Abstract
The current study uses functional magnetic resonance imaging (fMRI) to examine whether regulating negative bias to stigmatized individuals has a unique neural activity profile from general emotion regulation. Participants were presented with images of stigmatized (e.g. homeless people) or non-stigmatized (e.g. a man holding a gun) social targets while undergoing fMRI and were asked either to maintain or regulate their emotional response. Their implicit bias toward these stigmatized group members was also measured. Analyses were conducted in both, an event-related fashion, considering the event to be the onset of regulation, and in a blocked-design fashion, considering the sustained activity throughout the 8-s regulatory period. In the event-related (onset) analyses, participants showed more activity throughout the prefrontal cortex when initiating a regulatory response to stigmatized as compared with non-stigmatized images. This neural activity was positively correlated with their implicit bias. Interestingly, in the block (sustained) analyses, general emotion regulation elicited a more widespread pattern of neural activity as compared with stigma regulation. This activity was largely posterior, suggesting that general emotion regulation may engage more visuo-spatial processing as compared with stigma regulation. These findings suggest that regulating negative affect toward stigmatized targets may occur relatively more quickly than regulating negative affect toward non-stigmatized targets.
Keywords: emotion regulation, fMRI, IAT, stigma
INTRODUCTION
A critical skill to successfully negotiating everyday social interactions is the ability to regulate negative bias to people who are stigmatized (e.g. someone who is homeless, deformed or a substance abuser). Emerging research in social neuroscience has demonstrated that when perceivers evaluate novel stigmatized targets, they engage cognitive control networks (Richeson et al., 2003; Amodio et al., 2004; Cunningham et al., 2004; Bartholow et al., 2006; Krendl et al., 2006), similar to those engaged in general emotion regulation (Ochsner et al., 2002, 2004). One important distinction between the extant stigma research and emotion regulation research is that participants are not instructed to regulate their negative bias in the former, whereas they are specifically instructed to regulate their negative emotions in the latter. Therefore, it remains an open question whether perceivers are actually regulating their negative bias to stigma (as has been suggested), and, if so, whether this process is distinct from general emotion regulation. In the current functional magnetic resonance imaging (fMRI) study, we instructed participants to actively regulate their negative emotions to images of stigmatized individuals and other non-stigmatized negative social targets in order to directly compare the neural activity engaged during stigma regulation to that engaged during more general emotion regulation.
Emerging social neuroscience research has identified a vast network of neural regions [including the anterior cingulate cortex, lateral prefrontal cortex (PFC), insula and amygdala], which is engaged when individuals make relatively brief (ranging from 500 to 2500 ms) implicit or explicit evaluations of stigmatized targets (Richeson et al., 2003; Amodio et al., 2004; Cunningham et al., 2004; Krendl et al., 2006, 2009; Harris and Fiske, 2006). These regions play a complementary role in regulation: the anterior cingulate cortex (ACC) is involved in identifying situations in which regulation and control are needed (MacDonald et al., 2000), particularly with respect to monitoring potentially biased responses (Amodio et al., 2004, 2006). Conversely, the lateral PFC is involved in actively engaging and maintaining regulation (Lieberman et al., 2002; Lieberman, 2003; Richeson et al., 2003; Cunningham et al., 2004; Krendl et al., 2006). Specifically, the ventrolateral PFC (VLPFC) is involved in downregulation (Vogeley et al., 2001; Samson et al., 2005; Satpute and Lieberman, 2006), whereas the dorsolateral prefrontal cortex (DLPFC) is associated with goal maintenance. Indeed, several studies have demonstrated that DLPFC activity is positively correlated with individual differences in implicit bias toward stigmatized individuals, suggesting it may play an important role in controlling prejudicial thoughts (Richeson et al., 2003; Knutson, et al., 2007).
It is important to note that although social neuroscience research has demonstrated that participants have increased activation in prefrontal regulatory networks when they evaluate stigmatized individuals, participants are not explicitly instructed to regulate their negative bias in these studies. It is therefore intriguing that similar prefrontal networks are also engaged in tasks where participants are specifically instructed to regulate their emotions to negative images not relevant to stigma (Ochsner et al., 2002, 2004). For instance, Ochsner et al. (2002) conducted an fMRI study in which participants were asked to either maintain their negative emotions (‘attend’) to highly negative images of non-stigmatized targets or to ‘reappraise’ (reinterpret the image in such a way that it would no longer elicit a negative response) the images. Reappraising the images led to decreased subjective negative affect, and this was coupled with increased activation in the ACC and lateral PFC.
Thus, the extant research on stigma and emotion regulation suggests that similar neural networks (e.g. the ACC, the VLPFC and the DLPFC) may play a critical role in both regulating negative bias to stigma in the absence of overt instructions to regulate and in regulating negative emotions to non-stigmatized targets in the presence of overt instructions to regulate. However, it remains an open question as to how similar these processes are. Specifically, are the same networks engaged to the same extent when regulating negative affect to stigmatized targets as they are in emotion regulation to negative stimuli? In the current study, we examine this question by directly comparing the neural activity engaged in emotion regulation to that engaged in stigma regulation when participants are explicitly instructed to regulate their negative emotions in both cases. There are three possible outcomes to this study: first, stigma regulation will engage different areas of the PFC than will emotion regulation to non-stigmatized targets, second, stigma regulation will engage the same areas of the PFC as in emotion regulation, but to a different extent and third, stigma regulation and emotion regulation will engage the same areas of the PFC to the same extent. The first two possible outcomes would indicate that stigma regulation has a unique activity profile from emotion regulation, whereas the third option would suggest that the brain regulates negative affect to stigma in the same way it regulates negative affect to non-stigmatized targets.
One potential confound in comparing neural activity between emotion regulation and stigma regulation is that perceivers may engage different mental processes to maintain their regulatory responses to these disparate stimuli over the sustained regulatory period. Moreover, the stimuli may also elicit different affective responses. For instance, an image of a homeless person may elicit pity while the perceiver attempts to reappraise the image by thinking of the unfortunate circumstances that could have led to the person's condition. In contrast, an image of a man with a gun is more likely to lead to reappraisal via reinterpretation of the image (e.g. thinking about a person at a shooting range rather than in combat). In order to best circumvent these potential confounds, we chose to examine how perceivers initiate as well as how they maintain their regulatory responses to stigmatized as compared with non-stigmatized targets. If differences emerge between stigma regulation and more general emotion regulation in both the onset of regulatory attempts, as well as in sustained regulatory attempts, it would suggest that stigma regulation has a unique activity profile from more general emotion regulation. Importantly, such differences would suggest that stigma regulation's unique activity profile is not primarily due to perceivers’ engaging different mental processes to regulate their emotions to the two different types of images.
In the current study, we selected two stigmatized groups that have been widely shown to elicit strong negative emotions—homeless individuals and substance abusers (Jones et al., 1984; Weiner et al., 1988; Harris and Fiske, 2006). We then asked participants to regulate or to maintain their negative emotions to these socially stigmatized targets as well as to non-stigmatized, affectively negative social targets (e.g. a couple standing at a cemetery, a soldier firing a gun). We also measured participants’ implicit bias to these groups to examine whether individual differences in bias might affect neural responses (Phelps et al., 2000; Richeson et al., 2003; Knutson et al., 2007). Analyses focus on the role of the PFC in the initial and sustained attempts to regulate negative affect to both socially stigmatized targets and to non-stigmatized, affectively negative social targets.
METHODS
A total of 20 neurologically normal, right-handed adults (Mage = 21.6 years, 10 females) were recruited from the greater Boston area to participate in this study. They participated for monetary compensation. Four participants were excluded due to excessive movement during the scanning session (>2 mm) or failure to perform the task, leaving 16 remaining participants. Anatomical and functional whole-brain imaging was performed on a 3.0 T Siemens Trio Scanner (Trio, Siemens Ltd., Enlargen, Germany) using standard data acquisition protocols. Anatomical images were acquired using a high-resolution 3D magnetization prepared rapid gradient echo sequence (MP-RAGE; 144 sagittal slices, TE = 7 ms, TR = 2200 ms, flip angle = 7°, 1 × 1 × 0.89 mm voxels). Functional images were collected in five functional runs of 153 time points each, using a fast field echo-planar sequence sensitive to blood–oxygen level-dependent contrast (T2*) (31 axial slices per whole-brain volume, 3 mm in-plane resolution, 4 mm thickness, 0 mm skip, TR = 2000 ms).
