 | 1, 249 | 0.022 | 0.211 | 3.82 | 1, 249 | 0.047 |
| Weekend | 0.046 | 2.25 | 1, 7382 | 0.134 | 0.124 | 15.99 | 1, 7382 | <0.001 |
| Alone | -0.108 | 4.23 | 1, 7382 | 0.040 | -0.346 | 10.34 | 1, 7382 | <0.001 |
| Location | ||||||||
| Bar/Restaurant | 0.152 | 5.91 | 1, 7382 | 0.015 | 0.162 | 8.64 | 1, 7382 | 0.014 |
| Home | 0.084 | 2.14 | 1, 7382 | 0.143 | -0.071 | 2.82 | 1, 7382 | 0.239 |
| Outside | -0.037 | 0.55 | 1, 7382 | 0.459 | 0.114 | 6.43 | 1, 7382 | 0.028 |
| Vehicle | -0.043 | 0.30 | 1, 7382 | 0.584 | 0.231 | 7.56 | 1, 7382 | 0.005 |
| Other Location | 0.095 | 2.50 | 1, 7382 | 0.114 | 0.092 | 3.42 | 1, 7382 | 0.146 |
| Work/Schoool (Ref) | -- | -- | -- | -- | -- | -- | -- | -- |
| Time of Day | ||||||||
| Midnight-4 am | 0.291 | 19.60 | 1, 7382 | <0.001 | -0.110 | 2.61 | 1, 7382 | 0.106 |
| 4 am – 8 am | 0.262 | 1.39 | 1, 7382 | 0.239 | -0.302 | 1.72 | 1, 7382 | 0.188 |
| 8am – Noon | -0.300 | 6.53 | 1, 7382 | 0.011 | -0.190 | 3.36 | 1, 7382 | 0.124 |
| Noon-4pm | -0.129 | 5.35 | 1, 7382 | 0.021 | -0.093 | 3.86 | 1, 7382 | 0.115 |
| 4 pm – 8 pm | -0.075 | 4.29 | 1, 7382 | 0.038 | 0.012 | 0.005 | 1, 7382 | 0.758 |
| 8 pm – Midnight (Ref) | -- | -- | -- | -- | -- | -- | -- | -- |
| eBAC | 10.411 | 202.36 | 1, 7382 | <0.001 | 1.378 | 6.49 | 1, 7382 | 0.009 |
| Descending | -0.035 | 0.56 | 1, 7382 | 0.455 | -0.144 | 15.87 | 1, 7382 | 0.001 |
| Smoked | 0.292 | 49.78 | 1, 7382 | <0.001 | 0.063 | 2.68 | 1, 7382 | 0.104 |
| eBAC × Descending | -6.539 | 42.69 | 1, 7382 | <0.001 | -6.181 | 40.33 | 1, 7382 | <0.001 |
| eBAC × Smoked | -1.828 | 7.54 | 1, 7382 | 0.006 | -0.043 | 0.03 | 1, 7382 | 0.943 |
| Descending × Smoked | -0.167 | 7.15 | 1, 7382 | 0.008 | 0.024 | 0.21 | 1, 7382 | 0.693 |
| eBAC × Descending × Smoked | 5.626 | 15.39 | 1, 7382 | <0.001 | 4.504 | 10.87 | 1, 7382 | 0.001 |
Statistical significance of the intercepts not reported because test assesses difference from zero and responses scales did not include a zero value.
Grand mean centered. b = unstandardized fixed effects coefficient estimate, Q/F = quantity/frequency.
Figure 2.
Predicted values from models of buzzed (top) and excited (bottom) as a function of smoking occurrence, eBAC level, and ascending vs. descending limb. Best fitting lines for smoking and nonsmoking moments are given in each panel.
Excitement ratings were lower among heavier smokers and when participants were alone, and elevated on the weekend, among heavier drinkers, and in several contexts. Excitement was positively related to eBAC and lower when eBAC was descending. An eBAC × Descending interaction emerged, suggesting excitement ratings were more weakly related to eBAC when alcohol concentrations were falling. A three-way interaction was also observed, indicating that the relation between eBAC and excitement on the descending limb differed when participants had smoked compared to when they had not smoked. The bottom portion of Figure 2 presents predicted values and trend lines for excitement to illustrate these interactions.
Table 2 summarizes results from models predicting dizzy and sluggish ratings. Dizziness ratings were low overall, lower when alone, and higher among males and between 4 and 8 am. Dizziness increased modestly with eBAC, momentary smoking, and descending eBAC. A Descending × Smoked interaction indicated that the effect of smoking differed on the descending limb. The top panel of Figure 3 illustrates these effects. Ratings of sluggish (bottom, Figure 3) were also low overall. Sluggishness varied by time of day, was modestly lower on the weekend and slightly higher when alone. Sluggishness increased modestly with eBAC and was higher when eBAC was descending.
Table 2.
