Abstract
Tyrosine autophosphorylation within the cytoplasmic tail of EGF-receptor is a key event, which in turn recruits several factors including Shc, Grb2 and Rin1 that are essential activities for receptor-mediated endocytosis and signaling. In this study, we demonstrated that treatment with AG1478, an EGF-receptor kinase inhibitor, blocked the formation of Rab5-positive endosomes as well as the activation of Rab5 upon addition of EGF. We also found that EGF-receptor catalytically inactive mutant failed to activate Rab5 upon EGF stimulation. Additionally, endosomal co-localization of Rab5 and EGF-receptor was inhibited by AG1478. Interestingly, AG1478 inhibitor did not block the formation of enlarged Rab5-positive endosomes in cells expressing Rab5 GTP hydrolysis defective mutant (Rab5: Q79L). AG1478 inhibitor also blocked the in vitro endosome fusion in a concentration-dependent manner, and more importantly, Rab5: Q79L mutant rescued it. Furthermore, addition of Rin1, a Rab5 guanine nucleotide exchange factor, partially restored enodosome fusion in the presence of AG1478 inhibitor. Consistent with these observations, we also observed that Rin1 was unable to localize to membranes upon EGF-stimulation in the presence of AG1478 inhibitor. These results constitute first evidence that the enzymatic activity of a tyrosine kinase receptor is required endosome fusion via the activation of Rab5.
Keywords: receptor tyrosine kinase, small GTPase, endosome fusion, receptor-mediated encoytosis, kinase inhibitors
INTRODUCTION
Interaction of epidermal growth factor (EGF) [1] with EGF-receptors leads to activation and tyrosine autophosphorylation of the receptor, which in turn creates binding sites for proteins containing Src homology 2 (SH2) and phosphotyrosine-binding domains (PTB) [2]. Thus, tyrosine kinase activity plays an essential role in signal transduction and membrane trafficking of growth factors. Consequently, the activation of EGF-receptor dramatically accelerates endocytosis of EGF-receptor complexes through clathrin-coated pits [3].
Several factors affect the endocytosis of activated EGF-receptors, including Shc, Grb2, Cbl, Rab5, and PI3-kinase [2–4]. After internalization, EGF-receptors are efficiently sorted to the lysosomal compartment via early and late endosomes. Thus, increased internalization and lysosomal targeting, results in down-regulation of EGF-receptor and leads to attenuation of the growth factor signaling cascade [4].
The early endosome is a key checkpoint in endocytic pathways, where a decision is made whether to be sorted to the late endosome/lysosome compartment or to be recycled back to the plasma membrane [5, 6]. Rab5 and its effectors, including EEA1, Rin1 and Rabex-5, together with other small GTPases (i.e., Rab 4, 7, 11, 15 and 22), are likely to tightly control the fusion and sorting of molecules that have entered the early endosome [7, 8]. Hence, early endosome fusion is dependent on Rab5 proteins and is selective, thereby allowing orderly modification of ligand-receptor interaction complexes and signaling in a sequential manner by altering the surroundings during ligand-receptor internalization [9–14]. In addition, several Rab5-asociated proteins are also required for endosome-endosome fusion [14–21].
Given the importance of tyrosine phosphorylation in both signaling and trafficking of growth factors, specific and selective inhibitors of tyrosine kinase activity are important tools for studying EGF-receptor function [1, 4, 6, 22]. Thus, the analysis of the effects of tyrosine kinase inhibitors on EGF-receptor trafficking and signaling may provide important information about the mechanisms by which the inhibitor(s) function(s) at the cellular level.
In this study we demonstrate that tyrosine kinase activity of the EGF-receptor is required for the formation for enlarged Rab5-positive endosomes as well as for the activation of Rab5 in intact cells. We also observed that AG1478 inhibitor blocked endosome fusion, whose inhibitory effect is linked to the activation of Rab5. Furthermore, we found that AG1478 inhibitor blocked the endosome fusion reaction stimulated by Rin1. More importantly, we have also observed that the addition of a constitutively active mutant of Rab5 (e.g. Rab5: Q79L), reversed the inhibitory effect of AG1478 inhibitor, suggesting a mechanism by which the tyrosine kinase activity of the receptor modulates early endosome fusion.
MATERIAL AND METHODS
Cell culture and Materials
NR6 cells expressing the human EGF-receptor (NR6-E) and human EGF-receptor catalytically inactive mutant (K712M) (NR6-K) were grown in Dulbecco’s Modified Eagle’s medium supplemented with 5% (w/v) fetal bovine serum containing 0.3–0.5 mg/ml G418. Kinase inhibitors were purchased from EMD Biosciences (La Jolla, CA). PY-100, EEA-1 and Rab5 antibodies were from Cell Signaling Technology (Beverly, MA). Rin1 antibodies were from Abcam Inc. Biotin-EGF and Avidin β-galactosidase were purchased from Sigma-Aldrich (St. Louis, MO). All secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). All other chemicals were obtained from Sigma unless otherwise stated.
In Vitro Endosome Fusion Assay
Early endosomes were loaded with either Biotin-EGF (B-EGF) or Avidin β-galactosidase (Av-GAL) by a 5-min uptake at 37°C in the NR6-E cells [23]. Cells were washed and homogenized in homogenization buffer (HB, 0.25 M sucrose, 20 mM HEPES, 0.5 mM EGTA, pH 7.0). The homogenates were centrifuged at 600 × g for 5 min to eliminate nuclei and cell debris. The supernatants were centrifuged at 35,000 × g for 1 min, followed by another centrifugation at 45,000 g for 5 min in a Beckman L-100 ultracentrifuge. The pellets, enriched with early endosomes, were suspended in fusion buffer (250 mM sucrose, 0.5 mM EGTA, 1mM dithiothreitol, 1.5 mM MgCl2, 0.025% (w/v) Biotin-BSA, 50 mM KCl, 20 mM Hepes-KOH, pH 7.0) containing Sephadex G-25-filtered cytosol (1 mg/ml), protein and phosphatase inhibitors, and an ATP-regeneration system (1 mM ATP-8 mM phosphocreatine with creatine kinase at 40 units/ml). Fusion reactions were conducted at 37°C for 1 hr in the absence or in the presence of AG1478 inhibitor [24] or its inactive analog (AG9) [25] at the concentration indicated in each figure, and quantified by measuring the β-Galactosidase activity of the immunocomplex [23]. Typical homogenizations yielded >80% of endocytosed marker in the post nuclear supernatant (PNS) as determined by either radioactivity or immunoprecipitation of ligand. PNSs could then be used fresh for fractionation studies or stored frozen in liquid nitrogen for later use. Endosome-enriched and supernatant fractions were obtained as previously described [23, 26].
