Abstract
The immune system undergoes profound age-related changes, including a gradual increase in the production and circulation of proinflammatory cytokines. Despite the known capacity of fibroblasts to produce cytokines, little is known so far about the inflammatory response of fibroblasts to cellular stress such as viral and/or bacterial infection in the context of aging. Therefore the aim of this study was to analyze the levels of IL6 and IL8 secretion in supernatants of human skin fibroblasts from young and elderly persons. Cytokine and chemokine secretion was analyzed before and after in vitro infection of the cells with Cytomegalovirus (CMV) and/or stimulation with Lipopolysaccharide (LPS). The exposure of fibroblasts to these agents caused inflammatory changes, reflected by the secretion of both the cytokine IL6 and the chemokine IL8 by fibroblasts from young as well as elderly persons. The cytokine/chemokine production induced by either agent alone could be further increased by co-stimulation of the cells with both stimuli. The level of protein secretion was dependent on the chronological age of the fibroblasts. Stimulated human skin fibroblasts from elderly donors produced higher amounts of IL6 as well as IL8 than fibroblasts from young donors. These differences were more pronounced for IL6 than for IL8. The inflammatory response of fibroblasts to stimulation differed among donors and did not correspond to the responsiveness of whole blood derived from the same person.
In summary lifelong CMV-infection may act as an in vivo trigger for inflammatory changes by increasing the inflammatory response to bacterial products such as LPS. It may thus contribute to age-related inflammatory processes, referred to as ‘inflamm-aging’.
Keywords: Cytomegalovirus, Lipopolysaccharide, Interleukin-6, Interleukin-8, Fibroblasts, Aging
Highlights
► CMV and LPS induced cytokine production of skin fibroblasts depends on donor age. ► Stimulated fibroblasts from elderly donors produce higher amounts of IL6 and IL8. ► CMV-infection can increase the inflammatory response to bacterial products (LPS). ► The inflammatory response to LPS stimulation is different in individual cell types.
1. Introduction
As individuals age their immune system undergoes profound age-related changes, termed immunosenescence. This is of particular interest as immunological changes with aging contribute to a higher incidence and severity of infectious diseases as well as a decreased efficacy of vaccines in elderly persons (Weinberger et al., 2008). These age-related changes include a gradual increase in the production and circulation of proinflammatory cytokines such as Interleukin-6 (IL6), Interferon gamma (IFNγ) and Tumor necrosis factor alpha (TNFα) with age, leading to a systemic chronic low-grade state of inflammation. This phenomenon is referred to as ‘inflamm-aging’ (Franceschi et al., 2000). Cell types involved in ‘inflamm-aging’ can be innate immune cells, such as monocytes or macrophages, but also highly differentiated, antigen-experienced T cells (Arnold et al., 2011). However, the release of proinflammatory cytokines by non-immune cells such as fibroblasts has also been suggested to play a role in ‘inflamm-aging’ (Mohan et al., 2011). Fibroblasts are ubiquitous, differentiated cells of mesenchymal origin. Due to their capacity to produce cytokines, chemokines (Smith et al., 1997), growth factors (Kahari and Saarialho-Kere, 1997) and other biologically active molecules they are involved in local inflammatory and immune responses. Because of their great importance for the whole organism fibroblasts are an interesting and frequently studied cell type for in vitro investigation (Darby and Hewitson, 2007). In addition to host cells pathogens have been claimed to contribute to ‘inflamm-aging’. The chronic exposure to persistent viruses such as cytomegalovirus (CMV) seems to play an important role (Franceschi, 2007; Vasto et al., 2007). Chronic bacterial infections may also promote inflammation in elderly persons (Gavazzi and Krause, 2002). Despite the known capacity of fibroblasts to produce cytokines, particularly during replicative senescence (Coppe et al., 2008), the effects of aging on the inflammatory response of fibroblasts to cellular stress such as viral and/or bacterial infection have only scarcely been elucidated. In addition it is not known whether some elderly persons have a ‘proinflammatory phenotype’ reflected by high cytokine production in all cell types of the body or whether inflammatory activity varies from organ to organ in old age. To answer these questions we evaluated the production of the cytokine IL6 and the chemokine IL8 by human skin fibroblasts from young and elderly persons following CMV-infection and LPS stimulation.
