Abstract
The EphA2 receptor tyrosine kinase (RTK) is an attractive therapeutic target that is commonly overexpressed on solid tumors, with the degree of overexpression associated with disease progression, metastatic potential and poor prognosis. Agonistic monoclonal antibodies or ligand (ephrinA1)-Fc fusion protein are capable of inducing EphA2 internalization and degradation, thereby (at least transiently) eliminating the influence of this oncoprotein. We and others have also shown that EphA2 contains multiple peptide epitopes that can be recognized by effector CD4+ and CD8+ T cells isolated from tumor-bearing patients. Herein, we show that “agonist” reagents that trigger the proteasome-dependent degradation of tumor cell EphA2 result in the improved presentation of peptides derived from (both the extracellular and intracellular domains of) EphA2 in MHC class I complexes expressed on the tumor cell membrane for at least 48h, as manifest by increased recognition by EphA2-specific CD8+ T cells in vitro. We also observed that while delivery of ephrinA1-Fc fusion protein or agonist mAb into EphA2+ tumor lesions promotes EphA2 degradation in situ, this single administration of agent does not dramatically alter tumor progression in a Hu-SCID model. However, when combined with the adoptive transfer of normally non-therapeutic (human) anti-EphA2 CD8+ cytotoxic T lymphocytes (CTL), this dual agent regimen results in complete tumor eradication. These results suggest that strategies targeting the conditional proteasome-mediated destruction of tumor cell EphA2 may enable EphA2-specific CD8+ T cells (of modest functional avidity) to realize improved therapeutic potential.
Keywords: EphA2, Receptor Tyrosine Kinase, Proteasome, CD8+ T Lymphocyte Cancer Immunotherapy
INTRODUCTION
The EphA2 RTK plays a pivotal role in disease and development (1). EphA2 is a 130kDa (Type-1) glycoprotein that is expressed at low levels on non-transformed epithelial tissues (2). In these cells, EphA2 localizes to epithelial cell-to-cell contacts, and is believed to contribute to the well-known phenomenon of contact inhibition of cell growth and motility (3, 4). In contrast to its role in non-transformed cells, dysregulation in EphA2 expression and function causes EphA2 to support tumor progression and metastasis (4, 5). High levels of EphA2 expression have been observed in a range of malignant cell models and in clinical specimens of many different solid tumors, including metastatic melanoma and carcinomas of the bladder, breast, colon, esophagus, kidney, lung, mesothelium, ovary, prostate and pancreas, among others (5–14). The highest levels of EphA2 are found on the most aggressive tumors, with tumor cell EphA2 expression levels being predictive of increased metastatic potential and decreased patient survival (7–11, 13–16).
The prevalence of EphA2 overexpression on tumor cells has sparked interest in its use for the development of novel targeted therapeutics. In particular, a class of agonistic EphA2 antibodies has been developed that can induce EphA2 internalization and degradation, thereby reducing expression of this powerful oncoprotein. Repeated administration of these reagents has proven successful at inhibiting tumor cell growth in both in vitro and in vivo models, and in enhancing the survival of tumor-bearing mice (17, 18). Based on its overexpression on multiple epithelial tumor cell types, EphA2 may represent a pan-tumor associated antigen for the generalized immune targeting of carcinomas. In this light, we and others (15, 19, 20) have recently identified peptide epitopes derived from human and murine EphA2 that are competent to activate specific CD4+ and CD8+ T cells capable of recognizing tumor cells that constitutively (over)express the EphA2 protein. Notably, dendritic cell-based vaccines incorporating mEphA2 peptides have been reported to promote protective T cell responses in murine melanoma and colon cancer models (21, 22).
However, the clinical expectation would be that vaccines based on EphA2 epitopes would fail to be optimally efficacious in the cancer setting as they would likely elicit only moderate-to-low avidity T cells in patients with EphA2+ cancers, given tolerance mechanisms imposed against the self (non-mutated) EphA2 protein as well as immune deviation that is known to occur in these individuals (23). Herein, we investigated whether treatment of EphA2+ human tumor cells with specific agonists would induce proteasome-dependent degradation of EphA2 protein, thereby increasing tumor cell surface expression of MHC class I/EphA2 peptide complexes, resulting in improved recognition of tumor cells by anti-EphA2 CD8+ T cells. We determined that recombinant ligand (i.e. EphrinA1-Fc) and agonist anti-EphA2 mAb208 are both competent to promote the enhanced recognition of EphA2+ tumor cells by specific CD8+ T cells in vitro and in vivo. Such conditional augmentation in immune recognition of EphA2+ tumor cells by recombinant ligand or agonist mAb, in concert with active immunization or adoptive transfer of ex vivo expanded anti-EphA2 T cells, may serve to define novel and effective combinational immunotherapeutic strategies relevant to a large cohort of patients harboring EphA2+ malignancies.
MATERIALS AND METHODS
Cell Lines and Media
The T2 (HLA-A2+, EphA2−; refs. 24, 25) cell line (kindly provided by Dr. Janice Blum, Indiana University School of Medicine, Indianapolis, IN) was used as the peptide-presenting cell in ELISPOT assays. The EphA2+, HLA-A2− PC-3 prostate carcinoma cell line (5) was used as positive control for Western Blot analyses of EphA2 protein expression and was also used as a negative control target (along with the EphA2+, EGFR+, HLA-A2-SLR20 renal cell carcinoma line; ref. 15) in ELISPOT assays. SLR24, an EphA2+, EGFR+, HLA-A2+ cell line (15) was tested in Western Blot and ELISPOT assays and was also employed in the Hu-SCID treatment model. Additional target cells analyzed in this study included the HLA-A2 cDNA transfectants SLR20.A2 (generated for this study by recombinant retroviral transduction, data not shown). All cell lines were free of mycoplasma contamination and were maintained in RPMI-1640 culture medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin and 10 mM L-glutamine (all reagents from Invitrogen, Carlsbad, CA) in a humidified atmosphere under 5% CO2 tension at 37°C.
Peptides
The HLA-A2 presented EphA258-66 (IMNDMPIYM; ref. 19), EphA2883-891 (TLADFDPRV; ref. 15) and HIV-nef180-189 (VLEWRFDSRL; ref. 15) peptides were synthesized using FMOC chemistry by the University of Pittsburgh Cancer Institute’s (UPCI) Peptide Synthesis Facility, as previously described (15). Peptides were >96% pure based on high-performance liquid chromatography, with identities validated by mass spectrometric (MS/MS) analyses performed by the UPCI Protein Sequencing Facility.
Mice
Six-to-eight week old female C.B-17 scid/scid mice were purchased from Taconic Labs (Germantown, NY), and maintained in micro-isolator cages. Animals were handled under aseptic conditions as per an Institutional Animal Care and Use Committee (IACUC)-approved protocol and in accordance with recommendations for the proper care and use of laboratory animals.
