Abstract
Although it is well established that prior experience with faces determines their subsequent social–emotional evaluation, recent work shows that top-down inhibitory mechanisms, including response inhibition, can lead to social devaluation after even a single, brief exposure. These rapidly induced effects indicate interplay among perceptual, attentional, response-selection and social–emotional networks; yet, the brain mechanisms underlying this are not well understood. This study used functional magnetic resonance imaging (fMRI) to investigate the neural mechanism mediating the relationship between inhibitory control and emotional devaluation. Participants performed two tasks: (i) a Go/No-Go task in response to faces and (ii) a trustworthiness rating task involving the previously seen faces. No-Go faces were rated as significantly less trustworthy than Go faces. By examining brain activations during Task 1, behavioral measures and brain activations obtained in Task 2 could be predicted. Specifically, activity in brain areas during Task 1 associated with (i) executive control and response suppression (i.e. lateral prefrontal cortex) and (ii) affective responses and value representation (i.e. orbitofrontal cortex), systematically covaried with behavioral ratings and amygdala activity obtained during Task 2. The present findings offer insights into the neural mechanisms linking inhibitory processes to affective responses.
Keywords: cognitive control, emotion, fMRI, motor inhibition
INTRODUCTION
Coordination between cognition and emotion is necessary to guide perception and action. Indeed, converging evidence indicates that the systems subserving cognitive and emotional processes are integrated and interact strongly in the brain (Pessoa, 2008). Consistent with this perspective, there is now substantial evidence that emotionally salient stimuli modulate selective attention (Vuilleumier et al., 2001; Armony and Dolan, 2002; Pessoa et al., 2002) and response inhibition (Shafritz et al., 2006; Goldstein et al., 2007). Interestingly, the possibility of a reciprocal effect, that is, whether these cognitive processes also influence emotional responses, has also been demonstrated (see Raymond, 2009 for a review). Raymond et al. (2003) showed that selectively ignoring a distracting stimulus during a simple visual selection task produces an affective devaluation of those stimuli relative to previously attended stimuli. Raymond et al. (2003) proposed that the links between attentional inhibition and distractor stimuli are established during visual selection, stored, and then later reinstated, leading to more negative affective judgments. Behavioral (Fenske et al., 2004, 2005; Raymond et al., 2005; Veling et al., 2007; Griffiths and Mitchell, 2008; Goolsby et al., 2009a,b) and electrophysiological studies (Kiss et al., 2007, 2008) have consistently replicated this effect of attention on emotional evaluation using different experimental tasks and stimuli, and have provided evidence that distractor devaluation is specifically mediated by inhibition applied to distractors.
Importantly, behavioral evidence has revealed that these emotional devaluation effects can also arise from other types of top-down inhibitory mechanisms, such as response inhibition (Fenske et al., 2005). Using event-related potentials in a task combining Go/No-Go response contingencies followed by emotional evaluation, Kiss et al. (2008) found that a frontal electrophysiological correlate of response inhibition (No-Go N2 potential) covaried with the subsequent emotional evaluation of stimuli. The No-Go N2 recorded over frontocentral sites was larger in response to faces that later received a low trustworthiness rating as compared to faces that later received a more positive rating. This pattern of results supports the hypothesis that the efficiency of response inhibition triggered by individual No-Go faces has a systematic impact on their subsequent emotional evaluation. Based on these findings, Kiss et al. (2008) proposed that the strength of cortical response inhibition mediated by medial prefrontal areas involved in top-down motor control directly determines subsequent affective evaluation of stimuli. Furthermore, they proposed that the mechanism linking inhibitory processes to affective evaluation could rely on brain areas mediating the encoding of the value of stimuli (i.e. amygdala and orbitofrontal cortex), by which the inhibitory signals associated with the representation of faces would be used to reduce the value code associated with those faces, resulting in their emotional devaluation when reencountered (see also Fragopanagos et al., 2009).
To test these hypotheses, and to investigate the neural mechanisms mediating the relationship between top-down inhibitory control and emotional devaluation, we carried out an event-related functional magnetic-resonance imaging (fMRI) study using a similar paradigm as Kiss et al. (2008). Participants performed two tasks: in Task 1 they performed a series of manual Go/No-Go trials in response to novel Asian and Caucasian faces (with race determining Go or No-Go status). Then in Task 2, they provided trustworthiness ratings for each face seen in the previous Go/No-Go task. Ratings for faces previously seen as Go faces were compared to those presented as No-Go faces. Of specific interest here were the brain events surrounding the presentation of No-Go faces that were rated positively vs. those rated negatively. Like Kiss et al. we divided trials in the first task into two categories based on the evaluative ratings obtained in the second task. Specifically, we contrasted activations to No-Go faces (i.e. faces associated with response inhibition in Task 1) that were subsequently given low (negative) ratings (referred to here as No-Go-Low-Trust) vs. high ratings (referred to here as No-Go-High-Trust) on Task 2.
Based on previous work, the following predictions were made. First, if differential activation of areas involved in top-down motor control is directly associated with subsequent social-emotional devaluation, then: (i) Faces associated with response inhibition (No-Go faces) should be rated as less trustworthy than faces associated with a manual response (Go faces), (ii) during Task 1, activation in motor-control areas (reflecting response suppression) should be larger for No-Go-Low-Trust trials than for No-Go-High-Trust trials, and (iii) in Task 2, a contrast between No-Go-Low-Trust faces and No-Go-High-Trust faces should reveal differential activation in areas involved in evaluation of trustworthiness of faces, such as the amygdala (Winston et al., 2002; Engell et al., 2007; see Todorov, 2008 for a review).
Secondly, based on the hypothesis that the mechanisms linking inhibitory processes and emotional evaluation rely on brain areas involved in value encoding, we specifically interrogated the role of the orbitofrontal cortex (OFC), an area thought to play an important role in the interface between emotion and cognition (Pessoa, 2008; Rolls and Grabenhorst, 2008), in mediating the emotional consequences of inhibitory top-down control. Brain-imaging, lesion and neurophysiology research have implicated the OFC in both inhibitory control, including inhibition of inappropriate responses (Elliott and Dolan, 1999; Nobre et al., 1999; Horn et al., 2003; see Elliott et al., 2000 for a review), and in different aspects of emotional behavior such as the representation of affective value of stimuli and in using value to influence subsequent behavior (Kringelbach and Rolls, 2004; Rolls and Grabenhorst, 2008) as well as in social and emotional responses to faces (Rolls, 2007). We predicted that if the OFC mediates the emotional consequences of inhibitory control, OFC responses during the Go/No-Go task should be larger to No-Go faces subsequently rated as less trustworthy (No-Go-Low-Trust trials) than to No-Go faces with higher trustworthiness ratings (No-Go-High-Trust trials). Importantly, if this differential activation represents value signals that are mediating the affective response to these faces when they are reencountered, then this pattern of OFC activity should also be present during the evaluation task, when the emotional judgment of these faces is required.