Behavioral tasks
Implicit association test
Prior to the MRI task, participants completed two Implicit Association Tasks (IAT; Greenwald et al., 1998) to measure their implicit attitudes toward the homeless and toward alcoholism. In both IATs, 12 images (e.g. of homeless individuals, alcoholics or controls) and 12 words (that were either pleasant or unpleasant) were presented. The images of the stigmatized targets used in the IAT were different from the images presented during the MRI task. Participants then categorized words or pictures as belonging to one of four categories: (e.g. pleasant words, unpleasant words, images of homeless individuals or alcoholics, or images of controls). In both tasks, the control images were of age and gender matched individuals in similar poses to the stigmatized targets, but who were not stigmatized.
In the IAT, participants categorized words and pictures in a manner that is stereotypically congruent (e.g. ‘unpleasant’ and ‘homeless’ are paired on the same side of the screen) or stereotypically incongruent (e.g. ‘pleasant’ and ‘homeless’ are presented on the same side of the screen). The congruent and incongruent blocks were presented in a pseudorandom manner.
Regulation task during the scan
During the scanning session, participants were instructed to decrease or maintain their negative emotional response to 32 images of stigmatized targets (substance abusers and homeless individuals), 16 images of non-stigmatized targets (e.g. neutral images of people with no visible stigmas) and 32 negative images portraying negative social, but non-stigmatized, targets (e.g. a man holding a gun, a couple at a cemetery) from the International Affective Picture Set (IAPS; Lang et al., 2005).1 Our main purpose for including the IAPS images was to present images with similar negative affect as the stigmatized targets, so we could determine if regulating negative affect to stigma engaged a unique activity profile from more general emotion regulation.
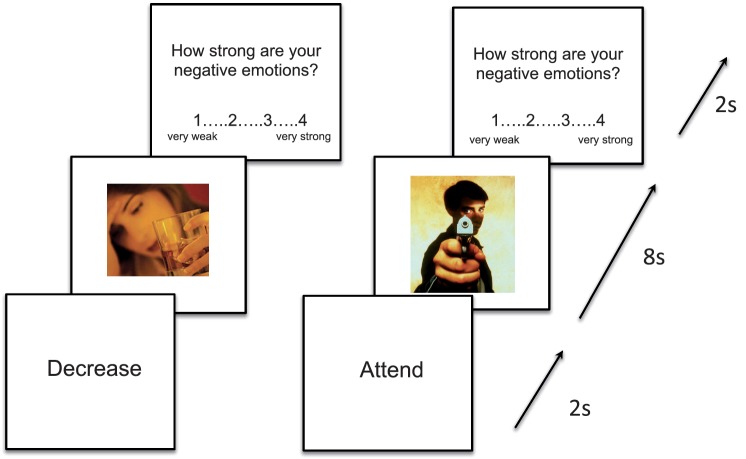
Prior to each image, participants saw a prompt on the screen for 2 s instructing them to ‘decrease’ or ‘attend’ to the image (Figure 1). The image then appeared on the screen for 8 s, during which time participants either ‘attended’ to the image by actively maintaining (i.e. not changing) their emotional response to the image, or ‘decreased’ their emotional response. After the 8-s presentation interval elapsed, participants rated the relative strength of their negative emotions to the image they had just seen (1 = very weak negative emotion, 4 = very strong negative emotion). After each set of ratings, pseudorandomly jittered fixation was presented for 4–8 s.
Fig. 1.
A sample trial of the MRI task using an image of a stigmatized target (an alcoholic woman) and a negative, non-stigmatized IAPS image (a man holding a gun). Participants were given a 2-s instruction to attend or decrease, and then the 8-s regulation period began. Finally, participants rated their negative emotions to the image.
Data analysis
The fMRI data were analyzed using the general linear model in SPM8 (Wellcome Department of Cognitive Neurology, London, UK). Data underwent standard pre-processing to remove sources of noise and artifact. Functional data were spatially smoothed [6mm full-width-at-half-maximum (FWHM)] using a Gaussian kernel. We used a general linear model incorporating task effects for the two different image types of interest (stigmatized individuals and IAPS images), and covariates of no interest (a session mean, a linear trend and six movement parameters derived from realignment corrections) to compute parameter estimates (β) and t-contrast images (containing weighted parameter estimates) for each comparison at each voxel and for each subject.
Our analyses focused on two types of neural activity in regulation: onset-related activity and activity over the sustained regulatory period. We measured onset-related neural activity to elucidate the mechanisms engaged in initiating regulation to images of stigmatized individuals and non-stigmatized negative controls. We also examined neural activity engaged over the entire regulatory period to determine whether the onset of regulatory attempts paralleled sustained regulatory attempts.
We conducted two separate types of analyses to best capture the neural response in the onset of regulation and sustained regulation. To identify neural activity engaged in the onset of regulation, we used an event-related design that modeled the first 2 s from trials in which participants were asked to decrease their affect to stigmatized face minus trials in which they were asked to decrease their affect to IAPS images. In order to examine the effects over the sustained regulatory period, we modeled each 8-s regulatory period as a block. Here, we created separate regressors for the trials for decrease stigma, decrease IAPS, attend stigma and attend IAPS. For both analyses, average parameter estimates were extracted from these peak activations by using the contrast from each condition relative to baseline fixation to conduct a region of interest (ROI) analysis. ROIs were extracted using the functional ROIs tool in SPM8 (marsbar). All significant voxels (P < 0.001 uncorrected, 5 voxel extent threshold) within 8 mm of a peak location were included in each ROI. Due to the relatively small number of trials in each condition, we also examined the results at a more liberal threshold of P < 0.05 corrected. In order to calculate the corrected threshold, we used a Monte Carlo conversion script from Slotnick et al. (2003) to determine the extent threshold required to convert P < 0.005 uncorrected to P < 0.05 corrected. We chose 1000 iterations of the Monte Carlo to select the most conservative threshold (13 cluster extent threshold).
RESULTS
Behavioral results
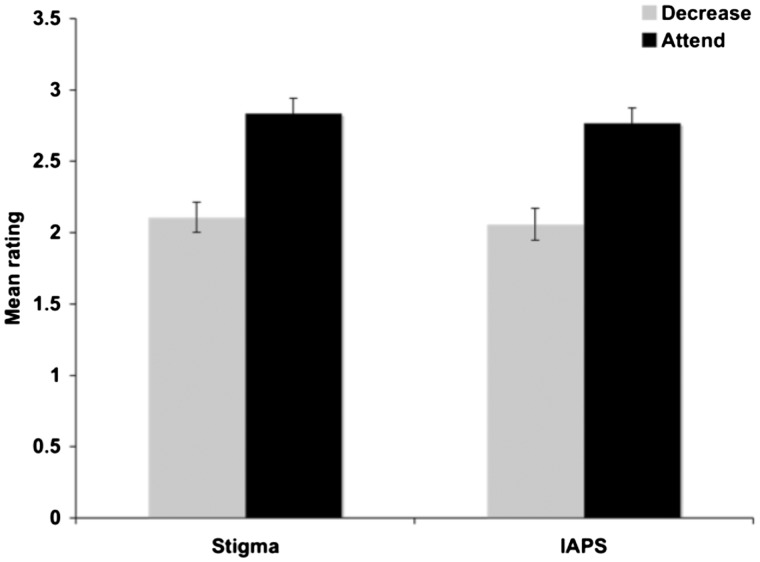
We entered participants’ behavioral ratings of their negative affect toward the stigmatized images and the IAPS images into a 2 (instruction: decrease or attend) × 2 (image type: stigma vs IAPS) ANOVA. Results revealed a main effect of instruction [F(1,16) = 39.88, P < 0.001], but no effect of image type [F(1,16) = 1.39, P = 0.26] or interaction (F < 1). Subsequent analyses demonstrated that the effect of instruction emerged because participants expressed stronger negative affect toward both the stigmatized target images and the IAPS images in the attend condition than they did in the decrease condition (P < 0.001 for both). However, affect ratings for the two sets of images did not differ in either the attend or the decrease condition (P > 0.28 for both). Thus, participants reported being able to regulate their negative affect toward both the stigmatized and the IAPS images in the decrease condition with equal efficacy (Figure 2).
Fig. 2.
Mean ratings (and s.e.m) of participants’ negative affect toward the stigma and IAPS images during the ‘decrease’ and ‘attend’ conditions (1 = very weak negative emotion, 4 = very strong negative emotion). Ratings indicate that participants successfully decreased their negative affect when instructed to do so for both the stigma and the IAPS images.
