Abstract
We and others have previously demonstrated that (chronic) IL-12 gene therapy delivered intratumorally via ex vivo gene-engineered DC is competent to promote the regression of established murine tumors. In this report, we have developed a conditional expression system (rAd.RheoIL12) in order to determine the temporal requirements of transgenic IL-12p70 production by administered DC on therapeutic outcome in a subcutaneous B16 melanoma model. DC infected with rAd.RheoIL12 (DC.RheoIL12) secreted IL-12p70 in a tightly-regulated fashion in response to a synthetic diacylhydrazine small molecule ligand in vitro, and the treatment benefit of DC.RheolIL12 delivered into B16 lesions was strictly ligand-dependent in vivo. Indeed, DC.RheoIL12-based therapy promoted the regression of established day 7 B16 tumor lesions after intratumoral injection, provided that ligand administration occurred within 24h of DC injection and was sustained for approximately 5 or more days. Treatment efficacy was correlated to the magnitude of systemic anti-B16 CD8+ T cells cross-primed in vivo, which in turn, appeared dependent on the early enhanced in vivo survival of adoptively-transferred DC.RheoIL12 in tumor and tumor-draining lymph nodes. The unique safety feature of DC.RheoIL12 application was emphasized in a combined treatment model with rIL-2, where profound TNF-α-associated toxicity could be ameliorated upon discontinuation of activating ligand administration.
Keywords: melanoma, gene therapy, IL-12, conditional, DC
INTRODUCTION
IL-12 remains a promising cancer therapeutic agent based on its potent supportive activity on Type-1 anti-tumor NK, CD4+ T cells and CD8+ T cells (1). However, given the reported toxicity of recombinant human IL-12 (rhIL-12) in patients (2), and limited sources of GMP-grade rhIL-12 for clinical application, gene therapy approaches may represent safer, more tenable treatment options. Indeed, phase I clinical trials implementing intra- or peri-tumoral delivery of recombinant viral- (3, 4) or plasmid- (5) based IL-12 cDNA, or IL-12 gene modified autologous fibroblasts (6) or DC (7) have been found both safe and well-tolerated. However, objective clinical responses in patients with melanoma or a diverse range of carcinomas receiving these gene therapies have been rare, variable (between disease histologies), transient and largely focused at the site of treatment (3–6). In cases where disease resolution was partial or complete, increased frequencies of tumor-infiltrating lymphocytes (4, 5) and elevated levels of circulating tumor-specific CD8+ T cells (5) were observed, consistent with the improved cross-priming of specific immunity in these patients.
The cross-priming of specific T cells appears to be best accomplished by DC that serve as a natural, but regulated, source of IL-12 (8), with recent reports suggesting the superior pre-clinical efficacy of DC-based IL-12 gene therapy (9–11). In particular, we have shown that intratumoral (i.t.) injection of DC engineered to produce IL-12p70 (via recombinant adenovirus infection) results in the dramatically improved cross-priming of a broadly-reactive, tumor-specific CD8+ T cell repertoire in concert with tumor rejection in a murine sarcoma model (11).
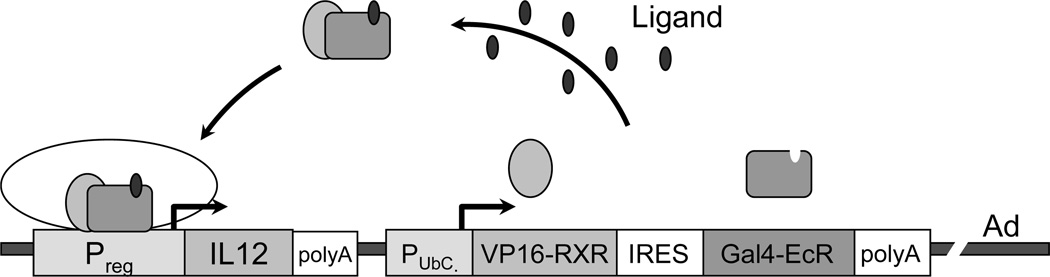
As this previous system used a recombinant adenovirus encoding mIL-12 under a CMV-based promoter (rAd.cIL12; ref. 11) to engineer DC, where IL-12p70 production was constitutive, the immunologic impact of this cytokine on early (i.e. within the tumor lesion) vs. late (i.e. within tumor-draining lymph nodes or the tumor at the time of primed T cell infiltration, etc.) immune events could not be resolved. In an attempt to address this issue, we have developed the novel RheoSwitch® Therapeutic System (RTS) that was incorporated into a rAd vector (rAd.RheoIL12) for expression of mIL-12p70 driven off a promoter that is conditionally activated by a small molecule diacylhydrazine ligand (12). Using this system, we could effectively turn on/off transgene expression in engineered DC (DC.RheoIL12) after i.t. injection (by regulating ligand administration) and investigate the temporal requirements of ectopic IL-12p70 production for optimal treatment safety and efficacy.
MATERIALS AND METHODS
Mice
Female 6–8 week old C57BL/6 wild-type and C57BL/6-TgN(ACTbEGFP)1Osb/J EGFP Tg mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in micro-isolator cages. Animals were handled under aseptic conditions per an Institutional Animal Care and Use Committee (IACUC)-approved protocol and in accordance with recommendations for the proper care and use of laboratory animals.
Cell Lines
The B16 melanoma, MC38 colon carcinoma and EL-4 thymoma H-2b cell lines, syngenic to C57BL/6 mice have been described previously (13). Cell lines were maintained in CM [RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, 100 µg/ml streptomycin, and 10 mM L-glutamine; all reagents from Invitrogen, Carlsbad, CA] in a humidified incubator at 5% CO2 and 37°C.
Generation of DC
DC were generated from murine bone marrow, as previously described (11). Briefly, wild-type or EGFP Tg mouse BM was cultured in CM supplemented with 1000 units/ml recombinant murine granulocyte/macrophage colony-stimulating factor and recombinant mIL-4 (Peprotech, Rocky Hill, NJ) at 37°C in a humidified, 5% CO2 incubator for 7 days. CD11c+ DCs were then isolated using specific MACS™ beads, per the manufacturer’s protocol (Miltenyi Biotec, Auburn, CA). CD11c+ DCs produced in this manner were >95% pure based on morphology and co-expression of the CD11b, CD40, CD80, and class I and class II MHC Ags (data not shown).