MATERIALS AND METHODS
Participants
Twelve healthy volunteer participants (8 females; age range 20–31) were recruited among students and staff at the University of Oxford. All were right handed and Caucasian, and had normal or corrected-to-normal vision. All participants gave written informed consent and were paid for their time. The study protocol was approved by the local ethics committee.
Behavioral task
Stimuli
Stimuli consisted of 160 colored face images (headshots, frontal views, gaze direction was directly forward) of young adults collected from five different databases [AR Face Database (Martinez and Benavente, 1998); Caltech Frontal Face Dataset, 1999; Georgia Tech Face Database, 1999; Asian Face Image Database PF01, 2001; PAL Database (Minear and Park, 2004)]. Photographs were adjusted to match in size and background. Half of the faces were Asian, and the other half were Caucasian, and both sets contained an equal number of female and male faces. Each face subtended ∼4.8° × 6.6° visual angle and was presented on a light gray background at the center of the screen. Stimuli were presented using Presentation (Neurobehavioral Systems, Albany, CA).
Experimental task
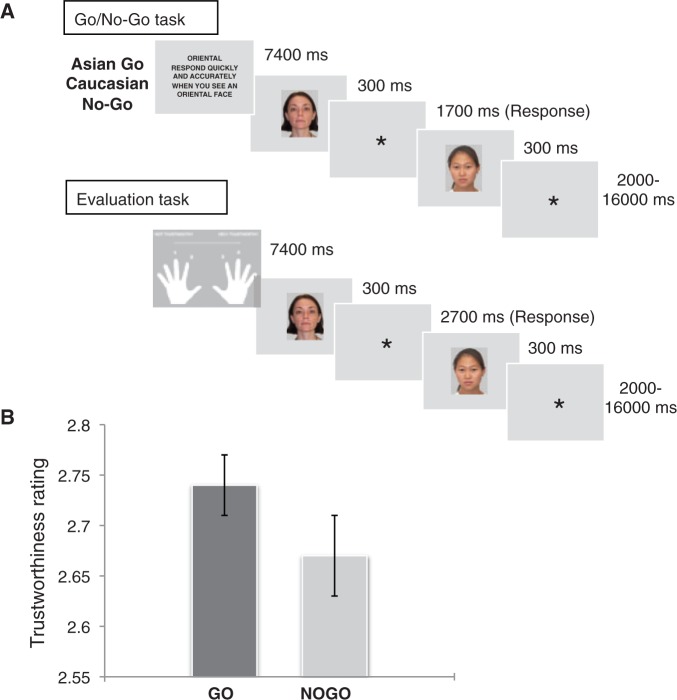
The basic experimental design is illustrated in Figure 1. The experiment consisted of 16 experimental blocks. In each block, participants performed a Go/No-Go task (Task 1) followed by an evaluation task (Task 2). In the Go/No-Go task, 10 faces (5 Asian, 5 Caucasian) were presented against a gray background for 300 ms at the center of the screen followed by a response window of 1700 ms and a random inter-stimulus interval (ISI) lasting 2–16 s, during which only a fixation cross was displayed. Participants were instructed to respond to faces of one race (Go stimuli) and to refrain from responding to faces of the other race (No-Go stimuli). Go and No-Go faces appeared unpredictably, with 50% probability. Like in the previous study, Go and No-Go stimuli were made equiprobable to reduce the likelihood that differences in brain activity between these two types of trials can be attributed to the detection of low-probability stimuli or differences in response conflict between them (i.e. Nieuwenhuis et al., 2003). Electrophysiological (i.e. Eimer, 1993; Bruin and Wijers, 2002; Nieuwenhuis et al., 2003) and neuroimaging (i.e. Liddle et al., 2001; Rubia et al., 2001) studies have shown that inhibitory processes are also engaged when Go and No-Go trials occur with equal frequency.
Fig. 1.
(A) Example of the sequence of events in each experimental block. Participants were instructed to perform a Go/No-Go task in response to Asian and Caucasian faces (with race determining their Go/No-Go status). In a subsequent evaluation task, they were required to rate the trustworthiness of the same faces viewed during the previous Go/No-Go task on a 4-points scale from 1 (‘not trustworthy’) to 4 (‘very trustworthy’). (B) Emotional devaluation behavioral effect. The graph shows the mean trustworthiness ratings (±SD) for faces previously presented as Go and No-Go stimuli. Previous No-Go faces were rated as less trustworthy than previous Go faces.
In the subsequent evaluation task, participants were required to rate the trustworthiness of the same 10 faces viewed during the previous Go/No-Go task on a 4-point scale from 1 (‘not trustworthy’) to 4 (‘very trustworthy’) by pressing the corresponding button on the button box with the index or middle finger of the left or right hand. They were asked to respond as quickly as possible, using a ‘gut feeling’. Faces appeared for 300 ms, followed by a 2700-ms response window and a 2–16-s random ISI. The durations for the ISIs used in Tasks 1 and 2 were drawn from a logarithmic distribution that was skewed toward the shorter intervals (50% 2–6 s, 25% 6–10 s, 25% 10–16 s). These had the advantages of shortening the overall trial durations, whilst enabling the individuation of hemodynamic responses to individual faces and maintaining a constant level of temporal expectation (see Nobre et al., 2004 for a similar approach).
The designated Go faces were Asian in half of the blocks and Caucasian in the other half, with order counterbalanced across participants. Each face was only shown once in each task and was never repeated across blocks. Face stimuli were counterbalanced across participants according to their status (Go/No-Go).
Participants received written instructions and a practice session before they entered the scanner, in order to become familiar with the task requirements. Prior to each task, instructions appeared reminding participants of the task to be performed.
Behavioral analysis
For the Go/No-Go task, reaction times (RTs) and the percentage of hits to Go faces were measured. Trials with response errors (i.e. failures to respond on Go trials, or False Alarms on No-Go trials) were excluded. Trials where RTs exceed ± 3 SD were also excluded. For the evaluation task, a t-test was carried out to compare the mean trustworthiness ratings to previous Go and No-Go stimuli.
