Abstract
Mycoplasma and Ureaplasma species are well-known human pathogens responsible for a broad array of inflammatory conditions involving the respiratory and urogenital tracts of neonates, children, and adults. Greater attention is being given to these organisms in diagnostic microbiology, largely as a result of improved methods for their laboratory detection, made possible by powerful molecular-based techniques that can be used for primary detection in clinical specimens. For slow-growing species, such as Mycoplasma pneumoniae and Mycoplasma genitalium, molecular-based detection is the only practical means for rapid microbiological diagnosis. Most molecular-based methods used for detection and characterization of conventional bacteria have been applied to these organisms. A complete genome sequence is available for one or more strains of all of the important human pathogens in the Mycoplasma and Ureaplasma genera. Information gained from genome analyses and improvements in efficiency of DNA sequencing are expected to significantly advance the field of molecular detection and genotyping during the next few years. This review provides a summary and critical review of methods suitable for detection and characterization of mycoplasmas and ureaplasmas of humans, with emphasis on molecular genotypic techniques.
The bacteria commonly referred to as mycoplasmas are included within the phylum Tenericutes, class Mollicutes, which is composed of four orders, five families, eight genera, and approximately 200 known species distributed among humans, animals, insects, and plants.1 Mollicutes are smaller than conventional bacteria, in cellular dimensions and genome size, making them the smallest free-living organisms known. Lack of a cell wall, coupled with their extremely small genome and limited biosynthetic capabilities, explains the parasitic or saprophytic existence of these organisms, their sensitivity to environmental conditions, resistance to β-lactam antibiotics, and fastidious growth requirements. Among the mollicutes that are the most important human pathogens, there are one or more type strains for which the genome has been completely sequenced and annotated. Genome sizes range from 580 to 2200 kbp, with Mycoplasma genitalium being the smallest.2 Mollicutes require enriched growth medium supplemented with nucleic acid precursors, fatty acids, and amino acids. Most mollicutes require sterols in growth media, supplied by the addition of horse or bovine serum.
There are 16 mollicute species that have been isolated from humans, excluding those of animal origin that have been detected occasionally in humans, usually in immunosuppressed hosts, but which are generally considered transient colonizers. There are at least six species considered to be of pathological significance, either as primary pathogens or opportunists: Mycoplasma pneumoniae, Mycoplasma hominis, M. genitalium, Mycoplasma fermentans, Ureaplasma urealyticum, and Ureaplasma parvum. A newly described species, Mycoplasma amphoriforme, has been detected in the lower respiratory tracts of several immunodeficient people with respiratory disease, but there is no conclusive evidence that this mycoplasma is a significant pathogen of humans.1 Other species, such as Mycoplasma penetrans and Mycoplasma pirum, have been studied because of a possible association with HIV and AIDS, but there is no evidence that either species is independently pathogenic in humans.3 Although M. fermentans can be considered an uncommon opportunistic pathogen of humans,3 it is rarely sought; methods for detection by culture are not well described, and few laboratories offer molecular-based tests for its detection. This article focuses on molecular methods for detection and identification of M. pneumoniae, M. genitalium, M. hominis, U. parvum, and U. urealyticum, because these are the mollicute species that are most important clinically and for which diagnostic testing is most commonly performed.
Major Pathogenic Mollicutes of Humans
A brief summary describing important biological characteristics of each species and salient clinical features of the infections they cause is provided. Readers are referred to other reference texts and reviews for more detailed descriptions.3–9
M. pneumoniae
This mycoplasma is a common cause of upper and lower respiratory tract infections in children and adults. The organism is easily spread through respiratory droplets and can cause a variety of clinical manifestations, including pharyngitis, tracheobronchitis, and pneumonia. Extrapulmonary manifestations sometimes occur after primary respiratory tract infection, either by direct spread or autoimmune effects.4,8 Attachment of M. pneumoniae to host cells in the respiratory tract is required for colonization and infection. Cytadherence is mediated by the P1 adhesin and other accessory proteins, followed by induction of chronic inflammation, and cytotoxicity is mediated by hydrogen peroxide, which also acts as a hemolysin. M. pneumoniae stimulates B and T lymphocytes and induces formation of autoantibodies that react with a variety of host tissues and the I antigen on erythrocytes, which is responsible for production of cold agglutinins.4 An ADP-ribosylating toxin, known as the community-acquired respiratory distress syndrome (CARDS) toxin, causes vacuolation and ciliostasis in cultured host cells and is becoming appreciated as a significant virulence factor.10 Although mycoplasmas are generally considered to be extracellular organisms, intracellular localization is appreciated for M. pneumoniae and other species, including M. genitalium.11,12 Intracellular localization may be responsible for protecting the organisms from antibodies and antibiotics, as well as contributing to disease chronicity and difficulty in cultivation. The genome of M. pneumoniae M129 (type strain for subtype 1) consists of 816 kbp with 687 protein-coding genes.13 The 811-kbp genome sequence of the FH strain (type strain for subtype 2) was published in 2010,14 and the third complete genome of M. pneumoniae strain 309 from Japan, classified as subtype 2a variant, consists of 817 kbp.15 A brief comparison of the three genomes indicated that they are similar, with variations in a region involving insertion changes in the putative lipoprotein genes.15 Comparative analysis of genomic differences among the type strains and the inclusion of clinical strains representing all of the major P1 subtypes could provide useful information in developing diagnostic tests and treatment strategies.
M. genitalium
This mycoplasma was initially isolated from men with urethritis and is a significant cause of this condition and female cervicitis and pelvic inflammatory disease.9 Unlike other genital mycoplasmas that are rather common as commensals in the lower urogenital tract of many healthy adults, M. genitalium is less commonly detected in the absence of clinical infection. M. genitalium possesses a terminal structure, the MgPa adhesin, that facilitates its attachment to epithelial cells,16 attaches to spermatozoa and erythrocytes, and invades epithelial cells with evidence of nuclear localization.9 A family of repetitive DNA elements homologous to the MgPa adhesin gene is believed to contribute to variation in the protein of the MgPa adhesin gene. Sequence divergence among strains of M. genitalium and antigenic variation may help avoid the host immune response and optimize adhesion.17 The 580-kbp genome of M. genitalium contains only 485 protein-coding genes, making it the smallest known free-living microorganism.
M. hominis
Approximately 21% to 53% of asymptomatic sexually active women are colonized with this mycoplasma in the cervix or vagina, but the occurrence is somewhat lower in the male urethra.3 M. hominis is often present concurrently with Ureaplasma species and is transmissible venereally and vertically. M. hominis is associated with a variety of conditions, including pyelonephritis, pelvic inflammatory diseases, chorioamnionitis, postpartum endometritis bacterial vaginosis, arthritis, osteoarthritis, wound infections, and several conditions in neonates (eg, congenital pneumonia, meningitis, bacteremia, and abscesses). Systemic infections sometimes occur in neonates, older children, and adults. Such extragenital infections outside of the neonatal period are usually, but not always, associated with an immunocompromised host status.3 Henrich et al18 demonstrated the presence of the variable adherence-associated antigen, which displays high-frequency phase and size variation that is believed to be a major adhesin of M. hominis and may also assist in evasion of host immune responses. Additional surface proteins, such as OppA, which is an oligopeptide permease substrate-binding protein, are also believed to be involved in cytadherence and may also induce ATP release from cells, resulting in apoptosis.19 The M. hominis genome contains 665 kbp and 527 protein-coding genes, placing it second, behind M. genitalium, as the smallest known self-replicating free-living organism.19 Genome analysis indicates that M. hominis has undergone horizontal gene transfer with Ureaplasma species.19
U. parvum and U. urealyticum
Members of the genus Ureaplasma hydrolyze urea and use it as a metabolic substrate for generation of ATP. This genus has seven recognized species, with U. parvum and U. urealyticum being the two species found in humans. As many as 40% to 80% of healthy adult women may harbor ureaplasmas in their cervix or vagina. Their occurrence is somewhat less in the lower urogenital tract of healthy men.3 The organisms are readily transmitted venereally and vertically, either in utero or at delivery of the neonate.3 U. parvum is more common than U. urealyticum as a colonizer of the male and female urogenital tracts and in the neonatal respiratory tract.3 Although detected less frequently than U. parvum in most patient populations, U. urealyticum may be more pathogenic in male urethritis.3,20 Ureaplasmas reside primarily on the mucosal surfaces of the urogenital tracts of adults or the respiratory tracts in infants. Despite their frequent occurrence in healthy people, Ureaplasma species may cause or be associated with a variety of clinical conditions in adults, including urethritis, arthritis, chorioamnionitis, postpartum endometritis as well as preterm birth, pneumonia, bacteremia, abscesses, meningitis, and chronic lung disease in neonates.3 Systemic spread is uncommon beyond the neonatal period unless there is an immunosuppressed condition, such as hypogammaglobulinemia.3,21 Ureaplasmas are capable of attaching to a variety of cell types, such as urethral epithelial cells, spermatozoa, and erythrocytes.3 The adhesins of ureaplasmas have not been characterized completely, but evidence suggests that the receptors are sialyl residues and/or sulfated compounds.3 A major family of surface proteins, the multiple-banded antigens, is immunogenic during ureaplasmal infections. Ureaplasmas produce an IgA protease and release ammonia through urea hydrolysis, both of which are considered possible virulence factors.3 Variation in surface antigens may be related to persistence of these organisms at invasive sites. Details of the pathogenic mechanisms through which ureaplasmas mediate human disease have been described elsewhere.3 Ureaplasma genomes range from 750 kbp (594 genes) for U. parvum to 947 kbp (711 genes) for U. urealyticum.
Limitations of Culture and Serological Analysis as Diagnostic Tests for Mycoplasmas and Ureaplasmas
Culture
Ureaplasma species grow rapidly in media containing urea, such as 10 B broth and A 8 agar, producing colonies visible with a stereomicroscope within 1 to 3 days.1 The appearance of brown granular colonies on A 8 agar is sufficient for the diagnosis of Ureaplasma species, but culture alone cannot distinguish between the two species. M. hominis grows well in SP 4 broth or SP 4 agar supplemented with arginine, but it will also grow on A 8 agar and in 10 B broth.1 Colonies appear on agar within 2 to 3 days, visible with a stereomicroscope. To confirm species identity for mycoplasmas growing on agar, additional procedures (eg, a PCR assay) must be performed because there are no phenotypic tests that can distinguish them.