2. Material and methods
2.1. Human skin fibroblasts
Human skin fibroblasts from elderly donors (n = 8, median age 91 years, range 90–92 years, 3 males, 5 females), obtained from participants of the Leiden 85-plus study (der Wiel et al., 2002), and healthy young donors (n = 5, median age 24 years, range 21–26 years, 2 males, 3 females) were obtained from skin biopsies taken from the inner side of the upper arm and prepared as previously described (Maier et al., 2007). The fibroblast strains from the elderly donors were chosen based on their cytokine production capacity of LPS stimulated whole blood samples, which were classified as ‘high proinflammatory responders’ or ‘low proinflammatory responders’ (van den Biggelaar et al., 2004). These fibroblasts were cultivated in D-MEM:F-12 (1:1) (Gibco Invitrogen Corporation, Paisley, Scotland) supplemented with 10% FCS (Sigma–Aldrich, Vienna, Austria), 1 mM sodium pyruvate, 10 mM HEPES, 2 mM Glutamax I and antibiotics (100 Units per mL penicillin, 100 μg/mL streptomycin and 2.5 μg/mL amphotericin B) at 37 °C and 5% carbon dioxide (all supplements were obtained from Invitrogen, Lofer, Austria if not stated differently).
2.2. Virus
Human cytomegalovirus (strain Town-eGFP) was obtained from the University of Regensburg, Institute for Medical Microbiology and Hygiene (Michael Nevels and Christina Paulus) and propagated in human diploid fetal lung fibroblasts (Mrc-5), which were cultured in Dulbecco's modified Eagle medium (DMEM, Gibco Invitrogen Corporation, Paisley, Scotland) supplemented with 10% FCS (Sigma–Aldrich, Vienna, Austria), penicillin/streptomycin (100 Units per mL, 100 μg/mL streptomycin) (Invitrogen, Lofer, Austria) and 2 mM L-glutamine (Sigma–Aldrich, Vienna, Austria). Infectious virus particles in the virus stock were quantified by a standard plaque assay. Briefly, 500 μL of varying virus dilutions was added to confluent Mrc-5 cells in a 12-well plate (Techno Plastic Products AG, Trasadingen, Switzerland). After an incubation period of 2 h (37 °C, 5% CO2) the virus suspension was removed and the cell monolayers were covered with 2 mL DMEM containing 1% methylcellulose (Sigma–Aldrich, Vienna, Austria) and incubated for 10 days. Then the plaques were counted under a fluorescence microscope. The viral concentration is expressed as plaque-forming units per mL (pfu/mL). All in vitro experiments with the virus were performed with a multiplicity of infection (MOI) of 1. For control experiments CMV was inactivated by UV irradiation (3 × 30 s, 120 mJ/cm2 in 15 cm cell culture dishes) in a cross-linking chamber (Stratalinker®, Heidelberg, Germany).
2.3. CMV-infection of fibroblasts and treatment with LPS
Human skin fibroblasts were seeded in 6-well plates (Techno Plastic Products AG, Trasadingen, Switzerland) and maintained in supplemented DMEM at 37 °C and 5% CO2 until they reached 100% confluency. At this time point the culture medium was removed and replaced by the virus-containing medium or medium containing 10 ng/mL LPS (Sigma–Aldrich, Vienna, Austria) for six days. Untreated fibroblasts (mock-infected) as well as fibroblasts infected with the UV-inactivated CMV were used as controls. Conditioned cell culture supernatants were collected as indicated and used for cytokine and chemokine profiling by ELISA. All experiments were performed with human skin fibroblasts of the same passage of cultivation, namely passage 21.
2.4. Cytokine and chemokine profiling
Supernatants of human skin fibroblasts were analyzed in duplicates for the presence of IL1A, IL1B, IL6, IL8, TNFα, IL10 and IFNγ by commercially available ELISA kits (MabTech, Hamburg, Germany; BioLegend, Fell, Germany or PromoKine, Heidelberg, Germany), each according to the manufacturer's instructions. Results are presented as ng/mL.
