Abstract
Alexithymia is a trait characterized by a diminished capacity to describe and distinguish emotions and to fantasize; it is associated with reduced introspection and problems in emotion processing. The default mode network (DMN) is a network of brain areas that is normally active during rest and involved in emotion processing and self-referential mental activity, including introspection. We hypothesized that connectivity of the DMN might be altered in alexithymia. Twenty alexithymic and 18 non-alexithymic healthy volunteers underwent a resting state fMRI scan. Independent component analysis was used to identify the DMN. Differences in connectivity strength were compared between groups. Within the DMN, alexithymic participants showed lower connectivity within areas of the DMN (medial frontal and temporal areas) as compared to non-alexithymic participants. In contrast, connectivity in the high-alexithymic participants was higher for the sensorimotor cortex, occipital areas and right lateral frontal cortex than in the low-alexithymic participants. These results suggest a diminished connectivity within the DMN of alexithymic participants, in brain areas that may also be involved in emotional awareness and self-referential processing. On the other hand, alexithymia was associated with stronger functional connections of the DMN with brain areas involved in sensory input and control of emotion.
Keywords: alexithymia, connectivity, default mode network, fMRI, resting state
INTRODUCTION
Alexithymia has been conceptualized as a personality trait that is associated with difficulties in emotion processing (Taylor et al., 1997). More specifically, alexithymia is characterized by difficulties in verbalizing one’s emotions, diminished affect-related fantasy and imagery, difficulty to distinguish emotions from bodily sensations and a tendency to focus on external events rather than internal experiences (Sifneos, 1973; Taylor et al., 1991). The prevalence was ∼10% in a Finnish sample (Salminen et al., 2007). Alexithymia has been associated with increased risk for psychosomatic complaints, anxiety disorders and depression (Taylor et al., 1997) and the emotion regulation difficulties characteristic of alexithymia have been hypothesized to play a mediating role in these (Taylor, 2000; Waller and Scheidt, 2006). Unraveling the neurocognitive mechanisms underlying alexithymia may improve our understanding of this trait with possible clinical and societal implications.
In this study, we started from the observation that alexithymia is associated with difficulties in emotion processing, e.g. recognizing emotional facial expressions and deducing emotions of others from narratives (Swart et al., 2009; Meltzer and Nielson, 2010), which may reflect a more general reduction of emotional awareness (Lane et al. 1997).The ability to recognize and experience emotions allows an individual to form a representation of his own emotions (Damasio et al., 2003; Northoff et al., 2006). Such self-referential emotional processing and imagery have been suggested to take place in a network of brain areas called the ‘Cortical Midline Structures’ (CMS) (Northoff et al., 2006), which is a key part of the default mode network (DMN) (Gusnard and Raichle, 2001). Indeed, parts of the DMN have been associated with emotion processing in general (Kober et al., 2008). The main regions within the DMN are the precuneus, posterior cingulate cortex (PCC), anterior cingulate cortex (ACC), inferior parietal lobule (IPL) and medial prefrontal cortex (MPFC) (Gusnard and Raichle, 2001). In a broader definition of the network, middle temporal gyrus (MTG), middle, superior and inferior frontal gyrus (MiFG, SFG and IFG), hippocampal formation and cerebellar regions are also included (He et al., 2004; Buckner et al., 2008). The DMN is also highly active during rest when self-referential processing apparently takes place (Gusnard and Raichle, 2001) and shows synchronized slow fluctuations across its brain areas (Gusnard and Raichle, 2001; Fransson, 2006). Schilbach et al. (2008) proposed in their review that brain activation of DMN regions during the resting state is related to self-consciousness and self-processing and may thus be relevant for introspection. Indeed, D’Argembeau et al. (2005) showed that activation in the anterior part of the DMN is correlated to self-referential thoughts.
Several studies have shown a relation between emotional awareness, which may be impaired in alexithymia, and DMN brain areas (Gusnard and Raichle, 2001; Northoff et al., 2006). Lower activation of the ACC and its connectivity to other brain areas have been related to lower emotional awareness and alexithymia (Lane et al., 1997, 1998). In alexithymic participants, the ACC and functionally related areas were less activated whereas the somatosensory cortex was more activated by emotionally valenced videos, emotional pictures, or imagery (Berthoz et al., 2002; Kano et al., 2003; Mantani et al., 2005; Moriguchi et al., 2006; Karlsson et al., 2008). Likewise, a structural MRI study found lower ACC and precuneus volumes in alexithymic participants (Borsci et al., 2008). Therefore, we hypothesized that alexithymic participants would show lower brain connectivity in areas implicated in emotional processing such as the ACC and higher somatosensory connectivity during rest, which may be related to less emotional awareness and a more action-oriented emotional coping style.
Since the DMN is highly active during rest and related to self-awareness, resting state analysis might, provide relevant information about the neural background of alexithymia. Resting state functional connectivity is considered as a more natural measure of brain function than task-based fMRI (Raichle and Gusnard, 2005), because it reflects intrinsic brain interactions (Van de Ven et al., 2004). These interactions may provide knowledge about overall brain function (Fox and Lancaster, 1994) and predict task performance or behavior (Fox and Raichle, 2007), without being biased by differences in task performance during the scanning (Calhoun et al., 2001; Van de Ven et al., 2004). If people with alexithymia in general devote less time to thinking about their feelings, this may have consequences for connectivity of relevant resting state areas, resulting in lower DMN connectivity.