Confocal microscopy
NR6 cells were seeded on glass coverslips at 1.0 × 105 per well. The following day, cells were transfected using FugeneHD with either pEGFP-Rab5: WT or pEGFP-Rab5: Q79L mutant. Cells were starved in serum-free Dulbecco’s Modified Eagle’s Medium supplemented with 5% (w/v) BSA Fraction V the next day overnight, and treated as indicated with 100 nM AG9 or 100 nM AG1478 for 30 minutes at 4°C, after which they were treated with 100 ng/ml EGF for 1hr at 4°C and then stimulated to uptake the ligand for 5 min at 37°C. They were then washed with PBS and fixed in 4% (w/v) paraformaldehyde for 20 minutes. Fixed cells were probed with the rabbit polyclonal EGF-receptor antibodies for 1h. Secondary antibody used was Alexa594-conjugated goat anti-rabbit IgG. Coverslips were mounted with Prolong and viewed on a Leica TCS SP2 confocal microscope.
Image analysis
Images of fluorescent Rab5-positive endosomes were obtained using Leica TCS software. Endosome size distribution of at least 1712 Rab5: WT and 1320 Rab5: Q79L endosomes from either EGFR: WT (NR6-E) or EGFR: K721M (NR6-K) cells were analyzed by measuring both endosome diameter and perimeter using NIH Image J software (obtained from http://rsweb.nih.gov/ij/).
EEA1 pull-down assay
NR6 cells were lysed using a buffer containing 20 mM HEPES (pH 7.5), 100 mM NaCl, 1 mM DTT, 5 mM MgCl2, 5% (v/v) glycerol and 1% (v/v) Triton-X-100 supplemented with 1 mM PMSF. Lysates (1 ml) were then incubated with 100 μl of glutathione beads containing ~10 μg of GST-EEA1 at 4°C while rocking for 1 hr. After incubation, the beads were washed three times using the lysis buffer. The pull-downs were subjected to SDS-PAGE and analyzed by immunoblotting using an anti-Rab5 antibody.
Immunoprecipitation and Western Blot analysis
NR6 cells were cultured in a 6-well plate in growth medium and serum-starved for 16 hrs. After starvation, cells were treated with EGF and AG1478 inhibitor. After treatment, cells were lysed in ice-cold lysis buffer (20 mM Tris-HCL (pH 7.5), 150 mM NaCl and 1% (v/v) Triton X-100) containing 10 mM NaF, 1mM Na pyrophosphate, 1 mM Na orthovanadate and 1% protease inhibitor mixture solution (Sigma). The lysates were clarified by centrifugation and subjected to SDS-PAGE and analyzed by Western blotting using specific antibodies. Relative units of proteins were determined by densitometry using the ratio of Grb2, Shc, Rin1, or Rabex-5 to total-EGF-receptor and tubulin, respectively. For immunoprecipitation, 100 μg of pre-cleared cell lysate was incubated with antibodies (0.5 μg) overnight at 4°C. The immune complexes were precipitated with protein G (Sigma) for 1 hr at 4° C, and the beads were washed extensively with lysis buffer before solubilization in SDS sample loading buffer.
Statistical analysis
All experiments presented were repeated a minimum of three times. The data represent the mean ± SD. Student’s t test was performed to calculate statistical significance.
RESULTS
EGF-receptor tyrosine kinase inhibitor blocks fusion between endosomes in intact cells
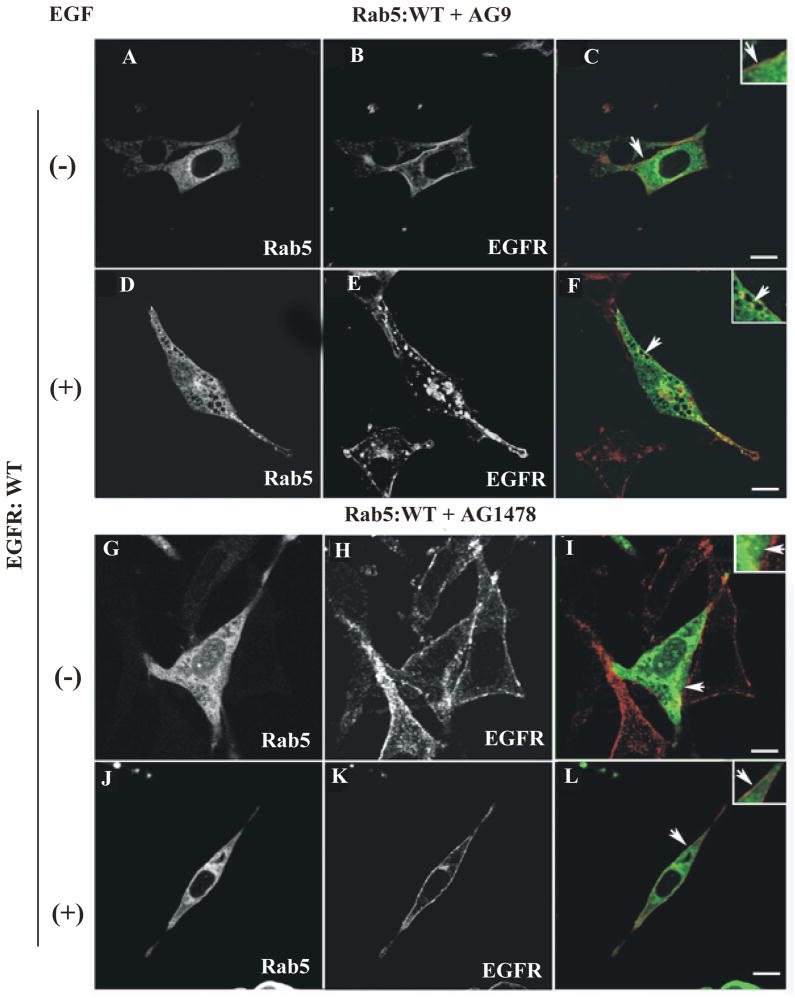
EGF-receptor activation leads to tyrosine autophosphorylation of the cytoplasmic tail and the subsequent recruitment of several factors including Shc, Grb2, PLC-γ, Rin1 and Rabex-5 [27–29]. To determine whether EGF-receptor kinase activity directly affects the activity of Rab5 in intact cells, we examined whether the addition of AG1478 inhibitor affects the changes in distribution and/or localization of Rab5 upon stimulation of EGF in intact cells. As expected, in the absence of EGF, Rab5: wild type appears diffuse and typically in punctate endosomal structures (see arrows in Figure 1C). However, upon addition of EGF, Rab5: wild type was found in enlarged vesicles (see arrows in Figure 1F). In these cells, the enlarged Rab5-positive endosomes were comparable in size to those endosomes observed in cells overexpressing Rab5: Q79L mutant (compare Figure 1F with Suppl. Figure 1A–R). Furthermore, we observed a significant decrease in size of Rab5-positive endosomes in cells expressing Rab5: wild type when treated with AG1478 (Figure 1L), but not in cells expressing the Rab5: Q79L mutant (Suppl. Figure 1G–L). We also observed that in the absence of AG1478 inhibitor, EGF-receptor co-localizes with Rab5 on endosomes when cells are stimulated with EGF, but not in the presence of the inhibitor (compare Figure 1F and Figure 1L). However, in cells expressing the Rab5: Q79L mutant, addition of the AG1478 inhibitor failed to affect either the formation of enlarged Rab5-positive endosomes or the co-localization of internalized EGF-receptor with Rab5: Q79L mutant-positive endosomes, when cells were treated with the AG1478 inhibitor (Suppl. Figure 1).
