Abstract
Brain circuits undergo distributed rearrangements throughout life: development, experience and behavior constantly modify synaptic strength and network connectivity. Despite these changes, neurons and circuits need to preserve their functional stability. Single neurons maintain their spontaneous firing rate within functional working ranges by regulating the efficacy of their synaptic inputs. But how do networks maintain a stable behavior? Is network homeostasis a consequence of cell autonomous mechanisms? In this article we will review recent evidence showing that network homeostasis is more than the sum of single-neuron homeostasis and that high-order network stability can be achieved by coordinated inter-cellular interactions.
Homeostatic regulation of pyramidal cell firing
In healthy circuits where excitatory and inhibitory inputs are balanced, excitability and spontaneous firing frequency are maintained within a sensitive working range, the optimal state for input processing [1–3]. Prolonged changes in driving inputs alter the balance between excitation and inhibition and push neurons away from their functional equilibrium, potentially inducing pathological states [4–6]. To readjust neuronal sensitivity, several compensatory mechanisms are called into place. Synaptic scaling, the ability of neurons to rescale their synaptic inputs in response to perturbations of activity, is one of the best known [7,8].
In a series of groundbreaking experiments, Turrigiano and collaborators showed that pharmacological perturbation of activity in cortical cultures results in compensatory modifications of excitatory currents in pyramidal neurons. Chronic activity blockade with tetrodotoxin (TTX) increases the amplitude of miniature excitatory postsynaptic currents (mEPSC), a measure of the strength of excitatory inputs; bicuculline driven increase in network activity, on the other hand, induces the opposite effect. In both conditions pyramidal neuron firing rates are restored within control levels [7]. Synaptic scaling is not specific to neocortical-cultured neurons as it can also be induced in hippocampal cultures [9,10]. The mechanisms, loci of expression and time courses, however, can vary. Neocortical neurons regulate AMPA receptor-mediated mEPSC amplitude without significantly affecting neurotransmitter release [7,11]; whereas hippocampal neurons modify their excitability through changes in release probability [9], by increasing the size of presynaptic terminals [10] and through modulation of extracellular levels of tumor necrosis factor α (TNFα) by glial cells [12]. Synaptic scaling can involve all glutamatergic synapses of a neuron if spiking activity is inhibited locally at the axon initial segment [7,13•], or can be limited to specific synapses if their activity is selectively blocked by insertion of hyper-polarizing channels [14]. The time course of induction can also vary: depending on the activity manipulation a fast NMDA-mediated [14,15•] and slow AMPA component of synaptic scaling have been reported and other molecular mechanisms have been recently identified [9,16,17]. The apparent lack of generalization may be partly explained by differences in neuronal types, maturation of the culture [18], and methods for activity manipulation [9,11,13•,14,15•,18,19] and may also reflect the robustness and diversity of homeostatic synaptic mechanisms [9,10,14–16,18,20].
Pyramidal neurons do not scale only their excitatory inputs. Chronic activity blockade with TTX, besides upscaling excitatory synapses, decreases the strength of inhibition by reducing the average amplitude of miniature inhibitory postsynaptic currents (mIPSC) and the size of GABAergic synaptic boutons [21]. A complementary effect is observed when networks are overstimulated: the strength of inhibitory connections increases by fast regulation of GABAergic synapses and prevents runaway excitation [22].
The implication of these experiments is clear and important: pyramidal neurons can autonomously regulate their operational range. They sense their own activity through changes in intracellular calcium levels [13•] or extracellular levels of neurotrophins [23] or cytokines [12,24], and adjust their synapses accordingly (Figure 1).
Figure 1.

Diagram of homeostatic circuit rearrangements. Pyramidal neurons (red) and inhibitory interneurons (blue) contact each other to form active circuits. Low activity: chronic incubation with TTX, blockers of neurotransmission or sensory deprivation increase overall circuit excitability by strengthening of excitatory connections (large upward red arrow). TTX incubation also weakens inhibitory synapses (small downward blue arrow). Increased activity: hyperactivation of circuits with bicuculline or electrical stimulation boosts spontaneous firing rates. To regain stability excitatory synapses are downscaled (small downward red arrow) while the inhibitory synapses and excitatory synapses onto inhibitory neurons are potentiated (large upward blue arrow: increased inhibition onto pyramidal neurons; rightmost large upward red arrow: increased excitatory drive onto inhibitory interneurons).