Finally, alexithymia has been conceptualized as a disorder of emotion regulation, which warrants special interest for prefrontal areas known to be involved in emotion regulation (Taylor et al., 1997; Ochsner et al., 2002). Participants with alexithymia tend more to suppression of emotions than to use emotion reappraisal strategies (Swart et al., 2009). In reappraisal, participants use cognitive–linguistic strategies to downregulate emotional responses to arousing stimuli (Goldin et al., 2008; Reker et al., 2009; Silani et al., 2009). Effective control of emotions by reappraisal strategies has been related to activation of the medial and lateral prefrontal areas (Ochsner et al., 2002; Phan et al., 2005; Kim et al., 2007; Wager et al., 2008), which prevent excessive experience of negative emotions (Urry et al., 2009; Abler et al., 2010). Emotion regulation strategies such as suppression may involve right sided lateral frontal areas, but for a longer time period because suppression works on later stages of emotion processing than reappraisal (Ochsner et al., 2002; Goldin et al., 2008; Abler et al., 2010). We hypothesize that participants with alexithymia may show higher connectivity in the right lateral frontal and lower connectivity in the ventromedial prefrontal cortex, related to hampered emotion regulation.
In this study, the resting state DMN connectivity of high- vs low-alexithymic participants was investigated. Since no such study has been conducted before, this study has an exploratory nature. We hypothesized that alexithymic participants would show diminished connectivity in areas implicated in awareness (DMN areas) and verbalizing of emotions (left frontal areas). In addition, we explored whether there might be higher connectivity in alexithymic participants of areas implicated in emotion control (lateral prefrontal areas) and action-oriented processing (sensory and motor areas).
METHODS
Participants
The study was approved by the local medical ethical committee and carried out in accordance with the latest version of the Declaration of Helsinki. A total of 493 right-handed students of different disciplines from the local university filled out the verbalizing subscale of the Bermond–Vorst Alexithymia Questionnaire (BVAQ). This subscale was chosen because reduced ability to verbalize emotions has been considered a central deficit of alexithymia (Aleman, 2005). Further details of the questionnaire are given in the next section. Subjects scoring at the upper and lower extremes (25%) of the verbalizing subscale of the BVAQ, i.e. showing high or low levels of alexithymia based on this measure, participated in the study.
All participants were normally functioning and showed no signs of psychiatric illness. Persons with a history of psychiatric or neurologic disorder for which they had received treatment were excluded from the study. Further exclusion criteria consisted of MRI incompatible implants, aged >50 years, pregnancy, claustrophobia, left-handedness and being a non-native Dutch speaker.
A total of 20 participants with high- and 18 participants with low-verbalizing score that fulfilled the inclusion criteria participated. All participants gave oral and written informed consent prior to testing after the procedure had been fully explained. An overview of the participant characteristics is given in Table 1. Groups did not differ in age (t-test; T = −0.28; P < 0.78). Since female participants may have stronger verbalizing skills, dissimilarity in general language skills due to different male/female ratios between groups might confound interpretation of findings and therefore our groups were matched on gender distribution (Chi-square test; χ2 = 1.03; P < 0.16).
Table 1.
Demographical data
| Demographic characteristic | Low alexithymia, mean (s.d.) | High alexithymia, mean (s.d.) | P-value |
|---|---|---|---|
| Age (years) | 22.3 (8.1) | 21.6 (6.7) | 0.77 |
| Males/females | 11/9 | 6/12 | 0.16 |
| Cognitive component | 42.9 (8.1) | 63.2 (9.7) | < 0.0005 |
| Emotional component | 36.1 (8.1) | 40.0 (9.7) | 0.19 |
| PANAS positive affect | 33.8 (5.5) | 30.5 (6.3) | 0.059 |
| PANAS negative affect | 15.1 (3.5) | 14.7 (4.1) | 0.77 |
Mean and standard deviation of the demographic data of both subject groups, and the P-values of the t-test. The mean score on the BVAQ components and the two PANAS subscales are also shown. Subjects did differ significantly on the cognitive component of the BVAQ, but not on other subscales or demographic characteristics.
On the day of the MRI-session participants filled out the complete BVAQ and the Positive And Negative Affect Schedule (PANAS; Watson et al., 1988).
Questionnaires
For assessment of alexithymia, we used the BVAQ. Several studies have supported the criterion validity of the BVAQ in clinical samples, e.g. for eating disorders (Deborde et al. 2008), alcoholism (Sauvage and Loas, 2006), autism spectrum disorders (Berthoz and Hill, 2005), schizophrenia (Van ’t Wout et al. 2007) and high risk for schizophrenia (Van Rijn et al. 2011). However, because the BVAQ includes self-assessment, which may be compromised in certain clinical samples, its validity may be attenuated in clinical groups characterized by reduced insight in their psychological functioning. This is not a problem in the present study, because we investigated nonclinical participants.
The BVAQ is a validated 40-item self-report scale that consists of five subscales: verbalizing, fantasizing, identifying (cognitive component), emotionalizing and analyzing (emotional component) (Bermond and Vorst, 1993). Higher scores indicate a stronger degree of alexithymic characteristics. The scale measures alexithymic features as defined by Sifneos (1973). Previous studies have shown that the BVAQ has good psychometric properties (Berthoz et al., 2000, 2007; Zech et al., 1999).
The PANAS measures current affective positive and negative state (Watson et al., 1988). Positive affect refers to the extent to which a person feels enthusiastic, active and alert, negative affect addresses distress. The scale consists of ten positive and ten negative items, which can be scored on a 5-point scale (1 = certainly does not apply to me, up to 5 = certainly applies to me). The PANAS has shown good reliability and validity to measure positive (Cronbach’s α = 0.89) and negative (α = 0.85) current mood states (Watson et al., 1988).
