Abstract
Previous studies have shown that healthy participants learn to control local brain activity with operant training by using real-time functional magnetic resonance imaging (rt-fMRI). Very little data exist, however, on the dynamics of interaction between critical brain regions during rt-fMRI-based training. Here, we examined self-regulation of stimulus-elicited insula activation and performed a psychophysiological interaction (PPI) analysis of real-time self-regulation data. During voluntary up-regulation of the left anterior insula in the presence of threat-related pictures, differential activations were observed in the ventrolateral prefrontal cortex, the frontal operculum, the middle cingulate cortex and the right insula. Down-regulation in comparison to no-regulation revealed additional activations in right superior temporal cortex, right inferior parietal cortex and right middle frontal cortex. There was a significant learning effect over sessions during up-regulation, documented by a significant improvement of anterior insula control over time. Connectivity analysis revealed that successful up-regulation of the activity in left anterior insula while viewing aversive pictures was directly modulated by dorsomedial and ventrolateral prefrontal cortex. Down-regulation of activity was more difficult to achieve and no learning effect was observed. More extensive training might be necessary for successful down-regulation. These findings illustrate the functional interactions between different brain areas during regulation of anterior insula activity in the presence of threat-related stimuli.
Keywords: emotion, real-time fMRI, psychophysiological interaction, insula, prefrontal cortex
INTRODUCTION
Recently, physiological self-regulation of circumscribed brain regions and networks has become feasible using real-time functional magnetic resonance imaging (rt-fMRI) (Weiskopf et al., 2007; Sitaram et al., 2008, 2009; Caria et al., 2011). Several studies have investigated learned modulation of neural activity in areas primarily implicated in emotional processes such as the amygdala (Posse et al., 2003; Johnston et al., 2010), the rostral anterior cingulate cortex (rACC; Weiskopf et al., 2003), the subgenual ACC (Hamilton et al., 2011), the anterior insula (Caria et al., 2007, 2010; Johnston et al., 2010) and it has been shown that support vector machine (SVM) classification of spatial patterns of activation in emotional networks can be used for real-time feedback (Sitaram et al., 2010). It is also increasingly being recognized that the ability to regulate activity of localized cortical areas can be useful in the treatment of various disorders including depression (Hamilton et al., 2011), chronic pain (deCharms et al., 2005), tinnitus (Haller et al., 2010) and schizophrenia (Ruiz et al., 2008, 2011), and for movement rehabilitation after stroke (Sitaram et al., 2011).
The anterior insula is a key structure in the emotional circuitry and its activity has been shown to correlate with subjective feelings of emotional states (Craig, 2002, 2003, 2009). Studies on emotion perception have revealed that insula activity correlates with the aversive valence of stimuli (Anders et al., 2004), sadness (Lane et al., 1997), fear (Morris et al., 1996) and disgust (Calder et al., 2001). Positively valenced responses were also reported to correlate with activations of left anterior insula (Craig, 2009). A review of PET (positron emission tomography) and fMRI studies (Phan et al., 2002) demonstrated that both the ACC and insula were recruited during emotion induction using emotional recall/imagery and during performance of emotional tasks with concurrent cognitive demands. By playing a critical role in mediating self-awareness, body integrity (Craig, 2009), and in the influence of peripheral autonomic arousal on consciously experienced emotional states (Critchley et al., 2002, 2004), the insula serves as a strategic neural node in the appraisal of emotional responses (Craig, 2009). Using rt-fMRI, our group previously demonstrated that untrained participants can learn to up-regulate activity of the anterior insula within three training sessions (Caria et al., 2007) and that the amount of up-regulation correlates with subsequent valence ratings of aversive pictures (Caria et al., 2010). A follow-up investigation of the same data (Lee et al., 2011) with multivariate pattern classification and Granger causality modelling (GCM) revealed that self-regulation training of the anterior insula caused an initial increase and subsequent pruning of the network density and a strengthening of the insula's connections with other regions involved in emotional processing (amygdala, medial prefrontal cortex). In the present study, we sought to extend these findings by examining the larger networks engaged during the self-regulation of insula activity.
Neuroimaging research on neural correlates of affect regulation often comprises either emotion suppression or reappraisal of the evocative stimuli, with the aim of reducing negative affect or increasing positive affect (Ochsner and Gross, 2005; Quirk and Beer, 2006; Goldin et al., 2008; Mak et al., 2009). Functional brain imaging of both these regulation strategies has shown that they engage specific frontal brain regions such as the orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC) and ACC (Beauregard et al., 2001; Ochsner et al., 2002, 2004; Phan et al., 2005; Urry et al., 2006; Eippert et al., 2007; Mak et al., 2009; Johnston et al., 2010). The importance of these frontal areas in emotion regulation is underscored by studies showing that the initial appraisal of negative emotional stimuli similarly engages VLPFC, DLPFC and dorsomedial prefrontal cortex (DMPFC) (Hariri et al., 2000, 2003; Taylor et al., 2003). Moreover, ACC and VLPFC are activated during inhibition of cognitive–emotional interference (Whalen et al., 1998; Bush et al., 2000; Etkin et al., 2006; Shafritz et al., 2006) and when participants divert their attention away from threatening and/or painful stimuli (Bantick et al., 2002; Tracey et al., 2002; Bishop et al., 2004).
Here, we investigate rt-fMRI-supported self-regulation of activity in the left anterior insula while subjects viewed threat-related pictures. To examine which brain areas are functionally connected during successful regulation of the targeted area, we employ psychophysiological interaction (PPI) analysis. We hypothesize that successful modulation of activity of the anterior insula is mediated by prefrontal areas that have previously been shown to subserve emotion regulation, as reviewed above. The present study thus aims to extend the understanding of the neural circuitry involved in rt-fMRI based self-regulation.
MATERIALS AND METHODS
Participants
Eleven healthy volunteers (aged 21–28 years, 8 females, 3 males) participated in the study. All participants were right-handed as assessed by the Edinburgh handedness inventory and had normal or corrected-to-normal vision. None of the participants had a history of psychiatric, medical or neurological illness. They were given written instructions, and informed consent was obtained from each. The study was approved by the ethics committee of the Faculty of Medicine of the University of Tübingen.
fMRI data acquisition
Functional images were acquired on a 1.5-T whole-body scanner with a standard 8-channel head coil (Siemens Magnetom Trio Tim, Siemens, Erlangen, Germany). A standard echo-planar imaging sequence (EPI), adapted for real-time image reconstruction to generate images at the end of each volume, was used [TR (repetition time) = 1.5 s, matrix size = 64 × 64, effective TE (echo time) = 40 ms, flip angle α = 70°, bandwidth = 1.954 kHz/pixel]. Sixteen slices (voxel size = 3.3 × 3.3 × 5.0 mm3, slice gap = 1 mm), AC/PC (anterior commissure/posterior commisure) aligned in axial orientation were acquired. For superposition of functional maps upon brain anatomy, a high resolution T1-weighted structural scan of the whole brain was collected from each participant (MPRAGE, matrix size = 256 × 256, 160 partitions, 1 mm3 isotropic voxels, TR = 2300 ms, TE = 3.93 ms, T1 = 1100 ms, α = 8°).