Viral Vectors
The control adenoviral vector rAd.ψ5 and rAd.cIL12, encoding mIL-12 driven off a CMV promoter, were produced and provided by the University of Pittsburgh Cancer Institute’s Vector Core Facility and have been described previously (11). The rAd.RheoIL12 vector was produced in the following manner. The coding sequences for VP16-RXR and Gal4-EcR separated by the EMCV internal ribosome entry site (IRES) sequence were inserted into the adenoviral shuttle vector under the control of the human ubiquitin C promoter. Subsequently, the coding sequences for the p40 and p35 subunits of mIL12 separated by IRES sequence, placed under the control of a synthetic inducible promoter, were inserted upstream of the ubiquitin C promoter. The shuttle vector carrying these transcription units for the two fusion proteins and inducible IL12 subunits was recombined with the Adenoviral backbone (AdEasy1; Stratagene, La Jolla, CA) in E.coli BJ5183 cells. After verifying the recombinant clone, the plasmid carrying the rAd.RheoIL12 genome was grown in and purified from XL10-Gold cells, digested off the plasmid backbone and was then packaged by transfection into HEK293 cells. The resulting primary viral stock was amplified by re-infection of HEK293 cells and was purified by CsCl density-gradient centrifugation. In brief, the RTS (12) incorporates two fusion proteins, the DEF domains of a mutagenized ecdysone receptor (EcR) fused with a Gal4 DNA binding domain and the EF domains of a chimeric RXR fused with a VP16 transcription activation domain, expressed under a constitutive promoter (Fig. 1). The binding of the small-molecule (pharmacologically-inert diacylhydrazine) ligand (12) to EcR causes heterodimerization between the two fusion proteins, forming an active transcription factor that induces expression of the mIL12 subunit genes placed under the control of an inducible promoter containing Gal4-binding sites.
Figure 1. Conditional production of IL-12p70 in DC using a recombinant adenovirus incorporating the RheoSwitch® Therapeutic System (RTS).
In DC infected with Ad.RheoIL12 (see Materials and Methods and (11) for construction details), the Gal4-EcR and VP16-RXR fusion proteins are expressed under the constitutive Ubiquitin C promoter. These proteins form heterodimers in the presence of the activator ligand, resulting in the conditional activation of mIL12p70 transcription (from a responsive/inducible promoter). The box labeled “IL12” represents the IL-12p40 and IL-12p35 coding sequences separated by IRES.
mIL-12p70 ELISA
Day 7 cultured DCs were untreated, were infected with recombinant Ads encoding murine IL-12p70 driven off a constitutive (rAd.cIL12) or inducible (rAd.RheoIL12) promoter, or were infected with mock, control vector rAdψ5, over a range of MOIs for 48h. At various time points after this (0 – 48h), DC were then cultured in the absence or presence of the activating ligand (10 – 200 ng/ml; ref. 12) for an additional 24h prior to analysis of IL-12p70 secretion using a specific ELISA kit (BD-Bioscience, San Diego, CA; lower level of detection = 62.5 pg/ml). In some cases, to discern the stringency of conditional cytokine production, DC infected with rAd.RheoIL12 (i.e. DC.RheoIL12), that had been pretreated for with ligand, were washed free of ligand and cultured in control media for an additional 24h prior to analysis of IL-12p70 secretion.
Flow Cytometry
For phenotypic analysis of adenovirus infected DCs, PE- or FITC-conjugated mAbs against mouse cell surface molecules [CD11b, CD11c, CD40, CD54, CD80, CD86, H-2Kd, I-Ad (all from BD-Bioscience)] and appropriate isotype controls were used, and flow cytometric analysis was performed using a FACscan (Becton Dickinson, San Jose, CA) flow cytometer.
B16 Tumor Model
B6 mice received s.c. injection with 1 × 105 B16 melanoma cells in the right flank on day 0. On day 7, tumors reached a size of approximately 20–30 mm2 and mice were treated with i.t. injections of PBS or 1 × 106 control vs. adenoviral transduced (MOI = 100) DCs in a total volume of 50 µl of PBS. Mice also received i.p. injections of 30–50 mg/kg ligand (in 50 µl DMSO) vs. DMSO carrier control that were initiated at 0h, 24h or 48h post-DC administration, as indicated. After initiation, mice received a total of 5 consecutive daily i.p. injections of activator ligand at this dose. In additional experiments, ligand was administered beginning on the day of DC injection and then terminated 1, 3 or 5 days post-DC injection to discern whether early cessation of IL-12p70 transgene promotion reduced the therapeutic benefits of this approach. In all cases, tumor size was assessed every 3 or 4 days and recorded in mm2 by determining the product of the largest perpendicular diameters measured by vernier calipers. In indicated experiments, animals rendered tumor-free (45 days) post-therapy were rechallenged with the B16 melanoma (105 cells injected on the left flank, i.e. contralateral to the original B16 challenge site) and MC38 colon carcinoma (105 cells on the right flank) cells in order to discern the presence and specificity of memory immunity in these mice. All data are reported as the average tumor area ± SD. All animal cohorts contained 5 mice/group.
Imaging of Injected DC
To assess the fate and function of injected DCs, we generated day 7 BM-derived, CD11c+ DCs from C57BL/6-TgN(ACTbEGFP)1Osb/J EGFP Tg mice. EGFP+ CD11c+ DC were left uninfected or they were infected with rAd viruses, as indicated above. Forty-eight hours after infection, 1 × 106 control or virally-infected DCs were harvested, washed in PBS, and injected into day 7 B16 tumor lesions established in syngeneic B6 mice. Three days after DC injection, tumors and draining inguinal lymph nodes (LN) were resected, fixed for 1h in 2% paraformaldehyde (in PBS), and then cryoprotected in 30% sucrose in PBS before being shock frozen in liquid nitrogen-cooled isopentane. Five micron frozen sections were then generated and counterstained with 2 µg/ml Hoechst 33258 (Sigma-Aldrich, St. Louis, MO) for 3 min. The washed sections were then mounted in Gelvatol (Monsanto Chemical Co., St. Louis, MO) and observed using an Olympus BX51 microscope equipped with a cooled charge-coupled device color camera.
Assessment of specific CD8+ T cell responses against B16 melanoma
Pooled CD8+ T cells were isolated to a purity of >95% from the spleens of 2 treated mice/group 25 days after tumor inoculation using magnetic bead cell sorting (MACS™; Miltenyi Biotec) and then co-cultured (1 × 105/well) with 1 × 104 irradiated (100 Gy) B16 or EL-4 tumor cells. After 48h incubation, culture supernatants were collected and analyzed for IFN-γ release using a commercial ELISA (BD-Bioscience) with a lower limit of detection of 31.5 pg/ml. Data are reported as the mean ± SD of triplicate determinations.
