Abstract
Macrophage migration inhibitory factor (MIF) has been shown to be involved in the pathogenesis of severe malaria. Malaria parasites express an MIF homolog that may play a role in regulating host immune responses and a recent study showed that overexpression of MIF reduced parasitemia in a mouse malaria model. Another recent study showed migration of monocytes to the spleen contributed to the control of blood stage infection. However, there are few papers describing the effect of MIF on monocyte recruitment/activation during the infection. We generated recombinant P. yoelii MIF (rPyMIF) and investigated its function on purified mouse CD11b+ cells in vitro and monocyte responses in vivo. The result shows that rPyMIF protein bound to mouse CD11b+ cells and inhibited their random migration in vitro. On the other hand, rPyMIF did not induce cytokine release from the cells directly or modulate LPS-induced cytokine release. Mice immunized with rPyMIF showed transient, but significantly lower parasitemia than the control mice at day 3 after lethal Py17XL challenge. The total number of CD11b+ cells in the spleens was significantly higher in rPyMIF-immunized group. Further investigation revealed that there were significantly higher numbers of recruited and activated monocytes in the spleens of rPyMIF immunization group on day 3. These results indicate that PyMIF potentially modulates monocyte recruitment and activation during infection of P. yoelii erythrocytic stages.
Keywords: malaria, macrophage migration inhibitory factor, mouse, monocyte, antibody
Introduction
Most malaria infections either remain asymptomatic or progress to disease without lethal complications in people who live in malaria endemic areas due to the acquisition of clinical but nonsterilizing immunity after repeated exposure. Viewed in an evolutionary context, malaria may have co-evolved with host immune responses and developed strategies to circumvent host defenses like other microorganisms.
Macrophage migration inhibitory factor (MIF) is a highly evolutionarily conserved 12.5-kDa protein that can inhibit random migration of macrophages (Baugh and Bucala 2002). MIF is a multifunctional, immunoregulatory, and proinflammatory cytokine secreted by many cell types including macrophages and T cells (Baugh and Bucala 2002; de Jong et al. 2001; Leech et al. 2000) and has been shown to be involved in many human inflammatory diseases (Santos et al. 2001; Takahashi et al. 2001). Host MIF has been reported to be involved in the pathogenesis of severe malarial anemia in humans (McDevitt et al. 2006; Awandare et al. 2007). In recent years, homologs of human MIF have been identified from Plasmodium falciparum (PfMIF), P. berghei (PbMIF), and P. yoelii (PyMIF) (Shao et al. 2008 and 2010; Augustijn et al. 2007; Cordery et al. 2007; Thorat et al. 2010). Interestingly, the malaria-derived MIF exhibits biochemical and immunostimulatory features similar to those of host MIF and may play a role in regulating host immune responses to help parasites survive within their hosts. PfMIF was shown to have chemotactic activity on human monocytes and reduce surface expression of Toll-like receptor (TLR) 2, TLR4, and CD86 (Cordery et al. 2007). PyMIF has a three-dimensional structure similar to that of mouse MIF and is capable of activating the MAPK/ERK and PI3K/AKT pathways in the NIH/3T3 cell line (Shao et al. 2010). While PbMIF knockout (KO) parasites exhibited no significant difference in parasitemia in vivo compared to wild-type parasites (Augustijn et al. 2007), a recent study with transgenic virulent Py17XL parasites that constitutively overexpressed PyMIF showed reduction of mortality (Thorat et al. 2010).
Recently, the roles of monocytes in malaria pathogenesis have received increasing attention. Monocytes have been reported to be important in the first line of innate defense against malaria (Mohan and Stevenson 1998; Urquhart 1994). Activation of monocytes can induce production of parasiticidal mediators and receptor-mediated phagocytosis (Greve et al. 1999; Patel et al. 2004; Sponaas et al. 2009). Monocytes have also been found to be associated with sequestration of infected erythrocytes in cerebral post-mortem specimens in P. falciparum (Jenkins et al. 2006). In the P. chabaudi challenge model, it has been shown that transgenic mice lacking a chemokine receptor CCR2, which is known to be involved in monocyte recruitment to the spleen, showed prolonged high parasitemia compared to wild type mice (Sponaas et al. 2009). However, the effect of malaria MIF on monocyte recruitment/activation during malaria infection has not been studied yet. To answer this question, we generated recombinant P. yoelii MIF (rPyMIF) protein and investigated its ability to modulate function of mouse CD11b+ cells in vitro. CD11b is widely used as a marker of mouse monocyte and monocyte lineage cells, but other types of cells, such as natural killer (NK) cells, granulocytes, also express the marker to a lower extent. Therefore, using additional markers, we explored the effect of rPyMIF immunization on the monocyte response during Py17XL infection.
