Summary
Neuron electrical properties are critical to function and generally subtype specific, as are patterns of axonal and dendritic projections. Specification of motoneuron morphology and axon pathfinding has been studied extensively, implicating the combinatorial action of Lim-homeodomain transcription factors. However, the specification of electrical properties is not understood. Here, we address the key issues of whether the same transcription factors that specify morphology also determine subtype specific electrical properties. We show that Drosophila motoneuron subtypes express different K+ currents and that these are regulated by the conserved Lim-homeodomain transcription factor Islet. Specifically, Islet is sufficient to repress a Shaker-mediated A-type K+ current, most likely due to a direct transcriptional effect. A reduction in Shaker increases the frequency of action potential firing. Our results demonstrate the deterministic role of Islet on the excitability patterns characteristic of motoneuron subtypes.
Highlights
► Embryonic motoneurons show subtype-specific electrical properties ► Common developmental mechanisms predetermine morphology and electrical properties ► Islet suppresses A-type K+ channel expression in ventral motoneurons ► Absence of the A-type K+ channel increases action potential firing frequency
Wolfram et al. report that early expression of the Lim-homeodomain transcription factor Islet represses the Shaker-dependent fast potassium channel in developing motoneurons. This is indicative that subtype-specific electrical properties in developing neurons are set by intrinsic mechanisms.
Introduction
Diversity in neuronal signaling is critical for emergence of appropriate behavior. This diversity is reflected in dendrite morphology, axon pathfinding, choice of synaptic partners, transmitter phenotype, and cocktail of ion channels expressed by individual neurons. Many aspects of vertebrate (e.g., chick, zebrafish, and mouse) motoneuron development, including cell specification, axonal pathfinding, and neurotransmitter choice are regulated through expression of LIM-homeodomain transcription factors, including Islet1/2, Lim1/3, and Hb9 (Appel et al., 1995; Hutchinson et al., 2007; Pfaff et al., 1996; Segawa et al., 2001; Song et al., 2009; Thaler et al., 2004). Homologous proteins, and additional homeodomain (HD) proteins such as Even-skipped (Eve), serve similar functions in invertebrate motoneurons (e.g., C. elegans and Drosophila) (Certel and Thor, 2004; Esmaeili et al., 2002; Fujioka et al., 2003; Landgraf et al., 1999; Landgraf and Thor, 2006; Odden et al., 2002; Thor and Thomas, 1997, 2002). However, the extent to which neuronal electrical properties are similarly predetermined as part of cell-intrinsic developmental mechanisms remains unknown.
Neurons grown in culture often express their normal complement of both voltage- and ligand-gated ion channels (O'Dowd et al., 1988; Ribera and Spitzer, 1990; Spitzer, 1994). This suggests a significant degree of cell autonomy in the determination of electrical properties that presumably facilitates initial network formation. Once part of a circuit, however, such neurons become exposed to synaptic activity. As a result, predetermined electrical properties are modified by a variety of well-described mechanisms (Davis and Bezprozvanny, 2001; Spitzer et al., 2002). Such tuning ensures consistency of network output in response to potentially destabilizing activity resulting from Hebbian-based synaptic plasticity (Turrigiano and Nelson, 2004). The formation of functional neural circuits would seem, therefore, critically reliant on both intrinsic predetermination and subsequent extrinsic activity-dependent mechanisms to shape neuronal electrical properties. Key to understanding how intrinsic and extrinsic mechanisms are integrated will be the identification of factors that regulate predetermination.
The fruitfly, Drosophila, has been central to studies that have identified intrinsic determinants of neuronal morphology. Within the Drosophila central nervous system (CNS) the transcription factor Islet is expressed in the RP1, RP3, RP4, and RP5 motoneurons (termed ventral motoneurons, vMNs) that project to ventral muscles (Broihier and Skeath, 2002; Landgraf and Thor, 2006; Thor et al., 1999). By contrast, motoneurons projecting to dorsal muscles (e.g., aCC and RP2, termed dorsal motoneurons, dMNs) express a different homeodomain transcription factor, Even-skipped (Eve) (Broihier and Skeath, 2002; Landgraf et al., 1999). Misregulation of these transcription factors is sufficient to alter subtype-specific axonal projections (Broihier and Skeath, 2002; Landgraf et al., 1999). Thus, Eve and Islet constitute what might be considered a bimodal switch with each being deterministic for either dorsal or ventral-projecting motor axon trajectories, respectively.
Here, we report that the presence of Islet is also deterministic for expression of Shaker (Sh)-mediated outward A-type K+ current. The vMN and dMN subgroups differ in magnitude of outward K+ currents recorded by whole-cell patch clamp. We show that this difference is maintained by endogenous expression of islet in the vMNs. We also show that Islet is sufficient to repress expression of a Sh-mediated K+ current. By contrast, dMNs, which do not express islet, exhibit a robust Sh-mediated K+ current. The deterministic function of Islet is evidenced first by the fact that loss of function results in a transformation of total outward K+ current in the vMNs to mirror that present in dMNs. Second, ectopic expression of islet in dMNs or body wall muscle is sufficient to repress expression of the endogenous Sh-mediated K+ current. Thus, in addition to being sufficient to predetermine aspects of neuronal connectivity, Islet is sufficient to specify electrical properties in those neurons in which it is expressed.
Results
Dorsal and Ventral Motoneuron Subgroups Show Specific K+ Current Profiles
A crucial test of the hypothesis that Islet regulates ion channel gene expression is the demonstration that membrane electrical properties of Islet-expressing vMNs differ to those of Eve-expressing dMNs. To determine if this is true, we recorded total K+ currents from both motoneuron subtypes in first-instar larvae (1–4 hr after hatching; see Figure 1A). Motoneurons were initially identified on the basis of their medial dorsal position in the ventral nerve cord; following electrophysiological patch clamp recordings precise subtype was confirmed on the basis of axonal projection that was visualized by dye filling. We did not observe differences within either subgroups; therefore, recordings have been pooled for the vMN or dMN subtypes.
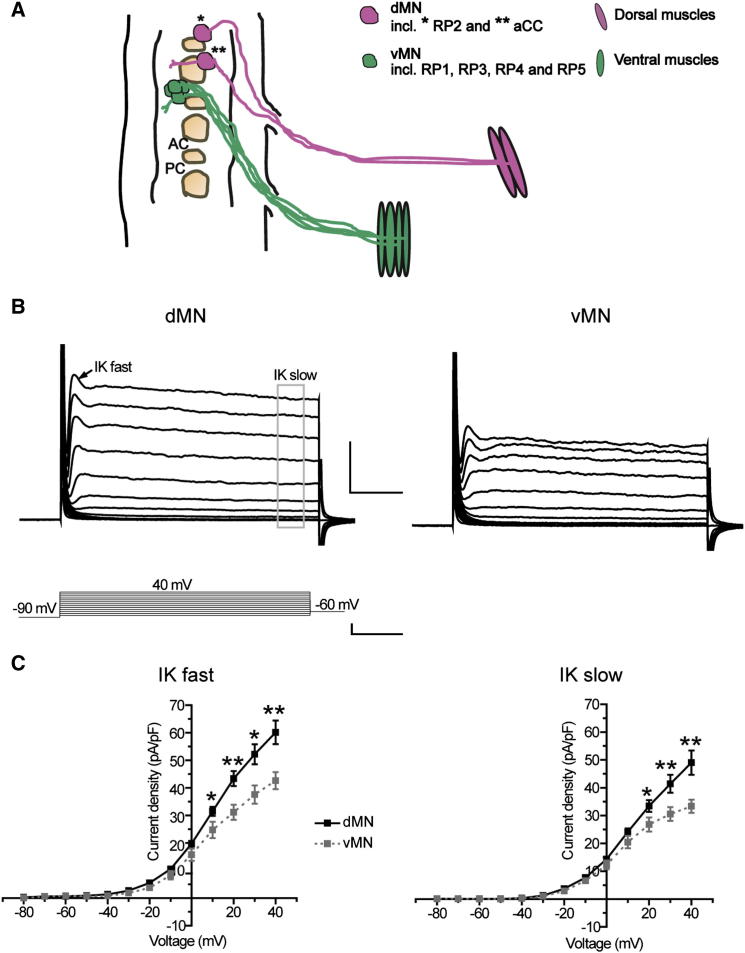
Figure 1.
Motoneurons Have Subtype-Specific K+ Current Profiles
(A) Schematic representation of dorsal and ventral motoneurons (dMNs and vMN, respectively) within the ventral nerve cord of young first-instar larvae and their muscle targets in one half segment. dMNs (magenta) comprise the two Eve positive motoneurons aCC (∗) and RP2 (∗∗), that project to dorsal muscles (magenta). vMNs (green) comprise the Islet positive motoneurons RP1, RP3, RP4, and RP5 (not individually indicated), that project to ventral muscles (green). AC, anterior commissure; PC, posterior commissure.