Image acquisition
Imaging was performed using a Siemens Trio 3T scanner equipped with an 8-channel head coil. Visual stimuli were projected onto a translucent screen at the rear bore of the magnet. Participants viewed the screen via an angled mirror and responded using an MRI-compatible button box. Functional images were obtained with a single-shot T2*-weighted echo-planar imaging (EPI) sequence (TE = 30 ms, TR = 3 s, flip angle = 87°, matrix = 64 × 64; FOV = 192 mm; voxel size = 3 × 3 × 3 mm3). Forty-five contiguous transversal slices covered the whole brain. The task was conducted in one run consisting of 1044 volumes (∼52.3 min). The first 4 images were discarded to avoid T1 equilibrium effects. In addition, a structural image was acquired for each participant, using a high-resolution T1-weighted sequence (MP Rage pulse sequence; TR = 2040 ms, TE = 4.7 ms; flip angle = 8°; orientation of slice = transversal; slice thickness = 1 mm; FOV = 192 mm; voxel size = 1 × 1 × 1 mm3).
Image processing and analysis
Data were analyzed using SPM5 software (Wellcome Trust Centre for Neuroimaging, London, UK). After the deletion of the first four volumes, images were corrected for slice timing, and subsequently realigned and unwarped to correct for movement artifacts. High-resolution anatomical T1 images were coregistered with the realigned functional images, and structural and functional images were spatially normalized into a standardized anatomical framework [Montreal Neurological Institute (MNI)-space] using the default EPI template provided in SPM5. Functional images were spatially smoothed using a 7-mm Gaussian kernel.
Statistical analyzes were conducted based upon the General Linear Model of SPM5. Our predictions, following previous behavioral and electrophysiological findings (Fenske et al., 2005; Kiss et al., 2008), are based on testing the brain areas modulated in relation to devaluation of stimuli associated with response inhibition. Accordingly, the analysis concentrated on differentiating brain activations to No-Go faces subsequently rated as low (1 or 2) or high (3 or 4) in trustworthiness. For each task, we separated trials according to the No-Go vs. Go status of the face and to its low vs. high rating, leading to four trial types: No-Go-Low-Trust, No-Go-High-Trust, Go-Low-Trust or Go-High-Trust trials.
Instruction screens and response errors (i.e. failures to respond on Go trials or False Alarms on No-Go trials) were modeled in each task as effects of no interest. Initially, first-level single-subject contrasts were carried out separately for Tasks 1 and 2. Events were modeled using canonical hemodynamic response functions (HRFs) together with their temporal derivatives. Individual t-contrasts were generated for each individual participant and then entered into a second-level group analysis (random-effect analysis), in which a one-sample t-test was conducted at the whole-brain level.
Because we had a priori hypotheses about the response inhibition- and affective-related regions mediating the distractor devaluation effect, analyzes focused on regions of interest (ROIs) defined from coordinates reported in previous studies, and used a small-volume correction (SVC) approach with a corrected significance threshold of P < 0.05. Mean amplitudes were extracted from ROIs using MarsBaR (Brett et al., 2002) (http://marsbar.sourceforge.net/).
Specifically, for the Go/No-Go task, we predicted that No-Go-Low-Trust faces (vs. No-Go-High-Trust faces) would be associated with higher activations in frontal regions involved in response inhibition (Kiss et al., 2008). Accordingly, we performed ROI analyzes based on frontal areas defined from a recent meta-analysis study of Go/No-Go tasks (Simmonds et al., 2008; see Supplementary Table 1 for a list of ROIs coordinates). Spheres were centered within 5 mm-diameter of the previously reported coordinates after their transformation from Talairach space into MNI space (tal2mni algorithm; Matthew Brett, http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
In addition, building on findings for the involvement of the OFC in inhibitory control and affective value representation, we specifically asked if this region plays a critical role in the emotional–devaluation effect. Coordinates were derived from a previous study by Nobre et al. (1999), in which OFC activations were associated with inhibition of inappropriate automatic response tendencies (see Supplementary Table 2). Because of the proximity of the OFC ROI to the cortical surface, box-shaped ROIs (5 mm-width) were used for this area, which enabled to respect the anatomical boundaries of the OFC.
To ensure that variations in inhibitory processes actually affected subsequent emotional ratings regardless of the stimulus content, the differential activations to Go-Low-Trust vs. Go-High-Trust faces were also analyzed. If the greater activation of brain regions that mediate response inhibition during the Go/No-Go task is responsible for subsequent low affective ratings, as opposed to specific characteristics of the face stimuli themselves, then these differential responses should be present only for No-Go stimuli but not for Go stimuli.
In the evaluation task, our prediction of differential amygdala activations for No-Go-Low-Trust faces (relative to No-Go-High-Trust faces) was tested using 5 mm-spheres ROIs in the amygdala, defined based on two previous studies where the trustworthiness evaluation of faces was investigated (Winston et al., 2002: −16, −3, −24 and 18, 1, −28; Engell et al., 2007: −16, −5, −23 and 24, 0, −21).
Next, in order to identify other voxels in the brain that showed greater BOLD responses to No-Go-Low-Trust stimuli relative to No-Go-High-Trust stimuli during the evaluation task, and about which we did not have any a priori hypothesis, we conducted a whole-brain analysis at a threshold of P < 0.05 (FDR corrected).
Finally, we addressed the role of the OFC in mediating the emotional devaluation of previously inhibited faces, by testing whether increases in OFC activation to No-Go-Low-Trust stimuli also occurred during the evaluation task.
To confirm that the emotional devaluation effect was directly linked to prior variations in inhibitory processes during Task 1, and not simply due to different affective judgments of faces during Task-2 ratings, differences in activation between Go-Low-Trust vs. Go-High-Trust trials during Task 2 were also analyzed.
To characterize the pattern of differential activity across the contrasts for No-Go and Go stimuli in the hypothesis-driven ROIs, in both Task 1 and Task 2, we tested the interaction between response (No-Go vs. Go) and trustworthiness ratings (Low vs. High) by examining the contrast [(No-Go-Low-Trust > No-Go-High-Trust) > (Go-Low-Trust > Go-High-Trust)].