Materials and methods
Cloning and expression of rPyMIF
Messenger RNA (mRNA) was isolated from blood-stage P. yoelii 17XL parasites with TRIZOL agent (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized using a commercial kit (Invitrogen). Sequence coding for PyMIF was PCR amplified using PyMIF-specific primers: PyMIF forward (5'-catggatccatgccttgctgcgaatta-3' with a BamHI restriction site) and PyMIF reverse (5'-atcgtcgacttagccaaatagtgaacc-3' with a SalI restriction site). After amplification, PCR products were digested with the restriction enzymes and cloned into plasmid vector pET32a (Invitrogen). Plasmids containing inserts were purified and sequenced to verify correct coding sequence and reading frame and were introduced into Escherichia coli (strain BL21; New England Biolabs, Ipswich, MA, USA). Bacteria with the plasmid from an overnight culture were diluted (1:100) and grown to an optical density of 1 OD. Expression of rPyMIF was induced at 37°C by addition of 0.1 mM IPTG for 5 h. The recombinant protein, expressed as an rPyMIF-trxA (thioredoxin) fusion protein, was purified using 6X His-tag/Ni-NTA affinity chromatography (Qiagen, Valencia, CA, USA). Eluted proteins were dialyzed against loading buffer (25 mM Tris-HCl, pH 7.8, and 50 mM NaCl). To remove the trxA fusion protein, the purified protein was cleaved with enterokinase (Roche, Indianapolis, IN, USA) and the trxA protein (with his-tag) was removed using a 6X His-tag/Ni-NTA affinity chromatography. Endotoxin in either rPyMIF-trxA or rPyMIF protein solution was removed using Detoxi-Gel endotoxin removing columns (Pierce, Rockford, IL, USA).
Western blot
A P. yoelii 17XL parasite pellet was dissolved in 1X sample loading buffer containing 0.5 M Tris-HCl (pH 6.8), 4.4% (w/v) SDS, 20% (v/v) glycerol, 2% (v/v) 2-mercaptoethanol, and 0.1% (w/v) bromophenol blue in deionized water and was further denatured by placing the proteins in boiling water for 10 min. Mouse MIF (mMIF) protein was purchased from R&D Systems (Minneapolis, MN, USA). SDS-PAGE gels were run under 180 V until the tracking dye reached the bottom of the gel. The proteins were transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA); and the membranes were blocked with blocking buffer (5% skim milk in 1Xphosphate-buffered saline Tween-20) for 2 h at 22°C. The membranes were probed with sera from mice immunized with rPyMIF-trxA fusion protein, rabbit anti-mouse MIF antibody (Invitrogen), or normal mouse sera. Sera were diluted appropriately and incubated with the membrane at 22°C for 2 h. After washing three times with washing buffer, the membranes were again incubated with anti-mouse IgG (R&D) or anti-rabbit IgG conjugated with horseradish peroxidase at 22°C for 1 h. The membranes were washed five times in washing buffer and developed in SuperSignal West Pico chemiluminescent substrate (Thermo, Pittsburgh, PA, USA).
Binding analysis
All animal studies were done in compliance with National Institutes of Health (NIH) guidelines and under the auspices of an Animal Care and Use Committee-approved protocol (LMVR 10E). The rPyMIF protein was conjugated with fluorescent dye PE/Cy5 using Lightning-Link™ PE-Cy5 tandem conjugation kit (Innova Bioscience, Cambridge, UK). Splenocytes (2 × 106) from naïve 4- to 6-week-old BALB/c mice (Taconic, Germantown, NY, USA) were incubated with 5 μg/ml 2.4G2 (Fc block; BD Bioscience, San José, CA, USA) for 10 min at 4°C. The cells were incubated for 45 min at 4°C with 5 μg/ml FITC rat anti-mouse CD11b (BD Bioscience, San jose, CA) and/or 200 ng PE/Cy5-labeled rPyMIF. Cell acquisition was performed in a FACSCalibur (BD Immunocytometry, San jose, CA), and data analysis was performed using FlowJo (TreeStar Inc., Ashland, OR).
Mouse CD11b+ cell migration assay and cytokine production in vitro
Mouse CD11b+ cell were purified from the spleens of naïve BALB/c mice using anti-CD11b magnetic beads (MACS, Auburn, CA). Migration assays were performed using the QCM chemotaxis 5 μm 24-well cell migration assay kit (Millipore, Billerica, MA, USA). The purified CD11b+ cells (2.5 × 105) in 250 μl medium with or without rPyMIF were added to the upper chamber, and 400 μl medium was added to the lower chamber. Plates were incubated immediately at 37°C in 5% CO2 for 4 h. The experiment was performed according to the manufacturer's instructions, and the samples were read on a Synergy HT multi-mode microplate reader (BioTek, Winooski, VT, USA).
For cytokine experiments, 1 × 106/ml purified CD11b+ cells were incubated with 100 ng/ml or 500 ng/ml of rPyMIF or 100 pg/ml of LPS (Sigma, St. Louis, MO, USA) at 37°C in 5% CO2 for 18 h. After incubation, supernatants were collected and analyzed using a CBA mouse inflammation kit (BD Bioscience, San José, CA, USA).
Mouse immunization and P. yoelii challenge
One group of 15 4- to 6-week-old BALB/c mice was immunized subcutaneously at three sites with 50 μg of rPyMIF-trxA protein. Another group of 15 mice was immunized with the same molar concentration of trxA protein, as a control. The protein antigens were given with complete Freund's adjuvant (Sigma) for the initial immunization; the second and third immunizations were given in incomplete Freund's adjuvant (Sigma) at 3-week intervals. Sera were collected before the first immunization (pre-immune sera) and 2 weeks after the third immunization for ELISA. Two weeks after the third immunization, the mice were infected intraperitoneally with 1 × 105 P. yoelii 17XL parasites. Three mice from each group were sacrificed to harvest spleens for cellular assays at days 0, 3, 5, and 7 post infection. Serum samples were also collected from each group for cytokine assay and ELISA. Blood smears were prepared daily from day 3 post infection. Parasitemia was determined microscopically by counting percentage of parasitized erythrocytes in 1000 red blood cells. Mice were euthanized when they were moribund.