(B) Average total K+ current recorded from dMNs and vMNs are shown. Currents shown are the composite averages made by combining currents obtained from at least eight individual neurons that were normalized for cell capacitance. The voltage-clamp protocol (bottom trace) was −90 mV for 100 msecs prior to voltage jumps of Δ10 mV increments/50 ms duration. Two parameters are measured from the current traces: IKfast (arrow) was measured at the beginning of the response and IKslow (gray box) was measured at the end of the voltage step. Scale bars 20 pA/pF and 10 ms for currents and 50 mV/10 ms for the voltage clamp protocol.
(C) Current-voltage (IV) plots show significant differences in magnitude of IKfast and IKslow in the two motoneuron populations. Both IKfast and IKslow are larger in dMNs (black lines) compared to vMNs (gray lines). Values shown are means ± SEM (n ≥ 8).
Figure 1B shows averaged total outward K+ currents recorded from both the dMNs and vMNs. The outward K+ current is composed of a fast-activating and inactivating component, (IKfast, indicated by the arrow in Figure 1B) and a slower-activating, noninactivating component, (IKslow, indicated by the box in Figure 1B). Analyzing current densities for IKfast and IKslow (Figure 1C) shows that dMNs have significantly larger outward K+ currents compared to vMNs (Figure 1C; at holding potential of +40 mV IKfast: 60.1 ± 4.3 versus 42.6 ± 3.1 pA/pF; IKslow: 49.0 ± 4.4 versus 33.3 ± 2.4 pA/pF, dMNs versus vMNs, respectively, p ≤ 0.01). Thus, vMNs and dMNs differ in their electrical properties.
The CNS of a first-instar larva is a mature functional neural network in which synaptic transmission is active. Hence, the differences we observe in K+ currents could be established entirely due to network activity. Alternatively, subtype specificity might be determined prior to neuronal network formation and, as such, could be considered an intrinsic property of the specific motoneurons. To determine this experimentally, we repeated our analysis following complete block of synaptic transmission (i.e., absence of network activity), achieved through expressing tetanus toxin light chain (TeTxLC) throughout the entire CNS. Using the GAL41407 driver, TeTxLC was expressed pan-neuronally starting at the early neuroblast stage. Since TeTxLC-expressing embryos do not hatch, we recorded K+ currents just prior to expected hatching (at late embryonic stage 17). At this stage motoneurons have become fully functional components of the motor network (Baines and Bate, 1998). We found that IK was not significantly perturbed, in either dMNs or vMNs, by blockade of synaptic release. Moreover the difference in K+ currents between the dMNs and vMNs was maintained for both IKfast and IKslow. That differences in IK levels between dMNs and vMNs are established and maintained in the absence of synaptic release strongly suggests that they arise from intrinsic developmental mechanisms independent of evoked synaptic transmission.
Islet Determines the Electrical Properties of Ventrally Projecting Motoneurons
Drosophila larval motoneurons that project axons to ventral muscles express Islet, while those that innervate dorsal muscles express Eve. Loss of islet is sufficient to direct ventral-projecting axons dorsally and loss of eve to direct dorsal-projecting axons ventrally (Landgraf et al., 1999; Thor and Thomas, 1997). These two distinct motoneuron subtypes provide, therefore, a tractable system to test whether the differences we observe in K+ conductance is also intrinsically determined. In order to test whether Islet is able to influence K+ currents we recorded from vMNs in an islet null (−/−) mutant. This analysis indicated that Islet is sufficient to regulate K+ conductance in these motoneurons. Thus, peak current density for IKfast was significantly increased in homozygous islet−/− mutants (Figure 2A; WT 42.6 ± 3.1 versus islet 62.6 ± 5.8 pA/pF p ≤ 0.05). By contrast, IKfast in heterozygous siblings (+/−) did not differ from WT (data not shown). To better measure IKslow we inactivated IKfast by applying a −20mV prepulse (100 ms; see Baines and Bate, 1998). Figure 2B shows that loss of islet had no effect on IKslow (WT 24.2 ± 2.3 versus islet 28 ± 3.9 pA/pF p = 0.45). We also compared voltage-gated inward currents (i.e., INa and ICa) in vMNs of heterozygous islet+/− and homozygous islet−/− mutants. Loss of islet did not affect the peak current densities of either current (INa: −23.4 ± 2.7 versus −19.7 ± 2.4 and ICa: −19.69 ± 1.68 versus −21.03 ± 2.43, islet+/− versus islet−/−). Thus, loss of islet results in a selective increase in only IKfast in the vMNs.
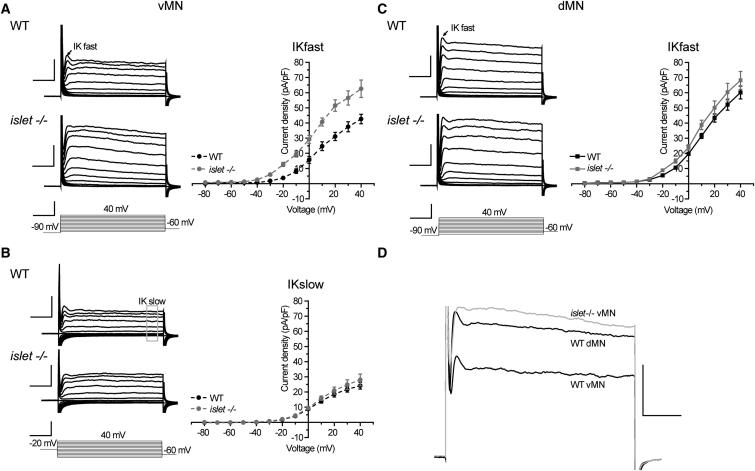
Figure 2.
Islet Regulates K+ Currents in Ventral, but Not Dorsal, Motoneurons
(A) Shows composite averaged K+ currents (representing the average from at least eight individual neurons) and respective IV plots for WT and islet−/− mutant vMNs. Voltage-clamp protocol as in Figure 1. Current density of IKfast of vMNs (obtained from a prepulse of −90mV) is significantly larger in islet−/− compared to WT at all test potentials above −40 mV.
(B) Neurons were subjected to a prepulse of −20 mV to inactivate IKfast. The remaining IKslow of vMNs is indistinguishable between islet−/− and WT.
(C) Measurement of IKfast (obtained from a prepulse of −90mV) in dMNs in islet−/− and WT are not different. Values shown are means ± SEM (n ≥ 8).
(D) Averaged responses of WT dMNs, WT vMNs, and islet−/− vMNs evoked by the highest test potential (−90 mV prepulse and +40 mV test) are superimposed. The absence of islet from vMNs increases K+ current magnitude to WT dMNs levels. Scale bars are 20 pA/pF and 10 ms for voltage-clamp responses and 100 mV/10 ms for voltage-clamp protocol.
To test for autonomy of effect, we also recorded from dMNs in the islet−/− mutant. Dorsal MNs do not express islet, and IKfast currents of WT and mutant larvae were statistically indistinguishable (Figure 2C; WT 60.1 ± 4.3 pA/pF versus islet−/− 68.2 ± 5.9 pA/pF p = 0.28). We conclude that loss of islet only affects IKfast in vMNs in which it is normally expressed, but not in dMNs that lack expression of this transcription factor. We further noted that loss of islet from the vMNs resulted in a transformation of IKfast to recapitulate the magnitude of this same current recorded in dMNs. When averaged responses of islet−/− vMNs and WT dMNs were superimposed, only small kinetic differences remain (Figure 2D). Such an observation is entirely consistent with, and indeed predictive of, the magnitude of IKfast being regulated by endogenous expression of Islet.
Islet Represses a DTx-Sensitive Current
Fast K+ currents in Drosophila neurons are encoded by one or more of at least three different genes: two voltage-gated fast-activating and inactivating channels (A-currents) termed Shal and Shaker (Sh) and a Ca2+-activated BK channel termed slowpoke (Baker and Salkoff, 1990; Elkins et al., 1986; Singh and Wu, 1990). To determine which K+ current is increased in vMNs following loss of islet, we used specific blockers of these individual currents. We first explored whether IKslowpoke is repressed by Islet. To do so we added Cd2+ to the bath solution. Cd2+ blocks Ca2+ entry and, as a consequence, prevents activation of Ca2+-activated K+ channels. Addition of Cd2+ did not diminish the increase in IKfast observed in the vMNs in islet−/− mutants (data not shown). We conclude from this that Islet does not influence IKslowpoke.