Fixed effects from mixed models predicting ratings of dizzy and sluggish.
| Predictor |
Dizzy
|
Sluggish
|
||||||
|---|---|---|---|---|---|---|---|---|
| b | F | df | p | b | F | df | p | |
| Intercepta | 1.106 | -- | -- | -- | 1.678 | -- | -- | -- |
| Daily Smoker | -0.067 | 1.56 | 1, 249 | 0.212 | -0.165 | 3.44 | 1, 249 | 0.065 |
| Cigarettes per Dayb | 0.001 | 0.19 | 1, 249 | 0.665 | 0.001 | 0.08 | 1, 249 | 0.771 |
| Alcohol Use Q/Fb | -0.005 | 0.81 | 1, 249 | 0.370 | -0.018 | 3.61 | 1, 249 | 0.059 |
| Male | 0.140 | 8.39 | 1, 249 | 0.004 | 0.001 | 0.00 | 1, 249 | 0.994 |
| Weekend | 0.029 | 2.99 | 1, 7382 | 0.084 | -0.074 | 6.72 | 1, 7382 | 0.010 |
| Alone | -0.064 | 5.11 | 1, 7382 | 0.024 | 0.139 | 8.18 | 1, 7382 | 0.004 |
| Location | ||||||||
| Bar/Restaurant | -0.006 | 0.03 | 1, 7382 | 0.860 | -0.089 | 2.34 | 1, 7382 | 0.126 |
| Home | -0.031 | 0.96 | 1, 7382 | 0.328 | 0.070 | 1.76 | 1, 7382 | 0.185 |
| Outside | 0.006 | 0.05 | 1, 7382 | 0.824 | -0.081 | 3.10 | 1, 7382 | 0.078 |
| Vehicle | -0.064 | 2.24 | 1, 7382 | 0.134 | 0.001 | 0.00 | 1, 7382 | 0.984 |
| Other Location | 0.033 | 0.98 | 1, 7382 | 0.322 | -0.012 | 0.04 | 1, 7382 | 0.833 |
| Work/Schoool (Ref) | -- | -- | -- | -- | -- | -- | -- | -- |
| Time of Day | ||||||||
| Midnight-4 am | 0.024 | 0.43 | 1, 7382 | 0.511 | 0.181 | 8.90 | 1, 7382 | 0.003 |
| 4 am – 8 am | 0.353 | 8.12 | 1, 7382 | 0.004 | 0.345 | 2.86 | 1, 7382 | 0.091 |
| 8am – Noon | 0.049 | 0.58 | 1, 7382 | 0.445 | -0.028 | 0.07 | 1, 7382 | 0.798 |
| Noon-4pm | 0.019 | 0.41 | 1, 7382 | 0.520 | 0.043 | 0.67 | 1, 7382 | 0.415 |
| 4 pm – 8 pm | -0.004 | 0.05 | 1, 7382 | 0.832 | -0.070 | 4.27 | 1, 7382 | 0.039 |
| 8 pm – Midnight (Ref) | -- | -- | -- | -- | -- | -- | -- | -- |
| eBAC | 2.438 | 30.55 | 1, 7382 | <0.001 | 1.101 | 5.31 | 1, 7382 | 0.021 |
| Descending | 0.066 | 5.91 | 1, 7382 | 0.015 | 0.164 | 14.94 | 1, 7382 | <0.001 |
| Smoked | 0.067 | 7.06 | 1, 7382 | 0.008 | 0.040 | 1.30 | 1, 7382 | 0.255 |
| eBAC × Descending | -0.327 | 0.30 | 1, 7382 | 0.585 | 0.747 | 0.71 | 1, 7382 | 0.398 |
| eBAC × Smoked | 0.179 | 0.20 | 1, 7382 | 0.655 | -0.317 | 0.34 | 1, 7382 | 0.562 |
| Descending × Smoked | -0.081 | 4.71 | 1, 7382 | 0.030 | -0.051 | 0.81 | 1, 7382 | 0.368 |
| eBAC × Descending × Smoked | -0.308 | 0.13 | 1, 7382 | 0.721 | 0.644 | 0.26 | 1, 7382 | 0.612 |
Statistical significance of the intercepts not reported because test assesses difference from zero and responses scales did not include a zero value.
Grand mean centered. b = unstandardized fixed effects coefficient estimate, Q/F = quantity/frequency.
Figure 3.
Predicted values from models of dizzy (top) and sluggish (bottom) as a function of smoking occurrence, eBAC level, and ascending vs. descending limb. Best fitting lines for smoking and nonsmoking moments are given in each panel.
Appraisals of Alcohol and Cigarette Effects
Table 3 summarizes analyses of appraisals of cigarettes. As the intercepts indicate, smokers tended to rate the last cigarette as fairly pleasurable, moderately negatively reinforcing, and not very punishing. Cigarettes were rated as less pleasurable and less negatively reinforcing when eBAC was descending. Ratings of cigarette punishment were higher when the participant had been in a vehicle, but were unrelated to eBAC level or limb.
Table 3.
Fixed effects from mixed models predicting cigarette appraisals.