Toxicity Model
The ability to ameliorate toxicity associated with DC.RheoIL12-based treatment via agonist ligand removal was assessed in an IL-2 co-administration model based on a report by Carson et al. (14). Female 6–8 week old C57BL/6 wild-type mice (n = 5 mice/group) were injected i.p. with 106 DC.RheoIL12 (or control DC.ψ5) and co-treated with daily i.p. injections of rhIL-2 (3 × 105 IU/day in PBS; Peprotech) or PBS. Activating ligand (30 mg/kg/day in 50 µl DMSO) was injected i.p. daily beginning on the day of DC administration for up to 7 days as indicated. Mice were monitored daily for toxicity. In addition, peripheral blood was obtained daily via tail-vein venipuncture for analysis of serum TNF-α levels using a commercial ELISA (eBioscience; San Diego, CA) with a lower limit of detection of 8 pg/ml, per the manufacturer’s instructions.
Statistical Analyses
All experiments were analyzed as previously described (11), with comparisons yielding p values < 0.05 considered significant.
RESULTS
Murine BM-derived DC infected with Rheo-IL12 conditionally produce high levels of IL-12p70 when treated with ligand in vitro
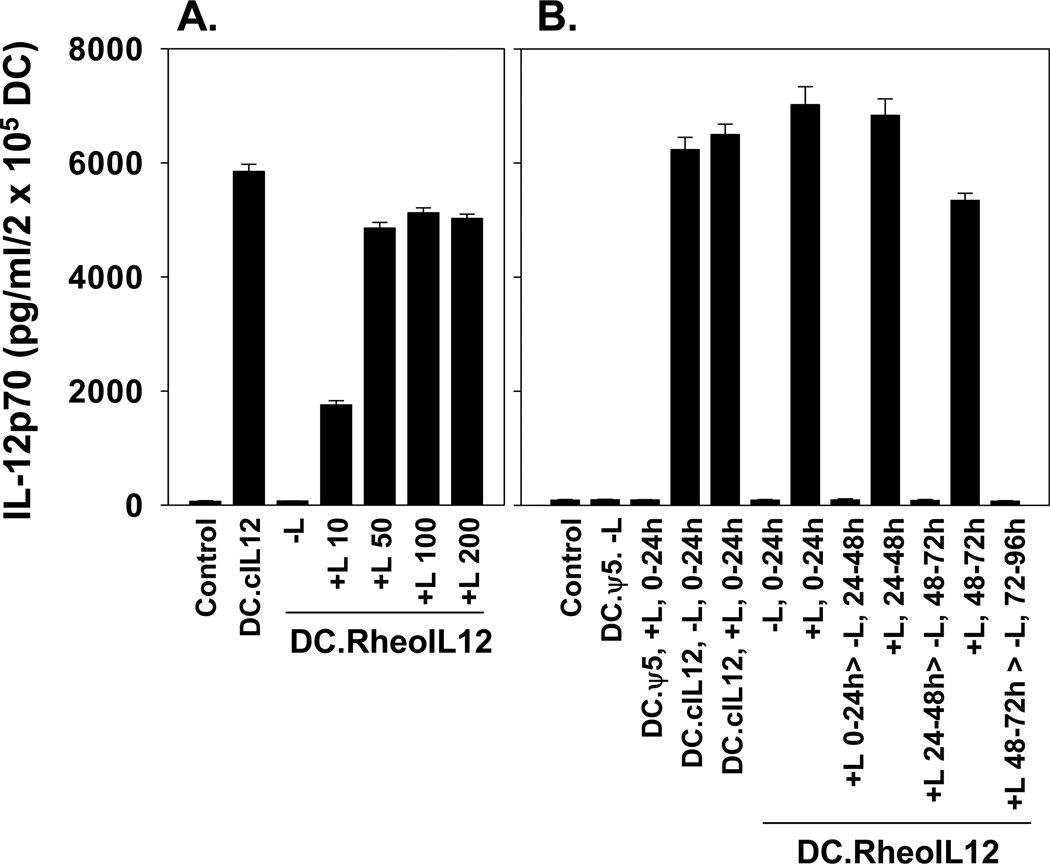
DC cultured from C57BL/6 (B6) mouse BM for 7 days in the presence of rmIL-4 and rmGM-CSF were left untreated, or infected at various MOIs with control rAd.ψ5, rAd.cIL-12 (encoding mIL-12p70 under a constitutive CMV promoter) or rAd.RheoIL12 (encoding IL-12 p70 under a conditional promoter responsive to the small molecule ligand; Fig.1). Forty-eight hours after infection, DCs were cultured in the absence or presence of ligand for an additional 24h, at which time, culture supernatants were harvested for quantitation of IL-12p70 production by ELISA. As shown in Fig. 2, control uninfected DC or DC infected with Ad.ψ5 in the absence or presence of exogenous drug failed to produce elevated levels of IL-12p70 when compared with DC infected with rAd.cIL12 (DC.cIL12). DC infected with rAd.RheoIL-12 (DC.RheoIL12) only produced IL-12p70 after treatment with activating ligand. Based on the results of “criss-cross” experiments, optimal infected DC production of IL-12p70 appears to occur using an MOI of 100, with cells treated with 50–100 ng/ml activating ligand (Fig.2A and data not shown). Delayed provision of ligand to DC.RheoIL12 for up to 48h did not result in any significant reduction in IL-12p70 production when compared to addition of ligand at the 0h time point in vitro (Fig.2B). Finally, removal of ligand acutely silenced the ability of DC.RheoIL12 (previously activated by ligand) to continue to produce elevated levels of IL-12p70 in vitro (Fig. 2B).
Figure 2. DC.RheoIL12 conditionally secrete high levels of IL-12p70 in response to activator ligand in vitro.
(A.) Day 7 cultured, BM-derived CD11c+ DC were infected with no virus, rAd.ψ5 control virus, rAd.cIL12 virus or rAd.RheoIL12 virus at MOI = 100 for 48h. Ligand was then added to cultures, as indicated, over a range of doses 10–200 ng/ml for an additional 24h, at which time culture supernatants were harvested and IL-12p70 levels quantitated by specific ELISA. (B.) Ligand was provided at various time points after rAd infection of DC. In all DC.RheoIL12 cohorts receiving ligand, supernatants were harvested 24h after provision of the drug. Washout of ligand after 24h of ligand activation, resulted in the loss of IL-12p70 secretion from DC.RheoIL12 over a consequent 24h period. Data are reported as the mean ± SD of triplicate determinations and are representative of 3 independent experiments performed in each instance.