By contrast, the presence of α-Dentrotoxin (DTx), a potent and specific blocker for Sh-mediated K+ currents (Ryglewski and Duch, 2009; Wu et al., 1989), completely abolishes the increase of IKfast seen in the vMNs in islet−/− (Figure 3A; control 58.5 ± 6.9 versus DTx 43.1 ± 2.7 pA/pF p ≤ 0.05). Indeed, IKfast values obtained in the presence of DTx closely mirrored untreated WT vMNs (43.1 ± 2.7 versus 42.6 ± 3.1 p = 0.9). That DTx negates the islet−/− phenotype is consistent with Islet inhibiting a Sh-mediated K+ current in WT vMNs. To verify this prediction, we recorded IKfast in a Sh;islet double mutant. Similarly, under these conditions, peak current density of IKfast in the double mutant was indistinguishable from WT vMNs (Figure 3A; p = 0.24).
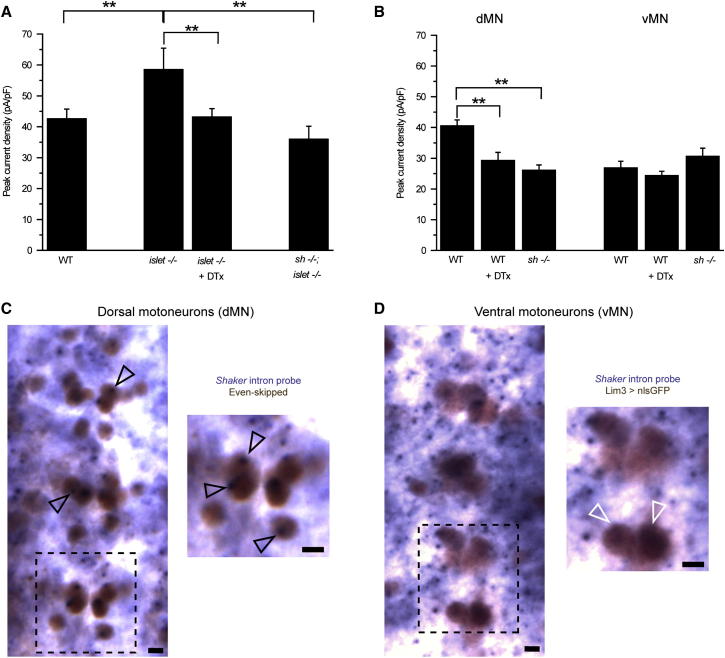
Figure 3.
Islet Expression in Ventral Motoneurons Represses a Sh-Mediated K+ Current
(A) The increase in IKfast observed in vMNs in the islet−/− mutant is effectively blocked by the presence of 200 nM DTx in the bath saline indicative that the increased K+ current is Sh mediated. This conclusion is further supported by the observation that the effect of removing islet on IKfast requires the presence of Sh; no increase is seen in a Sh;islet double mutant.
(B) The presence of DTx significantly reduces IKfast in dMNs indicative that this neuron subgroup expresses an endogenous Sh-mediated K+ current. This is confirmed by a similar reduction in IKfast observed in a Sh null mutant (Sh−/−). By contrast, IKfast is unaffected in WT vMNs either by exposure to DTx or loss of Sh. All recordings are done in the presence of Cd2+. Values shown are means ± SEM (n ≥ 8).
(C and D) In situ hybridization with Sh intron probes. Intron probes detect pre-mRNA at the site of transcription within the nucleus, but not fully processed mRNA in the cytoplasm. The black arrowheads (C) indicate staining for Sh transcript in dMN nuclei, labeled with anti-Eve. White arrowheads (D) indicate vMN nuclei, labeled with nuclear GFP, which do not express Sh. Early stage 17 embryos were analyzed. Scale bar is 5μm.
Sh Is Differentially Expressed in dMNs versus vMNs
Our data are consistent with Islet acting to repress expression of Sh in vMNs. Moreover, removal of this repression results in expression of Sh-mediated K+ channels that confer “dorsal-like” electrical properties. This model posits, therefore, that dMNs normally express a Sh-mediated K+ current.
To test this, we compared IKfast in dMNs between WT and in the presence of either DTx or in a Sh null mutant (Sh[14]). We performed these recordings in the presence of external Cd2+ to block Ca2+-activated fast K+ currents. Both acute block of Sh activity (DTx) and loss of function of Sh expression significantly reduced IKfast (Figure 3B; WT 40.5 ± 1.9 versus WT + DTx 29.3 ± 2.7 versus Sh[14] 26.1 ± 1.7 pA/pF; p ≤ 0.01 and p ≤ 0.01, respectively). Moreover, the IKfast recorded in dMNs under both conditions (WT + DTx 29.3 ± 2.7 and Sh[14] 26.1 ± 1.7 pA/pF) was indistinguishable from that of vMNs in WT (26.1 ± 2.3 pA/pF, DTx p = 0.38, Sh p = 1), which is in full agreement with our model. To further support the notion that the difference in IKfast that exists between dMNs and vMNs is due, at least in part, to expression of Sh in dMNs, we recorded IKfast in vMNs under the same conditions. As expected, neither the presence of DTx, nor loss of Sh, had any marked effect on IKfast in vMNs (p = 0.51 and 0.23, respectively; Figure 3B).
To further verify the differential expression of Sh in dMNs versus vMNs we assessed transcription of Sh in these two cell types by in situ hybridization. We designed probes that specifically recognize the Sh pre-mRNA. These intron probes label the unspliced Sh transcript at the site of transcription within the nucleus, but not the fully mature message in the cytoplasm. We detected Sh transcription in dMNs, labeled with Eve antibody (Figure 3C, black arrowheads), but not in vMNs, labeled by expression of GFP (Lim3 > nlsGFP; Figure 3D, white arrowheads). Taken together, both electrophysiology and in situ hybridization are consistent with dMNs expressing Sh while the vMNs do not.
Islet Is Both Necessary and Sufficient to Repress Sh-Mediated K+ Currents
Next, we tested whether Islet is sufficient to repress Sh-mediated K+ currents in cells where Sh, but not islet, is normally expressed. We used two different preparations for these experiments. First, we ectopically expressed islet in dMNs. Driving a UAS-islet transgene with GAL4RN2-0 significantly reduced IKfast (34.4 ± 2.6 versus 41.2 ± 1.9 pA/pF, experimental versus controls which consisted of WT and heterozygous GAL4 driver line, p ≤ 0.05; Figure 4A). These recordings were carried out in the presence of external Cd2+ to eliminate Ca2+-dependent K+ currents. The observed reduction in IKfast in dMNs could, however, be due to a reduction in either Sh- or Shal-mediated K+ currents. To distinguish between these two possibilities, we tested for DTx sensitivity, which is observed in WT dMNs and is an indicator for the presence of Sh currents. DTx sensitivity was lost when islet was ectopically expressed in dMNs (Figure 4A). In addition, when we expressed ectopic islet in dMNs in a Sh−/− background, there was no further reduction in IKfast compared to ectopic islet expression in a WT background (Figure 4A). We conclude from this that ectopic expression of islet in dMNs is sufficient to downregulate Sh-mediated IKfast.
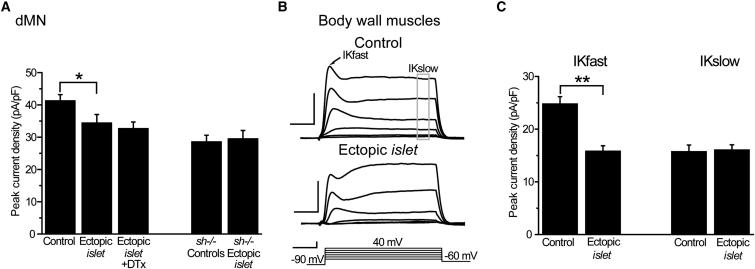
Figure 4.
Ectopic Expression of islet Is Sufficient to Reduce a Sh-Mediated K+ Current
(A) Selective expression of islet in dMNs is sufficient to significantly decrease IKfast compared to controls (average of WT and heterozygous GAL41407). Simultaneous application of DTx did not further reduce IKfast. Ectopic islet expression also had no effect on IKfast in a Sh−/− mutant. Taken together, this data is indicative that islet decreases a Sh-mediated K+ current in dMNs. Recordings were carried out in the presence of external Cd2+ to block Ca2+-activated K+ currents.
(B) Expression of islet in body wall muscle results in a significant reduction in IKfast. In low external Ca2+ (0.1 mM) IKfast in these muscles is mediated solely by Sh (see text for details). Traces show averaged composite K+ currents, obtained from at least eight individual muscle 6 recordings, in control and islet overexpression background. The prominent IKfast (arrow) of control muscles is significantly reduced when islet is ectopically expressed. Scale bar 10 pA/pF 10 ms for current and 50 mV/10 ms for voltage protocol.
(C) Averaged peak current densities of IKfast and IKslow are shown. Ectopic expression of islet significantly reduces IKfast but has no effect on IKslow. Values shown are means ± SEM (n ≥ 8).