Groups were tested on differences in the cognitive and emotional component of the BVAQ and the positive and negative subscale of the PANAS with a two sample t-test (α < 0.05).
Behavioral data
Participants also performed a language processing task in the same fMRI session. We report the performance on this task to provide an indication of language processing differences between groups. The task (adapted from Aleman et al., 2005) required participants to evaluate bisyllabic Dutch words for metrical stress followed by indicating the syllable that carried the metrical stress. In a second condition, participants rated the valence of a word (positive or negative).
Data acquisition
A resting state scan of 460 s was acquired at the end of an MRI session that additionally consisted of three tasks and an anatomical scan. During the resting state scan, participants were asked to close their eyes, relax and try to not fall asleep. There were no constraints on the content of their thoughts.
A 3 T Philips Intera MRI scanner (Best, The Netherlands) equipped with a eight-channel SENSE head coil was used to acquire 200 whole-brain echo-planar functional images (EPIs) with a TR of 2.3 s and TE 28 ms. The volumes contained 39 (n = 3 participants), 41 (n = 29) or 43 slices (n = 4); 3.8 × 3.8 × 3 mm), interleaved, with a 0-mm slice gap and a 85° flip-angle (FOV = 220 × 117 × 220 mm). The reason for a different number of slices is unspecified, but this is not expected to influence study outcome. A high-resolution, transverse T1 anatomical was also acquired for overlay of statistic images (160 slices; voxel size 1 × 1 × 1 mm; FOV 256 × 220 × 256 mm).
Preprocessing
The raw images were converted to ANALYZE format and analyzed using Statistical Parametric Mapping (SPM5; Wellcome Department of Imaging Neuroscience, London, UK) running on Matlab 7.1. Images were first corrected for slice-time differences and realigned to the first functional image. The mean image created during realignment was co-registered to the anatomy, together with the functional images, and the anatomy and functional images were normalized to the T1 template of SPM (voxel size 3 × 3 × 3 mm). Finally, images were smoothed with a 10-mm FWHM isotropic Gaussian kernel.
ICA procedure
Independent component analysis (ICA) is a data-driven method that can separate the fMRI signal into spatially independent networks (independent component; IC) that show shared temporal fluctuations (Calhoun et al., 2001; Jafri et al., 2008). An independent component consists of a time series and a spatial map per participant, which shows the contribution of every voxel in the brain to that time series, i.e. to that network (component). Those networks show a close correspondence to networks identified by activation studies (Smith et al., 2009). The size and strength of the identified networks may differ between individuals and groups sharing a specific trait (Calhoun et al., 2001; Van der Ven et al., 2005), i.e. a smaller network could represent altered connectivity of (the brain areas within) the network.
Images of all participants were decomposed into a set of independent components by the Group ICA FMRI Toolbox (GIFT) using the Infomax algorithm (Calhoun et al., 2001). We estimated the number of components by using the maximum likelihood and Akaike’s criteria (Li et al., 2006), to prevent splitting or merging of components (Smith et al., 2009). The Infomax algorithm is a commonly used method to unmix the signal (Calhoun et al., 2001). To perform group ICA, dimensionality of the data was first reduced using Principle Component analysis, and afterward the reduced subject data were concatenated over the time domain. Afterward, individual image maps were reconstructed from the aggregated data based on matrices stored during PCA. Resulting image maps and time courses were converted into Z-scores to normalize the signal. Though the overall architecture of the networks generated by spatial ICA is similar due to the separation based on spatial location, subtle differences between individuals may be present. These can be investigated in a voxel-wise group comparison (Calhoun et al., 2001). Independent components were first sorted based on the white matter and gray matter masks of SPM for exclusion of components with artifacts. Artifacts will generally represented be by separate components, which give the additional advantage of noise reduction in the data (Calhoun et al., 2001; Van der Ven et al., 2004). Selection of the component(s) of the DMN was based on spatial overlap with an anatomical mask of the DMN created with the WFU pickatlas (http://www.nitrc.org/projects/wfu_pickatlas/; Maldjian et al., 2003). The mask consisted of the PCC, precuneus, IPL, ACC, MPFC and MTG. See Supplementary Material for a figure of the mask. Components with a substantial overlap were visually inspected for DMN brain areas also described in the introduction. Stability of the components of interest, i.e. whether components had the tendency to split or merge with another component, was confirmed by running the ICASSO toolbox (Himberg and Hyvärinen 2003; Himberg et al., 2004), which ran 20 iterations of the ICA. If the same component was identified during all iterations, this indicates the stable presence of that component in the data.
Group differences on questionnaires
Statistical analyses on questionnaires were performed using Statistical Package for Social Sciences (SPSS 16) and all tests were two tailed. The cognitive component: the sum of the verbalizing, identifying and analyzing subscale and emotional component: the sum of the emotionalizing and fantasizing subscales, of the BVAQ were calculated. A two-sample t-test was applied to test for significant differences between groups.
The positive and negative affect subscales of the PANAS were also calculated and compared between groups.
Group differences on behavioral data
Reaction time and accuracy on the language task were compared between groups. A two-way ANOVA was conducted separately on reaction times and accuracy, and separately for both task conditions. The analyses had reaction time or accuracy on the metrical stress or valence condition as independent variable, and group and gender as independent variables (α < 0.05).
Statistical analysis of functional imaging data
The individual image maps of the identified components (networks) were entered into separate second level analyses of SPM5 per component, with a two sample t-test. First, a contrast was made of the main effect of interest, independent of group (FWE; P < 0.05; k > 15). Second, contrasts between both groups were made with a threshold of P < 0.001 uncorrected and a cluster-size threshold of 15 voxels. A mask of the contrast of the main effect of interest was used to restrict the analysis to DMN areas and to prevent false positive findings outside this area (inclusive mask; P < 0.05), as previously described (Garrity et al., 2007).