Intratumoral administration of DC.cIL12 alone or DC.RheoIL12 combined with i.p. administration of activator ligand promotes the regression of established s.c. B16 melanoma lesions
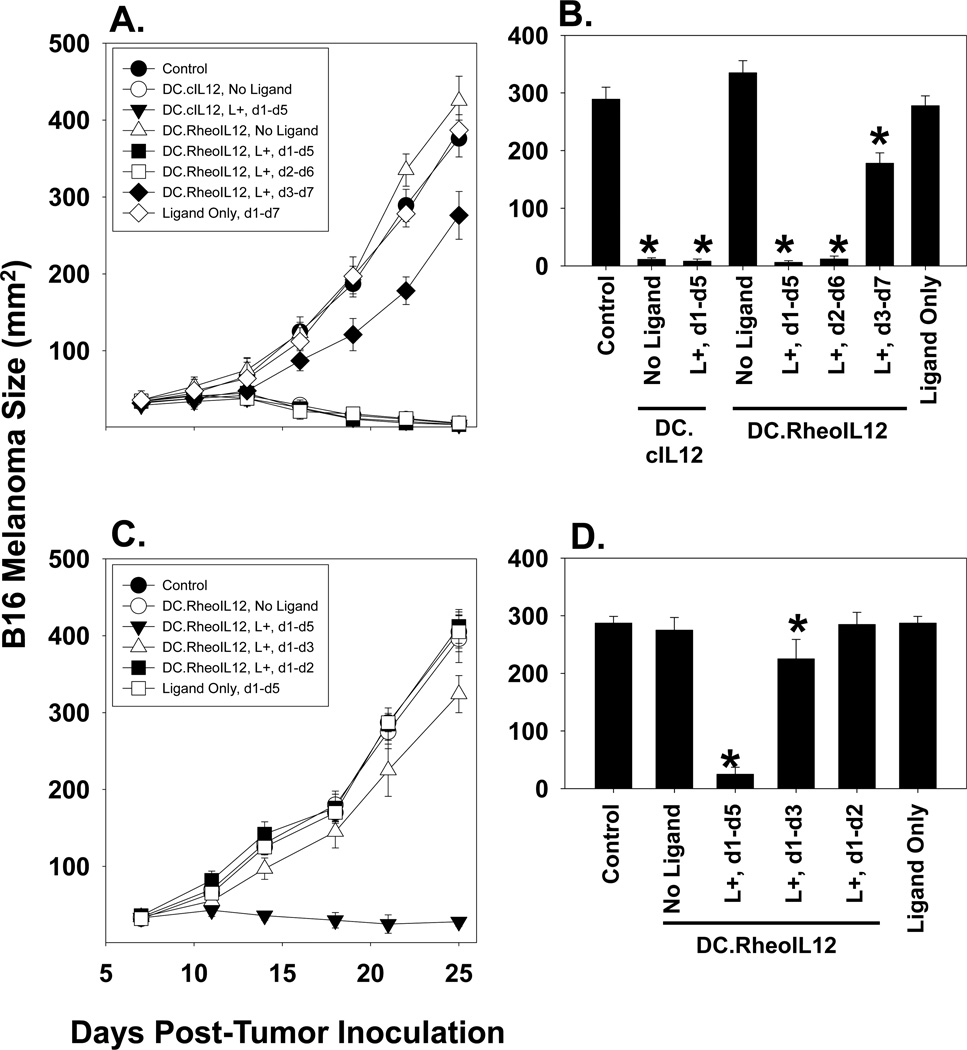
B16 melanoma cells (1 × 105) were injected s.c. in the right flank of syngenic H-2b B6 mice and allowed to establish. On day 7, mice were randomized into cohorts of 5 animals each, with a mean cohort tumor size of approximately 20–30 mm2. Mice then received intratumoral (i.t.) injections of PBS or 106 DC (pre-infected ex vivo for 48h with rAd.ψ5, rAd.cIL12 or rAd.RheoIL12) in a total volume of 50 µl PBS. Animals also received DMSO or activator ligand (in DMSO) i.p. injections at the time of DC administration (i.e. day 1 of treatment), or at 24h (i.e. day 2 of treatment) or 48h (i.e. day 3 of treatment) post-DC administration. As depicted in Fig. 3A and 3B, the treatment of mice with ligand alone or DC.RheoIL12 in the absence of ligand failed to yield any therapeutic benefit. In marked contrast, tumors treated with DC.cIL-12 or DC.RheoIL-12 (in concert with a 5 day course of ligand administration) regressed in size over the following 3 weeks, following ligand activation within 24h of DC injection (Fig. 3A, 3B). Indeed, these therapies were statistically indistinguishable based on tumor size measurements, and yielded 100% (5/5 mice) tumor regression rates in each of these instances. Interestingly, if ligand administration was delayed until 48h after i.t. DC injection (at a time point when this agent can still efficiently induce IL-12p70 production from DC.RheoIL-12 in vitro, i.e. Fig. 2B), DC.RheoIL12-based therapy resulted in only slight inhibition of tumor growth rate (p< 0.05 for all time points after day 10), and all animals exhibited progressive lesions that required sacrifice by day 30. This suggests that therapeutic benefit of i.t. DC.IL12 treatment is dependent upon IL-12p70 production at early time points (occurring presumably within the tumor lesion and/or draining lymph nodes).
Figure 3. DC.RheoIL12 i.t. therapy promotes tumor regression if activator ligand is provided i.p. within 24h of DC administration.
C57Bl/6 mice bearing established 7 day B16 s.c. tumors were injected with no DC or 106 CD11c+ DC (control, DC.cIL12, DC.RheoIL12) as indicated. In panel A., activator ligand (in 50 µl DMSO) or control DMSO was injected i.p. on a daily basis for the indicated periods of time (i.e. d1-d5 refers to ligand injection beginning on the day of DC administration (d1) for 5 consecutive days). Tumor growth was then monitored every 3 days until tumors became ulcerated or attained a size of approximately 400 mm2, at which time they were sacrificed. All animals in which treated tumors regressed after day 10 were ultimately cured of disease. Panel A data on day 22 are plotted in a histogram format in panel B, * p < 0.05 vs. control. In panel C., mice bearing day 7 B16 lesions were injected with no DC or 106 CD11c+ DC.RheoIL12 in the absence or presence of co-delivered activator ligand (provided over days 1–2, days 1–3 or days 1–5). Panel C data on day 21 are plotted in a histogram format in panel D, *p < 0.05 vs. control. All cohorts contained 5 mice/group, with data reported as the mean tumor size ± SD. Data are representative of 2 independent experiments performed.
Additional experiments were performed in which activator ligand was administered to DC.RheoIL-12 injected mice for 1, 3 or 5 days post-DC injection (Fig. 3C, 3D). The results of these studies suggest that early termination of ligand administration impacts the anti-tumor efficacy of i.t. delivered DC.RheoIL-12, with inhibition of tumor growth limited or ablated if ligand is not provided to mice for approximately 5 or more days after provision of gene-modified DC. These findings are consistent with data provided in Fig. 2B, and support the tight (ligand-dependent) regulation of therapeutic impact resulting from injected DC.RheoIL-12 in this model. Furthermore, when taken together, the results depicted in Fig. 3 strongly suggest that optimal anti-melanoma efficacy associated with i.t. delivery of DC.RheoIL-12 results from provision of the ligand during the day 1–5 period post-DC injection into B16 tumors.