Figure 1. Confocal immunofluorescence analysis of cells co-expressing EGF-receptor and Rab5: wild-type in the presence of EGF-receptor tyrosine kinase inhibitor.
NR6 cells expressing either EGF-receptor wild type (A–L) or EGF-receptor K721M mutant (M–R) were transfected with plasmids encoding GFP-Rab5: wild type (A–R) in the absence (A–C, G–I, M–O) or in the presence of EGF (D–F, J–L, P–R). Cells were also supplemented with 100 nM AG9 inactive analog (A–F) or 100 nM AG1478 inhibitors (G–L). 100 ng/ml EGF was bound to the cells at 4°C for 60 min. Cells were then washed with ice-cold PBS, incubated at 37°C for 8 min, washed three times with ice-cold PBS, fixed with 4% (w/v) paraformaldehyde and then permeabilized with 0.1% (v/v) Triton X-100 prior to incubation with antibodies. The primary antibody used was polyclonal rabbit anti-EGF-receptor. The secondary antibody used was Alexa564-labelled donkey anti-rabbit IgG. Yellow color indicates areas of co-localization between the internalized EGF-receptor (B, E, H, K, N and Q) and the overexpressed GFP-Rab5 proteins (A, D, G, J, M and P). An inactive analog (AG9) was used as control. The optical sections viewed are 0.4 μm. Size bars, 10 μm.
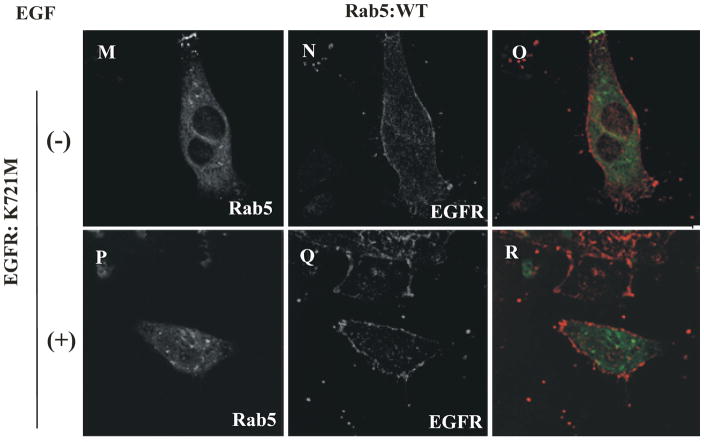
In addition, we found that in the absence or in the presence of EGF, Rab5: wild type appears diffuse and typically in punctate endosomal structures in cells expressing EGF-receptor catalytic inactive mutant (see arrows in Figure 1O–R). In contrast, we found enlarged Rab5-positive endosomes in cells expressing both Rab5: Q79L mutant and EGF-receptor catalytically inactive mutant (Suppl. Figure 1O–R). As expected, EGF-receptor: wild type, but not the catalytically inactive mutant of EGF-receptor, was tyrosine phosphorylated in the presence of EGF in these cells (Suppl. Figure 2). These observations are consistent with the idea that the EGF-receptor kinase activity is required for formation of enlarged Rab5-positive endosomes as well as the subsequent co-localization for Rab5 and EGF-receptor on the endosomes. Thus, it is possible to speculate that the AG418 inhibitor should alter the size distribution of endosomes in cells expressing Rab5: WT, but not in cell expressing Rab5: Q79L mutant. To test this hypothesis, we expressed GFP-Rab5: Q79L and GFP-Rab5: WT proteins respectively in NR6 cells. Rab5-positive endosomes were identified by confocal immunofluorescence microscopy and then quantified using the NIH Image J software. We observed that in NR6-E cells expressing Rab5: WT, the endosomes were generally smaller with an average perimeter of 1.51 μm (or 0.48 μm in diameter) and a relative variance of 1.70 In contrast upon stimulation with EGF, Rab5-positive endosomes were enlarged with an average perimeter of 3.29 μm (or 1.05 μm in diameter) and a relative variance of 1.41 (Suppl. Figure 3A). We also observed that the addition of AG418 inhibitor to NR6-E cells expressing Rab5: WT, which were then stimulated with EGF, clearly decreased size of the endosomes (average perimeter of 1.69 μm [or 0.54 μm in diameter] and a relative variance of 1.61) (Suppl. Figure 3B). As expected, the addition of AG9 an inactive analog, did not affect neither endosome size nor endosome distribution (Suppl. Figure A–B). These observations are in agreement with the inhibitory effect of AG418 on the activation of Rab5 in NR6-E cells upon stimulation of EGF.
Importantly, we observed that in NR6-E cells expressing Rab5: Q79L the size distribution of the endosomes showed an average perimeter of 5.74 μm (or 1.83 μm in diameter) and a relative variance of 1.44 and were not affected by the addition of either EGF or AG418 inhibitor (Suppl. Figure 3 C). These observations are consistent with the fact that the amount of GTP-bound form of Rab5 was not affected in NR6-E cells expressing Rab5: Q79L mutant.