PCR is more sensitive than culture for diagnostic purposes, even for organisms such as M. hominis and Ureaplasma species, which are relatively easily and quickly cultivated. Data from the University of Alabama at Birmingham Diagnostic Mycoplasma Laboratory showed that real-time PCR detected ureaplasmal DNA in 52 (39.4%) of 132 specimens versus 32 (24.2%) detected by culture.22 Even though culture was considered the reference method, PCR is theoretically able to detect fewer organisms; therefore, PCR-positive, culture-negative specimens likely represent true positives.
Despite PCR being more sensitive for detection of M. hominis and Ureaplasma species, culture remains the most economical and practical means for detection of these organisms for most laboratories with a low to moderate test volume. Cultures can be set up one at a time, whereas the most efficient and cost-effective use of PCR is to run the assays in batches of several specimens. However, batching lengthens the turnaround time for reporting results. Culture also has additional advantages in that it provides an isolate on which antimicrobial susceptibility testing can be performed. Highly sensitive PCR assays are useful for research studies when turnaround time is not so critical and when samples can be batch tested for cost-effectiveness. Although various modalities have been developed to enhance the ability to detect M. genitalium in culture, the high failure rate and extremely slow growth make culture impractical and rarely attempted now that molecular-based assays have been developed.
M. pneumoniae can be cultivated in SP 4 glucose broth, although its slow growth, requiring several days to weeks, and low sensitivity make culture unattractive for diagnostic purposes.1 Colonies growing on agar must undergo additional testing, typically by PCR, to confirm their identity because several commensal mycoplasmal species often inhabit the human oropharynx. Isolation of the organism from the respiratory tract or detection of its DNA is clinically significant in most instances. This should be correlated with the presence of clinical respiratory tract disease, because occasional asymptomatic carriers may exist.
Detailed methods for obtaining specimens, culturing, and identifying mycoplasmas and ureaplasmas of humans in vitro using culture-based methods have been described in other reference texts.1,23 Whether culture- or non–culture-based detection methods should be used for diagnostic purposes depends on the resources and facilities available in individual laboratories and the species being sought.
Serological Analysis
Serological testing was the first method developed for detection of M. pneumoniae infections. Complement fixation assays were used for many years until the development of alternative serological methods sold commercially, such as enzyme immunoassays, immunofluorescence, and particle agglutination assays. More recently, molecular-based nucleic acid amplification tests (NAATs) have further reduced the need for serological diagnosis. Despite its widespread use, at least partly because of the expense and limited availability of NAATs, serological analysis has far more limitations than advantages for detection of acute M. pneumoniae infections. The main disadvantages of serological analysis are the requirement for acute and convalescent serum samples that are tested simultaneously for IgM and IgG to confirm seroconversion, difficulty in distinguishing current or recent infection from past infection, and need to wait 1 to 2 weeks from onset of the infection until detectable antibody develops.24 Adults may never develop a measurable IgM response, presumably because of reinfections.4 Moreover, IgM antibodies can persist for weeks to months, making it risky to base diagnosis of acute infection on a single assay for IgM.4 In addition, as a result of repeated infections, many healthy adults can also have a high background seropositivity for IgG25; thus, there is a need to measure antibody increase and decrease in acute and convalescent specimens. Antibody production may also be delayed in some infections or even absent if the patient is immunosuppressed.
Problems with sensitivity and/or specificity have been reported for practically all serological assay formats and commercial products when rigorously compared with other detection methods, such as PCR.24 However, many serological tests have never undergone extensive comparative evaluations and comparison with other diagnostic methods, so their accuracy is unknown. Serological testing of M. pneumoniae and the various commercial methods available for antibody measurement have been described in other reference texts and reviews.1,4,23,24
Serological test methods for M. hominis, Ureaplasma species, and M. genitalium include microimmunofluorescence, metabolism inhibition, and enzyme immunoassay,1 but the ubiquity of ureaplasmas and M. hominis in healthy people makes interpretation of antibody titers against these organisms difficult. No serological assays for the genital mycoplasmas have been standardized, and they are not available in the United States for diagnostic purposes.
Overview of Molecular-Based Tests for Detection of Mollicutes
Interest in non–culture-based detection of mycoplasmas dates back to the 1980s when assays for M. pneumoniae antigen detection and nonamplified DNA probes were developed, driven by the drawbacks of culture and serological analysis. Once NAATs began to be developed, interest in nonamplified DNA probes and antigen detection assays waned, and there are no products of this nature sold commercially in the United States for detection of M. pneumoniae.
Since 1989, hundreds of publications have described various NAATs and their applications to detect mycoplasmas and ureaplasmas in clinical specimens. These assays enable detection of extremely fastidious species, such as M. genitalium, that might never be detected otherwise. NAATs are also useful for the identification of organisms grown in culture to the species level, replacing older and cumbersome technologies.
PCR is the most widely applied NAAT for detection of mycoplasmas and ureaplasmas. PCR has also been adapted to detect antimicrobial resistance determinants and to analyze genetic relatedness of clinical isolates. Conventional PCR measures the end-stage PCR products using gels or other methods, whereas real-time PCR detects and quantifies the products simultaneously with amplification. Nested PCR can increase sensitivity through re-amplification of a PCR product with a second set of primers.26 However, the nested PCR method may also enhance the risk of contamination.27 Publications describing real-time PCR for detection and characterization of mycoplasmas and ureaplasmas have used the ABI Prism 7900HT (Applied Biosystems, Carlsbad, CA), the iCycler iQ (Bio-Rad, Hercules, CA), and the LightCycler 2.0 (Roche Diagnostics, Indianapolis, IN). Detection systems include agarose gel electrophoresis, SYBR Green, TaqMan probes, hybridization probes, molecular beacons, and microchip electrophoresis (Foster City, CA).28 The UAB Diagnostic Mycoplasma Laboratory has eliminated conventional PCR in favor of real-time PCR using the Roche LightCycler for detection and identification of all of the major mollicute pathogens of humans because of its advantages in accuracy, quantitation, and turnaround time. The improved specificity of real-time PCR compared with conventional PCR is mainly because of the use of a third oligonucleotide probe that binds to the target sequence. The use of a labeled probe minimizes the probability of cross-reaction and detection of undesired amplicons. Another feature of real-time PCR that contributes to the specificity of the assay is that the amplicon melting temperature is determined at the end of the assay, so it can be used to verify whether the desired PCR product is being detected.
Because organism viability does not have to be maintained for NAAT-based detection, specimen collection, handling, and transport are somewhat simpler than for culture, in that a specialized nutritive transport medium is not required. Although conventional PCR methods can take 2 to 3 days, real-time PCR can potentially provide results the same day a specimen is received and provide quantitative data to determine the bacterial load. This can be important for interpretation of results for organisms that are known to colonize asymptomatic people. Two studies have reported that the bacterial load of M. pneumoniae in throat swabs was significantly greater in patients requiring hospitalization than in those who were not hospitalized, indicating the potential importance of such quantitative data.29,30 The pros and cons of NAATs, culture, and serological analysis, as they apply to detection of mycoplasmas and ureaplasmas in clinical specimens, are summarized in Table 1.
Table 1.
Advantages of Molecular-Based Methods Compared with Culture and Serological Analysis for Detection of Mycoplasmas and Ureaplasmas in Humans
| Criteria | Molecular-based assays | Culture | Serological analysis |
|---|---|---|---|
| Availability | Not commercially available in the United States, a few PCR kits are available in Europe and Asia. Nonproprietary PCR assays are available in a few US reference laboratories. | Commercially prepared SP 4, 10 B, and A 8 media and test kits for M. hominis and Ureaplasma species are available. Culture is available in many large hospital and reference laboratories. Additional immunoserological or genotypic tests are required to confirm species identity of large-colony mycoplasmas. | Commercial qualitative and quantitative antibody assays are available for M. pneumoniae. No such assays are available for other Mycoplasma or Ureaplasma species in the United States. |
| Cost | Cost of equipment and reagents is significant, and personnel trained in molecular diagnosis are required. Costs are less if equipment, facilities, and personnel can be used for other molecular diagnostic testing. | Media are somewhat expensive to obtain or prepare. Equipment used for general microbiology purposes is usually sufficient. Length of time cultures have to be held for slow-growing mycoplasmas adds to personnel costs. | Commercial serological kits for M. pneumoniae vary in cost. Some assay formats are suitable for testing single specimens, whereas others are more practical for batches. Cost per test depends on the volume of specimens and equipment requirements. |
| Turnaround time | Real-time PCR can be completed in a few hours. Batching specimens and running the assays once or twice each week decrease the costs but delay turnaround time. | M. hominis and Ureaplasma species can be grown in culture within 1–3 days, whereas M. pneumoniae requires from 5 days up to several weeks. M. genitalium cannot be reliably grown in culture. | Hands-on time varies from a few minutes to a few hours. Acute and convalescent serum sample collection time spans 2–3 weeks. |
| Analytical sensitivity | High: most assays detect <100 CFU/mL organisms or 100 genome copies. | May detect 100–1000 viable organisms per test. | Serological tests do not measure the presence of the microorganism, but instead measure the host immune response. Compared with PCR, serological analysis may miss many infected individuals. |
| Specificity | PCR assays that are carefully validated with targets chosen for diagnostic accuracy and lack of cross-reactivity are specific. | Culture is 100% specific when positive. | Older complement fixation tests had problems distinguishing M. pneumoniae from M. genitalium. Newer commercial ELISAs do not have this problem. |
| Specimen requirements | Organisms do not have to be viable. The same specimen types used for culture can be submitted for PCR assays. Specimens require frozen storage until processing. Formalin-fixed tissue can also be processed by PCR. | Properly collected specimens require appropriate transport media, frozen storage, and shipment to maintain viability. | Serum is the only required specimen type. No special handling or storage, other than refrigeration, is needed. |
ELISA, enzyme-linked immunosorbent assay.