Delayed activation of conditional DC.RheoIL12 therapy appears ineffective due to the apparent failure of injected DC to survive in vivo
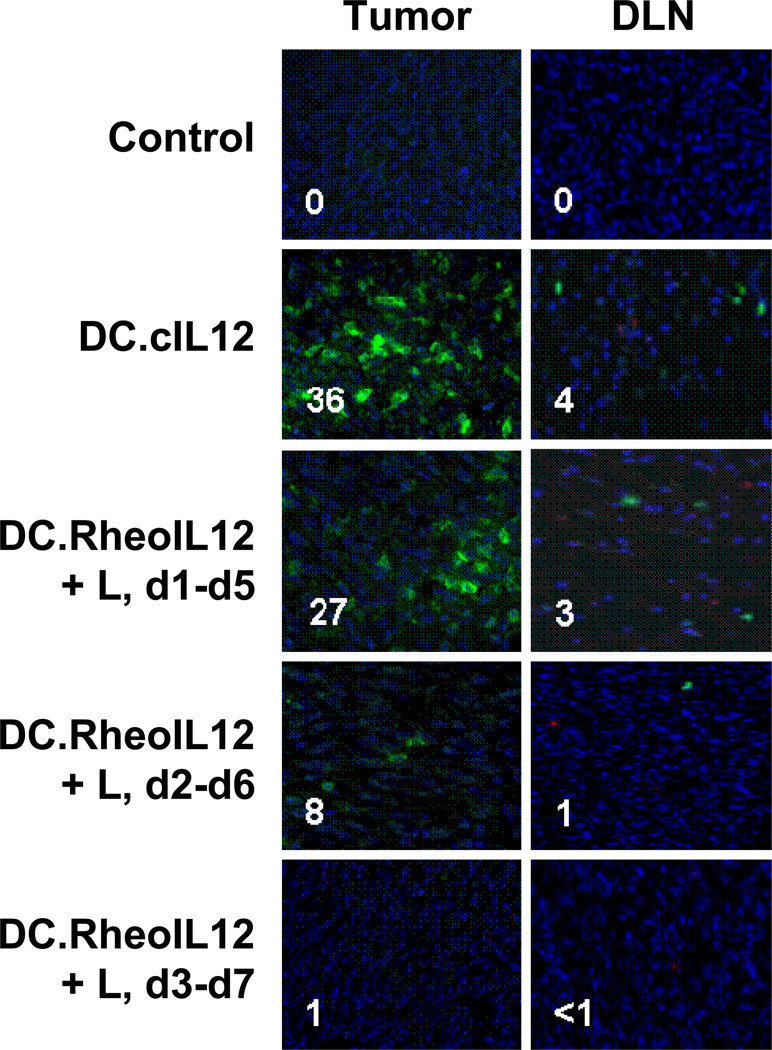
Our previous report (10) suggested that IL-12 gene insertion into DC promotes the enhanced survival of these cells after injection into the tumor microenvironment and the consequent capacity of these cells to cross-prime anti-tumor CD8+ T cells and conceivably recruit circulating effector T cells into the tumor microenvironment in vivo. Hence, we next attempted to discriminate whether unsuccessful DC-RheoIL12 therapy initiated (by i.p. ligand administration) 48h after DC injection was due to the inability of DC to persist in the tumor microenvironment, the inability of these cells to traffic to tumor-draining LN and/or the inability of specific CD8+ T cells to be cross-primed as a result of treatment. Experiments as outlined in Fig. 3A were recapitulated, with 2 mice per cohort sacrificed 72h after intratumoral injection of DC, with the exception that EGFP Tg (H-2b) mice were used as the source of bone marrow for DC generation. Tumor and LN were resected and tissue sections prepared for analysis of EGFP+ DC by fluorescence microscopy. The remaining 3 animals/cohort were followed until day 25, when they were sacrificed and pooled splenocytes isolated for analysis of B16-specific CD8+ T cell responses.
As depicted in Fig. 4, the ability to resolve EGFP+ DC in tumor or LN 72h after i.t. injection was dependent on the activation of the IL-12 transgene within 24–48h of in vivo administration of these cells. EGFP+ DC.cIL12 and DC.RheoIL12 could be readily observed in B16 lesions, and were seen more rarely within draining LNs, in mice injected i.t. with DC.cIL12 or DC.RheoIL12 (if activating ligand was provided i.p. at 0h or 24h post-DC administration). Very few or no EGFP+ DC were detectable in tissues harvested from mice treated with control (uninfected) DC or DC.RheoIL12 (where ligand administration was delayed for 48h post-DC injection). When comparing the tissues isolated from mice treated with DC.RheoIL12 and ligand provided at 0h vs. 24h, there were more EGFP+ DC in both the tumor (p = 0.001) and draining LN (p = 0.02) when the activating drug was provided earlier.
Figure 4. DC expressing transgene IL-12p70 exhibit prolonged survival in tumor and tumor-draining lymph node after i.t. injection.
Day 7 DC were developed from the bone marrow of EGFP Tg mice and not infected, or infected with the indicated rAd at an MOI = 100 for 48h. DC (106) were then harvested and injected i.t into established day 7 B16 tumor lesions. Ligand was administered i.p. at the indicated time points after DC injection as outlined in the Fig. 3A legend. Tumors and tumor-draining lymph nodes were harvested 72h after DC injection and 5 micron cryosections analyzed for the presence of EGFP+ DC. Inset numbers reflect the average number of EGFP+ cells per 10 fields analyzed for a given tissue. Data are representative of 2 animals analyzed per cohort in each of 2 independent experiments performed.