2.5. Western blot analysis
Cells were washed with cold PBS and whole cell pellets were resuspended in TNE lysis buffer (50 mM Tris HCL pH 8.0, 150 mM NaCL and 1% TritonX100, supplemented with protease inhibitor (1:100, Roche Diagnostics, Basel, Switzerland) and phosphatase inhibitors (1:25, Sigma–Aldrich). After removing unlysed cell components by centrifugation (6000 g, 10 min, 4 °C) protein concentration of lysates was assessed using the Bradford protein assay (Bio-Rad, Vienna, Austria). Samples were mixed with 5 × loading buffer (50% glycerol, 5% ß-mercaptoethanol, 8.3% SDS, 31.25% 1 M Tris pH 6.8, 0.017% bromophenol blue and 16.67% Aqua dest.), heated for 5 min at 95 °C and loaded onto 4–20% gradient Tris-Glycine precast polyacrylamide gels (Lonza, Basel, Switzerland). Electrophoresis was performed at constant current (20 mA) until adequate separation of the protein molecular marker (Bio-Rad) was achieved. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA) using a wet-blot transfer system. Membranes were then incubated with primary Abs against p16 (BD Pharmingen, Franklin Lakes, NJ, USA) and GAPDH (Abcam, Cambridge, UK). Appropriate secondary Abs conjugated with horseradish-peroxidase were used and the immune complexes were visualized using SuperSignal Western Femto substrate (Thermo Scientific, Waltham, MA, USA) and an Alpha Innotech Fluor Chem HD2 chemiluminescence detection unit with AlphaEase FC software (Alpha Innotech). For p16 expression of human fibroblasts densitometrical evaluation of obtained grayscale values normalized to GAPDH was performed. Human foreskin fibroblasts of early and late passages were provided by the Molecular and Cell Biology Division of the Institute for Biomedical Aging Research, Innsbruck, Austria and were used as controls for low and high p16 expression, respectively.
2.6. Statistical analysis
The arithmetic means ± S.E.M. were calculated for each examined group and time point. Comparisons between two groups were analyzed by Mann–Whitney-U-Test using SPSS version 19.0 (SPSS Inc., Chicago, Illinois, USA). Probability values (p) were calculated and a level of p < 0.05 was considered as statistically significant.
3. Results and discussion
3.1. Human skin fibroblasts secrete proinflammatory cytokines and chemokines
First, conditioned cell culture supernatants of untreated as well as LPS treated human skin fibroblasts from young and elderly persons were investigated after 48 h of cultivation for the presence of IL1A, IL1B, IL6, IL8, TNFα, IL10 and IFNγ. Whereas human skin fibroblasts were able to produce IL6 and IL8, they failed to produce detectable amounts of IFNγ, TNFα, IL10, IL1A and IL1B (data not shown). Based on these results we investigated whether the secretion of IL6 and IL8 depends on the chronological age of the fibroblast donors. Basal IL6 protein secretion was found to be slightly, but not significantly elevated in the supernatants of fibroblasts from old (0.9 ± 0.3 ng/mL) versus young donors (0.3 ± 0.1 ng/mL), indicating that fibroblasts may contribute to the chronic low-grade inflammatory state found in elderly people, a condition referred to as ‘inflamm-aging’ (Franceschi et al., 2000). Unlike IL6 the spontaneous production of IL8 was found to be slightly, but not significantly lower in human skin fibroblasts of elderly (0.06 ± 0.02 ng/mL) compared to young donors (0.17 ± 0.09 ng/mL).