Use of Molecular-Based Tests for Mollicute Detection
M. pneumoniae
Gene targets for PCR assays have included 16S ribosomal RNA (rRNA), P1, tuf, parE, dnak, pdhA, ATPase operon, CARDS toxin gene (mpn372), and the noncoding repetitive element repMp1.6,26,28,31–33 Analytical sensitivity is generally high, with some assays being capable of detecting a single organism when purified DNA is used.
The UAB Diagnostic Mycoplasma Laboratory adapted the real-time PCR assay published by Dumke et al,32 targeting the repMp1 noncoding DNA sequence for routine diagnostic use. Its theoretical advantage is the fact that sensitivity may be improved by amplification of a gene present in multiple copies. This assay provided acceptable sensitivity and specificity when tested against several M. pneumoniae reference strains, detecting as few as seven DNA molecules per microliter, including both major P1 subtypes. The assay also detected M. pneumoniae DNA in a large group of clinical specimens obtained from patients with radiologically proved pneumonias who were positive for M. pneumoniae by serological analysis, culture, and/or conventional PCR. No other mollicute species or other respiratory pathogens tested positive by this method.32,34
Use of PCR for detection of M. pneumoniae infection in extrapulmonary sites can also be helpful because cultures from nonpulmonary sites are rarely positive as a result of low organism load. PCR has also been advantageous when insufficient time has elapsed since onset of illness for an antibody response to develop and for testing fixed lung tissue obtained at biopsy.6 The advantage of real-time PCR over conventional PCR in detection of systemic infection was demonstrated in a study that found 15 (52%) of 29 patients with serological evidence of M. pneumoniae infection had a positive assay result when serum samples were tested by real-time PCR. Conventional PCR assay results for those specimens were entirely negative.35
Although PCR may theoretically be more sensitive than culture, some studies have yielded contradictory results.6 For PCR-positive, culture-negative patients, it is important to ascertain whether clinically significant respiratory disease is actually present, because the positive PCR assay result may reflect asymptomatic carriage with a low bacterial load, prior antibiotic therapy, persistence of mycoplasmal DNA after resolution of infection, organisms residing in an intracellular location not amenable to culture, or the possibility that the PCR target was nonspecific. A positive PCR assay result in a patient who is serologically negative may indicate that the specimen was obtained too early in the infection for measurable antibody to have developed, antimicrobial therapy that may have blunted the immune response, or an inadequate immune response because of immunosuppression. Negative PCR results in patients who are culturally and/or serologically positive could indicate technical problems with the PCR assay or inhibitors. Using a PCR assay with a different gene target may resolve the problem. One issue that can be problematic with PCR assays for M. pneumoniae is that they may not have been tested adequately for specificity for this organism by making certain there is no reactivity with the numerous commensal mycoplasmal species of the human respiratory tract. The use of a highly specific PCR for M. pneumoniae has been particularly useful in our experience when mycoplasma species are detected in respiratory tract cultures because they frequently turn out to be one of the common commensal species and not M. pneumoniae. Performing PCR assays with two different gene targets is theoretically the best diagnostic approach for M. pneumoniae infections, but using the second target increases costs and takes more time. Combining PCR with serological analysis has also been advocated as a possible means to distinguish colonization from active disease, but this also adds to the cost of testing and will not overcome the problem common in older people who often fail to mount an acute-phase IgM response, necessitating testing paired serum samples and prolonging the time until diagnosis can be confirmed.
There is no universal consensus regarding what constitutes the best respiratory specimen to be tested by PCR. Combining nasopharyngeal and oropharyngeal specimens may provide the greatest diagnostic yield.36 One study reported that sputum was superior to oropharyngeal or nasopharyngeal specimens in young adults with serologically proved M. pneumoniae infection.37 However, young children and many adults with mild illness often do not produce sputum, so nasopharyngeal or oropharyngeal samples may be the only specimen types available. In our experience, many children with severe pneumonia requiring hospitalization will have positive PCR results for M. pneumoniae on bronchoalveolar lavage fluid obtained by bronchoscopy.34
There have been few side-by-side comparisons to determine whether one assay format or gene target is better than another. Many of the comparisons that have been done compared PCR using culture or serological analysis as a reference method, and predictably yielded disparate results in some cases. The Centers for Disease Control and Prevention compared three real-time PCR assays to detect M. pneumoniae.31 They performed triplicate PCR assays using the Applied Biosystems ABI 7500 system using two different TaqMan primer-probe sets targeting the ATPase gene and a new assay targeting the CARDS toxin gene on 54 respiratory samples from an outbreak in a college setting. Eighteen cases had positive results with all three assays. When dilutions of M. pneumoniae reference strains were tested, the CARDS toxin PCR assay consistently detected 1 to 5 colony-forming units (CFUs), whereas the other two assays targeting the ATPase genes detected 5 to 50 CFUs. Two multicenter comparisons of various NAATs for M. pneumoniae detection38,39 reported significant variations in test performance among participating laboratories, making a strong case for an organized proficiency test program, which is available in some European countries.40 In the United States, where there are no commercially sold Food and Drug Administration–approved PCR assays for any Mycoplasma or Ureaplasma species, reference laboratories that offer their own internally developed NAAT should participate in alternative proficiency testing, in which clinical specimens are exchanged in a blinded manner, or use the M. pneumoniae proficiency testing specimens that are available through the College of American Pathologists.
Both conventional and real-time nucleic acid sequence-based amplification (NASBA) have been used to detect M. pneumoniae RNA.41 NASBA can provide rapid results with sensitivity as good as or better than PCR, with a detection threshold as low as 5 to 50 CFUs.41,42 This assay has been described in monoplex and multiplex format and has been developed as a commercial kit (NucliSENS; bioMérieux, Marcy l'Etoile, France) sold in various European countries. Multiplex PCR and NASBA assays have been developed in a variety of formats for detection of M. pneumoniae, along with Chlamydophila pneumoniae and Legionella pneumophila.43–46 Generally speaking, some loss in analytical sensitivity occurs in multiplex assays when compared with monoplex assays that may be related to incompatible amplification conditions for multiple targets and the high concentration of primers that can cause elevated background readings and reduced efficiency. Although the multiplex NASBA has potential to detect M. pneumoniae and C. pneumoniae and Legionella species, it had a slightly lower sensitivity than monoplex NASBA when applied to dilutions of wild-type RNA generated in vitro.47 Other techniques, including reverse line blot assays and microarrays, have been used alone or in combination with multiplex PCR assays for detection of M. pneumoniae and other respiratory pathogens.33,45,46,48,49
The loop-mediated isothermal amplification assay has been applied to detection of M. pneumoniae in clinical specimens using P1 sequences for primers in direct comparison to real-time PCR.50 The assay was specific, with a detection limit of 200 copies, and had 100% concordance with PCR when applied to 95 nasopharyngeal specimens. The development of commercial assays using this type of technology for M. pneumoniae is anticipated because instrumentation and reagents are undergoing clinical trials.
Commercial PCR assays have been available in Europe for several years, and additional products are still in development. Limited evaluations have shown they work in a comparable manner to noncommercial assays.45,46,51 These assays include the Artus RepMp1 (Qiagen, Valencia, CA), Venor Mp-QP (Minerva Biolabs, Berlin, Germany), Chamylege (Argene Inc., Shirley, NY), and Pneumoplex (Prodesse Inc., Waukesha, WI).
A comprehensive review of diagnostic methods for M. pneumoniae respiratory tract infection, published by Loens et al,28 indicated that, as of 2010, there were at least 61 published in-house PCR assays for M. pneumoniae, many of which have been validated only for their analytical sensitivity and not tested against many clinical samples, or against one another. Some of the more recently described real-time PCR assays for detection of M. pneumoniae and other species are listed in Table 2.
Table 2.
Examples of Real-Time PCR Assays for Detecting Mycoplasma and Ureaplasma Species in Clinical Samples
| Specificity | Target gene/region | Analytical sensitivity (genome copies or CFU/reaction) | Ref. no. |
|---|---|---|---|
| Up and Uu | 16S rRNA | 10 copies | Yoshida et al52 |
| Up | Urease gene subunits and adjacent regions | 5 copies | Mallard et al53 |
| Up | UU063 | 0.6 CFU | Xiao et al22 |
| Up | Urease gene subunits and adjacent regions | 10 copies | Cao et al54 |
| Uu | Urease gene subunits and adjacent regions | 10 copies | Cao et al54 |
| Uu | Urease gene subunits and adjacent regions | 5 copies | Mallard et al53 |
| Uu | UUR10_0680 | 0.8 CFU | Xiao et al22 |
| Mp | P1 | 10 copies | Pitcher et al55 |
| Mp | repMp1 in P1 | 0.2 CFU | Dumke et al32 |
| Mp | CARDS toxin (mpn372) | 1–5 CFU | Winchell et al31 |
| Mp | ATPase operon | 5–50 CFU | Winchell et al31 |
| Mp | 16S rRNA | 1 copy | Khanna et al45 |
| Mp | 16S rRNA | 5 CFU | Raggam et al44 |
| Mg | gap | 5 copies | Svenstrup et al56 |
| Mg | 16S rRNA | 10 copies | Yoshida et al57 |
| Mh | gap | 10 copies | Baczynska et al58 |
| Mh | yidC | 7 copies | Ferandon et al59 |
Mg, M. genitalium; Mh, M. hominis; Mp, M. pneumoniae; Up, U. parvum; Uu, U. urealyticum.