Homeostasis in small circuits
The studies discussed above focus on pyramidal neurons and their synapses. Do inhibitory interneurons equally scale their inputs to maintain their own firing rates? Experiments show that inhibitory interneurons behave quite differently. The same manipulation that effectively up-scales mEPSCs in pyramidal neurons does not affect excitatory synapses onto inhibitory interneurons [23]. Differently from pyramidal neurons, inhibitory inter-neurons do not preserve their firing rates within an optimal working range when faced with decreased overall activation [23]. From a network perspective, this behavior is functional to maintaining circuit homeostasis. In a network where excitatory and inhibitory neurons form recurrent circuits, a cell-autonomous homeostatic inter-neuron would counteract the homeostatic response of pyramidal neurons. If interneurons scaled their inputs and firing rates up following periods of inactivation, for example, they would likely enhance inhibition onto pyramidal cells and force them to climb an even steeper compensatory slope. In the context of a network, the homeostasis of inhibitory interneurons is sacrificed to preserve the activity of pyramidal neurons that is to preserve the output of the neural circuit. Thus the stability of a neural network does not come simply from the sum of cell-autonomous mechanisms, but also from the coordination of multiple inter-cellular processes aimed at maintaining a higher order function.
A good example of how coordination of multiple neurons and processes maintains network stability comes from the study of the stomatogastric ganglion (STG) of crabs and lobsters, a central pattern generator controlling rhythmic pyloric contraction [25••,26,27]. Its extensively characterized architecture and well-defined electrophysiological behavior [26] make this preparation an ideal model for studying network homeostasis. Neurons in the STG show a triphasic rhythm extremely well-conserved among individuals and robust to developmental and experimental perturbations [25••,28,29••]. In the first years of life lobsters grow ~20-fold in size and yet STG neurons manage to preserve the mean phase relationship of their rhythmic bursting, the core behavior of this network. Phase relationship is also conserved across adult subjects, regardless of differences in other rhythm parameters and in the number of neurons forming the circuit [28]. Converging modeling and experimental evidence suggests that this stability can be achieved with multiple network configurations, and that different combinations of neurons with dramatically different intrinsic properties and synaptic conductance produce the same collective rhythm [25••,29••]. Thus, the maintenance of individual neuron firing rates and tight constraints on synaptic currents do not appear to be strict requirements for STG network homeostasis; what matters is the coordination of parameters that regulate circuit interactions [25••].
Network plasticity in cortical circuits
To what extent do the cellular and network mechanisms described above operate in the dauntingly complex networks of the mammalian brain?
Cell autonomous synaptic scaling has been consistently observed in excitatory neurons following manipulation of sensory experience. Scaling of mEPSCs onto pyramidal neurons is induced in visual cortex following visual deprivation [30–32]; in the olfactory bulb following odor deprivation [8] and in the auditory cortex, induced by sensorineural hearing loss [33].
A similar mechanism also mediates sleep-dependent cortical homeostasis. During wakefulness overall cortical excitability increases [34,35]: as rats explore the environment, the amplitude of sensory evoked local field potentials is enhanced along with the expression of AMPA receptors and other markers associated with synaptic potentiation [34,35,36••]. While prolonged wakefulness due to sleep deprivation increases cortical excitability further, sleep’s deepest phase, slow wave sleep (SWS), restores activity to basal levels. Sleep-dependent down-scaling of AMPA receptor expression appears to mediate the renormalization of cortical activity and the maintenance of energetic sustainability [36••,37]. SWS-dependent downscaling shows intriguing similarities to bicuculline-induced downscaling of AMPA mEPSC in dissociated cultures [7], and highlights a possible connection between the homeostasis of excitatory neurons and the conservation of the brain metabolic state (Figure 2).
Figure 2.

Sleep-dependent homeostasis network behavior. Wakefulness increases brain circuit activity globally. The deep phase of sleep (slow wave sleep, SWS) contributes to normalize synaptic weights and decrease global excitability.