Implicit attitudes
In order to determine participants’ implicit attitudes toward homeless individuals and alcoholics, we compared participants’ mean reaction time for the congruent block (in which the stigma category was paired with unpleasant words) to their mean reaction times on the incongruent block (in which the stigma category was paired with pleasant words).
Participants demonstrated IAT bias against the stigmatized targets on both tasks, with reaction times for the stereotypically incongruent blocks (homeless: MRT = 871.35 ms; alcoholics: MRT = 853.10 ms) being significantly longer than reaction times for the stereotypically congruent blocks (homeless: MRT = 666.19 ms; alcoholics: MRT = 753.00 ms, P < 0.03 for both).
We also examined whether participants’ implicit bias affected their ability to decrease their negative bias to the stigmatized targets. That is, did individuals with more bias toward homeless people and alcoholics express more negative affect toward these targets in the decrease and attend conditions? To examine this question, we correlated each participant's IAT bias for homeless individuals and substance abusers with his or her negative affect ratings for the respective stigma groups during the decrease and attend conditions. We found no significant correlation between participants’ reported negative affect and their implicit bias for either homeless people or alcoholics in any condition (P > 0.5 for all). These results suggest that greater negative implicit bias did not affect participants’ behavioral performance on the task.
In order to examine whether participants’ IAT bias affected their pattern of neural activity when regulating their negative bias, we calculated an overall IAT bias score for each participant to compare with their imaging results. The overall IAT bias score was calculated by averaging the two IAT scores together.
Imaging results
In order to verify that we replicated previous findings in the emotion regulation research, we first examined the decrease IAPS > attend IAPS contrast, as well as the attend IAPS > decrease IAPS contrast over the full 8-s regulatory period.2 Results were consistent with previous findings (Ochsner et al., 2002, 2004). Specifically, we found heightened activation throughout the lateral and medial PFC in the decrease IAPS > attend IAPS contrast (Table 1 for complete list of activations). However, in the attend IAPS > decrease IAPS contrast, we found heightened activation in the amygdala, and throughout the temporal and parietal cortices (Table 1 for complete list of activations).
Table 1.
Full list of activations that were significantly active in the decrease IAPS > attend IAPS and attend IAPS > decrease IAPS
| Brain region | x | y | z | K extent | t-score |
|---|---|---|---|---|---|
| Decrease IAPS > attend IAPS | |||||
| Left precentral gyrus (BA 4) | −39 | −27 | 72 | 2931 | 7.24 |
| Right middle frontal gyrus (BA 10) | 30 | 51 | 0 | 102 | 5.12 |
| Left superior frontal gyrus (BA 11) | −18 | 57 | −18 | 233 | 3.16 |
| Right inferior temporal gyrus (BA 20) | 63 | −36 | −21 | 60 | 3.64 |
| Left inferior frontal gyrus (BA 45) | −57 | 18 | 6 | 28 | 3.52 |
| Right superior frontal gyrus (BA 9) | 15 | 57 | 18 | 27 | 3.52 |
| Left middle frontal gyrus (BA 46/10) | −30 | 45 | 9 | 199 | 3.41 |
| Left middle frontal gyrus (BA 8/9) | −24 | 12 | 45 | 208 | 3.37 |
| Left inferior parietal lobule | −18 | −63 | −51 | 39 | 3.32 |
| Right middle temporal lobe (BA 37) | 33 | −63 | 18 | 42 | 3.28 |
| Right superior frontal lobe (BA 6) | 30 | 12 | 54 | 39 | 3.21 |
| Left superior parietal lobule | −9 | −66 | 54 | 18 | 2.97 |
| Right superior frontal gyrus (BA 9) | 21 | 42 | 36 | 49 | 2.86 |
| Right orbitofrontal cortex (BA 11) | 24 | 48 | −24 | 27 | 2.81 |
| Right inferior frontal gyrus (BA 47) | 60 | 21 | −15 | 26 | 2.76 |
| Right superior frontal gyrus (BA 11) | 9 | 66 | −9 | 20 | 2.23 |
| Attend IAPS > decrease IAPS | |||||
| Left precentral gyrus (BA 4) | −39 | −27 | 72 | 2931 | 7.24 |
| Right cerebellum | 9 | −54 | −12 | 640 | 5.36 |
| Left brainstem, midbrain | −18 | −24 | −6 | 73 | 5.32 |
| Left inferior parietal lobe (BA 40) | 24 | −33 | 30 | 28 | 4.40 |
| Right middle frontal gyrus (BA 45) | 51 | 48 | 12 | 53 | 4.31 |
| Right hypothalamus | 3 | −3 | −12 | 24 | 3.74 |
| Left cerebellum | −18 | −78 | −36 | 294 | 3.59 |
| Left middle frontal gyrus (BA 11) | −24 | 42 | −6 | 33 | 3.53 |
| Right hippocampus | 27 | −9 | −24 | 69 | 3.28 |
| Right brainstem, midbrain | 9 | −12 | −21 | * | 2.92 |
| Right parahippocampal gyrus (BA 34) | 15 | −9 | −27 | * | 2.74 |
| Right amygdala | 20 | −3 | −27 | * | 2.47 |
| Right hippocampus | 33 | −18 | −24 | * | 2.27 |
| Left precentral gyrus (BA 6) | −63 | −3 | 39 | 23 | 3.21 |
| Right postcentral gyrus (BA 3) | 18 | −39 | 69 | 20 | 3.05 |
| Left brainstem, midbrain | −6 | −18 | −18 | 19 | 3.02 |
| Left occipital gyrus (BA 19) | −36 | −72 | −3 | 92 | 2.88 |
| Left inferior parietal (BA 40) | −27 | −30 | 27 | 25 | 2.84 |
| Left caudate nucleus | −15 | 0 | 24 | 60 | 2.83 |
| Left superior temporal gyrus (BA 38) | −51 | 15 | −21 | 31 | 2.79 |
| Left inferior frontal gyrus (BA 45) | −39 | 21 | 9 | 32 | 2.79 |
| Left middle frontal gyrus (BA 10) | −15 | 42 | 15 | 24 | 2.76 |
| Right precuneus | 21 | −63 | 42 | 37 | 2.73 |
| Left inferior parietal lobule | −48 | −42 | −30 | 30 | 2.70 |
| Right anterior cingulate cortex (BA 32) | 12 | 3 | 30 | 16 | 2.63 |
| Left middle temporal lobe (BA 21/22) | −42 | −48 | 3 | 26 | 2.50 |
| Right middle frontal gyrus (BA 10) | 15 | 54 | 0 | 32 | 2.43 |
| Left middle occipital gyrus (BA 19) | −27 | −87 | 12 | 19 | 2.43 |
| Right middle occipital gyrus (BA 19) | 27 | −90 | −15 | 30 | 2.42 |
| Right middle frontal gyrus (BA 10) | 36 | 54 | 24 | 16 | 2.09 |
Values are contrast at P < 0.05, corrected. All co-ordinates are MNI. For attend IAPS > decrease IAPS, we also report subclusters extending into the amygdala.
Having established that we replicated previous findings in emotion regulation research, we next focused our analyses on our central question: does the regulation of initial negative affect to stigmatized targets have a unique activity profile from more general emotion regulation? In order to best examine this question, we conducted two contrasts of interest to dissociate the neural mechanisms engaged in sustained regulatory attempts from those engaged in the onset of regulatory attempts: the former compared the difference in neural activation between stigma regulation and more general emotion regulation during the sustained 8-s regulatory period, the second compared stigma regulation with more general emotion regulation using an event-related design tethered to the onset of regulatory attempts (first 2 s). The findings for each of the two time points are discussed below.