Macrolide resistance in M. pneumoniae is a major problem in Asia and is spreading to Europe and North America.6 PCR assays have been developed to detect three major mutations in domain V of 23S rRNA that confer high-level macrolide resistance in isolates of M. pneumoniae or directly in clinical specimens.34,60,61 A rapid and inexpensive method that combines nested PCR, single-stranded conformation polymorphisms, and capillary electrophoresis can also detect macrolide-resistant mutants directly from throat swabs.62 Finally, pyrosequencing has also been applied for detection of macrolide resistance in M. pneumoniae and for strain typing.63
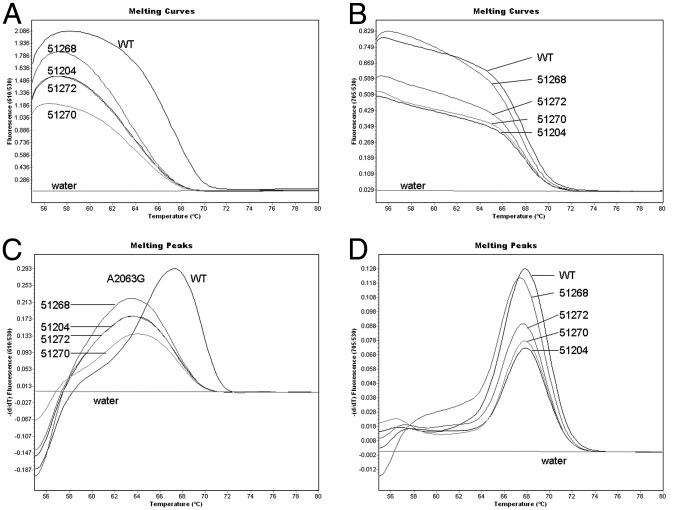
The UAB Diagnostic Mycoplasma Laboratory performs a multiplex real-time PCR assay to detect point mutations in all three positions of the 23S rRNA gene (2063, 2064, and 2617) that are related to the macrolide resistance on all clinical samples that are positive for M. pneumoniae in the repMp1 real-time PCR assay. This assay uses fluorescence resonant energy transfer hybridization probes and the Roche LightCycler 2.0 instrument. This method is based on the fact that nucleic acid will melt at a precise temperature that is related to the nucleotide base composition. The presence of one or more point mutations in 23S rRNA that impair drug attachment to the bacterial ribosome will be detected by this extremely sensitive method, which can be completed in just a few hours. The detection limit is as low as seven mutant molecules per microliter in the PCR mixture.34 Figure 1 illustrates how this assay detects and distinguishes the mutants.
Figure 1.
Real-time PCR detection of macrolide-resistant M. pneumoniae in clinical samples. Genomic DNA samples of two patients containing the A2063G mutation, verified by sequencing, are purified and tested together with a wild-type (WT) control (M. pneumoniae strain M129, ATCC number 29342). Melting curves (A and B) and corresponding melting peaks (C and D) are shown. A2063/A2064 mutations are analyzed in channel 610 (A and C). The WT melting peak is 67.31°C, whereas the melting temperature (Tm) of A2063G mutants is 63.25°C ± 0.04°C. Thus, a 4°C difference between WT and mutant is observed. The C2617 assay is shown in channel 705 (B and D). Because all samples do not have mutations at this position, they show similar WT Tm values of approximately 68°C, as predicted.
Data are reproduced from Xiao et al,34 with permission of Walters Kluwer Health (copyright 2009).
Given the relatively mild clinical course of most mycoplasmal respiratory tract infections, lack of widespread availability of rapid diagnostic tests, and their associated costs, many clinicians choose to treat empirically when infection with this organism is strongly suspected. Testing for M. pneumoniae would almost certainly become more widespread in the United States if rapid real-time PCR assays become more readily available so that test results can play a greater role in guiding initial patient management. Even if specimens have to be sent to a reference laboratory with a longer turnaround time, it is reasonable to pursue a comprehensive microbiological diagnosis for patients with lower respiratory tract infections, including testing for M. pneumoniae by PCR, when respiratory illness is of sufficient severity to warrant hospitalization and no other causative microorganism is readily apparent. Testing for M. pneumoniae should also be considered if there is an unsatisfactory clinical response to empirical treatment with macrolides because this may indicate the presence of a macrolide-resistant strain, in patients with an underlying comorbid condition or immunodeficiency that would make severe and disseminated disease more likely, and when there are significant extrapulmonary symptoms present.
M. genitalium
Although M. pneumoniae and M. genitalium are structurally and antigenically related, they are genomically different. Most of the early PCR assays targeted various regions of the MgPa operon, but some have used the 16S rRNA gene.64,65 Additional molecular-based assays, including transcription-mediated amplification, have also been used for epidemiological purposes.66–68 Quantitative, rapid, real-time PCR assays have used targets such as MgPa operon, 16S rRNA, the 115-kDa gene, and gap encoding glyceraldehyde-3-phosphate dehydrogenase.56,57,68–72 It has become apparent that not all primers used in various studies react with all M. genitalium strains, especially those using the MgPa target. Ma et al73 examined the three genes of the M. genitalium MgPa operon (mgpA, mgpB, and mgpC) and nine repetitive sequences (termed MgPars) dispersed throughout the genome in 15 geographically diverse strains. They discovered that all operon sequences and all MgPars differed from each other more than from the published G37 operon sequence at both the nucleotide and deduced amino acid levels. They determined that one of 19 primers contained up to 19 variable nucleotides and that the target for one of two typing systems was located in a hypervariable region, indicating the likelihood of erroneous results with some assays. This has been demonstrated in studies using primers MGS-2 and MgPa-903.73 These data suggest that there is an efficient recombination system enabling generation of numerous variants that may facilitate evasion of host defenses and that additional research and development must be undertaken to identify and validate the most appropriate PCR primers for diagnostic testing.
In view of concerns for use of MgPa, and 98% identity of the 16S rRNA gene for M. pneumoniae and M. genitalium, the UAB Diagnostic Mycoplasma Laboratory has adapted the real-time PCR assay described by Svenstrup et al56 for detection of M. genitalium in clinical specimens. This protocol targets the conserved housekeeping gene gap (National Center for Biotechnology Information accession number U39710) in a primer and probe system. This target is different from other species, including the gap homologue in M. pneumoniae (72.3% identity) and is present in the genome as a single copy.56 PCR-based assays have also been developed to detect mutations in DNA gyrase and/or topoisomerase IV, mediating fluoroquinolone resistance in M. genitalium, thereby circumventing the need for culture in vitro to determine antimicrobial susceptibilities to these agents.74
Gen-Probe (San Diego, CA) has developed a transcription-mediated amplification real-time PCR assay that performs well compared with other methods, but it is available in the United States only for research purposes.68,75,76 Multiplex PCR-based systems for detection of M. genitalium, along with Chlamydia trachomatis, Neisseria gonorrhoeae, and other urogenital mycoplasmas and ureaplasmas, are sold as kits in several European countries by multiple companies, including Bio-Rad (Hercules, CA), Amplex Biosystems (Giessen, Germany), PCR Diagnostics.eu (Bratislava, Slovak Republic), and Seegene, Inc. (Rockville, MD), using various formats and instrument platforms.
Because the requirements for marketing diagnostic products in Europe are not as stringent as those in the United States, mandated by the Food and Drug Administration, many products sold there have not been subjected to large and rigorous clinical trials or comparisons with existing assays.
Lack of commercial NAATs for detection of M. genitalium in the United States has greatly limited interest among physicians in seeking this mycoplasma in clinical specimens. Given the importance of this mycoplasma in conditions such as urethritis, cervicitis, and pelvic inflammatory disease, and the likelihood of venereal transmission of disease to other individuals, use of accurate molecular-based assays with a rapid turnaround time would undoubtedly increase if they become available. If M. genitalium is detected in the lower urogenital tract of a symptomatic patient, it should be considered medically significant.
M. hominis
Conventional PCR assays for M. hominis have mainly used 16S rRNA as a gene target.77,78 Because some heterogeneity has been reported in the 16S rRNA gene of M. hominis,79 other targets, including gap, fstY, and yidC, have been developed.58,59,80 The UAB Diagnostic Mycoplasma Laboratory uses a real-time PCR assay to detect M. hominis in clinical specimens targeting gap (National Center for Biotechnology Information accession number AJ243692) using the Roche LightCycler 2.0, as adapted from Baczynska et al.58
Because M. hominis is a common commensal in the female cervix and vagina and the male urethra, a positive PCR result on specimens from such sites may not be meaningful in the absence of clinical manifestations known to be associated with this organism. However, if the M. hominis load reaches ≥104 CFU/mL, this can be a crucial criterion for urogenital infections in women.81 In the case of extragenital specimens in adults or neonates, a positive M. hominis PCR assay or culture result should be considered clinically significant. There are many reports of systemic infections that were caused by M. hominis and for which diagnosis was delayed simply as a result of delays in appropriate testing because no one attempted to test.
Ureaplasma Species
PCR assays are important to detect and identify the individual Ureaplasma species for research purposes. Gel-based conventional PCR assays targeted sequences of 16S rRNA and 16S rRNA to 23S rRNA intergenic spacer regions, the urease gene, and mba,82–90 whereas real-time PCR assays have mainly targeted the urease genes and their subunits or mba.22,53,54,57,91,92 Yoshida et al78 described a conventional PCR assay applied to urine specimens of patients with urethritis targeting 16S rRNA genes of M. genitalium, M. hominis, U. parvum, and U. urealyticum. After amplification, PCR products were then subjected to hybridization assays using four species-specific capture probes to detect the targets. When compared with direct sequencing, this technique produced similar results and no cross-reactivity.
The UAB Diagnostic Mycoplasma Laboratory performs a real-time PCR assay for detection and differentiation of Ureaplasma species based on UU063 (NP_077893), which encodes a conserved hypothetical protein that is identical in all four U. parvum serovars and a 15,072-bp open reading frame, UUR10_0680 (NC_011374.1), that is conserved (>99.97%) in all 10 U. urealyticum serovars.22 This assay detected more positive clinical specimens than a conventional PCR assay based on a urease gene target in intralaboratory method evaluations.