| Predictor | Cigarette Pleasurable |
Cigarette Relievedc |
Cigarette Made Feel Worse |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | F | df | p | b | F | df | p | b | F | df | p | |
| Intercepta | 3.796 | -- | -- | -- | 2.279 | -- | -- | -- | 1.245 | -- | -- | -- |
| Daily Smoker | 0.030 | 0.06 | 1, 241 | 0.803 | -0.104 | 0.45 | 1, 241 | 0.502 | -0.041 | 0.36 | 1, 241 | 0.548 |
| Cigarettes per dayb | -0.003 | 0.51 | 1, 241 | 0.476 | 0.006 | 1.17 | 1, 241 | 0.281 | -0.004 | 2.99 | 1, 241 | 0.085 |
| Alcohol Use Q/Fb | 0.020 | 2.57 | 1, 241 | 0.111 | 0.022 | 1.89 | 1, 241 | 0.171 | -0.010 | 2.09 | 1, 241 | 0.149 |
| Male | -0.182 | 3.21 | 1, 241 | 0.074 | -0.164 | 1.52 | 1, 241 | 0.219 | 0.045 | 0.62 | 1, 241 | 0.430 |
| Weekend | 0.037 | 1.42 | 1, 3920 | 0.233 | 0.011 | 0.10 | 1, 3919 | 0.752 | -0.007 | 0.11 | 1, 3919 | 0.741 |
| Alone | 0.006 | 0.01 | 1, 3920 | 0.903 | 0.057 | 0.93 | 1, 3919 | 0.334 | 0.043 | 1.51 | 1, 3919 | 0.219 |
| Location | ||||||||||||
| Bar/Restaurant | 0.063 | 1.06 | 1, 3920 | 0.303 | 0.112 | 2.62 | 1, 3919 | 0.105 | -0.046 | 1.31 | 1, 3919 | 0.252 |
| Home | -0.020 | 0.13 | 1, 3920 | 0.717 | 0.032 | 0.26 | 1, 3919 | 0.607 | 0.014 | 0.14 | 1, 3919 | 0.704 |
| Outside | -0.066 | 2.00 | 1, 3920 | 0.158 | -0.045 | 0.71 | 1, 3919 | 0.400 | 0.035 | 1.22 | 1, 3919 | 0.270 |
| Vehicle | 0.067 | 0.78 | 1, 3920 | 0.377 | -0.049 | 0.33 | 1, 3919 | 0.566 | 0.141 | 7.90 | 1, 3919 | 0.005 |
| Other Location | 0.041 | 0.50 | 1, 3920 | 0.481 | 0.034 | 0.28 | 1, 3919 | 0.599 | -0.019 | 0.26 | 1, 3919 | 0.613 |
| Work/Schoool (Ref) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Time of Day | ||||||||||||
| Midnight-4 am | 0.103 | 2.52 | 1, 3920 | 0.113 | 0.132 | 3.23 | 1, 3919 | 0.072 | -0.031 | 0.52 | 1, 3919 | 0.470 |
| 4 am – 8 am | -0.203 | 0.92 | 1, 3920 | 0.337 | 0.136 | 0.33 | 1, 3919 | 0.568 | 0.057 | 0.17 | 1, 3919 | 0.682 |
| 8am – Noon | -0.185 | 2.70 | 1, 3920 | 0.100 | 0.100 | 0.62 | 1, 3919 | 0.432 | -0.064 | 0.72 | 1, 3919 | 0.396 |
| Noon-4pm | -0.082 | 2.22 | 1, 3920 | 0.136 | 0.019 | 0.09 | 1, 3919 | 0.764 | -0.025 | 0.45 | 1, 3919 | 0.503 |
| 4 pm – 8 pm | -0.012 | 0.10 | 1, 3920 | 0.753 | -0.015 | 0.12 | 1, 3919 | 0.726 | -0.005 | 0.04 | 1, 3919 | 0.837 |
| 8 pm – Midnight (Ref) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| eBAC | 0.429 | 1.49 | 1, 3920 | 0.222 | -0.362 | 0.84 | 1, 3919 | 0.358 | 0.400 | 2.54 | 1, 3919 | 0.111 |
| Descending | -0.187 | 24.60 | 1, 3920 | <0.001 | -0.161 | 15.46 | 1, 3919 | <0.001 | 0.054 | 3.02 | 1, 3919 | 0.082 |
| eBAC × Descending | -0.131 | 0.02 | 1, 3920 | 0.878 | 1.667 | 3.13 | 1, 3919 | 0.077 | -0.074 | 0.01 | 1, 3919 | 0.903 |
Statistical significance of the intercept not reported because test assesses difference from zero and response scales did not include a zero value.
Grand mean centered.
To facilitate model convergence, the random slope for the descending indicator was omitted from this model. b = unstandardized fixed effect coefficient estimate, Q/F = quantity/frequency.
Drinks were also appraised as highly pleasurable, modestly relieving, and not punishing (Table 4). Drinks were rated as more pleasurable when participants reported being in a bar/restaurant or vehicle within 15 minutes of the triggering record. Higher eBAC was associated with decreased ratings of relief and increased ratings of punishment from drinking.
Table 4.
Fixed effects from mixed models predicting drink appraisals.