In an additional analysis, PANAS and gender were entered as a covariate into the group analysis. The affect states were investigated to check for an effect of mood state on the group differences. Gender was controlled for because this might also have an effect on default mode connectivity (Bluhm et al., 2008).
RESULTS
Questionnaire results
The scores on the two components of the BVAQ subscales and the positive and negative affect subscales of the PANAS are shown in Table 1. A two-sample t-test showed that groups differed significantly on the cognitive component [t (36) = 4.7, P < 0.0005], but not on the emotional component [t (36) = 1.3, P = 0.19]. Furthermore, there was a strong association between the cognitive component and the verbalizing scale used for selection (r = 0.76, P < 0.0005). Groups did not differ significantly on positive [t (36) = 1.9, P = 0.059] nor on negative affect [t (36) = 0.3, P = 0.78] as measured with the PANAS, although high-alexithymic participants had a higher negative and lower positive affect than the low-alexithymic participants.
Behavioral results
Reaction times and accuracy of the language task are presented in Table 2, separated for males and females. For the metrical stress condition, there was no significant effect of group [F(1,34) = 0.11, P = 0.75] or gender [F(1,34) = 0.053, P = 0.82] on reaction times, and also no interaction [F(1,34) = 0.18, P = 0.68]. There was also no significant effect on accuracy of group [F(1,34) = 0.013, P = 0.91], gender [F(1,34) = 0.076, P = 0.79] or interaction [F(1,34) = 0.28, P = 0.60].
Table 2.
Performance on metrical stress task
| Performance measure | High alexithymia, mean (s.d.) |
Low alexithymia, mean (s.d.) |
||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Reaction time (s) metrical stress | 1.53 (0.46) | 1.45 (0.36) | 1.44 (0.27) | 1.46 (0.51) |
| Accuracy (%) metrical stress | 78.1 (19) | 76.4 (24) | 75.3 (22) | 80.8 (19) |
| Reaction time (s) valence evaluation | 1.07 (0.43) | 1.11 (0.47) | 1.20 (0.16) | 1.18 (0.42) |
| Accuracy (%) valence evaluation | 78.1 (32.6) | 68.4 (37.6) | 87.4 (19.4) | 80.0 (34.5) |
Mean and s.d. of the reaction times and accuracy on the metrical stress task. Results are shown separately for males and females in both groups.
For the valence evaluation condition, there was no significant effect or interaction of neither group nor gender on either reaction times or accuracy. The main effect of group on reaction times was F(1, 34) = 0.56, P = 0.46, the effect of gender was F(1,34) = 0.003, P = 0.95, and their interaction was F(1,34) = 0.057, P = 0.81. The effect of group on accuracy was F(1,34) = 0.10, P = 0.96, of gender F(1,34) = 0.63, P = 0.43, and their interaction F(1,34) = 0.012, P = 0.93.
Imaging results
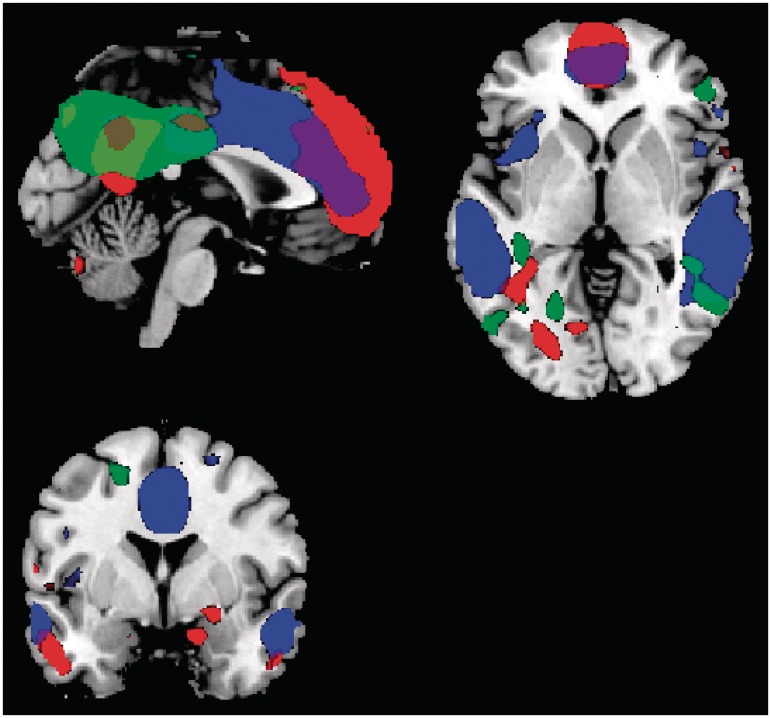
After running the ICA, the DMN was unexpectedly represented in three separate components. The DMN was split in (i) an anterior part with ACC, medial frontal gyri and lateral frontal areas, (ii) a mainly middle/lateral part containing MTG, cingulate, insula, hippocampal formation and (iii) a posterior part containing mainly PCC, precuneus and IPL. We will refer to these subnetworks as (i) ‘anterior’ component, (ii) ‘middle’ component and (3) ‘posterior’ component (Figure 1). The anterior component had a spatial overlap of 21% with the DMN template while it contained mostly frontal areas but also some PCC, the middle component of 25% and the posterior component 27%. One additional component showed an overlap of 23% but this component contained blood vessel artifacts. These components of interest were entered in the group comparison.
Fig. 1.