Some molecular-based assays that include detection of Ureaplasma species are commercially available in various European countries, but not in the United States. Seegene, Inc. markets their products STD6 and STD6B ACE Detection, which simultaneously detect Trichomonas vaginalis, M. hominis, M. genitalium, C. trachomatis, N. gonorrhoeae, and Ureaplasma species in endocervical/urethral swabs. The novel feature of the Seegene, Inc., technology is a dual-priming oligonucleotide system that contains two separate priming regions linked by a polydeoxyinosine spacer.93 The Seegene, Inc., STD6 ACE kit works with any thermocycler, and the post-PCR assay is designed for either manual or automated gel electrophoresis. The STD6 Ureaplasma assay amplifies a 130-bp region of the ureD cassette.94 Their new version, STD6B, differentiates U. urealyticum from U. parvum ureC genes. Other companies, including PCR Diagnostics.eu and Amplex Biosystems, have PCR-based diagnostic products to detect Ureaplasma species in clinical specimens. PCR Diagnostics.eu has an assay in traditional PCR format with detection of PCR products on agarose gels that will differentiate the two Ureaplasma species. The multiplex PCR/reverse line blotting assay method has been used to detect numerous respiratory pathogens, including M. pneumoniae and urogenital pathogens, including both Ureaplasma species, M. genitalium, and M. hominis.95–97 Examples of real-time PCR assays used for detection and differentiation of Ureaplasma species are shown in Table 2.
Such as M. hominis, Ureaplasma species are also common commensals in the lower urogenital tracts of healthy people. Therefore, a positive PCR assay or culture result from specimens from these sites is usually expected. It is the increased bacterial load determined by real-time PCR or quantitative culture that can sometimes be valuable as a clinical indication of infection.52 Positive PCR or culture results for Ureaplasma species from the urethra in men with urethritis, from tracheal aspirates of neonates with respiratory distress, from the bloodstream or cerebrospinal fluid in neonates with pleocytosis, and from normally sterile extragenital sites should be considered diagnostic of clinically significant infection. Ureaplasma species can be relatively common opportunistic pathogens causing systemic infections involving various body sites in immunosuppressed people; many of them are missed initially because they are not considered, and diagnostic tests are performed only after treatment with antimicrobials that are not active against these organisms has failed.
Xiao et al98 evaluated 1061 clinical isolates of Ureaplasma species from various patient populations and found that U. urealyticum, but not U. parvum, was associated with male urethritis, as noted by others.20,52 Even though there have been conflicting reports regarding differential pathogenicity of the two Ureaplasma species, there is no compelling necessity to identify ureaplasmas to the species level in most circumstances because antimicrobial treatment would not differ. The only possible exception would be cases of male urethritis, in which ureaplasmas were detected, because one could argue that U. parvum, if detected alone by PCR, might not warrant treatment. However, because the real-time PCR assay we use for diagnostic purposes uses gene targets that enable species distinction, the specific organisms that are detected are reported whenever there is a positive result. As mentioned earlier, our laboratory still provides culture as the primary means for Ureaplasma detection for clinical purposes, and we perform PCR only by special request or for research studies.
Important Technical Aspects of Molecular-Based Assays Used for Mollicute Detection
Specimen Collection
All clinical specimens suitable for culture are acceptable for diagnostic testing for mycoplasmas and ureaplasmas by molecular methods if they are collected, stored, and processed correctly. Blood should be collected in a tube containing acid citrate dextrose. Other specimens can be inoculated into transport media, culture media, or PBS at collection or as soon as possible thereafter. For swab specimens, use of only Dacron or polyester swabs as calcium alginate and cotton swabs can be inhibitory. The 10 B or SP 4 broths used for culture do not have any deleterious effect on performance of the real-time PCR assay using the Roche LightCycler 2.0 (Roche Applied Science, Penzberg, Germany), but it is possible that culture broth could be inhibitory when using other primers or reaction conditions, or thermocyclers. Thus, it is mandatory to verify that culture broth or any liquid other than a designated PCR transport buffer is not inhibitory before using it for PCR transport.
DNA Extraction
Lysis and proteinase K treatment usually yield PCR-detectable DNA, unless the specimen is inhibitory.99 Suitable specimens for this procedure include body fluids (other than blood) and transport systems containing material obtained from swabs. Potentially inhibitory specimens, including blood, tissue samples, lower respiratory tract secretions, and subcultures, may be purified by the QIAamp DNA Blood Mini Kit (Qiagen) or other commercial genomic DNA purification kits. Automated or semiautomated nucleic acid isolation methods, such as Qiagen BioRobot EZ1 (Qiagen), NucliSENS (bioMérieux), easyMAG (bioMérieux), or MagNaPure LC (Roche Applied Science), can also be used to prepare samples. Some automated extraction systems appear to work as well as manual systems. The easyMAG nucleic acid extractor actually enabled superior amplification results for M. pneumoniae when applied retrospectively to clinical specimens when compared with the QIAgen blood mini kit and the NucliSENS miniMAG systems.100,101
PCR Programs and Operating Conditions
Most aspects of the real-time PCR procedures are instrument and protocol specific, such that analytic sensitivity, specificity, primer selection, and all aspects of the operating program must be validated separately for each method and instrument. Each laboratory must perform an evaluation of every assay component, from sample type, transport media, and extraction method, to final PCR amplification and detection procedures using the specific primers and probes, reaction conditions, and controls applicable to the assay to ensure that the techniques are valid and that there is no PCR inhibition at any step.
Quality Control
Careful attention to quality control procedures should limit the risk of false-positive and false-negative results. False-positive results from contamination can be a major problem for conventional PCR, but are less problematic with real-time PCR. In addition to human errors, reasons for false-negative results include the presence of PCR inhibitors in the specimen, suboptimal reagent preparation and reaction conditions, and inefficient extraction of the target DNA. Inhibitory factors and suboptimal PCR conditions can be detected by adding a positive control DNA after purification. However, this external control strategy cannot reveal inefficient DNA extraction. Use of an internal control added directly to the crude sample and coprocessed for purification and amplification is the most accurate method to monitor the important steps of diagnostic PCR protocols.
Determination of Analytical Sensitivity and Specificity
The PCR analytical sensitivity test should be performed against serial dilutions of template DNA, either bacterial genomic DNA from a defined inoculum titer or a plasmid containing the target sequence, and can be expressed in terms of amount of DNA detected or numbers of organisms (CFUs). Published or commercially sold PCR assays used for diagnostic purposes can reasonably be expected to detect <102 CFUs of the targeted organism.
The analytical specificity of PCR assays should be tested against all other human mollicutes, including commensal mycoplasmal species and other bacteria that may appear in the same body locations or show sequence similarities to the targets. An assay should be validated against various type strains and low-passage clinical isolates. For M. pneumoniae, strains representing both of the main P1 subtypes should be included. For U. parvum, the four recognized serovars should be included; and for U. urealyticum, the 10 known serovars should be tested. Human genomic DNA should always be included in the evaluation because of its presence in clinical specimens and possible inhibitory effects. The assay reproducibility should be verified by testing the same samples multiple times.
Additional PCR Assay Validation
It is important to perform PCR assays on well-characterized clinical specimens, with or without the organism of interest, that have been tested by other acceptable methods, such as culture and other PCR assays using different gene targets. Before a laboratory can begin routine PCR-based diagnostic work, it must be able to demonstrate that its molecular results compare favorably or exceed the detection ability of conventional culture-based techniques to establish a clinical sensitivity for the assay. The lack of comprehensive published comparative data for validation of the wide variety of PCR assays used for detection of human mycoplasmas and ureaplasmas remains a significant shortcoming.
Summary and Conclusions
Development and application of molecular-based methods during the past two decades has significantly improved the ability to detect and identify mycoplasmas and ureaplasmas in clinical specimens, enabled expansion of knowledge about the diseases they may cause, and provided more rapid and accurate diagnosis. It has also heightened interest in obtaining diagnostic testing for these organisms among many clinicians. This has been especially true for M. pneumoniae and M. genitalium, for which real-time PCR methods are clearly the diagnostic methods of choice. NAAT-based detection methods have lessened the reliance on the problematic serological detection systems. The enthusiasm for development of NAAT-based systems for application in mycoplasmology has resulted in dozens of published assays using a broad array of gene targets and methods. When used for diagnostic or epidemiological purposes in a clinical setting, there is justifiable concern over accuracy because most assays have never been sufficiently validated against other molecular- or culture-based methods to ensure their accuracy. Because none of these assays has thus far been evaluated or approved by the Food and Drug Administration, much is still unknown about their sensitivity and specificity. The few comparative clinical studies of various NAATs and preliminary studies of interlaboratory proficiency testing have indicated that there are considerable differences with these assays for detection of mycoplasmal infections, as well as the capabilities of the individual testing laboratories. The future of diagnostic mycoplasmology and epidemiological research rests with molecular-based technology, although culture, phenotypic methods, and traditional antimicrobial susceptibility testing will still have an important role, especially for M. hominis and Ureaplasma species. Therefore, it is important that large-scale comparisons must be performed to compare reproducibility and accuracy of NAATs. This must include side-by-side comparisons of new assay formats and gene targets with existing assays using the same, and different, targets and with other established methods, including culture. Such comparisons should ideally include a broad selection of specimen sources from different geographic areas. Eventually, it seems likely that commercial development of NAATs for all of the important pathogenic mycoplasmal and ureaplasmal species that infect humans will come to the United States, as they have to Europe. Standardization of reagents and rigorous quality control would then be more realistic.
Footnotes
Supported in part by federal funds from the National Institute of Allergy and Infectious Diseases (grant RO1A1072577).
CME Disclosure: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interest to disclose.