The rt-fMRI system is based on Turbo-BrainVoyager 1.1 software (Brain Innovation, Maastricht, The Netherlands) in combination with in-house written scripts running on Matlab 6.5 (The Math Works, Natick, MA, USA) as previously described by Weiskopf et al. (2003).
Localizer session
Before feedback training, a localizer session consisting of 70 scans in total was performed to functionally identify the left anterior insula. After a 10-s baseline period, a set of highly aversive pictures from the International Affective Picture System (IAPS) (mutilation and burn victims) were presented in two blocks (22.5 s) alternating with resting periods of the same length. To mark the anterior insula region of interest (ROI) (ROI1), the final t-map of the activations during the localizer session was used to draw a rectangular box comprising 4*5 voxels centred on the voxel showing highest activation within the anterior part of the insula (Figure 2). The reference ROI (ROI2) was a slightly larger square (6*6 voxels). The placement of ROI2 was superior to ROI1, with at least two intervening slices between the two ROIs. We specifically ensured that no activation was present in the reference ROI during the localizer session. The purpose of using a reference ROI was to cancel out changes in BOLD (Blood Oxygen Level Dependent) signal due to global effects of movement and other task-unspecific changes.
Fig. 2.
Illustration of ROI definition in the left anterior insula. The red rectangular box delineates the ROI chosen for the rt-fMRI neurofeedback.
rt-fMRI task procedure and feedback calculation
The experimental paradigm required participants to actively regulate BOLD activity in the left anterior insula while viewing emotional stimuli. The conceptual scheme of the experiment and the picture set used were adapted from Eippert et al. (2007). The stimulus set consisted of 18 aversive (average arousal: 6.22 s.d. 0.59; average valence: 2.73 s.d. 0.61) and six neutral pictures based on IAPS (Lang et al., 1999). Moderately aversive or threatening pictures were chosen to avoid ceiling effects and allow differential regulation.
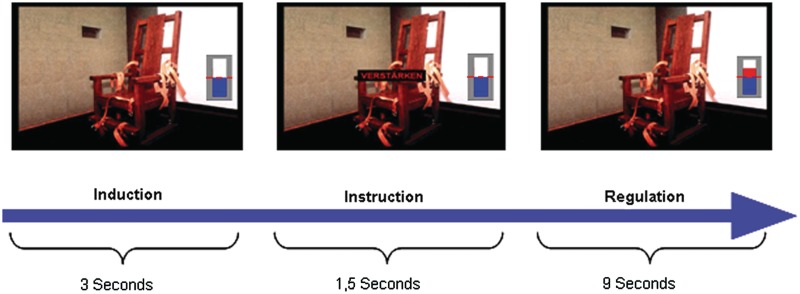
The protocol consisted of three sessions. Each session was 8 min 9 s long and comprised the following conditions: emotion induction, up-regulation, down-regulation and no-regulation. The trials started with a 3-s induction period and were followed by a 1.5-s task instruction superimposed on the pictures using the single words ‘increase’ for up-regulation, ‘decrease’ for down-regulation and ‘view’ for no-regulation. Each regulation block lasted 9 s (Figure 1) and was followed by a 7.5-s resting period. Feedback was provided in the form of a graphical thermometer whose bars increased or decreased in number from the baseline value (represented by the dashed red line in the middle of the thermometer, see Figure 1), in proportion to the differential BOLD signal in the target (ROI1) and reference (ROI2) ROIs. The baseline value was calculated from the last two scans of the resting period at the end of the preceding trial. Feedback was computed from a temporally smoothed BOLD signal. Temporal smoothing was achieved by averaging the BOLD signal within a moving window that included the BOLD value for the current TR and values from the past three TRs. Thus, the BOLD signal during the induction and instruction period plus the first scan of the regulation period formed the basis of the first feedback signal. More specifically, the number of bars was computed using the following equation (equation 1):
| (1) |
Fig. 1.
Schematic overview of the experimental design. Pictures were shown to the participants for 3 s while in the MR scanner (emotion induction). According to task instructions (1.5 s), the participants had to regulate (increase or decrease) signal in the left anterior insula in response to the image shown for additional 9 s (with passive viewing as control condition). BOLD response from left anterior insula was fed back in the form of a graphical thermometer.
If the computed number of bars was positive, the bars of the thermometer were shown in red colour above the baseline, and if the value was negative, the bars were shown in blue colour below the baseline. In addition, we implemented a real-time artefact correction that detected and ignored sudden changes in BOLD signal due to swallowing or movement of the tongue (Sitaram et al., 2010). Such movements can cause signal increases five times higher than the normal BOLD increase in a single TR. The thermometer display with increasing and decreasing bars representing feedback of activation in the target region was presented during all conditions, except the no-regulation condition where a thermometer with static bars was presented. Participants were informed about the data processing delay of 1.5 s and the intrinsic physiological haemodynamic response delay of ∼4–6 s.
To extend classical emotion regulation paradigms with the advantages of rt-fMRI-based neurofeedback, participants were instructed to use cognitive strategies that would help them learn to control the activity of the target ROI. Specifically, for up-regulation, participants were instructed to imagine themselves being personally involved in the situation depicted in the picture. For down-regulation, participants were required to cognitively and emotionally distance themselves from the situation displayed in the image. During the no-regulation condition, participants were required to passively view the images presented. After each session, they were also required to rate their success in regulation on a scale from 1 to 8 (1 = very good; 8 = very bad).
rt-fMRI analysis of feedback regulation
The difference of the baseline-adjusted signal changes in ROI1 and ROI2 averaged over the regulation period (equation 1) was computed individually for each subject on a trial-by-trial basis. The mean signal changes and corresponding standard deviations in each regulation task and session were calculated. The extracted signal changes were further analysed using SPSS 13.0. Paired t-test was performed to analyse the signal changes between the first and last training session for each task. Furthermore, a linear regression analysis using the individual trial-specific signal changes as dependent variable and time as independent variable was conducted to evaluate possible learning effects over time. Results were considered significant at P < 0.05 (one tailed).