In order to test for a functional link between inhibitory control-related activity during the Go/No-Go task and emotion-related areas during the evaluation task in mediating subsequent emotional behavior, we (i) tested the correlation between motor-related and OFC activity during Task 1 with emotion-related areas (amygdala and OFC) during Task 2, and (ii) the correlation between behavior (affective ratings to faces) and the brain areas recruited during Task 1 and Task 2. The correlational analysis (Pearson’s r) was conducted using the effect size for each ROI.
RESULTS
Behavioral results
Performance on the Go/No-Go task was highly accurate (Go trial mean RT (±SD) = 550 ± 18 ms, correct detections = 97%, false alarms = 6%). The mean trustworthiness ratings are displayed in Figure 1. Previous No-Go faces were rated as significantly less trustworthy than previous Go faces [t(11) = 2.405, P = 0.035], thus confirming that response inhibition affected subsequent emotional responses.
fMRI results
Go/No-Go task
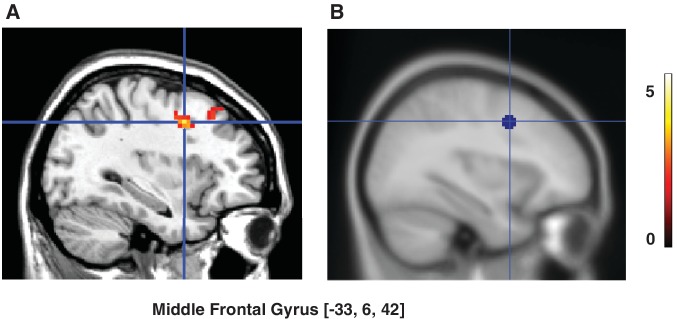
Analysis of frontal ROIs associated with response inhibition (Simmonds et al., 2008) revealed that there was a marginally significant interaction between response (No-Go vs. Go) and rating (Low vs. High) [(No-Go-Low-Trust > No-Go-High-Trust) > (Go-Low-Trust > Go-High-Trust) contrast] (t = 2.69, P = 0.08), with a significant simple contrast for No-Go faces (No-Go-Low-Trust vs. No-Go-High-Trust) in the left middle frontal gyrus (MFG) (peak coordinate: −33, 6, 42; t = 4.14, P = 0.016; Figure 2). There was no significant activation in this ROI when Go-Low-Trust vs. Go-High-Trust trials were compared. No significant activations were found within the other frontal ROIs.
Fig. 2.
Go/No-Go task: Prefrontal activity related to emotional devaluation of No-Go faces. (A) Activity in the left middle frontal gyrus (MFG) was larger for No-Go faces that were later rated as less trustworthy (No-Go-Low-Trust: ratings 1–2) relative to No-Go faces rated more positively (No-Go-High-Trust: ratings 3–4) (P < 0.05, small-volume-corrected). (B) Location of the ROI. The color scale represents t-values.
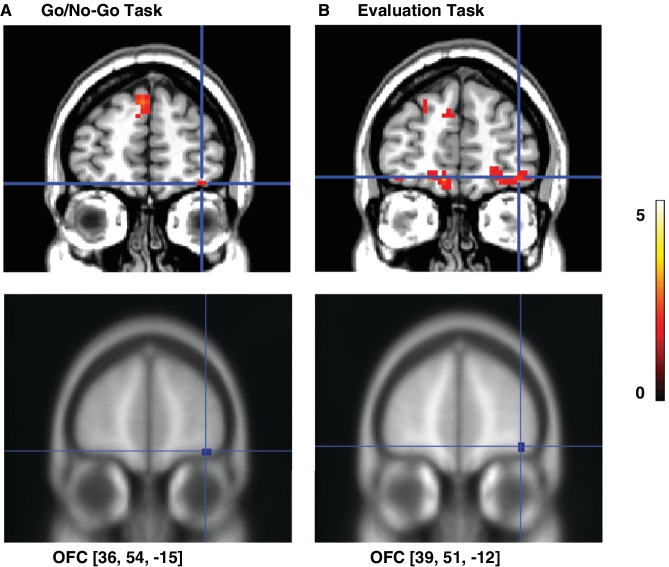
Analysis of the OFC ROI showed a significant interaction response x rating (t = 2.15, P = 0.021) that was accompanied by a significant simple contrast No-Go-Low-Trust vs. No-Go-High-Trust in the right lateral OFC (peak coordinate 36, 54, −15; t = 2.05, P = 0.033, Figure 3). Activation in this ROI was not significant for the contrast Go-Low-Trust vs. Go-High-Trust.
Fig. 3.
OFC activity related to emotional devaluation of No-Go faces. (A) During the Go/No-Go task, activity in the right lateral OFC was significantly larger for No-Go faces that were later rated as less trustworthy (No-Go-Low-Trust: ratings 1–2) relative to No-Go faces rated more positively (No-Go-High-Trust: ratings 3–4) (P < 0.05, small-volume-corrected). (B) The activation in this region persisted during the evaluation task, with higher responses to No-Go faces with lower emotional ratings relative to No-Go faces rated more positively (P < 0.05, small-volume-corrected). The location of the ROIs in each task is shown in the bottom images. The color scale represents t-values.
Evaluation task
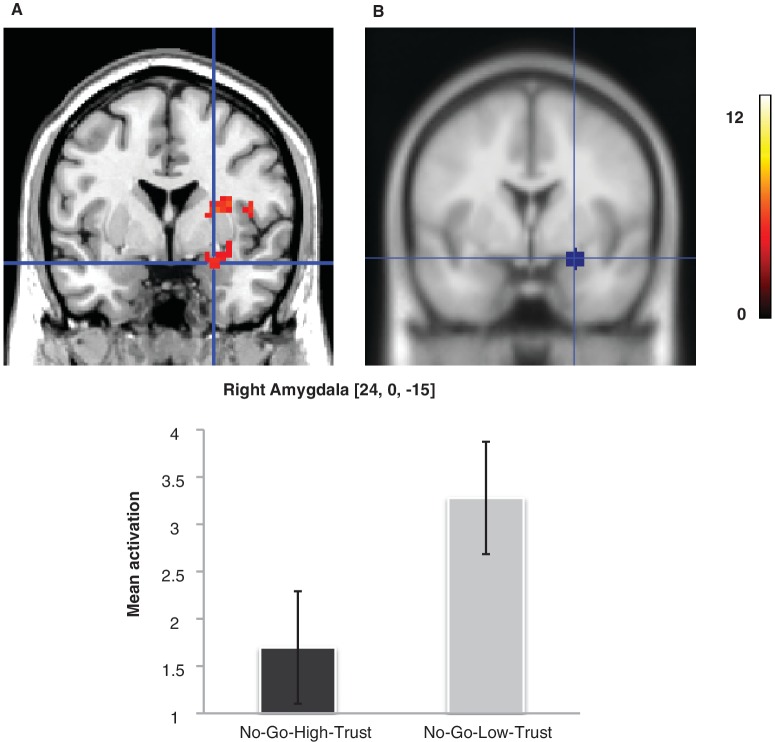
We first examined the role of the amygdala in the emotional devaluation effect for No-Go faces, given that previous studies have shown its critical involvement during evaluation of face trustworthiness (Winston et al., 2002; Engell et al., 2007). The interaction contrast response x rating yielded a non-significant activation in the right amygdala ROI (t = 2.06, P = 0.352), which reached significance when the simple contrast for No-Go stimuli was examined (peak coordinate 24, 0, −15; t = 4.97, P = 0.001, Figure 4), revealing a larger activation in the right amygdala to No-Go-Low-Trust faces (3.28 ± 2.06 SD) relative to No-Go-High-Trust faces (1.70 ± 2.06 SD). However, there was no significant activation in this ROI when Go-Low-Trust faces (2.13 ± 2.22 SD) vs. Go-High-Trust faces (1.20 ± 1.82 SD) were compared (P = 0.19).