Therapeutic benefits of DC.RheoIL12 administration are associated with the induction of specific CD8+ T cells and durable anti-tumor immunity
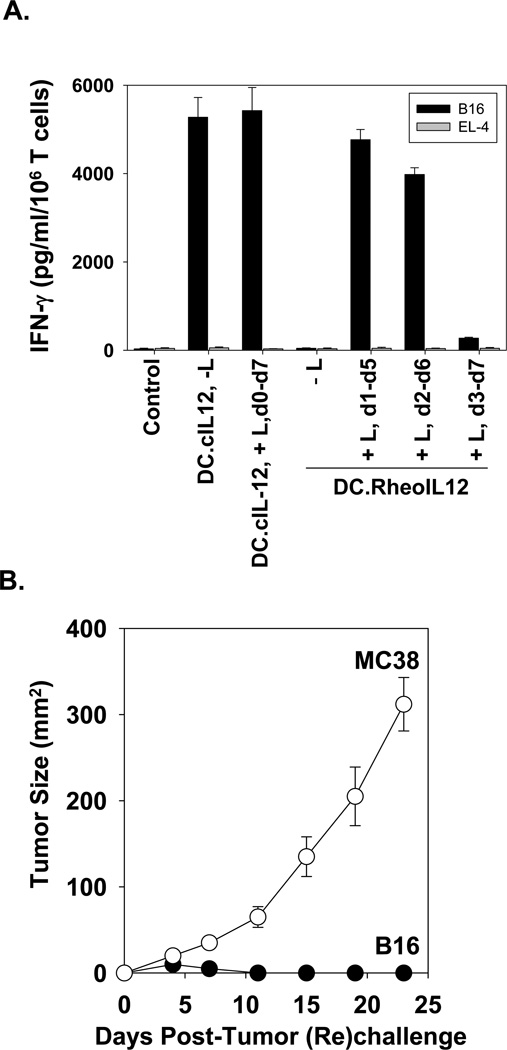
Given the apparent dependency of injected DC vitality on the timing of ligand injection, we would have predicted a superior degree of specific CD8+ T cell cross-priming in the case of mice receiving DC.RheoIL12 activated on day 1 vs. later time points by activator ligand. Interestingly, while this was certainly observed for the DC.RheoIL-12 treatment cohorts in which ligand was provided for days 1–5 vs. days 3–7, it was not the case when comparing those cohorts receiving ligand on days 1–5 vs. days 2–6 (Fig. 5A). Indeed, the in vitro splenic CD8+ T cell responses (IFN-γ secretion) against relevant B16 vs. irrelevant EL-4 tumor targets was comparable for both of these cohorts, and these each approximated that detected in mice treated with DC.cIL12. Overall, these anti-B16 CD8+ T cell response profiles appeared to directly correlate with the therapy outcome (Fig. 3A).
Figure 5. Conditional DC.RheoIL12 therapy promotes strong peripheral activation of anti-B16 CD8+ T cells if activating ligand is provided within 24h of DC injection and results in durable, specific protection against B16 melanoma rechallenge.
In panel A., spleens were harvested and pooled from 3 animals per cohort on day 25 after mice had received the indicated i.t. therapy. Purified CD8+ T cells were then stimulated in vitro with irradiated B16 (relevant) vs. EL4 (irrelevant) tumor cells, as outlined in Materials and Methods, and levels of mIFN-γ secretion quantitated by specific ELISA. Data are reported as the mean ± SD of triplicate determinations and are representative of 2 independent experiments performed. In panel B., all mice (n=5) rendered tumor-free after treatment with i.t. DC.RheoIL12 and the d1-d5 regimen of ligand were re-challenged on day 45 (post-initial tumor injection) with 105 B16 melanoma vs. 105 MC38 colon carcinoma cells (placed on contralateral flanks) and tumor sizes (mean mm2 ± SD) measured every 3–4 days.
To address whether effective DC.RheoIL12-based therapy was associated with the development of durable anti-tumor immunity, animals rendered tumor-free as a conmsequence of therapy were (re)challenged with relevant B16 melanoma cells or irrelevant MC38 colon carcinoma cells on day 45 (post-initial B16 challenge). As shown in Fig. 5B, all mice previously cured of their melanomas exhibited specific protection against B16 tumor cells, whereas, MC38 tumor lesions grew progressively.
Discontinuation of the administration of activating ligand mitigates IL-12-associated toxicity in vivo
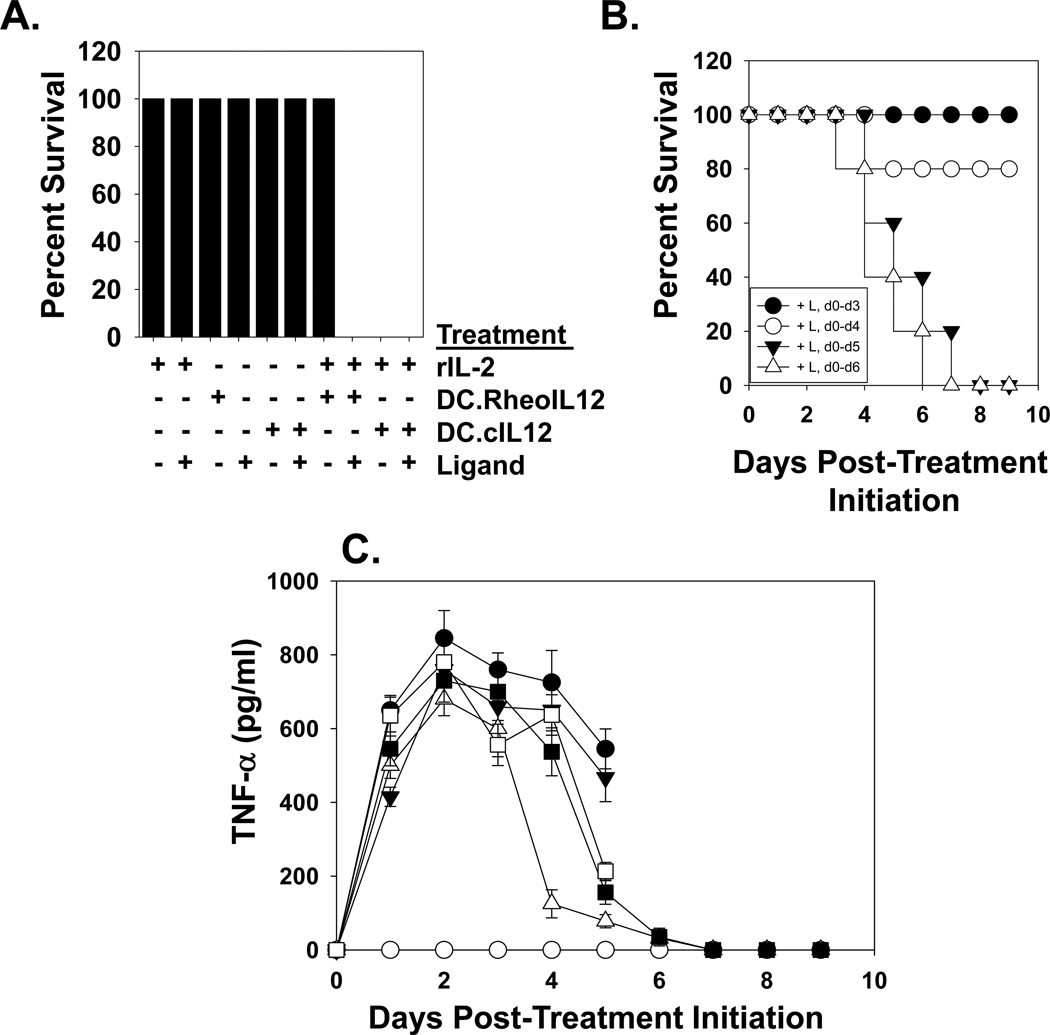
Conceptually, a major safety benefit in the therapeutic use of DC.RheoIL12 (vs. DC.cIL12) is the ability to silence cytokine production in vivo upon discontinuation of activating ligand administration. Based on the reported lethality of treating wild-type B6 mice with rIL-2 + rIL-12 (14), we developed a co-treatment model in which B6 mice were administered rIL-2 and either DC.RheoIL12 or control DC.cIL12 (as sources of IL-12). Consistent with the report of Carson et al. (14), treatment of mice with single agents (+/− ligand) or rIL-2 + DC.RheoIL12 (in the absence of ligand) proved safe (Fig. 6A). In contrast, co-treatment with rIL-2 + DC.cIL12 or DC.RheoIL12 (activated by ligand co-injection) proved highly-toxic (Fig. 6A). However, discontinuation of ligand administration before day 5 of rIL-2 + DC.RheoIL12 co-treatment reduced such toxicity (Fig. 6B), in concert with a normalization of serum TNF-α levels (Fig. 6C).