Time-series analysis
Peri-stimulus time histogram (PSTH) plots were extracted using the NOD Lab toolbox NERT4SPM (http://www.hih-tuebingen.de/en/sensorimotor-lab/nod-lab/). Based on the individual GLM analysis, a spherical ROI of 6 mm centred on the maximally activated voxel in the left anterior insula during aversive induction was used. This ROI definition differs slightly from the rectangular ROI used for the neurofeedback training, but has the advantage that only grey matter voxels showing significant activity are used for the time-course extraction. The average time course across all voxels in the ROI was calculated and normalized to percent signal change separately for each regulation condition and session. To test for signal changes during the regulation periods, the time course was scaled to 1.5 s pre-stimulus baseline (time zero represents the time-point after emotion induction). Repeated-measurement ANOVA with factors time (six time-points in the regulation condition) and session (three sessions) were performed for each regulation condition. Paired t-tests were used to compare session-wise differences between corresponding time-points separately for each regulation condition.
Whole-brain analysis of emotion induction and feedback regulation
The SPM5 statistical parametric mapping software package (Wellcome Department of Imaging Neuroscience, London) was used to perform off-line image pre-processing and data analysis. Before whole-brain statistical analysis, functional EPI volumes were spatially re-aligned. The images were normalized to the Montreal Neurological Institute (MNI) space and spatially smoothed (9-mm Gaussian kernel). A temporal filter (0.0088 Hz) was applied to remove low-frequency artefacts. For each participant, a general linear model (GLM) with the conditions ‘induction aversive’, ‘induction neutral’, ‘up-regulation aversive’, ‘down-regulation aversive’, ‘no-regulation aversive’ and ‘no-regulation neutral’ was created. All conditions were modelled with a canonical haemodynamic response function (HRF) using standard SPM5 settings. The duration of the regulation trials was set to 9 s. Movement regressors were included as confounds in the general linear model to account for possible head movement related variance. The following contrasts were computed: induction aversive vs induction neutral, aversive up-regulation vs aversive no-regulation and aversive down-regulation vs aversive no-regulation. Random-effects t-statistics across participants were calculated separately for the main contrasts. Effects were considered significant based on a whole-brain false discovery rate of P < 0.05 (FDR; Genovese et al., 2002).
PPI analysis
PPI is a functional connectivity analysis method that describes activity in a ROI based on its interaction with other brain regions and a psychological factor (Friston et al., 2003). Essentially, PPI implies that this interaction between brain regions is significantly modulated by the experimental or psychological context (e.g. attention vs no attention; up-regulation vs down-regulation). By integrating the physiological and experimental influences on regional responses, PPI allows one to confer a degree of functional specificity when making inferences about functional integration or interactions between cortical areas. Kim and Horwitz (2008) compared PPI analysis with correlation measures between fMRI signals for computing functional connectivity using simulated data reflecting synaptic activity. Their main finding was that PPI results better reflect interregional connections between areas compared to simple correlations between fMRI signals from two regions. Our aim was to examine how the left anterior insula interacts with other brain regions while participants regulate left anterior insula activity using rt-fMRI feedback.
In the first step, we extracted time courses for each training session using the first eigenvariate of the volume of interest, i.e. a spherical ROI of 6 mm centred on the maximally activated voxel in the left anterior insula, as described in the time-course analysis. The PPI toolbox of SPM5 was then employed to generate differential contrasts of both up-regulation vs no-regulation and down-regulation vs no-regulation. The GLM for each PPI consists of the interaction vector of the corresponding psychological or context factor (up-regulation or down-regulation in comparison to no-regulation), the time course of the BOLD signal in the target ROI using the first eigenvariate, and the context-specific contrast vector (up-regulation vs no-regulation, down-regulation vs no-regulation). The motion parameters were used as confounds in the design matrix. A contrast vector weighting the interaction contrast with one and the other regressors with zero results in a statistical parametric map with voxels showing a positive coactivation or interaction with the seed ROI, whereas a contrast weight of minus one yields a statistical map with voxels revealing a negative covariation with the target ROI.
For second-level analysis, we used the contrast images obtained from the first-level PPI analysis representing the interaction of brain regions with the left anterior insula during up- and down-regulation. This was followed by separate t-tests for each condition and session. An uncorrected threshold of P < 0.001 was applied for the group PPI analyses.
Self-report analysis
Statistical analysis of behavioural data (i.e. success ratings for regulation) were computed using the statistical package SPSS 13.0 (SPSS Inc., Chicago, IL, USA). A repeated-measurements ANOVA with the factors session and condition was carried out. Significant effects were further analysed using paired t-tests. A t-value exceeding a threshold of P < 0.05 was considered significant.
RESULTS
ROI analysis of feedback regulation
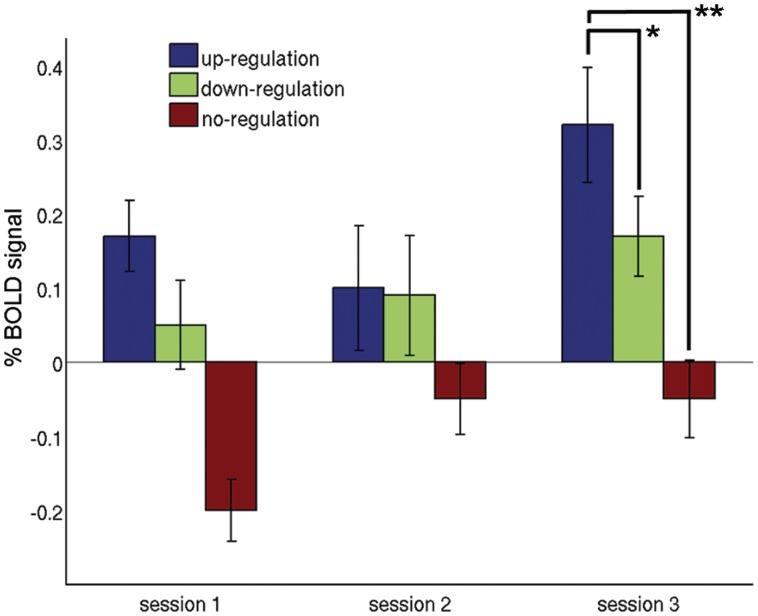
The comparison between tasks showed that the BOLD signal changes were significantly increased during up-regulation in comparison to down-regulation [t(10) = 2.02 P = 0.035 one-tailed] and no-regulation [t(10) = 5.83 P < 0.001] in the last training session (Table 1 and Figure 3). The comparison of BOLD signal changes during up-regulation based on the difference of baseline-corrected individual activities in ROI1 and ROI2 used for rt-fMRI training revealed a marginally significant increase between the first and last session [session 1: 0.17 (s.d. 0.17), session 3: 0.32, (s.d. 0.26), t(10) = 1.68 P = 0.07, one tailed]. Similarly, during down-regulation there was a small but non-significant increase between sessions 1 and 3 [session 1: 0.05 (s.d. 0.20), session 3: 0.17 s.d. (0.19), t(10) = 1.21 P = 0.25]. In the no-regulation condition, there was a significant change between the first and last training session [session 1: −0.27 (s.d. 0.15), session 3: −0.05 (s.d. 0.18), t(10) = 3.27 P = 0.008].