Fig. 4.
Evaluation task: modulation of amygdala responses to emotionally devaluated No-Go faces. (A) Activity in the right amygdala was significantly larger for No-Go-Low-Trust faces than for No-Go-High-Trust faces (P < 0.05, small-volume-corrected). (B) Location of the ROI. The color scale represents t-values.
The whole-brain analysis showed No-Go-Low-Trust faces to be associated with greater activation than No-Go-High-Trust faces of premotor areas (precentral gyrus and supplementary motor area in the right hemisphere), somatosensory areas (right post-central gyrus and insula), and subcortical areas including right putamen, right thalamus, and left cerebellum (Table 1). A significant activation was also found in the right amygdala (x, y, z = 27, 3, −21), as suggested by the significant activations associated with affectively devaluated faces revealed by our ROI analysis in this area.
Table 1.
Regions activated at whole-brain analysis in the contrast No-Go-Low-Trust faces minus No-Go-High-Trust faces in the evaluation task
| Region | MNI coordinates (x, y, z) | Z peak |
|---|---|---|
| R amygdala | 27, 3, −21 | 3.59 |
| R supplementary motor area (SMA) | 6, −3, 54 | 3.28 |
| R middle cingulate | 9, −12, 45 | 3.80 |
| R precentral gyrus | 45, −18, 54 | 5.26 |
| R precentral gyrus/insula | 48, 3, 12 | 3.69 |
| 45, −21, 63 | 5.12 | |
| R post-central gyrus | 39, −39, 60 | 4.90 |
| R post-central gyrus/insula | 51, −15, 15 | 4.32 |
| R insula | 36, −18, 6 | 4.45 |
| R superior parietal lobe | 27, −60, 60 | 3.78 |
| 21, −51, 75 | 3.66 | |
| R transverse temporal gyrus (Rolandic operculum) | 63, −12, 12 | 4.04 |
| R putamen | 27, 0, 12 | 3.96 |
| R claustrum/putamen | 33, −3, −6 | 3.46 |
| R thalamus | 12, −18, 6 | 4.75 |
| L cerebellum anterior lobe | −21, −48, −30 | 5.15 |
| −9, −51, −21 | 4.76 | |
| L cerebellum posterior lobe | −27, −60, −24 | 4.72 |
Threshold P < 0.05 (FDR corrected for multiple comparisons for the whole brain). R, right; L, left.
A whole-brain analysis comparing Go-Low-Trust faces vs. Go-High-Trust faces only showed significant activations in the right precentral gyrus and left cerebellum (Table 2).
Table 2.
Regions activated at whole-brain analysis in the contrast Go-Low-Trust faces minus Go-High-Trust faces in the evaluation task.
| Region | MNI coordinates (x, y, z) | Z peak |
|---|---|---|
| R precentral gyrus | 33, −21, 57 | 4.96 |
| 33, −24, 66 | 4.51 | |
| 24, −21, 75 | 4.44 | |
| L cerebellum | −24, −54, −21 | 4.82 |
| −12, −48, −24 | 4.24 | |
| −21, −36, −27 | 4.17 |
Threshold P < 0.05 (FDR corrected for multiple comparisons for the whole brain).
R, right; L, left.
Finally, we analyzed the role of the OFC in the emotional devaluation effect. Our results showed that the activation in the right lateral OFC observed in Task 1 and related to subsequent emotional devaluation of No-Go faces, was significantly activated during Task 2, as revealed by a significant interaction response × rating (t = 1.90, P = 0.035) together with a significant simple contrast for No-Go stimuli (No-Go-Low-Trust vs. No-Go-High-Trust) (activation peak at coordinates 39, 51, −12; t = 2.33, P = 0.020, Figure 3). This activation was not significant when Go-Low-Trust vs. Go-High-Trust stimuli were compared.
Relationship between activations in the Go/No-Go and evaluation task: correlation analysis
The correlation analyzes showed that (i) activation in motor inhibition-related areas (left MFG) to No-Go-Low-Trust faces (relative to No-Go-High-trust faces) significantly correlated with activation in right OFC during Task 1 (r = 0.81, P = 0.001), (ii) activity in OFC during Task 1 was coupled with activity in amygdala during Task 2 (r = 0.51; P = 0.046), (iii) activity in OFC during Task 2 to No-Go-Low-Trust faces (relative to No-Go-High-trust faces) significantly correlated with amygdala activation in Task 2 (r = 0.50, P = 0.048), and (iv) OFC activation and MFG activity during Task 1 correlated with lower ratings of No-Go faces relative to Go faces in Task 2 (OFC: r = 0.57, P = 0.025; MFG: r = 0.58, P = 0.025).
DISCUSSION
The aim of the present study was to investigate the underlying neural substrate linking inhibitory processes to affective responses. Our first prediction was that if inhibitory control mechanisms modulate social-emotion appraisal, then inhibition of a motor response associated with No-Go faces in a Go/No-Go task should directly affect the subsequent affective evaluation of these faces. As expected, we found that previous No-Go stimuli were rated more negatively relative to previous Go stimuli, confirming that top-down motor control can reliably modulate affective responses. This pattern of results is consistent with previous behavioral findings by Fenske et al. (2005), Veling et al. (2008) and Kiss et al. (2008), who observed emotional devaluation effects as a consequence of response inhibition using similar tasks.