Lastly, we observed that in NR6-K cells expressing Rab5: Q79L, the size distribution of the endosomes were comparable to those observed in NR6-E cells expressing Rab5: Q79L (Suppl. Figure 3 D). Furthermore, when EGF was added to NR6-K cells expressing Rab5: WT, Rab5-positive endosomes were not enlarged (average perimeter of 1.60 μm [or 0.51 μm in diameter], and a relative variance of 1.71) (Suppl. Figure 3 D). Taken together, this quantitative difference between Rab5: WT either in the absence or in the presence of EGF, are significant, especially reflected by the extended tail of Rab5: WT endosome distribution in cells stimulated with EGF.
EGF-receptor tyrosine kinase inhibitor blocks the activation of Rab5 in intact cells
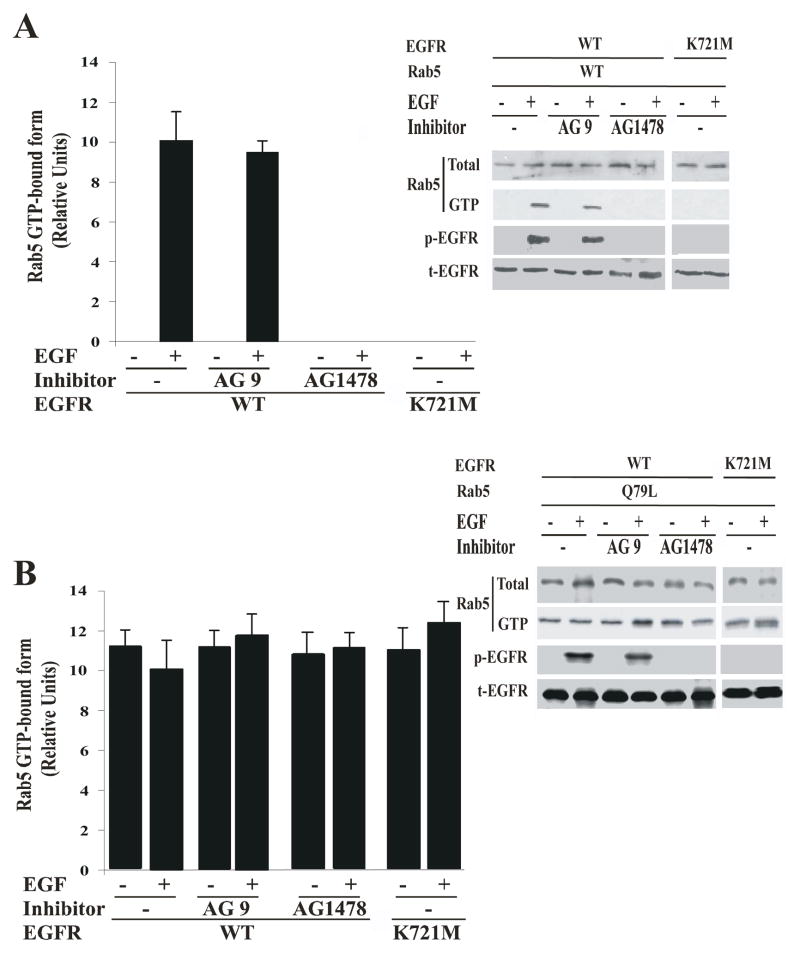
To further examine the role of EGF-receptor kinase activity on the activation of Rab5 upon stimulation with EGF, cells expressing either Rab5: wild type or Rab5: Q79L mutant were incubated with or without the AG1478 inhibitor in the absence or presence of EGF. After incubation, active or GTP-bound form of Rab5 was precipitated with GST-EEA1 domain as described in Materials and Methods. As shown in Figure 2A, the addition of AG1478 inhibitor significantly diminished the amount of GTP-bound form of Rab5 when the cells expressing both Rab5: wild type and EGF-receptor: wild type were stimulated with EGF. However, addition of the AG1478 inhibitor to cells expressing the Rab5: Q79L mutant had no effect at all (Figure 2B). Furthermore, we also found that in cells expressing EGF-receptor catalytically inactive mutant and Rab5: wild type, Rab5 was not activated and the catalytically inactive mutant of EGF-receptor was not tyrosine phosphorylated upon addition of EGF (Figure 2A and B). These data demonstrate that the inhibition of the EGF-receptor activity is linked to the inactivation of Rab5.
Figure 2. AG1478 inhibitor blocks the activation of Rab5 in intact cells.
NR6 cells expressing either EGF-receptor wild type or EGF-receptor K721M mutant were transfected with plasmids encoding GFP-Rab5: wild type (A) and GFP-Rab5: Q79L mutant (B) in the absence or in the presence of EGF and 100 nM AG1478 inhibitor as indicated in the Figure. 100 ng/ml EGF was bound to the cells at 4°C for 60 min. The cells were washed and then incubated at 37°C for 8 min. Subsequently, the cells were washed three times with ice-cold PBS, lysed and incubated with glutathione beads either in the presence of GST alone or GST-EEA1 at 4°C for 60 min. After incubation, the beads were washed and the presence of activated Rab5 was analyzed by Western blotting. The data are presented as means ± SD of four independent experiments.