Table 1.
Online analysis of the BOLD signal changes during the rt-fMRI training (mean ± s.d.)
| Up-regulation | Down-regulation | No-regulation | |
|---|---|---|---|
| Session 1 | 0.17 (0.16)a | 0.05 (0.20)a | −0.20 (0.14)a |
| Trial 1 | 0.53 (0.69) | 0.09 (0.54) | −0.08 (0.54) |
| Trial 2 | −0.06 (0.25) | 0.27 (0.21) | −0.08 (0.42) |
| Trial 3 | 0.00 (0.46) | 0.11 (0.26) | −0.21 (0.42) |
| Trial 4 | 0.05 (0.65) | −0.03 (0.33) | −0.15 (0.34) |
| Trial 5 | 0.23 (0.64) | −0.16 (0.76) | −0.34 (0.54) |
| Trial 6 | 0.29 (0.39) | 0.06 (0.48) | −0.36 (0.42) |
| Session 2 | 0.10 (0.28)b | 0.09 (0.27)b | −0.05 (0.16)b |
| Trial 1 | 0.02 (0.79) | 0.36 (0.42) | 0.12 (0.26) |
| Trial 2 | 0.09 (0.54) | 0.25 (0.30) | −0.01 (0.32) |
| Trial 3 | −0.01 (0.58) | 0.08 (0.56) | −0.11 (0.47) |
| Trial 4 | 0.24 (0.33) | 0.26 (0.44) | −0.27 (0.44) |
| Trial 5 | 0.20 (0.47) | −0.36 (0.55) | 0.01 (0.52) |
| Trial 6 | 0.03 (0.49) | −0.01 (0.74) | −0.05 (0.44) |
| Session 3 | 0.32 (0.26)c | 0.17 (0.18)c | −0.05 (0.17)c |
| Trial 1 | 0.65 (0.64) | 0.23 (0.38) | −0.17 (0.33) |
| Trial 2 | 0.26 (0.42) | 0.29 (0.31) | 0.09 (0.36) |
| Trial 3 | 0.22 (0.51) | 0.39 (0.46) | −0.11 (0.38) |
| Trial 4 | 0.12 (0.42) | 0.25 (0.29) | 0.02 (0.67) |
| Trial 5 | 0.27 (0.44) | −0.22 (0.39) | 0.17 (0.30) |
| Trial 6 | 0.41 (0.30) | 0.06 (0.37) | −0.28 (0.33) |
(ROI1reg − ROI1base) – (ROI2reg – ROI2base).
aMean s1.
bMean s2.
cMean s3.
Fig. 3.
Differential BOLD percent signal change (±s.e.m.) computed on the individual selected target and control ROI during the rt-fMRI training. A significant difference was found between up- and down-regulation as well as between up- and no-regulation in the last training session (*P < 0.05, **P < 0.001).
A linear regression analysis using the signal changes of every single trial as dependent variable revealed a trend for a progressive increase in performance during up-regulation over the course of training (y = 0.10 + 0.283x, t = 1.181, P = 0.257). When excluding the first trial under the assumption that participants were initially unfamiliar with the feedback display and the delay of the haemodynamic response, a significant learning effect was observed for up-regulation (y = −0.017 + 0.550x, t = 2.55, P = 0.022). In contrast, during down-regulation, no learning took place (y = 0.103 + 0.016x, t = 0.06, P = 0.951).
Overall, there was a large variability in BOLD signal changes across trials and subjects especially in the first two sessions (Table 1). However, during up-regulation in the last training session, we found consistent increases in all trials. Interestingly, during down-regulation there was a tendency of improved down-regulation ability in the last compared to the first trials in each session.
Time-series analyses
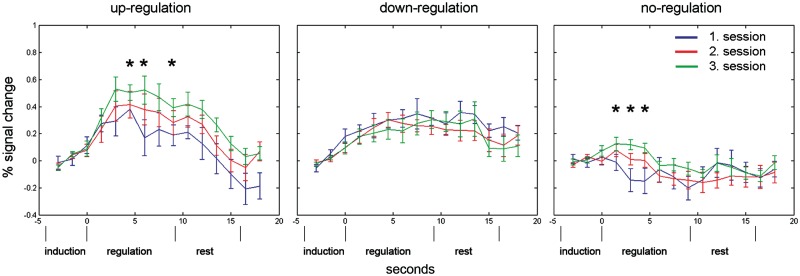
The direct comparison of the time courses between tasks revealed that there was a significantly decreased activation in the anterior insula during down-regulation compared to up-regulation in the last training session at three consecutive time-points [paired t-tests: Second 3: t(10) = 2.94, P = 0.015, Second 4.5: t(10) = 3.82, P = 0.003, Second 6: t(10) = 2.39, P = 0.037; Figure 5]. Within task, repeated-measurements ANOVA of left anterior insula activity with time-bin and session as factors revealed a significant effect of time [F(5,50) 5.15, P < 0.001] during up-regulation. The session effect was not significant [F(2,20) 1.69, P = 0.219]. However, exploratory pair-wise comparisons showed higher BOLD activity in the third compared to the first session (mean BOLD amplitude session 1: 0.244; mean BOLD amplitude session 3: 0.407; P = 0.037, one sided). Post hoc paired t-tests between corresponding peri-stimulus time-points in Sessions 1 and 3 revealed significantly higher BOLD responses in Session 3 at time 4.5 s (P = 0.034), 6 s (P = 0.022) and 9 s (P = 0.036) (Figure 5).
Fig. 5.
PSTH plots of the BOLD response in the left anterior insula during up-regulation (left), down-regulation (middle) and no-regulation (right) in the first (blue lines), second (red lines) and third (green lines) training session averaged over participants (±s.e.m.). Each trial consisted of an induction/instruction period (4.5 s), a regulation period (starts at time 0 s and lasts 9 s) and a resting period (7.5 s). There was a significant learning effect during up-regulation over sessions. Significant differences between corresponding time-points in Sessions 1 and 3 are indicated with black stars.
During down-regulation, there was also a significant effect of time [F(5,50) 3.712, P = 0.006], but no session effect [F(2,20) 0.322, P = 0.728]. Pair-wise comparisons revealed no differences between Sessions 1 and 3. There was a tendency towards a difference in BOLD levels between sessions in the no-regulation condition [F(2,20) 2.939, P = 0.104]. Pair-wise comparisons showed a significant difference between Sessions 1 and 3 (P = 0.018). Post hoc paired t-tests yielded significantly reduced BOLD responses during no-regulation in the first session compared to the third session at time 1.5 s (P = 0.036), 3 s (P = 0.023) and marginally at 4.5 s (P = 0.054).