The second preparation we used takes advantage of the fact that IKfast in body wall muscle is solely due to Sh and Slowpoke (the latter of which can be easily blocked [Singh and Wu, 1990]). We recorded from muscle 6 in abdominal segments 3 and 4 in first-instar larvae. To remove the IKslowpoke component and hence isolate the Sh-mediated IKfast, recordings were done in low calcium (0.1 mM) external saline. Figure 4B depicts the averaged responses from voltage-clamp recordings in control muscle (heterozygous GAL424B driver, upper trace) and muscle expressing islet (lower trace). Peak current densities of IKfast (entirely due to Sh-mediated K+ current) and the slow noninactivating currents recorded at +40 mV are shown in Figure 4C. Ectopic expression of islet in muscle is sufficient to produce a significant reduction in IKfast (control 26.6 ± 2.4 versus 24B > islet 15.8 ± 1.0 pA/pF, p ≤ 0.01) while no effect was seen on the slow current. Thus, expression of islet in dMNs is sufficient to reduce a DTx-sensitive component of IKfast. Similar expression in muscle clearly demonstrates that Islet is sufficient to downregulate a Sh-mediated fast K+ current.
Islet Binds Directly to the Sh Locus
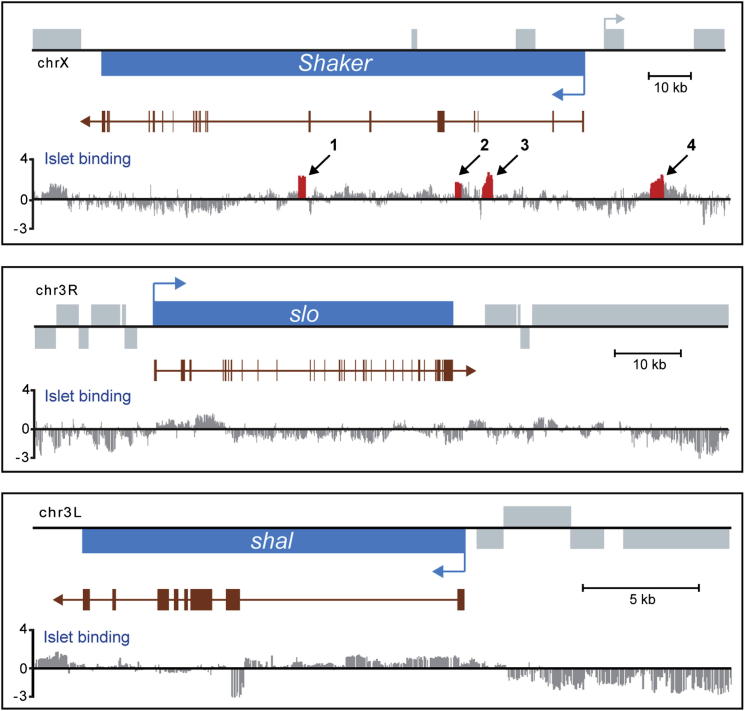
Our electrophysiology indicates that Islet is able to repress Sh-mediated K+ current. To identify putative targets of Islet we used DamID, a well-accepted technique for demonstrating direct binding to chromatin or DNA in vivo (Choksi et al., 2006; Filion et al., 2010; Southall and Brand, 2009; van Steensel and Henikoff, 2000). Our analysis identifies 1,769 genes (exhibiting one or more peaks of Islet binding within 5 kb of the transcriptional unit) as direct targets of Islet (FDR < 0.1%). Consistent with our model of Islet regulating a Sh-mediated K+ current, we find three significant binding sites within introns of the Sh locus (arrows 1 to 3 in Figure 5). Intragenic binding of transcription factors is common in both vertebrates (Robertson et al., 2007) and invertebrates (Southall and Brand, 2009). A fourth significant peak is found upstream of Sh (arrow 4 in Figure 5). Binding of Islet at this site could regulate the expression of either Sh and/or CG15373 an adjacent, divergently transcribed, gene. By contrast, Shal and slowpoke, which also encode fast neuronal K+ currents, were not identified as putative targets (Figure 5). Thus, these data show that Islet binds to the Sh locus and is likely to regulate transcription of the Sh gene directly.
Figure 5.
DamID Demonstrates Direct Binding of Islet to the Sh Locus In Vivo
Of the genes encoding the three known fast K+ current channels in Drosophila, Islet binds to Sh, but not Slo or Shal. The transcription units of Sh, Slo, and Shal are shown in blue with blue arrows indicating the direction of transcription. Grey vertical bars indicate the position of oligonucleotide probes on the genomic microarray. Bar heights show the average of normalized log2-transformed ratios from 3 independent DamID experiments with those in red, and indicated with arrows, showing a significant peak within the data set (FDR < 0.1%). The Sh, Slo, and Shal transcripts are shown in brown with vertical bars/boxes representing exons. Additional transcription units within the region are shown as gray boxes.
To confirm that Islet binds Sh and regulates its transcription, we used qRT-PCR to quantify levels of Sh transcripts. We compared Sh transcript levels in larval CNS between control, islet−/− and panneuronal islet expression (1407 > islet). In comparison to control, the absence of islet−/− resulted in a 27% increase in Sh (1.27 ± 0.01, n = 2, p < 0.05). By contrast, panneuronal expression of transgenic islet resulted in a 45% decrease in Sh transcript (0.45 ± 0.06, n = 2, p < 0.05). We also measured Sh transcript level in body wall muscle following ectopic expression of islet (24B > islet). Similar to the CNS, Sh transcripts were reduced by 31% relative to control (0.31 ± 0.01, n = 2, p < 0.05). Taken together with the results obtained by DamID, this strongly suggests that Islet binds to, and represses transcription of, the Sh gene.
Sh Regulates Action Potential Frequency
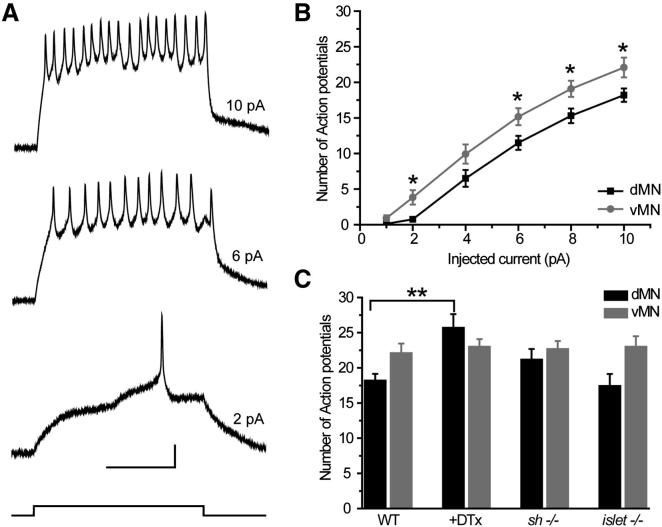
Voltage-dependent K+ currents, such as those mediated by Sh, contribute to setting membrane excitability (and thus the ability to fire action potentials) (Goldberg et al., 2008; Peng and Wu, 2007). These currents are therefore critical for network function and the generation of appropriate behaviors (Smart et al., 1998). It has been shown that modulation of Sh-mediated current, using dominant-negative transgenes, can bring about significant changes in excitability (Mosca et al., 2005). We were interested in whether and how excitability differs between motoneurons that express a Sh-mediated K+ current (dMNs) and those that do not (vMNs). We recorded excitability in current clamp. Typical responses are shown in Figure 6A. We found that dMNs fired significantly fewer action potentials than vMNs at most current steps (Figure 6B; 10 pA: 18.2 ± 0.9 versus 22.1 ± 1.4 p = 0.04; 8 pA: 15.3 ± 1.0 versus 19.1 ± 1.1 p = 0.02; 6 pA: 11.5 ± 1.0 versus 15.2 ± 1.2 p = 0.04; 4 pA: 6.5 ± 1.2 versus 9.9 ± 1.4 p = 0.09; 2 pA: 0.8 ± 0.3 versus 3.8 ± 1.0 p = 0.03; 1 pA: 0.1 ± 0.1, versus 0.9 ± 0.4: p = 0.13; dorsal versus ventral, respectively). The above results suggest that the Sh-mediated K+ current (expressed only in dMNs) reduces action potential (APs) firing when present.
Figure 6.
Membrane Excitability Differs between dMNs and vMNs
(A) Example of a whole cell current clamp recordings obtained from a dMN (aCC). Responses to 500 ms depolarizing current pulses of 2, 6, and 10 pA are shown. An example of a current step is shown underneath the responses. Scale bar is 10 mV/200 ms.
(B) Number of action potentials fired per 500 ms current step by dMNs and vMNs are plotted against the amplitude of injected current. dMNs fire significantly less action potentials than vMNs at most current steps.