References
- 1.Waites K.B., Taylor-Robinson D. Mycoplasma and ureaplasma. In: Versalovic J., Carroll K., Funke G., Jorgensen J., Landry M., Warnock D.W., editors. Manual of Clinical Microbiology. ed 10. ASM Press; Washington, DC: 2011. pp. 970–985. [Google Scholar]
- 2.Su C.J., Baseman J.B. Genome size of Mycoplasma genitalium. J Bacteriol. 1990;172:4705–4707. doi: 10.1128/jb.172.8.4705-4707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waites K.B., Katz B., Schelonka R.L. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18:757–789. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waites K.B., Talkington D.F. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. doi: 10.1128/CMR.17.4.697-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor-Robinson D., Jensen J.S. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498–514. doi: 10.1128/CMR.00006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waites K.B., Balish M.F., Atkinson T.P. New insights into the pathogenesis and detection of Mycoplasma pneumoniae infections. Future Microbiol. 2008;3:635–648. doi: 10.2217/17460913.3.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen J.S. Mycoplasma genitalium infections: diagnosis, clinical aspects, and pathogenesis. Dan Med Bull. 2006;53:1–27. [PubMed] [Google Scholar]
- 8.Atkinson T.P., Balish M.F., Waites K.B. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008;32:956–973. doi: 10.1111/j.1574-6976.2008.00129.x. [DOI] [PubMed] [Google Scholar]
- 9.Jensen J.S. Mycoplasma genitalium: the aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1–11. doi: 10.1111/j.1468-3083.2004.00923.x. [DOI] [PubMed] [Google Scholar]
- 10.Kannan T.R., Baseman J.B. ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proc Natl Acad Sci U S A. 2006;103:6724–6729. doi: 10.1073/pnas.0510644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dallo S.F., Baseman J.B. Intracellular DNA replication and long-term survival of pathogenic mycoplasmas. Microb Pathog. 2000;29:301–309. doi: 10.1006/mpat.2000.0395. [DOI] [PubMed] [Google Scholar]
- 12.Baseman J.B., Tully J.G. Mycoplasmas: sophisticated, reemerging, and burdened by their notoriety. Emerg Infect Dis. 1997;3:21–32. doi: 10.3201/eid0301.970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Himmelreich R., Hilbert H., Plagens H., Pirkl E., Li B.C., Herrmann R. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res. 1996;24:4420–4449. doi: 10.1093/nar/24.22.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnakumar R., Assad-Garcia N., Benders G.A., Phan Q., Montague M.G., Glass J.I. Targeted chromosomal knockouts in Mycoplasma pneumoniae. Appl Environ Microbiol. 2010;76:5297–5299. doi: 10.1128/AEM.00024-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenri T., Horino A., Matsui M., Sasaki Y., Suzuki S., Narita M., Ohya H., Okazaki N., Shibayama K. Complete genome sequence of Mycoplasma pneumoniae type 2a strain 309, isolated in Japan. J Bacteriol. 2012;194:1253–1254. doi: 10.1128/JB.06553-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu P.C., Schaper U., Collier A.M., Clyde W.A., Jr, Horikawa M., Huang Y.S., Barile M.F. A Mycoplasma genitalium protein resembling the Mycoplasma pneumoniae attachment protein. Infect Immun. 1987;55:1126–1131. doi: 10.1128/iai.55.5.1126-1131.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson S.N., Bailey C.C., Jensen J.S., Borre M.B., King E.S., Bott K.F., Hutchison C.A., 3rd Characterization of repetitive DNA in the Mycoplasma genitalium genome: possible role in the generation of antigenic variation. Proc Natl Acad Sci U S A. 1995;92:11829–11833. doi: 10.1073/pnas.92.25.11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henrich B., Feldmann R.C., Hadding U. Cytoadhesins of Mycoplasma hominis. Infect Immun. 1993;61:2945–2951. doi: 10.1128/iai.61.7.2945-2951.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereyre S., Sirand-Pugnet P., Beven L., Charron A., Renaudin H., Barre A., Avenaud P., Jacob D., Couloux A., Barbe V., de Daruvar A., Blanchard A., Bebear C. Life on arginine for Mycoplasma hominis: clues from its minimal genome and comparison with other human urogenital mycoplasmas. PLoS Genet. 2009;5:e1000677. doi: 10.1371/journal.pgen.1000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deguchi T., Yoshida T., Miyazawa T., Yasuda M., Tamaki M., Ishiko H., Maeda S. Association of Ureaplasma urealyticum (biovar 2) with nongonococcal urethritis. Sex Transm Dis. 2004;31:192–195. doi: 10.1097/01.olq.0000114653.26951.71. [DOI] [PubMed] [Google Scholar]
- 21.Gelfand E.W. Unique susceptibility of patients with antibody deficiency to mycoplasma infection. Clin Infect Dis. 1993;17(Suppl 1):S250–S253. [PubMed] [Google Scholar]
- 22.Xiao L., Glass J.I., Paralanov V., Yooseph S., Cassell G.H., Duffy L.B., Waites K.B. Detection and characterization of human Ureaplasma species and serovars by real-time PCR. J Clin Microbiol. 2010;48:2715–2723. doi: 10.1128/JCM.01877-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waites K.B., Bebear C.M., Robertson J.A., Talkington D.F., Kenny G.E., editors. American Society for Microbiology; Washington, DC: 2001. (Cumitech 34: Laboratory Diagnosis of Mycoplasmal Infections). [Google Scholar]
- 24.Beersma M.F., Dirven K., van Dam A.P., Templeton K.E., Claas E.C., Goossens H. Evaluation of 12 commercial tests and the complement fixation test for Mycoplasma pneumoniae-specific immunoglobulin G (IgG) and IgM antibodies, with PCR used as the “gold standard.”. J Clin Microbiol. 2005;43:2277–2285. doi: 10.1128/JCM.43.5.2277-2285.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Csango P.A., Pedersen J.E., Hess R.D. Comparison of four Mycoplasma pneumoniae IgM-, IgG- and IgA-specific enzyme immunoassays in blood donors and patients. Clin Microbiol Infect. 2004;10:1094–1098. doi: 10.1111/j.1469-0691.2004.00993.x. [DOI] [PubMed] [Google Scholar]
- 26.Daxboeck F., Krause R., Wenisch C. Laboratory diagnosis of Mycoplasma pneumoniae infection. Clin Microbiol Infect. 2003;9:263–273. doi: 10.1046/j.1469-0691.2003.00590.x. [DOI] [PubMed] [Google Scholar]
- 27.Loens K., Ursi D., Goossens H., Ieven M. Molecular diagnosis of Mycoplasma pneumoniae respiratory tract infections. J Clin Microbiol. 2003;41:4915–4923. doi: 10.1128/JCM.41.11.4915-4923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loens K., Goossens H., Ieven M. Acute respiratory infection due to Mycoplasma pneumoniae: current status of diagnostic methods. Eur J Clin Microbiol Infect Dis. 2010;29:1055–1069. doi: 10.1007/s10096-010-0975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorigo-Zetsma J.W., Zaat S.A., Wertheim-van Dillen P.M., Spanjaard L., Rijntjes J., van Waveren G., Jensen J.S., Angulo A.F., Dankert J. Comparison of PCR, culture, and serological tests for diagnosis of Mycoplasma pneumoniae respiratory tract infection in children. J Clin Microbiol. 1999;37:14–17. doi: 10.1128/jcm.37.1.14-17.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsson A.C., Björkman P., Welinder-Olsson C., Widell A., Persson K. Clinical severity of Mycoplasma pneumoniae (MP) infection is associated with bacterial load in oropharyngeal secretions but not with MP genotype. BMC Infect Dis. 2010;10:39. doi: 10.1186/1471-2334-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winchell J.M., Thurman K.A., Mitchell S.L., Thacker W.L., Fields B.S. Evaluation of three real-time PCR assays for detection of Mycoplasma pneumoniae in an outbreak investigation. J Clin Microbiol. 2008;46:3116–3118. doi: 10.1128/JCM.00440-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumke R., Schurwanz N., Lenz M., Schuppler M., Luck C., Jacobs E. Sensitive detection of Mycoplasma pneumoniae in human respiratory tract samples by optimized real-time PCR approach. J Clin Microbiol. 2007;45:2726–2730. doi: 10.1128/JCM.00321-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lodes M.J., Suciu D., Wilmoth J.L., Ross M., Munro S., Dix K., Bernards K., Stover A.G., Quintana M., Iihoshi N., Lyon W.J., Danley D.L., McShea A. Identification of upper respiratory tract pathogens using electrochemical detection on an oligonucleotide microarray. PLoS One. 2007;2:e924. doi: 10.1371/journal.pone.0000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X., Atkinson T.P., Hagood J., Makris C., Duffy L.B., Waites K.B. Emerging macrolide resistance in Mycoplasma pneumoniae in children: detection and characterization of resistant isolates. Pediatr Infect Dis J. 2009;28:693–696. doi: 10.1097/INF.0b013e31819e3f7a. [DOI] [PubMed] [Google Scholar]
- 35.Daxboeck F., Khanakah G., Bauer C., Stadler M., Hofmann H., Stanek G. Detection of Mycoplasma pneumoniae in serum specimens from patients with mycoplasma pneumonia by PCR. Int J Med Microbiol. 2005;295:279–285. doi: 10.1016/j.ijmm.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Michelow I.C., Olsen K., Lozano J., Duffy L.B., McCracken G.H., Hardy R.D. Diagnostic utility and clinical significance of naso- and oropharyngeal samples used in a PCR assay to diagnose Mycoplasma pneumoniae infection in children with community-acquired pneumonia. J Clin Microbiol. 2004;42:3339–3341. doi: 10.1128/JCM.42.7.3339-3341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raty R., Ronkko E., Kleemola M. Sample type is crucial to the diagnosis of Mycoplasma pneumoniae pneumonia by PCR. J Med Microbiol. 2005;54:287–291. doi: 10.1099/jmm.0.45888-0. [DOI] [PubMed] [Google Scholar]