The three networks identified, the whole network is shown irrespective of group. All main areas of the default mode network are visible, including the anterior cingulate, posterior cingulate, medial middle/MTG, prefrontal cortex, precuneus and IPL. The anterior component is indicated in red, the middle DMN component in blue and the posterior component in green.
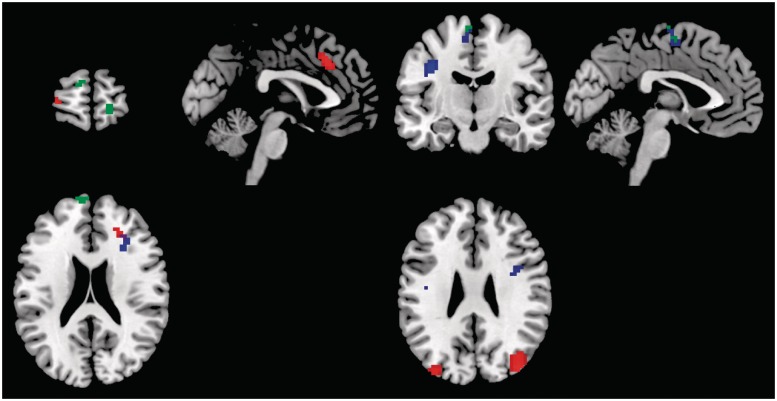
The contrast of alexithymic vs non-alexithymic participants showed lower connectivity within the DMN for the following areas in alexithymic individuals; anterior component: cingulate gyrus, superior frontal gyri and right medial temporal gyrus (MTG); middle component: right MiFG; posterior component: left medial frontal gyrus (MeFG) and right SFG (Table 3 and Figure 2).
Table 3.
Results of group comparison
| Voxels | T | Z | x | y | z | Area |
|---|---|---|---|---|---|---|
| High vs low alexithymia | ||||||
| Anterior | ||||||
| 36 | 5.23 | 4.48 | −33 | −84 | 24 | L superior occipital gyrus |
| 87 | 5.22 | 4.47 | 39 | −81 | 27 | R superior occipital gyrus |
| 22 | 4.08 | 3.67 | −27 | −75 | −27 | L declive |
| Middle | ||||||
| 44 | 5.66 | 4.75 | −36 | −15 | 36 | L precentral gyrus |
| 37 | 4.83 | 4.21 | −6 | −9 | 60 | L precentral gyrus |
| 27 | 4.55 | 4.02 | 33 | 0 | 30 | R IFG |
| Posterior | ||||||
| 30 | 3.93 | 3.56 | −6 | −9 | 60 | L precentral gyrus |
| 20 | 3.83 | 3.48 | 57 | 0 | 45 | R MiFG |
| Low alexithymia vs high alexithymia | ||||||
| Anterior | ||||||
| 47 | 4.93 | 4.28 | 0 | 24 | 39 | Cingulate gyrus |
| 23 | 4.59 | 4.05 | 27 | 36 | 24 | R SFG |
| 16 | 4.33 | 3.86 | −30 | 63 | 3 | L SFG |
| 33 | 4.21 | 3.77 | 39 | −3 | −36 | R MTG |
| Middle | ||||||
| 42 | 4.61 | 4.06 | 30 | 27 | 27 | R MiFG |
| Posterior | ||||||
| 16 | 4.34 | 3.87 | −9 | 66 | 18 | L MeFG |
| 15 | 4.04 | 3.65 | 15 | 66 | −6 | R SFG |
Overview of clusters showing connectivity differences between groups (P < 0.001; k > 15, inclusive mask at P < 0.05). The first part of the table shows areas that are more connected in alexithymia, and the second half areas of decreased connectivity. Anterior, middle and posterior refer to the different components of the DMN (L = left, R = right).
Fig. 2.
Left: brain regions that show decreased connectivity in subjects with high alexithymia compared to low alexithymic subjects. Different components are indicated in different colors. Green: anterior part, red: middle part; blue: posterior part. Right: brain regions that show increased connectivity in subjects with alexithymia compared to control subjects. Different components are indicated in different colors. Green: anterior part; red: middle part; blue: posterior part.
In contrast, higher connectivity with the DMN was observed for the following areas in alexithymic individuals; anterior component: occipital gyri and declive; middle component: precentral gyrus and right IFG; posterior component: precentral gyrus and right MiFG (P < 0.001; k > 15; Table 3 and Figure 2).
Adding gender, positive or negative affect as a covariate did not influence the outcome of the study. Thus group differences could not be explained by these variables.
DISCUSSION
The aim of this study was to investigate differences between alexithymic and non-alexithymic participants in default mode connectivity. Participants with high levels of alexithymia showed lower connectivity of DMN areas, including the cingulate gyrus, MeFG and MTG. In contrast, they showed higher connectivity to sensory areas and right lateral frontal areas. These group differences were not caused by differences in affect or gender distribution. Moreover, participants showed no difference in performance on a language task involving semantic emotional evaluation and stress placement, indicating that differences in such skills were not confounding interpretation of group differences. Networks identified with a resting state analysis have been shown to converge with networks resulting from task-induced activation (Fox and Lancaster, 1994; Smith et al., 2009). However, analysis of resting state networks is not biased by differences in task performance between groups (Van der Ven et al., 2004).
The high- and low-alexithymic group showed no effect of positive or negative affect on imaging results. The TAS-20, measuring a trait, often shows a relation with negative affect, which is a state characteristic (Lane et al., 1997). Since the TAS-20 may also tap aspects of affective state, the use of the BVAQ and the absence of an effect of the PANAS on our results may indicate that this study specifically measured trait alexithymia.