(C) Number of action potentials evoked by a 10 pA current injection. WT dMNs fire significantly less action potentials than vMNs. Removal of Sh-dependent K+ current by DTx or Sh−/− increases action potential firing in dMNs to levels seen in vMNs. Action potential firing in vMNs remains unaffected. Removal of islet (islet−/−) also has no effect on firing in either dMns or vMNs. Values shown are means ± SEM (n ≥ 8).
To validate this conclusion, we reduced Sh current in dMNs acutely by adding DTx to the bath and recorded AP firing. AP firing increased from 18.2 ± 0.9 APs (WT) to 25.7 ± 1.9 APs (DTx, p < 0.05; Figure 6C). A similar result, although not significant, was obtained when APs were recorded from dMNs in a Sh mutant (18.2 ± 0.9 to 21.2 ± 1.5 APs, p = 0.07; Figure 6C). Indeed, in both treatments, firing rates between dMNs and vMNs were indistinguishable (Sh−/− 21.2 ± 1.5 versus 22.7 ± 1.1; DTx 25.7 ± 1.9 versus 23.0 ± 1.8 APs, dMNs versus vMNs respectively, p > 0.05; Figure 6C). As predicted, vMN excitability was not affected by either DTx or loss of Sh (22.1 ± 1.4 versus 23.0 ± 1.8 versus 22.7 ± 1.1, WT, DTX, Sh−/−, respectively, p > 0.05; Figure 6C). Perhaps unexpectedly, the increase in IKfast in vMNs, which results from the loss of islet, did not influence AP firing. Loss of islet also had no effect on APs fired in dMNs which is predictable because dMNs do not express this protein (Figure 6C). Finally, determination of AP firing in a Sh;islet double loss of function mutant revealed no additional effects: AP firing is increased in dMNs and unaffected in vMNs (data not shown). Why loss of islet, which increases IKfast in vMNs, does not influence AP firing in these neurons is unknown, but may be indicative of additional homeostatic mechanisms.
Discussion
Diversity in neuronal electrical properties is dictated by the type, location, and number of ion channels expressed in individual neurons. While activity-dependent mechanisms that act to adjust these properties in mature neurons have been studied in detail (Davis and Bezprozvanny, 2001; Spitzer et al., 2002), the mechanisms that specify electrical properties in embryonic neurons, prior to network formation, are not understood. These mechanisms are, however, likely to be part of cell-intrinsic programs of specification. The demonstration of differential expression of transcription factors between neuronal cell types underpins the proposal of a combinatorial code sufficient to determine key aspects of neuron specification, including axon guidance and neurotransmitter phenotype (Polleux et al., 2007; Shirasaki and Pfaff, 2002; Thor and Thomas, 2002). However, whether these same factors are sufficient to set cell-specific electrical characteristics remains unknown.
A wealth of studies on motoneuron specification, from flies to mammals, has shown that early developmental decisions, such as subclass identity, is dictated, at least in part, by a code of transcription factors (Dasen et al., 2005, 2008; De Marco Garcia and Jessell, 2008; Landgraf et al., 1999; Landgraf and Thor, 2006; Thor and Thomas, 1997). With its relatively simple CNS and powerful molecular genetics, Drosophila has been central to these studies. Embryonic Drosophila motoneurons express a stereotypic mix of identified transcription factors which are evolutionary conserved with mammals (Thaler et al., 1999, 2002; Thor and Thomas, 1997). Motoneurons which predominantly innervate ventral muscles express islet, Lim3, and dHb9. Motoneurons which project dorsally express eve (Landgraf et al., 1999; Landgraf and Thor, 2006; Thor and Thomas, 1997). A first indication that ion channel genes may also be targets of these transcription factors was provided by our demonstration that overexpression of eve was sufficient to alter the outward voltage-gated K+ current through transcriptional repression of slowpoke (encoding a BK Ca2+-activated K+ channel) in Drosophila motoneurons (Pym et al., 2006). However, while a common developmental regulation of neuronal morphology and function, at least in motoneurons, might be inferred from this study, only Eve-positive cells were investigated. This leaves open the question, whether Eve, or for that matter any of the other transcription factors, is deterministic for specific membrane currents.
The principle of duality in role for transcription factors such as Eve and Islet is significant because it is predictive that neuron morphology and electrical signaling are, at least in part, determined by common developmental mechanisms. Studies of vertebrate homologs of these transcription factors, widespread in the mammalian CNS, provide additional support for such a scenario. For example, Islet-1 and Islet-2 are known to regulate neuron identity, axonal guidance and choice of neurotransmitter in vertebrate CNS (Hutchinson and Eisen, 2006; Segawa et al., 2001; Thaler et al., 2004). Associated microarray analysis on murine mutant tissue identifies ion channels as putative targets of Islet-1, including Shal-related K+ channel Kcnd2 and Na+ channel Nav1.8. Regulation of expression has, however, yet to be demonstrated (Sun et al., 2008). It is conceivable that in zebrafish recently reported differences in outward K+ currents between two embryonic motoneurons, dorsal MiP and ventral CaP (Moreno and Ribera, 2009), may be regulated by the differential expression of Islet1/2 in these neurons (Appel et al., 1995).
We provide substantial evidence that differential expression of islet in vMNs versus dMNs is critical for determining subtype-specific differences in Sh-mediated K+ currents. Because these Sh-mediated K+ currents regulate action potential frequency, they will contribute to network function. Comparable to our findings in Drosophila, in both the mouse cochlea and cortex, neurons that fire only a small number of action potentials to a given current pulse (termed rapidly adapting) express a DTx-sensitive Kv1 (Sh-like) K+ current. By contrast, neurons that fire many action potentials (slowly adapting) do not. The firing pattern of rapidly adapting neurons can be transformed into that of slowly adapting neurons by application of the Sh-specific blocker DTx (Miller et al., 2008). Our own data are consistent with such a role for Sh because we show that dMNs which express Sh, fire fewer action potentials than vMNs. Moreover, the number of action potentials fired by dMNs is increased by genetic or pharmacological block of the Sh-mediated K+ current. We envisage, therefore, that regulation of action potential firing, through Islet-mediated transcriptional control of a Sh-like K+ current, might be well conserved.
While the presence of early factors able to regulate ion-channel gene expression is predictive of predetermination of electrical signaling properties in embryonic neurons, a challenge remains to understand how individual neurons decode this information. In the Drosophila ventral nerve cord, we find that the presence or absence of a Sh-mediated K+ current is determined by whether islet is expressed or not. Thus, Islet seems to act as a binary switch; when present it prevents expression of Sh and vice versa. However, it seems unlikely that all combinatorial factors act in this way. For example, the activity of Eve seems to be related to its relative level of expression, since endogenous Eve only partially represses transcription of slowpoke (a Ca2+-dependent K+ channel) in the dorsal motoneuron aCC (Pym et al., 2006). It remains to be determined whether efficacy of regulatory activity is specific to individual transcription factors or to target genes.
We show here that the Lim-homeodomain transcription factor Islet forms part of an intrinsic “decision-making” process that is critical to specifying subtype-specific electrical properties in developing motoneurons. It might be argued that input from pre- and postsynaptic partners is involved in setting early electrophysiological differences between neurons. Indeed such inputs play a pivotal role during axonogenesis and synapse development. Blocking all synaptic transmission showed that neural network activity is not required to establish early electrophysiological differences between motoneuron subgroups. Motoneurons also receive instructive cues from their postsynaptic muscle targets during NMJ development (Fitzsimonds and Poo, 1998). In this regard it is significant that the difference in IKfast we observe between dMNs and vMNs is abolished in a myosin heavy chain mutant (mhc1) that fails to produce contractile muscles. Indeed, IKfast is decreased in dMNs to the level seen in WT vMNs (V.W. and R.A.B., unpublished observations). This is, perhaps, indicative that the dMNs require an instructive signal from their muscle targets in order to follow a different path of electrical development. Whether this path suppresses islet expression in dMNs remains to be determined. Significantly, vMNs were not affected in the Mhc1 mutant suggesting that repression of Sh-dependent IK by Islet is independent of muscle derived input.
Why do motoneurons differ in their electrical properties and what is the functional implication? dMNs and vMNs receive differential synaptic drive (Baines et al., 2002) and innervate distinct muscle targets, dorsal obliques and ventral longitudinals, respectively (Landgraf et al., 1997). During larval crawling ventral muscles are recruited prior to dorsal muscles (Fox et al., 2006) to, probably, facilitate coordinated movement. Interestingly, synaptic strength, based on EJP amplitude, is largest between vMNs and their target muscles. While the precise underlying mechanism is unknown, pharmacology suggests that terminals of dMNs express a larger Sh-dependent K+ current compared to vMNs. This current disproportionately reduces presynaptic neurotransmitter release and hence regulates synaptic strength (Lee et al., 2008). Whether this alone can account for the delay of dorsal muscle contraction is not known. Differences in electrical properties, specifically delay to first spike, have also been observed between Drosophila motoneurons (Choi et al., 2004). While the precise reasons for these differences remain speculative, they are consistent with differential contribution to muscle activity that underlies locomotion in Drosophila larvae.