Modulation of motor inhibition during the Go/No-Go task related to subsequent devaluation
Our second prediction was that affective devaluation of No-Go faces is mediated by a differential activation of frontal areas involved in motor inhibition. We compared brain activations in response to No-Go and Go faces during the Go/No-Go task separately as a function of their emotional ratings in a subsequent evaluation task. The interaction between response (No-Go vs. Go) and rating (Low vs. High) yielded a marginally significant activation in the left middle frontal gyrus, which points to a trend that the differential activity in frontal areas involved in motor inhibition for subsequently devaluated faces is larger for No-Go relative to Go stimuli. Importantly, decomposing the interaction into simple effects confirmed the differential activity in the MFG between faces with lower emotional ratings vs. those with higher emotional ratings to be significant only for No-Go faces. This pattern of results provides further support for a specific enhancement of No-Go inhibition-related activity to faces subsequently rated low in trustworthiness.
The focus of activation at the MFG associated with No-Go faces is consistent with findings demonstrating the involvement of the prefrontal cortex in response inhibition (Sasaki et al., 1989, 1993; Liddle et al., 2001; Rubia et al., 2001; Watanabe et al., 2002; Picton et al., 2007; Simmonds et al., 2008). In particular, engagement of the left MFG is consistent with previous reports of activation in this region associated with motor inhibition during Go/No-Go tasks (Liddle et al., 2001; Rubia et al., 2001; Simmonds et al., 2008). The activation of the left lateral prefrontal cortex is consistent with its role in mediating executive control processes necessary to perform inhibition tasks (Rubia et al., 2001; see also Mostofsky and Simmonds, 2008), and supports our hypothesis that the affective devaluation of stimuli associated with motor inhibition is mediated by prefrontal areas involved in top-down inhibitory processes.
A recent proposal by Pessoa (2008) is consistent with our finding of a systematic covariation between brain activation patterns related to inhibitory motor control and subsequent emotional judgments. He suggested that lateral prefrontal cortex signals involved in inhibitory processes reflect both cognitive and affective information, proposing an important role for this area in the integration of cognitive and emotional information for guiding action. Based on previous evidence as to how the brain codes value in order to guide behavior, we suggest that inhibitory signals are encoded with the representation of faces (Raymond et al., 2003; Fenske et al., 2004; Raymond et al., 2005), and are used to reduce the value code associated with those faces, resulting in their emotional devaluation when reencountered (Raymond, 2009). Support for this hypothesis is derived from the correlation that is seen between activity in the MFG to No-Go faces during the Go/No-Go phase of the task and the degree of behavioral affective devaluation, consistent with the proposal that direct links exist between motor control mechanisms and emotional responses.
Modulation of limbic activity to No-Go stimuli during the evaluation task
Our third prediction was that if inhibitory control mechanisms modulate affective appraisal of stimuli, No-Go faces with lower trustworthiness ratings should be associated with a differential activation in areas involved in evaluation of trustworthiness of faces, such as the amygdala (Winston et al., 2002; Engell et al., 2007; see Todorov 2008 for a review) during the subsequent evaluation task. Examining the interaction between response and ratings revealed a non-significant activation in the right amygdala. Analysis of simple contrasts enabled us to observe that, in line with our predictions, a significant larger activation in the right amygdala was present to affectively devaluated No-Go faces (relative to No-Go faces rated more positively). The different level of amygdala activity to faces rated low vs. high in trustworthiness was not, however, significant for previous Go faces. Overall, the activity pattern in the amygdala indicates that, as expected, much of the variance in its activation levels could be attributed to trustworthiness ratings. Previous research has consistently demonstrated its important role in complex social judgments including trustworthiness (Adolphs et al., 1998; Winston et al., 2002; Adolphs, 2003; Engell et al., 2007). Adolphs et al. (1998) showed that patients with bilateral amygdala damage were impaired in discriminating between trustworthy and untrustworthy-looking faces. The critical involvement of amygdala in decisions on trustworthiness has been confirmed by accumulating evidence of larger responses in the amygdala to untrustworthy faces as compared with trustworthy faces (Winston et al., 2002; Engell et al., 2007; for a review see Todorov, 2008). Moreover, the right-lateralized response in the amygdala in our study is in line with previous reports of larger responses in the right amygdala for untrustworthy faces (Todorov, 2008).
Finally, we noted modulation of activity in other brain areas during the evaluation of previously inhibited faces. Other areas differentially modulated for No-Go-Low-Trust stimuli vs. No-Go-High-Trust stimuli included motor-related regions and somatosensory areas (right post-central gyrus and insula), as well as subcortical regions such as right thalamus and left cerebellum. These activations coincide with areas previously linked to social-emotional evaluation of stimuli (Adolphs, 2002, 2003; Winston et al., 2002; Cunningham and Zelazo, 2007). For instance, it has been proposed that the somatosensory cortex, by coding visceral bodily changes, enables a representation of the current autonomic state and, consequently, the perception of one’s own emotional response to stimuli (Damasio, 1999; Adolphs, 2002; Cunningham and Zelazo, 2007). Consistent with the present results, Winston et al. (2002), in an fMRI study investigating the neural substrate mediating trustworthiness judgments, found larger activations in bilateral amygdala and right insula to untrustworthy vs. trustworthy faces, proposing a role for the insula in mapping the autonomic bodily changes produced as a consequence of amygdala activation. The present findings confirm our third prediction that the affective devaluation effect of faces previously associated with inhibition of a motor response is mediated by brain areas known to be involved in face-related social–emotional judgments.
Modulation of OFC activity during the Go/No-Go task related to subsequent devaluation persists during the evaluation task
Finally, we specifically interrogated the role of OFC in allowing variations in inhibitory control at one point in time to impact selectively on emotional responses later on. We predicted that if a differential OFC activation to subsequently devaluated No-Go faces is present during the Go/No-Go phase of the task and represents a means of coding and storing value for a specific face, then this orbitofrontal activity should also be present during the evaluation task, when faces are reencountered and an explicit emotional judgment was required. Indeed, we found a significant interaction response x rating in the right lateral OFC during Task 1, which persisted to Task 2. This was further qualified by simple effects tests showing that the differential level of activity in the right lateral OFC to affectively devaluated faces was significant for No-Go faces, but not for Go faces, during both Task 1 and Task 2. These findings strongly support the proposed role of the OFC in linking inhibitory to emotional processes.