- 38.Ursi D., Ieven M., Noordhoek G.T., Ritzler M., Zandleven H., Altwegg M. An interlaboratory comparison for the detection of Mycoplasma pneumoniae in respiratory samples by the polymerase chain reaction. J Microbiol Methods. 2003;53:289–294. doi: 10.1016/s0167-7012(02)00230-0. [DOI] [PubMed] [Google Scholar]
- 39.Loens K., Beck T., Ursi D., Pattyn S., Goossens H., Ieven M. Two quality control exercises involving nucleic acid amplification methods for detection of Mycoplasma pneumoniae and Chlamydophila pneumoniae and carried out 2 years apart (in 2002 and 2004) J Clin Microbiol. 2006;44:899–908. doi: 10.1128/JCM.44.3.899-908.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loens K., Mackay W.G., Scott C., Goossens H., Wallace P., Ieven M. A multicenter pilot external quality assessment programme to assess the quality of molecular detection of Chlamydophila pneumoniae and Mycoplasma pneumoniae. J Microbiol Methods. 2010;82:131–135. doi: 10.1016/j.mimet.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Loens K., Ieven M., Ursi D., Beck T., Overdijk M., Sillekens P., Goossens H. Detection of Mycoplasma pneumoniae by real-time nucleic acid sequence-based amplification. J Clin Microbiol. 2003;41:4448–4450. doi: 10.1128/JCM.41.9.4448-4450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Templeton K.E., Scheltinga S.A., Graffelman A.W., Van Schie J.M., Crielaard J.W., Sillekens P., Van Den Broek P.J., Goossens H., Beersma M.F., Claas E.C. Comparison and evaluation of real-time PCR, real-time nucleic acid sequence-based amplification, conventional PCR, and serology for diagnosis of Mycoplasma pneumoniae. J Clin Microbiol. 2003;41:4366–4371. doi: 10.1128/JCM.41.9.4366-4371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loens K., Beck T., Ursi D., Overdijk M., Sillekens P., Goossens H., Ieven M. Evaluation of different nucleic acid amplification techniques for the detection of M. pneumoniae, C pneumoniae and Legionella spp in respiratory specimens from patients with community-acquired pneumonia. J Microbiol Methods. 2008;73:257–262. doi: 10.1016/j.mimet.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Raggam R.B., Leitner E., Berg J., Muhlbauer G., Marth E., Kessler H.H. Single-run, parallel detection of DNA from three pneumonia-producing bacteria by real-time polymerase chain reaction. J Mol Diagn. 2005;7:133–138. doi: 10.1016/S1525-1578(10)60019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khanna M., Fan J., Pehler-Harrington K., Waters C., Douglass P., Stallock J., Kehl S., Henrickson K.J. The pneumoplex assays, a multiplex PCR-enzyme hybridization assay that allows simultaneous detection of five organisms, Mycoplasma pneumoniae, Chlamydia (Chlamydophila) pneumoniae, Legionella pneumophila, Legionella micdadei, and Bordetella pertussis, and its real-time counterpart. J Clin Microbiol. 2005;43:565–571. doi: 10.1128/JCM.43.2.565-571.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ginevra C., Barranger C., Ros A., Mory O., Stephan J.L., Freymuth F., Joannes M., Pozzetto B., Grattard F. Development and evaluation of Chlamylege, a new commercial test allowing simultaneous detection and identification of Legionella, Chlamydophila pneumoniae, and Mycoplasma pneumoniae in clinical respiratory specimens by multiplex PCR. J Clin Microbiol. 2005;43:3247–3254. doi: 10.1128/JCM.43.7.3247-3254.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loens K., Beck T., Ursi D., Overdijk M., Sillekens P., Goossens H., Ieven M. Development of real-time multiplex nucleic acid sequence-based amplification for detection of Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella spp in respiratory specimens. J Clin Microbiol. 2008;46:185–191. doi: 10.1128/JCM.00447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y., Kong F., Yang Y., Gilbert G.L. A multiplex PCR-based reverse line blot hybridization (mPCR/RLB) assay for detection of bacterial respiratory pathogens in children with pneumonia. Pediatr Pulmonol. 2008;43:150–159. doi: 10.1002/ppul.20749. [DOI] [PubMed] [Google Scholar]
- 49.Roth S.B., Jalava J., Ruuskanen O., Ruohola A., Nikkari S. Use of an oligonucleotide array for laboratory diagnosis of bacteria responsible for acute upper respiratory infections. J Clin Microbiol. 2004;42:4268–4274. doi: 10.1128/JCM.42.9.4268-4274.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saito R., Misawa Y., Moriya K., Koike K., Ubukata K., Okamura N. Development and evaluation of a loop-mediated isothermal amplification assay for rapid detection of Mycoplasma pneumoniae. J Med Microbiol. 2005;54:1037–1041. doi: 10.1099/jmm.0.46071-0. [DOI] [PubMed] [Google Scholar]
- 51.Dumke R., Jacobs E. Comparison of commercial and in-house real-time PCR assays used for detection of Mycoplasma pneumoniae. J Clin Microbiol. 2009;47:441–444. doi: 10.1128/JCM.01989-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshida T., Deguchi T., Meda S., Kubota Y., Tamaki M., Yokoi S., Yasuda M., Ishiko H. Quantitative detection of Ureaplasma parvum (biovar 1) and Ureaplasma urealyticum (biovar 2) in urine specimens from men with and without urethritis by real-time polymerase chain reaction. Sex Transm Dis. 2007;34:416–419. doi: 10.1097/01.olq.0000243621.89212.40. [DOI] [PubMed] [Google Scholar]
- 53.Mallard K., Schopfer K., Bodmer T. Development of real-time PCR for the differential detection and quantification of Ureaplasma urealyticum and Ureaplasma parvum. J Microbiol Methods. 2005;60:13–19. doi: 10.1016/j.mimet.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Cao X., Wang Y., Hu X., Qing H., Wang H. Real-time TaqMan polymerase chain reaction assays for quantitative detection and differentiation of Ureaplasma urealyticum and Ureaplasma parvum. Diagn Microbiol Infect Dis. 2007;57:373–378. doi: 10.1016/j.diagmicrobio.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Pitcher D., Chalker V.J., Sheppard C., George R.C., Harrison T.G. Real-time detection of Mycoplasma pneumoniae in respiratory samples with an internal processing control. J Med Microbiol. 2006;55:149–155. doi: 10.1099/jmm.0.46281-0. [DOI] [PubMed] [Google Scholar]
- 56.Svenstrup H.F., Jensen J.S., Bjornelius E., Lidbrink P., Birkelund S., Christiansen G. Development of a quantitative real-time PCR assay for detection of Mycoplasma genitalium. J Clin Microbiol. 2005;43:3121–3128. doi: 10.1128/JCM.43.7.3121-3128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshida T., Deguchi T., Ito M., Maeda S., Tamaki M., Ishiko H. Quantitative detection of Mycoplasma genitalium from first-pass urine of men with urethritis and asymptomatic men by real-time PCR. J Clin Microbiol. 2002;40:1451–1455. doi: 10.1128/JCM.40.4.1451-1455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baczynska A., Svenstrup H.F., Fedder J., Birkelund S., Christiansen G. Development of real-time PCR for detection of Mycoplasma hominis. BMC Microbiol. 2004;4:35. doi: 10.1186/1471-2180-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferandon C., Peuchant O., Janis C., Benard A., Renaudin H., Pereyre S., Bebear C. Development of a real-time PCR targeting the yidC gene for the detection of Mycoplasma hominis and comparison with quantitative culture. Clin Microbiol Infect. 2011;17:155–159. doi: 10.1111/j.1469-0691.2010.03217.x. [DOI] [PubMed] [Google Scholar]
- 60.Wolff B.J., Thacker W.L., Schwartz S.B., Winchell J.M. Detection of macrolide resistance in Mycoplasma pneumoniae by real-time PCR and high resolution melt analysis. Antimicrob Agents Chemother. 2008;52:3542–3549. doi: 10.1128/AAC.00582-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peuchant O., Menard A., Renaudin H., Morozumi M., Ubukata K., Bebear C.M., Pereyre S. Increased macrolide resistance of Mycoplasma pneumoniae in France directly detected in clinical specimens by real-time PCR and melting curve analysis. J Antimicrob Chemother. 2009;64:52–58. doi: 10.1093/jac/dkp160. [DOI] [PubMed] [Google Scholar]
- 62.Lin C., Li S., Sun H., Zhao H., Feng Y., Cao L., Yuan Y., Zhang T. Nested PCR-linked capillary electrophoresis and single-strand conformation polymorphisms for detection of macrolide-resistant Mycoplasma pneumoniae in Beijing, China. J Clin Microbiol. 2010;48:4567–4572. doi: 10.1128/JCM.00400-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spuesens E.B., Hoogenboezem T., Sluijter M., Hartwig N.G., van Rossum A.M., Vink C. Macrolide resistance determination and molecular typing of Mycoplasma pneumoniae by pyrosequencing. J Microbiol Methods. 2010;82:214–222. doi: 10.1016/j.mimet.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 64.Eastick K., Leeming J.P., Caul E.O., Horner P.J., Millar M.R. A novel polymerase chain reaction assay to detect Mycoplasma genitalium. Mol Pathol. 2003;56:25–28. doi: 10.1136/mp.56.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jensen J.S., Borre M.B., Dohn B. Detection of Mycoplasma genitalium by PCR amplification of the 16S rRNA gene. J Clin Microbiol. 2003;41:261–266. doi: 10.1128/JCM.41.1.261-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huppert J.S., Mortensen J.E., Reed J.L., Kahn J.A., Rich K.D., Hobbs M.M. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis. 2008;35:250–254. doi: 10.1097/OLQ.0b013e31815abac6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wroblewski J.K., Manhart L.E., Dickey K.A., Hudspeth M.K., Totten P.A. Comparison of transcription-mediated amplification and PCR assay results for various genital specimen types for detection of Mycoplasma genitalium. J Clin Microbiol. 2006;44:3306–3312. doi: 10.1128/JCM.00553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hardick J., Giles J., Hardick A., Hsieh Y.H., Quinn T., Gaydos C. Performance of the gen-probe transcription-mediated [corrected] amplification research assay compared to that of a multitarget real-time PCR for Mycoplasma genitalium detection. J Clin Microbiol. 2006;44:1236–1240. doi: 10.1128/JCM.44.4.1236-1240.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deguchi T., Yoshida T., Yokoi S., Ito M., Tamaki M., Ishiko H., Maeda S. Longitudinal quantitative detection by real-time PCR of Mycoplasma genitalium in first-pass urine of men with recurrent nongonococcal urethritis. J Clin Microbiol. 2002;40:3854–3856. doi: 10.1128/JCM.40.10.3854-3856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jensen J.S., Bjornelius E., Dohn B., Lidbrink P. Use of TaqMan 5′ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J Clin Microbiol. 2004;42:683–692. doi: 10.1128/JCM.42.2.683-692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jurstrand M., Jensen J.S., Fredlund H., Falk L., Molling P. Detection of Mycoplasma genitalium in urogenital specimens by real-time PCR and by conventional PCR assay. J Med Microbiol. 2005;54:23–29. doi: 10.1099/jmm.0.45732-0. [DOI] [PubMed] [Google Scholar]