| Predictor | Drink Pleasurable |
Drink Relieved |
Drink Made Feel Worse |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | F | df | p | b | F | df | p | b | F | df | p | |
| Intercepta | 4.094 | -- | -- | -- | 2.686 | -- | -- | -- | 1.104 | -- | -- | -- |
| Daily Smoker | -0.080 | 0.74 | 1, 246 | 0.391 | -0.183 | 1.37 | 1, 246 | 0.242 | 0.031 | 0.61 | 1, 246 | 0.436 |
| Cigarettes per dayb | -0.002 | 0.34 | 1, 246 | 0.560 | 0.010 | 2.60 | 1, 246 | 0.108 | -0.001 | 0.84 | 1, 246 | 0.360 |
| Alcohol Use Q/Fb | 0.020 | 3.75 | 1, 246 | 0.054 | 0.028 | 2.83 | 1, 246 | 0.094 | -0.006 | 1.99 | 1, 246 | 0.159 |
| Male | -0.125 | 2.25 | 1, 246 | 0.135 | -0.193 | 1.96 | 1, 246 | 0.163 | 0.024 | 0.43 | 1, 246 | 0.513 |
| Weekend | 0.003 | 0.01 | 1, 4357 | 0.917 | -0.050 | 1.86 | 1, 4357 | 0.173 | 0.005 | 0.08 | 1, 4357 | 0.780 |
| Alone | -0.074 | 1.92 | 1, 4357 | 0.166 | 0.060 | 0.81 | 1, 4357 | 0.368 | -0.001 | 0.00 | 1, 4357 | 0.986 |
| Location | ||||||||||||
| Bar/Restaurant | 0.140 | 5.25 | 1, 4357 | 0.022 | 0.018 | 0.06 | 1, 4357 | 0.810 | -0.020 | 0.27 | 1, 4357 | 0.601 |
| Home | 0.012 | 0.05 | 1, 4357 | 0.831 | -0.024 | 0.11 | 1, 4357 | 0.739 | -0.002 | 0.00 | 1, 4357 | 0.949 |
| Outside | -0.041 | 0.73 | 1, 4357 | 0.392 | -0.026 | 0.19 | 1, 4357 | 0.664 | -0.027 | 0.84 | 1, 4357 | 0.359 |
| Vehicle | 0.166 | 4.43 | 1, 4357 | 0.035 | 0.014 | 0.02 | 1, 4357 | 0.889 | 0.032 | 0.43 | 1, 4357 | 0.510 |
| Other Location | 0.037 | 0.38 | 1, 4357 | 0.537 | -0.029 | 0.15 | 1, 4357 | 0.697 | 0.024 | 0.42 | 1, 4357 | 0.515 |
| Work/Schoool (Ref) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Time of Day | ||||||||||||
| Midnight-4 am | 0.031 | 0.28 | 1, 4357 | 0.599 | 0.047 | 0.41 | 1, 4357 | 0.524 | 0.010 | 0.08 | 1, 4357 | 0.784 |
| 4 am – 8 am | -0.067 | 0.04 | 1, 4357 | 0.842 | 0.259 | 0.38 | 1, 4357 | 0.538 | -0.069 | 0.10 | 1, 4357 | 0.747 |
| 8am – Noon | -0.262 | 3.55 | 1, 4357 | 0.060 | -0.003 | 0.00 | 1, 4357 | 0.987 | -0.038 | 0.19 | 1, 4357 | 0.661 |
| Noon-4pm | -0.080 | 1.78 | 1, 4357 | 0.182 | -0.010 | 0.02 | 1, 4357 | 0.894 | -0.059 | 2.55 | 1, 4357 | 0.110 |
| 4 pm – 8 pm | 0.019 | 0.29 | 1, 4357 | 0.592 | -0.027 | 0.39 | 1, 4357 | 0.530 | -0.005 | 0.06 | 1, 4357 | 0.809 |
| 8 pm – Midnight (Ref) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| eBAC | -0.321 | 0.39 | 1, 4357 | 0.531 | -1.448 | 5.41 | 1, 4357 | 0.020 | 1.180 | 9.10 | 1, 4357 | 0.003 |
| Smoked | 0.021 | 0.28 | 1, 4357 | 0.598 | -0.044 | 0.94 | 1, 4357 | 0.333 | -0.004 | 0.03 | 1, 4357 | 0.861 |
| eBAC × Smoked | 0.232 | 0.18 | 1, 4357 | 0.676 | 1.242 | 3.45 | 1, 4357 | 0.063 | 0.176 | 0.21 | 1, 4357 | 0.644 |
Statistical significance of the intercept not reported because test assesses difference from zero and response scales did not include a zero value.
Grand mean centered. b = unstandardized fixed effect coefficient estimate, Q/F = quantity/frequency.
Tests of Moderation by Gender
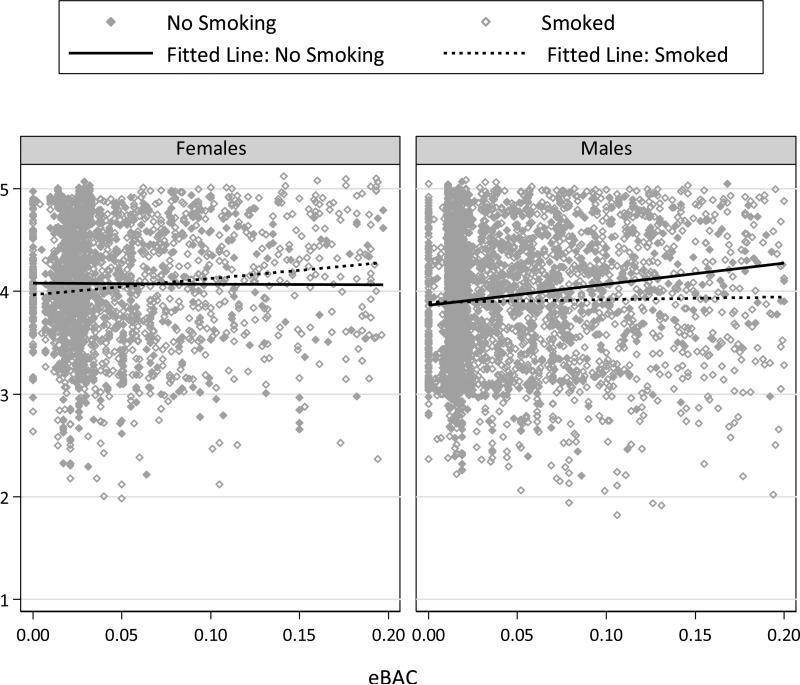
Including additional interaction terms involving gender yielded nonsignificant effects in all but one analysis. In the model predicting appraisals of pleasure from the last drink, all of the additional interaction terms were significant: Male × eBAC (b = 3.041, F (1,4354) = 8.24, p = .004), Male × Smoked (b = .207, F (1,4354) = 6.55, p =.011), and Male × eBAC × Smoked (b = -3.94, F (1,4354) = 11.66, p =.001). Figure 4 depicts predicted values and trend lines for drinking pleasure as a function of gender, eBAC, and smoking since last report.