Blocked-design analyses: neural mechanisms engaged when viewing stigma and IAPS images over 8s
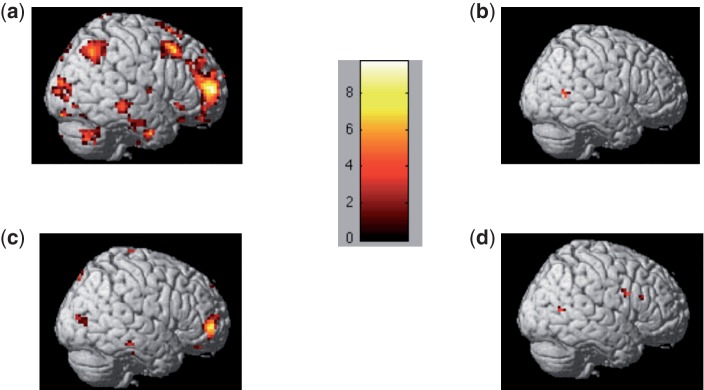
We compared neural activation for stigma and IAPS images in the two instruction conditions (attend and decrease) over the entire 8-s regulatory block. Here, we found more widespread activation in response to the IAPS as compared with the stigma images, regardless of regulation instruction. In fact, no region was more active in the stigma > IAPS contrast over the 8-s regulatory block in either the decrease or attend conditions (Figure 3A and C; Table 2). Conversely, in both the decrease and attend IAPS > stigma conditions, a vast network of heightened activity was observed over the 8-s regulatory block (Figure 3B and D; Table 2). Interestingly, in both instruction conditions, these activations were centered primarily around the temporal and occipital lobes, with only moderate activation differences in the PFC.
Fig. 3.
Neural activation in the initial 2 s for the contrasts for (A) decrease stigma > decrease IAPS, (B) decrease IAPS > decrease stigma, (C) attend stigma > attend IAPS, (D) attend IAPS > attend stigma. Results demonstrate that participants have heightened activation in the PFC when they engage initial image processing to stigmatized as compared with non-stigmatized IAPS images. Contrasts are thresholded at P < 0.001, uncorrected. Color-coded bar shows the t-values for the contrast analyses.
Table 2.
The full list of significant activations that emerged over the full 8-s time regulation period
| Brain region | x | y | z | K extent | t-score |
|---|---|---|---|---|---|
| Decrease stigma vs decrease IAPS | |||||
| None | |||||
| Decrease IAPS vs decrease stigma | |||||
| Right precentral gyrus (BA 4) | −39 | −15 | 48 | 23 | 5.86 |
| Left superior temporal sulcus (BA 22) | −45 | −21 | −3 | 30 | 5.82 |
| Left superior temporal gyrus (BA 22) | −48 | −60 | 15 | 13 | 5.17 |
| Right middle temporal lobe (BA 37) | 39 | −63 | 18 | 18 | 5.03 |
| Left middle temporal lobe (BA 37) | −51 | −66 | 6 | 29 | 5.02 |
| Right optic radiation | 36 | −24 | −6 | 5 | 4.98 |
| Right inferior parietal lobe (BA 40) | 45 | −33 | 27 | 12 | 4.96 |
| Right parahippocampal gyrus | 27 | −42 | −6 | 15 | 4.90 |
| Left paracentral lobule (BA 5) | −9 | −30 | 54 | 12 | 4.88 |
| Right superior temporal gyrus (BA 38) | 33 | 9 | −30 | 10 | 4.78 |
| Left fusiform gyrus (BA 20) | −33 | −39 | −24 | 11 | 4.67 |
| Left middle frontal gyrus (BA 10) | −3 | 54 | −9 | 9 | 4.50 |
| Left orbitofrontal gyrus (BA 11) | −18 | 60 | −18 | 5 | 4.32 |
| Right middle temporal gyrus (BA 21) | 42 | −15 | −12 | 17 | 4.32 |
| Left inferior frontal gyrus (BA 46) | −36 | 33 | 12 | 5 | 4.31 |
| Right fusiform gyrus (BA 37) | 39 | −45 | −18 | 9 | 4.31 |
| Left superior frontal gyrus (BA 6) | −18 | −9 | 69 | 15 | 4.31 |
| Right superior temporal sulcus (BA 22) | 48 | 0 | −9 | 6 | 4.22 |
| Left putamen | −30 | −6 | 3 | 5 | 3.99 |
| Right inferior frontal gyrus (BA 45/46)* | 36 | 39 | 6 | 25 | 4.15 |
| Right putamen* | 21 | 12 | −12 | 22 | 4.06 |
| Left precentral gyrus (BA 4)* | −15 | −30 | 72 | 23 | 4.01 |
| Right precentral gyrus (BA 4)* | 15 | −27 | 48 | 14 | 4.00 |
| Left inferior frontal gyrus (BA 44/45)* | −36 | 18 | 21 | 39 | 4.00 |
| Left putamen* | −30 | −6 | 3 | 29 | 3.99 |
| Right middle frontal gyrus (BA 46)* | 51 | 21 | 24 | 20 | 3.97 |
| Right precentral gyrus (BA 4)* | 30 | −30 | 69 | 25 | 3.91 |
| Right occipital lobe (BA 7)* | 24 | −81 | 30 | 32 | 3.71 |
| Left insula (BA 13)* | −39 | −15 | 18 | 13 | 3.69 |
| Left insula (BA 13)* | −42 | 9 | 3 | 15 | 3.60 |
| Left inferior occipital gyrus (BA 19)* | −39 | −78 | −9 | 27 | 3.60 |
| Right precentral gyrus (BA 6)* | 57 | 6 | 9 | 13 | 3.36 |
| Attend stigma vs attend IAPS | |||||
| None | |||||
| Attend IAPS vs attend stigma | |||||
| Right superior temporal sulcus (BA 22) | 45 | −3 | −12 | 91 | 7.39 |
| Right occipital lobe (BA 7) | 24 | −84 | 30 | 51 | 6.56 |
| Right inferior frontal gyrus (BA 46) | 54 | 42 | 9 | 27 | 5.54 |
| Right inferior frontal gyrus (BA 45) | 45 | −9 | 21 | 19 | 5.59 |
| Left superior temporal gyrus (BA 38) | −51 | −3 | −9 | 22 | 5.54 |
| Right inferior frontal gyrus (BA 44) | 39 | 3 | 36 | 28 | 5.43 |
| Left superior temporal gyrus (BA 41) | −45 | −30 | 18 | 12 | 5.35 |
| Right inferior temporal gyrus (BA 19) | 45 | −66 | −6 | 184 | 5.19 |
| Left cerebellum | −6 | −78 | −33 | 36 | 5.06 |
| Right superior temporal gyrus (BA 22) | 60 | −27 | 15 | 57 | 4.99 |
| Right lingual gyrus | 21 | −60 | −9 | 29 | 4.88 |
| Left fusiform gyrus (BA 37) | −36 | −45 | −21 | 10 | 4.86 |
| Left lingual gyrus | −12 | −69 | −6 | 8 | 4.84 |
| Right superior temporal gyrus (BA 42) | 63 | −18 | 6 | 7 | 4.81 |
| Left middle frontal gyrus (BA 6) | −9 | −30 | 69 | 17 | 4.73 |
| Left middle frontal gyrus (BA 6) | −54 | 6 | 48 | 13 | 4.72 |
| Left middle frontal gyrus (BA 9)* | −48 | 36 | 36 | 91 | 4.56 |
| Right parahippocampal gyrus | 24 | −45 | −6 | 17 | 4.71 |
| Right paracentral lobule (BA 4) | 18 | −39 | 51 | 8 | 4.59 |
| Left middle occipital lobe (BA 19) | −51 | −75 | −15 | 8 | 4.59 |
| Left inferior temporal gyrus (BA 37) | −51 | −66 | −3 | 24 | 4.53 |
| Left postcentral gyrus (BA 2) | −60 | −21 | 27 | 13 | 4.47 |
| Left middle frontal gyrus (BA 9) | −51 | 27 | 33 | 6 | 4.42 |
| Right transverse temporal lobe (BA 41) | 33 | −33 | 3 | 9 | 4.37 |
| Left insula* | −39 | 12 | 3 | 30 | 4.22 |
| Right posterior cingulate (BA 31) | 12 | −66 | 15 | 8 | 4.21 |
| Right fusiform gyrus (BA 37) | 39 | −54 | −24 | 11 | 4.14 |
| Right middle temporal gyrus (BA 21) | 39 | 3 | −36 | 16 | 4.09 |
| Right cingulate gyrus (BA 23)* | 12 | −66 | 15 | 16 | 4.06 |
| Right putamen | 27 | 3 | 3 | 6 | 4.04 |
| Right fusiform gyrus (BA 37) | 36 | −54 | −9 | 5 | 4.00 |
| Left putamen* | −27 | 9 | 0 | 20 | 3.94 |
| Left middle occipital lobe (BA 19)* | −24 | −87 | 21 | 18 | 3.65 |
| Right precentral gyrus (BA 6)* | 27 | −21 | 66 | 19 | 3.64 |
| Left precuneus* | −18 | −75 | 36 | 23 | 3.63 |
| Right inferior parietal lobe (BA 40)* | 51 | −33 | 30 | 23 | 3.62 |
| Left putamen* | −30 | −6 | 0 | 17 | 3.60 |
| Left inferior frontal gyrus (BA 45)* | −39 | 18 | 21 | 44 | 3.59 |
All co-ordinates are MNI. Unless note with *, all activations reported here are significant at P < .001 uncorrected with 5 voxel extent threshold. * denotes additional significant activations at P < .05 corrected. Tables include: decrease stigma > decrease IAPS; decrease IAPS > decrease stigma; attend stigma > attend IAPS; attend IAPS > attend stigma.