Regulating pyramidal neuron excitability via cell autonomous scaling is not the only strategy for maintaining homeostasis in cortical networks. The cortex is composed of complex neuronal circuits [38,39] organized in multiple layers. In a widely agreed upon simplified connection scheme layer 4 is the input stage, where most thalamic axons terminate, layer 2/3 integrates inputs from layer 4 and other cortical areas and projects to layer 5, the cortical output [40]. Co-regulation of excitation and inhibition within and between layers is necessary to maintain the balance of such a complex network [2,3,41]. The study of rat’s visual cortex plasticity following sensory deprivation is a good example of how cell autonomous mechanisms integrate with coordinated intra and inter-laminar network changes [42,43••]. Sensory deprivation significantly affects connectivity and excitation/inhibition balance in cortical circuits [31,44–48].
Layer 4 behaves similarly to cultured networks when visual deprivation is started early in development (during the so-called pre-critical period). Pyramidal neurons upscale their excitatory inputs, downscale inhibitory ones, and the overall excitability of the cortical circuit increases. A complex series of changes in different populations of interneurons results in an overall decrease of inhibitory load onto principal cells that, together with the increased strength of glutamatergic synapses, leads to a substantial shift in the excitation/inhibition balance toward a more excitable state [48]. A similar decrease in inhibitory synaptic transmission is observed in layer 4 of the barrel cortex following whisker deprivation [46], however, it is unclear whether this decrease in inhibition is part of a compensatory response of the circuit or whether it is the first step toward the enlargement of spared barrels at the expenses of the deprived one.
Monocular deprivation started just a few days after eye opening, at the beginning of the critical period for visual cortical plasticity, produces an even more elaborate cascade of events. The homeostatic response of the circuit now depends on the interaction of the recurrent and feedforward circuit in layer 4 and 2/3. More specifically, the excitability of layer 4 pyramidal neuron is now reduced by potentiation of inhibitory synapses. Inhibitory neurons no longer act for the greater good of circuit excitability: instead of decreasing their activity to maintain pyramidal neuron output, interneurons upscale their own excitatory inputs [47]. As a consequence layer 2/3, the main recipient of layer 4, receives a weaker input and compensates for it by increasing the excitability of its own pyramidal neurons [31]. This compensatory response of layer 2/3 is also confirmed by experiments in vivo, which show that visual responses in neurons receiving inputs from the visually deprived eye are maintained [42,43••].
Layer 2/3 homeostasis is specific to the critical period: it is in fact absent during the pre-critical period when layer 4 adjusts its excitability homeostatically [48]. The developmental mechanisms underlying the inability of layer 4 to compensate and the consequent switch of homeostatic plasticity to layer 2/3 are not known. The maturation of inhibitory circuits, which are substantially refined after eye opening and well into the critical period [49–52,53••], is likely a determinant factor.
A closer look at the mechanisms of layer 2/3 homeostasis provides further support for the importance of coordinated network adjustments. Here the same circuit behavior can be achieved through multiple forms of plasticity at different sites. TTX injection and eyelid suture, two standard methods for visual deprivation, increase the excitability of pyramidal neurons to the same extent, but through different sets of changes. Alterations of intrinsic excitability, up and down scaling of intra and inter-layer excitatory synapses, and modifications of inhibition all contribute in different proportions to the regulation of layer 2/3 excitability (Figure 3) [31]. This ability to produce the same output using different configurations resembles the strategy adopted by the STG (see above), and is likely a general property of neural circuits [54,55].
Figure 3.