Figure 6. Discontinuation of activating ligand administration ameliorates toxicity associated with DC.RheoIL12 application in an IL-2 + IL-12 co-treatment model.
B6 mice were treated with i.p. injections of PBS, DC.RheoIL12 (+/− rIL-2 +/− activating ligand) or DC.cIL12, as outlined in the Materials and Methods. In panel A., no deaths were observed in cohorts receiving rhIL-2 only (+/− activating ligand for days 0–7), DC.RheoIL12 (+/− activating ligand for days 0–7), DC.cIL12 only (+/− activating ligand for 7 days) or rIL-2 + DC.RheoIL12 (in the absence of activating ligand). However, mice receiving either rIL-2 + DC.cIL12 or rIL-2 + DC.RheoIL12 + activating ligand for days 0–6 displayed severe toxicity. Data are reported on day 8 of the study. In panel B., discontinuation of activating ligand prevents the death of mice receiving IL-2 + DC.RheoIL12 injections. Activating ligand was provided during the indicated time intervals. In panel C., TNF-α was analyzed in serum obtained from the indicated animals by ELISA. Symbols represent mice treated with rhIL-2 plus: DC.cIL12 (●), DC.RheoIL12 without ligand (○); DC.RheoIL12 + ligand for days 0–3 only (Δ); DC.RheoIL12 + ligand for days 0–4 only (■); DC.RheoIL12 + ligand for days 0–5 only (□); DC.RheoIL12 + ligand on all days (▼). Data represent the mean +/− SD of data determined from triplicate ELISA determinations using all viable animals/cohort on a given day of analysis. For all panels, the reported data are representative of 2 independent experiments performed.
DISCUSSION
The application of cytokine gene therapy for the treatment of cancer has attracted significant attention over the past 15 years, largely owing to the possibility of providing paracrine production of an immunologic response modifier via cDNA insertion, thus obviating systemic toxicities and frequent drug administration due to the typically short biologic half-lives of recombinant cytokines in vivo (2, 15, 16). IL-12 in particular has demanded intensive scrutiny given its potent capacity to enhance inflammatory, Type-1 T cell-mediated immunity—a preferred response associated with tumor regression (1, 11, 17, 18).
IL-12 gene therapy has demonstrated profound anti-tumor efficacy in numerous animal model studies when applied as a recombinant cDNA vector (19, 20), but even more so, when applied in the context of gene-modified DC (9–11). To date, however, human phase I trials of IL-12 gene therapy implementing plasmids or viral vectors have failed to achieve durable, objective clinical responses in the cancer setting (3–6, 19). DC-based IL-12 gene therapy has thus far only been attempted in a single phase I trial, in which intratumoral injection of 1 – 5 × 107 DC infected with adenovirus encoding IL-12p70 was investigated in 17 patients with colorectal, hepatic or pancreatic carcinoma (7). In this phase I trial, treatment was deemed well-tolerated (with the notable therapy-associated adverse events being lymphopenia (grade 1–3) and fever (grade 1–2) occurring in 65–76% of patients) and effective in promoting 1 partial response (pancreatic carcinoma) and stabilization of disease in 2 patients with hepatocellular carcinoma (7).
Given residual concerns that are both clinical (i.e. unanticipated toxicities associated with DC-based IL-12 gene therapy and potential IL-12-dependent limitations in therapeutic DC.IL12 migration after intratumoral administration) and basic (i.e. when is IL-12 production in transduced DC most important for therapeutic efficacy?) in nature, we have developed a conditional IL-12 gene therapy model in the current report. This model allows for delayed turn-on of IL-12p70 production by injected DC (at a time when these cells may have acquired tumor Ags and migrated to tissue-draining lymph nodes) and/or turn-off of IL-12p70 production at any consequent time (i.e. when toxicity might be observed in patients).
Using this conditional DC.RheoIL12 gene therapy in the aggressive B16 melanoma model in C57Bl/6 mice, we suggest the following conclusions: 1) elevated levels of IL-12p70 are only secreted from DC.RheoIL12 in the presence of the activating ligand and is silenced upon ligand removal; 2) i.t. DC.RheoIL12-based therapy is as effective as i.t. DC.cIL12-based therapy when ligand is administered to tumor-bearing animals within 24h of DC injection and activation is sustained for approximately 5 or more days; 3) IL12 transgene expression in DC appears to prolong the survival of these cells in the tumor microenvironment and is associated with higher numbers of i.t. injected DC that migrate to tumor-draining lymph nodes; 4) the strongest immune correlate to therapy outcome is the level of tumor-specific CD8+ T cells cross-primed by the therapy and not the number of injected DC sustained in the tumor microenvironment; and 5) animals rendered free of disease by DC.RheoIL12-based therapy exhibit durable protective immunity against B16 melanoma rechallenge.