3.2. Secretion of IL6 and IL8 by human skin fibroblasts after CMV-infection and/or stimulation with LPS is dependent on the chronological age
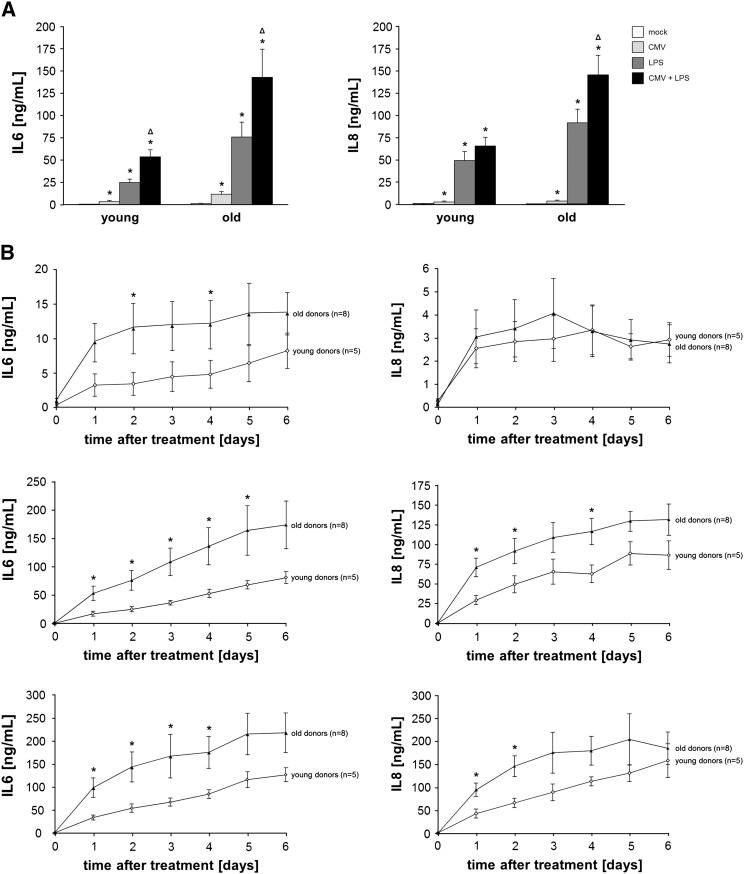
After our initial studies we wanted to analyze the effect of viral infection and bacterial exposure on the inflammatory responses of human skin fibroblasts. For this reason we infected fibroblasts in vitro with CMV and compared the secretion of IL6 and IL8 to that of LPS treated cells after 48 h of treatment (Fig. 1A). A much stronger reaction to LPS than CMV was observed, but viral infection also induced a significant release of these inflammatory markers. This is in accordance with earlier reports which describe that HCMV stimulates the production of IL8 and IL6 in human fibroblasts as well as endothelial cells (Browne et al., 2001; Craigen et al., 1997; Grundy et al., 1998; Zhu et al., 1998). Interestingly, cytokine and chemokine production, particularly of IL6, could be further increased by co-stimulation of the cells with LPS and CMV together compared to separate exposure to each of them, suggesting that latent CMV-infection can increase the inflammatory response to bacterial products such as LPS (Fig. 1A).
Fig. 1.
(A) Secretion of the inflammatory proteins IL6 and IL8 by human skin fibroblasts from young and elderly donors following infection with CMV and/or stimulation with LPS.
IL6 (left) and IL8 (right) concentration in the supernatant of human skin fibroblasts after infection with CMV (MOI = 1) and/or stimulation with LPS (10 ng/mL) was determined by ELISA after 48 h. The data represent mean ± S.E.M of n = 5 (young)/n = 8 (old) donors. Statistical analyses were carried out using the Mann–Whitney-U-Test and p values < 0.05 were considered as statistically significant.
* significant differences compared to mock-infected cells.
Δ significant differences between LPS stimulated and co-stimulated (CMV/LPS) fibroblasts.
(B) Human skin fibroblasts from elderly donors secrete higher amounts of the inflammatory proteins IL6 and IL8 following infection with CMV and/or stimulation with LPS.
IL6 (left panels) and IL8 (right panels) concentration in the supernatant of human skin fibroblasts after infection with CMV (MOI = 1) (upper part) or stimulation with 10 ng/mL LPS (middle part) or a combination of both (lower part) was determined by ELISA every 24 h for six days. The data represent mean ± S.E.M of n = 5 (young)/n = 8 (old) donors. Significant differences (Mann–Whitney-U-Test) between young and old human skin fibroblasts are marked by * (p < 0.05).