EGF-receptor tyrosine kinase inhibitor blocks the recruitment of Rin1 in intact cells
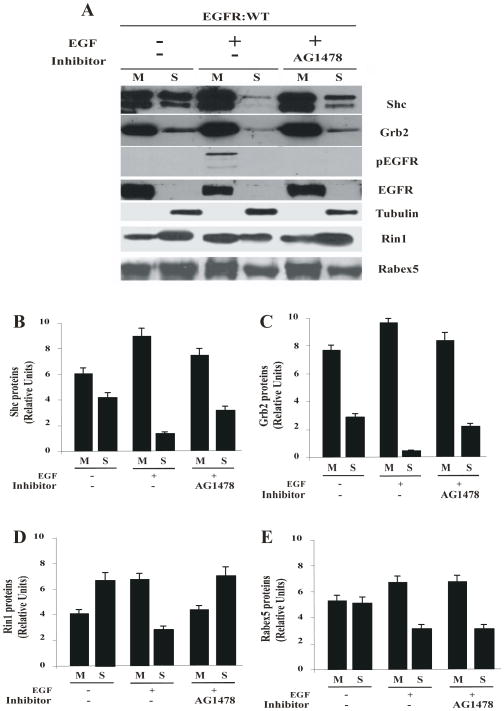
Given the effect of AG1478 inhibitor on the activation of Rab5 in intact cells, we examined whether the addition of AG1478 inhibitor can affect the changes in membrane distribution of several factors associated with EGF-receptor: wild type, upon stimulation with EGF in intact cells. As expected, either in the absence or presence of EGF, EGF-receptor was always found in the membrane fraction (Figure 3A). EGF-receptor was also tyrosine phosphorylated only in the presence of EGF, while the addition of the AG1478 inhibitor blocked the phosphorylation of EGF-receptor (Figure 3A). Consistent with this data, in the absence (but not in presence) of AG1478 inhibitor, Shc and Grb2 were recruited to the membrane fraction upon stimulation with EGF (Figure 3B and C). Furthermore, Rin1 was recruited to the membrane upon EGF stimulation. In contrast, Rin1 was poorly localized on the membrane in the presence of AG1478 and upon stimulation with EGF (Figure 3D). Interestingly, Rin1 was also tyrosine phopshorylated upon EGF stimulation (Suppl. Figure 4), but not in the presence of AG1478 inhibitor and in cells expressing EGF-receptor catalytically inactive mutant (data not shown). As an extension of our observation, we also investigated the effect of AG1478 inhibitor on the membrane distribution of Rabex-5. As shown in Figure 3E, Rabex-5 was recruited to the membrane upon stimulation with EGF. However, the addition of AG1478 upon stimulation with EGF had no effect on the recruitment of Rabex-5 to the membrane. These data demonstrate that in addition to being able to block the recruitment of Shc and Grb2 factors, the inhibition of the tyrosine kinase activity of EGF-receptor is linked to a selective membrane targeting of Rin1. Consistent with these observations we found that Rin1, Rabex-5 and Shc were not translocated to membranes upon EGF stimulation in cells expressing EGF-receptor catalytically inactive mutant (Suppl. Figure 4). As expected, EGF-receptor catalytically inactive mutant was not tyrosine phosphorylated upon addition of EGF (Suppl. Figure 5). Taken together, these observations suggest that both Rin1 and Rabex-5 are targeted to membrane upon EGF stimulation but they are not translocated either in cells expressing EGF-receptor catalytically inactive mutant or selectively affected in the presence of AG1478 inhibitor.
Figure 3. Effect of AG1478 inhibitor on the tyrosine phosphorylation of EGF-receptor and recruitment of Rin1 in intact cells.
NR6 cells expressing EGF-receptor wild type were incubated with 100 ng/ml EGF and 100 nM AG1478 inhibitor as described in Materials and Methods. After treatment, cells were washed with ice cold PBS and incubated at 37°C for 8 minutes. Cells were then washed again using ice-cold PBS, homogenized and membrane fractions were prepared as described in Materials and Methods. Membrane [M] and cytosol [S] fractions (A) were treated with sample buffer and proteins were subject to SDS-PAGE, blotted to a nitrocellulose membrane, and antibodies specific to Shc (B), Grb2 (C), Rin1 (D), Rabex-5 (E), phospho(p)-EGF-receptor, total(t)-EGF-receptor, and tubulin (A) were used to visualize these proteins by Western blot analysis. Relative levels of these proteins were determined by densitometry. Data represents the mean ± SD of four independent experiments.
EGF-receptor tyrosine kinase inhibitor inhibits in vitro endosome fusion
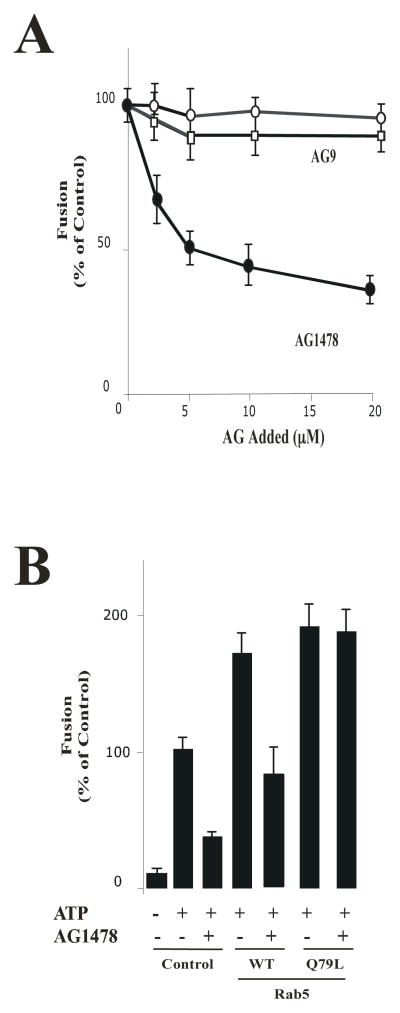
We have previously demonstrated that different factors (i.e., Rab5, Rin1 and PI3-kinase) were required for the fusion reaction between endosomes containing EGF-receptor [21]. Interestingly, these activities have also been linked to the activated EGF-receptor, which in turn involve the formation of enlarged Rab5-positive endosomes in intact cells upon stimulation with EGF [13]. Therefore, we raised the question of whether tyrosine kinase activity of the EGF-receptor is also required in the fusion between endosomes. To investigate this requirement, we first examined the effect of AG1478 inhibitor [24, 25] on the fusion reaction. As a control, we also examined the effect of an inactive tyrosine kinase inhibitor (AG9). Five-min vesicles containing either Biotin (B)-EGF or Avidin (Av)-GAL were mixed in fusion buffer supplemented with 1 mg/ml of cytosol containing an ATP-regenerating system either in the absence or in the presence of different concentrations of AG9 and AG1478 (Figure 4A). Samples were then transferred to 37°C for the indicated times and processed as described in Materials and Methods to determine the percentage forming the immune complex. When the fusion reaction was conducted in the presence of AG1478, we observed a strong inhibition of the fusion reaction that occurred in a concentration dependent manner. However, the addition of AG9 did not affect the fusion reaction in comparison to the control, which argues for the specificity of the exhibited inhibitory effect (Figure 4A).
Figure 4. Effect of AG1478 inhibitor on fusion between endosomes.