Whole-brain analysis of emotion induction and feedback regulation
The contrast ‘induction-aversive’ compared to ‘induction-neutral’ yielded activations in supplementary motor area (SMA), paracingulate cortex, anterior insula bilaterally extending into the frontal operculum, ACC, thalamus, left putamen, caudate nucleus bilaterally and left inferior parietal cortex, together suggesting successful emotion induction.
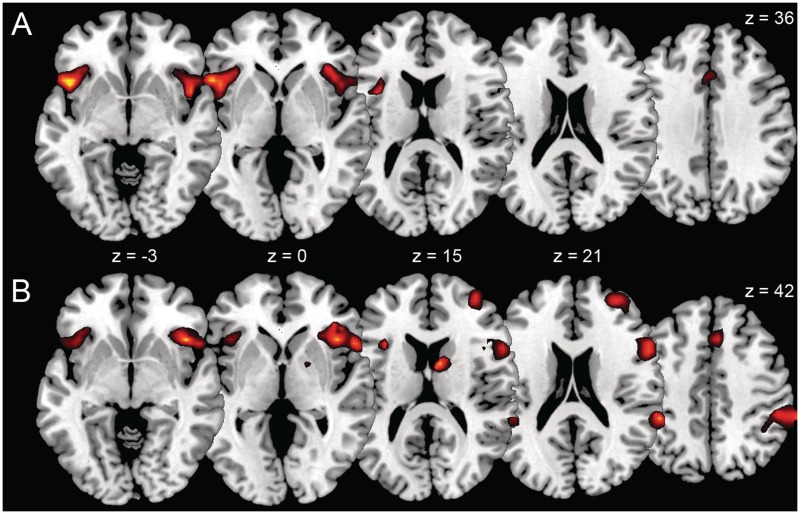
Up-regulation in comparison to no-regulation in the presence of threat-related stimuli revealed activation in the frontal operculum bilaterally, right anterior insula, left VLPFC, left ACC and right middle cingulate cortex (Table 2 and Figure 4A). In contrast to the no-regulation condition, down-regulation activated the right insula extending into the right VLPFC, right superior temporal cortex, right caudate nucleus, right inferior parietal cortex, right middle frontal cortex (MFC), right superior occipital cortex, left VLPFC, right superior medial frontal cortex and right ACC (Table 3 and Figure 4B).
Table 2.
Regions showing increased activation during up-regulation compared to no-regulation of the left anterior insula
| Region (Brodmann's area) | t-value | MNI coordinates |
||
|---|---|---|---|---|
| x | y | z | ||
| Ventrolateral PFC/insula L (BA 47) | 9.55 | −48 | 18 | −3 |
| Frontal inferior opercularis L | 6.16 | −48 | 6 | 6 |
| Insula R (BA 22) | 7.56 | 48 | 12 | −6 |
| Frontal inferior opercularis R | 4.81* | 39 | 15 | 9 |
| Middle cingulate cortex R (BA 32) | 5.26 | 3 | 21 | 36 |
| ACC L (BA 24) | 4.33 | −3 | 24 | 30 |
FDR P < 0.05 corrected for the amount of false positive activations of the whole brain. *P < 0.001 (uncorrected for multiple comparisons).
L = left; R = right.
Fig. 4.
Differential activation in the contrast up-regulation vs no-regulation (A) and in the contrast down-regulation vs no-regulation (B) rendered on a canonical single-subject brain (p<0.001).
Table 3.
Regions showing increased activation during down-regulation in comparison to no-regulation of the left anterior insula
| Region (Brodmann's area) | t-value | MNI coordinates |
||
|---|---|---|---|---|
| x | y | z | ||
| Ventrolateral PFC/insula R (BA 47) | 8.76 | 42 | 21 | −9 |
| Superior temporal R (BA 40) | 8.44 | 66 | −39 | 21 |
| Nucleus caudate R | 8.07 | 9 | 3 | 15 |
| Inferior parietal R | 7.75 | 51 | −36 | 48 |
| Frontal inferior opercularis L | 6.68 | −33 | 18 | 15 |
| Frontal middle R (BA 10) | 6.61 | 33 | 51 | 18 |
| Frontal superior medial R (BA 32) | 6.19 | 6 | 21 | 42 |
| Frontal middle R | 5.88 | 36 | 6 | 57 |
| Superior occipital R (BA 19) | 5.84 | 36 | −81 | 42 |
| Ventrolateral PFC L (BA 47) | 5.57 | −39 | 21 | −3 |
| ACC R (BA 32) | 4.66 | 6 | 36 | 24 |
FDR P < 0.05 corrected for the amount of false positive activations of the whole brain.
L = left; R = right.
PPI analysis
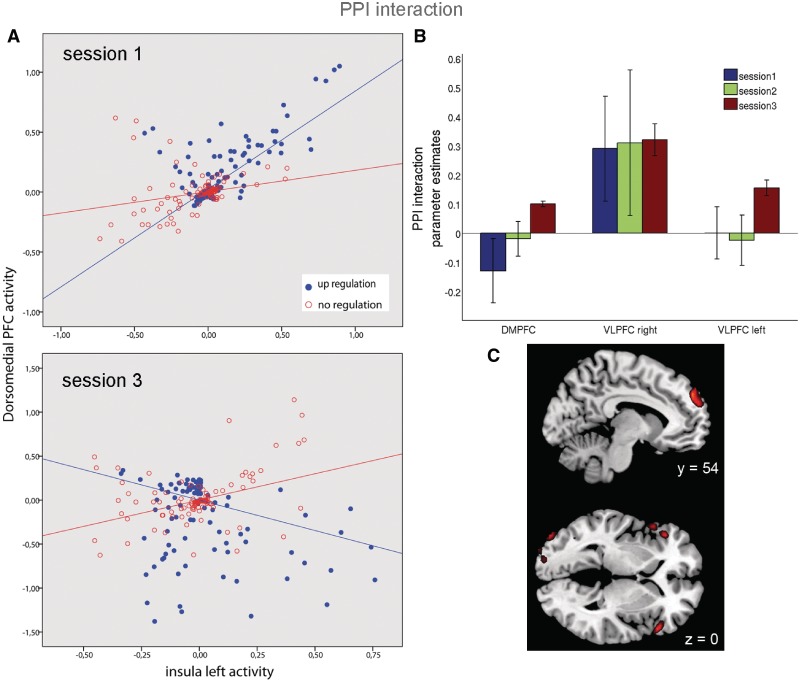
PPI analysis for up-regulation vs no-regulation in the first session showed a right-lateralized connectivity pattern with left anterior insula activity in the lingual gyrus, anterior insula, VLPFC and frontal inferior operculum. In the second session, we found positive coactivation in the left inferior orbitofrontal, left middle frontal and left middle OFC. Analysis of the third training session revealed that successful up-regulation of the BOLD response in the left anterior insula was positively linked to activity in left DMPFC and bilateral VLPFC, presumably as an effect of the prolonged training (Table 4 and Figure 6). The parameter estimates of the PPI interaction analyses for all three sessions in these areas as displayed in Figure 6B confirm that a robust positive interaction was only found in the third training session. While a negative interaction was present in the first session, a significantly positive interaction was observed in the third session (the interaction in one representative subject is plotted in Figure 6A). Another area that showed significant interaction with left anterior insula was the bilateral inferior occipital cortex.