The focus of activation in the right lateral OFC is close to that seen in studies requiring inhibition of inappropriate responses (see Elliott et al., 2000, for a review), including Go/No-Go tasks (Horn et al., 2003), spatial and temporal orienting tasks using invalid cues (Nobre et al., 1999), and delayed non-matched to sample (Elliott and Dolan, 1999). Furthermore, the involvement of the lateral OFC in making choices on the basis of response suppression (Elliott et al., 2000) has been reported for emotional behavior (Blair et al., 1999; O’Doherty et al., 2001; Kringelbach and Rolls, 2004). It has been proposed that the engagement of the lateral OFC in response inhibition reflects the involvement in complex operations linked to behavioral flexibility and decision-making (Kringelbach, 2005).
The differential response to affectively devalued No-Go faces during Task 1, which was significantly larger than that observed for Go stimuli and persisted to Task 2, is consistent with the proposal that variations in executive processes mediating suppression of inappropriate responses covary with low emotional ratings during subsequent affective evaluations. Additional support for this proposal comes from the correlation analysis, which revealed that the OFC activity to No-Go faces during the Go/No-Go task correlated with the differential activation in motor inhibition-related regions (lateral prefrontal cortex) to subsequently devaluated No-Go faces and with the behavioral affective devaluation effect. These results thus provide strong support for the hypothesis that devaluation is coded at the time of inhibition and remains accessible at least until the rating period, where it manifests as a low rating score.
The pattern of activations observed during the evaluation task revealed that the larger activation of the OFC to No-Go-Low-Trust (compared to No-Go-High-Trust) stimuli was associated with higher responses in the amygdala. This is consistent with evidence showing reciprocal connections between these areas and their interactive role in decision-making and emotion (Elliott et al., 2000; Kringelbach and Rolls, 2004). Critically, we found that differential activation in the amygdala to No-Go faces rated low vs. high in trustworthiness correlated with differential activity in the OFC during the Go/No-Go task. This indicates that the interaction between OFC and amygdala mediates, at least partially, the effect of top-down inhibition on affective responses to faces.
Taken together, the present results provide evidence demonstrating that variations in inhibitory processes during Task 1 in areas mediating executive control and response suppression (lateral prefrontal cortex) as well as areas known to be involved in affective responses and value representation (OFC), systematically covary with subsequent behavioral measures of emotional evaluation and with a differential activation of brain regions involved in emotional responses to faces (i.e. amygdala) during Task 2. Several implications could be proposed from this pattern of results: (i) BOLD signal in frontal regions during Task 1 reflects the social-emotional judgments of faces that subjects are likely to make later on during Task 2, and (ii) it is consistent with the hypothesis that the emotional devaluation effect of visual stimuli previously submitted to inhibitory control is mediated by the reinstantiation of value signals (reflected by the reactivation of OFC) encoded with the representation of these stimuli when reencountered. These value signals would be thus available to the affective mechanisms that control explicit rating behavior (i.e. amygdala), leading thus to more negative emotional judgments.
Although previous fMRI studies have reported that response inhibition is modulated by the emotional significance of stimuli (Hare et al., 2005; Shafritz et al., 2006; Goldstein et al., 2007), this is the first study, to our knowledge, showing that top-down inhibitory motor control mechanisms actually have affective ‘consequences’, providing evidence for a reciprocal relationship between response-induced top-down inhibitory control and subsequent emotional processes.
Based on these findings and those of earlier studies showing emotional devaluation effects as a result of attentional inhibition of distractors during target selection (Fenske et al., 2004, 2005; Raymond et al., 2005; Kiss et al., 2007; Goolsby et al., 2009a,b), we propose that emotional devaluation reflects a general mechanism whereby top-down inhibitory processes, including attentional suppression and response inhibition, give rise to emotional consequences for the purpose of regulating goal-directed behavior.
SUPPLEMENTARY DATA
Supplementary Data are available at SCAN Online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgement
This work was supported by Integrative Analysis of Brain and Behaviour (IABB) (initiative grant BBS/B/16178) from the Biotechnology and Biological Sciences Research Council (BBSRC), UK (to J.E.R., K.S., M.E. and A.C.N.). S.D. was supported by a post-doctoral contract from the Spain's Ministry of Education and Science and the Spanish Science and Technology Foundation (FECYT) at the Department of Experimental Psychology in the University of Oxford and by a current post-doctoral contract from the Isidro Parga Pondal program (Xunta de Galicia, Spain). Our thanks to Mark Stokes for advice on the analysis.