(A) Effect of cytosol and energy on the endosome-endosome fusion. Five-min vesicles containing either Biotin (B)-EGF or Avidin (Av)-GAL were mixed in fusion buffer supplemented with 1 mg/ml cytosol containing an ATP-regenerating system either in the absence (
 ) or in the presence of different amounts of AG9 (
) or in the presence of different amounts of AG9 (
 ) and AG1478 (●). Samples were then transferred to 37°C for the indicated times and processed as described in Materials and Methods in order to determine the percentage forming the immune complex formation. The data are presented as means ± SD of four independent experiments. (B) Fusion assay was performed under standard conditions as described in Figure 1A, either in the absence or in the presence of Rab5: wild type or Rab5: Q79L mutant, supplemented with either 20 μM AG1478 or 0.5 mg/ml of cytosol in the presence of ATP. The data are presented as means ± SD of four independent experiments.
) and AG1478 (●). Samples were then transferred to 37°C for the indicated times and processed as described in Materials and Methods in order to determine the percentage forming the immune complex formation. The data are presented as means ± SD of four independent experiments. (B) Fusion assay was performed under standard conditions as described in Figure 1A, either in the absence or in the presence of Rab5: wild type or Rab5: Q79L mutant, supplemented with either 20 μM AG1478 or 0.5 mg/ml of cytosol in the presence of ATP. The data are presented as means ± SD of four independent experiments.
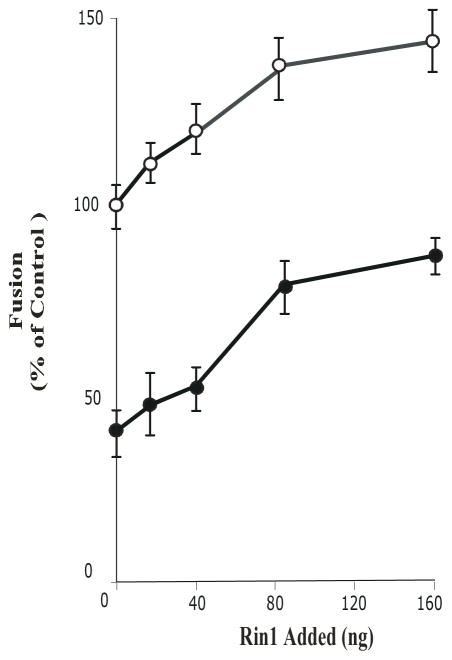
Because of the inhibitory pattern of AG1478 exhibited in the fusion reaction (Figure 4A) and because Rab5 is a key factor required for fusion between endosomes directed by the EGF-receptor [9, 12], we tested whether the AG1478 inhibitor would affect endosome fusion stimulated Rab5. Early endosome and cytosol preparation were pre-incubated with 20 μM AG1478 for 15 minutes prior to the initiation of the fusion reaction. The addition of AG1478 inhibited early endosome fusion in the presence of Rab5: wild type, however, the addition of Rab5: Q79L mutant reversed the inhibitory effect and thus stimulated endosome fusion (Figure 4B).
Based on previous data [21, 30] and the results reported here, we then investigated the effect of the tyrosine kinase enzymatic activity of the EGF-receptor on the endosome fusion stimulated by Rin1. Rin1 is a guanine nucleotide exchange factor for Rab5 that supports endosome fusion [21, 23]. As shown in Figure 5, Rin1 stimulated endosome fusion. As expected, the addition of AG9 did not affect the endosome fusion. However, AG1478 inhibitor partially blocked endosome fusion stimulated by Rin1. Collectively, these results reinforce the conclusion that tyrosine kinase activity of EGF-receptor may be involved in the activation of Rab5, at least in part, via Rin1.
Figure 5. AG1478 blocks endosome fusion stimulated by Rin1.
Fusion assay was performed under standard conditions as described in Figure 4, either in the absence or in the presence of different concentrations of Rin1 supplemented with 20 μM AG1478 (●) and 0.5 mg/ml cytosol. Empty circle (
 ) indicates addition of AG9. The data are presented as means ± SD of four independent experiments.
) indicates addition of AG9. The data are presented as means ± SD of four independent experiments.
DISCUSSION
Given the importance of tyrosine phosphorylation, which is produced upon stimulation with growth factors in intact cells, specific and selective inhibitors of tyrosine kinase activity are an important means for investigating receptor tyrosine kinases (i.e., EGF-receptor) [1]. Therefore, the utilization of these inhibitors may be critical for elucidation of the role that tyrosine kinases play in membrane trafficking and signaling [4]. Furthermore, the activation of intrinsic or associated tyrosine kinases during the internalization of growth factor receptors is a key feature of both endocytic and signaling processes. Indeed, the studies on endocytosis of kinase-inactive mutants of several growth factors, including the EGF-receptor, suggested that kinase activity is necessary for maximal internalization rate, endosomal localization and down regulation of the receptor [22, 28, 29, 31].
Here we demonstrated that AG1478, an EGF-receptor tyrosine kinase inhibitor, blocked the fusion between endosomes. This inhibitory effect was reversed by the addition of Rab5: Q79L mutant, but not by the addition of Rab5: wild type. Consistent with this observation, AG1478 blocked the formation of enlarged Rab5-positive endosomes upon EGF stimulation in intact cells. This observation is further supported by the significant reduction in the perimeter of Rab5-positive endosomes (Suppl. Figure 3). Thus, these data suggest a positive role of receptor tyrosine kinase activity on endosome fusion that is mediated by the activation of Rab5, which is in agreement with an increase in early endosome size observed upon EGF stimulation.
Interestingly, we also observed that the presence of AG1478 inhibitor blocked the activation of Rab5 in intact cells expressing Rab5: wild type (Figure 2). However, the addition of AG1478 inhibitor neither blocked the intracellular localization of EGF-receptor nor the formation of enlarged Rab5-positve endosomes in cells expressing Rab5: Q79L mutant (Suppl. Figure 1). These results are supported by the observation that the perimeter of Rab5-positive endosomes in cells expressing Rab5: Q79L mutant were not significantly affected by the addition of either EGF or AG418 inhibitor (Suppl. Figure 3). Thus, these data establish that tyrosine kinase activity of the EGF-receptor is required for the formation of enlarged Rab5-positive endosomes, and more importantly, they also demonstrate that the inhibition of the tyrosine kinase activity of the EGF-receptor is linked to the inactivation of Rab5.
Fluorescence microscopy analysis also revealed that the accumulation of EGF-receptor in early endosomes is dependent on tyrosine kinase activity, since AG1478 inhibitor also blocked the internalization of the EGF-receptor (compare Figure 1E and H). Similarly, it has been demonstrated that PD158780, another EGF-receptor tyrosine kinase inhibitor, also affected early steps of internalization [22, 32]. Thus, it is possible that these inhibitors may work at different steps during endocytosis of EGF-receptor by affecting the recruitment of selective factors [24, 33]. PD158780 prevented the targeting of EGF-receptor into coated pits by altering the recruitment of subunits of the AP2 complex [22]. However, here we showed that Rin1, a Rab5-GEF, was not recruited to the membrane upon EGF stimulation in the presence of AG1478.