Table 4.
Regions showing increased functional connectivity during up-regulation of the left anterior insula during training
| Region (Brodmann's area) | t-value | MNI coordinates |
||
|---|---|---|---|---|
| x | y | z | ||
| Session 1 | ||||
| Lingual gyrus R (BA 18) | 4.4 | 30 | −93 | −18 |
| Insula R | 4.09 | 42 | 9 | −3 |
| Ventrolateral PFC R (BA 47) | 3.93 | 54 | 18 | −6 |
| Frontal inferior operculum R | 3.89 | 42 | 27 | −6 |
| Session 2 | ||||
| Inferior orbitofrontal L (BA 47) | 6.95 | −36 | 30 | −18 |
| Middle frontal L | 3.91 | −33 | 48 | 30 |
| Middle orbitofrontal L (BA 11) | 3.92 | −27 | 48 | −15 |
| Session 3 | ||||
| Occipital inferior R (BA 19) | 9.48 | 45 | −69 | −18 |
| Dorsal medial PFC L (BA 9) | 8.62 | −12 | 54 | 27 |
| Occipital inferior L (BA 19) | 4.74 | −45 | −78 | −12 |
| Ventrolateral PFC R (BA 47) | 5.75 | 54 | 24 | 0 |
| Ventrolateral PFC L (BA 47) | 5.63 | −45 | 33 | 3 |
All P < 0.001 (uncorrected for multiple comparisons).
L = left; R = right.
Fig. 6.
(A) PPI interaction displayed as different regression slopes of DMPFC activity on left anterior insula activity during up-regulation compared to no-regulation in one representative subject. While a negative interaction was present in the first session, a significantly positive interaction was observed in the third session. (B) Parameter estimates reflecting the positive or negative connectivity between DMPFC/bilateral ventrolateral PFC and left anterior insula. (C) Regions showing effective connectivity with left anterior insula during emotional up-regulation while viewing threat-related pictures in the last training session, overlaid on a canonical single-subject brain. Significant positive modulations were found in the DMPFC and bilateral VLPFC (p<0.001).
In the first two training sessions, there was no significant connectivity with the target ROI during down-regulation. Analysis of down-regulation data in the third session showed a positive covariation with activity in the left fusiform gyrus (x = −39, y = −60, z = −15, t = 7.42). However, we found no involvement of prefrontal areas.
Subjective ratings
There was a significant session effect [F(2,22) = 4.184, P < 0.05) in subjective ratings, indicating improved success to regulate over the course of the experiment. There was a tendency towards up-regulation being judged easier than down-regulation [success rating up-regulation: 4.47 ± 1.03; success rating down-regulation: 4.81 ± 1.31 (mean ± s.d.)], but the effect was not significant F(1,11) = 1.609, P = 0.23).
DISCUSSION
The present study aimed to explore the neural circuitry involved when healthy participants self-regulate BOLD activity of the left anterior insula while viewing emotional pictures. Our findings corroborate and extend previous studies that trained participants to self-regulate anterior insula activity without emotion induction by external stimuli (Caria et al., 2007, 2010). Whereas both emotion induction and self-regulation activated a number of predominantly frontal regions, PPI analysis uncovered a more focused network specifically involved in self-regulation of anterior insula activity.
Whole-brain analyses during up-regulation showed higher activity in the left insula, left VLPFC, right insula, left frontal operculum and anterior and middle cingulate cortex. Group analysis of the differential activation between the individual anterior insula and control ROI during up-regulation used for the neurofeedback training revealed that subjects learned to increase their BOLD acitivity from the first to the last training session. In fact, robust up-regulation ability over all trials was found in the last training session. Additional ROI time-course analysis showed enhanced BOLD activation in left anterior insula over training sessions, indicating a learning effect. This is in line with the studies of Caria et al. (2007, 2010), where feedback was supplied without preceding emotion induction by external stimuli. Our results thus support previous findings that self-regulation of localized brain areas can be learned within a few training sessions.
During down-regulation, we observed increased activation in specific frontal areas including left frontal inferior operculum, bilateral MFC, superior medial frontal cortex, bilateral VLPFC, right anterior insula and ACC. In addition to these frontal regions, we found activations in superior temporal cortex, in the caudate nucleus and in right inferior parietal cortex. However, no decrease of left anterior insula activity and no learning effect across training sessions were observed in this condition. Interestingly, a tendency of improved down-regulation ability in the last training trails of each session was observed. In contrast, during the no-regulation condition we consistently found no significant BOLD signal increases, in line with other rt-fMRI studies using passive viewing as a baseline condition (Caria et al., 2007, 2010). The relative signal increase from session 1 to sessions 2 and 3 during no-regulation cannot be attributed to differential activations in the control area across sessions, but rather—as suggested by time-series and rt-fMRI analyses—the effect of emotional induction on anterior insula activity varies over trials.
In up- and down-regulation conditions, we found increased activation in the dorsal part of the ACC and bilaterally in the anterior insula, the adjacent frontal inferior operculum and VLPFC (BA 47). The dorsal ACC activity can be interpreted in terms of ongoing monitoring of regulation performance, as previously reported in emotion regulation paradigms (Ochsner et al., 2002, 2004). Conjoint activation of ACC and anterior insula is often reported in participants experiencing emotional feelings (Craig, 2009). VLPFC is specifically involved in the reappraisal of negative emotions (Ochsner and Gross, 2005), to support the selection and application of reappraisal strategies and to decrease, increase or maintain activity in appraisal systems such as the amygdala or insula in accordance with the goal of reappraisal (Beauregard et al., 2001; Ochsner et al., 2002; Schaefer et al., 2002; Levesque et al., 2003; Kim et al., 2004; Ochsner et al., 2004; Phan et al., 2005). The present results thus corroborate previous research showing that increased frontal cortex activation supports the top–down processes required to exert control over emotion-related insula activity (Davidson et al. 2000).