ELISA
The standardized methodology for performing the ELISA has been described previously (Miura et al. 2008). A 96-well plate was coated with 100 ng/well of purified rPyMIF (without trxA) or trxA protein. An ELISA unit of a standard was assigned as the reciprocal of the dilution giving an OD 405 of 1 in a standardized assay. The OD of individual test samples was converted into ELISA units using a standard curve generated from a serially diluted standard; ELISA units of the standard were fixed once assigned, regardless of actual OD 405 value of a standard curve in a plate.
Surface and intracellular cytokine staining and flow cytometry
Single-cell suspensions of splenocytes from P. yoelii-challenged mice were prepared by passing the cells through a 70 μm cell strainer (BD Biosciences), and live cells were enumerated by trypan blue exclusion (Lonza, Walkersville, MD, USA). Splenocytes from the mice were stained with antibodies immediately. Activation status and cell phenotype of monocytes were determined using flow cytometry and the following antibodies: FITC rat anti-mouse CD80, PerCP rat anti-mouse CD11b, APC rat anti-mouse TNF-α, PE hamster anti-mouse CD54, FITC rat anti-mouse CD40, PE rat anti-mouse IL-6, APC rat anti-mouse IL-12(p40/p70), PE rat anti-mouse Ly6C, FITC rat anti-mouse CD62L, and APC rat anti-mouse F4/80. All antibodies were purchased from BD Bioscience except F4/80 (Biolegend, San Diego, CA, USA). Cell acquisition was performed in a FACSCalibur, and data analysis was performed using FlowJo.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA). An unpaired t-test was used to compare the difference between two groups; probability values less than 0.05 were considered significant.
Results
Characterization of rPyMIF protein and anti-rPyMIF antibody
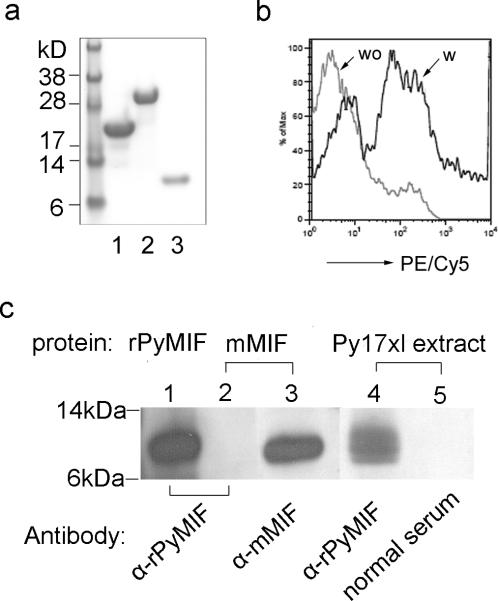
Recombinant PyMIF-trxA fusion protein (rPyMIF-trxA) was expressed in E. coli, and the soluble protein was purified using an Ni-NTA affinity column. The fusion protein was digested with enterokinase to remove trxA-Tag protein. We successfully produced rPyMIF-trxA, rPyMIF, and trxA as a control protein with more than 95% purity (Fig. 1a). Purified rPyMIF was conjugated with fluorescent dye PE/Cy5 for binding assays. Using flow cytometry, we found that PE/Cy5-rPyMIF could bind to mouse CD11b+ monocytes (Fig. 1b). Crossreactivity between mouse and malaria MIF proteins and anti-mouse MIF and anti-rPyMIF antibodies was evaluated using Western blot (Fig. 1c). The anti-rPyMIF-trxA serum from immunized mice recognized both rPyMIF and native PyMIF protein in malaria extracts, but it did not react with mMIF protein (Fig. 1c). These results suggest that anti-PyMIF antibody may not neutralize host-derived MIF, while PyMIF can bind to host monocytes.
Fig. 1. Characterization of rPyMIF protein and anti-rPyMIF antibody.
a) The rPyMIF-trxA protein was expressed using pET-32a vector in Escherichia coli (strain BL21) and purified using a nickel-agarose column. rPyMIF (without trxA) was prepared by digesting rPyMIF-trxA protein with enterokinase and separating the components by binding to a nickel-agarose column: lane 1, trxA tag produced by vector the pET-32a; lane 2, rPyMIF-trxA fusion protein; lane 3, rPyMIF without trxA protein. b) Flow cytometry analysis of the binding of PE/Cy5-rPyMIF to mouse splenic CD11b+ cells. Splenocytes were prepared from a naïve mouse, and CD11b+ cells were gated. wo, mouse CD11b+ cells without rPyMIF incubation; w, mouse CD11b+ cells incubated with PE/Cy5-rPyMIF. c) Reactivity of anti-rPyMIF-trxA antibody and anti-mMIF antibody was tested on Western blot: lane 1, rPyMIF protein incubated with anti-rPyMIF-trxA antibody; lane 2, mMIF protein incubated with anti-rPyMIF-trxA antibody; lane 3, mMIF protein incubated with anti-mMIF antibody; lane 4, Py17XL parasite extract incubated with anti-rPyMIF-trxA antibody; lane 5, Py17XL parasite extract incubated with normal mouse serum.