In the decrease IAPS > decrease stigma conditions, we observed heightened activation in the temporal lobes, including bilateral BA 22 and bilateral BA 37 (left and right BA 22; left and right BA 37), bilateral fusiform gyrus, including left BA 20 and right BA 37 and right inferior parietal lobe (BA 40) (Table 2). There was also heightened activation in this contrast in some areas of the PFC, specifically left VLPFC (BA 46), left medial PFC (BA 10) and left orbitofrontal cortex (BA 11).
In the attend IAPS > attend stigma contrast, there was heightened activation in the bilateral temporal lobe (right BA 22, left BA 38, left BA 41, right BA 19), bilateral fusiform gyrus (left and right BA 37), the bilateral occipital lobe (left BA 19 and right BA 7), as well as increased activation in select areas of the PFC, including right VLPFC (BA 44, BA 45 and BA 46) and left middle frontal gyrus (BA 9).
Together, these results suggest that over the 8-s span, there was increased activation in IAPS > stigma images, regardless of regulation instruction. These activations were predominantly posterior (including the temporal and occipital lobes), but included some increased activation in the PFC as well.
Event-related analyses: neural mechanisms engaged at the onset of the regulatory period for stigma and IAPS images
We next examined whether the processes engaged during the initial regulation attempts of negative affect to stigmatized targets are unique from those engaged in initial regulation of negative affect to non-stigmatized negative targets. Specifically, we identified neural activation in the onset of regulation (which we defined as the activity that arose in an event-related analysis centered on the first TR (i.e. the first 2 s) following image presentation for stigma regulation as compared to more general emotion regulation. Imaging data identified widespread neural activations that were greater during the first 2 s of the decrease to stigma condition as compared with the decrease to IAPS condition (Table 3 and Figure 4A). Much of this activation was centered in the PFC, notably bilateral DLPFC (right BA 10, right BA 9 and left BA 9), bilateral orbitofrontal cortex (right and left BA 11, and right BA 10), bilateral VLPFC (right BA 46, right BA 46/10, right and left BA 47), right ACC (right BA 32) and bilateral medial prefrontal cortex (MPFC) (right and left BA 10).
Table 3.
The full list of significant activations that emerged in the 2-s analysis
| Brain region | x | y | z | K extent | t-score |
|---|---|---|---|---|---|
| Decrease stigma vs decrease IAPS | |||||
| Right middle frontal gyrus (BA 10) | 39 | 57 | 6 | 718 | 9.75 |
| Right orbitofrontal cortex (BA 11) | 21 | 24 | −12 | * | 7.43 |
| Right superior frontal gyrus (BA 10) | 18 | 54 | 0 | * | 7.18 |
| Right middle frontal gyrus (BA 46) | 48 | 45 | 18 | * | 6.47 |
| Right orbitofrontal gyrus (BA 10) | 15 | 51 | −9 | * | 5.86 |
| Right middle frontal gyrus (BA46/10) | 51 | 48 | 21 | * | 5.80 |
| Right middle frontal gyrus (BA 10) | 0 | 51 | 0 | * | 5.59 |
| Right orbitofrontal gyrus (BA 11) | 15 | 48 | −21 | * | 5.42 |
| Right anterior cingulate cortex (BA 32) | 12 | 45 | −6 | * | 5.40 |
| Right superior frontal gyrus (BA 10) | 18 | 57 | 21 | * | 5.38 |
| Right orbitofrontal cortex (BA10/11) | 24 | 48 | −6 | * | 5.37 |
| Right anterior cingulate cortex (BA 32) | 15 | 45 | 6 | * | 5.25 |
| Right middle frontal gyrus (BA 11) | 36 | 51 | −12 | * | 5.23 |
| Right middle frontal gyrus (BA 10) | 33 | 48 | −9 | * | 5.20 |
| Left middle frontal gyrus (BA 10) | −9 | 60 | 6 | * | 5.01 |
| Right middle frontal gyrus (BA 8) | 45 | 21 | 51 | 112 | 7.60 |
| Left middle frontal gyrus (BA 6) | −6 | 27 | 39 | 75 | 7.13 |
| Left cingulate gyrus (BA 23) | −6 | −27 | 27 | 391 | 6.75 |
| Left superior frontal gyrus (BA 9) | −42 | 33 | 33 | 354 | 6.44 |
| Right inferior parietal lobe (BA 7) | 42 | −63 | 45 | 150 | 5.84 |
| Right middle temporal gyrus (BA 21) | 66 | −30 | −6 | 47 | 5.83 |
| Left inferior parietal lobe (BA 40) | −33 | −42 | 30 | 7 | 5.75 |
| Left cerebellum | −24 | −84 | −27 | 27 | 5.40 |
| Right parahippocampal gyrus (BA 34) | 18 | 3 | −15 | 41 | 5.35 |
| Left parietal lobe (BA 40) | −24 | −33 | 33 | 5 | 5.31 |
| Left parahippocampal gyrus (BA 34) | −18 | 3 | −18 | 19 | 5.31 |
| Right cerebellum | 39 | −69 | −33 | 71 | 5.30 |
| Left orbitofrontal gyrus (BA 11) | −12 | 54 | −24 | 34 | 5.30 |
| Right cerebellum | 21 | −48 | −24 | 21 | 5.29 |
| Left superior frontal gyrus (BA 8) | −9 | 27 | 60 | 97 | 5.29 |
| Left inferior frontal gyrus (BA 47) | −48 | 21 | −9 | 68 | 5.28 |
| Left cerebellum | −36 | −38 | −42 | 79 | 5.23 |
| Right inferior frontal gyrus (BA 47) | 48 | 24 | −9 | 15 | 5.22 |
| Right inferior occipital gyrus (BA 18) | 51 | −78 | −6 | 20 | 5.08 |
| Left caudate | −36 | −30 | −3 | 16 | 4.99 |
| Left cerebellar tonsil | −6 | −48 | −51 | 35 | 5.13 |
| Left parietal lobe (BA 40) | −30 | −72 | 42 | 40 | 4.88 |
| Right superior frontal gyrus (BA 6) | 21 | 27 | 60 | 10 | 4.79 |
| Right inferior frontal gyrus (BA 47) | 27 | 18 | −21 | 10 | 4.72 |
| Right superior frontal gyrus (BA 9) | 21 | 48 | 36 | 25 | 4.71 |
| Left cerebellum | −27 | −45 | −24 | 7 | 4.69 |
| Right cerebellum | 3 | −69 | −36 | 6 | 4.58 |
| Right middle occipital gyrus (BA 19) | 45 | −84 | 0 | 15 | 4.56 |
| Right superior temporal gyrus (BA 22) | 63 | −3 | 6 | 5 | 4.52 |
| Left lingual gyrus (BA 18) | −6 | −81 | −15 | 9 | 4.49 |
| Right cerebellum | 3 | −60 | −51 | 9 | 4.42 |
| Right superior frontal gyrus (BA 8) | 15 | 51 | 45 | 6 | 4.39 |
| Left precuneus | −12 | −69 | 42 | 7 | 4.38 |
| Left middle frontal gyrus (BA 8) | −18 | 30 | 42 | 6 | 4.32 |
| Left middle frontal gyrus (BA 8)* | −21 | 24 | −15 | 29 | 4.22 |
| Left inferior parietal (BA 40) | −45 | −60 | 51 | 5 | 3.98 |
| Left cingulate gyrus (BA 24)* | −15 | −6 | 27 | 30 | 4.11 |
| Left inferior parietal lobe (BA 40)* | −45 | −54 | 42 | 65 | 4.10 |