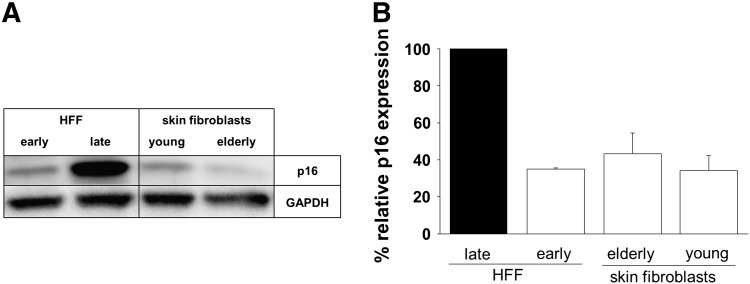
To investigate the kinetics of age-related changes in the production of IL6 and IL8 by human skin fibroblasts in response to different cellular stimuli, human skin fibroblasts from young and elderly persons were infected with CMV and/or stimulated with LPS and cultured for six days. Supernatants were collected every 24 h for six days and analyzed by ELISA. After in vitro infection with CMV and/or stimulation with LPS IL6 and IL8 secretion by human skin fibroblasts from young and elderly persons increased rapidly within 24 h and then steadily within the following five days of culture (Fig. 1B). Human skin fibroblasts from elderly donors produced higher amounts of IL6 and IL8 than cells from young donors in response to stimulation. The difference was particularly pronounced for IL6, but also significant at some time points for IL8 following LPS stimulation. Increased cytokine production has been described for senescent fibroblasts. We therefore analyzed the expression of the senescence marker p16 in the human skin fibroblasts. Evaluation of p16 protein expression by Western Blot analysis, as shown in Fig. 2, revealed low levels of p16 indicating that the skin fibroblasts were not senescent at the time of our experiments.
Fig. 2.
p16 protein expression of cultivated human skin fibroblasts of young and elderly persons as well as early and late passage human foreskin fibroblasts.
The protein expression of p16 was assessed in cultivated human skin fibroblasts of young and elderly donors by Western Blot analysis. As controls early and late passage (replicative senescent) human foreskin fibroblasts (HFF) were used. (A) The image shows one representative Western blot analysis of p16 expression of early and late HFF and human skin fibroblasts of young and elderly donors. (B) The graph shows the relative expression of p16 compared to late passage (senescent) HFF. Shown are mean values ± S.E.M; n = 3 (early HFF)/n = 5 (young)/n = 8 (elderly). All values were normalized to expression of the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase).
No difference in p16 expression was observed in skin fibroblasts from young and elderly donors. Human foreskin fibroblasts (HFF) of early and late passages were used as controls. Our results are in concordance with observations by Maier et al. showing that skin fibroblasts of elderly donors reach senescence only after at least 50 population doublings, corresponding to approximately 30 passages of in vitro cultivation (Maier et al., 2007). These results support the concept that the secretion of IL6 and IL8 by human skin fibroblasts following infection with CMV and/or stimulation with LPS is affected by the chronological age of the fibroblast donor. Furthermore these findings corroborate the hypothesis of an increased proinflammatory status of aged cells (Coppe et al., 2008; Shelton et al., 1999). Although the molecular mechanisms responsible for the effect are not yet clear, the ‘overproduction’ of IL6 and IL8 by fibroblasts of elderly persons might be the result of in vivo priming with potential epigenetic changes as a consequence. This possibility has also been previously addressed for other cell types (Clark and Peterson, 1994). Control experiments performed with UV-inactivated virus, which can be internalized into the cells but is not able to replicate, additionally demonstrated that the induction of IL6 and IL8 requires active viral replication (data not shown). Lifelong CMV-infection may thus represent an in vivo trigger for changes leading to an inappropriate production of proinflammatory molecules, chronic inflammation and an imbalance in the cytokine network between pro- and antiinflammatory cytokines. Increased occurrence or progression of age-related diseases may be the consequence. However, inflammation may not be only detrimental since high proinflammatory activity of peripheral blood cells of persons older than 85 years has been linked with a survival benefit (van den Biggelaar et al., 2004). Under which conditions detrimental effects and under which beneficial ones dominate, remains yet to be elucidated.