Migration and activation of CD11b+ cells mediated by rPyMIF
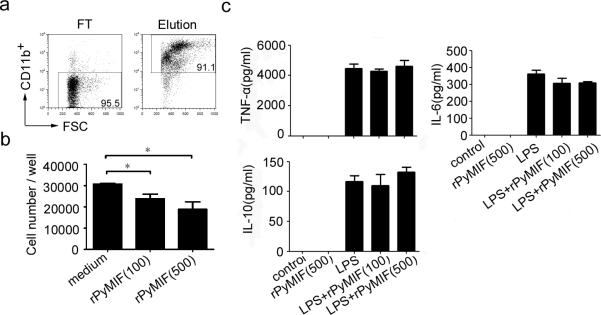
We next investigated whether rPyMIF showed any activity on migration of CD11b+ cells in vitro (Fig. 2). Migration assays showed that random migration of CD11b+ cells was significantly inhibited by treatment with 100 ng/ml or 500 ng/ml rPyMIF compared with control (p = 0.0335 and 0.0286, respectively; unpaired t-test) (Fig. 2b). Cytokine production in CD11b+ cells by rPyMIF stimulation was determined (Fig. 2c). Treatment with 500 ng/ml rPyMIF did not result in any increase in production of TNF-α, IL-6 or IL-10 within 18 h. Similarly, no production of IL-12 and MCP-1 was observed by rPyMIF (data not shown). Moreover, there was no effect of rPyMIF on LPS-induced production of TNF-α, IL-6, or IL-10. The results indicate that rPyMIF protein produced in this study could modulate migration of CD11b+ cells but did not elicit cytokine production.
Fig. 2. In vitro migration and cytokine production of mouse splenic CD11b+ cells treated with rPyMIF.
a) Mouse CD11b+ cells were purified by use of anti-CD11b magnetic beads from the spleens of naïve BALB/c mice. FT, flow through faction; Elution, collected CD11b+ cells fraction. b) Random migration of CD11b+ cells across a membrane was assessed in presence of rPyMIF. Purified mouse CD11b+ cells (2.5 × 105) were added to the upper chamber with 100 or 500 ng/ml of rPyMIF, and the number of migrated cells in the bottom chamber was determined 4 h after incubation. The mean and SEMs of numbers of CD11b+ cells in triplicate wells are shown. *, p < 0.05 (unpaired t-test). c) Culture supernatant from 1 × 106/ml monocytes treated with rPyMIF (100 or 500 ng/ml) and/or LPS (100 pg/ml) for 18 h was assessed for TNF-α, IL-6 and IL-10 release using a BD CBA mouse inflammation kit. The mean and SEM of cytokine levels in triplicate wells are shown. Data are representative of two independent experiments.
rPyMIF immunization recruited activated-monocytes to the spleen during Py17XL parasite infection
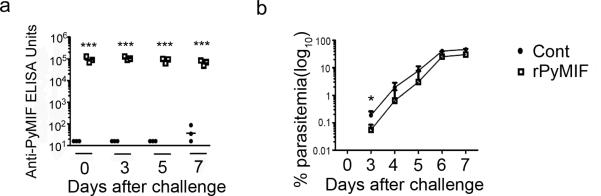
To evaluate the effect of PyMIF in vivo, we conducted a challenge study with P. yoelii 17XL (lethal) parasites. Before challenge, we first immunized BALB/c mice with 50 μg rPyMIF-trxA fusion protein (rPyMIF group) or the same molar concentration of trxA (control group). To confirm that the immunization induced anti-PyMIF antibody in mice, the antibody titer was determined in each mouse using ELISA plates coated with rPyMIF (without trxA) protein two weeks after the third immunization (day 0 of challenge). The rPyMIF group showed significantly higher anti-rPyMIF antibody titers than the control group (p < 0.0001 by unpaired t-test) (Fig. 3a). Both groups showed almost the same level of anti-trxA antibody (data not shown). After the immunization, we challenged the mice with 1 × 105 P. yoelii 17XL parasites. The rPyMIF group showed significantly lower parasitemia than controls at day 3 after challenge (p = 0.0472 by unpaired t-test); however, the differences in parasitemia between rPyMIF-immunized and trxA-immunized groups were not significant after day 3 (Fig. 3b). Anti-rPyMIF antibodies were sustained at high levels in the rPyMIF group until 7 days after challenge (Fig. 3a).
Fig. 3. rPyMIF-trxA vaccination changed Py17XL parasitemia significantly at the early stage of infection.
BALB/c mice (n = 15 per group) were immunized three times with rPyMIF-trxA or trxA formulated with Freund's adjuvant or incomplete Freund's adjuvant. Two weeks after the third immunization, the mice were challenged with 1 × 105 Py17XL parasites. a) At days 0, 3, 5, and 7 after challenge, sera were collected from three mice from each group to determine the anti-rPyMIF ELISA units. Individual ELISA data and the geometric mean of the group are shown. ***, p < 0.0001 (unpaired t-test). b) Parasitemia was monitored every day starting at day 3 after challenge. Mean parasitemia and the standard error of the group are shown. There was a significant difference between the two groups at day 3 (p = 0.0472, unpaired t-test). Similar data were obtained in two independent experiments.