| Right parahippocampal gyrus (BA 28)* | 21 | −21 | −21 | 23 | 4.02 |
| Left middle frontal gyrus (BA 6/8)* | −36 | 12 | 45 | 20 | 3.95 |
| Decrease IAPS vs decrease stigma | |||||
| Left inferior parietal lobe (BA 39) | −48 | −66 | 15 | 75 | 6.29 |
| Right middle temporal gyrus (BA 37) | 54 | −57 | 6 | 11 | 4.32 |
| Attend stigma vs attend IAPS | |||||
| Right middle frontal gyrus (BA 10) | 39 | 54 | 3 | 73 | 9.25 |
| Left middle frontal gyrus (BA 10) | −33 | 54 | 15 | 126 | 6.89 |
| Right caudate nucleus | 12 | 21 | 0 | 51 | 6.44 |
| Left inferior temporal gyrus (BA 20) | −57 | −30 | −18 | 36 | 6.40 |
| Left caudate nucleus | −21 | 18 | 9 | 5 | 5.81 |
| Left putamen | −18 | 19 | −9 | 31 | 4.20 |
| Right middle occipital gyrus (BA 19) | 33 | −72 | 3 | 31 | 5.89 |
| Right pulvinar nucleus | 18 | −36 | 12 | 11 | 5.34 |
| Right lingual gyrus | 9 | −75 | −6 | 26 | 4.80 |
| Left cerebellum | −57 | −60 | −33 | 10 | 4.64 |
| Right inferior temporal gyrus (BA 20) | 63 | −30 | −18 | 6 | 4.89 |
| Left cingulate gyrus (BA 23)* | −3 | −24 | 33 | 142 | 4.72 |
| Left middle frontal gyrus (BA 8)* | −24 | 30 | 42 | 67 | 4.39 |
| Right inferior frontal gyrus (BA 45) | 27 | 36 | 12 | 22 | 4.84 |
| Left middle frontal gyrus (BA 10) | −9 | 48 | 6 | 5 | 4.39 |
| Right orbitofrontal gyrus (BA 11) | 18 | 36 | −12 | 18 | 4.79 |
| Left middle frontal gyrus (BA 8) | −24 | 30 | 42 | 18 | 4.70 |
| Left cerebellum | −42 | −84 | −33 | 8 | 4.59 |
| Right anterior cingulate gyrus (BA 32) | 6 | 39 | −6 | 22 | 4.59 |
| Left parahippocampal gyrus (BA 37) | −33 | −39 | −12 | 6 | 4.54 |
| Right inferior temporal lobe (BA 20)* | 42 | −33 | −9 | 21 | 4.35 |
| Left hippocampus | −33 | −27 | −15 | 6 | 4.30 |
| Left parahippocampal gyrus* | −27 | −48 | 3 | 27 | 4.27 |
| Left middle frontal gyrus (BA 8) | −36 | 15 | 48 | 14 | 4.14 |
| Left orbitofrontal gyrus (BA 11)* | −18 | 36 | −27 | 11 | 4.11 |
| Left caudate nucleus* | −24 | −36 | 18 | 20 | 4.08 |
| Right precentral gyrus (BA 6)* | 27 | 0 | 33 | 25 | 4.05 |
| Left inferior parietal cortex (BA 40) | 30 | −36 | 24 | 14 | 4.02 |
| Left cingulate cortex (BA 23) | −9 | −57 | 18 | 13 | 3.67 |
| Attend IAPS vs attend stigma | |||||
| Left superior temporal gyrus (BA 20) | −54 | −63 | 18 | 94 | 6.55 |
| Right inferior frontal gyrus (BA 44) | 48 | 6 | 27 | 26 | 4.34 |
| Right middle temporal gyrus (BA 39) | 54 | −60 | 9 | 8 | 4.23 |
| Right superior temporal gyrus (BA 22)* | 63 | −39 | 9 | 16 | 4.15 |
| Left precentral gyrus (BA 6)* | −45 | 9 | 30 | 62 | 3.87 |
| Right inferior parietal lobe (BA 40)* | 60 | −45 | 21 | 15 | 3.36 |
All co-ordinates are MNI. Unless note with *, all activations reported here are significant at P < .001 uncorrected with 5 voxel extent threshold. * denotes additional significant activations at P < .05 corrected. Tables include: decrease stigma > decrease IAPS; decrease IAPS > decrease stigma; attend stigma > attend IAPS; attend IAPS > attend stigma.
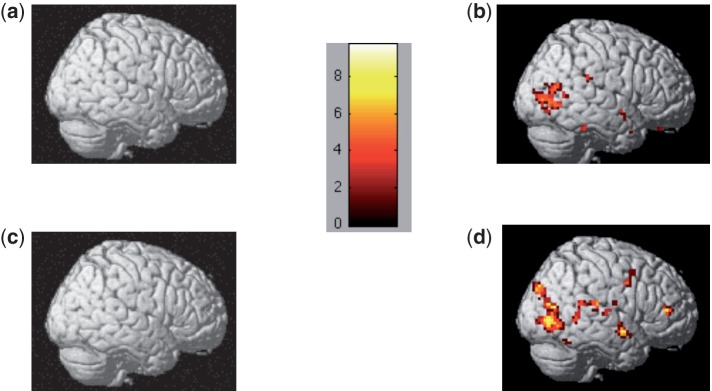
Fig. 4.
Neural activation over the entire 8-s regulatory window for the contrasts for (A) decrease stigma > decrease IAPS, (B) decrease IAPS > decrease stigma, (C) attend stigma > attend IAPS, (D) attend IAPS > attend stigma. Results demonstrate that participants have heightened activation in posterior cortical regions when they engage image processing to non-stigmatized IAPS as compared to stigmatized images. Color-coded bar shows the t-values for the contrast analyses.
We next conducted the reverse contrast: decrease IAPS > decrease stigma. Here, we found a greatly reduced activation pattern than what we observed in the stigma > IAPS contrasts for both instruction conditions. Specifically, for decrease IAPS > decrease stigma (Figure 4C), results revealed heightened activation only in the left inferior parietal lobe (BA 39) and right middle temporal gyrus (BA 37; Table 3).
In the event-related (onset) analysis of the attend stigma > attend IAPS condition, participants also had heightened activation throughout the PFC (Figure 4B and Table 3). Just as in the decrease stigma > decrease IAPS contrast, much of this activity was localized to the PFC, including bilateral DLPFC (right and left BA 10), right VLPFC (BA 45), left MPFC (BA 10), right orbitofrontal gyrus (BA 11) and right ACC (BA 32). In the attend IAPS > attend stigma contrast, however, we found heightened activation only in the bilateral temporal gyrus (left BA 20 and right BA 39) as well as the right inferior frontal gyrus (BA 44; Figure 4C and Table 3).
These results demonstrated that participants had an increased neural response throughout the PFC when initially evaluating an image of a stigmatized individual than when initially evaluating an image of a non-stigmatized, negative image.
IAT bias and neural activation
Finally, we examined whether implicit bias towards the stigmatized groups could explain the increased activation we observed in the PFC regions that were more active in the decrease stigma than the decrease IAPS conditions as revealed in the event-related (onset) analyses. Our primary interest in this analysis was to determine whether individual differences in implicit bias were related to some of the activity we observed in the prefrontal regions that were unique to initiating regulatory responses to stigmatized targets. Thus, we focused our analyses on the peak activations observed in the PFC in the decrease stigma > decrease IAPS condition. This analysis isolated neural regions unique to perceiving stigma over IAPS images during the onset of regulatory attempts. We calculated a difference score between the neural activity in the decrease stigma condition and the neural activity in the attend stigma condition to isolate neural activity that was unique to decreasing negative affect to stigma.3 The resulting scores were entered into a Pearson bivariate correlation with each participant's respective IAT bias score.