In line with the studies of Caria et al. (2007, 2010), we found bilateral insula activation during volitional up-regulation of left anterior insula. However, we found strong right-lateralized anterior insula activation during down-regulation. Gray et al. (2007) reported right anterior insula activity when false physiological feedback was provided. They concluded that the right anterior insula acts as a superordinate appraisal system for bodily arousal. It should be noted that participants were not aware that they were regulating activity of the anterior insula, as they were simply informed about their general ‘regulation success’ by the increasing or decreasing thermometer bar. Similarly, Lee et al. (2006) have shown that incongruent emotional states (smiling while viewing sad movies) activate the right anterior insula. Therefore, one could speculate that particularly during the early regulation period where the BOLD signal is enhanced after emotion induction, a mismatch between one's own feelings and the increasing feedback signal could cause a conflict during down-regulation but not during up-regulation. The heightened right anterior insula activity during down-regulation which we observed here could thus be interpreted as emotional conflict monitoring.
The multitude of additional activations in frontal, temporal and parietal areas during down-regulation may imply that more cognitive effort was involved during down-regulation. The greater difficulty to decrease negative emotions has been described previously (Ochsner et al., 2004; Kim et al., 2007). The middle frontal gyrus is involved in the selection and control of behavioural and emotional strategies during task performance and regulates selective attention (Garavan et al., 2006). Koenigsberg et al. (2010) have shown that emotional distancing from negatively valenced pictures activates inferior parietal gyrus, as well as the middle and superior temporal gyri. We found activations in these areas only during down-regulation and this supports the assumption that participants tried to distance themselves from the negative pictures.
A closer examination of activation time courses revealed different temporal dynamics during up- and down-regulation. While activity after up-regulation declined to baseline values during the resting period, activity after down-regulation kept increasing before the next trial. Interestingly, Goldin et al. (2008) reported a bilateral increase of insula activity when participants engaged in expressive suppression to regulate emotions. It is possible that different participants used different strategies for up- and down-regulation (i.e. reappraisal vs suppression) and this may partly explain our findings. It is also possible that the presence of threat-related images along with frustration about one's inability to down-regulate led to an unwanted increase in arousal and mental effort. Thus, while participants tried to reduce insula activity, they may have ended up being aroused by the self-regulation process, and their poor performance during down-regulation may have paradoxically activated the insula and other emotion-related areas. In this sense, down-regulation would become more difficult to achieve than up-regulation without previous training. In several studies, participants were successful at down-regulation of their emotional responses, but only after extensive training (Ochsner et al., 2004; Eippert et al., 2007). It can be assumed that without the conflicting feedback information, participants are better at controlling their emotional involvement. The fact that decreased anterior insula activity during down-regulation was observed in all sessions during the last trials supports the assumption that prolonged training periods might yield successful down-regulation.
The PPI analysis for the different training sessions during up-regulation revealed that in the first session, left anterior insula activity was functionally linked to right insula, right VLPFC and right frontal operculum, while in the second session, the left VLPFC and left middle OFC covaried with the seed region. The PPI analysis for up-regulation in the last training session, when most participants showed improved up-regulation of left anterior insula activity, revealed strengthened connectivity between left DMPFC and bilateral VLPFC and left anterior insula. Ochsner and Gross (2005) reported that top–down control of emotional responses via reappraisal activated the lateral and medial PFC and others have reported similar results (Quirk and Beer, 2006). Self-knowledge, i.e. the monitoring of one's own emotional state, has been associated with activation of MFC (Bush et al., 2000; Phan et al., 2002; Ochsner et al., 2004; Steele et al., 2004) and DMPFC. Pollatos et al. (2007) investigated brain areas involved in interoceptive awareness and demonstrated that the amount of interoceptive awareness correlated strongly with activity in DMPFC. A recent study using extended rt-fMRI training of the bilateral insula in schizophrenic patients showed that during successful up-regulation, the medial prefrontal cortex exhibited strong effective connectivity (outflow) to the insula using GCM (Ruiz et al., 2011). Based on these findings, our results suggest that the DMPFC plays a key role in the perception of feelings and feedback monitoring during up-regulation of the anterior insula.
A limitation of our study is that participants lacked training in self-regulation before the measurements were made. This prevents us from drawing firm conclusions about the effects of down-regulation, as no significant decrease in anterior insula activation was seen across subjects. Also, while rt-fMRI neurofeedback studies usually use regulation periods of 20–30 s, emotion regulation studies frequently apply regulation periods of only 11 s on average (Kalisch, 2009). It could be argued that a period of 9 s employed here was too short, because the BOLD signal is delayed relative to the onset of the task and the computed feedback signal during initial self-regulation is thus contaminated by late signals from the induction phase. It is possible that during the induction period, different pictures elicited stronger or weaker emotional responses and therefore modified the activation and hence the feedback signal. However, the BOLD signal during no-regulation trials reveals that the signal from the anterior insula can decrease even below baseline values in the presence of aversive pictures, making this confound appear less problematic. Nevertheless, optimized training protocols for rt-fMRI may be necessary when appraisal-relevant regions like the amygdala or anterior insula are the targets of self-regulation of stimulus-elicited activity. It is conceivable that longer regulation periods or the presentation of feedback signals only during the later parts of self-regulation periods are more suitable in these cases. The additional recording of peripheral data like breathing would further enhance the neural specificity of the feedback signal, although whole-brain and PPI analyses argue that respiratory effects do not account for the reported findings. Moreover, improved neurofeedback systems allowing the selection of ROIs solely in the grey matter over several slices, as well as advanced online artefact correction could increase the reliability of the feedback signal.
CONCLUSION
This study investigated the brain regions involved in the regulation of left anterior insula activity using rt-fMRI while subjects viewed aversive pictures. PPI connectivity analysis showed that during up-regulation, left anterior insula interacts positively with DMPFC and VLPFC. Our findings extend previous rt-fMRI work that focused on the targeted ROI, without addressing the question of how other regions may support or inhibit regulation success. Our results further demonstrate that rt-fMRI-based neurofeedback training may augment classical emotion regulation paradigms by providing direct feedback of activity in emotional brain networks and thus improving regulation success. Such an approach may prove especially useful in patients with impaired emotional control mechanisms such as in schizophrenia and psychopathy.
Conflict of Interest
None declared.
Acknowledgments
The Deutsche Forschungsgemeinschaft (DFG) (BI 195/56-1, BI 195/59-1 and KU 1453/3-1).
The research was supported by grants from the The Deutsche Forschungsgemeinschaft (DFG, German Research Foundation; grant BI 195/56-1, BI 195/59-1 and KU 1453/3-1) and the European Union (grant EU-FP7, CEEDs #258749).