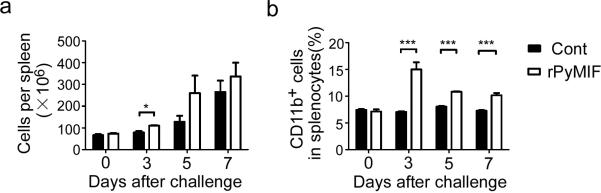
To investigate the effects of anti-PyMIF antibody on mouse splenic monocytes during infection, splenocytes were harvested from both rPyMIF-immunized and control trxA groups. There were more splenocytes per spleen in the rPyMIF-immunized group compared with the control at day 3 after challenge (p = 0.0170 by unpaired t-test) (Fig. 4a). The percentage of CD11b+ cells in the spleen also significantly increased in the rPyMIF group during infections (p < 0.0001 at day 3, 5, and 7; Fig. 4b), and the biggest difference was observed at day 3 after challenge.
Fig. 4. rPyMIF immunization modulated number of CD11b+ cells in the spleen during Py17XL infection.
BALB/c mice were immunized and challenged with Py17XL parasites as described in the Fig. 3 legend. At days 0, 3, 5, and 7 after challenge, spleens were harvested from three mice for each group. The splenocytes per spleen were counted and stained with various surface makers. a) Means and SEMs of numbers of splenocytes per spleen are shown. There was a significant difference between the two groups (p = 0.0170, unpaired t-test) at day 3. b) Means and SEMs of percentage of CD11b+ cells in total splenocytes are shown. There was a significant difference between the two groups (p < 0.0001, unpaired t-test) at days 3, 5, and 7. Similar data were obtained in two independent experiments.
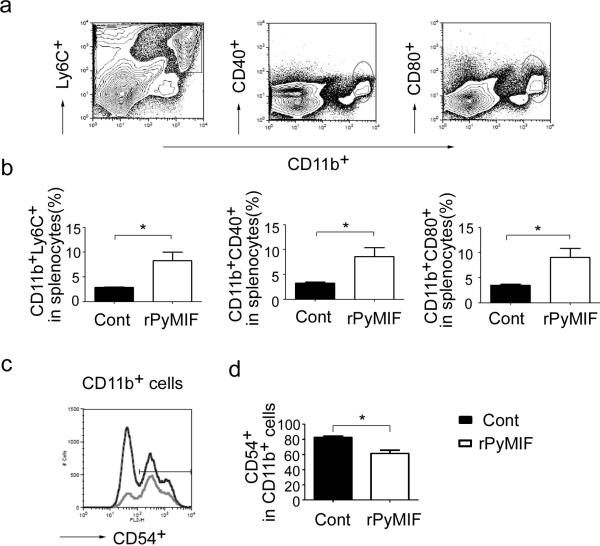
Because there were significant differences between the two groups at day 3 after challenge in terms of parasitemia and total number of CD11b+ cells in the spleen, we further analyzed the characteristics of CD11b+ cells at day 3 using flow cytometry (Fig.5). Additional markers were used to classify monocyte subpopulation and also to differentiate them from other CD11b+ cells. Ly6C, a marker of inflammatory monocytes, has been identified as a marker of monocytes that migrate from the bone marrow (BM) into sites of inflammation. In addition, such cells have been reported to be associated with protective responses in P. chabaudi infection (Sponaas et al. 2009). The percentage of CD11b+Ly6C+ cells per spleen in the rPyMIF-immunized group was 2.9-fold higher than that in the control group (Fig. 5b). Similarly, there were more activated monocytes per spleen in the rPyMIF group as judged by activation markers CD40 and CD80 (Fig. 5b). We also analyzed other activation or subpopulation markers of monocytes such as CD86, CD62L, and F4/80 and found that the percentage of these subpopulations of monocytes per spleen was higher in the rPyMIF-immunized group (data not shown); however, when the percentage of these monocyte subpopulations in the CD11b+ cells was calculated (e.g., the number of CD11b+Ly6C+ cells was divided by the number of CD11b+ cells, instead of dividing by the number of total splenocytes, etc.), there was no difference in rPyMIF-immunized and trxA control groups (data not shown). The only exception was that the proportion of CD11b+CD54+ cells in CD11b+ cells was lower in the rPyMIF-immunized group than in the control (Fig. 5d). The results suggest that rPyMIF immunization modulates host monocyte responses during a malaria infection.
Fig.5. rPyMIF immunization recruited activated-monocytes to the spleen during Py17XL infection.
a) Gating profiles of CD11b+Ly6C+, CD11b+CD40+, CD11b+CD80+ cells in mouse spleen are shown. b) Means and SEMs of the percentage of CD11b+Ly6C+, CD11b+CD40+, CD11b+CD80+ cells in splenocytes at day 3 after challenge are shown. There was a significant difference between the groups immunized with rPYMIF and the trxA controls (p < 0.05, unpaired t-test). c) Gating profile of CD54+ population is shown. The curve with dark line represents the rPyMIF immunized group, and the lighter line represents the trxA immunized group. d) Means and SEMs of the percentage of CD54+ cells in CD11b+ cells at day 3 after challenge are shown. There was a significant difference between the rPyMIF immunized group and the trxA immunized group (p = 0.0121, unpaired t-test).