EphA2 Agonists
EphrinA1-Fc (R & D Systems, Minneapolis, MN) is a chimeric protein consisting of the ligand binding domain of the EphA2 ligand ephrinA1 fused with the Fc portion of a mouse IgG antibody. mAb208 (kindly provided by MedImmune Inc., Gaithersburg, MD) is a mouse IgG monoclonal antibody specific for hEphA2 (16). EphrinB1-Fc (Sigma-Aldrich, St. Louis, MO) and MOPC21 mAb (mouse IgG; Sigma-Aldrich) were employed as specificity controls for EphrinA1-Fc and mAb208, respectively.
Western Blot Analyses
Tumor cells were grown to 80–90% confluency, then treated with agonists where indicated for up to 48h prior to analysis. In addition, resected SLR24 lesions were obtained pre- and 24h post-intratumoral injection with EphrinA1-Fc, EphrinB1-Fc or mAb208, as in text and the Fig. 5 legend. Tumor samples were analyzed for EphA2 expression via Western blots using the rabbit anti-human EphA2 polyclonal antibody (clone C-20), Santa Cruz Biotechnology, Inc., Santa Cruz, CA). SLR20.A2 and SLR24 cells were also analyzed for expression of the epidermal growth factor receptor (EGFR) using rabbit anti-human polyclonal antibody sc-03 (Santa Cruz Biotechnology). Single tumor cell suspensions isolated from confluent tissue culture flasks or from the enzymatic digestion of resected tumor lesions were lysed using 500 μl lysis buffer (1% Triton–X, 150 nM NaCl, 10 mM Tris pH7.4, 1 mM EDTA, 0.2 mM SOV, 0.5% NP-40; all from Sigma-Aldrich) in PBS containing protease inhibitors (Complete, Roche Diagnostic, Mannheim, Germany) for 30 min at 4°C. After centrifugation at 13,500 × g for 20m, the supernatant was mixed 1:1 with SDS-PAGE running buffer and proteins separated on 7.5% PAGE gels, prior to electro-blotting onto PVDF membranes (Millipore, Bedford, MA). Blots were imaged on Kodak X-Omat Blue XB-1 film (NEN Life Science Products, Boston, MA) after using horseradish peroxidase (HRP)-conjugated goat anti-rabbit Ig (Santa Cruz Biotechnology) and the Western Lighting chemiluminescence detection kit (Perkin-Elmer, Boston, MA). Immunoprecipitations for EphA2 were performed using the anti-EphA2 antibody D7 (Millipore). Anti-phosphotyrosine antibodies (Clone py99, Santa Cruz Biotechnology) were used to assess pEphA2 content. Mouse anti-β-actin antibody (clone AC-15, Abcam, Cambridge, MA) was used as a loading control.
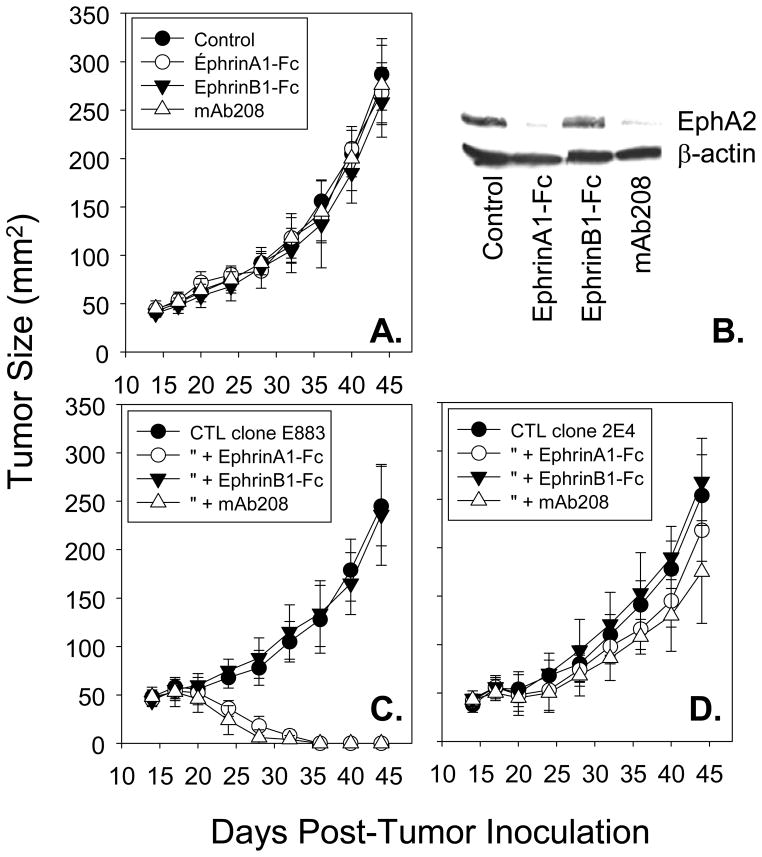
Figure 5. EphrinA1-Fc and mAb208 promote EphA2 down-regulation in situ and sensitize EphA2+, HLA-A2+ SLR24 tumors to enhanced eradication mediated by adoptively transferred anti-EphA2, but not anti-HLA2 allo-specific, CD8+ T cell clones in vivo.
In panel A, Female CB17-scid/scid mice were injected with 1 × 106 human SLR24 (HLA-A2+/EphA2+) RCC cells s.c. in the right flank and allowed to establish to a size of approximately 30 mm2 (i.e. d18). Animals were then randomized into 4 cohorts (6 animals each) receiving no treatment (control), or intratumoral injections of EphrinA1-Fc (50 μg) or EphrinB1-Fc (50 μg) or mAb208 (50 μg) on d18. Tumor size was evaluated every 3–4 days, with results reported in mean mm2 +/− SD. In panel B, tumors were resected from 1 mouse/cohort on day 19 (i.e. 24 hours after treatment and Western Blots performed to validate EphA2 degradation in situ. In panels C and D, 5 × 106 CD8+ T cells (either anti-EphA2883-891 clone E883 or anti-HLA-A2 allo-specific clone 2E4, respectively) were adoptively transferred by tail-vein injection on day 19 post-tumor inoculation, alone or in combination with prior day 18 intratumoral injections of EphrinA1-Fc, EphrinB1-Fc or mAb208 (50 μg each). Tumor size was evaluated every 3–4 days, with results reported in mean mm2 +/− SD. Data are representative of 3 independent experiments performed.
T Cell Lines and Clones
Bulk CD8+ human T cell lines and clones specific for EphA258-66 (i.e. 15/9) and EphA2883-891 (i.e. E883, 3C1) were generated as previously described (15). The HLA-A2 allo-specific CD8+ T clone 2E4 was generated by three rounds of in vitro stimulation of HLA-A2-negative (HLA-DR4+) normal donor T cells with irradiated (100 Gy) T2 cells, followed by limiting-dilution cloning. All T cell lines and clones were specifically re-stimulated every 7–10 days and were maintained in IMDM media (Invitrogen) containing 10% Human AB serum (Sigma-Aldrich), 100 U/ml penicillin, 100 μg/ml streptomycin and 10 mM L-glutamine (all from Invitrogen), and rhIL-2 (100 IU/ml; Peprotech, Rocky Hill, NJ). All donor specimens were obtained with written consent under an IRB-approved protocol.