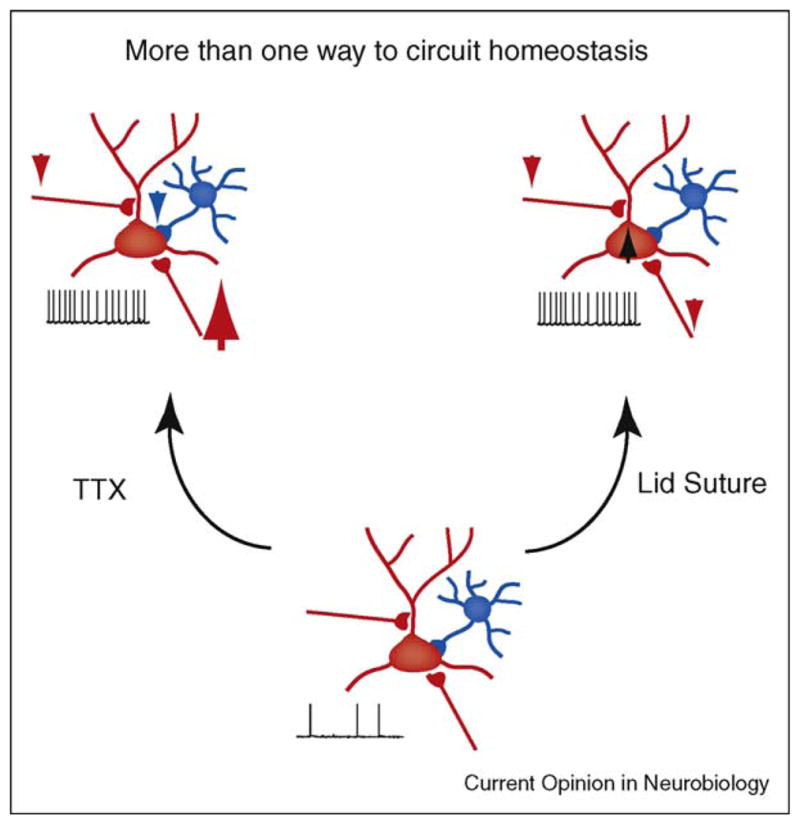
Multiple routes to circuit homeostasis. In layer 2/3 during the critical period intraocular TTX injection and eyelid suture produce similar increases in circuit excitability through dramatically different patterns of circuit rewiring. TTX: intraocular TTX injection (leftmost panel) induces scaling of excitatory synapses onto pyramidal neurons (large upward red arrow), depression of recurrent excitatory connections between pyramidal neurons (small downward red arrow) and decreased inhibitory synaptic transmission (small downward blue arrow). Eyelid suture (rightmost panel): all of the excitatory inputs onto pyramidal neurons are weakened (small downward red arrows); inhibitory synaptic transmission is unchanged but pyramidal neurons intrinsic excitability is increased (black upward black arrow).
Conclusions
In this review we looked at homeostasis from a network perspective. While cell-autonomous mechanisms play certainly a key role in maintaining network excitability, additional mechanisms seem to be involved. Interactions and co-regulations between multiple elements of the circuit are determinant factors that shape compensatory network responses to persistent modifications of their input.
The study of coordinated changes regulating circuit homeostasis is just beginning. Are there specific patterns of network rearrangements? How is the coordination of the different forms of plasticity achieved and regulated? At the moment we can only speculate. Network homeostasis might indeed arise from a combination of local regulations – coordinated by the release of neuromodulators and growth factors – and interactions with other areas of the brain that might act as supervisors (i.e. higher order cortical areas and/or neuromodulatory nuclei).
The use of multielectrode and imaging techniques for recording the simultaneous activation of multiple components of the circuit, together with experimental approaches aimed at describing their synaptic interactions, will provide fundamental help in facing this ambitious set of questions.
Acknowledgments
We wish to thank Martha Stone, Matthew PH Gartner, Sudipto Chakrabortty and Dr Shaoyu Ge for useful comments. This work was supported by National Institute on Deafness and Other Communication Disorders Grant DC-008885 to A. Fontanini.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Turrigiano G, Nelson S. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 2.Haider B, Duque A, Hasenstaub A, McCormick D. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haider B, Duque A, Hasenstaub A, Yu Y, McCormick D. Enhancement of visual responsiveness by spontaneous local network activity in vivo. J Neurophysiol. 2007;97:4186–4202. doi: 10.1152/jn.01114.2006. [DOI] [PubMed] [Google Scholar]
- 4.Ramocki M, Zoghbi H. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 2008;455:912–918. doi: 10.1038/nature07457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fröhlich F, Bazhenov M, Sejnowski T. Pathological effect of homeostatic synaptic scaling on network dynamics in diseases of the cortex. J Neurosci. 2008;28:1709–1720. doi: 10.1523/JNEUROSCI.4263-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trasande C, Ramirez J. Activity deprivation leads to seizures in hippocampal slice cultures: is epilepsy the consequence of homeostatic plasticity? J Clin Neurophysiol. 2007:24. doi: 10.1097/WNP.0b013e318033787f. [DOI] [PubMed] [Google Scholar]
- 7.Turrigiano G, Leslie K, Desai N, Rutherford L, Nelson S. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 8.Tyler W, Petzold G, Pal S, Murthy V. Experience-dependent modification of primary sensory synapses in the mammalian olfactory bulb. J Neurosci. 2007;27:9427–9438. doi: 10.1523/JNEUROSCI.0664-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiagarajan T, Lindskog M, Tsien R. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 10.Murthy V, Schikorski T, Stevens C, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- 11.Wierenga C, Ibata K, Turrigiano G. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stellwagen D, Beattie E, Seo J, Malenka R. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Ibata K, Sun Q, Turrigiano G. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008:57. doi: 10.1016/j.neuron.2008.02.031. This paper demonstrates that neurons can regulate the strength of their synapses by sensing their own firing rate. This is a particularly efficient mechanism that efficiently normalizes neuronal excitability without affecting the induction of synapse specific forms of plasticity. [DOI] [PubMed] [Google Scholar]