Discussion
In this study, we expressed a recombinant PyMIF protein that was shown to bind to mouse CD11b+ cells and inhibit their random migration. We found that mice immunized with rPyMIF-trxA protein had significantly lower parasitemia at day 3 after challenge compared with mice immunized with trxA alone. Corresponding to the reduction of parasitemia on day 3, higher numbers of recruited and activated monocytes in spleens were observed in mice immunized with rPyMIF-trxA protein. The results suggest that malaria MIF may suppress monocyte recruitment and activation during the infection.
Orthologs of MIF have been identified in numerous invertebrate species, including nematodes, protozoa, and ticks (Falcone et al. 2001; Tan et al. 2001; Pastrana et al. 1998; Augustijn et al. 2007; Miska et al. 2007; Marson et al. 2001; Wu et al. 2003; Umemiya et al. 2007), which indicates the importance of this cytokine in a wide variety of organisms. The amino acid sequence of PyMIF shares only 30% identity with murine MIF (Shao et al. 2010), and our Western blot experiment showed that antibodies against rPyMIF did not recognize mouse MIF. The result indicates their immunoreactive epitopes may be different, and anti-rPyMIF antibody did not interfere with mMIF in the rPyMIF-trxA immunization study. Despite the low homology, we showed that PyMIF could bind to mouse CD11b+ cells and inhibit random migration of these cells in vitro. A similar phenomenon was reported by Cordery et al. (2007), where random migration of human monocytes was inhibited by treatment with PfMIF. These results suggest that the activation motif of MIF may be conserved in different species. In addition to the random migration assay, we also examined the function of rPyMIF on cytokine production. The rPyMIF protein did not directly induce TNF-α, IL-6, IL-10, or IL-12 cytokine production. A similar phenomenon was reported by Cordery—that human monocytes treated with PfMIF did not release different levels of IL-8, TNF-α, or IL-13 compared with control (Cordery et al. 2007). In addition, a recent paper by Thorat, et al., also showed that rPyMIF alone did not induce significant TNF-α release from peritoneal macrophages compared with the medium control (Thorat et al. 2010). These previous results, along with our current study, indicate that malaria MIF by itself may not have the capacity to induce cytokines from host cells (at least from CD11b+ cells).
We also immunized mice with rPyMIF-trxA protein with the goal of generating neutralizing antibodies that target PyMIF protein produced by P. yoelii during the infection. At day 3 after challenge, there was a small but significant reduction of parasitemia in the rPyMIF-trxA immunized group compared with trxA (control) group; however, reduction of parasitemia was observed only at this early stage of the infection, and there was no significant differences in parasitemias after day 4 of challenge, while the level of anti-PyMIF antibodies in the serum was sustained during infection. The result suggests that PyMIF may have only minor effects on parasite replication in vivo in this very virulent infection, likely due to rapid increase in the number of parasites. In addition, a recent report by Thorat, et al. (Thorat et al. 2010) also showed that there was marginal or no difference in parasitemia between transgenic P. yoelii 17XL parasites that overexpress PyMIF and control mice through day 8 of infection. Similarly, PbMIF knockout (KO) parasites exhibited no significant difference in parasitemia in vivo compared to wild-type parasites (Augustijn et al. 2007). Taken together, these results suggest that parasite-derived MIF has only minor effects on parasitemia.
Monocytes derived from bone marrow (BM) hematopoiesis play an important role in host defense against many types of pathogens, including Plasmodium (Serbina et al. 2008). CD11bhiLy6C+ cells have been described as inflammatory monocytes that migrate from the BM to sites of infection in the periphery. It has been reported that these migrating monocytes recruited to the spleen play an important role in clearance of P. chabaudi parasites (Sponaas et al. 2009). These inflammatory monocytes also express the F/40 and CD62L antigens (Sponaas et al. 2009; Serbina et al. 2008). In this study, we show that while P. yoelii infection increased the total number of splenocytes in the spleen in both groups, significantly higher % of CD11b+ cells in splenocytes were seen in the PyMIF-immunization group, a procedure that presumably reduces the level of PyMIF during infection. When we analyzed this subpopulation of monocytes further, the percentage of CD11bhiLy6C+ and CD40+, CD80+ activated monocytes in splenocytes also increased in the presence of anti-PyMIF antibodies after challenge. These results suggest that PyMIF protein may play a role in downregulating the host immune response through reduction in inflammatory monocyte recruitment from bone marrow and downregulation of monocytes during parasite infection. While it may limit the immune response, it may also result in less pathology for the host.
We also observed increases in another subpopulation of monocytes (CD62L, F4/80, CD54) in total splenocytes in rPyMIF group (data not shown). When the percent of each subpopulation of monocytes in total CD11b+ cells was calculated, however, there was no difference between rPyMIF and control groups, indicating that PyMIF neutralization (by anti-rPyMIF antibody) can increase total number of monocytes in spleen but does not change the composition of monocytes. The exception was CD54+ (ICAM-1) monocytes: the percentage of CD54+ cells in the CD11b+ monocyte population was lower in the rPyMIF-immunized group than that in the control group. Human MIF has been reported to upregulate expression of ICAM-1 on vascular endothelial cells and was associated with monocyte adhesion (Lue et al. 2002). Expression of ICAM-1 on antigen-presenting cells has been identified as a co-stimulatory ligand during antigen presentation (Lebedeva et al. 2005), and children with severe malaria have been found to have increased expression of ICAM-1 on monocytes (Jenkins et al. 2006). Other studies show that hemozoin-loaded monocytes have decreased ICAM-1 expression (Schwarzer et al. 1998; Skorokhod et al. 2004). These results suggest the importance of ICAM-1 (CD54+) expression on monocytes (or the lineage cells) during malaria infection. Additionally, we performed intracellular cytokine staining with the splenocytes harvested from both groups of mice. While we detected a few TNF-α-, IL-6-, IL-10-, or IL-12-producing CD11b+ cells, there were no differences between rPyMIF and control groups (data not shown). This is consistent with our in vitro stimulation results (i.e., no cytokine induction by rPyMIF stimulation and no modification under LPS stimulation conditions). Therefore, it was suggested that the reduction of parasitemia at day 3 after challenge in rPyMIF group was not due to modification of cytokine produced from monocytes by PyMIF. Monocyte recruitment and activation induced by the rPyMIF immunization might contribute to the slight modulation of parasitemia at the early stage of infection. Alternatively, immunization with PyMIF may prime or stimulate the host innate immune system, leading to the transient lower parasitemia.