ELISA and ELISPOT Assays
In vitro T cell responses were evaluated by commercial hIFN-γ ELISA (BD OptEIA™; BD Biosciences, San Jose, CA; limit of detection 4.7 pg/ml) per the manufacturer’s instructions and by IFN-γ ELISPOT assays, as previously described (15).
Preoteasome dependency assessment
The impact of proteasome inhibition in Western Blot (evaluating agonist-induced EphA2 degradation in tumor cells) and T cell ELISPOT (evaluating enhanced anti-EphA2 T cell recognition of agonist-treated tumor cells) assays was assessed by treatment of tumor cells with either MG-132 (Sigma-Aldrich) or clasto-lactacystin β-lactone (hereafter designated as lactacystin; Biomol International LP, Plymouth Meeting, PA), as outlined in relevant text and figure legends.
Cytotoxicity Assays
CD8+ T cell clones E883 (anti-EphA2883-891) and 2E4 (anti-HLA-A2) were evaluated for their capacity to lyse EphA2+, HLA-A2+ SLR24 tumor target cells using standard 4h51Cr-release assays, as previously described (26).
Flow Cytometry
For phenotypic analyses of control or ligand agonist-treated tumor cells, PE-or FITC-conjugated monoclonal antibodies against total HLA class I complexes (W6/32; pan-class I specific; Serotec Inc., Raleigh, NC), HLA-A2 complexes (American Type Culture Collection, ATCC, Rockville, MD), or empty HLA-A molecules (HC-A2, ref. 27, the kind gift of Dr. H. Ploegh, MIT) and appropriate isotype controls (purchased from BD Biosciences, San Jose, CA) were used, and flow cytometric analyses were performed using a FACscan (Becton Dickinson, San Jose, CA) flow cytometer. Cell surface expression of EphA2 protein was analyzed using direct immunofluorescence staining monitored by flow cytometry. After treatment for 0–24h at 37°C with 10 μg/ml EphrinA1-Fc, EphrinB1-Fc, mAb208 or the MOPC21 mAb, tumor cells were stained for 30m at 4°C with FITC-conjugated anti-EphA2 mAb B2D6 (Millipore; note: this mAb is not sterically inhibited by the binding of EphrinA1-Fc or mAb208 to EphA2, data not shown), prior to washing using PBS and analysis by flow cytometry. The results of these assays are reported as percent control (untreated) tumor cell expression based on a comparison of arbitrary mean fluorescence intensity (MFI) units obtained for experimental vs. control specimens.
Hu-SCID Tumor Model
C.B17-scid/scid mice were injected s.c. in the right flank with 1 × 106 SLR24 (EphA2+, HLA-A2+) RCC cells and tumors allowed to establish to a size of approximately 30 mm2 (i.e. day 18 post-injection). The tumor-bearing mice were then randomized into groups (5 animals each with comparable tumor sizes) that received either no treatment, a single intratumoral injection of 50 μg of EphrinA1-Fc, EphrinB1-Fc or mAb208 (in 50 μl saline) on day 18, a single tail-vein injection with 5 × 106 CD8+ T cells (Clone E883 specific for HLA-A2 presented EphA2883-891 or allo-specific anti-HLA-A2 Clone 2E4) in 100 μl saline on day 19, or the combined d18 (EphA2 agonist) plus d19 (CD8+ T cell adoptive transfer) regimen. Animals were evaluated every 3–4 days for tumor size, with tumor-free status noted on day 44 post-tumor inoculation. For the analyses of EphA2 content in SLR24 tumor lesions pre-and post-administration of agonists or control proteins, tumors were surgically resected from euthanized mice, digested into single-cell suspensions using a DNAse, hyaluronidase, DNAse cocktail (all reagents purchased from Sigma), as previously described (28), and filtered through Nitex mesh (Tetko, Kansas City, MO), prior to generating lysates for Western Blotting analyses, as outlined above.
Statistical Analyses
Statistical differences between groups were evaluated using a two-tailed Student’s t test, with p values < 0.05 considered significant.
RESULTS
EphrinA1-Fc and mAb208 Induce EphA2 Phosphorylation and Proteasome-Dependent Degradation in Tumor Cell Lines
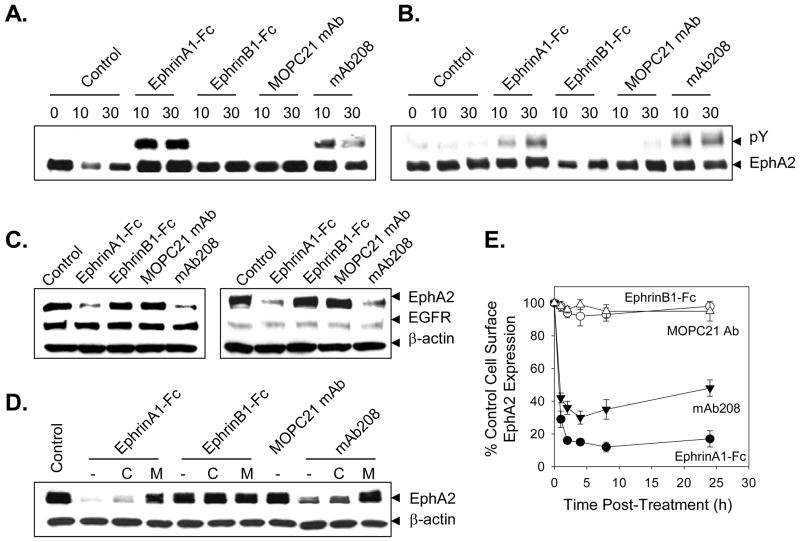
Previous studies have demonstrated that tumor cells exhibit unstable cell-cell contacts and that this impairs the ability of EphA2 to interact with its EphrinA1 ligand on neighboring cells (29–31). Consequently, the EphA2 protein in malignant cells is generally observed to be in a hypo-phosphorylated state (5). Consistent with these previous reports, our Western Blot analyses verified that EphA2 protein expressed in a series of renal cell (SLR20.A2, SLR24) carcinoma cell lines is constitutively non-phosphorylated, but that treatment of these cells with EphA2 agonists (EphrinA1-Fc, anti-EphA2 mAb208) is sufficient to rapidly increase EphA2 phosphotyrosine content (Fig. 1A, 1B). As negative controls, treatment of the EphB1+ SLR20.A2 or SLR24 tumor cells with EphrinB1-Fc (a ligand for EphB1, but not EphA2) or control mIgG MOPC21 failed to induce EphA2 phosphorylation (Fig. 1A, 1B). Immunoblotting of tumor cell lysates (after 24h of treatment) verified that EphrinA1-Fc and mAb208, but not EphrinB1-Fc or MOPC21 mAb induces substantial EphA2 protein degradation (Fig. 1C, 1D). These treatments did not alter tumor cell expression of control proteins, including the epidermal growth factor receptor (EGFR) and β-actin (Fig. 1C, 1D). A re-analysis, in the absence or presence of chloroquine or MG-132, suggested that EphA2 degradation in SLR20.A2 cells was predominantly 26S proteasome-dependent (Fig. 1E), consistent with a previous report for the proteasomal dependency of EphA2 destruction in breast and prostate carcinoma cell lines (32). Flow cytometric analysis of agonist-treated SL20.A2 tumor cells indicated that cell surface EphA2 is rapidly lost (i.e. internalized) within hours after treatment with EphrinA1-Fc and mAb208, but not EphrinB1-Fc or MOPC21 mAb (Fig. 1F).