We can recapitulate the repressive effect of ectopic islet expression on Sh-mediated K+ current in body wall muscle. This is important for two reasons. First, it provides unequivocal support for the hypothesis that Islet is deterministic for expression of Sh in excitable cells, regardless of whether those cells are neurons or muscle. Second, body wall muscles are isopotential and do not therefore suffer from issues of space clamp (Broadie and Bate, 1993). Analysis of ionic currents in neurons can be complicated by such factors, which becomes more serious for analysis of those currents located further away from the cell body in the dendritic arbor. Hence electrophysiological-tractable muscles may offer the possibility to derive a more complete understanding of the differential activity of codes of transcription factors on the regulation of ion channel development within the developing nervous system.
Experimental Procedures
Fly Stocks
For larval collections, flies were transferred into laying pots and allowed to lay eggs onto grape juice agar plates. Laying pots were kept at 25°C and 18°C for motoneuron and muscle experiments, respectively. The following fly strains were used: Canton-S as wild-type (WT), islet mutant tup[isl-1] rdo[1] hk[1] pr[1]/Cyo act::GFP (rebalanced from Bloomington 3556), Shaker mutant Sh[14] (Bloomington 3563, carries the KS133 mutation). The Shaker and islet mutations were combined in a double mutant Sh[14];tup[Isl-1]/CyO act::GFP. The islet mutants and Sh;islet double mutants are embryonic lethal; however, a few homozygous escapers are viable up until the first-instar larval stage. Transgenes were expressed in a tissue-specific manner using the GAL4/UAS system (Brand and Perrimon, 1993). The driver line GAL41407 (homozygous viable on the second chromosome) was used to express UAS containing transgenes carrying the active (UAS-TNT-G) or inactive (UAS-TNT-VF) form of tetanus toxin light chain (TeTxLC) in all CNS neurons (Sweeney et al., 1995). GAL4Lim3 was used to express GFP in vMNs for in situ hybridization. GAL4RN2-0 (homozygous viable on the second chromosome) or GAL4RRa (homozygous viable on the 3rd chromosome) were used to express islet (UAS-islet x2) in dMNs. GAL424B (homozygous viable on the second chromosome) was used to express islet (UAS-islet x2) body wall muscle. The dMN driver GAL4RRa as well as the UAS-islet construct were crossed into the Sh[14] mutant background.
Embryo and Larval Dissection
Newly hatched larvae or late stage 17 embryos were dissected and central neurons were accessed for electrophysiology as described by Baines and Bate (1998). For muscle recordings newly hatched larvae were dissected as for CNS electrophysiology, but the CNS was removed. The muscles were treated with 1 mg/ml collagenase (Sigma) for 0.5 to 1 min prior to whole cell patch recording. Larvae were visualized using a water immersion lens (total magnification, 600×) combined with DIC optics (BX51W1 microscope; Olympus Optical, Tokyo, Japan).
Electrophysiology
Recordings were performed at room temperature (20°C to 22°C). Whole-cell recordings (current and voltage clamp) were achieved using borosilicate glass electrodes (GC100TF-10; Harvard Apparatus, Edenbridge, UK), fire-polished to resistances of between 15 - 20 MΩ for neurons and between 5 and 10 MΩ for muscles.
Neurons were identified based on their position within the ventral nerve cord. Neuron type was confirmed after recording by filling with 0.1% Alexa Fluor 488 hydrazyde sodium salt (Invitrogen), which was included in the internal patch saline. Recordings were made using a Multiclamp 700B amplifier controlled by pClamp 10.2 (Molecular Devices, Sunnyvale, CA). Only neurons with an input resistance > 1 GΩ were accepted for analysis. Traces were sampled at 20 kHz and filtered at 2 kHz. The voltage-clamp protocols used to record total K+ currents were as follows: for neurons, from the resting potential of −60 mV neurons were hyperpolarized to −90 mV for 100 ms, the voltage was then stepped from −80 mV to +40 mV in increments of Δ10 mV for 50 ms. To isolate slow K+ currents a prepulse of −20mV for 100 ms was used (Baines and Bate, 1998). For muscles a maintained holding potential of −60 mV was used and a −90 mV prepulse for 200 ms and voltage jumps of Δ20 mV increments were applied from −40 to +40 mV. Leak currents were subtracted off-line for central neuron recordings. For muscle recordings, however, on line leak subtraction (P/4) was used. Recordings were done in at least four animals and at least eight neurons/muscles were recorded from in total for each experiment. Individual recordings were averaged, following normalization relative to cell capacitance, to produce one composite average representative of that group of recordings. Cell capacitance was determined by integrating the area under the capacity transients evoked by stepping from −60 to −90 mV (checked before and after recordings).
Membrane excitability (i.e., action potential firing) was determined using injection of depolarizing current (1, 2, 4, 6, 8, 10 pA/500 ms) from a maintained membrane potential (Vm) of −60 mV. Vm was maintained at −60 mV by injection of a small amount of hyperpolarizing current.
Solutions
Motoneuron Recordings
External saline for dissection and current clamp analysis of excitability consisted of the following (in mM): 135 NaCl, 5 KCl, 4 MgCl2·6H2O, 2 CaCl2·2H2O, 5 N-Tris [hydroxymethyl]methyl-2-aminoethanesulfonic acid (TES), 36 sucrose, pH 7.15. For isolation of total K+ currents 1 μM TTX (Alomone Labs, Jerusalem, Israel) was added to the external solution. For most recordings Ca2+-activated K+ currents were eliminated by adding Cd2+ (0.2 mM) to the saline. Sh-mediated K+ current was blocked using dendrotoxin (DTx, Sigma, 200 nM). Current clamp recordings were done in the presence of mecamylamine (1 mM, Sigma) to block endogenous cholinergic synaptic currents. Internal patch solution consisted of (in mM): 140 K+ gluconate, 2 MgCl2·6H2O, 2 EGTA, 5 KCl, and 20 HEPES, pH 7.4.
Muscle Recordings
External saline (Stewart et al., 1994) for dissection and voltage-clamp analysis consisted of the following (in mM): 70 NaCl, 5 KCl, 0.1 CaCl2, 20 MgCl2·6H2O, 10 NaHCO3, 5 HEPES, 115 sucrose, 5 trehalose (pH 7.2). The calcium concentration was kept low (0.1 mM) to prevent activation of Ca2+-dependent K+ currents. Internal patch saline was the same as for neurons.
In Situ Hybridization
In situ hybridization was performed as previously described (Choksi et al., 2006), using a hybridization temperature of 65°C. Five separate probes were generated to target an intron of Sh common to all splice isoforms (second intron of Sh-RB). The probes were equally mixed before use. The primers used to generate the RNA probes are as follows:
Sh_int1_FW (CTTCTTTCTTGGATTGAAGGACAT), Sh_int1_RVT7 (CAGTAATACGACTCACTATTATAATGCAACAAAATTGAAGCAGAT), Sh_int2_FW (TAGGCATCATTGCACTGTCTTATT), Sh_int2_RVT7 (CAGTAATACGACTCACTATTATAGTAGCCACTCTGAGCACTATGG),
Sh_int3_FW (CACTTTGAGAGTCCTGCAGTTTTA), Sh_int3_RVT7 (CAGTAATACGACTCACTATTATTTGGGTCATTTGTCAAACATATC),
Sh_int4_FW (GCCAAAGAAAACGTGTTAAAATCT), Sh_int4_RVT7 (CAGTAATACGACTCACTATTAGTACCAAGTTTGTTTTTGCATCTG),
Sh_int5_FW (AAAGCAATTCAAGGCACTAAAATC), Sh_int5_RVT7 (CAGTAATACGACTCACTATTAGCTATTTGAAACTTTTCGTCGTTT).
Immunohistochemistry was performed after the in situ protocol using an anti-Eve antibody (1:5,000; Frasch et al., 1987) or an anti-GFP antibody (1:2,000; Abcam ab6556) and developed using DAB.
Real-Time RT-PCR
Muscle tissue and CNS were collected from newly hatched larvae or late stage 17 embryos. Between 100 and 180 animals were dissected for each genotype. Following RNA extraction (QIAGEN RNaesy Micro kit) cDNA was synthesized using the Fermentas Reverse Aid H minus First strand cDNA synthesis kit, according to the manufacturer's protocol. RNA concentration was matched for control and experimental sample prior cDNA synthesis. qPCR was performed on the Roche LightCycler 1.5 (Roche, Lewes, UK) using the Roche LightCycler FastStart DNA Master SYBR Green reaction mix. The thermal profile used was 10 min at 95°C followed by 40 cycles of 10 s at 95°C, followed by 4 s at 59°C, and finally 30 s at 72°C. Results were recorded using the delta delta Ct method and are expressed as Fold difference compared to control (isl−/− compared to isl+/−, 1407 > islet to 1407 > GFP, 24 B > islet to 24B > GFP). Ct values used were the means of duplicate replicates. Experiments were repeated twice. PCR primers (forward and reverse primers in 5′ to 3′ orientation) were as follows: rp49 CTAAGCTGTCGCACAAATGG and GGAACTTCTTGAATCCGGTG; Sh CAACACTTTGAACCCATTCC and CAAAGTACCGTAATCTCCGA.