- 14.Hou Q, Zhang D, Jarzylo L, Huganir R, Man H. Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc Natl Acad Sci U S A. 2008;105:775–780. doi: 10.1073/pnas.0706447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Sutton M, Ito H, Cressy P, Kempf C, Woo J, Schuman E. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. The experiments in this paper show the existence of different time courses for homeostatic plasticity. The authors provide evidence for slow and fast forms of synaptic scaling that rely on different induction mechanisms and can coexist in the same neuron. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd J, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir R, Worley P. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoto J, Nam C, Poon M, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wierenga C, Walsh M, Turrigiano G. Temporal regulation of the expression locus of homeostatic plasticity. J Neurophysiol. 2006;96:2127–2133. doi: 10.1152/jn.00107.2006. [DOI] [PubMed] [Google Scholar]
- 19.Buckby L, Jensen T, Smith P, Empson R. Network stability through homeostatic scaling of excitatory and inhibitory synapses following inactivity in CA3 of rat organotypic hippocampal slice cultures. Mol Cell Neurosci. 2006;31:805–816. doi: 10.1016/j.mcn.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Karmarkar U, Buonomano D. Different forms of homeostatic plasticity are engaged with distinct temporal profiles. Eur J Neurosci. 2006;23:1575–1584. doi: 10.1111/j.1460-9568.2006.04692.x. [DOI] [PubMed] [Google Scholar]
- 21.Kilman V, van Rossum M, Turrigiano G. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartmann K, Bruehl C, Golovko T, Draguhn A. Fast homeostatic plasticity of inhibition via activity-dependent vesicular filling. PLoS ONE. 2008;3:e2979. doi: 10.1371/journal.pone.0002979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutherford L, Nelson S, Turrigiano G. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- 24.Stellwagen D, Malenka R. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 25••.Marder E, Tobin A, Grashow R. How tightly tuned are network parameters? Insight from computational and experimental studies in small rhythmic motor networks. Progress Brain Res. 2007;165:193–200. doi: 10.1016/S0079-6123(06)65012-7. The authors demonstrate that a given pattern of neurons activity can arise from a variable set of underlying conductance whose distribution and amplitude widely vary in individual identified neurons. [DOI] [PubMed] [Google Scholar]
- 26.Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- 27.Golowasch J, Casey M, Abbott L, Marder E. Network stability from activity-dependent regulation of neuronal conductances. Neural Comput. 1999;11:1079–1096. doi: 10.1162/089976699300016359. [DOI] [PubMed] [Google Scholar]
- 28.Bucher D, Prinz A, Marder E. Animal-to-animal variability in motor pattern production in adults and during growth. J Neurosci. 2005;25:1611–1619. doi: 10.1523/JNEUROSCI.3679-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Prinz A, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7:1345–1352. doi: 10.1038/nn1352. This paper shows that virtually indistinguishable network activity can arise from widely disparate sets of underlying mechanisms, suggesting that many different combinations of synaptic strengths and intrinsic membrane properties can be consistent with appropriate network performance. [DOI] [PubMed] [Google Scholar]
- 30.Desai N, Cudmore R, Nelson S, Turrigiano G. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- 31.Maffei A, Turrigiano G. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28:4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goel A, Lee H. Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J Neurosci. 2007;27:6692–6700. doi: 10.1523/JNEUROSCI.5038-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotak V, Fujisawa S, Lee F, Karthikeyan O, Aoki C, Sanes D. Hearing loss raises excitability in the auditory cortex. J Neurosci. 2005;25:3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber R, Tononi G, Cirelli C. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep. 2007;30:129–139. doi: 10.1093/sleep/30.2.129. [DOI] [PubMed] [Google Scholar]