The two groups were initially selected based on the verbalizing subscale of the BVAQ. However, the high- and low-alexithymic group differed strongly on the cognitive component at the day of scanning. Thus, selection based on the verbalizing item of the BVAQ was considered adequate for creating two rather extreme groups. Interestingly, the cognitive component has shown a high correlation with the TAS-20 (Zech et al., 1999; Berthoz et al., 2000, 2007), thus this selection may be comparable to selection with the TAS-20.
The DMN was split into three subnetworks, while a test for component stability (ICASSO) showed a good stability of selected components. We did not expect the DMN to split into separate components. However, different components containing part of the DMN have been described by other studies as well, e.g. (Damoiseaux et al., 2006; Garrity et al., 2007; Jafri et al., 2008). Indeed, our identified networks may fit the DMN model of Laird et al. (2010) and three subdomains described by Kim (2010). The part containing MTG quite closely resembles the ‘action subnetwork’ defined by Laird et al. (2010) and is involved in salience according to Kim et al. (2010). The anterior component (Laird: ‘emotion subnetwork’) could be the core domain of the DMN involved in cognitive emotional processing, and the posterior component (Laird: ‘perception subnetwork’) could be involved in monitoring of external cues and top–down memory (Laird et al., 2009; Kim, 2010).
A key finding in the group comparison was that the alexithymic participants showed lower connectivity in anterior parts of the DMN, including cingulate gyrus, medial frontal regions and MTG (Gusnard and Raichle, 2001; Buckner et al., 2008). This finding is in accordance with our hypothesis of decreased emotional awareness in alexithymia (Lane et al., 1997), and is consistent with earlier approaches (Lane et al., 1998).
The DMN is believed to reflect the baseline ‘idling’ state of the brain that diminishes during specific goal-directed behaviors (Raichle et al., 2001). It has functions related to attending internal versus external state and consciousness (Raichle et al., 2001; Spreng et al., 2009). Slow-wave fluctuations of the brain have shown to be related to short gaps of inattentiveness during task performance, which could be interpreted as a transient more internally oriented focus (Singh and Fawcett, 2009). Resting state fluctuations of the DMN may thus be related to the degree to which persons engage in introspective thinking (Singh and Fawcett, 2009), which may be less pronounced in alexithymia. In a similar vein, research on subjects with meditation experience showed increased connectivity within attentional networks, as well as between attentional regions and medial frontal regions (Hasenkamp and Barsalou, 2012). The authors suggested that these neural relationships may be involved in the development of cognitive skills, such as maintaining attention and disengaging from distraction, that are often reported with meditation practice. Thus, prolonged mental training or habits may have lasting influences on resting state networks.
Interestingly, lower DMN connectivity has also been shown in ASD (Assaf et al., 2010), implying that it may reflect a trait characteristic associated with socioemotional difficulties. Furthermore, lower connectivity of the MTG may relate to impaired ability to link external events to self-referential mental activity (Laird et al., 2009). Like the anterior cingulate, prefrontal regions and temporal areas have also been implicated in language aspects of emotions (Anderson et al., 2010; Hesling et al., 2010), disturbances in these areas may also reflect more specific difficulties to put feelings into words (Aleman et al., 2005).
In addition, the alexithymic group showed stronger connectivity to areas implicated in sensory input, namely the precentral gyrus and occipital areas. One could speculate that higher connectivity with sensory areas is consistent with the action-oriented tendencies of alexithymic people and a tendency toward strong bodily expressions of emotions (Sifneos, 1973; Taylor et al., 1991; Taylor et al., 1997). Supporting our findings, alexithymic participants showed higher activation in sensory and motor areas (Karlsson et al., 2008) and altered activation of visual areas (Mantani et al., 2005; Karlsson et al., 2008) during viewing of emotional pictures. Participants with lower emotional complexity showed higher activation in action-oriented brain areas, such as the precentral gyrus, during animated ‘social’ interactions (Tavares et al., 2010).
The alexithymia group also showed higher connectivity in right-sided prefrontal regions. These areas have been implicated in emotional control including suppression (Goldin et al., 2008; Reker et al., 2009; Silani et al., 2009). Ochsner (2002) hypothesized that cognitive reappraisal may involve verbalizing strategies resulting in more left sided activation whereas other emotion regulation strategies such as suppression—more often used by alexithymic persons (Swart et al., 2009)—may lead to right sided frontal involvement (Ochsner et al., 2002). Of note, it has been shown that reduction of emotional arousal by affect labeling is mediated by the right vLPF (Lieberman et al., 2005, 2007), which does not fit our hypothesis. However, this topic should need further investigation in relation to alexithymia.
On a final note, some brain areas that showed altered connectivity in our study were not core parts of the DMN. Supporting our results, He et al. reported that these areas, such as precentral gyrus and lateral frontal areas, show a synchronized activation with the DMN (He et al., 2004).
In conclusion, the alexithymic group demonstrated a higher connectivity with right-sided prefrontal regions and sensory areas. These areas have been associated with emotion suppression and a more action-oriented focus. However, the high alexithymic group showed less connectivity with frontal areas of the DMN. We suggest that such distinct patterns of connectivity may be related to the diminished emotional awareness of alexithymic people.
FUNDING
This work was supported by a EURopean Young Investigator award (N.W.O. number 044035001) awarded to A.A.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This work was supported by a EURopean Young Investigator award (N.W.O. number 044035001) awarded to A.A.