REFERENCES
- Adolphs R. Trust in the brain. Nature Neuroscience. 2002;5:192–3. doi: 10.1038/nn0302-192. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behavior. Nature Review Neuroscience. 2003;4:165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–4. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Armony JL, Dolan RJ. Modulation of spatial attention by fear-conditioned stimuli: an event-related fMRI study. Neuropsychologia. 2002;40:817–26. doi: 10.1016/s0028-3932(01)00178-6. [DOI] [PubMed] [Google Scholar]
- Asian Face Image Database PF01. Collected at the Intelligent Multimedia Lab, Pohang University of Science and Technology, Korea. 2001. http://nova.postech.ac.kr/∼dkim/new_imlab/ (15 March, 2007, date last accessed)
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–93. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an spm toolbox. NeuroImage. 2002;16:S497. [Google Scholar]
- Bruin KJ, Wijers AA. Inhibition, response mode, and stimulus probability: a comparative event-related potential study. Clinical Neurophysiology. 2002;113:1172–82. doi: 10.1016/s1388-2457(02)00141-4. [DOI] [PubMed] [Google Scholar]
- Caltech Frontal Face Dataset. Collected by Markus Weber at California Institute of Technology, USA. 1999. http://www.vision.caltech.edu/html-files/archive.html (15 March, 2007, date last accessed)
- Cunningham WA, Zelazo PD. Attitudes and evaluations: a social cognitive neuroscience perspective. Trends in Cognitive Sciences. 2007;11:97–104. doi: 10.1016/j.tics.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Damasio A. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- Eimer M. ERPs elicited by Go and Nogo stimuli: effects of attention and stimulus probability. Biological Psychology. 1993;35:123–38. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ. Differential neural responses during performance of matching and nonmatching to sample tasks at two delay intervals. Journal of Neuroscience. 1999;19:5066–73. doi: 10.1523/JNEUROSCI.19-12-05066.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cerebral Cortex. 2000;10:308–17. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Engell AD, Haxby JV, Todorov A. Implicit trustworthiness decisions: automatic coding of face properties in the human amygdala. Journal of Cognitive Neuroscience. 2007;19:1508–19. doi: 10.1162/jocn.2007.19.9.1508. [DOI] [PubMed] [Google Scholar]
- Fenske MJ, Raymond JE, Kessler K, Westoby N, Tipper SP. Attentional inhibition has social-emotional consequences for unfamiliar faces. Psychological Science. 2005;16:753–58. doi: 10.1111/j.1467-9280.2005.01609.x. [DOI] [PubMed] [Google Scholar]
- Fenske MJ, Raymond JE, Kunar MA. The affective consequences of visual attention in preview search. Psychonomic Bulletin & Review. 2004;11:1055–61. doi: 10.3758/bf03196736. [DOI] [PubMed] [Google Scholar]
- Fragopanagos N, Cristescu T, Goolsby BA, Kiss M, Eimer M, Nobre AC, et al. Modelling distractor devaluation (DD) and its neurophysiological correlates. Neuropsychologia. 2009;47:2354–66. doi: 10.1016/j.neuropsychologia.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Georgia Tech Face Database. Collected at the Georgia Institute of Technology, USA. 1999. http://www.anefian.com/face_reco.htm (15 March, 2007, date last accessed)
- Goldstein M, Brendel G, Tuescher O, Pan H, Epstein J, Beutel M, et al. Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: an emotional linguistic go/no-go fMRI study. NeuroImage. 2007;36:1026–40. doi: 10.1016/j.neuroimage.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Goolsby BA, Shapiro KL, Raymond JE. Distractor devaluation requires visual working memory. Psychonomic Bulletin & Review. 2009a;16:133–8. doi: 10.3758/PBR.16.1.133. [DOI] [PubMed] [Google Scholar]
- Goolsby BA, Shapiro KL, Silvert L, Kiss M, Fragopanagos N, Taylor JG, et al. Feature-based inhibition underlies the affective consequences of attention. Visual Cognition. 2009b;17:500–530. [Google Scholar]
- Griffiths O, Mitchell CJ. Negative priming reduces affective ratings. Emotion. 2008;22:1119–29. [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry. 2005;57:624–32. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JFW, Woodruff PWR. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–66. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Kiss M, Goolsby BA, Raymond JE, Shapiro KL, Silvert L, Nobre AC, et al. Efficient attentional selection predicts distractor devaluation: event-related potentials evidence for a direct link between attention and emotion. Journal of Cognitive Neuroscience. 2007;19:1316–22. doi: 10.1162/jocn.2007.19.8.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss M, Raymond JE, Westoby N, Nobre AC, Eimer M. Response inhibition is linked to emotional devaluation: behavioural and electrophysiological evidence. Frontiers in Human Neuroscience. 2008;2:1–14. doi: 10.3389/neuro.09.013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12:100–9. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez AM, Benavente R. CVC Technical Report 24. Barcelona, Spain: Computer Vision Center, Universitat Autònoma de Barcelona; 1998. The AR Face Database. [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behavior Research Methods, Instruments, & Computers. 2004;36:630–3. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. Journal of Cognitive Neuroscience. 2008;20:751–61. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulated function in a go/no-go task: effects of response conflict and trial-type frequency. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Frith CD, Mesulam MM. Orbitofrontal cortex is activated during breaches of expectation in tasks of visual attention. Nature Neuroscience. 1999;2:11–12. doi: 10.1038/4513. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Maquet P, Frith CD, Vandenberghe R, Mesulam MM. Orienting attention to locations in perceptual versus mental representations. Journal of Cognitive Neuroscience. 2004;16:363–73. doi: 10.1162/089892904322926700. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9:148–58. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neutral and emotional stimuli. Brain Research Cognitive Brain Research. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of focal frontal lesions on response inhibition. Cerebral Cortex. 2007;17:826–38. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- Raymond JE. Interactions of attention, emotion, and motivation. In: Srinivasan N, editor. Progress in Brain Research: Attention. Amsterdam: Elsevier; 2009. pp. 293–308. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Fenske MJ, Tavassoli NT. Selective attention determines emotional responses to novel visual stimuli. Psychological Science. 2003;14:537–42. doi: 10.1046/j.0956-7976.2003.psci_1462.x. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Fenske MJ, Westoby N. Emotional devaluation of distracting patterns and faces: a consequence of attentional inhibition during visual search? Journal of Experimental Psychology: Human Perception and Performance. 2005;31:1404–15. doi: 10.1037/0096-1523.31.6.1404. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The representation of information about faces in the temporal and frontal lobes. Neuropsychologia. 2007;45:124–43. doi: 10.1016/j.neuropsychologia.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Progress in Neurobiology. 2008;86:216–44. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, et al. Mapping motor inhibition: conjunctive brain activations across different versions of Go/No-Go and Stop tasks. NeuroImage. 2001;13:250–61. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Gemba H, Nambu A, Matsuzaki R. No-go activity in the frontal association cortex of human subjects. Neuroscience Research. 1993;18:249–52. doi: 10.1016/0168-0102(93)90062-u. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Gemba H, Tsujimoto T. Suppression of visually initiated hand movement by stimulation of the prefrontal cortex in the monkey. Brain Research. 1989;495:100–7. doi: 10.1016/0006-8993(89)91222-5. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. Neuroimage. 2006;31:468–75. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-Go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–32. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A. Evaluating faces on trustworthiness. An extension of systems for recognition of emotions signaling approach/avoidance behaviors. Annals of the New York Academy of Sciences. 2008;1124:208–24. doi: 10.1196/annals.1440.012. [DOI] [PubMed] [Google Scholar]
- Veling H, Holland RW, van Knippenberg A. Devaluation of distracting stimuli. Cognition & Emotion. 2007;21:442–8. [Google Scholar]
- Veling H, Holland RW, van Knippenberg A. When approach motivation and behavioral inhibition collide: behavior regulation through stimulus devaluation. Journal of Experimental Social Psychology. 2008;44:1013–19. [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effect of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–41. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Sugiura M, Sato K, Sato Y, Maeda Y, Matsue Y, et al. The human prefrontal and parietal association cortices are involved in NO-GO performances: an event-related fMRI study. NeuroImage. 2002;17:1207–16. doi: 10.1006/nimg.2002.1198. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5:277–83. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.