Figure 1. EphA2 agonists induce the phosphorylation and proteasome-dependent degradation of EphA2 in tumor cells in vitro.
In order to determine whether agonist treatment promotes EphA2 phosphorylation, SLR20.A2 (panel A) and SLR24 (panel B) renal carcinoma cells (2–4 × 106) were left untreated or were treated for 10m or 30m with EphrinA1-Fc, EphrinB1-Fc, MOPC21 mAb or mAb208 (each at 10 μg/ml) at 37°C. Cellular lysates were resolved by SDS-PAGE and EphA2 protein was immunoprecipitated using the anti-EphA2 antibodies D7 in pull-down assays. Western blot analyses were then performed using anti-EphA2 and anti-phosphotyrosine antibodies, respectively. To determine whether agonist treatment induces EphA2-specific degradation, SLR20.A2 (panel C) and SLR24 (panel D) tumor cells were treated as in panel A above for 24h hours with consequent cell lysates resolved by SDS-PAGE and Western blot analyses performed using polyclonal anti-EphA2 and control anti-β-actin antibodies. Anti-EGFR antibody was used to image identically-prepared lysates as a specificity (negative) control in these experiments. To assess the proteasome-dependence of agonist-induced EphA2 degradation, MG-132 (50 μM) or chloroquine (Chl.; 100 μM) were also added to SLR20.A2 cell cultures, where indicated, 30m prior to the addition of Ephrin-Fc proteins or mAb208 (panel E). After 24 h, cell lysates were generated and resolved using SDS-PAGE. Western blot analyses were then performed using anti-EphA2 antibodies and negative control anti-β-actin antibodies. In panel F, the kinetics of EphA2 down-modulation on the surface of treated (with the indicated agents) SLR20.A2 cells was investigated by flow cytometry. Data are reported as % control EphA2 cell surface expression (vs. untreated cells) based on mean fluorescence intensity values obtained. All data are representative of 3 independent experiments performed.
EphrinA1-Fc and mAb208 Treatment Enhances CD8+ T Cell Recognition of EphA2+ Tumors In Vitro
Since agonistic antibodies triggered the proteasomal destruction of EphA2, we hypothesized that this could preferentially increase presentation of EphA2 peptides in tumor cell surface HLA class I complexes. If correct, it would then logically follow that EphA2 agonists could selectively enhance tumor cell recognition by EphA2-specific CD8+ T cells. To address this question, EphA2+ tumor cell lines were incubated with mAb208 for 24h or 48h prior to evaluating the ability of these target cells to be recognized by HLA-A2-restricted CD8+ T cell lines and clones specific for the EphA258-66 (ref. 19 located in EphA2 ECD) or EphA2883-891 (ref. 15; located in the EphA2 ICD) peptide epitopes. Rather than initially assessing differential tumor sensitivity to T cell killing (which could involve changes in both T cell and tumor cell functions induced by agonists), we instead chose to more directly interrogate changes in T cell functional recognition of treated tumor cells using the IFN-γ ELISPOT assay as a readout of effector T cell reactivity.
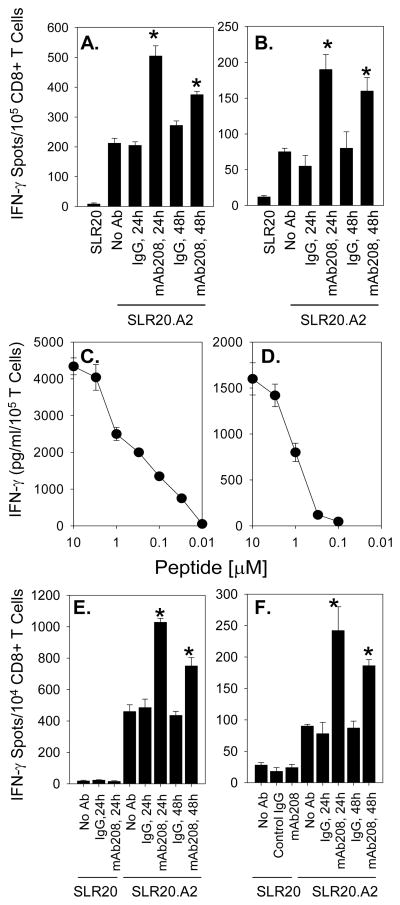
Pre-treatment of SLR20.A2 (EphA2+, HLA-A2+) tumor cells with mAb208 significantly enhanced their recognition by both anti-EphA2 CD8+ T cell lines (Fig. 2A, 2B) and moderate avidity (Fig. 2C, 2D) CD8+ T cell clones 15/9 and 3C1 (Fig. 2E, 2F, respectively). Notably, improved T cell recognition of treated tumor cells was sustained for a period of at least 48h (Fig. 2A, 2B, 2E and 2F). SLR20 (EphA2+, HLA-A2−) tumor cells failed to be recognized by any of these T cell populations, even after treatment with mAb208 (that promotes EphA2 degradation, data not shown). Furthermore, treatment of SLR20.A2 cells with control IgG (MOPC21 mAb) failed to enhance tumor cell recognition by anti-EphA2 CD8+ T cells (Figs. 2A, 2B, 2E, 2F).
Figure 2. Anti-EphA2 mAb208 sensitizes the EphA2+, HLA-A2+ tumor cell line SLR20.A2 to recognition by anti-EphA2 CD8+ T cells in vitro.
Bulk CD8+ T cell lines were developed from HLA-A2+ normal donors against the EphA258-66 (panel A) and EphA2883-891 (panel B) peptides, as described in the Materials and Methods, and evaluated for their differential recognition of SLR20 (EphA2+, HLA-A2−) and SLR20.A2 (EphA2+, HLA-A2+) tumor cell targets using IFN-γ ELISPOT assays. SLR20.A2 cells were pre-treated with no Ab, control IgG (MOPC21 mAb) or mAb208 (10 μg/ml each) for 24h or 48h, as indicated, prior to ELISPOT analyses. Data are reported as mean IFN-γ specific spots/105 CD8+ T cells +/− SD from triplicate determinations. The HLA-A2 restricted CD8+ T cell clones 15/9 (specific for EphA258-66; panel C) and 3C1 (specific for EphA2883-891; panel D) were developed, as described in the Materials and Methods, and exhibited moderate-to-low functional avidity against specific peptide-pulsed T2 cells (EC50 approximately 1 μM peptide for both clones) as assessed by IFN-γ ELISA (mean +/− SD from triplicate determinations). These clones recognize SLR20.A2 tumor cells selectively in IFN-γ ELISPOT assays, with recognition increased at 24h and 48h by pretreatment of tumor cells with mAb208, but not control IgG. Data are reported as mean IFN-γ specific spots/104 CD8+ 15/9 (panel E) and 3C1 (panel F) T cells +/− SD from triplicate determinations. All data are representative of 3 independent experiments performed. *p < 0.05 vs. control IgG-treated tumor cells.