- 35.Tsanov M, Manahan-Vaughan D. The adult visual cortex expresses dynamic synaptic plasticity that is driven by the light/dark cycle. J Neurosci. 2007;27:8414–8421. doi: 10.1523/JNEUROSCI.1101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Vyazovskiy V, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. This paper shows that many cellular mechanisms involved in increasing or decreasing neuronal excitability are active during wakefulness and sleep respectively, providing strong support for the role of sleep in the renormalization of synaptic weights. [DOI] [PubMed] [Google Scholar]
- 37.Faraguna U, Vyazovskiy V, Nelson A, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28:4088–4095. doi: 10.1523/JNEUROSCI.5510-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Markram H, Goodman P, Berger T, Ma J, Goldman-Rakic P. Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci. 2006;9:534–542. doi: 10.1038/nn1670. [DOI] [PubMed] [Google Scholar]
- 40.Burkhalter A. Intrinsic connections of rat primary visual cortex: laminar organization of axonal projections. J Comp Neurol. 1989;279:171–186. doi: 10.1002/cne.902790202. [DOI] [PubMed] [Google Scholar]
- 41.Mishra J, Fellous J, Sejnowski T. Selective attention through phase relationship of excitatory and inhibitory input synchrony in a model cortical neuron. Neural Netw. 2006;19:1329–1346. doi: 10.1016/j.neunet.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaneko M, Stellwagen D, Malenka R, Stryker M. Tumor necrosis factor-α mediates one component of competitive experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Mrsic-Flogel T, Hofer S, Ohki K, Reid R, Bonhoeffer T, Hübener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. Short MD durations decreased deprived-eye responses in neurons with binocular input, while leaving unaffected the activation of deprived monocular neurons. This suggests a homeostatic regulation of the responsiveness of deprived eye activated neurons. [DOI] [PubMed] [Google Scholar]
- 44.Bender K, Allen C, Bender V, Feldman D. Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex. J Neurosci. 2006;26:4155–4165. doi: 10.1523/JNEUROSCI.0175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takasaki C, Rieko Okada R, Akira Mitani A, Fukaya M, Yamasaki M, Fujihara Y, Shirakawa T, Tanaka K, Watanabe M. Glutamate transporters regulate lesion-induced plasticity in the developing somatosensory cortex. J Neurosci. 2008;28:4995–5006. doi: 10.1523/JNEUROSCI.0861-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiao Y, Zhang C, Yanagawa Y, Sun Q. Major effects of sensory experiences on the neocortical inhibitory circuits. J Neurosci. 2006;26:8691–8701. doi: 10.1523/JNEUROSCI.2478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maffei A, Nataraj K, Nelson S, Turrigiano G. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- 48.Maffei A, Nelson S, Turrigiano G. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- 49.Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott G, Kuhlman S, Welker E, Huang Z. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hensch T. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 51.Maffei A, Turrigiano G. The age of plasticity: developmental regulation of synaptic plasticity in neocortical microcircuits. Progress Brain Res. 2008;169:211–223. doi: 10.1016/S0079-6123(07)00012-X. [DOI] [PubMed] [Google Scholar]
- 52.Kotak V, Takesian A, Sanes D. Hearing loss prevents the maturation of GABAergic transmission in the auditory cortex. Cereb Cortex. 2008;18:2098–2108. doi: 10.1093/cercor/bhm233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Sugiyama S, Di Nardo A, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch T. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. These groundbreaking results link for the first time the experience-dependent transfer of the homeoprotein Otx2 during the development of primary visual cortex. Otx2 transfer likely establishes the physiological environment for plasticity during postnatal circuit refinement. [DOI] [PubMed] [Google Scholar]
- 54.Leonardo A. Degenerate coding in neural systems. J Comp Physiol. 2005;191:995–1010. doi: 10.1007/s00359-005-0026-0. [DOI] [PubMed] [Google Scholar]
- 55.Tononi G, Sporns O, Edelman G. Measures of degeneracy and redundancy in biological networks. Proc Natl Acad Sci U S A. 1999;96:3257–3262. doi: 10.1073/pnas.96.6.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]