Figure 4.
Predicted values from the model predicting pleasurable drink appraisals as a function of ascending eBAC level, smoking occurrence, and gender. Best fitting lines for smoking and nonsmoking moments are given in each panel.
Discussion
The current research examined real-world responses to alcohol and tobacco reported by frequent drinkers who were not trying to quit smoking. Whereas most existing EMA studies have examined momentary snapshots of the effects of alcohol and tobacco, we intensively oversampled post-drinking moments to permit a more comprehensive analysis of alcohol and tobacco effects. The findings reproduced important phenomena observed in laboratory alcohol challenge research, increasing confidence in the validity of the assessment and analytic strategies. For example, we found that a descending eBAC was associated with less hedonically positive states (lower excitement, higher sluggishness and dizziness). We also observed significant, negative eBAC × Descending interactions in analyses of buzz and excitement, indicating weaker relations between eBAC and these states when eBAC was falling. These observations are congruent with laboratory observations of acute tolerance to alcohol with respect to self-reported stimulant effects or subjective intoxication (e.g., Martin and Moss 1993; Cromer et al. 2010).
The findings suggest cigarettes may extend the pleasurable or stimulant effects of alcohol beyond the BAC peak. Buzz reports were the subjective states most strongly influenced by alcohol and tobacco. A significant three-way interaction indicated that, on the descending limb, eBAC was more strongly related to buzz in post-smoking moments compared to nonsmoking moments (Figure 2, top). A similar three-way interaction effect emerged for excitement, a BAES stimulant effects item (Figure 2, bottom).
Results from some laboratory studies are congruent with the current findings. Kouri et al. (2004) found that pretreatment with a 21mg nicotine patch accentuated reports of feeling drunk and feeling the effects of alcohol compared to a placebo patch condition. These effects were significant 60 minutes after beverage consumption, approximately 5-15 minutes after the observed peak BAC. Chi and deWit (2003) demonstrated the converse, finding that mecamylamine, a nicotinic receptor antagonist, decreased stimulant effects of alcohol at peak BAC and beyond. Contrary results have also been reported. For example, Perkins et al. (1995) found that repeated administration of nicotine nasal spray decreased both perceptions of intoxication and sedative-like responses when blood alcohol was descending. McKee et al. (2008) administered priming drinks to mildly tobacco-deprived smokers who wore either a 21mg nicotine patch or a placebo patch. In a subsequent choice period during which the BAC from a priming drink was descending, the active patch was associated with slower initiation of drinking and consumption of fewer drinks. Thus, the combination of nicotine and a descending BAC did not seem to motivate drinking behavior in the manner one might expect if these conditions acutely accentuated pleasurable alcohol effects. Future laboratory studies might be designed to more systematically assess the subjective and behavioral consequences of smoking or acute nicotine administration coinciding with a descending BAC.
A low level of response to alcohol is hypothesized to be associated with risk for alcohol use disorders. However, the evidence is inconsistent and there is unresolved controversy concerning whether this diminished response should be expected across the entire BAC curve or is more limited to the descending limb (e.g., Morean and Corbin 2010). Given this and some evidence that smoking or nicotine can diminish acute alcohol responses in the laboratory (e.g., Madden et al. 1995; McKee et al. 2008), we tentatively advanced the hypothesis that heavy smoking might be associated with decreased stimulant effects during ecologically-assessed drinking episodes, perhaps especially when eBAC was descending. The findings clearly did not support this hypothesis. Notably, recent evidence suggests that heightened stimulating alcohol effects at peak BAC may be predictive of risk for escalation of binge drinking (King et al. 2011). The current findings might be reconciled with known epidemiological associations between smoking and problem drinking via this account.
Analyses of drug appraisals suggested co-users normatively tended to rate both cigarettes and drinks as highly pleasurable, moderately relieving, and not very punishing. Contrary to predictions, reports of cigarette pleasure were not significantly related to eBAC and reports of drink pleasure were not predicted by having smoked since last report. A change in the frame of reference might account for the discrepancy with our prior findings. For example, Piasecki et al. (2011) compared ratings of cigarettes smoked with and without recent alcohol consumption, whereas the current analyses examined cigarette pleasure after drinking as a function of variations in estimated alcohol concentration. Smoking was rated as less pleasurable, less negatively reinforcing, and more punishing when eBAC was descending. These findings are perhaps congruent with the general expectation that hedonic states tend to be more unpleasant on the descending limb. It is notable, however, that there was no eBAC × Descending interaction in predicting cigarette pleasure or relief. Thus, the apparent enhancement of current buzz and excitement by smoking on the descending limb was not accompanied by conscious attributions of positive or negative reinforcing consequences to recently smoked cigarettes. Negative reinforcement from the last drink decreased and punishment increased with eBAC, suggesting diminished hedonic returns from drinking after heavy consumption.