While unlikely, we considered the trivial explanation that increased tumor cell recognition by anti-EphA2 CD8+ T cells could be the result of a general up-regulation in tumor cell expression of HLA-A2 class I molecules (and hence a compensatory increase in the cohort of HLA-A2 complexes containing EphA2-derived peptides). To address this possibility, the SLR20.A2 and SLR24 cell lines were treated with EphrinA1-Fc or mAb208 and analyzed by flow cytometry for cell surface expression levels of total HLA class I molecules (using the W6/32 mAb), peptide-loaded (monitored using the BB7.2 mAb) and empty (monitored using the HC-A2 mAb) HLA-A2 complexes. We noted no significant changes in the mean fluorescence intensities of any of these parameters as a consequence of tumor treatment with these EphA2 agonists (data not shown).
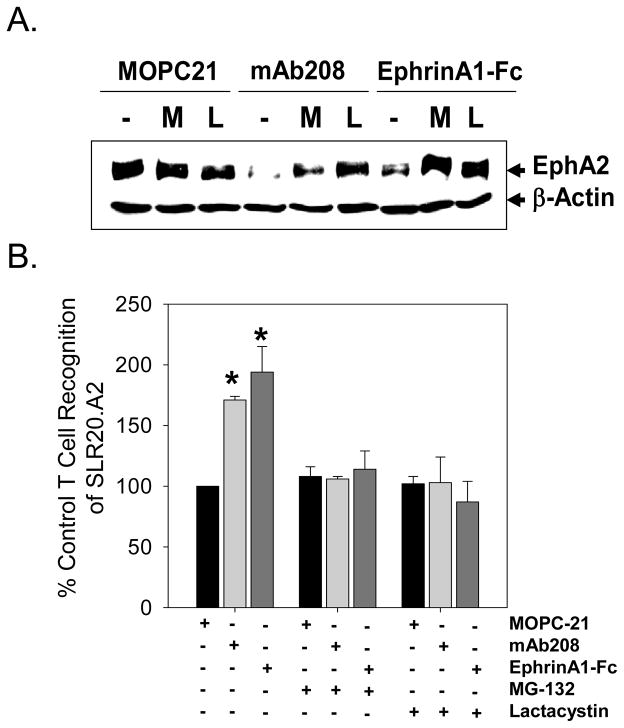
To demonstrate whether enhanced CD8+ T cell recognition of tumor cells was due to processing through the proteasome, SLR20.A2 tumor cells were pretreated with MG-132 or β-lactone to block proteasomal function, and then were cultured these cells with EphA2 agonists prior to use as targets for CD8+ T cell recognition. Due to concerns for the toxicity of proteasome inhibitors using a prolonged exposure, we chose a short 3h period for tumor pre-treatment, with no impact on tumor cell morphology or viability noted (data not shown). As a confirmation and extension of data depicted in Fig. 1D, application of MG-132 or lactacystin (clasto-lactacystin β-lactone) prevented agonist-induced EphA2 degradation (Fig. 3A). These proteasome inhibitors also completely abrogated any enhancement in recognition of SLR20.A2 cells by anti-EphA2 T cells resulting from treatment with the EphA2 agonists, mAb208 and EphrinA1-Fc (Fig. 3B).
Figure 3. EphA2 agonist enhanced in vitro recognition of SLR20.A2 tumor cells by anti-EphA258-66 T cell clone 15/9 is proteasome-dependent.
SLR20.A2 cells were pre-treated with MG-132 (10 μM) or lactacystin (clasto-lactacystin β-lactone; 20 μM) or media for 3 hours, before being treated with 1 μg/ml MOPC-21 (control IgG), 1 μg/ml mAb208 or 0.1 μg/ml EphrinA1-Fc for an additional 3 hours. No toxicity was observed under any conditions. The effect of proteasomal inhibitors on agonist-induced degradation of EphA2 expression was confirmed by Western Blot (panel A.). After harvesting and washing the treated tumor cells extensively, these cells were used as targets for clone 15/9 (anti-EphA258-66) in IFN-γ ELISPOT assays (panel B) as outlined in Materials and Methods. Data from a representative experiment are reported as % control (mean +/− SD) T cell response to SLR20.A2 versus tumor cells treated with control IgG (i.e. MOPC21). *p < 0.05 vs. control IgG-treated tumor cells.
EphrinA1-Fc and mAb208 Treatment Enhances the Therapeutic Efficacy of Adoptively Transferred Anti-EphA2 CD8+ T Cells in a Hu-SCID Tumor Model
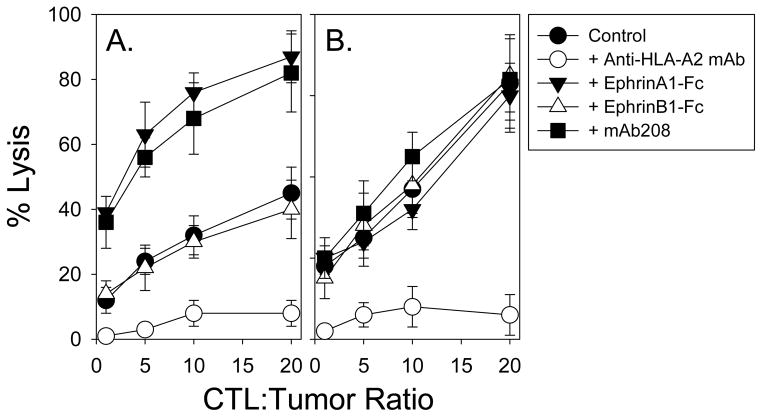
To determine whether the conditional (agonist-induced) enhancement of EphA2+ tumor cell recognition by anti-EphA2 CD8+ T cells could be of potential clinical significance, we established a Hu-SCID tumor model system for the analysis of combinational adoptive cellular immunotherapy. Human SLR24 (EphA2+, HLA-A2+) renal carcinoma cells were injected s.c. into the right flanks of C.B-17 scid/scid mice and allowed to progress to a size of approximately 30 mm2; at which time, animals were either left untreated, or treated with intratumoral injection of EphrinA1-Fc, EphrinB1-Fc or mAb208 and/or intravenous delivery of an HLA-A2-restricted, anti-EphA2883-891 CD8+ T cell clone (i.e. clone E883 was used vs. clone 3C1 due to its ability to be expanded to the high numbers of T cells required for these experiments). As depicted in Fig. 4A, Clone E883 mediates the lysis of SLR24 tumor cells in vitro in an HLA-A2-restricted manner, with cytolysis dramatically increased if the tumor cell line is pre-treated with either EphrinA1-Fc or mAb208, but not EphrinB1-Fc. Treatment-induced sensitization of SLR24 to T cell-mediated cytolysis is specific to anti-EphA2 CD8+ T cells, since alloreactive (anti-HLA-A2) CD8+ T cell clone 2E4 lysed SLR24 tumor cells to a comparable degree regardless of in vitro pretreatment conditions applied to the tumor cells (Fig. 4B).