- 72.Dupin N., Bijaoui G., Schwarzinger M., Ernault P., Gerhardt P., Jdid R., Hilab S., Pantoja C., Buffet M., Escande J.P., Costa J.M. Detection and quantification of Mycoplasma genitalium in male patients with urethritis. Clin Infect Dis. 2003;37:602–605. doi: 10.1086/376990. [DOI] [PubMed] [Google Scholar]
- 73.Ma L., Jensen J.S., Mancuso M., Hamasuna R., Jia Q., McGowin C.L., Martin D.H. Genetic variation in the complete MgPa operon and its repetitive chromosomal elements in clinical strains of Mycoplasma genitalium. PLoS One. 2010;5:e15660. doi: 10.1371/journal.pone.0015660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shimada Y., Deguchi T., Nakane K., Masue T., Yasuda M., Yokoi S., Ito S., Nakano M., Ishiko H. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255–258. doi: 10.1016/j.ijantimicag.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 75.Schwebke J.R., Rompalo A., Taylor S., Sena A.C., Martin D.H., Lopez L.M., Lensing S., Lee J.Y. Re-evaluating the treatment of nongonococcal urethritis: emphasizing emerging pathogens–a randomized clinical trial. Clin Infect Dis. 2011;52:163–170. doi: 10.1093/cid/ciq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mena L.A., Mroczkowski T.F., Nsuami M., Martin D.H. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649–1654. doi: 10.1086/599033. [DOI] [PubMed] [Google Scholar]
- 77.Grau O., Kovacic R., Griffais R., Launay V., Montagnier L. Development of PCR-based assays for the detection of two human mollicute species: Mycoplasma penetrans and M hominis. Mol Cell Probes. 1994;8:139–147. doi: 10.1006/mcpr.1994.1019. [DOI] [PubMed] [Google Scholar]
- 78.Yoshida T., Maeda S., Deguchi T., Miyazawa T., Ishiko H. Rapid detection of Mycoplasma genitalium, Mycoplasma hominis, Ureaplasma parvum, and Ureaplasma urealyticum organisms in genitourinary samples by PCR-microtiter plate hybridization assay. J Clin Microbiol. 2003;41:1850–1855. doi: 10.1128/JCM.41.5.1850-1855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mygind T., Birkelund S., Christiansen G. DNA sequencing reveals limited heterogeneity in the 16S rRNA gene from the rrnB operon among five Mycoplasma hominis isolates. Int J Syst Bacteriol. 1998;48(Pt 3):1067–1071. doi: 10.1099/00207713-48-3-1067. [DOI] [PubMed] [Google Scholar]
- 80.Menard J.P., Fenollar F., Henry M., Bretelle F., Raoult D. Molecular quantification of Gardnerella vaginalis and Atopobium vaginae loads to predict bacterial vaginosis. Clin Infect Dis. 2008;47:33–43. doi: 10.1086/588661. [DOI] [PubMed] [Google Scholar]
- 81.Bébéar C., Pereyre S., Bébéar C.M. Mycoplasma spp. In: Gillespie S.H., Hawkey P.W., editors. Principles and Practice of Clinical Bacteriology. ed 2. John Wiley & Sons Ltd; Hoboken, NJ: 2006. pp. 305–315. [Google Scholar]
- 82.Kong F., Zhu X., Wang W., Zhou X., Gordon S., Gilbert G.L. Comparative analysis and serovar-specific identification of multiple-banded antigen genes of Ureaplasma urealyticum biovar 1. J Clin Microbiol. 1999;37:538–543. doi: 10.1128/jcm.37.3.538-543.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robertson J.A., Vekris A., Bebear C., Stemke G.W. Polymerase chain reaction using 16S rRNA gene sequences distinguishes the two biovars of Ureaplasma urealyticum. J Clin Microbiol. 1993;31:824–830. doi: 10.1128/jcm.31.4.824-830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harasawa R., Kanamoto Y. Differentiation of two biovars of Ureaplasma urealyticum based on the 16S-23S rRNA intergenic spacer region. J Clin Microbiol. 1999;37:4135–4138. doi: 10.1128/jcm.37.12.4135-4138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blanchard A. Ureaplasma urealyticum urease genes: use of a UGA tryptophan codon. Mol Microbiol. 1990;4:669–676. doi: 10.1111/j.1365-2958.1990.tb00636.x. [DOI] [PubMed] [Google Scholar]
- 86.Kong F., Ma Z., James G., Gordon S., Gilbert G.L. Species identification and subtyping of Ureaplasma parvum and Ureaplasma urealyticum using PCR-based assays. J Clin Microbiol. 2000;38:1175–1179. doi: 10.1128/jcm.38.3.1175-1179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Povlsen K., Jensen J.S., Lind I. Detection of Ureaplasma urealyticum by PCR and biovar determination by liquid hybridization. J Clin Microbiol. 1998;36:3211–3216. doi: 10.1128/jcm.36.11.3211-3216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kong F., Ma Z., James G., Gordon S., Gilbert G.L. Molecular genotyping of human Ureaplasma species based on multiple-banded antigen (MBA) gene sequences. Int J Syst Evol Microbiol. 2000;50(Pt 5):1921–1929. doi: 10.1099/00207713-50-5-1921. [DOI] [PubMed] [Google Scholar]
- 89.Teng L.J., Zheng X., Glass J.I., Watson H.L., Tsai J., Cassell G.H. Ureaplasma urealyticum biovar specificity and diversity are encoded in multiple-banded antigen gene. J Clin Microbiol. 1994;32:1464–1469. doi: 10.1128/jcm.32.6.1464-1469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Teng L.J., Ho S.W., Ho H.N., Liaw S.J., Lai H.C., Luh K.T. Rapid detection and biovar differentiation of Ureaplasma urealyticum in clinical specimens by PCR. J Formos Med Assoc. 1995;94:396–400. [PubMed] [Google Scholar]
- 91.Yi J., Yoon B.H., Kim E.C. Detection and biovar discrimination of Ureaplasma urealyticum by real-time PCR. Mol Cell Probes. 2005;19:255–260. doi: 10.1016/j.mcp.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 92.Cao X., Jiang Z., Wang Y., Gong R., Zhang C. Two multiplex real-time TaqMan polymerase chain reaction systems for simultaneous detecting and serotyping of Ureaplasma parvum. Diagn Microbiol Infect Dis. 2007;59:109–111. doi: 10.1016/j.diagmicrobio.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 93.Ovyn C., van Strijp D., Ieven M., Ursi D., van Gemen B., Goossens H. Typing of Mycoplasma pneumoniae by nucleic acid sequence-based amplification. NASBA Mol Cell Probes. 1996;10:319–324. doi: 10.1006/mcpr.1996.0043. [DOI] [PubMed] [Google Scholar]
- 94.Kim T.H. Detection of cryptic microorganisms in patients with chronic prostatitis by multiplex polymerase chain reaction. Korean J Urol. 2007;3:304. doi: 10.4111/kju.2011.52.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McKechnie M.L., Hillman R., Couldwell D., Kong F., Freedman E., Wang H., Gilbert G.L. Simultaneous identification of 14 genital microorganisms in urine by use of a multiplex PCR-based reverse line blot assay. J Clin Microbiol. 2009;47:1871–1877. doi: 10.1128/JCM.00120-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y., Kong F., Gilbert G.L., Brown M., Gao W., Yu S., Yang Y. Use of a multiplex PCR-based reverse line blot (mPCR/RLB) hybridisation assay for the rapid identification of bacterial pathogens. Clin Microbiol Infect. 2008;14:155–160. doi: 10.1111/j.1469-0691.2007.01890.x. [DOI] [PubMed] [Google Scholar]
- 97.Wang H., Kong F., Jelfs P., James G., Gilbert G.L. Simultaneous detection and identification of common cell culture contaminant and pathogenic mollicutes strains by reverse line blot hybridization. Appl Environ Microbiol. 2004;70:1483–1486. doi: 10.1128/AEM.70.3.1483-1486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiao L., Paralanov V., Glass J.I., Duffy L.B., Robertson J.A., Cassell G.H., Chen Y., Waites K.B. Extensive horizontal gene transfer in ureaplasmas from humans questions the utility of serotyping for diagnostic purposes. J Clin Microbiol. 2011;49:2818–2826. doi: 10.1128/JCM.00637-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blanchard A., Hentschel J., Duffy L., Baldus K., Cassell G.H. Detection of Ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amniotic fluid, and in the respiratory tract of newborns. Clin Infect Dis. 1993;17(Suppl 1):S148–S153. doi: 10.1093/clinids/17.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 100.Loens K., Bergs K., Ursi D., Goossens H., Ieven M. Evaluation of NucliSens easyMAG for automated nucleic acid extraction from various clinical specimens. J Clin Microbiol. 2007;45:421–425. doi: 10.1128/JCM.00894-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Loens K., Ursi D., Goossens H., Ieven M. Evaluation of the NucliSens miniMAG RNA extraction and real-time NASBA applications for the detection of Mycoplasma pneumoniae and Chlamydophila pneumoniae in throat swabs. J Microbiol Methods. 2008;72:217–219. doi: 10.1016/j.mimet.2007.11.008. [DOI] [PubMed] [Google Scholar]