Overall, the findings were broadly similar for men and women. We found evidence for gender differences only in one model. Among women, smoking slightly accentuated the relation between eBAC and ratings of drinking pleasure, whereas this relation was somewhat weakened by smoking among men (Figure 4). Future studies are needed to evaluate whether this finding is replicable.
Several important limitations of the current work need to be highlighted. Due to concern over the potential for assessment burden during drinking episodes, our diary assessments were very brief. The current analyses focused on a handful of subjective states. These are defensible proxies for intoxication effects but wider assessment of sedative or stimulant effects of alcohol would have been preferable. In hindsight, the finding that drinkers responded to 84% of the signaled prompts delivered during drinking episodes assuages some concerns about burden; longer assessments may prove feasible in future studies.
We analyzed the diary records as a function of estimated BAC level and ascending vs. descending eBAC in order to probe the data with respect to known, meaningful determinants of alcohol response. However, both parameters are only estimates and their calculation involved several assumptions and idealizations. Therefore, we do not expect that our point estimates of eBAC or limb classifications are precise. We do believe, however, that they are likely to be statistically related to the true, unobserved values and provide a better proxy for level of intoxication than measures that fail to account for gender, drinking duration, and body weight. Future research could incorporate more detailed assessments of drink type, volume, and duration (cf. Ray et al. 2010) to potentially derive refined estimates of alcohol concentrations.
Misleading inferences could result if the accuracy of the eBAC equation varies as a function of the predictor variables. For example, some evidence suggests smoking slows the rate of gastric emptying, potentially delaying alcohol absorption (Gritz, et al. 1988; Johnston, et al. 1991). If so, this could lead to systematic misclassification of moments as ascending vs. descending and provide a rival explanation for the 3-way interactions found for buzzed and excitement. Field research using transdermal ethanol sensors (e.g., Sakai et al. 2006) and studies testing the validity of eBAC equations across variations in smoking status would permit greater insights into these concerns.
Many of the significant fixed effects were small in magnitude relative to the original response scale, potentially questioning their value in explaining alcohol or tobacco use. As Figures 2-4 make clear, there is a great deal of scatter of predicted values around the trend lines. Random effects terms (not shown) were typically significant, indicating the presence of substantial between-episode and between-persons variation underlying the fixed effects.
The diary data were observational, preventing strong causal inferences. Strictly speaking, the current analyses could not determine whether heavy smoking causes enhanced buzz or excitement on the descending limb or whether those individuals who experience heightened stimulant effects on the descending limb self-select into continued smoking. Descending limb observations may be more likely when “the party's over,” suggesting environmental or contextual influences should be considered as alternatives to pharmacological accounts for some of the major findings. Location and social contacts were not assessed during DFUs to minimize assessment burden. This prevented tests of intra-episode changes in context that could be related to eBAC limb, level, or smoking behavior. Finally, complex latent mixture or censoring issues inherent in the data potentially complicate the analyses. For example, our observations of the descending limb are probably both “right censored” (because participants could stop recording to retire for the night) and “left censored” (because observing high descending eBACs requires obtaining high peak eBAC).1 Mean eBAC levels were considerably lower in descending vs. ascending records. In supplementary analyses, the key effects involving the descending indicator reported in the main analyses remained significant after entering a dichotomized eBAC level (above or below .04) and various interactions of this variable with eBAC and smoking, Additionally, the descending variable was associated with higher sluggish ratings but the dichotomized eBAC level variable was not. These findings increase confidence that the obtained effects are related to descending alcohol levels per se rather statistical artifact.
Strengths include the use of an innovative diary assessment protocol and data analytic approach to examine data collected from smokers during more than 2,000 drinking episodes. The findings present new and potentially generative information about the experiences smokers have while drinking and smoking. The most notable findings suggested that smoking is associated with increased stimulant alcohol effects on the descending limb. Future laboratory studies might manipulate nicotine or tobacco administration on the descending limb of the BAC curve to evaluate the reliability of these observations and to extend our understanding of the motivational underpinnings of alcohol-tobacco co-use and comorbidity. There is also a need to identify additional explanatory factors accounting for variation in ecologically-assessed subjective states across drinkers and drinking episodes.
Acknowledgements
Supported by National Institutes of Health grants P50AA011998 (Heath), K05AA017688 (Heath), and K05AA017242 (Sher). Saul Shiffman is a cofounder of invivodata, inc., which provides electronic diary services for research and was responsible for the software used in this study.
Footnotes
We conducted additional analyses predicting buzzed and excited that were limited to the descending limb and included the intra-episode peak eBAC value as a covariate. A significant eBAC × Smoked interaction remained for buzzed, b = 4.69, F (1, 1586) = 10.56, p<.001, and the form of the interaction resembled the pattern in Figure 2. The eBAC × Smoked interaction was not significant in the model predicting excitement, b = 2.13, F (1, 1586) = 1.93, p = .16.