Figure 4. EphrinA1-Fc and mAb208 sensitize EphA2+, HLA-A2+ SLR24 tumor cells to enhanced lysis mediated by anti-EphA2, but not anti-HLA2 allo-specific, CD8+ T cell clones in vitro.
SLR24 tumor cells were untreated or pretreated for 24h with EphrinA1-Fc, EphrinB1-Fc or mAb208, prior to their use as target cells in 4h 51Cr-release assays. Anti-EphA2883-891 CD8+ T cell clone E883 (15) and anti-HLA-A2 allo-specific CD8+ T cell clone 2E4 (developed as described in the Materials and Methods) were used effector cells at the indicated effector-to-target cell ratios in panels A and B, respectively. Anti-HLA-A2 mAb BB7.2 was added to wells in order to demonstrate the HLA-A2-restricted nature of T cell recognition of SLR24 tumor cells. Data are reported as the mean +/− SD of triplicate determinations and are representative of 3 independent experiments performed.
In the Hu-SCID tumor model, intratumoral injection of 50 μg EphrinA1-Fc, EphrinB1-Fc or mAb208 (on day 18 post-tumor inoculation) had minimal effect on the continued progressive growth of SLR24 lesions (Fig. 5A), despite specific, acute reduction in in situ EphA2 expression 24h after injection with EphrinA1-Fc or mAb 208, but not EphrinB1-Fc (Fig. 5B). The adoptive transfer of 5 × 106 CD8+ T cells (either the anti-EphA2 Clone E883 or the anti-HLA-A2 Clone 2E4 on day 19 post-tumor inoculation) also failed to significantly alter consequent SLR24 lesional growth in vivo (Fig. 5C, 5D). However, combined application of EphrinA1-Fc or mAb208 (on day 18) along with the adoptive transfer of E883 T cells (on day 19), resulted in complete tumor eradication in all treated animals (Fig. 5C, Table 1). In contrast, combined use of EphrinB1-Fc and E883 T cells yielded a tumor growth curve that was indistinguishable from single agent controls, with no animals rejecting their tumors (Fig. 5C). Furthermore, even though the allospecific 2E4 CD8+ T cell clone efficiently kills SLR24 tumor cells in vitro (Fig. 4B), improved efficacy was not observed in combinational approaches using EphA2 agonists and 2E4 T cells (Fig. 5D). This result is consistent with the failure of SLR24 pre-treatment with EphrinA1-Fc or mAb208 to augment tumor sensitivity to 2E4 T cell-mediated lysis in vitro (Fig. 4B).
Table 1.
Combined application of EphA2 agonists and the adoptive transfer of anti-EphA2 CD8+ T cells results in the therapeutic regression of SLR24 tumors in C.B-17 mice.
| Treatment Group | Fraction of Tumor-Free Mice (d44)a |
|---|---|
| Control | 0/15 |
| EphrinA1-Fc only | 0/15 |
| EphrinB1-Fc only | 0/10 |
| mAb208 only | 0/10 |
| Clone E883 only | 0/15 |
| Clone 2E4 only | 0/15 |
| EphrinA1-Fc + Clone E883 | 14/15 |
| EphrinB1-Fc + Clone E883 | 0/10 |
| mAb208 + Clone E883 | 10/10 |
| EphrinA1-Fc + Clone 2E4 | 0/10 |
| EphrinB1-Fc + Clone 2E4 | 0/10 |
| mAb208 + Clone 2E4 | 0/10 |
Aggregate results obtained from 3 independent experiments performed as described in Fig. 5.
DISCUSSION
In the current age of cancer therapy, some of the most promising new therapies include antibodies and small molecule modulators of receptor tyrosine kinases (over)expressed by tumor cells (32). The mechanisms of action associated with therapeutic efficacy are varied (i.e. silencing of receptor signaling, promotion of receptor down-regulation, induction of tumor cell death mediated via ADCC, among others; ref. 32). The results of our studies suggest that additional immune-based mechanisms may be co-operational in the setting of such therapies, and that if optimized, such combinational treatments may yield enhanced clinical benefits to patients with many forms of (EphA2+) cancer.
The major finding of the present study is that the treatment of tumor cells with agonists that promote EphA2 autophosphorylation and proteasomal degradation/processing also result in improved recognition by EphA2-specific CD8+ T-cells both in vitro and in vivo. As a consequence, moderate-to-low functional avidity (i.e. approximately 1 μM ED50 for peptide recognition on T2 cells) EphA2-reactive CD8+ T cells are rendered more effective in reacting against, and mediating the regression of, EphA2+ tumor lesions in vivo. Notably, EphA2 was capable of yielding epitopes (EphA258-66 and EphA2883-891, located in the ECD and ICD of the target protein, respectively) which could consequently be presented by MHC Class I molecules for extended periods of time. However, the intermediate steps involved in this mechanism remain unclear, but likely depend upon alternate, non-classical mechanisms of antigen processing (33). Cytosolic proteins generally are thought to be the primary substrates for the proteasome, and are fed into the ER via TAP1/TAP2 transporters (33, 34). In contrast, EphA2 is a transmembrane protein, generally poised for degradation by lysosomes after ligand-induced internalization (35). It remains unknown whether the cohort of EphA2 protein associated with agonist-enhanced T cell recognition derives from EphA2 molecules that are retrotransported into the ER, or deposited into the cytoplasm via a loss of endocytic vesicle integrity or a “ratcheting” mechanism applied to ubiquitinated transmembrane protein substrates (33, 36–38). We are currently undertaking pharmacologic studies to begin to delineate such intermediate steps. A better understanding of the mechanism(s) involved in agonist-induced EphA2 molecule processing may allow for the accentuation of relevant pathways, allowing for even greater enhancement in therapeutic immune recognition of EphA2+ tumor cells.
The ability of agonistic reagents to conditionally trigger the proteasome-dependent degradation of overexpressed EphA2 molecules on tumor cells in vivo may provide opportunities for the development of new combinational therapeutic strategies for the treatment of patients with EphA2+ cancers. In particular, our present results suggest the potential therapeutic benefits of using “off-the-shelf” agonists to sensitize EphA2+ tumor cells to anti-EphA2 CD8+ T cells that could be pre-activated via specific immunization (15, 19) or provided by the adoptive transfer of antigen-specific ex vivo expanded, autologous CD8+ T cell populations. At present, however, many questions remain unanswered with regard to the optimal implementation of such a treatment strategy, including: 1) Must a tumor grossly overexpress EphA2 protein (relative to normal epithelia, etc.) in order for agonists to enable modest-to-low avidity anti-EphA2 CD8+ T cells to mediate improved therapeutic benefit?, 2) Can agonist-enhanced CD8+ T cell recognition of tumor cells be further enhanced by the co-application of IFN-α or IFN-γ (i.e. cytokines that upregulate the MHC class I antigen processing machinery; ref. 39), without destroying tumor-presented EphA2 epitopes due to the concomitant activation of the immunoproteasome?; and 3) Will this strategy sensitize normal EphA2+ tissues to the spectre of autoimmune pathology? Based on preliminary data, we can suggest that even tumor cells exhibiting only modestly overexpressed levels of (hypophosphorylated) EphA2 protein appear capable of being sensitized by agonists to specific CD8+ T cells (Fig. 4 and data not shown) and that the EphA2883-891 peptide (i.e. identical sequence occurs in both human and murine EphA2) elicits potent anti-tumor CD8+ T cell responses in the absence of autoimmune pathology in HLA-A2 Tg mice that constitutively express EphA2 protein in normal lung, liver, and kidney cells (20). Furthermore, based on analysis using a web-based algorithm (http://www.imtech.res.in/raghava/propred1/index.html), at least the EphA2883-891 peptide epitope is not predicted to be destroyed by the immunoproteasome. Hence, we believe that combinational immunotherapies targeting EphA2 will prove both safe and effective.