These data suggest the continued investigation of i.t. delivered DC.IL12-based gene therapy in the clinical setting, focusing on the objective clinical response as a primary study endpoint, and cross-primed anti-tumor CD8+ T cells (producing IFN-γ) as a secondary study endpoint. Although significant differences were not observed in tumor regression and the activation of tumor-specific CD8+ T cells as a consequence of treatment with DC.RheoIL12 (activated by the ligand within 24 hours of injection) vs. DC.cIL12, the ability to turn transgene IL12 expression on and off in vivo offers additional safety and potential therapeutic control to this treatment modality (in that both the timing and level of IL-12 expression may be regulated by the administration of ligand). This may be most salient in prospective therapies integrating the combined use of multiple cytokines (such as IL-2 + IL-12 or IL-12 + IL-18) to further optimize the induction of clinically-beneficial Type-1 anti-tumor immunity, where the risk of collateral toxicities may be anticipated (14, 21). Given these considerations, we are currently developing a phase I clinical trial implementing i.t. delivered DC.RheoIL12 gene therapy for patients with accessible, advanced stage melanoma at the University of Pittsburgh.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Amy Wesa for her careful review of this work and suggestions made during the preparation of this manuscript.
REFERENCES CITED
- 1.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 2.Atkins MB, Robertson MJ, Gordon M, Lotze MT, DeCoste M, DuBois JS, et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res. 1997;3:409–417. [PubMed] [Google Scholar]
- 3.Triozzi P, Allen KO, Carlisle RR, Craig M, LoBuglio AF, Conry RM. Phase I study of the intratumoral administration of recombinant canarypox viruses expressing B7.1 and interleukin 12 in patients with metastatic melanoma. Clin Cancer Res. 2005;11:4168–4175. doi: 10.1158/1078-0432.CCR-04-2283. [DOI] [PubMed] [Google Scholar]
- 4.Sangro B, Mazzolini G, Ruiz J, Harraiz M, Quiroga J, Herrero I, et al. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J Clin Oncol. 2004;22:1389–1397. doi: 10.1200/JCO.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 5.Heinzerling L, Burg G, Dummer R, Maier T, Oberbolzer PA, Schultz J, et al. Intratumoral injection of DNA encoding human interleukin 12 into patients with metastatic melanoma: clinical efficacy. Hum Gene Ther. 2005;16:35–48. doi: 10.1089/hum.2005.16.35. [DOI] [PubMed] [Google Scholar]
- 6.Kang WK, Park C, Yoon HL, Kim WS, Yoon SS, Lee MH, et al. Interleukin 12 gene therapy of cancer by peritumoral injection of transduced autologous fibroblasts: outcome of a phase I study. Hum Gene Ther. 2001;12:671–684. doi: 10.1089/104303401300057388. [DOI] [PubMed] [Google Scholar]
- 7.Mazzolini G, Alfaro C, Sangro B, Feijoo E, Ruiz J, Benito A, et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J Clin Oncol. 2005;23:999–1010. doi: 10.1200/JCO.2005.00.463. [DOI] [PubMed] [Google Scholar]
- 8.Berard F, Blanco P, Davoust J, Neidhart-Berard EM, Nouri-Shirazi M, Taquet N, et al. Cross-priming of naive CD8+ T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J Exp Med. 2000;192:1535–1544. doi: 10.1084/jem.192.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satoh Y, Esche C, Gambotto A, Shurin GV, Yurkovetsky ZR, Robbins PD, et al. Local administration of IL-12-transfected dendritic cells induces anti-tumor immune responses to colon adenocarcinoma in the liver in mice. J Exp Ther Oncol. 2002;2:337–349. doi: 10.1046/j.1359-4117.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka R, Zullo SA, Ramsey J, Yajima N, Tsuchiya N, Tanaka R, et al. Marked enhancement of antitumor immune responses in mouse brain tumor models by genetically modified dendritic cells producing Semliki Forest virus-mediated interleukin-12. J Neurosurg. 2002;97:611–618. doi: 10.3171/jns.2002.97.3.0611. [DOI] [PubMed] [Google Scholar]
- 11.Tatsumi T, Huang J, Gooding WE, Gambotto A, Robbins PD, Vujanovic NL, et al. Intratumoral delivery of dendritic cells engineered to secrete both interleukin (IL)-12 and IL-18 effectively treats local and distant disease in association with broadly reactive Tc1-type immunity. Cancer Res. 2003;63:6378–6386. [PubMed] [Google Scholar]
- 12.Kumar P, Katakam AK. RheoSwitch® System: a highly sensitive ecdysone receptor-based gene regulation system induced by synthetic small-molecule ligands. In: Friedman T, Rossi J, editors. Gene Transfer: Delivery and Expression of DNA and RNA. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2007. pp. 643–651. [Google Scholar]
- 13.Itoh T, Storkus WJ, Gorelik E, Lotze MT. Partial purification of murine tumor-associated peptide epitopes common to histologically distinct tumors, melanoma and sarcoma, that are presented by H-2Kb molecules and recognized by CD8+ tumor-infiltrating lymphocytes. J Immunol. 1994;153:1202–1215. [PubMed] [Google Scholar]
- 14.Carson WE, Yu H, Dierksheide J, Pfeffer K, Bouchard P, Clark R, et al. A fatal cytokine-induced systemic inflammatory response reveals a critical role for NK cells. J Immunol. 1999;162:4943–4951. [PubMed] [Google Scholar]
- 15.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 16.Chada S, Ramesh R, Mhashilkar AM. Cytokine- and chemokine-based gene therapy for cancer. Curr Opin Mol Ther. 2003;5:463–474. [PubMed] [Google Scholar]
- 17.Svane IM, Boesen M, Engel AM. The role of cytotoxic T-lymphocytes in the prevention and immune surveillance of tumors--lessons from normal and immunodeficient mice. Med Oncol. 1999;16:223–238. doi: 10.1007/BF02785868. [DOI] [PubMed] [Google Scholar]
- 18.Faure F, Even J, Kourilsky P. Tumor-specific immune response: current in vitro analyses may not reflect the in vivo immune status. Crit Rev Immunol. 1998;18:77–86. doi: 10.1615/critrevimmunol.v18.i1-2.90. [DOI] [PubMed] [Google Scholar]
- 19.Sangro B, Melero I, Qian C, Prieto J. Gene therapy of cancer based on interleukin 12. Curr Gene Ther. 2005;5:573–581. doi: 10.2174/156652305774964712. [DOI] [PubMed] [Google Scholar]
- 20.Wigginton JM, Wiltrout RH. IL-12/IL-2 combination cytokine therapy for solid tumours: translation from bench to bedside. Expert Opin Biol Ther. 2:513–524. doi: 10.1517/14712598.2.5.513. [DOI] [PubMed] [Google Scholar]
- 21.Carson WE, Dierksheide JE, Jabbour S, Anghelina M, Bouchard P, Ku G, et al. Coadministration of interleukin-18 and interleukin-12 induces a fatal inflammatory response in mice: critical role of natural killer cell interferon-γ production and STAT-mediated signal transduction. Blood. 2000;96:1465–1473. [PubMed] [Google Scholar]