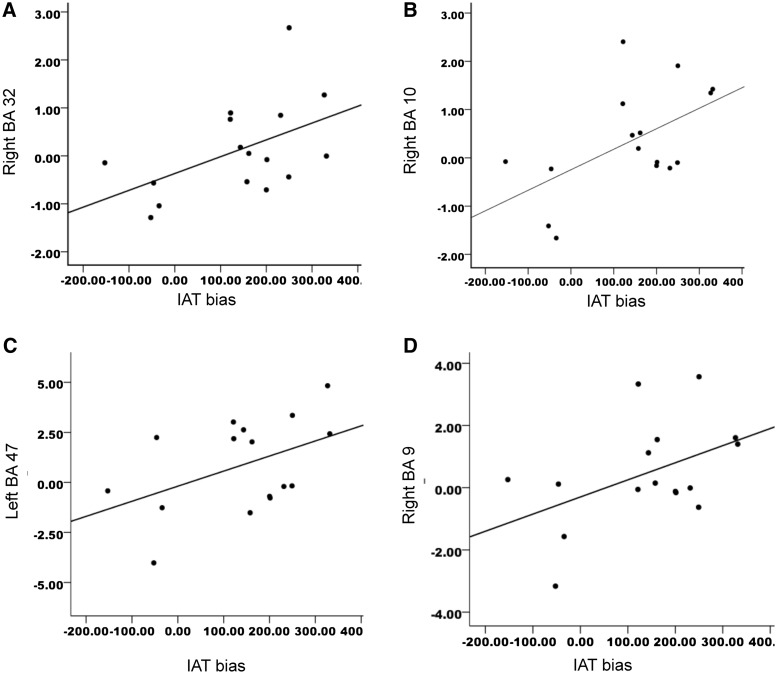
IAT bias correlated significantly with the right ACC (BA 32: 12, 45, − 6) and right superior frontal gyrus [BA 10: 18, 57, 21; right BA 32: r(16) = 0.50, P < 0.05; right BA 10: r(16) = 0.55, P < 0.03], with a trend in left VLPFC (BA 47: −48, 21, −9) and right superior frontal gyrus (left BA 47: r(16) = 0.46, P = 0. 07; right BA 9: r(16) = 0.47, P = 0.07), see Figure 5. Thus, the more implicit bias that the participants had toward the stigmatized groups, the greater their neural activation in certain prefrontal regions.
Fig. 5.
Correlations between neural activity for the first 2 s in the decrease stigma condition and IAT bias: (A) IAT bias is correlated with neural right ACC (BA 32); (B) IAT bias and right BA 10; trends between (C) IAT bias and activity in left VLPFC (BA 47) and (D) IAT bias and right superior frontal gyrus (BA 9).
DISCUSSION
There were two main findings from this study. First, the regulation of initial negative affect to stigmatized targets has a unique activity profile compared to more general emotion regulation. Specifically, participants had higher activity in the ACC, as well as the lateral and medial PFC during their initial attempts to regulate their negative affect to images of stigmatized individuals as compared to images of non-stigmatized individuals. Second, we found time course differences between stigma regulation and more general emotion regulation. Participants showed increased neural activity during stigma regulation as compared with general emotion regulation during the onset of regulation. However, general emotion regulation elicited more pronounced differences in activation as compared to stigma regulation over the sustained regulatory period.
Together, our findings demonstrate that stigma regulation has a unique activity profile from general emotion regulation. Specifically, the time course differences suggest that stigma regulation may be a more immediate response, whereas emotion regulation may be relatively more prolonged. Although these time course differences are intriguing, it is important to note that our study was designed to examine whether any region differences existed between stigma regulation and more general emotion regulation, and not to examine potential time course differences. In order to best interrogate these differences, future studies should examine the time course involved in stigma and emotion regulation more closely. However, it is important to note that previous research using electroencephalography provides converging evidence that regulating negative bias to stigma may be relatively automatic, consistent with the potential time course differences we observed in the present study. For instance, Amodio et al. (2004) found that the anterior cingulate cortex was more active when participants had the potential for making race-biased errors even before those errors occurred (see also Amodio et al., 2006, 2008).
Our main question concerned the neural activity profile in stigma regulation as compared to general emotion regulation. In the initial regulatory efforts, stigma regulation elicited heightened neural response primarily in the PFC as compared with more general emotion regulation. These prefrontal regions, notably the ACC, VLPFC and DLPFC, are all regions that have been previously implicated in evaluating stigma (Richeson et al., 2003; Cunningham et al., 2004; Wheeler and Fiske, 2005; Krendl et al., 2006). Our study extends these previous findings in two ways. First, we used a direct manipulation of regulation to demonstrate that these prefrontal areas do in fact play a critical role in downregulating negative bias (Vogeley et al., 2001; Cunningham et al., 2004; Samson et al., 2005; Krendl et al., 2006; Satpute and Lieberman, 2006). Second, our study establishes that these prefrontal areas are involved in downregulating negative bias to outgroups other than just the domain of race (Phelps et al., 2000; Richeson et al., 2003; Cunningham et al., 2004; Knutson et al., 2007).
Based on previous research, the activation we observed throughout the PFC plays a converging and supporting role in downregulating negative bias. For instance, the ACC is likely involved in identifying situations in which regulation and control are needed (MacDonald et al., 2000; Amodio et al., 2004), whereas the lateral PFC is involved in implementing and maintaining regulation (Lieberman et al., 2002; Lieberman, 2003; Richeson et al., 2003; Cunningham et al., 2004; Krendl et al., 2006). Specifically, the VLPFC is involved in downregulation and inhibition (Vogeley et al., 2001; Samson et al., 2005; Satpute and Lieberman, 2006), whereas the DLPFC is associated with goal maintenance, such as in controling unwanted prejudice thoughts (Richeson et al., 2003; Knutson et al., 2007).
Many of the same PFC activations that we observed in the decrease stigma > decrease IAPS conditions were also active in the attend stigma > attend IAPS condition (mPFC, ACC, orbitofrontal cortex and, to a lesser extent, VLPFC). One potential explanation for this finding is that even when attending to stigma, people may automatically regulate their negative affective response to stigma, even when they are not explicitly instructed to do so. Indeed, in a recent study, participants were asked to make explicit and implicit evaluations of stigmatized targets (Krendl et al., 2006). Regardless of judgment type, increased activity was observed in the VLPFC and ACC when participants evaluated stigmatized targets. However, during the implicit task, these PFC regions were more active to the more unpleasant targets. Conversely, during the explicit judgments, these regions were tonically active regardless of the unpleasantness of the target. In other words, when participants were instructed to make explicit judgments of stigmatized targets, they engaged the VLPFC and ACC throughout the task, perhaps to automatically help monitor and regulate their responses.
An additional key finding in this study was the correlation between the activation in some of the prefrontal PFC regions during stigma regulation and participants’ implicit bias. There are two plausible interpretations of this relationship. On the one hand, this correlation may reflect the fact that bias against the stigmatized groups evaluated in this study is associated with increased PFC activity during initial regulatory attempts, which would be consistent with the findings and interpretation by Richeson et al. (2003). Simply put, individuals with more bias may have higher PFC activity when evaluating negative stigmatized group members because it takes more regulatory effort to minimize their negative bias. Conversely, the relationship between the PFC activity and IAT bias may reflect individual differences in participants’ ability to regulate their bias. Emerging research in social cognition suggests that IAT performance may be driven by control-related factors, such as the ability to regulate bias (Conrey et al., 2005) and task-switching ability (Klauer et al., 2010). Thus, a larger IAT bias score would reflect the fact that the individual has weaker cognitive control ability, not necessarily more negative bias. In line with this interpretation, the relationship we observed between the PFC and IAT bias would therefore suggest that individuals with higher IAT scores have weaker cognitive control, and, parallel to that, less efficient PFC functioning. Participants may therefore have heightened activity in the PFC when trying to downregulate their negative bias because downregulating bias might be relatively more effortful for individuals with weaker cognitive controls and therefore may require more PFC activity.
Although stigma regulation engaged more PFC during initial regulatory attempts as compared to more general emotion regulation, more general emotion regulation elicited heightened activation in posterior neural regions (notably the temporal, parietal and occipital lobes) as compared to stigma regulation when activity was measured over the entire regulation period. One explanation for these findings is that perceivers may have used different strategies to regulate their bias to stigmatized images than they used to regulate their bias to negative, non-stigmatized images. For instance, perceivers may have more experience regulating negative affective responses to images of homeless people and substance abusers than they do to images of people in cemeteries or holding guns, as they may encounter stigmatized targets more frequently in everyday life. Thus, while inhibition or perspective taking may have been effective strategies to help perceivers reduce their negative bias to stigmatized individuals, it may not have been as useful when regulating their negative affective response to the less familiar non-stigmatized targets. For these images, they may have relied on more elaborate processing, such as mental imagery or shifts of visual attention, to help them reinterpret and subsequently regulate their negative emotions. Such processes rely heavily on the visuo-spatial networks in the temporal, parietal and occipital lobes (D’Esposito et al., 1997; Wang et al., 2010).