One surprising aspect in our work is that the combinational therapy works or fails in vivo, presumably based on an approximate 2–4 fold increase in tumor cell recognition by anti-EphA2 CD8+ T cells after agonist treatment in vitro. This may suggest that additional mechanisms of action are in play in vivo, only some of which relate to tumor presentation of EphA2 epitopes. Clearly, one might envision that tumor EphA2 processing in vivo could be more efficient than that observed in vitro for a given tumor cell line. We are currently attempting to address this possibility by performing mass spectrometry analyses for the EphA258-66 and EphA2833-891 peptide epitopes extrcated from HLA-A2 complexes of SLR24 tumor cells grown in vitro vs. in vivo +/− agonist treatment for 24h (via addition to culture or i.t. injection). Beyond this mechanism, we have not observed any effects of EphA2 agonists directly on T cells, and indeed, our T cell lines/clones fail to express discernable levels of EphA2 (data not shown). However, it is possible that EphA2 agonists could trigger alterations in production of inflammatory chemokines that would serve to enhance T cell recruitment or survival within the tumor microenvironment. Given the speciation of many chemokines, relevant alterations in our Hu-SCID model would likely be tumor cell (rather than stroma)-dependent. Hence, we are currently investigating whether EphA2 agonists promote alterations in expression of chemokines, such as IP-10, Mig and I-TAC, by SLR24 tumor cells that may facilitate CTL recruitment in vivo. Alternatively or additionally, the enhanced efficacy of the combinational therapy in vivo could relate to the direct targeting of the EphA2+ tumor-associated vasculature (40) by anti-EphA2 T cells after agonist administration. Indeed, we have recently shown that the vaccination of mice with peptides representing CD8+ T cell epitopes derived from mEphA2 protein inhibits the growth of EphA2-negative tumor cells in vivo and limits the neoangiogenesis of Matrigel implants containing VEGF (22). This suggests that combinational treatments using EphA2 agonists and T cell-based immunotherapy will likely have multiple strategic EphA2+ cellular targets within the tumor microenvironment, potentially opening patient accrual to individuals harboring any form of vascularized tumor.
Acknowledgments
The authors wish to thank Drs. Hassane Zarour, William Chambers and Russ Salter for their careful review and helpful comments provided during the preparation and revision of this manuscript.
Non-standard abbreviations used in this paper
- ECD
Extracellular Domain
- ECM
Extracellular Matrix
- FMOC
N-(9-fluorenyl)methoxycarbonyl
- ICD
Intracellular Domain
- RTK
Receptor Tyrosine Kinase
Footnotes
This work was supported by a National Institutes of Health (NIH) Immunology Training Grant 5T32 CA82084 (C.J.H.) and NIH R01 grant CA 114071 (W.J.S.).
Non-standard abbreviations used in this paper: ECD = Extracellular Domain; ECM = Extracellular Matrix; FMOC = N-(9-fluorenyl)methoxycarbonyl; ICD = Intracellular Domain; RTK = Receptor Tyrosine Kinase
REFERENCES CITED
- 1.Murai KK, Pasquale EB. ‘Eph’ective signaling: forward, reverse and crosstalk. J Cell Sci. 2003;116:2823–2832. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- 2.Lindberg RA, Hunter T. cDNA cloning and characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases. Mol Cell Biol. 1990;10:6316–6324. doi: 10.1128/mcb.10.12.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 1999;10:629–638. [PubMed] [Google Scholar]
- 4.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. [PubMed] [Google Scholar]
- 5.Walker-Daniels J, Coffman K, Azimi M, Rhim JS, Bostwick DG, Snyder P, Kerns BJ, Waters DJ, Kinch MS. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. 1999;41:275–280. doi: 10.1002/(sici)1097-0045(19991201)41:4<275::aid-pros8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Abraham S, Knapp DW, Cheng L, Snyder PW, Mittal SK, Bangari DS, Kinch M, Wu L, Dhariwal J, Mohammed SI. Expression of EphA2 and EphrinA1 in carcinoma of the urinary bladder. Clin Cancer Res. 2006;12:353–360. doi: 10.1158/1078-0432.CCR-05-1505. [DOI] [PubMed] [Google Scholar]
- 7.Saito T, Masuda N, Miyazaki T, Kanoh K, Suzuki H, Shimura T, Asao T, Kuwano H. Expression of EphA2 and E-cadherin in colorectal cancer: correlation with cancer metastasis. Oncol Rep. 2004;11:605–611. [PubMed] [Google Scholar]
- 8.Miyazaki T, Kato H, Fukuchi M, Nakajima M, Kuwano H. EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int J Cancer. 2003;103:657–663. doi: 10.1002/ijc.10860. [DOI] [PubMed] [Google Scholar]
- 9.Kinch MS, Moore MB, Harpole DH., Jr Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res. 2003;9:613–618. [PubMed] [Google Scholar]
- 10.D’Amico TA, Aloia TA, Moore MB, Conlon DH, Herndon JE, 2nd, Kinch MS, Harpole DH., Jr Predicting the sites of metastases from lung cancer using molecular biologic markers. Ann Thorac Surg. 2001;72:1144–1148. doi: 10.1016/s0003-4975(01)02979-4. [DOI] [PubMed] [Google Scholar]
- 11.Hendrix MJ, Seftor EA, Kirschmann DA, Quaranta V, Seftor RE. Remodeling of the microenvironment by aggressive melanoma tumor cells. Ann NY Acad Sci. 2003;995:151–161. doi: 10.1111/j.1749-6632.2003.tb03218.x. [DOI] [PubMed] [Google Scholar]
- 12.Nasreen N, Mohammed KA, Antony VB. Silencing the receptor EphA2 suppresses the growth and haptotaxis of malignant mesothelioma cells. Cancer. 2006;107:2425–2435. doi: 10.1002/cncr.22254. [DOI] [PubMed] [Google Scholar]
- 13.Lin YG, Han LY, Kamat AA, Merritt WM, Landen CN, Deavers MT, Fletcher MS, Urbauer DL, Kinch MS, Sood AK. EphA2 expression is associated with angiogenesis in ovarian cancer. Cancer. 2007;109:332–340. doi: 10.1002/cncr.22415. [DOI] [PubMed] [Google Scholar]
- 14.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene. 2004;23:1448–1156. doi: 10.1038/sj.onc.1207247. [DOI] [PubMed] [Google Scholar]