Acknowledgements
We thank Lubin Jiang and Cecilia Huaman for their technical help, and thank the animal facility in NIH Twinbrook 3 for taking care of the mice. We also thank intramural editor Brenda Rae Marshall for editorial assistance. This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health and by grants from the National Basic Research Program of China (973 Program) 2007CB513103, from the Science Planning Program of Fujian Province (2010J1008), and from 111 Project of Education of China (NO. B06016).
References
- Augustijn KD, Kleemann R, Thompson J, Kooistra T, Crawford CE, Reece SE, Pain A, Siebum AH, Janse CJ, Waters AP. Functional characterization of the Plasmodium falciparum and P. berghei homologues of macrophage migration inhibitory factor. Infect Immun. 2007;75:1116–1128. doi: 10.1128/IAI.00902-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awandare GA, Ouma Y, Ouma C, Were T, Otieno R, Keller CC, Davenport GC, Hittner JB, Vulule J, Ferrell R, Ong'echa JM, Perkins DJ. Role of monocyte-acquired hemozoin in suppression of macrophage migration inhibitory factor in children with severe malarial anemia. Infect Immun. 2007;75:201–210. doi: 10.1128/IAI.01327-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh JA, Bucala R. Macrophage migration inhibitory factor. Crit Care Med. 2002;30:S27–S35. [PubMed] [Google Scholar]
- Cordery DV, Kishore U, Kyes S, Shafi MJ, Watkins KR, Williams TN, Marsh K, Urban BC. Characterization of a Plasmodium falciparum macrophage-migration inhibitory factor homologue. J Infect Dis. 2007;195:905–912. doi: 10.1086/511309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong YP, Abadia-Molina AC, Satoskar AR, Clarke K, Rietdijk ST, Faubion WA, Mizoguchi E, Metz CN, Alsahli M, ten Hove T, Keates AC, Lubetsky JB, Farrell RJ, Michetti P, van Deventer SJ, Lolis E, David JR, Bhan AK, Terhorst C. Development of chronic colitis is dependent on the cytokine MIF. Nat Immunol. 2001;2:1061–1066. doi: 10.1038/ni720. [DOI] [PubMed] [Google Scholar]
- Falcone FH, Loke P, Zang X, MacDonald AS, Maizels RM, Allen JE. A Brugia malayi homolog of macrophage migration inhibitory factor reveals an important link between macrophages and eosinophil recruitment during nematode infection. J Immunol. 2001;167:5348–5354. doi: 10.4049/jimmunol.167.9.5348. [DOI] [PubMed] [Google Scholar]
- Greve B, Lehman LG, Lell B, Luckner D, Schmidt-Ott R, Kremsner PG. High oxygen radical production is associated with fast parasite clearance in children with Plasmodium falciparum malaria. J Infect Dis. 1999;179:1584–1586. doi: 10.1086/314780. [DOI] [PubMed] [Google Scholar]
- Jenkins NE, Chakravorty SJ, Urban BC, Kai OK, Marsh K, Craig AG. The effect of Plasmodium falciparum infection on expression of monocyte surface molecules. Trans R Soc Trop Med Hyg. 2006;100:1007–1012. doi: 10.1016/j.trstmh.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Lebedeva T, Dustin ML, Sykulev Y. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr Opin Immunol. 2005;17:251–258. doi: 10.1016/j.coi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Leech M, Metz C, Bucala R, Morand EF. Regulation of macrophage migration inhibitory factor by endogenous glucocorticoids in rat adjuvant-induced arthritis. Arthritis Rheum. 2000;43:827–833. doi: 10.1002/1529-0131(200004)43:4<827::AID-ANR13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Lue H, Kleemann R, Calandra T, Roger T, Bernhagen J. Macrophage migration inhibitory factor (MIF): mechanisms of action and role in disease. Microbes Infect. 2002;4:449–460. doi: 10.1016/s1286-4579(02)01560-5. [DOI] [PubMed] [Google Scholar]
- Marson AL, Tarr DE, Scott AL. Macrophage migration inhibitory factor (mif) transcription is significantly elevated in Caenorhabditis elegans dauer larvae. Gene. 2001;278:53–62. doi: 10.1016/s0378-1119(01)00706-5. [DOI] [PubMed] [Google Scholar]
- McDevitt MA, Xie J, Shanmugasundaram G, Griffith J, Liu A, McDonald C, Thuma P, Gordeuk VR, Metz CN, Mitchell R, Keefer J, David J, Leng L, Bucala R. A critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia. J Exp Med. 2006;203:1185–1196. doi: 10.1084/jem.20052398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska KB, Fetterer RH, Lillehoj HS, Jenkins MC, Allen PC, Harper SB. Characterisation of macrophage migration inhibitory factor from Eimeria species infectious to chickens. Mol Biochem Parasit. 2007;151:173–183. doi: 10.1016/j.molbiopara.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26:193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan K, Stevenson MM. Dyserythropoiesis and severe anaemia associated with malaria correlate with deficient interleukin-12 production. Brit J haematol. 1998;103:942–949. doi: 10.1046/j.1365-2141.1998.01126.x. [DOI] [PubMed] [Google Scholar]