We do not mean to imply that general emotion regulation does not require cognitive control. Indeed, we found heightened PFC activity in the decrease IAPS condition as compared to the attend IAPS condition over the 8-s regulation period, suggesting that cognitive control is required to regulate negative emotions. Our findings simply demonstrate that there is more PFC activity in the initial 2 s of the stigma regulation condition compared to more general emotion regulation. Similarly, over the 8-s regulatory period, there was heightened activity in visuo-spatial regions in the general emotion regulation condition as compared to the stigma regulation condition.
Several reasons could be offered for the different activity profile between stigma regulation and emotion regulation, although none of these are conclusive. One possibility is that although the images used in this study did not differ in valence, the negative emotions elicited by the stigma images may have been more complex than those elicited by the images used in the emotion regulation task. That is, viewing images of homeless individuals or substance abusers may have elicited pity, fear or even disgust, whereas the negative, non-stigmatized images may have elicited less complex negative emotions. This would not explain, however, why the PFC activation was correlated with participants’ implicit bias.
Alternatively, participants may not have wanted to appear prejudiced in the stigma regulation task. Thus, in addition to regulating their negative affect to the stigmatized targets, participants may have also been concerned with trying not to appear prejudiced, which might have led to increased activation in the PFC for the stigma regulation trials only. Future research should examine this possibility.
Together, these findings contribute to the growing social neuroscience literature on person perception. Extending previously reported work examining how stigma is perceived on a neural level, these results indicate that the initial regulation of negative affect toward stigmatized targets may be more cognitively effortful than regulating negative affect to other social and negative, but non-stigmatized targets.
Conflict of Interest
None declared.
Acknowledgments
The authors wish to thank Tammy Moran at the Harvard Center for Brain Sciences, Rebecca Mohr and Cindy Ko for assistance with data collection. We also thank the Kensinger lab for valuable feedback on study design. National Institutes of Health (R01 MH070833-02 to N.A.); NRSA training grant (1F32AG034039 to A.C.K.).
Footnotes
1 Pilot testing determined that the IAPS images and the stigma images did not differ in their respective valence or arousal.
2 In this analysis, we had many fewer trials than previous emotion regulation research (in our study, we had 16 IAPS images per instruction condition, compared with 38 used by Ochsner et al., 2002). Thus, in order to ensure that our results were sufficiently powered, we used a Monte Carlo conversion script from Slotnick et al. (2003) to determine the extent threshold required to convert P < 0.05 uncorrected to P < 0.05 corrected. We chose 1000 iterations of the Monte Carlo to select the most conservative threshold (20 cluster extent threshold). The corrected results are reported in this article. No other analysis reported here used that threshold.
3 In order to ensure our difference score was unbiased, we used the mean neural activity extracted from the region of interest (ROI) analyses. The ROI analyses calculated mean neural activity by comparing the task and baseline conditions. Thus, the mean neural activity we used in the decrease IAPS and decrease stigma conditions were extracted from the decrease IAPS > baseline and decrease stigma > baseline conditions.
REFERENCES
- Amodio DM, Devine PG, Harmon-Jones E. Individual differences in the regulation of intergroup bias: the role of conflict monitoring and neural signals for control. Journal of Personality of Social Psychology. 2008;94(1):60–74. doi: 10.1037/0022-3514.94.1.60. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Harmon-Jones E, Devine PG, Curtin JJ, Hartley S, Covert AE. Neural signals for the detection of unintentional race bias. Psychological Science. 2004;15(2):88–93. doi: 10.1111/j.0963-7214.2004.01502003.x. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Kubota JT, Harmon-Jones E, Devine PG. Alternative mechanisms for regulating racial responses according to internal vs external cues. Social Cognitive and Affective Neuroscience. 2006;1(1):26–36. doi: 10.1093/scan/nsl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD, Dickter CL, Sestir MA. Stereotype activation and control of race bias: Cognitive control and inhibition and its impairment by alcohol. Journal of Personality and Social Psychology. 2006;90(2):272–87. doi: 10.1037/0022-3514.90.2.272. [DOI] [PubMed] [Google Scholar]
- Conrey FR, Sherman JW, Gawronski B, Hugenberg K, Groom CJ. Separating multiple processes in implicit social cognition: The quad model of implicit task performance. Journal of Personality and Social Psychology. 2005;89(4):469–87. doi: 10.1037/0022-3514.89.4.469. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Gatenby CJ, Gore JC, Banaji MR. Separable neural components in the processing of black and white faces. Psychological Science. 2004;15(12):806–13. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Aguirre GK, et al. A functional MRI study of mental image generation. Neuropscyhologia. 1997;35(5):725–30. doi: 10.1016/s0028-3932(96)00121-2. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JLK. Measuring individual differences in implicit cognition: the implicit association test. Journal of Personality and Social Psychology. 1998;74(6):1464–80. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- Harris LT, Fiske ST. Dehumanizing the lowest of the low: neuroimaging responses to extreme out-groups. Psychological Science. 2006;17(10):847–53. doi: 10.1111/j.1467-9280.2006.01793.x. [DOI] [PubMed] [Google Scholar]
- Jones EE, Farina A, Hastorf AH, Markus H, Miller DT, Scott RA. Social Stigma: the Psychology of Marked Relationships. New York: W.H. Freeman and Company; 1984. [Google Scholar]
- Klauer KC, Schmitz F, Teige-Mocigemba S, Voss A. Understanding the role of executive control in the implicit association test: Why flexible people have small IAT effects. The Quarterly Journal of Experimental Psychology. 2010;63(3):595–619. doi: 10.1080/17470210903076826. [DOI] [PubMed] [Google Scholar]
- Knutson KM, Mah L, Manly CF, Grafman J. Neural correlates of automatic beliefs about gender and race. Human Brain Mapping. 2007;28:915–30. doi: 10.1002/hbm.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendl AC, Heatherton TF, Kensinger EA. Aging minds and twisting attitudes: An fMRI investigation of age differences in inhibiting prejudice. Psychology and Aging. 2009;24(3):530–41. doi: 10.1037/a0016065. [DOI] [PubMed] [Google Scholar]
- Krendl AC, Macrae CN, Kelley WM, Fugelsang JF, Heatherton TF. The good, the bad, and the ugly: An fMRI investigation of the functional anatomic correlates of stigma. Social Neuroscience. 2006;1(1):5–15. doi: 10.1080/17470910600670579. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. Gainesville, FL: Univeristy of Florida; 2005. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Technical Manual and Affective Ratings. [Google Scholar]
- Lieberman MD. Reflective and reflexive judgment processes: a social cognitive neuroscience approach. In: Forgas JP, Williams KR, von Hippel W, editors. Social Judgments: Implicit and Explicit Processes. New York: Cambridge University Press; 2003. pp. 44–67. [Google Scholar]
- Lieberman MD, Gaunt R, Gilbert DT, Trope Y. Reflection and reflexion: A social cognitive neuroscience approach to attributional inference. Advances in Experimental Social Psychology. 2002;34:199–249. [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner K, Ray RD, Cooper JC, et al. For better for for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;2:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Cunningham WA, et al. Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience. 2000;12(5):729–38. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Richeson JA, Baird AA, Gordon HL, et al. An fMRI investigation of the impact of interracial contact on executive function. Nature Neuroscience. 2003;6(12):1323–8. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Kathirgamanathan U, Humphreys GW. Seeing it my way: A case of selective deficit in inhibiting self-perspective. Brain. 2005;128:1102–11. doi: 10.1093/brain/awh464. [DOI] [PubMed] [Google Scholar]
- Satpute AB, Lieberman MD. Integrating automatic and controlled processing into neurocognitive models of social cognition. Brain Research. 2006;1079:86–97. doi: 10.1016/j.brainres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with % item memory and source memory for visual shapes. Cognitive Brain Research. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Wang J, Conder JA, Blitzer DN, Shinkareva SV. Neural representation of abstract and concrete concepts: a meta-analysis of neuroimaging studies. Human Brain Mapping. 2010;31(10):1459–68. doi: 10.1002/hbm.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner B, Perry RP, Magnusson J. An attributional analysis of reactions to stigma. Interpersonal relations and group processes. 1988;55(5):738–48. doi: 10.1037//0022-3514.55.5.738. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Fiske ST. Controlling racial prejudice: social-cognitive goals affect amygdala and stereotype activation. Psychological Science. 2005;16(1):56–63. doi: 10.1111/j.0956-7976.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, et al. Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14:170–81. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]