The possible mechanism of this inhibitory effect may be associated with the fact that Rin1 is associated with EGF-receptor upon ligand stimulation [31], and since Rin1 is also required for the fusion assay [21, 23], it raises the possibility that the tyrosine enzymatic activity of the EGF-receptor may be required during the fusion assay. To our surprise, we found that the addition of AG1478 inhibitor blocked the fusion reaction. This inhibitory effect was concentration-dependent and specific, since an inactive analog (i.e., AG9) did not affect the fusion reaction. More importantly, stimulation of the fusion reaction by Rin1 was also partially blocked by AG1478, suggesting that the presence of AG1478 may affect the recruitment of Rin1 to membrane, which is in strong agreement with the fact that Rin1 was found to be significantly less associated with membrane fraction in the presence of AG1478 upon EGF stimulation. Consistent with this idea, we also found that Rab5: wild type partially reversed the inhibitory effect of AG1478. Alternatively, it is possible that Rin1 may also activate Rab5 independently of its association with the activated EGF-receptor in our in vitro endosome fusion system. Therefore, once Rab5 is activated, it will increase the endosome fusion independently of the presence AG1478 inhibitor, which is consistent with the observation that Rin1 activates Rab5 in an in vitro system [21]. In support of this idea, the addition of AG1478 neither affects the endosome fusion stimulated by Rab5: Q79L mutant nor the formation of enlarged Rab5: Q79L mutant-positive endosomes in intact cells. Nevertheless, our in vitro and in vivo data support the idea that the tyrosine kinase enzymatic activity of the EGF-receptor, at least in part, is required during the fusion assay.
Another view of the fusion reaction between endosomes containing B-EGF and Av-GAL, is that AG1478 inhibitor affects the activation of Rab5 in one set of endosomes (i.e., B-EGF endosomes). Therefore, this model predicts that Rab5 must be present in both sets of endosomes. Our results are in strong agreement with previous observations that Rab5 was required for the fusion in both endosomes [34–36]. However, two questions remain to be answered: 1-whether Rab5 is present on endosomes containing Av-Galactosidase?, and 2-what is the nucleotide status of Rab5 on these endosomes? Several lines of evidence have shown that Rab5 localizes on early endosomes containing fluid phase markers [37–42] and more importantly, we have found that Rab5 is activated during the enodocytosis of HRP, a commonly used fluid phase marker. However, this activation of Rab5 during fluid phase endocytosis is less robust when compared with the activation of Rab5 during EGF stimulation (Suppl. Figure 6). Similarly, other fluid phase markers (i.e., Av-GAL and Dextran) poorly activated Rab5 as compared to EGF stimulation (data not shown). The activation of Rab5 during the endocytosis of fluid phase markers is currently under further investigation. For instance, dependent on cell type, overexpression of Rabex-5 induces the formation of enlarged endosome [15, 40], but not the overexpression of either Rin1 or RAP6 in mammalian cells; Rin1 requires tyrosine phosphorylation of the EGF-receptor [31], while Rabex-5 requires ubiquitination [45, 46] and RAP6 does not interact with EGF-receptor directly [47]. These Rab5-GEFs were also required in several in vitro endosome fusion reactions [15, 21, 42]. Thus, it is possible that several mechanisms of activation of Rab5 are taking place during EGF-receptor endocytosis and endosome fusion.
In conclusion, we have used in vivo and in vitro approaches to investigate the role of tyrosine phosphorylation during the fusion reaction. Specifically, we have demonstrated that tyrosine kinase inhibitor affects the formation of enlarged Rab5-positive endosomes and activation of Rab5 in intact cells. These data suggest that the receptor kinase activity may provide a link between early endosomes and signaling molecules, including Rin1, which in turn, will activate the small GTPase Rab5 as well as directly increase the fusion activity.
Supplementary Material
HIGHLIGHTS.
By inhibiting RTK activity, AG1478 diminishes endosome fusion and activation of Rab5.
Inhibition can be fully rescued by Rab5: Q79L mutant.
Rin1 is able to partially restore endosome fusion in presence of AG1478.
AG1478 causes a mislocalization of Rin1 upon EGF stimulation.
Enzymatic activity of RTK is required for fusion events via activation of Rab5.
Acknowledgments
This work was supported by the National Institutes of Health grant SC1DK084343 (to MAB).