3.3. The inflammatory responses to LPS stimulation differs between cell types
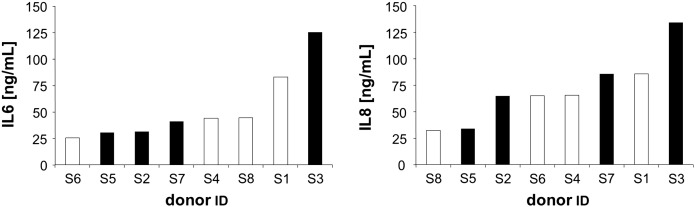
For the Leiden 85-plus study whole blood samples of 85-year-old donors were stimulated with 10 ng/mL LPS for 24 h and LPS induced secretion of IL6, TNFα and IL1B was determined in ELISA. Inter-individual differences in the inflammatory cytokine production capacity were observed and based on these individuals were subdivided into ‘high proinflammatory’ or ‘low proinflammatory’ responders (van den Biggelaar et al., 2004). The capacity to produce high quantities of proinflammatory cytokines was significantly linked with a survival benefit. In addition IFNγ production of LPS stimulated whole blood was determined. As skin fibroblasts were available from the same donors, it was of interest to assess whether a high/low proinflammatory cytokine production profile of LPS stimulated peripheral blood was accompanied by a corresponding proinflammatory potential of in vitro LPS stimulated fibroblasts. IL6 and IL8 secretion by fibroblasts was, however, different from the inflammatory activity of peripheral blood following LPS stimulation (Fig. 3). Fibroblasts from elderly persons, who were defined as ‘low proinflammatory responders’ – including lower levels of IL6 – based on their whole blood secreted similar amounts of IL6 and IL8 after 24 h of LPS stimulation as fibroblasts from persons who were defined as ‘high proinflammatory responders’. The level of IL6 and IL8 secreted by stimulated fibroblasts was not significantly correlated with IL6, TNFα, IL1B or IFNγ production of whole blood following stimulation with LPS (Spearman's rank correlation). These results indicate that the inflammatory response to LPS varies from cell type to cell type. Whether this implies that certain organs are more susceptible to the consequences of inflammation in some persons than in others and what role the genetic background plays versus environmental factors in this respect is currently subject to further investigations.
Fig. 3.
LPS induced IL6 and IL8 secretion of human skin fibroblasts from elderly donors participating in the Leiden 85-plus study.
IL6 (left panel) and IL8 (right panel) concentration in the supernatant of human skin fibroblasts from elderly donors was determined by ELISA after 24 h of stimulation with LPS (10 ng/mL).
White bars: ‘low proinflammatory’ responders, black bars: ‘high proinflammatory’ responders as defined on the basis of the production of proinflammatory cytokines (TNFα, IL1B and IL6) by blood cells following stimulation with LPS for 24 h (van den Biggelaar et al., 2004).
Conflict of interests
The authors declare that none of them has any conflict of interest related to this manuscript.
Acknowledgments
This work has been supported by and carried out within the EU funded Network of Excellence LifeSpan (project EUPO134) and the Austrian Science Fund (project S9308-B05). We want to thank Brigitte Jenewein and Michael Keller for the excellent technical assistance.
Section Editor: R. Effros
Contributor Information
Juliane Wolf, Email: juliane.wolf@assoc.oeaw.ac.at.
Birgit Weinberger, Email: birgit.weinberger@oeaw.ac.at.
Christoph R. Arnold, Email: christoph.arnold@oeaw.ac.at.
Andrea B. Maier, Email: A.B.Maier@lumc.nl.
Rudi G.J. Westendorp, Email: R.G.J.Westendorp@lumc.nl.
Beatrix Grubeck-Loebenstein, Email: beatrix.grubeck-loebenstein@oeaw.ac.at.