- 15.Tatsumi T, Herrem CJ, Olson WC, Finke JH, Bukowski RM, Kinch MS, Ranieri E, Storkus WJ. Disease stage variation in CD4+ and CD8+ T-cell reactivity to the receptor tyrosine kinase EphA2 in patients with renal cell carcinoma. Cancer Res. 2003;63:4481–4489. [PubMed] [Google Scholar]
- 16.Herrem CJ, Tatsumi T, Olson KS, Shirai K, Finke JH, Bukowski RM, Zhou M, Richmond AL, Derweesh I, Kinch MS, Storkus WJ. Expression of EphA2 is prognostic of disease-free interval and overall survival in surgically-treated patients with renal cell carcinoma. Clin Cancer Res. 2005;11:226–231. [PubMed] [Google Scholar]
- 17.Carles-Kinch K, Kilpatrick KE, Stewart JC, Kinch MS. Antibody targeting of the EphA2 tyrosine kinase inhibits malignant cell behavior. Cancer Res. 2002;62:2840–2847. [PubMed] [Google Scholar]
- 18.Dobrzanski P, Hunter K, Jones-Bolin S, Chang H, Robinson C, Pritchard S, Zhao H, Ruggeri B. Antiangiogenic and antitumor efficacy of EphA2 receptor antagonist. Cancer Res. 2004;64:910–919. doi: 10.1158/0008-5472.can-3430-2. [DOI] [PubMed] [Google Scholar]
- 19.Alves PM, Faure O, Graff-Dubois S, Gross DA, Cornet S, Chouaib S, Miconnet I, Lemonnier FA, Kosmatopoulos K. EphA2 as target of anticancer immunotherapy: identification of HLA-A*0201-restricted epitopes. Cancer Res. 2003;63:8476–8480. [PubMed] [Google Scholar]
- 20.Hatano M, Eguchi J, Tatsumi T, Kuwashima N, Dusak JE, Kinch MS, Pollack IF, Hamilton RL, Storkus WJ, Okada H. EphA2 as a glioma-associated antigen: a novel target for glioma vaccines. Neoplasia. 2005;7:717–722. doi: 10.1593/neo.05277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaguchi S, Tatsumi T, Takehara T, Sakamori R, Uemura A, Mizushima T, Ohkawa K, Storkus WJ, Hayashi N. Immunotherapy of murine colon cancer using receptor tyrosine kinase EphA2-derived peptide-pulsed dendritic cell vaccines. Cancer. 2007;110:1469–1177. doi: 10.1002/cncr.22958. [DOI] [PubMed] [Google Scholar]
- 22.Hatano M, Kuwashima N, Tatsumi T, Dusak JE, Nishimura F, Reilly KM, Storkus WJ, Okada H. Vaccination with EphA2-derived T cell-epitopes promotes immunity against both EphA2-expressing and EphA2-negative tumors. J Transl Med. 2004;2:40. doi: 10.1186/1479-5876-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mapara MY, Sykes M. Tolerance and cancer: mechanisms of tumor evasion and strategies for breaking tolerance. J Clin Oncol. 2004;22:1136–1151. doi: 10.1200/JCO.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 24.Salter RD, Howell DN, Cresswell P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21:235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- 25.Kierstead LS, Ranieri E, Olson W, Brusic V, Sidney J, Sette A, Kasamon YL, Slingluff CL, Jr, Kirkwood JM, Storkus WJ. gp100/pmel17 and tyrosinase encode multiple epitopes recognized by Th1-type CD4+ T cells. Br J Cancer. 2001;85:1738–1745. doi: 10.1054/bjoc.2001.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuting T, Wilson CC, Martin DM, Kasamon YL, Rowles J, Ma DI, Slingluff CL, Jr, Wagner SN, van der Bruggen P, Baar J, Lotze MT, Storkus WJ. Autologous human monocyte-derived dendritic cells genetically modified to express melanoma antigens elicit primary cytotoxic T cell responses in vitro: enhancement by cotransfection of genes encoding the Th1-biasing cytokines IL-12 and IFN-a. J Immunol. 1998;160:1139–1147. [PubMed] [Google Scholar]
- 27.Stam NJ, Vroom TM, Peters PJ, Pastoors EB, Ploegh HL. HLA-A- and HLA-B-specific monoclonal antibodies reactive with free heavy chains in western blots, in formalin-fixed, paraffin-embedded tissue sections and in cryoimmunoelectron microscopy. Int Immunol. 1990;2:113–125. doi: 10.1093/intimm/2.2.113. [DOI] [PubMed] [Google Scholar]
- 28.Itoh T, Storkus WJ, Gorelik E, Lotze MT. Partial purification of murine tumor-associated peptide epitopes common to histologically distinct tumors, melanoma and sarcoma, that are presented by H-2Kb molecules and recognized by CD8+ tumor-infiltrating lymphocytes. J Immunol. 1994;153:1202–1215. [PubMed] [Google Scholar]
- 29.Kinch MS, Carles-Kinch K. Overexpression and functional alterations of the EphA2 tyrosine kinase in cancer. Clin Exp Metastasis. 2003;20:59–68. doi: 10.1023/a:1022546620495. [DOI] [PubMed] [Google Scholar]
- 30.Coffman KT, Hu M, Carles-Kinch K, Tice D, Donacki N, Munyon K, Kifle G, Woods R, Langermann S, Kiener PA, Kinch MS. Differential EphA2 epitope display on normal versus malignant cells. Cancer Res. 2003;63:7907–7912. [PubMed] [Google Scholar]
- 31.Bogenrieder T, Herlyn M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene. 2003;22:6524–6536. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- 32.Yan L, Hsu K, Beckman RA. Antibody-based therapy for solid tumors. Cancer J 2008. 2008;14:178–83. doi: 10.1097/PPO.0b013e318172d71a. [DOI] [PubMed] [Google Scholar]
- 33.Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol Rev. 2005;207:145–57. doi: 10.1111/j.0105-2896.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 34.York IA, Rock KL. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14:369–396. doi: 10.1146/annurev.immunol.14.1.369. [DOI] [PubMed] [Google Scholar]
- 35.Wiley HS, Burke PM. Regulation of receptor tyrosine kinase signaling by endocytic trafficking. Traffic. 2001;2:12–18. doi: 10.1034/j.1600-0854.2001.020103.x. [DOI] [PubMed] [Google Scholar]
- 36.Marmor MD, Yarden Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene. 2004;23:2057–2070. doi: 10.1038/sj.onc.1207390. [DOI] [PubMed] [Google Scholar]
- 37.Nakatsukasa K, Huyer G, Michaelis S, Brodsky JL. Dissecting the ER-associated degradation of a misfolded polytopic membrane protein. Cell. 2008;132:101–112. doi: 10.1016/j.cell.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonifacino JS, Weissman AM. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Ann Rev Cell Dev Biol. 1998;14:19–57. doi: 10.1146/annurev.cellbio.14.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.David-Watine B, Israel A, Kourilsky P. The regulation and expression of MHC class I genes. Immunol Today. 1990;11:286–292. doi: 10.1016/0167-5699(90)90114-o. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19:6043–6052. doi: 10.1038/sj.onc.1204004. [DOI] [PubMed] [Google Scholar]