The abbreviations used are
- Rin1
Ras interference 1
- EGF-receptor
Epidermal growth factor-receptor
- EEA1
Early endosomal autoantigen 1
- SH2
Src homology 2 domain
- PTB
phosphotyrosine-binding domain
- PI3-Kinase
Phosphatylinositol 3-Kinase
- PLC-γ
Phospholipase C-gamma
- Grb2
Growth factor receptor-bound protein 2
- Shc
SH2 adaptor protein C
- HRP
Horseradish peroxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Citri A, Yarden Y. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 2.O’Bryan JP, Lambert QT, Der CJ. J Biol Chem. 1998;273:20431–20437. doi: 10.1074/jbc.273.32.20431. [DOI] [PubMed] [Google Scholar]
- 3.Huang F, Sorkin A. Mol Biol Cell. 2005;16:1268–1281. doi: 10.1091/mbc.E04-09-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke P, Schooler K, Wiley HS. Mol Biol Cell. 2001;12:1897–1910. doi: 10.1091/mbc.12.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeffer SR. Annu Rev Biochem. 2007;76:629–645. doi: 10.1146/annurev.biochem.76.061705.130002. [DOI] [PubMed] [Google Scholar]
- 6.Doherty GJ, McMahon HT. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 7.Scita G, Di Fiore PP. Nature. 2010;463:464–473. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- 8.Sorkin A, von Zastrow M. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 10.Brandhorst D, Zwilling D, Rizzoli SO, Lippert U, Lang T, Jahn R. Proc Natl Acad Sci U S A. 2006;103:2701–2706. doi: 10.1073/pnas.0511138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbieri MA, Hoffenberg S, Roberts R, Mukhopadhyay A, Pomrehn A, Dickey BF, Stahl PD. J Biol Chem. 1998;273:25850–25855. doi: 10.1074/jbc.273.40.25850. [DOI] [PubMed] [Google Scholar]
- 12.Barbieri MA, Li G, Colombo MI, Stahl PD. J Biol Chem. 1994;269:18720–18722. [PubMed] [Google Scholar]
- 13.Barbieri MA, Roberts RL, Gumusboga A, Highfield H, Alvarez-Dominguez C, Wells A, Stahl PD. J Cell Biol. 2000;151:539–550. doi: 10.1083/jcb.151.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li G, D’Souza-Schorey C, Barbieri MA, Roberts RL, Klippel A, Williams LT, Stahl PD. Proc Natl Acad Sci U S A. 1995;92:10207–10211. doi: 10.1073/pnas.92.22.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horiuchi H, Lippe R, McBride HM, Rubino M, Woodman P, Stenmark H, Rybin V, Wilm M, Ashman K, Mann M, Zerial M. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- 16.Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 17.McBride HM, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M. Cell. 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- 18.Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M. Nat Cell Biol. 1999;1:249–252. doi: 10.1038/12075. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, Hoflack B, Zerial M. J Cell Biol. 2000;151:601–612. doi: 10.1083/jcb.151.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lippe R, Miaczynska M, Rybin V, Runge A, Zerial M. Mol Biol Cell. 2001;12:2219–2228. doi: 10.1091/mbc.12.7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tall GG, Barbieri MA, Stahl PD, Horazdovsky BF. Dev Cell. 2001;1:73–82. doi: 10.1016/s1534-5807(01)00008-9. [DOI] [PubMed] [Google Scholar]
- 22.Sorkina T, Huang F, Beguinot L, Sorkin A. J Biol Chem. 2002;277:27433–27441. doi: 10.1074/jbc.M201595200. [DOI] [PubMed] [Google Scholar]
- 23.Galvis A, Balmaceda V, Giambini H, Conde A, Villasana Z, Fornes MW, Barbieri MA. Arch Biochem Biophys. 2009;482:83–95. doi: 10.1016/j.abb.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondratov KA, Chernorudskiy AL, Amosova AP, Kornilova ES. Cell Biol Int. 2010;34:81–87. doi: 10.1042/CBI20090159. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto N, Mammadova G, Song RX, Fukami Y, Sato K. J Cell Sci. 2006;119:4623–4633. doi: 10.1242/jcs.03236. [DOI] [PubMed] [Google Scholar]
- 26.Gruenberg JE, Howell KE. EMBO J. 1986;5:3091–3101. doi: 10.1002/j.1460-2075.1986.tb04615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayes N, Howard-Cofield E, Gullick W. Cancer Lett. 2004;206:129–135. doi: 10.1016/j.canlet.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Wells A, Ware MF, Allen FD, Lauffenburger DA. Cell Motil Cytoskeleton. 1999;44:227–233. doi: 10.1002/(SICI)1097-0169(199912)44:4<227::AID-CM1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 29.Wiley HS. Exp Cell Res. 2003;284:78–88. doi: 10.1016/s0014-4827(03)00002-8. [DOI] [PubMed] [Google Scholar]
- 30.Hunker CM, Giambini H, Galvis A, Hall J, Kruk I, Veisaga ML, Barbieri MA. Exp Cell Res. 2006;312:1106–1118. doi: 10.1016/j.yexcr.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Barbieri MA, Kong C, Chen PI, Horazdovsky BF, Stahl PD. J Biol Chem. 2003;278:32027–32036. doi: 10.1074/jbc.M304324200. [DOI] [PubMed] [Google Scholar]
- 32.Wolff M, Tetzlaff K, Nivens MC, Schneider FJ, Jung B, Hohlfeld J, Heilker R. Exp Cell Res. 2011;317:42–50. doi: 10.1016/j.yexcr.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Thomas CY, Chouinard M, Cox M, Parsons S, Stallings-Mann M, Garcia R, Jove R, Wharen R. Int J Cancer. 2003;104:19–27. doi: 10.1002/ijc.10880. [DOI] [PubMed] [Google Scholar]
- 34.Roberts RL, Barbieri MA, Pryse KM, Chua M, Morisaki JH, Stahl PD. J Cell Sci. 1999;112(Pt 21):3667–3675. doi: 10.1242/jcs.112.21.3667. [DOI] [PubMed] [Google Scholar]
- 35.Gorvel JP, Chavrier P, Zerial M, Gruenberg J. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- 36.Wainszelbaum MJ, Proctor BM, Pontow SE, Stahl PD, Barbieri MA. Exp Cell Res. 2006;312:2238–2251. doi: 10.1016/j.yexcr.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- 38.Mu FT, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo JP, Tock EP, Toh BH. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- 39.Singh SB, Tandon R, Krishnamurthy G, Vikram R, Sharma N, Basu SK, Mukhopadhyay A. EMBO J. 2003;22:5712–5722. doi: 10.1093/emboj/cdg557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu H, Liang Z, Li G. Mol Biol Cell. 2009;20:4720–4729. doi: 10.1091/mbc.E09-06-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu H, Zhu G, Liu J, Liang Z, Zhang XC, Li G. Mol Biol Cell. 2007;18:4119–4128. doi: 10.1091/mbc.E07-02-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunker CM, Galvis A, Kruk I, Giambini H, Veisaga ML, Barbieri MA. Biochem Biophys Res Commun. 2006;340:967–975. doi: 10.1016/j.bbrc.2005.12.099. [DOI] [PubMed] [Google Scholar]
- 43.Han L, Colicelli J. Mol Cell Biol. 1995;15:1318–1323. doi: 10.1128/mcb.15.3.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semerdjieva S, Shortt B, Maxwell E, Singh S, Fonarev P, Hansen J, Schiavo G, Grant BD, Smythe E. J Cell Biol. 2008;183:499–511. doi: 10.1083/jcb.200806016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattera R, Tsai YC, Weissman AM, Bonifacino JS. J Biol Chem. 2006;281:6874–6883. doi: 10.1074/jbc.M509939200. [DOI] [PubMed] [Google Scholar]
- 46.Penengo L, Mapelli M, Murachelli AG, Confalonieri S, Magri L, Musacchio A, Di Fiore PP, Polo S, Schneider TR. Cell. 2006;124:1183–1195. doi: 10.1016/j.cell.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 47.Su X, Kong C, Stahl PD. J Biol Chem. 2007;282:21278–21284. doi: 10.1074/jbc.M703725200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.