DamID Analysis
A pUASTattB-NDam vector was created (to allow integration of the Dam transgene into a specific site) by cloning the Dam-Myc sequence from pNDamMyc (van Steensel and Henikoff, 2000) into the multiple cloning site of pUASTattB (Bischof et al., 2007) using EcoRI and BglII sites. The full-length coding sequence of islet was PCR amplified from an embryonic cDNA library and cloned into pUASTattB-NDam using BglII and NotI sites. Transgenic lines were generated by injecting pUASTattB-NDam (control line) and pUASTattB-NDam-islet constructs (at 100ng/μl) into ΦX-22A (with phiC31 expressed in the germline and a docking site at 22A) blastoderm embryos (Bischof et al., 2007). Preparation of Dam-methylated DNA from stage 17 embryos was performed as previously described (Pym et al., 2006). The Dam-only and Dam-islet samples were labeled and hybridized together on a whole genome 2.1 million feature tiling array, with 50- to 75-mer oligonucleotides spaced at approximately 55 bp intervals (Nimblegen systems). Arrays were scanned and intensities extracted (Nimblegen Systems). Three biological replicates (with one dye-swap) were performed. Log2 ratios of each spot were median normalized.
A peak finding algorithm with false discovery rate (FDR) analysis was developed to identify significant binding sites (PERL script available on request). All peaks spanning 8 or more consecutive probes (>∼900 bp) over a 2-fold ratio change were assigned a FDR value. To assign a FDR value, the frequency of a range of small peak heights (from 0.1 to 1.25 log2 increase) were calculated within a randomized data set (for each chromosome arm) using 20 iterations for each peak size. This was repeated for a range of peak widths (6 to 15 consecutive probes). All of these data were used to model the exponential decay of the FDR with respect to increasing peak height and peak width, therefore enabling extrapolation of FDR values for higher and broader peaks. This analysis was performed independently for each replicate data set. Each peak was assigned the highest FDR value from the 3 replicates. Genes were defined as targets where a binding event (with a FDR < 0.1%) occurred within 5 kb of the transcriptional unit (depending on the proximity of adjacent genes).
Statistics
Statistical significance was calculated using a nonpaired t test with a confidence interval of p ≤ 0.05 (∗) and ≤ 0.01 (∗∗). All quantitative data shown are means ± SEM.
Acknowledgments
We would like to thank Drs. J Jaynes, M. Fujioka, K. Koh, J. Skeath, and S. Thor for providing flies and Matthias Landgraf for comments on the manuscript. This study was funded by grants from the Wellcome Trust to R.A.B. (083837 and 090798) and AHB (programme grants 068055 and 092545). A.H.B. acknowledges the core funding provided by the Wellcome Trust (092096) and CRUK (C6946/A14492). Work on this project benefited from the Manchester Fly Facility, established through funds from the University of Manchester and the Wellcome Trust (087742).
Published: August 22, 2012
References
- Appel B., Korzh V., Glasgow E., Thor S., Edlund T., Dawid I.B., Eisen J.S. Motoneuron fate specification revealed by patterned LIM homeobox gene expression in embryonic zebrafish. Development. 1995;121:4117–4125. doi: 10.1242/dev.121.12.4117. [DOI] [PubMed] [Google Scholar]
- Baines R.A., Bate M. Electrophysiological development of central neurons in the Drosophila embryo. J. Neurosci. 1998;18:4673–4683. doi: 10.1523/JNEUROSCI.18-12-04673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines R.A., Seugnet L., Thompson A., Salvaterra P.M., Bate M. Regulation of synaptic connectivity: levels of Fasciclin II influence synaptic growth in the Drosophila CNS. J. Neurosci. 2002;22:6587–6595. doi: 10.1523/JNEUROSCI.22-15-06587.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K., Salkoff L. The Drosophila Shaker gene codes for a distinctive K+ current in a subset of neurons. Neuron. 1990;4:129–140. doi: 10.1016/0896-6273(90)90449-p. [DOI] [PubMed] [Google Scholar]
- Bischof J., Maeda R.K., Hediger M., Karch F., Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Broadie K.S., Bate M. Development of larval muscle properties in the embryonic myotubes of Drosophila melanogaster. J. Neurosci. 1993;13:167–180. doi: 10.1523/JNEUROSCI.13-01-00167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broihier H.T., Skeath J.B. Drosophila homeodomain protein dHb9 directs neuronal fate via crossrepressive and cell-nonautonomous mechanisms. Neuron. 2002;35:39–50. doi: 10.1016/s0896-6273(02)00743-2. [DOI] [PubMed] [Google Scholar]
- Certel S.J., Thor S. Specification of Drosophila motoneuron identity by the combinatorial action of POU and LIM-HD factors. Development. 2004;131:5429–5439. doi: 10.1242/dev.01418. [DOI] [PubMed] [Google Scholar]
- Choi J.C., Park D., Griffith L.C. Electrophysiological and morphological characterization of identified motor neurons in the Drosophila third instar larva central nervous system. J. Neurophysiol. 2004;91:2353–2365. doi: 10.1152/jn.01115.2003. [DOI] [PubMed] [Google Scholar]
- Choksi S.P., Southall T.D., Bossing T., Edoff K., de Wit E., Fischer B.E., van Steensel B., Micklem G., Brand A.H. Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Dev. Cell. 2006;11:775–789. doi: 10.1016/j.devcel.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Dasen J.S., Tice B.C., Brenner-Morton S., Jessell T.M. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Dasen J.S., De Camilli A., Wang B., Tucker P.W., Jessell T.M. Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell. 2008;134:304–316. doi: 10.1016/j.cell.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Davis G.W., Bezprozvanny I. Maintaining the stability of neural function: a homeostatic hypothesis. Annu. Rev. Physiol. 2001;63:847–869. doi: 10.1146/annurev.physiol.63.1.847. [DOI] [PubMed] [Google Scholar]
- De Marco Garcia N.V., Jessell T.M. Early motor neuron pool identity and muscle nerve trajectory defined by postmitotic restrictions in Nkx6.1 activity. Neuron. 2008;57:217–231. doi: 10.1016/j.neuron.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins T., Ganetzky B., Wu C.F. A Drosophila mutation that eliminates a calcium-dependent potassium current. Proc. Natl. Acad. Sci. USA. 1986;83:8415–8419. doi: 10.1073/pnas.83.21.8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili B., Ross J.M., Neades C., Miller D.M., 3rd, Ahringer J. The C. elegans even-skipped homologue, vab-7, specifies DB motoneurone identity and axon trajectory. Development. 2002;129:853–862. doi: 10.1242/dev.129.4.853. [DOI] [PubMed] [Google Scholar]
- Filion G.J., van Bemmel J.G., Braunschweig U., Talhout W., Kind J., Ward L.D., Brugman W., de Castro I.J., Kerkhoven R.M., Bussemaker H.J., van Steensel B. Systematic protein location mapping reveals five principal chromatin types in Drosophila cells. Cell. 2010;143:212–224. doi: 10.1016/j.cell.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimonds R.M., Poo M.-M. Retrograde signaling in the development and modification of synapses. Physiol. Rev. 1998;78:143–170. doi: 10.1152/physrev.1998.78.1.143. [DOI] [PubMed] [Google Scholar]
- Fox L.E., Soll D.R., Wu C.-F. Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine beta hydroxlyase mutation. J. Neurosci. 2006;26:1486–1498. doi: 10.1523/JNEUROSCI.4749-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch M., Hoey T., Rushlow C., Doyle H., Levine M. Characterization and localization of the even-skipped protein of Drosophila. EMBO J. 1987;6:749–759. doi: 10.1002/j.1460-2075.1987.tb04817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka M., Lear B.C., Landgraf M., Yusibova G.L., Zhou J., Riley K.M., Patel N.H., Jaynes J.B. Even-skipped, acting as a repressor, regulates axonal projections in Drosophila. Development. 2003;130:5385–5400. doi: 10.1242/dev.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg E.M., Clark B.D., Zagha E., Nahmani M., Erisir A., Rudy B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron. 2008;58:387–400. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson S.A., Eisen J.S. Islet1 and Islet2 have equivalent abilities to promote motoneuron formation and to specify motoneuron subtype identity. Development. 2006;133:2137–2147. doi: 10.1242/dev.02355. [DOI] [PubMed] [Google Scholar]