References
- Acheson A, Mahler SV, Chi H, de Wit H. Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology. 2006;186:54–63. doi: 10.1007/s00213-006-0338-y. doi:10.1007/s00213-006-0338-y. [DOI] [PubMed] [Google Scholar]
- Babor TF, Biddle-Higgins JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care. World Health Organization; Geneva, Switzerland: 2001. [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. doi:10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Hernandez-Avila CA, Van Kirk J, Rose JE, Kranzler HR. Meamylamine modifies the pharmacokinetics and reinforcing effects of alcohol. Alcohol Clin Exp Res. 2002;26:326–331. doi:10.1111/j.1530-0277.2002.tb02541.x. [PubMed] [Google Scholar]
- Chi H, deWit H. Mecamylamine attenuates the subjective stimulant-like effects of alcohol in social drinkers. Alcohol Clin Exp Res. 2003;27:780–786. doi: 10.1097/01.ALC.0000065435.12068.24. doi:10.1097/01.ALC.0000065435.12068.24. [DOI] [PubMed] [Google Scholar]
- Cromer JR, Cromer JA, Maruff P, Snyder PJ. Perception of alcohol intoxication shows acute tolerance while executive functions remain impaired. Exp Clin Psychopharmacol. 2010;18:329–339. doi: 10.1037/a0019591. doi:10.1037/a0019591. [DOI] [PubMed] [Google Scholar]
- Drobes DJ. Concurrent alcohol and tobacco dependence: Mechanisms and treatment. Alcohol Res Health. 2002;26:136–142. [Google Scholar]
- Glautier S, Clements K, White JAW, Taylor C, Stolerman IP. Alcohol and the reward value of cigarette smoking. Behav Pharmacol. 1996;7:144–154. doi:10.1097/00008877-199603000-00005. [PubMed] [Google Scholar]
- Gritz ER, Ippolitti A, Jarvik ME, Rose JE, Shiffman S, Harrison A, Van Vunakis H. The effect of nicotine on the delay of gastric emptying. Aliment Pharmacol Ther. 1988;2:173–8. doi: 10.1111/j.1365-2036.1988.tb00685.x. doi:10.1111/j.1365-2036.1988.tb00685.x. [DOI] [PubMed] [Google Scholar]
- Harrison ELR, Hinson RE, McKee SA. Experimenting and daily smokers: Episodic patterns of alcohol and cigarette use. Addict Behav. 2008;34:484–486. doi: 10.1016/j.addbeh.2008.12.013. doi:10.1016/j.addbeh.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hustad JTP, Carey KB. Using calculations to estimate blood alcohol concentrations for naturally occurring drinking episodes: A validity study. J Stud Alcohol. 2005;66:130–138. doi: 10.15288/jsa.2005.66.130. [DOI] [PubMed] [Google Scholar]
- Johnston RD, Horowitz M, Maddox AF, Wishart JM, Shearman DJC. Cigarette smoking and rate of gastric emptying: Effect on alcohol absorption. BMJ. 1991;302:20–3. doi: 10.1136/bmj.302.6767.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. doi:10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–35. doi:10.1097/00000374-200206000-00012. [PubMed] [Google Scholar]
- King AC, McNamara P, Conrad M, Cao D. Alcohol-induced increases in smoking behavior for nicotinized and denicotinized cigarettes in men and women. Psychopharmacology. 2009;207:107–117. doi: 10.1007/s00213-009-1638-9. doi:10.1007/s00213-009-1638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner TR, Sayette MA. Effects of smoking abstinence and alcohol consumption on smoking-related outcome expectancies in heavy smokers and tobacco chippers. Nicotine Tob Res. 2007;9:365–376. doi: 10.1080/14622200701188893. doi:10.1080/14622200701188893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri EM, McCarthy EM, Faust AH, Lukas SE. Pretreatment with transdermal nicotine enhances some of ethanol's acute effects in men. Drug Alcohol Depend. 2004;75:55–65. doi: 10.1016/j.drugalcdep.2004.01.011. doi:10.1016/j.drugalcdep.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. 2nd ed. SAS Institute, Inc.; Cary, NC: 2006. [Google Scholar]
- Littleton J, Barron S, Prendergast M, Nixon SJ. Smoking kills (alcoholics)! Shouldn't we do something about it? Alcohol Alcohol. 2007;42:167–173. doi: 10.1093/alcalc/agm019. doi:10.1093/alcalc/agm019. [DOI] [PubMed] [Google Scholar]
- Madden PAF, Heath AC, Martin NG. Smoking and intoxication after alcohol challenge in women and men: Genetic influences. Alcohol Clin Exp Res. 1997;21:1732–1741. doi:10.1111/j.1530-0277.1997.tb04517.x. [PubMed] [Google Scholar]
- Madden PAF, Heath AC, Starmer GA, Whitfield JB, Martin NG. Alcohol sensitivity and smoking history in men and women. Alcohol Clin Exp Res. 1995;19:1111–1120. doi: 10.1111/j.1530-0277.1995.tb01588.x. doi:10.1111/j.1530-0277.1995.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. doi:10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Martin CS, Moss HB. Measurement of acute tolerance to alcohol in human subjects. Alcohol Clin Exp Res. 1993;17:211–216. doi: 10.1111/j.1530-0277.1993.tb00751.x. doi:10.1111/j.1530-0277.1993.tb00751.x. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Miller WR. Estimating blood alcohol concentration: Two computer programs and their applications in therapy and research. Addict Behav. 1979;4:55–60. doi: 10.1016/0306-4603(79)90021-2. doi:10.1016/0306-4603(79)90021-2. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, Shi J. Alcohol expectancy increases positive responses to cigarettes in young, escalating smokers. Psychopharmacology. 2010;210:355–364. doi: 10.1007/s00213-010-1831-x. doi:10.1007/s00213-010-1831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Hinson R, Rounsaville D, Petrelli P. Survey of subjective effects of smoking while drinking among college students. Nicotine Tob Res. 2004;6:111–117. doi: 10.1080/14622200310001656939. doi:10.1080/14622200310001656939. [DOI] [PubMed] [Google Scholar]