REFERENCES
- Anders S, Lotze M, Erb M, Grodd W, Birbaumer N. Brain activity underlying emotional valence and arousal: a response-related fMRI study. Human Brain Mapping. 2004;23:200–9. doi: 10.1002/hbm.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–9. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–8. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cogntive Sciences. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nature Reviews Neuroscience. 2001;2:352–63. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- Caria A, Sitaram R, Birabumer N. Real-time fMRI: a tool for local brain regulation. Neuroscientist. 2011 doi: 10.1177/1073858411407205. 7 June 2011 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Caria A, Sitaram R, Veit R, Begliomini C, Birbaumer N. Volitional control of insula activity modulates the response to aversive stimuli. A real-time functional magnetic resonance study. Biological Psychiatry. 2010;68:425–32. doi: 10.1016/j.biopsych.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Caria A, Veit R, Sitaram R, et al. Regulation of anterior insular cortex activity using real-time fMRI. Neuroimage. 2007;35:1238–46. doi: 10.1016/j.neuroimage.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annual Review of Neuroscience. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel - now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ. Volitional control of autonomic arousal: a functional magnetic resonance study. Neuroimage. 2002;16:909–19. doi: 10.1006/nimg.2002.1147. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Maeda F, Glover GH, et al. Control over brain activation and pain learned by using real-time functional MRI. Proceedings of the National Acadademy of Sciences USA. 2005;102:18626–31. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert. F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28:409–23. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Research. 2006;1105:130–42. doi: 10.1016/j.brainres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural basis of emotion regulation: reapparaisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Harrison NA, Wiens S, Critchley HD. Modulation of emotional appraisal by false physiological feedback during fMRI. PLoS One. 2007;2:e546. doi: 10.1371/journal.pone.0000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S, Birbaumer N, Veit R. Real-time fMRI feedback training may improve chronic tinnitus. European Radiology. 2010;20:696–703. doi: 10.1007/s00330-009-1595-z. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Glover GH, Hsu J-J, Johnson RF, Gotlib IH. Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Human Brain Mapping. 2011;32:22–31. doi: 10.1002/hbm.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–8. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Johnston SJ, Boehm SG, Healy D, Goebel R, Linden DE. Neurofeedback: a promising tool for the self-regulation of emotion networks. Neuroimage. 2010;49:1066–72. doi: 10.1016/j.neuroimage.2009.07.056. [DOI] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: time matters. Neuroscience and Biobehavioral Reviews. 2009;33:1215–26. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kim J, Horwitz B. Investigating the neural basis for fMRI-based functional connectivity in a blocked design: application to interregional correlations and psycho-physiological interactions. Magnetic Resonance Imaging. 2008;26:583–93. doi: 10.1016/j.mri.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, et al. Contextual modulation of amygdala responsivity to surprised faces. Journal of Cognitive Neuroscience. 2004;16:1730–45. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, et al. Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia. 2010;48:1813–22. doi: 10.1016/j.neuropsychologia.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. American Journal of Psychiatry. 1997;154:926–33. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- Lee TW, Josephs O, Dolan RJ, Critchley HD. Imitating expressions: emotion-specific neural substrates in facial mimicry. Social Cognitive Affective Neuroscience. 2006;1:122–35. doi: 10.1093/scan/nsl012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Ruiz S, Caria S, Veit R, Birbaumer N, Sitaram R. Detection of cerebral reorganization induced by real-time fMRI feedback training of insula activation. Neurorehabilitation and Neural Repair. 2011;25:259–67. doi: 10.1177/1545968310385128. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, et al. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003;53:502–10. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Mak AK, Hu ZG, Zhang JX, Xiao ZW, Lee TM. Neural correlates of regulation of positive and negative emotions: an fMRI study. Neuroscience letters. 2009;457:101–6. doi: 10.1016/j.neulet.2009.03.094. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–5. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Bioliogical Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Human Brain Mapping. 2007;28:9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posse S, Fitzgerald D, Gao K, et al. Real-time fMRI of temporolimbic regions detects amygdala activation during single-trial self-induced sadness. Neuroimage. 2003;18:760–8. doi: 10.1016/s1053-8119(03)00004-1. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ruiz S, Lee S, Soekader S, et al. Acquired self-control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Human Brain Mapping. 2011 doi: 10.1002/hbm.21427. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz S, Sitaram R, Lee S, et al. Learned control of insular activity and functional connectivity changes using a fMRI Brain Computer Interface in Schizophrenia. 38th annual meeting of the Society for Neuroscience. 2008 Washington. November [Abstract] [Google Scholar]
- Schaefer SM, Jackson DC, Davidson RJ, Aguirre GK, Kimberg DY, Thompson-Schill SL. Modulation of amygdalar activity by the conscious regulation of negative emotion. Journal of Cognitive Neuroscience. 2002;14:913–21. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. Neuroimage. 2006;31:468–75. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Sitaram R, Caria A, Birbaumer N. Hemodynamic brain-computer interfaces for communication and rehabilitation. Neural Networks. 2009;22:1320–8. doi: 10.1016/j.neunet.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Sitaram R, Lee S, Ruiz S, Rana M, Veit R, Birbaumer N. Real-time support vector classicfication and feedback of multiple emotional brain states. Neuroimage. 2010;56:753–65. doi: 10.1016/j.neuroimage.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Sitaram R, Veit R, Steven B, et al. Acquired control of ventral premotor cortex activity by feedback training: an exploratory real-time fMRI and TMS study. Neurorehabilitation and Neural Repair. 2011 doi: 10.1177/1545968311418345. 8 September 2011 (Epub ahead of print; doi:10.1177/1545968311418345) [DOI] [PubMed] [Google Scholar]
- Sitaram R, Weiskopf N, Caria A, Veit R, Erb M, Birbaumer N. fMRI Brain-Computer Interfaces. IEEE Signal Processing. 2008;25:95–106. [Google Scholar]
- Steele JD, Lawrie SM. Segregation of cognitive and emotional function in the prefrontal cortex: a stereotactic meta-analysis. Neuroimage. 2004;21:868–75. doi: 10.1016/j.neuroimage.2003.09.066. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18:650–9. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, et al. Imaging attentional modulation of pain in the periaqueductal gray in humans. Journal of Neuroscience. 2002;22:2748–52. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf N, Sitaram R, Josephs O, et al. Real-time functional magnetic resonance imaging: methods and applications. Magnetic Resonance Imaging. 2007;25:989–1003. doi: 10.1016/j.mri.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Veit R, Erb M, et al. Physiological self-regulation of regional brain activity using real-time functional magnetic resonance imaging (fMRI): methodology and exemplary data. Neuroimage. 2003;19:577–86. doi: 10.1016/s1053-8119(03)00145-9. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, et al. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biological Psychiatry. 1998;44:1219–28. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]