- Hutchinson S.A., Cheesman S.E., Hale L.A., Boone J.Q., Eisen J.S. Nkx6 proteins specify one zebrafish primary motoneuron subtype by regulating late islet1 expression. Development. 2007;134:1671–1677. doi: 10.1242/dev.02826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf, M., and Thor, S. (2006). Development and structure of Motoneurons. In International Review of Neurobiology (San Diego, CA: Academic Press), pp. 33-53. [DOI] [PubMed]
- Landgraf M., Bossing T., Technau G.M., Bate M. The origin, location, and projections of the embryonic abdominal motorneurons of Drosophila. J. Neurosci. 1997;17:9642–9655. doi: 10.1523/JNEUROSCI.17-24-09642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf M., Roy S., Prokop A., VijayRaghavan K., Bate M. even-skipped determines the dorsal growth of motor axons in Drosophila. Neuron. 1999;22:43–52. doi: 10.1016/s0896-6273(00)80677-7. [DOI] [PubMed] [Google Scholar]
- Lee J., Ueda A., Wu C.F. Pre- and post-synaptic mechanisms of synaptic strength homeostasis revealed by slowpoke and shaker K+ channel mutations in Drosophila. Neuroscience. 2008;154:1283–1296. doi: 10.1016/j.neuroscience.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.N., Okaty B.W., Nelson S.B. Region-specific spike-frequency acceleration in layer 5 pyramidal neurons mediated by Kv1 subunits. J. Neurosci. 2008;28:13716–13726. doi: 10.1523/JNEUROSCI.2940-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno R.L., Ribera A.B. Zebrafish motor neuron subtypes differ electrically prior to axonal outgrowth. J. Neurophysiol. 2009;102:2477–2484. doi: 10.1152/jn.00446.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca T.J., Carrillo R.A., White B.H., Keshishian H. Dissection of synaptic excitability phenotypes by using a dominant-negative Shaker K+ channel subunit. Proc. Natl. Acad. Sci. USA. 2005;102:3477–3482. doi: 10.1073/pnas.0406164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd D.K., Ribera A.B., Spitzer N.C. Development of voltage-dependent calcium, sodium, and potassium currents in Xenopus spinal neurons. J. Neurosci. 1988;8:792–805. doi: 10.1523/JNEUROSCI.08-03-00792.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odden J.P., Holbrook S., Doe C.Q. Drosophila HB9 is expressed in a subset of motoneurons and interneurons, where it regulates gene expression and axon pathfinding. J. Neurosci. 2002;22:9143–9149. doi: 10.1523/JNEUROSCI.22-21-09143.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng I.F., Wu C.F. Differential contributions of Shaker and Shab K+ currents to neuronal firing patterns in Drosophila. J. Neurophysiol. 2007;97:780–794. doi: 10.1152/jn.01012.2006. [DOI] [PubMed] [Google Scholar]
- Pfaff S.L., Mendelsohn M., Stewart C.L., Edlund T., Jessell T.M. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84:309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- Polleux F., Ince-Dunn G., Ghosh A. Transcriptional regulation of vertebrate axon guidance and synapse formation. Nat. Rev. 2007;8:331–340. doi: 10.1038/nrn2118. [DOI] [PubMed] [Google Scholar]
- Pym E.C., Southall T.D., Mee C.J., Brand A.H., Baines R.A. The homeobox transcription factor Even-skipped regulates acquisition of electrical properties in Drosophila neurons. Neural Dev. 2006;1:3. doi: 10.1186/1749-8104-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribera A.B., Spitzer N.C. Differentiation of IKA in amphibian spinal neurons. J. Neurosci. 1990;10:1886–1891. doi: 10.1523/JNEUROSCI.10-06-01886.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson G., Hirst M., Bainbridge M., Bilenky M., Zhao Y., Zeng T., Euskirchen G., Bernier B., Varhol R., Delaney A. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat. Methods. 2007;4:651–657. doi: 10.1038/nmeth1068. [DOI] [PubMed] [Google Scholar]
- Ryglewski S., Duch C. Shaker and Shal mediate transient calcium-independent potassium current in a Drosophila flight motoneuron. J. Neurophysiol. 2009;102:3673–3688. doi: 10.1152/jn.00693.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa H., Miyashita T., Hirate Y., Higashijima S., Chino N., Uyemura K., Kikuchi Y., Okamoto H. Functional repression of Islet-2 by disruption of complex with Ldb impairs peripheral axonal outgrowth in embryonic zebrafish. Neuron. 2001;30:423–436. doi: 10.1016/s0896-6273(01)00283-5. [DOI] [PubMed] [Google Scholar]
- Shirasaki R., Pfaff S.L. Transcriptional codes and the control of neuronal identity. Annu. Rev. Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Singh S., Wu C.F. Properties of potassium currents and their role in membrane excitability in Drosophila larval muscle fibers. J. Exp. Biol. 1990;152:59–76. doi: 10.1242/jeb.152.1.59. [DOI] [PubMed] [Google Scholar]
- Smart S.L., Lopantsev V., Zhang C.L., Robbins C.A., Wang H., Chiu S.Y., Schwartzkroin P.A., Messing A., Tempel B.L. Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–819. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- Song M.R., Sun Y., Bryson A., Gill G.N., Evans S.M., Pfaff S.L. Islet-to-LMO stoichiometries control the function of transcription complexes that specify motor neuron and V2a interneuron identity. Development. 2009;136:2923–2932. doi: 10.1242/dev.037986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southall T.D., Brand A.H. Neural stem cell transcriptional networks highlight genes essential for nervous system development. EMBO J. 2009;28:3799–3807. doi: 10.1038/emboj.2009.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N.C. Development of voltage-dependent and ligand-gated channels in excitable membranes. Prog. Brain Res. 1994;102:169–179. doi: 10.1016/S0079-6123(08)60538-5. [DOI] [PubMed] [Google Scholar]
- Spitzer N.C., Kingston P.A., Manning T.J., Conklin M.W. Outside and in: development of neuronal excitability. Curr. Opin. Neurobiol. 2002;12:315–323. doi: 10.1016/s0959-4388(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Stewart B.A., Atwood H.L., Renger J.J., Wang J., Wu C.F. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- Sun Y., Dykes I.M., Liang X., Eng S.R., Evans S.M., Turner E.E. A central role for Islet1 in sensory neuron development linking sensory and spinal gene regulatory programs. Nat. Neurosci. 2008;11:1283–1293. doi: 10.1038/nn.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney S.T., Broadie K., Keane J., Niemann H., O'Kane C.J. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Thaler J., Harrison K., Sharma K., Lettieri K., Kehrl J., Pfaff S.L. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron. 1999;23:675–687. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- Thaler J.P., Lee S.K., Jurata L.W., Gill G.N., Pfaff S.L. LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein-protein interactions. Cell. 2002;110:237–249. doi: 10.1016/s0092-8674(02)00823-1. [DOI] [PubMed] [Google Scholar]
- Thaler J.P., Koo S.J., Kania A., Lettieri K., Andrews S., Cox C., Jessell T.M., Pfaff S.L. A postmitotic role for Isl-class LIM homeodomain proteins in the assignment of visceral spinal motor neuron identity. Neuron. 2004;41:337–350. doi: 10.1016/s0896-6273(04)00011-x. [DOI] [PubMed] [Google Scholar]
- Thor S., Thomas J.B. The Drosophila islet gene governs axon pathfinding and neurotransmitter identity. Neuron. 1997;18:397–409. doi: 10.1016/s0896-6273(00)81241-6. [DOI] [PubMed] [Google Scholar]
- Thor S., Thomas J.B. Motor neuron specification in worms, flies and mice: conserved and ‘lost’ mechanisms. Curr. Opin. Genet. Dev. 2002;12:558–564. doi: 10.1016/s0959-437x(02)00340-4. [DOI] [PubMed] [Google Scholar]
- Thor S., Andersson S.G.E., Tomlinson A., Thomas J.B. A LIM-homeodomain combinatorial code for motor-neuron pathway selection. Nature. 1999;397:76–80. doi: 10.1038/16275. [DOI] [PubMed] [Google Scholar]
- Turrigiano G.G., Nelson S.B. Homeostatic plasticity in the developing nervous system. Nat. Rev. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- van Steensel B., Henikoff S. Identification of in vivo DNA targets of chromatin proteins using tethered dam methyltransferase. Nat. Biotechnol. 2000;18:424–428. doi: 10.1038/74487. [DOI] [PubMed] [Google Scholar]
- Wu C.F., Tsai M.C., Chen M.L., Zhong Y., Singh S., Lee C.Y. Actions of dendrotoxin on K+ channels and neuromuscular transmission in Drosophila melanogaster, and its effects in synergy with K+ channel-specific drugs and mutations. J. Exp. Biol. 1989;147:21–41. doi: 10.1242/jeb.147.1.21. [DOI] [PubMed] [Google Scholar]