REFERENCES
- Aleman A. Feelings you can't imagine: towards a cognitive neuroscience of alexithymia. Trends in Cognitive Science. 2005;9:553–55. doi: 10.1016/j.tics.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Aleman A, Formisano E, Koppenhagen H, Hagoort P, De Haan EH, Kahn RS. The functional neuroanatomy of metrical stress evaluation of perceived and imagined spoken words. Cerebral Cortex. 2005;15:221–8. doi: 10.1093/cercor/bhh124. [DOI] [PubMed] [Google Scholar]
- Abler B, Hofer C, Walter H, et al. Habitual emotion regulation strategies and depressive symptoms in healthy subjects predict fMRI brain activation patterns related to major depression. Psychiatry Research: Neuroimaging. 2010;183:105–13. doi: 10.1016/j.pscychresns.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Lange N, Froehlich A, et al. Decreased left posterior insular activity during auditory language in autism. American Journal of Neuroradiology. 2010;31:131–9. doi: 10.3174/ajnr.A1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. NeuroImage. 2010;53:247–56. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer DP. Spontaneous low-frequency blood oxygenation level-dependent fluctuations and functional connectivity analysis of the ‘resting’ brain. Magnetic Resonance Imaging. 2008;26:1055–64. doi: 10.1016/j.mri.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Bermond B, Vorst HCM, editors. Bermond-Vorst Alexithymia Questionaire. Amsterdam: Psychologisch Adviesbureau Bermond & Psychometric Service Interope; 1993. [Google Scholar]
- Berthoz S, Artiges E, Van de Moortele P-F, et al. Effect of impaired recognition and expression of emotions on frontolimbic cortices: an fMRI study of men with alexithymia. American Journal of Psychiatry. 2002;159:961–7. doi: 10.1176/appi.ajp.159.6.961. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Hill E. Reliability of the Bermond-Vorst Alexithymia Questionnaire: data from adults with autism spectrum disorder, their relatives and normal controls. European Psychiatry. 2005;20:291–8. doi: 10.1016/j.eurpsy.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Perdereau F, Godart N, Corcos M, Haviland MG. Observer- and self-rated alexithymia in eating disorder patients: levels and correspondence among three measures. Journal of Psychosomatic Research. 2007;62:341–7. doi: 10.1016/j.jpsychores.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Quhayoun B, Perez-Diaz F, Consoli SM, Jouvent R. Comparison of the psychometric properties of two self-report questionnaires measuring alexithymia: confirmatory factor analysis of the 20-item Toronto Alexithymia Scale and the Bermond-Vorst Alexithymia Questionnaire. European Review of Applied Psychology. 2000;50:359–68. [Google Scholar]
- Bluhm RL, Osuch EA, Lanius RA, et al. Default mode network connectivity: effects of age, sex, and analytic approach. Neuroreport. 2008;19:887–91. doi: 10.1097/WNR.0b013e328300ebbf. [DOI] [PubMed] [Google Scholar]
- Borsci G, Boccardi M, Rossi R, et al. Alexithymia in healthy women: a brain morphology study. Journal of Affective Disorders. 2008;114:208–15. doi: 10.1016/j.jad.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Buckner R, Andrews-Hanna J, Schacter D. The brain's default network anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from funcional MRI data using independent component analysis. Human Brain Mapping. 2001;14:140–51. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio A. Feelings of emotions and the self. Annals of the New York Academy of Sciences. 2003;1001:253–61. doi: 10.1196/annals.1279.014. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Collette F, Van der Linden M, et al. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage. 2005;25:616–24. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Deborde AS, Berthoz S, Wallier JM, et al. The Bermond-Vorst Alexithymia Questionnaire cutoff scores: a study in eating-disordered and control subjects. Psychopathology. 2008;41:43–9. doi: 10.1159/000109955. [DOI] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–45. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews in Neuroscience. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL. Neuroscience on the net. Science. 1994;266:994–6. doi: 10.1126/science.7973682. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. American Journal of Psychiatry. 2007;164:450–7. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel V, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D, Raichle M. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W, Barsalou LW. Effects of meditation experience on functional connectivity of distributed brain networks. Frontiers in Human Neuroscience. 2012;6:38. doi: 10.3389/fnhum.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zang YF, Jiang TZ, Lu YL, Weng XC. Detection of functional networks in the resting brain. Institute of Electrical and Electronics Engineers. 2004;1:980–3. [Google Scholar]
- Hesling I, Dilharreguy B, Peppé S, Amirault M, Bouvard M, Allard M. The integration of prosodic speech in high functioning autism: a preliminary fMRI study. PLoS One. 2010;5:e11571. doi: 10.1371/journal.pone.0011571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himberg, J., Hyvärinen, A. (2003) Icasso: software for investigating the reliability of ICA estimates by clustering and visualization. In: Proceeding 2003 IEEE Workshop on Neural Networks for Signal Processing (NNSP2003), Toulouse, France, pp. 259–68.
- Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time-series via clustering and visualization. NeuroImage. 2004;22:1214–22. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. NeuroImage. 2008;39:1666–81. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Fukudo S, Gyoba J, et al. Specific brain processing of facial expressions in people with alexithymia: an H2 15O-PET study. Brain. 2003;126:1474–84. doi: 10.1093/brain/awg131. [DOI] [PubMed] [Google Scholar]
- Karlsson H, Näätänen P, Stenman H. Cortical activation in alexithymia as a response to emotional stimuli. British Journal of Psychiatry. 2008;192:32–8. doi: 10.1192/bjp.bp.106.034728. [DOI] [PubMed] [Google Scholar]
- Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. NeuroImage. 2010;50:1648–57. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19:776–98. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. Journal of Neuroscience. 2009;29:14496–505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R, Kaszniak A, Ahern G, Schwartz G. Is alexithymia the emotional equivalent of blindsight? Biological Psychiatry. 1997;42:834–44. doi: 10.1016/s0006-3223(97)00050-4. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 1998;10:525–35. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Li Y, Adali T, Calhoun VD. In Proceedings ISBI. Washington, DC: 2006. Sample dependence correction for order selection in fMRI analysis. [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18:421–8. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Hariri A, Jarcho JM, Eisenberger NI, Bookheimer SY. An fMRI investigation on race-related amygdala activation in African-American and Caucasian-American individuals. Nature Neuroscience. 2005;8:720–2. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;9:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mantani T, Okamoto Y, Shirao N, Okado G, Yamawaki S. Reduced activation of posterior cingulate cortex during imagery in subjects with high degrees of alexithymia: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:982–90. doi: 10.1016/j.biopsych.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Meltzer MA, Nielson KA. Memory for emotionally provocative words in alexithymia: a role for stimulus relevance. Conscious Cognition. 2010;19:1062–8. doi: 10.1016/j.concog.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Lane RD, et al. Impaired self-awareness and theory of mind: an fMRI study of mentalizing in alexithymia. NeuroImage. 2006;32:1472–82. doi: 10.1016/j.neuroimage.2006.04.186. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, De Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain - a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard D, Shulman GL. A default mode of brain function. PNAS. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Gusnard DA. Intrinsic brain activity sets the stage for expression of motivated behavior. Journal of Comparative Neurology. 2005;493:167–76. doi: 10.1002/cne.20752. [DOI] [PubMed] [Google Scholar]
- Reker M, Ohrman P, Rauch AV, et al. Individual differences in alexithymia and brain response to masked emotion faces. Cortex. 2009;46:658–67. doi: 10.1016/j.cortex.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Salminen JK, Saarijarvi S, Aarela E, Toikka T, Kauhanen J. Prevalence of alexithymia and its association with sociodemographic variables in the general population of Finland. Journal of Psychosomatic Research. 2007;46:75–82. doi: 10.1016/s0022-3999(98)00053-1. [DOI] [PubMed] [Google Scholar]
- Sauvage L, Loas G. Criterion validity of Bermond-Vorst Alexithymia Questionnaire-20 Form B: a study of 63 alcoholic subjects. Psychological Reports. 2006;98:234–6. doi: 10.2466/pr0.98.1.234-236. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the ‘‘default system” of the brain. Consiousness and Cognition. 2008;17:457–67. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Shalom DB. The medial prefrontal cortex and integration in autism. The Neuroscientist. 2009;15:589–98. doi: 10.1177/1073858409336371. [DOI] [PubMed] [Google Scholar]
- Sifneos P. The prevalence of 'alexithymic' characteristic mechanisms in psychosomatic patients. Psychotherapy and Psychosomatics. 1973;21:133–6. doi: 10.1159/000286529. [DOI] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U. Levels of emotional awareness and autism: an fMRI study. Social Neuroscience. 2009;3:97–112. doi: 10.1080/17470910701577020. [DOI] [PubMed] [Google Scholar]
- Singh KD, Fawcett IP. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. NeuroImage. 2008;41:100–12. doi: 10.1016/j.neuroimage.2008.01.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart M, Kortekaas R, Aleman A. Dealing with feelings: characterization of trait alexithymia on emotion regulation strategies and cognitive-emotional processing. PLoS One. 2009;4:e5751. doi: 10.1371/journal.pone.0005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares P, Barnard PJ, Lawrence AD. Emotional complexity and the neural representation of emotion in motion. Social Cognitive and Affective Neuroscience. 2010;6:98–108. doi: 10.1093/scan/nsq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM, Parker JDA. The alexithymia construct: a potential paradigm for psychosomatic medicine. Psychosymatics. 1991;32:153–64. doi: 10.1016/s0033-3182(91)72086-0. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM, Parker JDA, editors. Disorders of Affect Regulation: Alexithymia in Medical and Psychiatric Illness. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Taylor GJ. Recent developments in alexithymia theory and research. Canadian Journal of Psychiatry. 2000;45:134–42. doi: 10.1177/070674370004500203. [DOI] [PubMed] [Google Scholar]
- Urry HL, Van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. NeuroImage. 2009;47:852–63. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Ven VG, Formisano E, Prvulovic D, Roeder CH, Linden DEJ. Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Human Brain Mapping. 2004;22:165–78. doi: 10.1002/hbm.20022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ven VG, Formisano E, Roder CH, et al. The spatiotemporal pattern of auditory cortical responses during verbal hallucination. NeuroImage. 2005;27:644–55. doi: 10.1016/j.neuroimage.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Van Rijn S, Schothorst P, Van ‘t Wout M, et al. Affective dysfunctions in adolescents at risk for psychosis: emotion awareness and social functioning. Psychiatry Research. 2011;187:100–5. doi: 10.1016/j.psychres.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Van ’t Wout M, Aleman A, Bermond B, Kahn RS. No words for feelings: alexithymia in schizophrenia patients and first-degree relatives. Comprehensive Psychiatry. 2007;48:27–33. doi: 10.1016/j.comppsych.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Neural mechanisms of emotion regulation: evidence for two independent prefrontal-subcortical pathways. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller E, Scheidt CE. Somatoform disorders as disorders of affect regulation: a development perspective. International Review of Psychiatry. 2006;18:13–24. doi: 10.1080/09540260500466774. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;47:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Zech E, Luminet E, Rime B, Wagner H. Alexithymia and its measurement: Confirmatory factor analyses of the 20-item Toronto Alexithymia Scale and the Bermond-Vorst Alexithymia Questionnaire. European Journal of Personality. 1999;13:511–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.