- McKee SA, O'Malley SS, Shi J, Mase T, Krishnan-Sarin S. Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacology. 2008;196:189–200. doi: 10.1007/s00213-007-0952-3. doi:10.1007/s00213-007-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective response to alcohol: A critical review of the literature. Alcohol Clin Exp Res. 2010;34:385–395. doi: 10.1111/j.1530-0277.2009.01103.x. doi:10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Renton RM. High risk groups often have higher levels of alcohol response than low risk: The other side of the coin. Alcohol Clin Exp Res. 2010;34:199–202. doi: 10.1111/j.1530-0277.2009.01081.x. doi:10.1111/j.1530-0277.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Combined effects of nicotine and alcohol on subjective, behavioral, and physiological responses in humans. Addict Biol. 1997;2:255–267. doi: 10.1080/13556219772552. doi:10.1080/13556219772552. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Grobe JE. Sex differences in the acute effects of cigarette smoking on the reinforcing value of alcohol. Behav Pharmacol. 2000;11:63–70. doi: 10.1097/00008877-200002000-00007. doi:10.1097/00008877-200002000-00007. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sexton JE, DiMarco A, Grobe J, Scierka A, Stiller RL. Subjective nd cardiovascular responses to nicotine combined with alcohol in male and female smokers. Psychopharmacology. 1995;119:205–212. doi: 10.1007/BF02246162. doi:10.1007/BF02246162. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jahng S, Wood PK, Robertson BM, Epler AJ, Cronk NJ, Rohrbaugh JW, Heath AC, Shiffman S, Sher KJ. The subjective effects of alcohol-tobacco co-use: An ecological momentary assessment investigation. J Abnorm Psychol. 2011;120:557–571. doi: 10.1037/a0023033. doi: 10.1037/a0023033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, McCarthy DE, Fiore MC, Baker TB. Alcohol consumption, smoking urge, and the reinforcing effects of cigarettes: An ecological study. Psychol Addict Behav. 2008;22:230–239. doi: 10.1037/0893-164X.22.2.230. doi:10.1037/0893-164X.22.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Miranda R, Jr, Tidey JW, McGeary JE, MacKillop J, Gwaltney CJ, Rohsenow DJ, Swift RM, Monti PM. Polymorphisms of the μ-opioid receptor and dopamine D4 receptor genes and subjective response to alcohol in the natural environment. J Abnorm Psychol. 2010;119:115–125. doi: 10.1037/a0017550. doi: 10.1037/a0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson BM, Piasecki TM, Slutske WS, Wood PK, Sher KJ, Shiffman S, Heath AC. Validity of the Hangover Symptoms Scale: Evidence from an electronic diary study. Alcohol Clin Exp Res. 2012;36:171–177. doi: 10.1111/j.1530-0277.2011.01592.x. doi:10.1111/j.1530-0277.2011.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Psychopharmacologic interactions between nicotine and ethanol. Nicotine Tob Res. 2004;6:133–144. doi: 10.1080/14622200310001656957. doi:10.1080/14622200310001656957. [DOI] [PubMed] [Google Scholar]
- Rutledge PC, Park A, Sher KJ. 21st birthday drinking: Extremely extreme. J Consult Clin Psychol. 2008;76:511–516. doi: 10.1037/0022-006X.76.3.511. doi:10.1037/0022-006X.76.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai JT, Mikulich-Gilbertson SK, Long RJ, Crowley TJ. Validity of transdermal alcohol monitoring: Fixed and self-regulated dosing. Alcohol Clin Exp Res. 2006;30:26–33. doi: 10.1111/j.1530-0277.2006.00004.x. doi:10.1111/j.1530-0277.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim RS. University of California, San Diego. Alcohol Clin Exp Res. 2010;34:203–205. doi:10.1111/j.1530-0277.2009.01082.x. [Google Scholar]
- Sher KJ, Wood MD, Richardson AE, Jackson KM. Subjective effects of alcohol I: Effects of the drink and drinking context. In: Earleywine M, editor. Mind Altering Drugs: Scientific Evidence for Subjective Experience. Oxford; New York: 2005. pp. 86–134. [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychol Assess. 2009;21:486–497. doi: 10.1037/a0017074. doi:10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Balabanis M. Alcohol and tobacco: From basic science to clinical practice. National Institute of Alcohol Abuse and Alcoholism Research Monograph No. 30, NIH Publication 95-3931; 1995. Associations between alcohol and tobacco. pp. 17–36. [Google Scholar]
- Shiffman S, Kirchner TR. Cigarette-by-cigarette satisfaction during ad libitum smoking. J Abnorm Psychol. 2009;118:348–359. doi: 10.1037/a0015620. doi:10.1037/a0015620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg P, Nichter M, Nichter M. Taking play seriously: Low-level smoking among college students. Cult Med Psychiatry. 2007;31:1–24. doi: 10.1007/s11013-006-9042-y. doi:10.1007/s11013-006-9042-y. [DOI] [PubMed] [Google Scholar]