References
- Arnold C.R., Wolf J., Brunner S., Herndler-Brandstetter D., Grubeck-Loebenstein B. Gain and loss of T cell subsets in old age–age-related reshaping of the T cell repertoire. J. Clin. Immunol. 2011;31:137–146. doi: 10.1007/s10875-010-9499-x. [DOI] [PubMed] [Google Scholar]
- Browne E.P., Wing B., Coleman D., Shenk T. Altered cellular mRNA levels in human cytomegalovirus-infected fibroblasts: viral block to the accumulation of antiviral mRNAs. J. Virol. 2001;75:12319–12330. doi: 10.1128/JVI.75.24.12319-12330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J.A., Peterson T.C. Cytokine production and aging: overproduction of IL-8 in elderly males in response to lipopolysaccharide. Mech. Ageing Dev. 1994;77:127–139. doi: 10.1016/0047-6374(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Coppe J.P., Patil C.K., Rodier F., Sun Y., Munoz D.P., Goldstein J., Nelson P.S., Desprez P.Y., Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigen J.L., Yong K.L., Jordan N.J., MacCormac L.P., Westwick J., Akbar A.N., Grundy J.E. Human cytomegalovirus infection up-regulates interleukin-8 gene expression and stimulates neutrophil transendothelial migration. Immunology. 1997;92:138–145. doi: 10.1046/j.1365-2567.1997.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby I.A., Hewitson T.D. Fibroblast differentiation in wound healing and fibrosis. Int. Rev. Cytol. 2007;257:143–179. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- der Wiel A.B., van Exel E., de Craen A.J., Gussekloo J., Lagaay A.M., Knook D.L., Westendorp R.G. A high response is not essential to prevent selection bias: results from the Leiden 85-plus study. J. Clin. Epidemiol. 2002;55:1119–1125. doi: 10.1016/s0895-4356(02)00505-x. [DOI] [PubMed] [Google Scholar]
- Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr. Rev. 2007;65:S173–S176. doi: 10.1111/j.1753-4887.2007.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Franceschi C., Bonafe M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Gavazzi G., Krause K.H. Ageing and infection. Lancet Infect. Dis. 2002;2:659–666. doi: 10.1016/s1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- Grundy J.E., Lawson K.M., MacCormac L.P., Fletcher J.M., Yong K.L. Cytomegalovirus-infected endothelial cells recruit neutrophils by the secretion of C-X-C chemokines and transmit virus by direct neutrophil-endothelial cell contact and during neutrophil transendothelial migration. J. Infect. Dis. 1998;177:1465–1474. doi: 10.1086/515300. [DOI] [PubMed] [Google Scholar]
- Kahari V.M., Saarialho-Kere U. Matrix metalloproteinases in skin. Exp. Dermatol. 1997;6:199–213. doi: 10.1111/j.1600-0625.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Maier A.B., le Cessie S., de Koning-Treurniet C., Blom J., Westendorp R.G., van Heemst D. Persistence of high-replicative capacity in cultured fibroblasts from nonagenarians. Aging Cell. 2007;6:27–33. doi: 10.1111/j.1474-9726.2006.00263.x. [DOI] [PubMed] [Google Scholar]
- Mohan S.V., Liao Y.J., Kim J.W., Goronzy J.J., Weyand C.M. Giant cell arteritis: immune and vascular aging as disease risk factors. Arthritis Res. Ther. 2011;13:231. doi: 10.1186/ar3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D.N., Chang E., Whittier P.S., Choi D., Funk W.D. Microarray analysis of replicative senescence. Curr. Biol. 1999;9:939–945. doi: 10.1016/s0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- Smith R.S., Smith T.J., Blieden T.M., Phipps R.P. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am. J. Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- van den Biggelaar A.H., Huizinga T.W., de Craen A.J., Gussekloo J., Heijmans B.T., Frolich M., Westendorp R.G. Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study. Exp. Gerontol. 2004;39:1407–1414. doi: 10.1016/j.exger.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Vasto S., Candore G., Balistreri C.R., Caruso M., Colonna-Romano G., Grimaldi M.P., Listi F., Nuzzo D., Lio D., Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Mech. Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Weinberger B., Herndler-Brandstetter D., Schwanninger A., Weiskopf D., Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin. Infect. Dis. 2008;46:1078–1084. doi: 10.1086/529197. [DOI] [PubMed] [Google Scholar]
- Zhu H., Cong J.P., Mamtora G., Gingeras T., Shenk T. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14470–14475. doi: 10.1073/pnas.95.24.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]