- Pastrana DV, Raghavan N, FitzGerald P, Eisinger SW, Metz C, Bucala R, Schleimer RP, Bickel C, Scott AL. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect Immun. 1998;66:5955–5963. doi: 10.1128/iai.66.12.5955-5963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SN, Serghides L, Smith TG, Febbraio M, Silverstein RL, Kurtz TW, Pravenec M, Kain KC. CD36 mediates the phagocytosis of Plasmodium falciparum-infected erythrocytes by rodent macrophages. J Infect Dis. 2004;189:204–213. doi: 10.1086/380764. [DOI] [PubMed] [Google Scholar]
- Santos L, Hall P, Metz C, Bucala R, Morand EF. Role of macrophage migration inhibitory factor (MIF) in murine antigen-induced arthritis: interaction with glucocorticoids. Clin Exp Immunol. 2001;123:309–314. doi: 10.1046/j.1365-2249.2001.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer E, Alessio M, Ulliers D, Arese P. Phagocytosis of the malarial pigment, hemozoin, impairs expression of major histocompatibility complex class II antigen, CD54, and CD11c in human monocytes. Infect Immun. 1998;66:1601–1606. doi: 10.1128/iai.66.4.1601-1606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D, Han Z, Lin Y, Zhang L, Zhong X, Feng M, Guo Y, Wang H. Detection of Plasmodium falciparum derived macrophage migration inhibitory factor homologue in the sera of malaria patients. Acta Trop. 2008;106:9–15. doi: 10.1016/j.actatropica.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Shao D, Zhong X, Zhou YF, Han Z, Lin Y, Wang Z, Bu L, Zhang L, Su XD, Wang H. Structural and functional comparison of MIF ortholog from Plasmodium yoelii with MIF from its rodent host. Mol Immunol. 2010;47:726–737. doi: 10.1016/j.molimm.2009.10.037. [DOI] [PubMed] [Google Scholar]
- Skorokhod OA, Alessio M, Mordmuller B, Arese P, Schwarzer E. Hemozoin (malarial pigment) inhibits differentiation and maturation of human monocyte-derived dendritic cells: a peroxisome proliferator-activated receptor-gamma-mediated effect. J Immunol. 2004;173:4066–4074. doi: 10.4049/jimmunol.173.6.4066. [DOI] [PubMed] [Google Scholar]
- Sponaas AM, Freitas do Rosario AP, Voisine C, Mastelic B, Thompson J, Koernig S, Jarra W, Renia L, Mauduit M, Potocnik AJ, Langhorne J. Migrating monocytes recruited to the spleen play an important role in control of blood stage malaria. Blood. 2009;114:5522–5531. doi: 10.1182/blood-2009-04-217489. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Nishihira J, Takahashi Y, Ikeda U, Shimada K. De Novo expression of macrophage migration inhibitory factor in atherogenesis. Circ Res. 2001;88:E31. doi: 10.1161/01.res.88.4.e31. [DOI] [PubMed] [Google Scholar]
- Tan TH, Edgerton SA, Kumari R, McAlister MS, Roe SM, Nagl S, Pearl LH, Selkirk ME, Bianco AE, Totty NF, Engwerda C, Gray CA, Meyer DJ. Macrophage migration inhibitory factor of the parasitic nematode Trichinella spiralis. Biochem J. 2001;357:373–383. doi: 10.1042/0264-6021:3570373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorat S, Daly TM, Bergman LW, Burns JM., Jr Elevated levels of the Plasmodium yoelii homologue of macrophage migration inhibitory factor attenuate blood-stage malaria. Infect Immun. 78:5151–5162. doi: 10.1128/IAI.00277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemiya R, Hatta T, Liao M, Tanaka M, Zhou J, Inoue N, Fujisaki K. Haemaphysalis longicornis: molecular characterization of a homologue of the macrophage migration inhibitory factor from the partially fed ticks. Exp Parasitol. 2007;115:135–142. doi: 10.1016/j.exppara.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Urquhart AD. Putative pathophysiological interactions of cytokines and phagocytic cells in severe human falciparum malaria. Clin Infect Dis. 1994;19:117–131. doi: 10.1093/clinids/19.1.117. [DOI] [PubMed] [Google Scholar]
- Wu Z, Boonmars T, Nagano I, Nakada T, Takahashi Y. Molecular expression and characterization of a homologue of host cytokine macrophage migration inhibitory factor from Trichinella spp. J Parasitol. 2003;89:507–515. doi: 10.1645/0022-3395(2003)089[0507:MEACOA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]